Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations
Abstract
1. Introduction
2. Risk Factors for the Development and Progression of MS
- Research: Identifying risk factors helps guide studies on MS pathogenesis and treatment [12].
3. Brain Plasticity
4. Neurodegeneration in MS
- Chronic inflammation in MS leads to the activation of microglia, which releases inflammatory mediators and generates oxidative stress. This can result in damage to neurons and axons [43,44,45]. Neuroinflammation, characterized by the infiltration of immune cells into the central nervous system, can contribute to neurodegeneration in MS. Immune-mediated mechanisms, such as the release of inflammatory cytokines and autoantibodies, can cause neuronal damage [46].
- Glutamate, an excitatory neurotransmitter, can accumulate in the extracellular space during inflammation and demyelination in MS. Excessive glutamate can lead to excitotoxicity, causing damage to neurons and axons [45].
- Additional mechanisms include the following:
- Altered sodium and calcium homeostasis contributes to axonal injury [44].
- Activated microglia release neurotoxic factors [45].
- Myelin debris accumulation impairs neurorepair and plasticity.
- Increased TNF signaling in neurons leads to programmed cell death [50].
- Impaired astrocytic support may contribute to axonal degeneration [49].
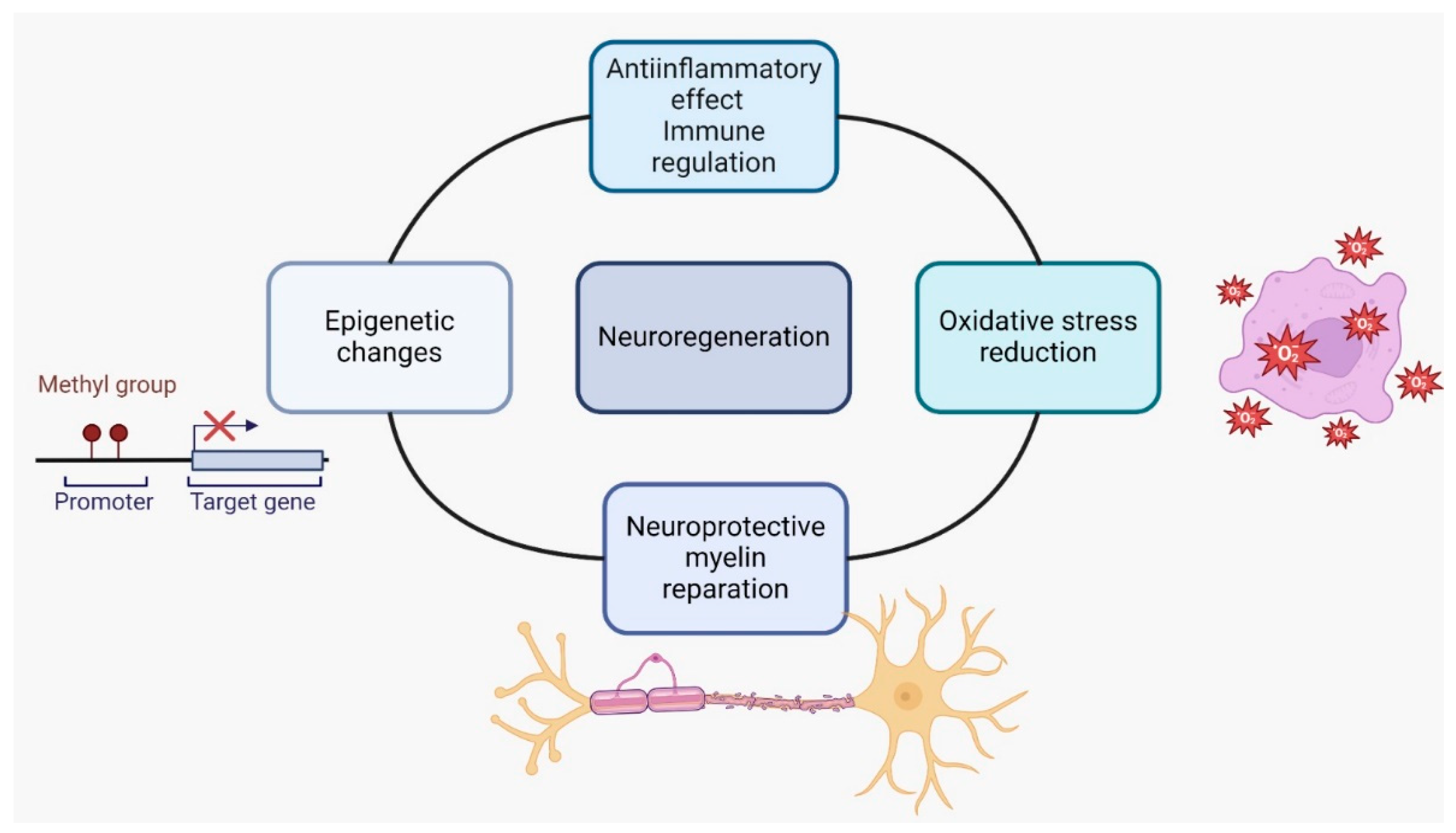
5. Nutraceuticals in MS
- Nutraceuticals such as polyunsaturated fatty acids (PUFAs), green tea flavonoids (epigallocatechin-3-gallate), curcumin, and scorpion toxins have been found to possess anti-inflammatory properties and can modulate the immune response in MS. They can inhibit proinflammatory signaling pathways, such as NF-κB or Toll-like receptors and reduce the activity of auto-aggressive immune cells. These effects may help reduce inflammation and immune-mediated damage in MS [56].
- Oxidative stress is implicated in the pathogenesis of MS. Nutraceuticals like green tea, curcumin, and resveratrol have antioxidative properties and can scavenge free radicals, reducing oxidative damage. By reducing oxidative stress, these compounds may protect against neuronal damage and inflammation in MS [61].
- Nutraceuticals such as flavonoids, terpenoids, and polyphenols have shown potential in promoting neuroprotection and myelin repair in animal models of MS. They may support the survival and function of neurons, promote remyelination, and enhance endogenous repair processes [62].
- Epigenetic modifications play a role in MS. Some nutraceuticals, such as plant polyphenols, Ω-3 and Ω-6 polyunsaturated fatty acids, and sulfur-containing compounds, can influence gene expression through epigenetic mechanisms. These compounds may regulate the production of proinflammatory proteins and modulate immune responses in MS [63].
5.1. Vitamin D3
5.2. Immunoglobulin Y
5.3. Homeopathy and Alternative Medicine
5.4. Ginkgo biloba
5.5. Alpha Lipoic Acid
5.6. Biotin
5.7. Flavonoids
- Flavonoids have been found to exert neuroprotective effects by reducing oxidative stress and inflammation, which are key factors in MS [90,91]. They have also been shown to promote synaptogenesis and neurogenesis, which are important processes for brain plasticity [90]. Additionally, flavonoids have been found to modulate signaling pathways involved in neuronal survival and synaptic plasticity [92,93].
