Assessing the Toxicity of Metal- and Carbon-Based Nanomaterials In Vitro: Impact on Respiratory, Intestinal, Skin, and Immune Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Characterisation of Nanomaterials
2.2. Cytotoxicity
2.3. Genotoxicity Assay
2.4. ROS Production
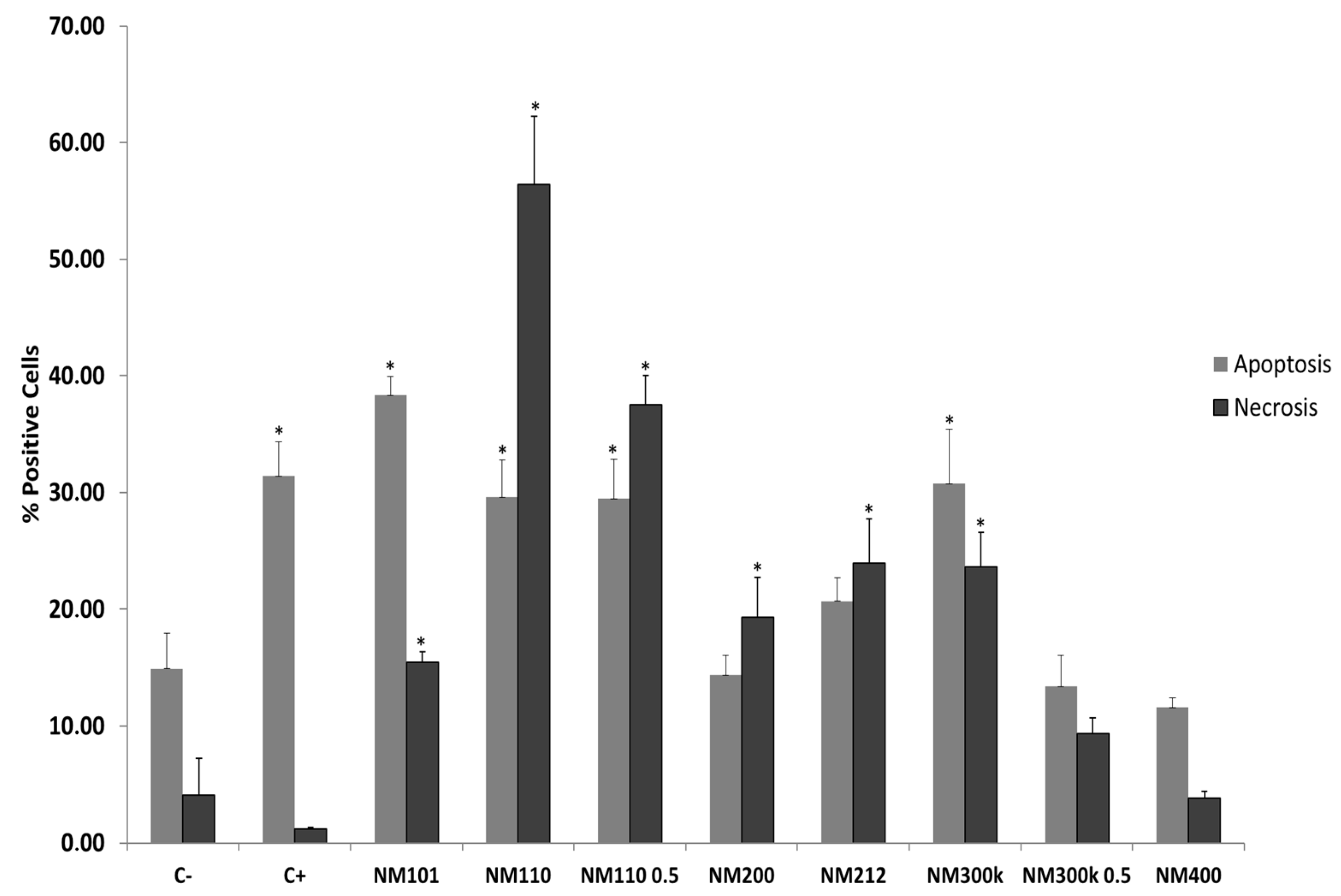
2.5. Necrosis/Apoptosis Assay
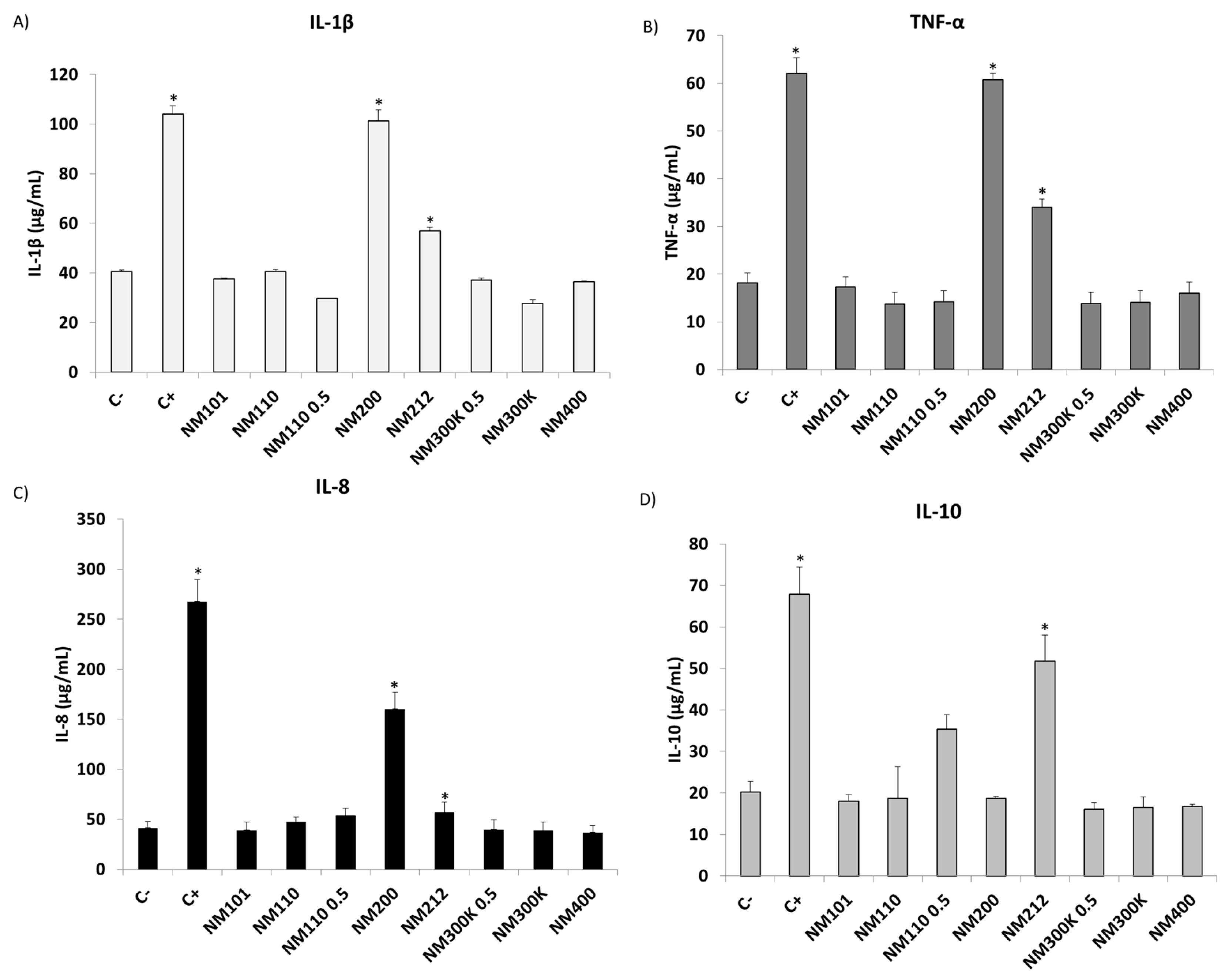
2.6. Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Characterisation of Nanomaterials
4.2. Cell Culture
4.3. Cell Viability Assay
4.3.1. MTS Assay
4.3.2. Alamar Blue Assay
4.3.3. Neutral Red Assay
4.4. ROS Detection Assay
4.5. Apoptosis/Necrosis Assay
4.6. Genotoxicity Assay
4.7. Cytokine Production Determination
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahamuni-Badiger, P.; Dhanavade, M.J. Challenges and toxicity assessment of inorganic nanomaterials in biomedical applications: Current status and future roadmaps. J. Drug Deliv. Sci. Technol. 2023, 87, 104806. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Kato, H.; Fujita, K.; Endoh, S.; Iwahashi, H. In vitro evaluation of cellular response induced by manufactured nanoparticles. Chem. Res. Toxicol. 2012, 25, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Bessa, M.J.; Brandão, F.; Fokkens, P.H.B.; Leseman, D.L.A.C.; Boere, A.J.F.; Cassee, F.R.; Salmatonidis, A.; Viana, M.; Vulpoi, A.; Simon, S.; et al. In vitro toxicity of industrially relevant engineered nanoparticles in human alveolar epithelial cells: Air–liquid interface versus submerged cultures. Nanomaterials 2021, 11, 3225. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon. Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Sufian, M.M.; Khattak, J.Z.K.; Yousaf, S.; Rana, M.S. Safety issues associated with the use of nanoparticles in human body. Photodiagnosis Photodyn. Ther. 2017, 19, 67–72. [Google Scholar] [CrossRef]
- Firme, C.P.; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles–friends or enemies? Int. J. Environ. Health Res. 2022, 32, 885–901. [Google Scholar] [CrossRef]
