Mystery of the Passerini Reaction for the Synthesis of the Antimicrobial Peptidomimetics against Nosocomial Pathogenic Bacteria
Abstract
1. Introduction
2. Results
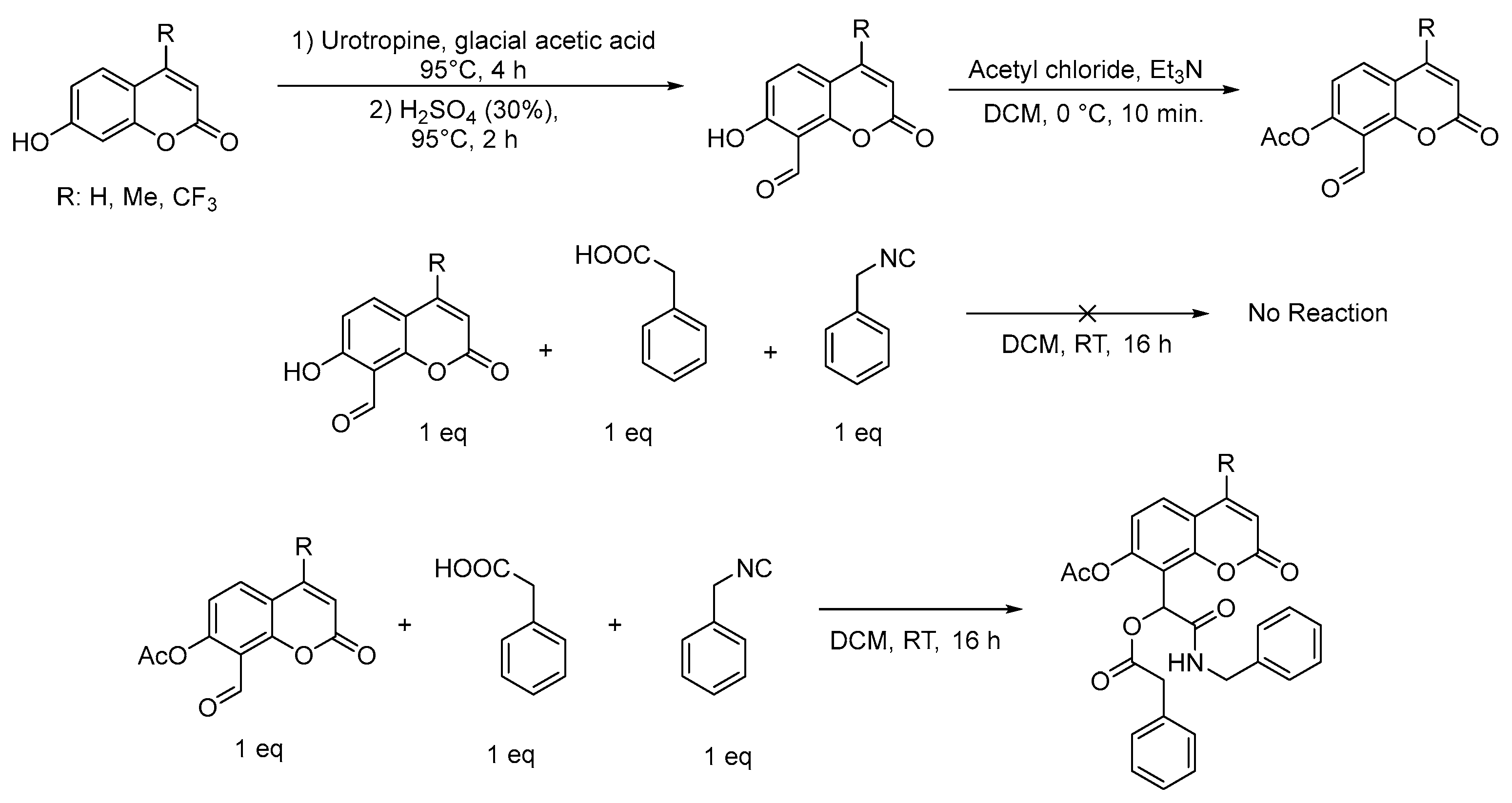
2.1. Chemistry
2.2. In Vitro Biological Studies of Synthesised Compounds
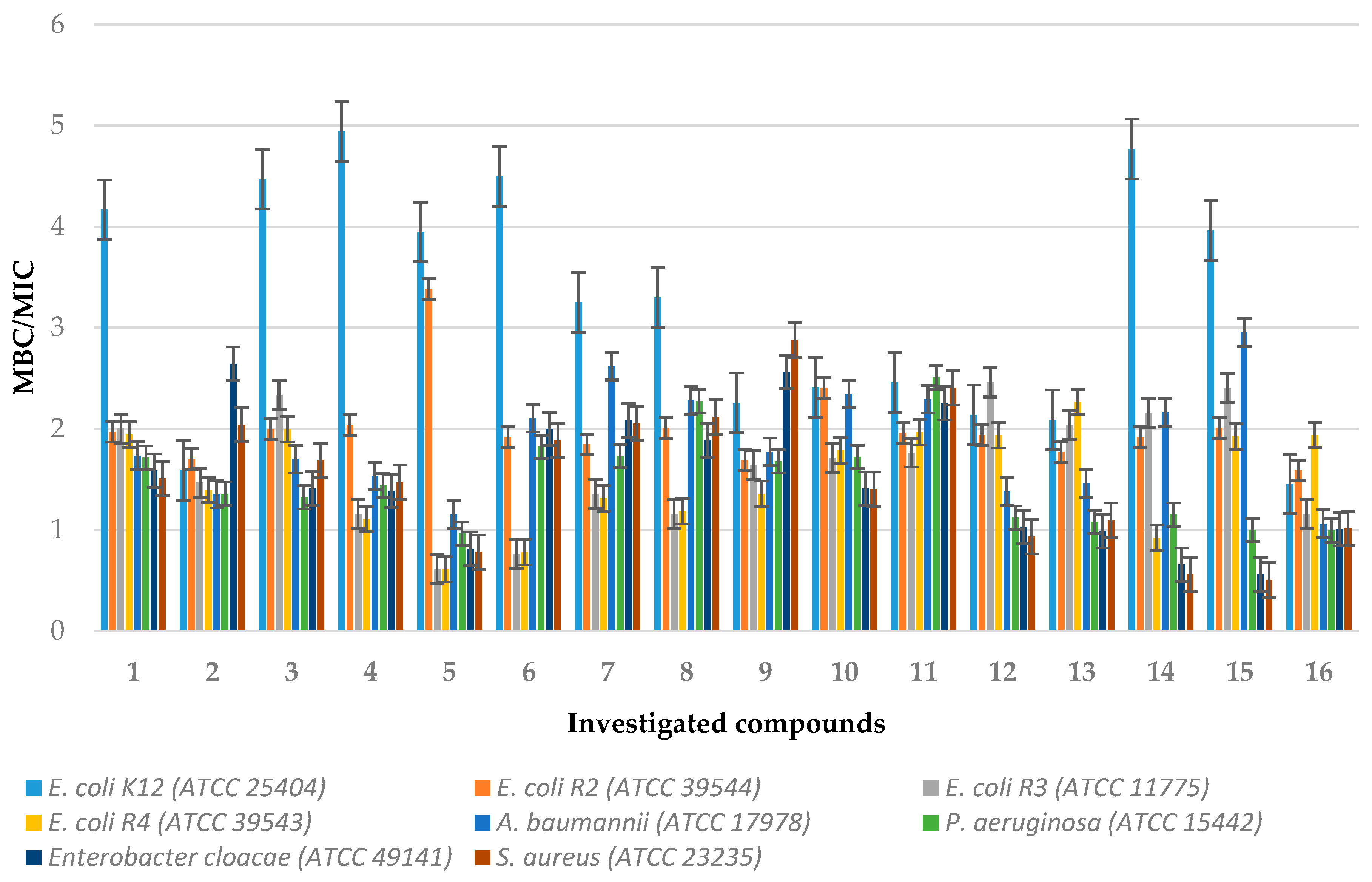
2.2.1. MIC and MBC Studies
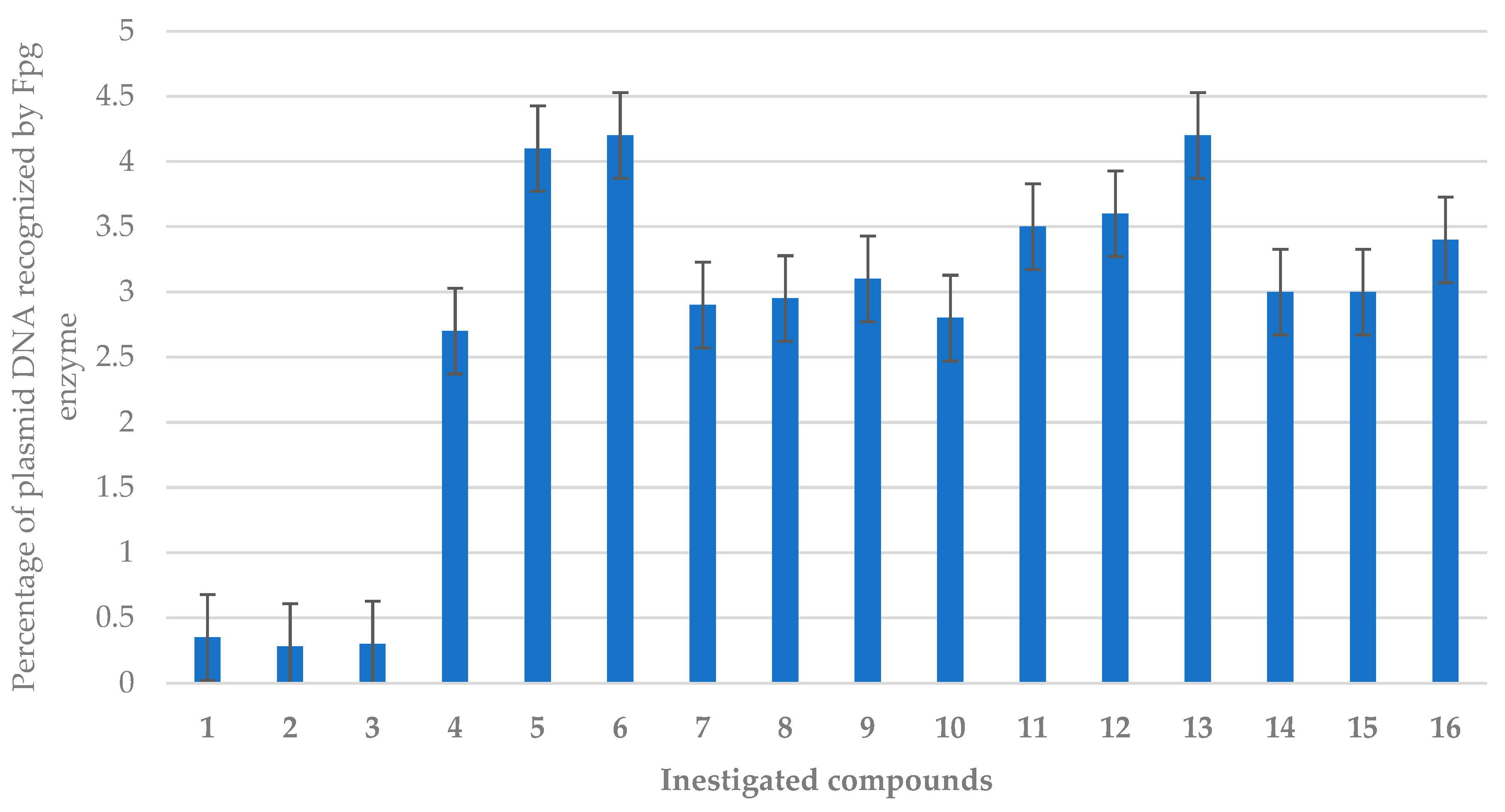
2.2.2. Oxidative DNA Damage Studies
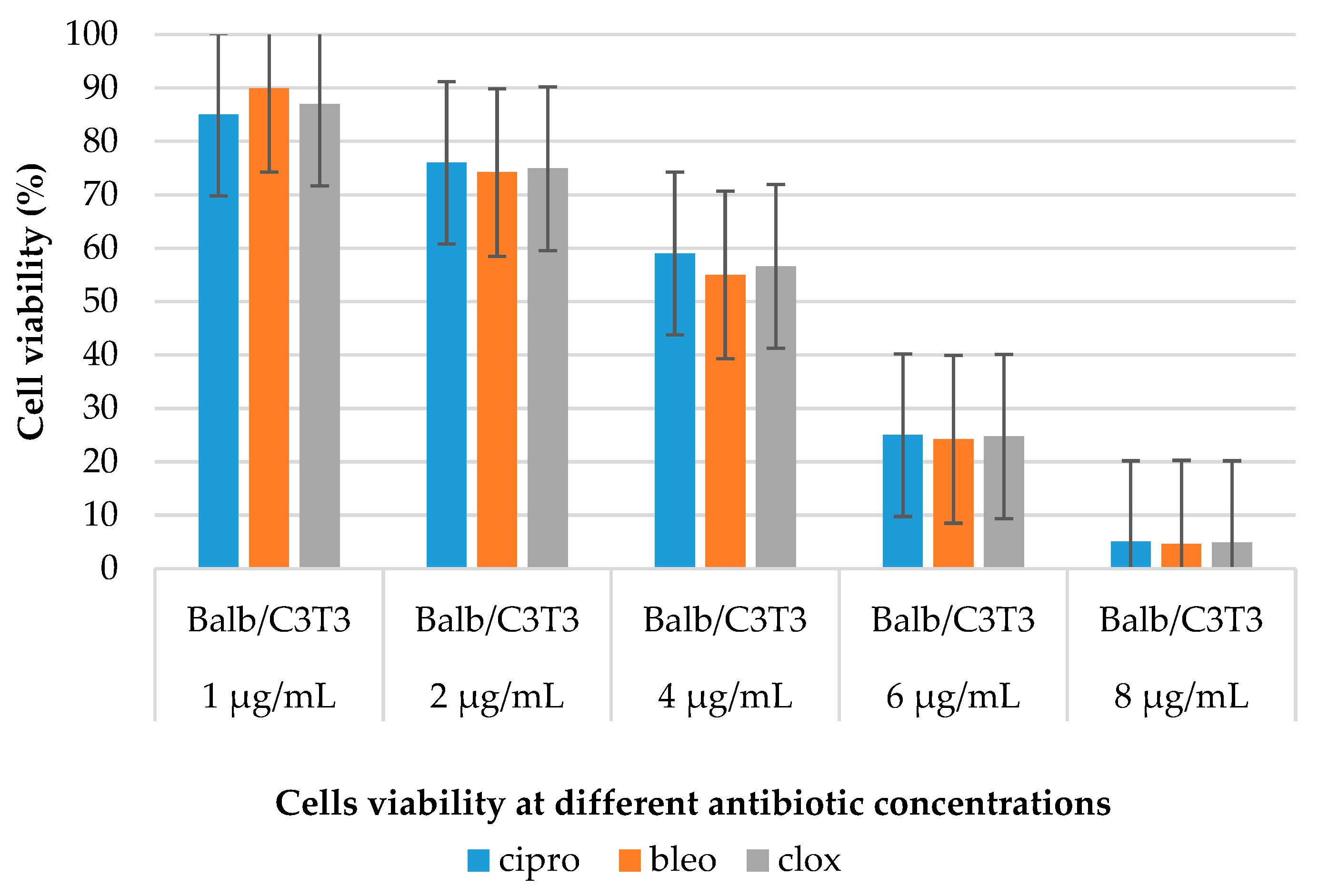
2.2.3. Cytotoxicity Studies
3. Materials and Methods
