Antibiofilm and Antimicrobial Potentials of Novel Synthesized Sulfur Camphor Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity
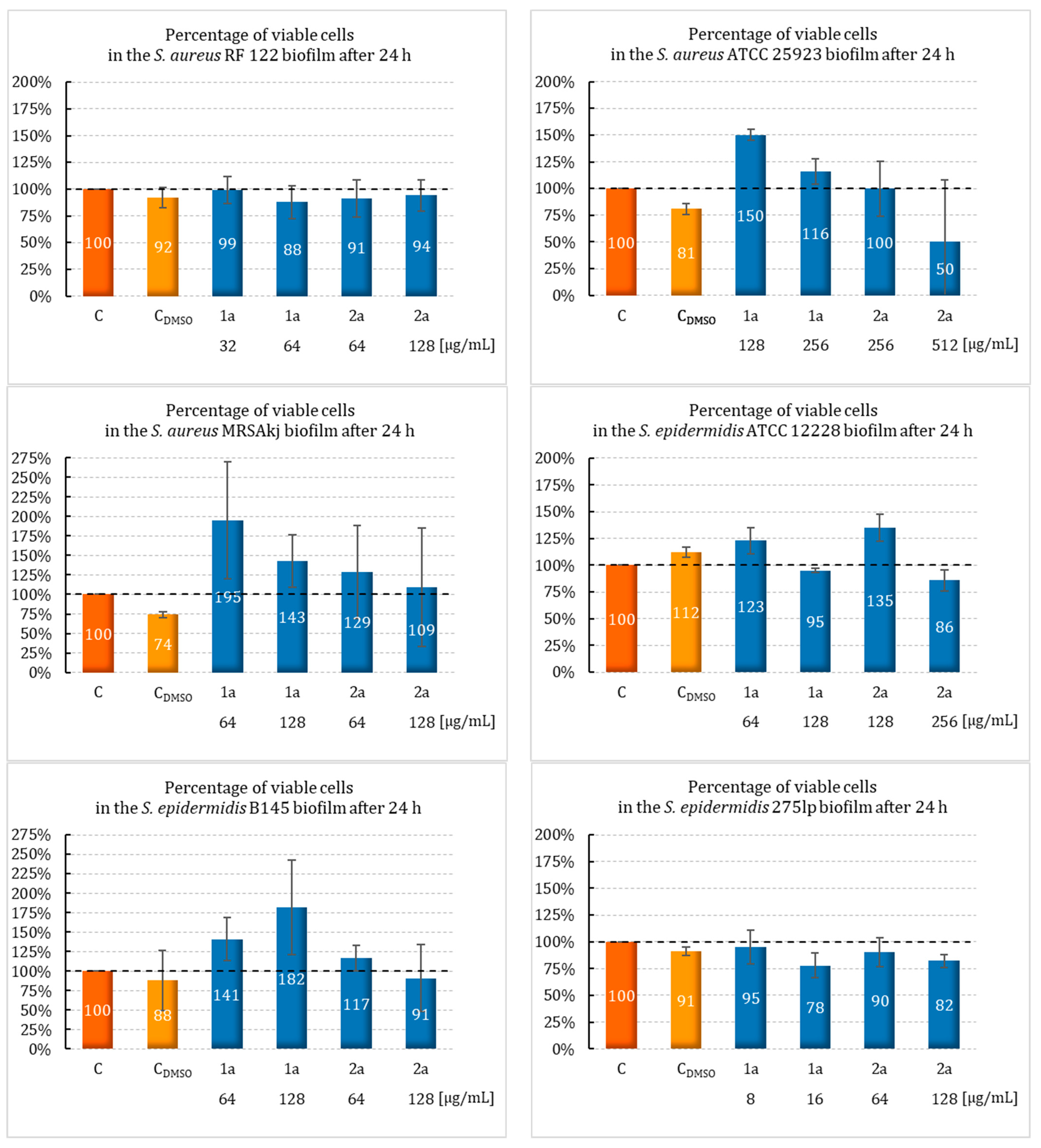
2.2. Effect of Sulfur Derivatives of Camphor on Biofilm Formation
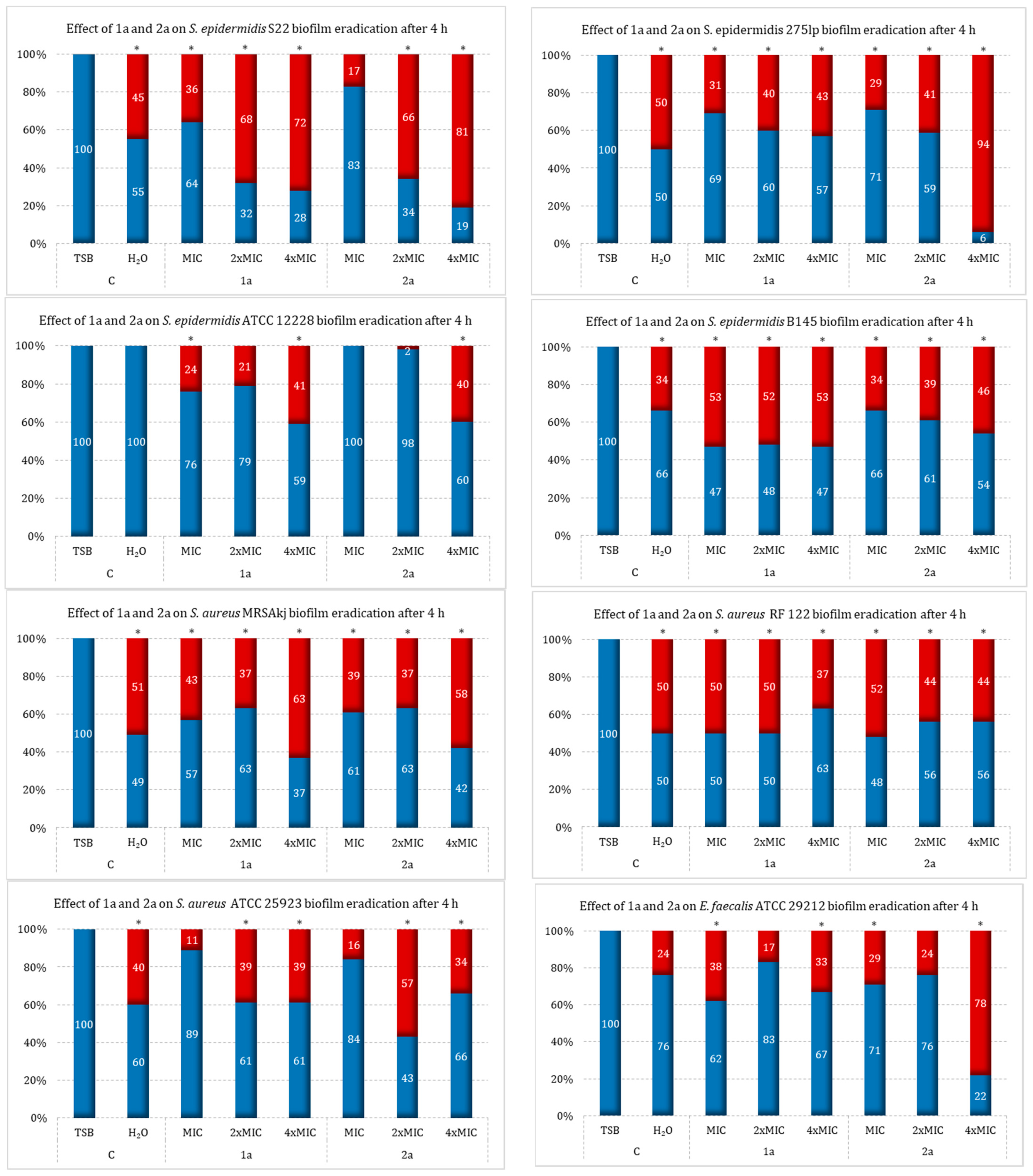
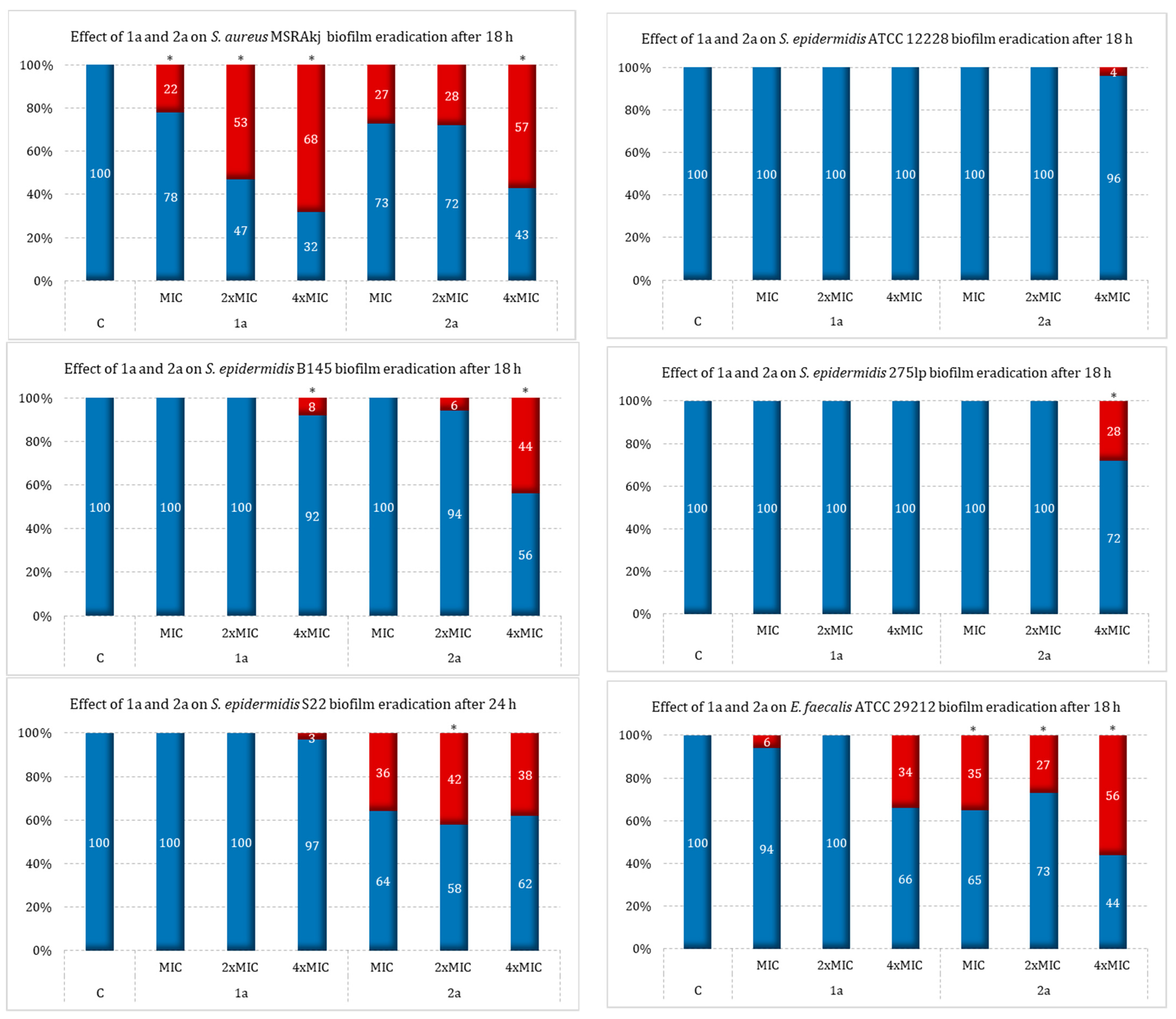
2.3. Effect of Sulfur Derivatives of Camphor on Eradication of Mature Bacterial Biofilm
2.4. Ability to Remove Biofilm under Flow Conditions
3. Materials and Methods
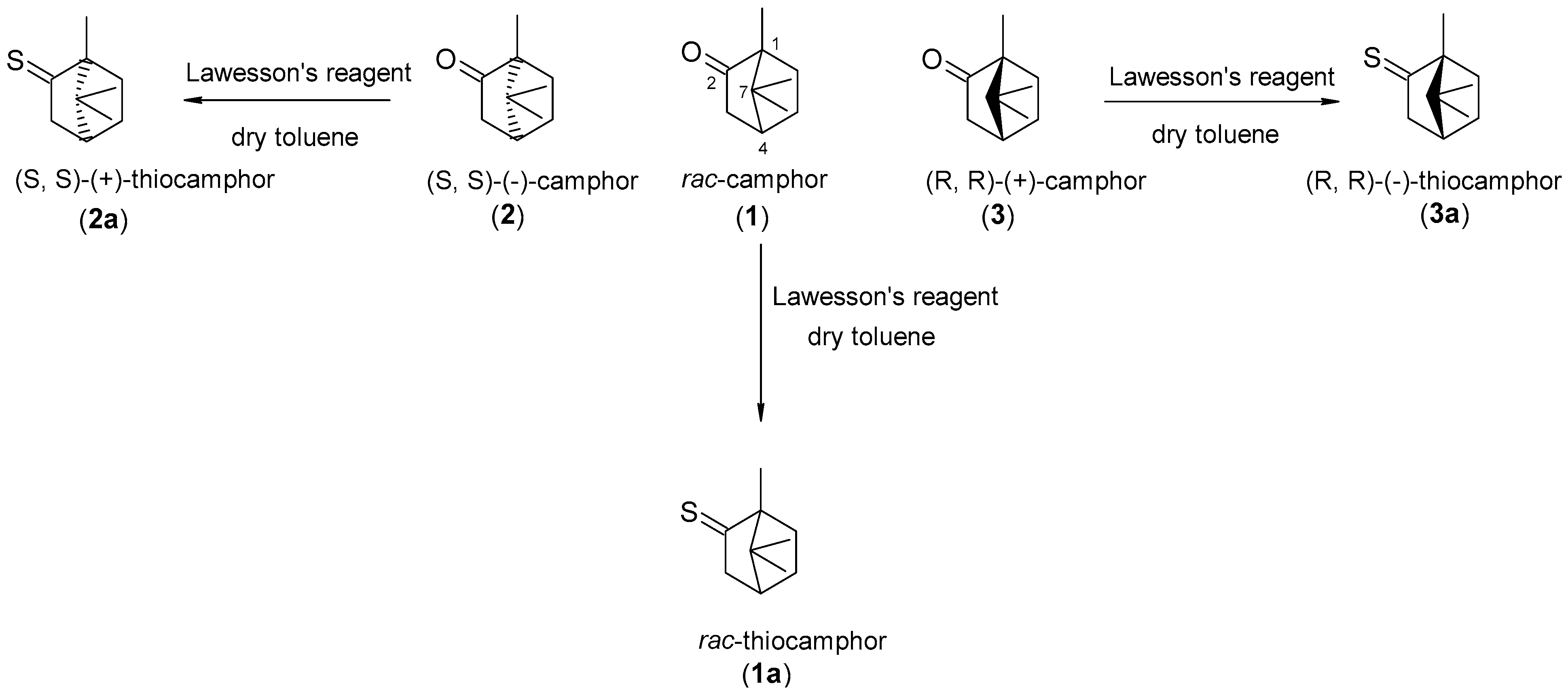
3.1. Derivation of Sulfur Derivatives of Camphor
3.2. Synthesis to Obtain Thiocamphor
3.3. Tested Bacterial Strains
3.4. Assay of the Minimum Inhibitory and Bactericidal Concentrations
3.5. Sulfur Derivatives’ Effect on Biofilm Formation
3.6. Effect of Sulfur Derivatives of Camphor on Bacterial Biofilm Eradication
3.7. Influence of Flow Conditions on Biofilm Eradication
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, H.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Chieosilapatham, P.; Kiatsurayanon, C.; Song, P.; Okumura, K.; Ogawa, H.; et al. Exogenous Factors in the Pathogenesis of Atopic Dermatitis: Irritants and Cutaneous Infections. Clin. Exp. Allergy 2021, 51, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128029459. [Google Scholar]
- Bouza, E.; De Rosa, F.G.; Guzek, A.; Dirschka, T.; Pellacani, G. Multidisciplinary Panel Opinion on the Management of Bacterial Skin Infections. JEADV Clin. Pract. 2022, 1, 165–175. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 3071. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-kaszewska, J.; Kraszewska, Z.; Wiktorczyk-kapischke, N.; Grudlewska-buda, K.; Kwiecińska-piróg, J.; Wałecka-zacharska, E.; Radtke, L.; Gospodarek-komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Nowicka, D.; Chilicka, K.; Dzieńdziora-Urbińska, I. Host-Microbe Interaction on the Skin and Its Role in the Pathogenesis and Treatment of Atopic Dermatitis. Pathogens 2022, 11, 71. [Google Scholar] [CrossRef]
- Ki, V.; Rotstein, C. Bacterial Skin and Soft Tissue Infections in Adults: A Review of Their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef]
- Golan, Y. Current Treatment Options for Acute Skin and Skin-Structure Infections. Clin. Infect. Dis. 2019, 68, S206–S212. [Google Scholar] [CrossRef]
- Dryden, M.S. Complicated Skin and Soft Tissue Infection. J. Antimicrob. Chemother. 2010, 65, 35–44. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Holder, I.P. Aeruginosa Burn Infections: Pathogenesis and Treatment. In Pseudomonas aeruginosa as an Opportunistic Pathogen. Infectious Agents and Pathogenesis; Campa, M., Bendinelli, M., Friedman, H., Eds.; Springer: Boston, MA, USA, 1993; ISBN 978-1-4615-3036-7. [Google Scholar]
- Hofstee, M.I.; Muthukrishnan, G.; Atkins, G.J.; Riool, M.; Thompson, K.; Morgenstern, M.; Stoddart, M.J.; Richards, R.G.; Zaat, S.A.J.; Moriarty, T.F. Current Concepts of Osteomyelitis: From Pathologic Mechanisms to Advanced Research Methods. Am. J. Pathol. 2020, 190, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Leng, X.; He, M.; Wang, J.; Cheng, S.; Wu, H. Roles of Reactive Oxygen Species in Cell Signaling Pathways and Immune Responses to Viral Infections. Arch. Virol. 2017, 162, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zegadło, K.; Gieroń, M.; Żarnowiec, P.; Durlik-Popińska, K.; Kręcisz, B.; Kaca, W.; Czerwonka, G. Bacterial Motility and Its Role in Skin and Wound Infections. Int. J. Mol. Sci. 2023, 24, 1707. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. The Anti-Inflammatory Potential of Cinnamomum camphora (L.) J.Presl Essential Oil in Vitro and in Vivo. J. Ethnopharmacol. 2021, 267, 113516. [Google Scholar] [CrossRef]