- In animal models of MS, flavonoids have demonstrated positive therapeutic effects. For example, flavonoid luteolin has been shown to suppress clinical symptoms, reduce inflammation, and prevent relapse in rats with EAE [94]. A systematic review of studies on EAE and MS also reported positive outcomes for the therapeutic effect of flavonoids on these conditions [95].
5.8. Polyunsaturated Fatty Acids
- A study on mice with induced CNS demyelination found that an increased n-3 PUFA status promoted remyelination after toxic injury to CNS oligodendrocytes. This effect may be mediated by n-3 PUFA-derived lipid metabolites [104].
- Omega-3 PUFAs, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), have been shown to modulate microglial responses to myelin pathology. They can inhibit inflammation while enhancing beneficial immune responses, such as microglial phagocytosis. In a mouse model of MS, n-3 PUFA supplementation reduced demyelination and shifted microglial polarization toward a beneficial phenotype [105].
- In vitro studies using oligodendroglia cells and primary oligodendrocytes have shown that supplementation with n-3 and n-6 PUFAs can promote oligodendrocyte differentiation. This was evidenced by an increased expression of markers of oligodendroglia differentiation and enhanced myelin sheet formation [105].
- A study on healthy older adults found that omega-3 PUFAs were associated with individual differences in functional brain connectivity. Specifically, they were linked to connectivity within regions supporting executive function, memory, and emotion. These regions were also found to predict general, fluid, and crystallized intelligence [106].
5.9. Curcumin
5.10. Resveratrol
5.11. Terpenoids
5.12. Polyphenols
- Polyphenols can scavenge and neutralize harmful free radicals, reducing oxidative stress and protecting cells from damage [135].
- Polyphenols have been investigated for their potential anticarcinogenic effects. They may inhibit tumor growth, induce apoptosis in cancer cells, and have antimutagenic properties [139].
- Some polyphenols, such as resveratrol and epigallocatechin-3-gallate, have shown promise in protecting against neurodegenerative disorders by reducing mitochondrial dysfunction and oxidative stress [140].
5.13. Sulfur-Containing Compounds
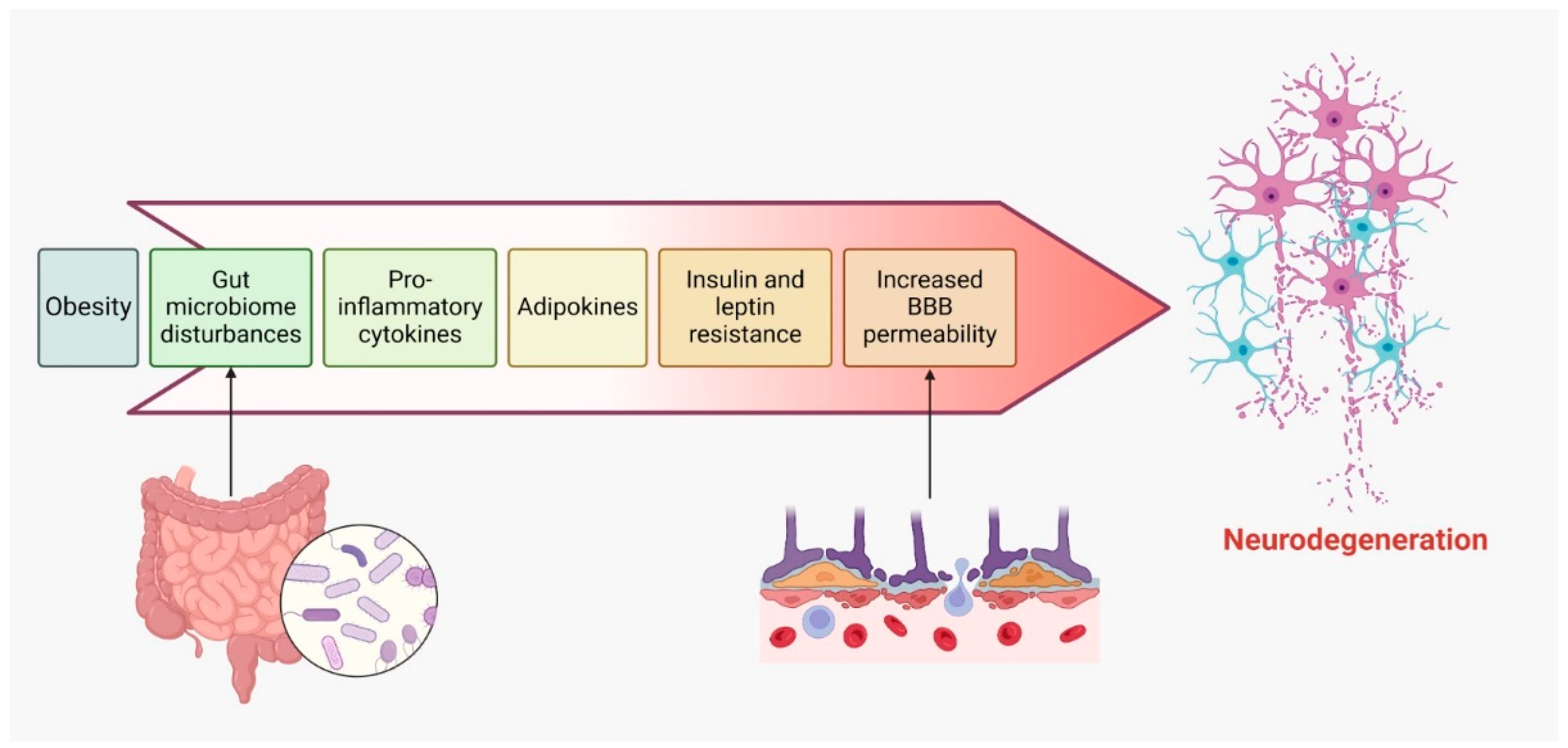
6. Obesity
- Obesity is linked to a chronic, low-grade inflammatory state, marked by elevated levels of proinflammatory cytokines. This persistent systemic inflammation may extend to the central nervous system (CNS), where it exacerbates neuroinflammation and accelerates neurodegeneration in individuals with MS [170,171].
- Adipose tissue in obese individuals secretes various adipokines, such as leptin, resistin, and visfatin, which are dysregulated in obesity. These adipokines can traverse the blood–brain barrier and activate immune cells within the CNS, thereby fostering inflammation and contributing to demyelination in MS [4,172].
- High-calorie diets and obesity are often associated with disruptions in gut microbiota. Emerging research suggests that gut dysbiosis may influence the onset and progression of MS, as altered microbiota can affect immune responses and neuroinflammation, thereby worsening neurodegeneration in obese individuals with MS [162,174].
- Obesity is commonly accompanied by insulin and leptin resistance, impairing neuroprotective signaling pathways. Resistance to these molecules may diminish their protective roles in the CNS, leading to increased neurodegeneration in MS patients [175].