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011, 11, 3744–3750. [Google Scholar] [CrossRef]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1658. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Moosavi, M.A.; Sharifi, M.; Ghafary, S.M.; Mohammadalipour, Z.; Khataee, A.; Rahmati, M.; Hajjaran, S.; Łos, M.J.; Klonisch, T.; Ghavami, S. Photodynamic N-TiO2 nanoparticle treatment induces controlled ROS-mediated autophagy and terminal differentiation of leukemia cells. Sci. Rep. 2016, 6, 34413. [Google Scholar] [CrossRef]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef]
- García-Salvador, A.; Katsumiti, A.; Rojas, E.; Aristimuño, C.; Betanzos, M.; Martínez-Moro, M.; Moya, S.E.; Goñi-De-Cerio, F. A complete in vitro toxicological assessment of the biological effects of cerium oxide nanoparticles: From acute toxicity to multi-dose subchronic cytotoxicity study. Nanomaterials 2021, 11, 1577. [Google Scholar] [CrossRef]
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Vanitha, N.S.; Radhika, K.; Sudarmozhi, G.; Kavitha, G.; Shenbagapriya, M. NanoTechnology in Cosmetics and Cosmeceuticals; 2023; pp. 130–155. [Google Scholar] [CrossRef]
- Velidandi, A.; Dahariya, S.; Pabbathi, N.P.P.; Kalivarathan, D.; Baadhe, R.R. A review on synthesis, applications, toxicity, risk assessment and limitations of plant extracts synthesized silver nanoparticles. NanoWorld J. 2020, 6, 35–60. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Jenkins, G.J.S.; Doak, S.H. An Alternative Perspective towards Reducing the Risk of Engineered Nanomaterials to Human Health. Small 2020, 16, e2002002. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried Out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Göbbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part. Fibre Toxicol. 2011, 8, 9. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, J.; Zhai, S. Mechanistic understanding of toxicity from nanocatalysts. Int. J. Mol. Sci. 2014, 15, 13967–13992. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Yacobi, N.R.; Fazllolahi, F.; Kim, Y.H.; Sipos, A.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Nanomaterial interactions with and trafficking across the lung alveolar epithelial barrier: Implications for health effects of air-pollution particles. Air Qual. Atmos. Heal. 2011, 4, 65–78. [Google Scholar] [CrossRef]
- Liu, L.; Kong, L. Research progress on the carcinogenicity of metal nanomaterials. J. Appl. Toxicol. 2021, 41, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lv, S.; Liu, Y.; Li, Y. Biomarkers for the adverse effects on respiratory system health associated with atmospheric particulate matter exposure. J. Hazard. Mater. 2022, 421, 126760. [Google Scholar] [CrossRef]