- Sounouvou, H.T.; Toukourou, H.; Catteau, L.; Toukourou, F.; Evrard, B.; Van Bambeke, F.; Gbaguidi, F.; Quetin-Leclercq, J. Antimicrobial Potentials of Essential Oils Extracted from West African Aromatic Plants on Common Skin Infections. Sci. African 2021, 11, e00706. [Google Scholar] [CrossRef]
- Orchard, A.; Van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef]
- Costa, J.; Sousa, S.; Galvão, A.; Mata, J.; Leitão, J.; Carvalho, M. Key Parameters on the Antibacterial Activity of Silver Camphor Complexes. Antibiotics 2021, 10, 135. [Google Scholar] [CrossRef]
- Santos, T.B.; Vieira, A.A.; Paula, L.O.; Santos, E.D.; Radi, P.A.; Khouri, S.; Maciel, H.S.; Pessoa, R.S.; Vieira, L. Flexible Camphor Diamond-like Carbon Coating on Polyurethane to Prevent Candida Albicans Biofilm Growth. J. Mech. Behav. Biomed. Mater. 2017, 68, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Grabarczyk, M.; Topola, E.; Kozłowska, J.; Mączka, W.; Wińska, K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals 2024, 17, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Ware, R.A.; Ghude, D.S.; Akolkar, M.P.; Bhise, M.G. The Therapeutic and Medicinal Use of Camphor in Ordinary Life. Int. J. Res. Eng. Sci. Manag. 2020, 3, 998–999. [Google Scholar]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, P.; Soldani, G. Camphor: Benefits and Risks of a Widely Used Natural Product Pharmacology Pharmacodynamics. Acta Biol. Szeged. 2009, 53, 77–82. [Google Scholar]