3.1. Chemicals
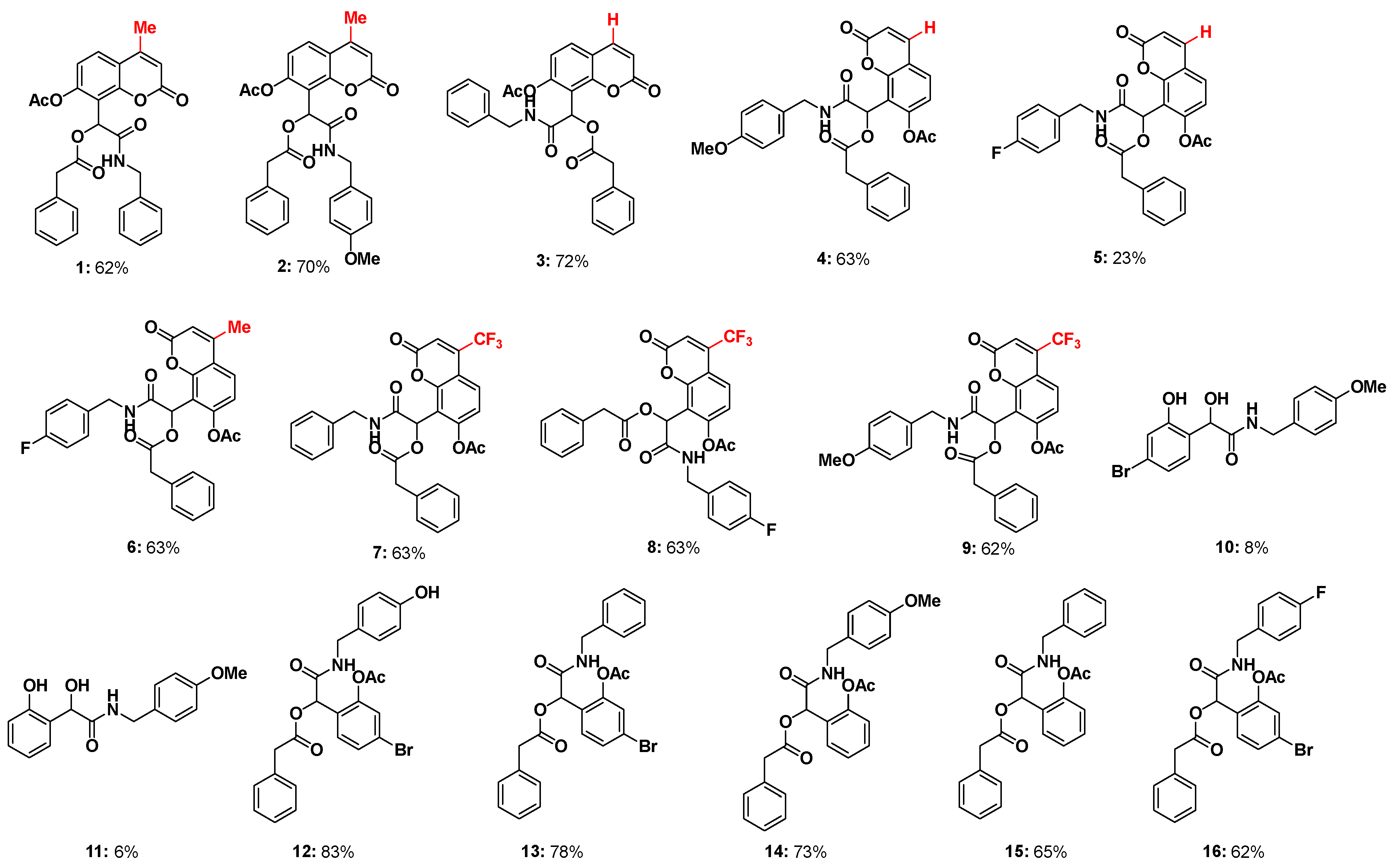
3.2. General Procedure (1) for the Synthesis of Coumarin α-Acyloxy Carboxamides 1–9
- 1-(7-Acetoxy-4-methyl-2-oxo-2H-chromen-8-yl)-2-((4-fluorobenzyl)amino)-2-oxoethyl 2-phenylacetate (1): Compound 1 was obtained according to the General method with a 62% yield (63 mg, 0.126 mmol) as an off-white solid; m.p.: 122–124 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 8.7 Hz, 1H), 7.37–7.25 (m, 5H), 7.20–7.09 (m, 6H), 6.76 (s, 1H), 6.36 (d, J = 5.4 Hz, 1H), 6.26 (d, J = 1.3 Hz, 1H), 4.38 (d, J = 5.7 Hz, 2H), 3.60 (s, 2H), 2.41 (d, J = 1.2 Hz, 3H), 2.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 174.8, 169.1, 168.8, 167.5, 159.2, 152.8, 151.98, 137.4, 133.7, 133.1, 129.3, 129.0, 128.7, 128.6, 128.5, 128.1, 127.5, 127.4, 127.1, 125.8, 119.2, 118.1, 116.9, 114.5, 66.0, 43.6, 41.2, 20.8, 18.8. HRMS calcd. for C29H25NO7Na [M+Na]+, 522.1526, found: 522.1529. Element. anal. for C29H25NO7 calc. C 69.73, H 5.04, N 2.80. found C 69.42, H 5.08, N 2.90.