- Portugal, J.; Bedia, C.; Amato, F.; Juárez-Facio, A.T.; Stamatiou, R.; Lazou, A.; Campiglio, C.E.; Elihn, K.; Piña, B. Toxicity of airborne nanoparticles: Facts and challenges. Environ. Int. 2024, 190, 108889. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Zaiter, T.; El-Basset, W.; Cornu, R.; Martin, H.; Diab-Assaf, M.; Béduneau, A. Interaction and toxicity of ingested nanoparticles on the intestinal barrier. Toxicology 2022, 481, 153353. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lai, X.; Shao, L.; Li, L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int. J. Nanomed. 2018, 13, 4445–4459. [Google Scholar] [CrossRef]
- Zaiter, T.; Cornu, R.; El Basset, W.; Martin, H.; Diab, M.; Béduneau, A. Toxicity assessment of nanoparticles in contact with the skin. J. Nanoparticle Res. 2022, 24, 149. [Google Scholar] [CrossRef]
- Colletta, A.D.; Pelin, M.; Sosa, S.; Fusco, L.; Prato, M.; Tubaro, A. CARBON-BASED nanomaterials and SKIN: An overview. Carbon 2022, 196, 683–698. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in vitro systems for nanotoxicology: Methodological considerations in vitro methods for nanotoxicology Vicki Stone et al. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef]
- Love, S.A.; Maurer-Jones, M.A.; Thompson, J.W.; Lin, Y.S.; Haynes, C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 2012, 5, 181–205. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Alba-González, A.; Fernández-Bertólez, N.; Touzani, A.; Ramos-Pan, L.; Reis, A.T.; Moreda-Piñeiro, J.; Yáñez, J.; Laffon, B.; Folgueira, M. Effects of Zinc Oxide Nanoparticle Exposure on Human Glial Cells and Zebrafish Embryos. Int. J. Mol. Sci. 2023, 24, 12297. [Google Scholar] [CrossRef] [PubMed]
- Katsumiti, A.; Arostegui, I.; Oron, M.; Gilliland, D.; Valsami-Jones, E.; Cajaraville, M.P. Cytotoxicity of Au, ZnO and SiO2 NPs using in vitro assays with mussel hemocytes and gill cells: Relevance of size, shape and additives. Nanotoxicology 2016, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Abudayyak, M.; Gurkaynak, T.A.; Özhan, G. In Vitro Toxicological Assessment of Cobalt Ferrite Nanoparticles in Several Mammalian Cell Types. Biol. Trace Elem. Res. 2017, 175, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Tanwir, F.; Yousefi Babadi, V. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, D.; Baranwal, M.; Chudasama, B. ROS-induced cytotoxicity of colloidal copper nanoparticles in MCF-7 human breast cancer cell line: An in vitro study. J. Nanoparticle Res. 2020, 22, 244. [Google Scholar] [CrossRef]
- De Mori, A.; Jones, R.S.; Cretella, M.; Cerri, G.; Draheim, R.R.; Barbu, E.; Tozzi, G.; Roldo, M. Evaluation of antibacterial and cytotoxicity properties of silver nanowires and their composites with carbon nanotubes for biomedical applications. Int. J. Mol. Sci. 2020, 21, 2303. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In vitro methods for assessing nanoparticle toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar] [CrossRef]
- Haniu, H.; Saito, N.; Matsuda, Y.; Kim, Y.-A.; Park, K.C.; Tsukahara, T.; Usui, Y.; Aoki, K.; Shimizu, M.; Ogihara, N.; et al. Elucidation mechanism of different biological responses to multi-walled carbon nanotubes using four cell lines. Int. J. Nanomed. 2011, 6, 3487–3497. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Kiliç, G.; Costa, P.M.; Fadeel, B. Cytotoxicity screening and cytokine profiling of nineteen nanomaterials enables hazard ranking and grouping based on inflammogenic potential. Nanotoxicology 2017, 11, 809–826. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Karlsson, H.L. Primary and Secondary Genotoxicity of Nanoparticles: Establishing a Co-Culture Protocol for Assessing Micronucleus Using Flow Cytometry. Front. Toxicol. 2022, 4, 845987. [Google Scholar] [CrossRef] [PubMed]
- Chetyrkina, M.R.; Fedorov, F.S.; Nasibulin, A.G. In vitro toxicity of carbon nanotubes: A systematic review. RSC Adv. 2022, 12, 16235–16256. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, M.S.; Friedman, A.E.; Finkelstein, J.N.; Oberdörster, G.; McGrath, J.L. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009, 30, 603–610. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Rubio, L.; Vila, L.; Xamena, N.; Velázquez, A.; Marcos, R.; Hernández, A. The comet assay as a tool to detect the genotoxic potential of nanomaterials. Nanomaterials 2019, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- eNanoMapper. Available online: https://www.enanomapper.net/data (accessed on 7 October 2024).