- Malabadi, R.B.; Kolkar, K.P.; Meti, N.T.; Chalannavar, R.K. An age old botanical weapon for herbal therapy: Camphor tree, Cinnamomum camphora. Int. J. Innov. Sci. Res. Rev. 2021, 3, 1518–1523. [Google Scholar]
- Shimada, K.; Kodaki, K.; Aoyagi, S.; Takikawa, Y.; Kabuto, C. Formation of Kinetically Stabilized Dithiiranes by Treating Thione S-Oxides Bearing a Bulky Substituent with Lawesson’s Reagent. Chem. Lett. 1999, 28, 695–696. [Google Scholar] [CrossRef]
- Raghav, N.; Kaur, R. Synthesis and Evaluation of Some Semicarbazone- and Thiosemicarbazone-Based Cathepsin B Inhibitors. Med. Chem. Res. 2014, 23, 4669–4679. [Google Scholar] [CrossRef]
- Olanrewaju, A.A.; Oke, D.G.; Adekunle, D.O.; Adeleke, O.A.; Akinola, O.T.; Emmanuel, A.V.; Oyeneyin, O.E. Synthesis, in-Vitro and in-Silico Antibacterial and Computational Studies of Selected Thiosemicarbazone-Benzaldehyde Derivatives as Potential Antibiotics. SN Appl. Sci. 2023, 5, 21. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Babijczuk, K.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Cofta, G.; Mrówczyńska, L. Indole Derivatives Bearing Imidazole, Benzothiazole-2-Thione or Benzoxazole-2-Thione Moieties—Synthesis, Structure and Evaluation of Their Cytoprotective, Antioxidant, Antibacterial and Fungicidal Activities. Molecules 2023, 28, 708. [Google Scholar] [CrossRef] [PubMed]
- Marinova, P.; Tamahkyarova, K. Synthesis and Biological Activities of Some Metal Complexes of Peptides: A Review. BioTech 2024, 13, 186–213. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Piccioni, M.; De Marco, S.; Pagiotti, R.; Nascimento, V.; Braga, A.L.; Santi, C.; Pietrella, D. Diphenyl Diselenide Derivatives Inhibit Microbial Biofilm Formation Involved in Wound Infection. BMC Microbiol. 