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Ward, M.; Goldman, M.D. Epidemiology and Pathophysiology of Multiple Sclerosis. Continuum 2022, 28, 988–1005. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- University of California, San Francisco MS-EPIC Team; Cree, B.A.; Gourraud, P.A.; Oksenberg, J.R.; Bevan, C.; Crabtree-Hartman, E.; Gelfand, J.M.; Goodin, D.S.; Graves, J.; Green, A.J.; et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann. Neurol. 2016, 80, 499–510. [Google Scholar]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of Viruses in the Pathogenesis of Multiple Sclerosis. Viruses 2020, 12, 643. [Google Scholar] [CrossRef]
- Vattoth, S.; Kadam, G.H.; Gaddikeri, S. Revised McDonald Criteria, MAGNIMS Consensus and Other Relevant Guidelines for Diagnosis and Follow Up of MS: What Radiologists Need to Know? Curr. Probl. Diagn. Radiol. 2021, 50, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Sorensen, P.S.; Cutter, G.; Reingold, S.C.; Trojano, M. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult. Scler. J. 2014, 21, 282–293. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Westergaard, T.; Frisch, M.; Rostgaard, K.; Wohlfahrt, J.; Koch-Henriksen, N.; Melbye, M.; Hjalgrim, H. Type 1 diabetes and multiple sclerosis: A Danish population-based cohort study. Arch. Neurol. 2006, 63, 1001–1004. [Google Scholar] [CrossRef]
- Barac, I.S.; Iancu, M.; Văcăraș, V.; Cozma, A.; Negrean, V.; Sâmpelean, D.; Mureșanu, D.F.; Procopciuc, L.M. Potential Contribution of IL-27 and IL-23 Gene Polymorphisms to Multiple Sclerosis Susceptibility: An Association Analysis at Genotype and Haplotype Level. J. Clin. Med. 2021, 11, 37. [Google Scholar] [CrossRef]
- Gupta, G.; Gelfand, J.M.; Lewis, J.D. Increased risk for demyelinating diseases in populations with inflammatory bowel disease. Gastroenterology 2005, 129, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.; Vandebergh, M.; McCauley, J.L.; Saarela, J.; Cotsapas, C. Genetics of multiple sclerosis: Lessons from polygenicity. Lancet Neurol. 2022, 21, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Vacaras, V.; Paraschiv, A.-C.; Iluț, S.; Vacaras, C.; Nistor, C.; Marin, G.-E.; Schiopu, A.M.; Nistor, D.-T.; Vesa, Ș.C.; Mureșanu, D.F. Brain-Derived Neurotrophic Factor in Multiple Sclerosis Disability: A Prospective Study. Brain Sci. 2024, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Multiple sclerosis: Autoimmunity and viruses. Curr. Opin. Rheumatol. 2013, 25, 496–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taan, M.; Al Ahmad, F.; Ercksousi, M.K.; Hamza, G. Risk Factors Associated with Multiple Sclerosis: A Case-Control Study in Damascus, Syria. Mult. Scler. Int. 2021, 2021, 8147451. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Giovannoni, G.; Cuzick, J.; Dobson, R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Mult. Scler. 2020, 26, 1281–1297. [Google Scholar] [CrossRef]
- Xu, Y.; A Smith, K.; Hiyoshi, A.; Piehl, F.; Olsson, T.; Montgomery, S. Hospital-diagnosed infections before age 20 and risk of a subsequent multiple sclerosis diagnosis. Brain 2021, 144, 2390–2400. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Noyce, A.J.; Giovannoni, G.; Dobson, R. BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian Randomization study. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e662. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrinia, A.; Tench, C.R.; Maxted, J.; Bibani, R.H.; Britton, J.; Constantinescu, C.S. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013, 136 Pt 7, 2298–2304. [Google Scholar] [CrossRef]
- Ramanujam, R.; Hedström, A.-K.; Manouchehrinia, A.; Alfredsson, L.; Olsson, T.; Bottai, M.; Hillert, J. Effect of Smoking Cessation on Multiple Sclerosis Prognosis. JAMA Neurol. 2015, 72, 1117–1123. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef]
- Chandler, L. Ethanol and brain plasticity: Receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol. Ther. 2003, 99, 311–326. [Google Scholar] [CrossRef]
- Kolb, B.; Whishaw, I.Q. Brain plasticity and behavior. Annu. Rev. Psychol. 1998, 49, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Freitas, C.; Oberman, L.; Horvath, J.C.; Halko, M.; Eldaief, M.; Bashir, S.; Vernet, M.; Shafi, M.; Westover, B.; et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011, 24, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Missitzi, J.; Gentner, R.; Geladas, N.; Politis, P.; Karandreas, N.; Classen, J.; Klissouras, V. Plasticity in human motor cortex is in part genetically determined. J. Physiol. 2011, 589 Pt 2, 297–306. [Google Scholar] [CrossRef]
- Tzounopoulos, T.; Kraus, N. Learning to encode timing: Mechanisms of plasticity in the auditory brainstem. Neuron 2009, 62, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, A.; Bisaz, R.; Cruz, E.; Alberini, C.M. Developmental changes in plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus. Neurobiol. Learn. Mem. 2016, 135, 125–138. [Google Scholar] [CrossRef]
- Johnston, M.V. Plasticity in the developing brain: Implications for rehabilitation. Dev. Disabil. Res. Rev. 2009, 15, 94–101. [Google Scholar] [CrossRef]
- Strettoi, E.; Di Marco, B.; Orsini, N.; Napoli, D. Retinal Plasticity. Int. J. Mol. Sci. 2022, 23, 1138. [Google Scholar] [CrossRef]
- Pearson-Fuhrhop, K.M.; Kleim, J.A.; Cramer, S.C. Brain plasticity and genetic factors. Top. Stroke Rehabil. 2009, 16, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Ying, Z.; Gómez-Pinilla, F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience 2011, 192, 773–780. [Google Scholar] [CrossRef]
- Ben-Zeev, T.; Shoenfeld, Y.; Hoffman, J.R. The Effect of Exercise on Neurogenesis in the Brain. Isr. Med. Assoc. J. 2022, 24, 533–538. [Google Scholar]
- Smith, A.E.; Goldsworthy, M.R.; Garside, T.; Wood, F.M.; Ridding, M.C. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp. Brain Res. 2014, 232, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Berthoud, H.R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Kalron, A. Physical activity: Positive impact on brain plasticity. Harefuah 2008, 147, 252–255, 276. [Google Scholar] [PubMed]
- Cirillo, J.; Lavender, A.P.; Ridding, M.C.; Semmler, J.G. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J. Physiol. 2009, 587 Pt 24, 5831–5842. [Google Scholar] [CrossRef]
- Mori, F.; Kusayanagi, H.; Buttari, F.; Centini, B.; Monteleone, F.; Nicoletti, C.G.; Bernardi, G.; Di Cantogno, E.V.; Marciani, M.G.; Centonze, D. Early treatment with high-dose interferon beta-1a reverses cognitive and cortical plasticity deficits in multiple sclerosis. Funct. Neurol. 2013, 27, 163–168. [Google Scholar]
- Parry, A.M.M.; Scott, R.B.; Palace, J.; Smith, S.; Matthews, P.M. Potentially adaptive functional changes in cognitive processing for populations with multiple sclerosis and their acute modulation by rivastigmine. Brain 2003, 126 Pt 12, 2750–2760. [Google Scholar] [CrossRef]
- Murphy, T.; Dias, G.P.; Thuret, S. Effects of diet on brain plasticity in animal and human studies: Mind the gap. Neural Plast. 2014, 2014, 563160. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 2010, 168, 130–137. [Google Scholar] [CrossRef]
- Xu, B.-L.; Wang, R.; Ma, L.-N.; Dong, W.; Zhao, Z.-W.; Zhang, J.-S.; Wang, Y.-L.; Zhang, X. Effects of Caloric Intake on Learning and Memory Function in Juvenile C57BL/6J Mice. BioMed Res. Int. 2015, 2015, 759803. [Google Scholar] [CrossRef]