- 1-(7-Acetoxy-4-methyl-2-oxo-2H-chromen-8-yl)-2-((4-methoxybenzyl)amino)-2-oxoethyl 2-phenylacetate (2): Compound 2 was obtained according to the General method with a 70% yield (75 mg, 0.142 mmol) as an off-white solid; m.p.: 66–68 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 8.6 Hz, 1H), 7.24–7.02 (m, 8H), 6.87 (d, J = 8.4 Hz, 2H), 6.74 (s, 1H), 6.33 (s, 1H), 6.26 (s, 1H), 4.32 (s, 2H), 3.83 (s, 3H), 3.60 (s, 2H), 2.41 (s, 3H), 2.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.1, 168.8, 167.3, 161.7, 159.1, 152.9, 151.9, 151.8, 133.1, 129.6, 129.5, 129.4, 129.0, 129.0, 128.8, 128.7, 128.5, 127.3, 125.7, 121.3, 119.2, 118.1, 114.6, 114.0, 66.0, 55.3, 43.1, 41.2, 20.8, 18.8. HRMS calcd. for C30H27NO8Na [M+Na]+, 552.1637, found: 552.1634. Element. anal. for C30H27NO8 calc. C 68.05, H 5.14, N 2.65. found C 67.91, H 5.24, N 2.85.
- 1-(7-Acetoxy-2-oxo-2H-chromen-8-yl)-2-(benzylamino)-2-oxoethyl 2-phenylacetate (3): Compound 3 was obtained according to the General method with a 72% yield (75 mg, 0.154 mmol) as an off-white solid; m.p.: 64–66 °C. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 9.6 Hz, 1H), 7.48 (d, J = 8.5 Hz, 1H), 7.39–7.26 (m, 5H), 7.21–7.12 (m, 5H), 7.08 (d, J = 8.5 Hz, 1H), 6.75 (s, 1H), 6.38 (d, J = 9.6 Hz, 2H), 4.43–4.34 (m, 2H), 3.61 (s, 2H), 2.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 173.2, 169.0, 168.7, 167.3, 153.4, 152.1, 142.8, 137.4, 133.0, 129.0, 129.0, 128.8, 128.6, 128.1, 127.5, 127.4, 119.6, 116.9, 116.2, 100.2, 65.8, 43.6, 41.2, 20.8. HRMS calcd. for C28H23NO7Na [M+Na]+, 508.1371, found: 508.1372. Element. anal. for C28H23NO7 calc. C 69.27, H 4.78, N 2.89. found C 69.10, H 4.79, N 2.92.
- 1-(7-Acetoxy-2-oxo-2H-chromen-8-yl)-2-((4-methoxybenzyl)amino)-2-oxoethyl 2-phenylacetate (4): Compound 4 was obtained according to the General method with a 63% yield (70 mg, 0.135 mmol) as an off-white solid; m.p.: 57–59 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 9.6 Hz, 1H), 7.47 (d, J = 8.5 Hz, 1H), 7.24–7.10 (m, 7H), 7.07 (d, J = 8.5 Hz, 1H), 6.88 (d, J = 8.6 Hz, 2H), 6.73 (s, 1H), 6.38 (d, J = 9.6 Hz, 1H), 6.32 (s, 1H), 4.32 (qd, J = 14.4, 5.5 Hz, 2H), 3.83 (s, 3H), 3.60 (s, 2H), 2.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.0, 168.7, 167.2, 159.1, 159.0, 153.4, 152.1, 142.8, 133.5, 133.0, 129.5, 129.4, 129.0, 128.9, 128.8, 128.6, 128.6, 127.4, 119.6, 117.0, 116.9, 116.1, 114.0, 109.7, 65.8, 55.3, 43.1, 41.2, 20.8. HRMS calcd. for C29H25NO8Na [M+Na]+, 538.1480, found: 538.1478. Element. anal. for C29H25NO8 calc. C 67.57, H 4.89, N 2.72. found C 67.78, H 5.10, N 2.96.