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Mikláš, R.; Miklášová, N.; Bukovský, M.; Horváth, B.; Kubincová, J.; Devínsky, F. Synthesis, Surface and Antimicrobial Properties of Some Quaternary Ammonium Homochiral Camphor Sulfonamides. Eur. J. Pharm. Sci. 2014, 65, 29–37. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of Camphor Tree Essential Oil by Steam Distillation and Supercritical CO2 Extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef]
- Laczkowski, K.; Misiura, K.; Biernasiuk, A.; Malm, A.; Siwek, A.; Plech, T.; Ciok-Pater, E.; Skowron, K.; Gospodarek, E. Synthesis, In Vitro Biological Screening and Molecular Docking Studies of Novel Camphor-Based Thiazoles. Med. Chem. 2015, 10, 600–608. [Google Scholar] [CrossRef]
- Mohanty, P.; Behera, S.; Behura, R.; Shubhadarshinee, L.; Mohapatra, P.; Barick, A.K.; Jali, B.R. Antibacterial Activity of Thiazole and Its Derivatives: A Review. Biointerface Res. Appl. Chem. 2022, 12, 2171–2195. [Google Scholar] [CrossRef]
- Naghiyev, F.N.; Asgarova, A.R.; Maharramov, A.M.; Rahimova, A.G.; Akhundova, M.A.; Mamedov, I.G. Synthesis and Antimicrobial Properties of Some Thiazole and Pyridine Derivatives. New Mater. Compd. Appl. 2020, 4, 5–9. [Google Scholar]
- Carvalho, M.F.N.N.; Leite, S.; Costa, J.P.; Galvão, A.M.; Leitão, J.H. Ag(I) Camphor Complexes: Antimicrobial Activity by Design. J. Inorg. Biochem. 2019, 199, 110791. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Galvão, A.M.; Guerreiro, S.I.; Leitão, J.H.; Suarez, A.C.; Carvalho, M.F.N.N. Antibacterial Activity of Silver Camphorimine Coordination Polymers. Dalt. Trans. 2016, 45, 7114–7123. [Google Scholar] [CrossRef]