- Kalantarzadeh, E.; Radahmadi, M.; Reisi, P. The impact of different dark chocolate dietary patterns on synaptic potency and plasticity in the hippocampal CA1 area of the rats under chronic isolation stress. Nutr. Neurosci. 2022, 26, 756–765. [Google Scholar] [CrossRef]
- Libner, C.D.; Salapa, H.E.; Levin, M.C. The Potential Contribution of Dysfunctional RNA-Binding Proteins to the Pathogenesis of Neurodegeneration in Multiple Sclerosis and Relevant Models. Int. J. Mol. Sci. 2020, 21, 4571. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; D’mello, C.; Pinsky, W.; Lozinski, B.M.; Kaushik, D.K.; Ghorbani, S.; Moezzi, D.; Brown, D.; Melo, F.C.; Zandee, S.; et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 2021, 24, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Gonsette, R. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Vyshkina, T.; Kalman, B. Autoantibodies and neurodegeneration in multiple sclerosis. Mod. Pathol. 2008, 88, 796–807. [Google Scholar] [CrossRef]
- Kalman, B.; Leist, T.P. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromol. Med. 2003, 3, 147–158. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Barnett, M.H.; Mathey, E.; Kiernan, M.C.; Pollard, J.D. Axonal damage in central and peripheral nervous system inflammatory demyelinating diseases: Common and divergent pathways of tissue damage. Curr. Opin. Neurol. 2016, 29, 213–221. [Google Scholar] [CrossRef]
- Zoupi, L.; Booker, S.A.; Eigel, D.; Werner, C.; Kind, P.C.; Spires-Jones, T.L.; Newland, B.; Williams, A.C. Selective vulnerability of inhibitory networks in multiple sclerosis. Acta Neuropathol. 2021, 141, 415–429. [Google Scholar] [CrossRef]
- Chapman, C.; Lucas, R.M.; Ponsonby, A.-L.; Taylor, B.; Ausimmune Investigator Group. Predictors of progression from a first demyelinating event to clinically definite multiple sclerosis. Brain Commun. 2022, 4, fcac181. [Google Scholar] [CrossRef]
- Alroughani, R.A.; Akhtar, S.; Ahmed, S.F.; Al-Hashel, J.Y. Clinical predictors of disease progression in multiple sclerosis populations with relapsing onset in a nation-wide cohort. Int. J. Neurosci. 2015, 125, 831–837. [Google Scholar] [CrossRef]
- Simmons, S.B.; Schippling, S.; Giovannoni, G.; Ontaneda, D. Predicting disability worsening in relapsing and progressive multiple sclerosis. Curr. Opin. Neurol. 2021, 34, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Dlugonski, D.; Pilutti, L.; Sandroff, B.; McAuley, E. Premorbid physical activity predicts disability progression in relapsing–remitting multiple sclerosis. J. Neurol. Sci. 2012, 323, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zarghami, A.; A Jelinek, G.; Simpson-Yap, S.; Neate, S.; Nag, N. Diet and omega-3 and vitamin D supplement use predict five-year fatigue and disability trajectories in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 86, 105615. [Google Scholar] [CrossRef]
- Schmitz, K.; Barthelmes, J.; Stolz, L.; Beyer, S.; Diehl, O.; Tegeder, I. “Disease modifying nutricals” for multiple sclerosis. Pharmacol. Ther. 2015, 148, 85–113. [Google Scholar] [CrossRef]
- O’Connor, K.; Weinstock-Guttman, B.; Carl, E.; Kilanowski, C.; Zivadinov, R.; Ramanathan, M. Patterns of dietary and herbal supplement use by multiple sclerosis populations. J. Neurol. 2012, 259, 637–644. [Google Scholar] [CrossRef]
- Bergien, S.; Petersen, C.; Lynning, M.; Kristiansen, M.; Skovgaard, L. Use of natural medicine and dietary supplements concomitant with conventional medicine among people with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102197. [Google Scholar] [CrossRef]
- Marx, W.; Hockey, M.; McGuinness, A.J.; Lane, M.; Christodoulou, J.; van der Mei, I.; Berk, M.; Dean, O.M.; Taylor, B.; Broadley, S.; et al. The effect of emerging nutraceutical interventions for clinical and biological outcomes in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2019, 37, 101486. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, P. Interactions Between Nutraceutical Supplements and Standard Acute Myeloid Leukemia Chemotherapeutics. J. Pharm. Pharm. Sci. 2015, 18, 339–343. [Google Scholar] [CrossRef]
- Szymaszkiewicz, A.; López-Gómez, L.; Zielińska, M.; Abalo, R. Nutraceuticals and peripheral glial cells: A possible link? J. Integr. Neurosci. 2022, 21, 1. [Google Scholar] [CrossRef]
- Yuan, J.; Tao, Y.; Wang, M.; Huang, F.; Wu, X. Natural compounds as potential therapeutic candidates for multiple sclerosis: Emerging preclinical evidence. Phytomedicine 2024, 123, 155248. [Google Scholar] [CrossRef]
- Rito, Y.; Torre-Villalvazo, I.; Flores, J.; Rivas, V.; Corona, T. Epigenetics in Multiple Sclerosis: Molecular Mechanisms and Dietary Intervention. Central Nerv. Syst. Agents Med. Chem. 2018, 18, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.G.; Manucha, W. Vitamin D as a Modulator of Neuroinflammation: Implications for Brain Health. Curr. Pharm. Des. 2024, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wergeland, S.; Torkildsen, Ø.; Myhr, K.-M.; Aksnes, L.; Mørk, S.J.; Bø, L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS ONE 2011, 6, e26262. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Jameie, S.B.; Mehdizadeh, M.; Keradi, M.; Masoumipoor, M.; Mehrabi, S. Vitamin D3 influence the Th1/Th2 ratio in C57BL/6 induced model of experimental autoimmune encephalomyelitis. Iran J. Basic Med. Sci. 2014, 17, 785–792. [Google Scholar] [PubMed]
- Dias da Silva, W.; Tambourgi, D.V. IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Vet. Immunol. Immunopathol. 2010, 135, 173–180. [Google Scholar] [CrossRef]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for Potential Diagnostic and Therapeutic Applications in Infectious Diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef]
- Lee, P.W.; Selhorst, A.; Lampe, S.G.; Liu, Y.; Yang, Y.; Lovett-Racke, A.E. Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity. Front. Neurol. 2020, 11, 19. [Google Scholar] [CrossRef]
- Paraschiv, A.C.; Văcăraș, V.; Nistor, C.; Văcăraș, C.; Nistor, D.T.; Vesa, Ș.C.; Silvina, I.; Mureșanu, D.F. Dysbiosis in Multiple Sclerosis: Can Immunoglobulin Y Supplements Help? J. Gastrointestin Liver Dis. 2024, 33, 115–122. [Google Scholar] [CrossRef]
- Shinto, L.; Calabrese, C.; Morris, C.; Sinsheimer, S.; Bourdette, D. Complementary and alternative medicine in multiple sclerosis: Survey of licensed naturopaths. J. Altern. Complement. Med. 2004, 10, 891–897. [Google Scholar] [CrossRef]
- Shinto, L.; Calabrese, C.; Morris, C.; Yadav, V.; Griffith, D.; Frank, R.; Oken, B.S.; Baldauf-Wagner, S.; Bourdette, D. A Randomized pilot study of naturopathic medicine in multiple sclerosis. J. Altern. Complement. Med. 2008, 14, 489–496. [Google Scholar] [CrossRef]
- Teixeira, M.Z. Immunomodulatory drugs (natalizumab), worsening of multiple sclerosis, rebound effect and similitude. Homeopathy 2013, 102, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, A.-C.; Vacaras, V.; Nistor, C.; Vacaras, C.; Strilciuc, S.; Muresanu, D.F. The effect of multiple sclerosis therapy on gut microbiota dysbiosis: A longitudinal prospective study. Microb. Cell 2024, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Alzahrani, T. Ginkgo Biloba [Internet]. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541024/ (accessed on 11 July 2024).