- 1-(7-Acetoxy-2-oxo-2H-chromen-8-yl)-2-((4-fluorobenzyl)amino)-2-oxoethyl 2-phenylacetate (5): Compound 5 was obtained according to the General method with a 23% yield (50 mg, 0.099 mmol) as a pale yellow solid; m.p.: 61–63 °C. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 9.6 Hz, 1H), 7.48 (d, J = 8.5 Hz, 1H), 7.23–7.14 (m, 7H), 7.08 (d, J = 8.5 Hz, 1H), 7.04–7.00 (m, 2H), 6.73 (s, 1H), 6.38 (d, J = 9.6 Hz, 1H), 6.34 (d, J = 6.0 Hz, 1H), 4.40–4.25 (m, 2H), 3.62 (s, 2H), 2.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.0, 168.7, 167.4, 163.5, 161.0, 159.0, 153.3, 152.1, 142.9, 133.2, 133.2, 133.1, 129.8, 129.7, 129.0, 129.0, 128.8, 127.4, 119.6, 116.9, 116.8, 116.1, 115.5, 115.3, 65.7, 42.9, 41.2, 20.8. HRMS calcd. for C28H22FNO7Na [M+Na]+, 526.1280, found: 526.1278. Element. anal. for C28H22FNO7 calc. C 66.80, H 4.40, N 2.78. found C 66.82, H 4.33, N 3.05.
- 1-(7-Acetoxy-4-methyl-2-oxo-2H-chromen-8-yl)-2-((4-fluorobenzyl)amino)-2-oxoethyl 2-phenylacetate (6): Compound 6 was obtained according to the General method with a 63% yield (66 mg, 0.127 mmol) as an off-white solid; m.p.: 56–59 °C. 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.7 Hz, 1H), 7.25–7.08 (m, 8H), 7.01 (t, J = 8.6 Hz, 2H), 6.74 (s, 1H), 6.35 (s, 1H), 6.26 (s, 1H), 4.33 (qd, J = 14.7, 5.7 Hz, 2H), 3.61 (s, 2H), 2.41 (s, 3H), 2.29 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.1, 168.7, 167.5, 163.5, 161.0, 159.1, 152.8, 152.0, 151.9, 133.2, 133.2, 133.1, 129.8, 129.7, 129.0, 128.8, 127.4, 125.8, 119.2, 118.1, 116.8, 115.5, 115.3, 114.5, 65.9, 42.9, 41.2, 20.8, 18.8. HRMS calcd. for C29H24FNO7Na [M+Na]+, 540.1437, found: 540.1434. Element. anal. for C29H24FNO7 calc. C 67.31, H 4.67, N 2.71. found C 67.29, H 4.67, N 2.55.
- 1-(7-Acetoxy-2-oxo-4-(trifluoromethyl)-2H-chromen-8-yl)-2-(benzylamino)-2-oxoethyl 2-phenylacetate (7): Compound 7 was obtained according to the General method with a 63% yield (66 mg, 0.127 mmol) as an off-white solid; m.p.: 54–57 °C. 1H NMR (400 MHz, CDCl3) δ 7.74 (dd, J = 8.9, 1.7 Hz, 1H), 7.40–7.28 (m, 3H), 7.26–7.07 (m, 8H), 6.76 (d, J = 3.0 Hz, 2H), 6.35 (t, J = 5.6 Hz, 1H), 4.38 (qd, J = 14.6, 5.7 Hz, 2H), 3.61 (d, J = 1.2 Hz, 2H), 2.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.0, 168.4, 167.1, 157.2, 153.7, 152.9, 137.3, 132.9, 128.9, 128.9, 128.8, 128.8, 128.7, 128.7, 128.7, 128.1, 127.6, 127.5, 126.6, 126.6, 120.2, 117.8, 115.4, 115.3, 111.6, 65.7, 43.7, 41.2, 20.8. HRMS calcd. for C29H22F3NO7Na [M+Na]+, 576.1241, found: 576.1246. Element. anal. for C29H22F3NO7 calc. C 62.93, H 4.01, N 2.53. found C 62.81, H 4.12, N 2.62.
- 1-(7-Acetoxy-2-oxo-4-(trifluoromethyl)-2H-chromen-8-yl)-2-((4-fluorobenzyl)amino)-2-oxoethyl 2-phenylacetate (8): Compound 8 was obtained according to the General method with a 63% yield (66 mg, 0.127 mmol) as a pale yellow solid; m.p.: 51–55 °C. 1H NMR (400 MHz, CDCl3) δ 7.74 (dd, J = 8.9, 1.6 Hz, 1H), 7.24–7.12 (m, 8H), 7.03 (t, J = 8.7 Hz, 2H), 6.76 (s, 1H), 6.74 (s, 1H), 6.30 (d, J = 5.7 Hz, 1H), 4.32 (ddd, J = 40.4, 14.7, 5.8 Hz, 2H), 3.61 (s, 2H), 2.30 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.0, 168.4, 167.2, 163.5, 157.2, 153.7, 153.0, 133.1, 132.9, 129.8, 129.7, 129.2, 129.0, 128.8, 127.5, 126.6, 126.6, 122.6, 120.2, 117.6, 115.6, 115.4, 115.3, 111.6, 65.6, 42.9, 41.2, 20.8. HRMS calcd. for C29H21F4NO7Na [M+Na]+, 594.1153, found: 594.1152. Element. anal. for C29H21F4NO7 calc. C 60.95, H 3.70, N 2.45. found C 60.79, H 3.83, N 2.63.
- 1-(7-Acetoxy-2-oxo-4-(trifluoromethyl)-2H-chromen-8-yl)-2-((4-methoxybenzyl)amino)-2-oxoethyl 2-phenylacetate (9): Compound 9 was obtained according to the General method with a 62% yield (60 mg, 0.107 mmol) as a pale yellow solid; m.p.: 54–58 °C. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.4 Hz, 1H), 7.22–7.09 (m, 8H), 6.88 (d, J = 8.7 Hz, 2H), 6.75 (d, J = 9.3 Hz, 2H), 6.30 (s, 1H), 4.31 (qd, J = 14.5, 5.7 Hz, 2H), 3.83 (s, 3H), 3.60 (s, 2H), 2.27 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.0, 168.4, 167.0, 159.2, 157.3, 153.7, 152.9, 141.3, 132.9, 129.5, 129.4, 129.3, 129.0, 128.8, 127.5, 126.6, 126.6, 126.6, 126.5, 120.2, 117.8, 115.4, 115.3, 114.0, 111.6, 65.7, 55.3, 43.1, 41.2, 20.8. HRMS calcd. for C30H24F3NO8Na [M+Na]+, 606.1357, found: 606.1352. Element. anal. for C30H24F3NO8 calc. C 61.75, H 4.15, N 2.40. found C 61.52, H 4.17, N 2.54.