- Peraman, R.; Tiwari, A.K.; Geetha Vani, M.; Hemanth, J.; Geetha Sree, Y.; Karthik, K.; Ashby, C.R.; Padmanabha Reddy, Y.; Pemmidi, R.V. New Camphor Hybrids: Lipophilic Enhancement Improves Antimicrobial Efficacy against Drug-Resistant Pathogenic Microbes and Intestinal Worms. Med. Chem. Res. 2018, 27, 1728–1739. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and Pharmacology of Synthetic Organoselenium Compounds: An Update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A Retention Index Calculator Simplifies Identification of Plant Volatile Organic Compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Gaur, P.; Hada, V.; Rath, R.S.; Mohanty, A.; Singh, P.; Rukadikar, A. Interpretation of antimicrobial susceptibility testing using European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) breakpoints: Analysis of agreement. Cureus 2023, 15, e36977. [Google Scholar] [CrossRef]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of Tetrazolium Salt Assay for Pseudomonas aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.J.; Kang, H.Y.; Seol, S.Y.; Cho, D.T.; Kim, J. A Simple Colorimetric Method for Testing Antimicrobial Susceptibility of Biofilmed Bacteria. J. Microbiol. 2010, 48, 709–711. [Google Scholar] [CrossRef]


| Bacterial Strain | MIC50/MIC90/MBC Values for Camphor Sulfur Derivatives (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1a | 2a | 3a | |
| S. aureus RF 122 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 64/128/128 | 128/>512/>512 | 512/>512/>512 |
| S. aureus ATCC 25923 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 256/>512/>512 | 512/512/>512 | >512/>512/>512 |
| S. aureus MRSAkj | >512/>512/>512 | >512/>512>512 | >512/>512>512 | 128/256/>512 | 128/>512>512 | 512/>512/>512 |
| S. epidermidis ATCC 12228 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 256/512/>512 | 512/>512/>512 |