- Lovera, J.F.; Kim, E.; Heriza, E.; Fitzpatrick, M.; Hunziker, J.; Turner, A.P.; Adams, J.; Stover, T.; Sangeorzan, A.; Sloan, A.; et al. Ginkgo biloba does not improve cognitive function in MS: A randomized placebo-controlled trial. Neurology 2012, 79, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Lovera, J.; Bagert, B.; Smoot, K.; Morris, C.D.; Frank, R.; Bogardus, K.; Wild, K.; Oken, B.; Whitham, R.; Bourdette, D. Ginkgo biloba for the improvement of cognitive performance in multiple sclerosis: A randomized, placebo-controlled trial. Mult. Scler. 2007, 13, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-J.; He, Y.; An, J.; Miao, Q.; Sui, R.-X.; Wang, Q.; Yu, J.-Z.; Xiao, B.-G.; Ma, C.-G. Dynamic Balance of Microglia and Astrocytes Involved in the Remyelinating Effect of Ginkgolide B. Front. Cell. Neurosci. 2020, 13, 572. [Google Scholar] [CrossRef]
- Seifar, F.; Khalili, M.; Khaledyan, H.; Amiri Moghadam, S.; Izadi, A.; Azimi, A.; Shakouri, S.K. α-Lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: A review. Nutr. Neurosci. 2019, 22, 306–316. [Google Scholar] [CrossRef]
- Rocamonde, B.; Paradells, S.; Barcia, J.; Barcia, C.; Verdugo, J.G.; Miranda, M.; Gómez, F.R.; Soria, J. Neuroprotection of lipoic acid treatment promotes angiogenesis and reduces the glial scar formation after brain injury. Neuroscience 2012, 224, 102–115. [Google Scholar] [CrossRef]
- Marracci, G.H.; McKeon, G.P.; Marquardt, W.E.; Winter, R.W.; Riscoe, M.K.; Bourdette, D.N. Alpha lipoic acid inhibits human T-cell migration: Implications for multiple sclerosis. J. Neurosci. Res. 2004, 78, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, A.I.; Remalante-Rayco, P.P.M. High-dose biotin for multiple sclerosis: A systematic review and meta-analyses of randomized controlled trials. Mult. Scler. Relat. Disord. 2021, 55, 103159. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Garcia-Diaz, B.; Sedel, F.; Evercooren, A.B.-V.; Mozafari, S. High Dose Pharmaceutical Grade Biotin (MD1003) Accelerates Differentiation of Murine and Grafted Human Oligodendrocyte Progenitor Cells In Vivo. Int. J. Mol. Sci. 2022, 23, 15733. [Google Scholar] [CrossRef]
- Sedel, F.; Bernard, D.; Mock, D.M.; Tourbah, A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 2016, 110 Pt B, 644–653. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Cutter, G.; Wolinsky, J.S.; Freedman, M.S.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Arnold, D.; Kuhle, J.; Block, V.; et al. Safety and efficacy of MD1003 (high-dose biotin) in populations with progressive multiple sclerosis (SPI2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2020, 19, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Ielpo, M.; Basile, A.; Miranda, R.; Moscatiello, V.; Nappo, C.; Sorbo, S.; Laghi, E.; Ricciardi, M.; Ricciardi, L.; Vuotto, M. Immunopharmacological properties of flavonoids. Fitoterapia 2000, 71, S101–S109. [Google Scholar] [CrossRef] [PubMed]
- Atucha, N.M.; Romecín, P.; Vargas, F.; García-Estañ, J. Effects of Flavonoids in Experimental Models of Arterial Hypertension. Curr. Top. Med. Chem. 2022, 22, 735–745. [Google Scholar] [CrossRef]
- Jaeger, B.N.; Parylak, S.L.; Gage, F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Asp. Med. 2017, 61, 50–62. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. food Sci. food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes. Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Hendriks, J.J.A.; Alblas, J.; van der Pol, S.M.A.; van Tol, E.A.F.; Dijkstra, C.D.; de Vries, H.E. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J. Exp. Med. 2004, 200, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Bayat, P.; Farshchi, M.; Yousefian, M.; Mahmoudi, M.; Yazdian-Robati, R. Flavonoids, the compounds with anti-inflammatory and immunomodulatory properties, as promising tools in multiple sclerosis (MS) therapy: A systematic review of preclinical evidence. Int. Immunopharmacol. 2021, 95, 107562. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Chénais, B.; Blanckaert, V. The janus face of lipids in human breast cancer: How polyunsaturated Fatty acids affect tumor cell hallmarks. Int. J. Breast Cancer 2012, 2012, 712536. [Google Scholar] [CrossRef]
- Davinelli, S.; Intrieri, M.; Corbi, G.; Scapagnini, G. Metabolic indices of polyunsaturated fatty acids: Current evidence, research controversies, and clinical utility. Crit. Rev. Food Sci. Nutr. 2020, 61, 259–274. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Baroselli, S.; Nadalini, E.; Lapenna, R.; Chiuch, A.; Sechi, L. Omega-3 fatty acids: From biochemistry to their clinical use in the prevention of cardiovascular disease. Recent. Patents Cardiovasc. Drug Discov. 2007, 2, 13–21. [Google Scholar] [CrossRef]
- Khandelwal, S.; Kelly, L.; Malik, R.; Prabhakaran, D.; Reddy, S. Impact of omega-6 fatty acids on cardiovascular outcomes: A review. J. Prev. Cardiol. 2013, 2, 325–336. [Google Scholar]
- D’archivio, M.; Scazzocchio, B.; Vari, R.; Santangelo, C.; Giovannini, C.; Masella, R. Recent Evidence on the Role of Dietary PUFAs in Cancer Development and Prevention. Curr. Med. Chem. 2018, 25, 1818–1836. [Google Scholar] [CrossRef]
- Mbarik, M.; Biam, R.S.; Robichaud, P.-P.; Surette, M.E. The impact of PUFA on cell responses: Caution should be exercised when selecting PUFA concentrations in cell culture. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102083. [Google Scholar] [CrossRef]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated fatty acids: Biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Siegert, E.; Paul, F.; Rothe, M.; Weylandt, K.H. The effect of omega-3 fatty acids on central nervous system remyelination in fat-1 mice. BMC Neurosci. 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, M.; Baron, W.; Beermann, C.; Dijkstra, C.; van Tol, E. Polyunsaturated fatty acid supplementation stimulates differentiation of oligodendroglia cells. Dev. Neurosci. 2006, 28, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, T.; Zamroziewicz, M.K.; Zwilling, C.E.; Barbey, A.K. Nutrient biomarkers shape individual differences in functional brain connectivity: Evidence from omega-3 PUFAs. Hum. Brain Mapp. 2018, 40, 1887–1897. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Sadek, M.A.; Rabie, M.A.; El Sayed, N.S.; Sayed, H.M.; Kandil, E.A. Neuroprotective effect of curcumin against experimental autoimmune encephalomyelitis-induced cognitive and physical impairments in mice: An insight into the role of the AMPK/SIRT1 pathway. Inflammopharmacology 2023, 32, 1499–1518. [Google Scholar] [CrossRef]