3.3. General Procedure (2) for the Synthesis of Coumarin α-Acyloxy Carboxamides 10–16
- 2-(4-Bromo-2-hydroxyphenyl)-2-hydroxy-N-(4-methoxybenzyl)acetamide (10): Compound 10 was obtained according to the General method with an 8% yield (29 mg, 0.079 mmol) as an off-white solid; m.p.: 96–100 °C. 1H NMR (400 MHz, CDCl3) δ 12.12 (s, 1H), 8.49 (d, J = 8.8 Hz, 1H), 7.47 (t, J = 9.1 Hz, 1H), 7.21 (dd, J = 10.3, 8.6 Hz, 3H), 7.19–7.10 (m, 1H), 7.06 (dd, J = 8.8, 1.8 Hz, 1H), 6.96–6.69 (m, 3H), 4.50 (d, J = 5.9 Hz, 2H), 3.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 188.5, 163.7, 161.5, 159.4, 134.6, 133.2, 129.3, 128.4, 123.2, 121.9, 114.3, 55.3, 43.3. Element. anal. for C16H16BrNO4 calc. C 52.48, H 4.40, N 3.82. found C 52.42, H 4.47, N 4.04.
- 2-Hydroxy-2-(2-hydroxyphenyl)-N-(4-methoxybenzyl)acetamide (11): Compound 11 was obtained according to the General method with a 17% yield (20 mg, 0.069 mmol) as a pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 11.84 (s, 1H), 8.60 (dd, J = 8.2, 1.7 Hz, 1H), 7.54 (ddd, J = 8.8, 7.3, 1.7 Hz, 1H), 7.38 (s, 1H), 7.28–7.26 (m, 1H), 7.25 (d, J = 2.1 Hz, 1H), 7.00 (dd, J = 8.5, 0.8 Hz, 1H), 6.91 (ddt, J = 9.5, 5.0, 2.0 Hz, 4H), 4.51 (d, J = 5.9 Hz, 2H), 3.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 189.8, 163.7, 161.8, 159.4, 138.1, 133.7, 129.3, 128.7, 119.5, 118.6, 117.7, 114.3, 55.3, 43.2. Element. anal. for C16H17NO4 calc. C 66.89, H 5.96, N 4.88. found C 66.78, H 5.54, N 5.22.
- 1-(2-Acetoxy-4-bromophenyl)-2-((4-methoxybenzyl)amino)-2-oxoethyl 2-phenylacetate (12): Compound 12 was obtained according to the General method with an 83% yield (90 mg, 0.170 mmol) as a pale yellow solid; m.p.: 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 7.36 (dd, J = 8.3, 1.9 Hz, 1H), 7.30 (d, J = 1.9 Hz, 1H), 7.25–7.18 (m, 6H), 7.07 (d, J = 8.6 Hz, 2H), 6.88–6.81 (m, 2H), 6.22 (s, 1H), 6.02 (t, J = 5.0 Hz, 1H), 4.25 (ddd, J = 47.3, 14.5, 5.8 Hz, 2H), 3.81 (s, 3H), 3.64 (t, J = 9.3 Hz, 2H), 2.14 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.2, 168.9, 166.9, 159.2, 151.6, 149.2, 136.9, 133.2, 130.9, 129.5, 129.2, 129.1, 128.8, 127.4, 127.0, 126.6, 123.4, 114.1, 70.2, 55.3, 42.9, 41.2, 20.7. Element. anal. for C26H24BrNO6 calc. C 59.33, H 4.60, N 2.66. found C 59.32, H 4.73, N 2.88.
- 1-(2-Acetoxy-4-bromophenyl)-2-(benzylamino)-2-oxoethyl 2-phenylacetate (13): Compound 13 was obtained according to the General method with a 78% yield (72 mg, 0.160 mmol) as a white solid; m.p.: 122–124 °C. 1H NMR (400 MHz, CDCl3) δ 7.38–7.29 (m, 5H), 7.25–7.18 (m, 6H), 7.17–7.12 (m, 2H), 6.24 (s, 1H), 6.09 (s, 1H), 4.32 (ddd, J = 44.5, 14.7, 5.8 Hz, 2H), 3.67 (t, J = 9.3 Hz, 2H), 2.14 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.3, 168.9, 167.0, 149.2, 137.4, 133.2, 130.9, 129.6, 129.1, 128.8, 128.7, 127.8, 127.7, 127.49, 127.0, 126.7, 123.4, 70.2, 43.4, 41.2, 20.7. Element. anal. for C25H22BrNO5 calc. C 60.50, H 4.47, N 2.82. found C 60.70, H 4.28, N 2.86.
- 1-(2-Acetoxyphenyl)-2-((4-methoxybenzyl)amino)-2-oxoethyl 2-phenylacetate (14): Compound 14 was obtained according to the General method with a 73% yield (100 mg, 0.222 mmol) as a pale yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.41–7.34 (m, 2H), 7.22 (dd, J = 9.0, 5.2 Hz, 6H), 7.09 (dd, J = 11.9, 8.4 Hz, 3H), 6.84 (d, J = 8.6 Hz, 2H), 6.29 (s, 1H), 6.18 (t, J = 5.5 Hz, 1H), 4.26 (ddd, J = 40.3, 14.5, 5.8 Hz, 2H), 3.79 (s, 3H), 3.65 (s, 2H), 2.15 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.5, 169.4, 167.4, 159.1, 148.8, 133.4, 130.3, 129.9, 129.7, 129.2, 129.1, 128.7, 127.8, 127.3, 126.3, 123.2, 114.0, 70.7, 55.3, 42.8, 41.1, 20.8. Element. anal. for C26H25NO6 calc. C 69.79, H 5.63, N 3.13. found C 69.38, H 5.71, N 3.02.
- 1-(2-Acetoxyphenyl)-2-(benzylamino)-2-oxoethyl 2-phenylacetate (15): Compound 15 was obtained according to the General method with a 65% yield (83 mg, 0.197 mmol) as a pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.45–7.25 (m, 6H), 7.25 (d, J = 1.2 Hz, 2H), 7.20 (d, J = 8.0 Hz, 2H), 7.17–7.10 (m, 3H), 6.32 (s, 1H), 6.21 (t, J = 5.4 Hz, 1H), 4.33 (ddd, J = 36.2, 14.7, 5.9 Hz, 2H), 3.67 (s, 2H), 2.15 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.5, 169.5, 167.5, 148.8, 137.6, 133.4, 130.4, 129.9, 129.2, 128.7, 128.7, 127.8, 127.6, 127.4, 126.4, 123.2, 70.7, 43.4, 41.1, 20.8. Element. anal. for C25H23NO5 calc. C 71.93, H 5.55, N 3.36. found C 71.52, H 5.73, N 3.14.