| S. epidermidis 275lp | 128/128/256 | >512/>512/>512 | >512/>512/>512 | 16/256/256 | 128/256/>512 | 512/512/>512 |
| S. epidermidis B145 | >512/>512/>512 | 512/>512/>512 | >512/>512/>512 | 128/>512/>512 | 128/256/>512 | 512/>512/>512 |
| S. epidermidis S22 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 64/64/128 | 256/512/>512 | 512/>512/>512 |
| E. faecalis ATCC 29212 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 64/128/128 | >512/>512/>512 | >512/>512/>512 |
| E. coli PCM 2427 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 512/>512/>512 | >512/>512/>512 |
| E. coli ATCC 35218 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| E. coli 1471 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| E. coli PA170 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| E. coli 27/2021 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| E. coli 105/2021 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| A. baumannii ATCC 19606 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 512/>512/>512 | >512/>512/>512 | >512/>512/>512 |
| P. aeruginosa ATCC 27853 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | >512/>512/>512 | 512/>512/>512 | >512/>512/>512 |
| Bacterial Strain | Antimicrobial Resistance Profile |
|---|---|
| S. aureus MRSAkj | FOXR, ER, CLDR, CIPR, GMS, TETS, SXTS, LZDS |
| S. epidermidis 275lp | FOXS, ES, CLDS, GMS, TETS, CIPS, SXTS, LZDS |
| S. epidermidis B145 | FOXR, ER, CLDR, GMR, TETR, CIPWZE, SXTS, LZDS, VAS, TECS |
| S. epidermidis S22 | FOXR, ER, CLDR, SXTR, CIPS, VANS, GMS, NETS, TECS, TETS |
| E. coli 1471 | CIPR, GMR, TOBR, CTXR, CAZR, CXMR, AMCR, ATMR, FEPR, TZPR, MEMR, AKNR, SAMR, SXTS, IMPS, ETPS, DORS |