- Elbini-Dhouib, I.; Manai, M.; Neili, N.-E.; Marzouki, S.; Sahraoui, G.; Ben Achour, W.; Zouaghi, S.; BenAhmed, M.; Doghri, R.; Srairi-Abid, N. Dual Mechanism of Action of Curcumin in Experimental Models of Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 8658. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Castillo, C.M.S.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Kulashekar, M.; Stom, S.M.; Peuler, J.D. Resveratrol’s Potential in the Adjunctive Management of Cardiovascular Disease, Obesity, Diabetes, Alzheimer Disease, and Cancer. J. Am. Osteopath. Assoc. 2018, 118, 596–605. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, M.; Jasińska, L.; Ogrodowczyk, M. Resveratrol in prostate diseases—A short review. Central Eur. J. Urol. 2013, 66, 144–149. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomas-Barberan, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Shamsher, E.; Khan, R.S.; Davis, B.M.; Dine, K.; Luong, V.; Somavarapu, S.; Cordeiro, M.F.; Shindler, K.S. Nanoparticles Enhance Solubility and Neuroprotective Effects of Resveratrol in Demyelinating Disease. Neurotherapeutics 2023, 20, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2016, 54, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Liu, Y. Terpenoids: Natural Compounds for Non-Alcoholic Fatty Liver Disease (NAFLD) Therapy. Molecules 2022, 28, 272. [Google Scholar] [CrossRef]
- Ahmad, A.; Tiwari, R.K.; Ansari, I.A. Revisiting the Antiviral Efficacy of Terpenoids: Plausible Adjunct Therapeutics for Novel SARS-CoV-2? Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2119–2130. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants 2022, 11, 1834. [Google Scholar] [CrossRef]
- Yang, H.; Dou, Q.P. Targeting apoptosis pathway with natural terpenoids: Implications for treatment of breast and prostate cancer. Curr. Drug Targets 2010, 11, 733–744. [Google Scholar] [CrossRef]
- Heras, B.d.L.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy-Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef]
- Tomassini, V.; D’Ambrosio, A.; Petsas, N.; Wise, R.G.; Sbardella, E.; Allen, M.; Tona, F.; Fanelli, F.; Foster, C.; Carnì, M.; et al. The effect of inflammation and its reduction on brain plasticity in multiple sclerosis: MRI evidence. Hum. Brain Mapp. 2016, 37, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A. Polyphenols Effect on Circulating Lipids and Lipoproteins: From Biochemistry to Clinical Evidence. Curr. Pharm. Des. 2018, 24, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: From diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.A.; Valenti, D.; Caccamese, S.; Daglia, M.; Braidy, N.; Nabavi, S.M. Plant polyphenols as natural drugs for the management of Down syndrome and related disorders. Neurosci. Biobehav. Rev. 2016, 71, 865–877. [Google Scholar] [CrossRef]
- Mabrouk, M.; El Ayed, M.; Démosthènes, A.; Aissouni, Y.; Aouani, E.; Daulhac-Terrail, L.; Mokni, M.; Bégou, M. Antioxidant effect of grape seed extract corrects experimental autoimmune encephalomyelitis behavioral dysfunctions, demyelination, and glial activation. Front. Immunol. 2022, 13, 960355. [Google Scholar] [CrossRef]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstić, D.Z. Sulphur-Containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef]
- Partti-Pellinen, K.; Marttila, O.; Vilkka, V.; Jaakkola, J.J.K.; Jäppinen, P.; Haahtela, T. The South Karelia Air Pollution Study: Effects of low-level exposure to malodorous sulfur compounds on symptoms. Arch. Environ. Health Int. J. 1996, 51, 315–320. [Google Scholar] [CrossRef]
- Jaakkola, J.J.; Partti-Pellinen, K.; Marttila, O.; Miettinen, P.; Vilkka, V.; Haahtela, T. The South Karelia Air Pollution Study: Changes in Respiratory Health in Relation to Emission Reduction of Malodorous Sulfur Compounds from Pulp Mills. Arch. Environ. Health Int. J. 1999, 54, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gurvitz, M.; Lui, G.K.; Marelli, A. Adult congenital heart disease—Preparing for the changing work force demand. Cardiol. Clin. 2020, 38, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Caylak, E.; Aytekin, M.; Halifeoglu, I. Antioxidant effects of methionine, alpha-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp. Toxicol. Pathol. 2008, 60, 289–294. [Google Scholar] [CrossRef]
- Ghaiad, H.R.; AAbd-Elmawla, M.; Gad, E.S.; A Ahmed, K.; Abdelmonem, M. Modulating miR-146a Expression by Hydrogen Sulfide Ameliorates Motor Dysfunction and Axonal Demyelination in Cuprizone-Induced Multiple Sclerosis. ACS Chem. Neurosci. 2023, 14, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yun, Y.; Sang, N. Differential effects between one week and four weeks exposure to same mass of SO2 on synaptic plasticity in rat hippocampus. Environ. Toxicol. 2014, 31, 820–829. [Google Scholar] [CrossRef]
- Munger, K.L.; Chitnis, T.; Ascherio, A. Body size and risk of MS in two cohorts of US women. Neurology 2009, 73, 1543–1550. [Google Scholar] [CrossRef]
- Neto, A.; Fernandes, A.; Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef] [PubMed]
- Faysal, M.; Dehbia, Z.; Zehravi, M.; Sweilam, S.H.; Haque, M.A.; Kumar, K.P.; Chakole, R.D.; Shelke, S.P.; Sirikonda, S.; Nafady, M.H.; et al. Flavonoids as Potential Therapeutics against Neurodegenerative Disorders: Unlocking the Prospects. Neurochem. Res. 2024, 49, 1926–1944. [Google Scholar] [CrossRef]
- Lopresti, A.L. Potential Role of Curcumin for the Treatment of Major Depressive Disorder. CNS Drugs 2022, 36, 123–141. [Google Scholar] [CrossRef]