- 1-(2-acetoxy-4-bromophenyl)-2-((4-fluorobenzyl)amino)-2-oxoethyl-2-phenylacetate (16): Compound 16 was obtained according to the General method with a 62% yield (60 mg, 0.107 mmol) as a pale yellow solid; m.p.: 122–124 °C; 1H NMR (400 MHz, CDCl3) δ 7.58 (s, 1H), 7.43–7.28 (m, 8H), 7.29–7.18 (m, 3H), 6.95 (dd, J = 9.1, 8.3 Hz, 2H), 6.25 (s, 1H), 3.75 (s, 2H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.4, 169.2, 165.0, 160.7, 158.3, 149.1, 133.4, 131.2, 129.9, 129.0, 127.6, 126.7, 126.6, 123.8, 121.4, 121.3, 115.7, 115.5, 70.0, 41.2, 20.8. Element. anal. for C25H21BrNO5 calc. C 58.38, H 4.12, N 2.72. found C 58.25, H 4.08, N 2.63.
3.4. Microorganisms and Media
3.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.6. MTT Assay
3.7. Estimation of Oxidised Damage Based on Bacterial DNA Digestion by the Fpg Protein
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DODAB | Dimethyldioctadecylammonium bromide |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| oc | open circle |
| ccc | covalently closed circle |
| lin | linear |
| BER | base excision repair |
| Fpg | DNA-formamidopyrimidine glycosylase |
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Hoban, D.J.; Harding, G.K. The postantibiotic effect: A review of in vitro and in vivo data. DICP 1991, 25, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Powderly, W.G. The post-antibiotic era is here. Science 2021, 373, 471. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.; Brenthel, A. Imagining a post-antibiotic era: A cultural analysis of crisis and antibiotic resistance. Med. Humanit. 2022, 48, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Modranka, J.; Nawrot, E.; Graczyk, J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur. J. Med. Chem. 2006, 41, 1301–1309. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Kramkowski, K.; Ostaszewski, R. Evaluation of Antibacterial Activity against Nosocomial Pathogens of an Enzymatically Derived α-Aminophosphonates Possessing Coumarin Scaffold. Int. J. Mol. Sci. 2023, 24, 14886. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Kowalczyk, P.; Brodzka, A.; Hrunyk, A.; Kramkowski, K.; Ostaszewski, R. Enzymatic Synthesis of a Novel Coumarin Aminophosphonates: Antibacterial Effects and Oxidative Stress Modulation on Selected E. coli Strains. Int. J. Mol. Sci. 2023, 24, 7609. [Google Scholar] [CrossRef] [PubMed]
- Penta, S. Chapter 2—Antimicrobial agents. In Advances in Structure and Activity Relationship of Coumarin Derivatives; Penta, S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 9–45. [Google Scholar]
- Li, B.; Pai, R.; Di, M.; Aiello, D.; Barnes, M.H.; Butler, M.M.; Tashjian, T.F.; Peet, N.P.; Bowlin, T.L.; Moir, D.T. Coumarin-Based Inhibitors of Bacillus Anthracis and Staphylococcus Aureus Replicative DNA Helicase: Chemical Optimization, Biological Evaluation, and Antibacterial Activities. J. Med. Chem. 2012, 55, 10896–10908. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Willcox, M.D. Antimicrobial resistance of ocular microbes and the role of antimicrobial peptides. Clin. Exp. Optom. 2021, 104, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Curr. Med. Chem. 2017, 24, 4303–4314. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, B.; Wang, Z. The revitalization of antimicrobial peptides in the resistance era. Pharmacol. Res. 2021, 163, 105276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Vyas, V.K.; Bhatt, S.; Ghate, M.D. Therapeutic potential of 4-substituted coumarins: A conspectus. Eur. J. Med. Chem. Rep. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides against Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Browne, K.; Kuppusamy, R.; Chen, R.; Willcox, M.D.P.; Walsh, W.R.; Black, D.S.; Kumar, N. Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 2952. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Samperio, P. Peptidomimetics as a new generation of antimicrobial agents: Current progress. Infect. Drug Resist. 2014, 7, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Grauer, A.; König, B. Peptidomimetics—A Versatile Route to Biologically Active Compounds. Eur. J. Org. Chem. 2009, 2009, 5099–5111. [Google Scholar] [CrossRef]
- Carofiglio, T.; Cozzi, P.G.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. Nonorganometallic pathway of the Passerini reaction assisted by titanium tetrachloride. Organometallics 1993, 12, 2726–2736. [Google Scholar] [CrossRef]
- Paprocki, D.; Koszelewski, D.; Walde, P.; Ostaszewski, R. Efficient Passerini reactions in an aqueous vesicle system. RSC Adv. 2015, 5, 102828–102835. [Google Scholar] [CrossRef]
- Koszelewski, D.; Kowalczyk, P.; Śmigielski, P.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Szymczak, M.; Ostaszewski, R. Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik–Fields Reaction. Materials 2022, 15, 3846. [Google Scholar] [CrossRef] [PubMed]
- Gentry, C.L.; Egleton, R.D.; Gillespie, T.; Abbruscato, T.J.; Bechowski, H.B.; Hruby, V.J.; Davis, T.P. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides 1999, 20, 1229–1238. [Google Scholar] [CrossRef]
- Molchanova, N.; Nielsen, J.E.; Sørensen, K.B.; Prabhala, B.K.; Hansen, P.R.; Lund, R.; Barron, A.E.; Jenssen, H. Halogenation as a tool to tune antimicrobial activity of peptoids. Sci. Rep. 2020, 10, 14805. [Google Scholar] [CrossRef]
- ISO 11133; Microbiology of Food, Animal Feed and Water—Preparation, Production, Storage and Performance Testing of Culture Media. Online Browsing Platform (OBP) Version 4.31.1; ISO: Geneva, Switzerland, 2014.