| E. coli PA 170 | CXMR, FEPR, CTXR, CAZR, GMR, AMXR, SAMR, AMCR, TIMR, ATMR, TOBR, AKNR, CIPR, SMXR, SXTR, IMPS, MEMS |
| E. coli 27/2021 | CXMR, FEPS, CAZS, GMS, AMXS, SAMS, AMCS, TIMS, ATMS, TOBS, AKNS, CIPS, SXTS, IMPS, MEMS |
| E. coli 105/2021 | CXMR, FEPS, CAZS, GMS, AMXS, SAMS, AMCS, TIMS, ATMS, TOBS, AKNS, CIPS, SXTS, IMPS, MEMS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Viscardi, S.; Pacyga, K.; Kupczyński, R.; Mączka, W.; Grabarczyk, M.; Pacyga, P.; Topola, E.; Ostrówka, M.; Bania, J.; et al. Antibiofilm and Antimicrobial Potentials of Novel Synthesized Sulfur Camphor Derivatives. Int. J. Mol. Sci. 2024, 25, 10895. https://doi.org/10.3390/ijms252010895
Duda-Madej A, Viscardi S, Pacyga K, Kupczyński R, Mączka W, Grabarczyk M, Pacyga P, Topola E, Ostrówka M, Bania J, et al. Antibiofilm and Antimicrobial Potentials of Novel Synthesized Sulfur Camphor Derivatives. International Journal of Molecular Sciences. 2024; 25(20):10895. https://doi.org/10.3390/ijms252010895
Chicago/Turabian StyleDuda-Madej, Anna, Szymon Viscardi, Katarzyna Pacyga, Robert Kupczyński, Wanda Mączka, Małgorzata Grabarczyk, Paweł Pacyga, Ewa Topola, Michał Ostrówka, Jacek Bania, and et al. 2024. "Antibiofilm and Antimicrobial Potentials of Novel Synthesized Sulfur Camphor Derivatives" International Journal of Molecular Sciences 25, no. 20: 10895. https://doi.org/10.3390/ijms252010895
APA StyleDuda-Madej, A., Viscardi, S., Pacyga, K., Kupczyński, R., Mączka, W., Grabarczyk, M., Pacyga, P., Topola, E., Ostrówka, M., Bania, J., Szumny, A., & Wińska, K. (2024). Antibiofilm and Antimicrobial Potentials of Novel Synthesized Sulfur Camphor Derivatives. International Journal of Molecular Sciences, 25(20), 10895. https://doi.org/10.3390/ijms252010895