- Seidell, J.C. Obesity, insulin resistance and diabetes—A worldwide epidemic. Br. J. Nutr. 2000, 83 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef]
- Mokry, L.E.; Ross, S.; Timpson, N.J.; Sawcer, S.; Davey Smith, G.; Richards, J.B. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2016, 13, e1002053. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.-T.; Yu, J.; Liu, Y.-L.; Qi, S.-F.; Zhao, J.-J.; Liu, D.-W.; Tian, Q.-B. Excess Body Weight during Childhood and Adolescence Is Associated with the Risk of Multiple Sclerosis: A Meta-Analysis. Neuroepidemiology 2016, 47, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. Body mass index during adolescence, rather than childhood, is critical in determining MS risk. Mult. Scler. J. 2016, 22, 878–883. [Google Scholar] [CrossRef]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult. Scler. J. 2012, 18, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Kavak, K.S.; E Teter, B.; Hagemeier, J.; Zakalik, K.; Weinstock-Guttman, B.; on behalf of the New York State Multiple Sclerosis Consortium. Higher weight in adolescence and young adulthood is associated with an earlier age at multiple sclerosis onset. Mult. Scler. J. 2014, 21, 858–865. [Google Scholar] [CrossRef]
- Kvistad, S.S.; Myhr, K.-M.; Holmøy, T.; Benth, J.Š.; Wergeland, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Lilleås, F.; Midgard, R.; et al. Body mass index influence interferon-beta treatment response in multiple sclerosis. J. Neuroimmunol. 2015, 288, 92–97. [Google Scholar] [CrossRef]
- Lutfullin, I.; Eveslage, M.; Bittner, S.; Antony, G.; Flaskamp, M.; Luessi, F.; Salmen, A.; Gisevius, B.; Klotz, L.; Korsukewitz, C.; et al. Association of obesity with disease outcome in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 57–61. [Google Scholar] [CrossRef]
- Harroud, A.; Manousaki, D.; Butler-Laporte, G.; Mitchell, R.E.; Davey Smith, G.; Richards, J.B.; Baranzini, S.E. The relative contributions of obesity, vitamin D, leptin, and adiponectin to multiple sclerosis risk: A Mendelian randomization mediation analysis. Mult. Scler. J. 2021, 27, 1994–2000. [Google Scholar] [CrossRef]
- Samara, A.; Cantoni, C.; Piccio, L.; Cross, A.H.; Chahin, S. Obesity, gut microbiota, and multiple sclerosis: Unraveling the connection. Mult. Scler. Relat. Disord. 2023, 76, 104768. [Google Scholar] [CrossRef]
- Hedström, A.K.; Bomfim, I.L.; Barcellos, L.; Gianfrancesco, M.; Schaefer, C.; Kockum, I.; Olsson, T.; Alfredsson, L. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014, 82, 865–872. [Google Scholar] [CrossRef]
- Pakpoor, J.; Schmierer, K.; Cuzick, J.; Giovannoni, G.; Dobson, R. Estimated and projected burden of multiple sclerosis attributable to smoking and childhood and adolescent high body-mass index: A comparative risk assessment. Leuk. Res. 2020, 49, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Lunghi, C.; Dardano, A.; Binda, P.; Ceccarini, G.; Santini, F.; Giusti, L.; Ciccarone, A.; Bellini, R.; Moretto, C.; et al. Bariatric surgery restores visual cortical plasticity in nondiabetic subjects with obesity. Int. J. Obes. 2021, 45, 1821–1829. [Google Scholar] [CrossRef]
- Lunghi, C.; Daniele, G.; Binda, P.; Dardano, A.; Ceccarini, G.; Santini, F.; Del Prato, S.; Morrone, M.C. Altered Visual Plasticity in Morbidly Obese Subjects. iScience 2019, 22, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Ridding, M.C.; Hordacre, B. Obesity is Associated with Reduced Plasticity of the Human Motor Cortex. Brain Sci. 2020, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalaeen, A.; Al-Domi, H. Does obesity put your brain at risk? Diabetes Metab. Syndr. 2022, 16, 102444. [Google Scholar] [CrossRef]
- Ji, Z.; Wu, S.; Xu, Y.; Qi, J.; Su, X.; Shen, L. Obesity Promotes EAE Through IL-6 and CCL-2-Mediated T Cells Infiltration. Front. Immunol. 2019, 10, 1881. [Google Scholar] [CrossRef]
- Bassi, M.S.; Iezzi, E.; Buttari, F.; Gilio, L.; Simonelli, I.; Carbone, F.; Micillo, T.; De Rosa, V.; Sica, F.; Furlan, R.; et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult. Scler. J. 2020, 26, 1237–1246. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef]
- Davanzo, G.G.; Castro, G.; Monteiro, L.d.B.; Castelucci, B.G.; Jaccomo, V.H.; da Silva, F.C.; Marques, A.M.; Francelin, C.; de Campos, B.B.; de Aguiar, C.F.; et al. Obesity increases blood-brain barrier permeability and aggravates the mouse model of multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 72, 104605. [Google Scholar] [CrossRef]
- Shahi, S.K.; Ghimire, S.; Lehman, P.; Mangalam, A.K. Obesity induced gut dysbiosis contributes to disease severity in an animal model of multiple sclerosis. Front. Immunol. 2022, 13, 966417. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 2014, 273, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzade, M.; Agha-Alinejad, H.; Motl, R.W.; Negaresh, R.; Baker, J.S.; Zimmer, P. Weight control and physical exercise in people with multiple sclerosis: Current knowledge and future perspectives. Complement. Ther. Med. 2019, 43, 240–246. [Google Scholar] [CrossRef]
- Bruce, J.M.; Cozart, J.S.; Shook, R.P.; Ruppen, S.; Siengsukon, C.; Simon, S.; Befort, C.; Lynch, S.; Mahmoud, R.; Drees, B.; et al. Modifying Diet and Exercise in MS (MoDEMS): Study design and protocol for a telehealth weight loss intervention for adults with obesity & Multiple Sclerosis. Contemp. Clin. Trials 2021, 107, 106495. [Google Scholar] [CrossRef]
- Bruce, J.M.; Cozart, J.S.; Shook, R.P.; Befort, C.; Siengsukon, C.F.; Simon, S.; Lynch, S.G.; Mahmoud, R.; Drees, B.; Posson, P.; et al. Modifying diet and exercise in multiple sclerosis (MoDEMS): A randomized controlled trial for behavioral weight loss in adults with multiple sclerosis and obesity. Mult. Scler. J. 2023, 29, 1860–1871. [Google Scholar] [CrossRef]