- Alves, C.B.C.; Segurado, M.N.; Dorta, M.C.L.; Dias, F.R.; Lenza, M.G.; Lenza, M.A. Evaluation of cytotoxicity and corrosion resistance of orthodontic mini-implants. Dent. Press J. Orthod. 2016, 21, 39–46. [Google Scholar] [CrossRef][Green Version]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef]
- Milovanovic, V.; Minic, R.; Vakic, J.; Ivanovic, S.; Cupic, V.; Borozan, S.; Nesic, A.; Zivkovic, I. *MTT based L-aminoacid oxidase activity test for determination of antivenom potency against Vipera ammodytes envenomation. Toxicon 2021, 192, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Modanloo, M. An overview of the most common methods for assessing cell viabilit. J. Res. Med. Dent. Sci. 2017, 5, 33–41. [Google Scholar] [CrossRef]

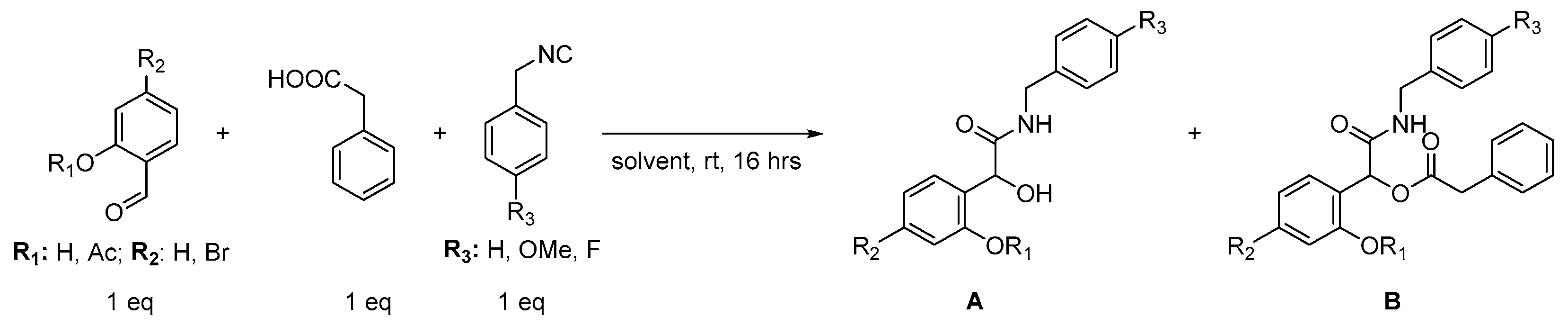





| Entry | R1 | R2 | R3 | Solvent | Yield [%] a A | Yield [%] a B |
|---|---|---|---|---|---|---|
| 1 | H | Br | OMe | DCM | 17 | - |
| 2 | H | Br | OMe | CHCl3 | 12 | - |
| 3 | H | Br | OMe | - | 16 | - |
| 4 | H | Br | OMe | Ethanol | - | - |
| 5 | H | Br | OMe | Toluene | 16 | - |
| 6 | H | Br | OMe | Dioxane | 8 | - |
| 7 | H | H | OMe | DCM | 6 | - |
| 8 | H | H | OMe | H2O, DODAB, 20% | 6 | - |
| 9 | Ac | Br | OMe | DCM | - | 86 |
| 10 | Ac | Br | OMe | H2O, DODAB, 20% | - | 64 |
| 11 | Ac | Br | H | DCM | - | 27 |
| 12 | Ac | Br | H | H2O, DODAB, 10% | - | 65 |
| 13 | Ac | Br | H | H2O, DODAB, 20% | - | 78 |
| 14 | Ac | Br | H | H2O, DODAB, 30% | - | 75 |
| 15 | Ac | Br | H | H2O, DODAB, 40% | - | 65 |
| 16 | Ac | H | H | DCM | - | 16 |
| 17 | Ac | H | H | H2O, DODAB, 20% | - | 65 |
| 18 | Ac | H | OMe | DCM | - | 64 |
| 19 | Ac | H | OMe | H2O, DODAB, 20% | - | 73 |
| 20 | Ac | Br | F | DCM | - | 70 |
| 21 | Ac | Br | F | H2O, DODAB, 20% | - | 6 |
| No. of Samples | 1, 2, 3 | 4–9 | 10–16 | Type of Test |
|---|---|---|---|---|
| K12 (ATCC 25404) | * | ** | ** | MIC |
| R2 (ATCC 39544) | * | ** | ** | MIC |
| R3 (ATCC 11775) | * | ** | ** | MIC |
| R4 (ATCC 39543) | * | ** | ** | MIC |
| K12 (ATCC 25404) | * | ** | *** | MBC |
| R2 (ATCC 39544) | * | ** | *** | MBC |
| R3 (ATCC 11775) | * | ** | *** | MBC |
| R4 (ATCC 39543) | * | ** | *** | MBC |
| A. baumannii (ATCC 17978) | * | ** | * | MIC |
| P. aeruginosa (ATCC 15442) | * | ** | * | MIC |
| E. cloacae (ATCC 49141) | ** | * | * | MIC |
| S. aureus (ATCC 23235) | * | * | * | MIC |
| A. baumannii (ATCC 17978) | * | ** | * | MBC |
| P. aeruginosa (ATCC 15442) | ** | ** | ** | MBC |
| E. cloacae (ATCC 49141) | ** | ** | * | MBC |
| S. aureus (ATCC 23235) | ** | ** | ** | MBC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wavhal, D.S.; Koszelewski, D.; Gulko, C.; Kowalczyk, P.; Brodzka, A.; Kramkowski, K.; Ostaszewski, R. Mystery of the Passerini Reaction for the Synthesis of the Antimicrobial Peptidomimetics against Nosocomial Pathogenic Bacteria. Int. J. Mol. Sci. 2024, 25, 8330. https://doi.org/10.3390/ijms25158330
Wavhal DS, Koszelewski D, Gulko C, Kowalczyk P, Brodzka A, Kramkowski K, Ostaszewski R. Mystery of the Passerini Reaction for the Synthesis of the Antimicrobial Peptidomimetics against Nosocomial Pathogenic Bacteria. International Journal of Molecular Sciences. 2024; 25(15):8330. https://doi.org/10.3390/ijms25158330
Chicago/Turabian StyleWavhal, Deepak S., Dominik Koszelewski, Cezary Gulko, Paweł Kowalczyk, Anna Brodzka, Karol Kramkowski, and Ryszard Ostaszewski. 2024. "Mystery of the Passerini Reaction for the Synthesis of the Antimicrobial Peptidomimetics against Nosocomial Pathogenic Bacteria" International Journal of Molecular Sciences 25, no. 15: 8330. https://doi.org/10.3390/ijms25158330
APA StyleWavhal, D. S., Koszelewski, D., Gulko, C., Kowalczyk, P., Brodzka, A., Kramkowski, K., & Ostaszewski, R. (2024). Mystery of the Passerini Reaction for the Synthesis of the Antimicrobial Peptidomimetics against Nosocomial Pathogenic Bacteria. International Journal of Molecular Sciences, 25(15), 8330. https://doi.org/10.3390/ijms25158330