- Stenberg, E.; Forsberg, L.; Hedström, A.; Hillert, J.; Näslund, E. Bariatric and metabolic surgery in populations with morbid obesity and multiple sclerosis—A nationwide, matched cohort study. Surg. Obes. Relat. Dis. 2021, 17, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Bencsath, K.; Jammoul, A.; Aminian, A.; Shimizu, H.; Fisher, C.J.; Schauer, P.R.; Rae-Grant, A.; Brethauer, S.A. Outcomes of Bariatric Surgery in Morbidly Obese Populations with Multiple Sclerosis. J. Obes. 2017, 2017, 1935204. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.; Collado Sánchez, E.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Allogmanny, S.; Probst, Y. Dietary Modification Combined with Nutrition Education and Counseling for Metabolic Comorbidities in Multiple Sclerosis: Implications for Clinical Practice and Research. Curr. Nutr. Rep. 2024, 13, 106–112. [Google Scholar] [CrossRef]


| Risk Factors | Protective Factors |
|---|---|
| Smoking | Smoking cessation |
| Obesity | Calorie restriction Physical activity |
| Childhood low sun exposure | Higher sun exposure Vitamin D3 supplementation |
| Oxidative diet | Antioxidant supplementation Diet re-assessment |
| Compound | Primary Function/Effect | Role in Brain Plasticity and MS | Key Findings and Studies |
|---|---|---|---|
| Vitamin D3 (Cholecalciferol) | Maintains calcium and phosphate balance for bone health, with emerging roles in brain health. | Immunomodulatory and neuroprotective effects; promotes neurogenesis and synaptic plasticity. | May reduce disease activity and demyelination in MS; early intervention in EAE mice controlled neuroinflammation [64,65,66]. |
| Immunoglobulin Y (IgY) | Avian antibody involved in immune defense; passive immunity to offspring via egg yolk. | Limited research in MS, but potential in modulating immune responses and gut microbiota. | Positive outcomes in clinical trials for MS, with reduced symptoms and altered immune responses; further research needed [68,69,70]. |
| Homeopathy | CAM approach using highly diluted substances for symptom relief based on individualized treatment. | Limited evidence; potential placebo effect. | Some individuals report symptom relief; more research is required to determine efficacy in MS [12,71,72,73,74]. |
| Ginkgo biloba extract (GBE) | Plant extract with neuroprotective and neuroregenerative properties; enhances cerebral blood flow. | Limited evidence in MS; potential effects on synaptic plasticity. | No significant cognitive improvement in MS; potential neuroprotective effects in neurological diseases [75,76,77,78]. |
| Alpha Lipoic Acid (ALA) | Antioxidant and anti-inflammatory compound with roles in mitochondrial energy production. | Promotes neuroprotection and repair after injury; potential therapy for MS. | Preclinical and clinical studies show promising effects; more research needed to establish efficacy in MS [79,80,81]. |
| Biotin (Vitamin B7) | Cofactor in metabolic processes, energy production, and fatty acid synthesis. | Potential in preventing brain neurodegeneration in MS. | High-dose biotin (MD1003) shows promise in slowing MS progression; mechanisms include enhanced energy production and myelin synthesis [4,60,82,83,84,85]. |
| Flavonoids | Plant polyphenols with antioxidant, anti-inflammatory, and immunomodulatory properties. | Neuroprotective and neuroplasticity-promoting effects; limited direct evidence in MS. | Positive therapeutic effects in EAE models; potential benefits for brain plasticity in MS, but more research needed [86,87,88,89,90,91,92,93,94,95,149]. |
| Polyunsaturated Fatty Acids (PUFA) | Essential fatty acids with anti-inflammatory properties and roles in brain function. | Modulate microglial responses; promote remyelination and oligodendrocyte differentiation. | Omega-3 PUFA supplementation reduces demyelination in MS models; associated with improved brain connectivity in humans [96,97,98,99,100,101,102,103,104,105,106,150]. |
| Curcumin | Natural compound with antioxidant, anti-inflammatory, and neuroprotective properties. | Potential benefits for brain health and plasticity in MS, though limited research specific to MS. | Demonstrated neuroprotective effects and reduced demyelination in MS animal models; more research needed [107,108,109,110,111,112,113,114,115]. |
| Resveratrol | Polyphenol with antioxidant, anti-inflammatory, and anti-cancer properties. | Limited research in MS; potential neuroprotective effects. | Improved outcomes in EAE mouse models; promotes remyelination and motor coordination in MS-like conditions in mice [116,117,118,119,120,121,122,123,124,125]. |
| Terpenoids | Natural compounds with diverse biological activities, including anti-inflammatory and antioxidant effects. | Studied for potential benefits in chronic illnesses, including neurological conditions. | Preclinical studies suggest neuropharmacological effects; further research needed to explore benefits in MS and brain plasticity [126,127,128,129,130,131,132,133]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciumărnean, L.; Sârb, O.-F.; Drăghici, N.-C.; Sălăgean, O.; Milaciu, M.-V.; Orășan, O.-H.; Vlad, C.-V.; Vlad, I.-M.; Alexescu, T.; Para, I.; et al. Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations. Int. J. Mol. Sci. 2024, 25, 10909. https://doi.org/10.3390/ijms252010909
Ciumărnean L, Sârb O-F, Drăghici N-C, Sălăgean O, Milaciu M-V, Orășan O-H, Vlad C-V, Vlad I-M, Alexescu T, Para I, et al. Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations. International Journal of Molecular Sciences. 2024; 25(20):10909. https://doi.org/10.3390/ijms252010909
Chicago/Turabian StyleCiumărnean, Lorena, Oliviu-Florențiu Sârb, Nicu-Cătălin Drăghici, Octavia Sălăgean, Mircea-Vasile Milaciu, Olga-Hilda Orășan, Călin-Vasile Vlad, Irina-Maria Vlad, Teodora Alexescu, Ioana Para, and et al. 2024. "Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations" International Journal of Molecular Sciences 25, no. 20: 10909. https://doi.org/10.3390/ijms252010909
APA StyleCiumărnean, L., Sârb, O.-F., Drăghici, N.-C., Sălăgean, O., Milaciu, M.-V., Orășan, O.-H., Vlad, C.-V., Vlad, I.-M., Alexescu, T., Para, I., Țărmure, S.-F., Hirișcău, E.-I., & Dogaru, G.-B. (2024). Obesity Control and Supplementary Nutraceuticals as Cofactors of Brain Plasticity in Multiple Sclerosis Populations. International Journal of Molecular Sciences, 25(20), 10909. https://doi.org/10.3390/ijms252010909