- Rosenkranz, P.; Fernández-Cruz, M.; Conde, E.; Ramírez-Fernández, M.; Flores, J.; Fernández, M.; Navas, J. Effects of cerium oxide nanoparticles to fish and mammalian cell lines: An assessment of cytotoxicity and methodology. Toxicol. Vitr. 2012, 26, 888–896. [Google Scholar] [CrossRef]
- Gábelová, A.; El Yamani, N.; Alonso, T.I.; Buliaková, B.; Srančíková, A.; Bábelová, A.; Pran, E.R.; Fjellsbø, L.M.; Elje, E.; Yazdani, M.; et al. Fibrous shape underlies the mutagenic and carcinogenic potential of nanosilver while surface chemistry affects the biosafety of iron oxide nanoparticles. Mutagenesis 2017, 32, 193–202. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Vranic, S.; Boland, S.; Moreau, K.; Baeza-Squiban, A.; Gaiser, B.K.; Andrzejczuk, L.A.; Stone, V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: Cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrol. 2013, 14, 96. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Rolim, C.M.B.; Vinardell, M.P. Mechanisms underlying cytotoxicity induced by engineered nanomaterials: A review of in vitro studies. Nanomaterials 2014, 4, 454–484. [Google Scholar] [CrossRef]
- Şeker, Ş.; Elçin, A.E.; Yumak, T.; Sinaǧ, A.; Elçin, Y.M. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. Vitr. 2014, 28, 1349–1358. [Google Scholar] [CrossRef]
- Li, L.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Gao, F. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Alkahtani, S.; Verma, A.; Ahamed, M.; Alhadlaq, H.A.; Ahamed, M. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int. J. Nanomed. 2013, 8, 983–993. [Google Scholar] [CrossRef]
- Patlolla, A.; Knighten, B.; Tchounwou, P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn. Dis. 2010, 20, S1. [Google Scholar] [PubMed]
- Browning, C.L. Titanium Dioxide Nanoparticles are not Cytotoxic or Clastogenic in Human Skin Cells. J. Environ. Anal. Toxicol. 2014, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chang, K.L.B.; Hwang, D.F.; Kong, Z.L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Bui, V.K.H.; Moon, J.-Y.; Lee, Y.-C. In-Vitro Cytotoxicity and Oxidative Stress Induced by Cerium Aminoclay and Cerium Oxide Nanoparticles in Human Skin Keratinocyte Cells. J. Nanosci. Nanotechnol. 2019, 19, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Borchert, P.; Zellmer-Bruhn, D.M. Evaluation of silver nanoparticle Toxicity in Skin in Vivo and Keratinocytes in Vitro. J. Allergy Clin. Immunol. 2010, 130, 556. [Google Scholar]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.-H.; Park, K.; Yi, J.; Ryu, D.-Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Chen, N.; Song, Z.-M.; Tang, H.; Xi, W.-S.; Cao, A.; Liu, Y.; Wang, H. Toxicological effects of Caco-2 cells following short-term and long-term exposure to Ag nanoparticles. Int. J. Mol. Sci. 2016, 17, 974. [Google Scholar] [CrossRef]
- Mello, D.F.; Trevisan, R.; Rivera, N.; Geitner, N.K.; Di Giulio, R.T.; Wiesner, M.R.; Hsu-Kim, H.; Meyer, J.N. Caveats to the use of MTT, neutral red, Hoechst and Resazurin to measure silver nanoparticle cytotoxicity. Chem. Biol. Interact. 2020, 315, 108868. [Google Scholar] [CrossRef]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef]
- Mittag, A.; Hoera, C.; Kämpfe, A.; Westermann, M.; Kuckelkorn, J.; Schneider, T.; Glei, M. Cellular uptake and toxicological effects of differently sized zinc oxide nanoparticles in intestinal cells. Toxics 2021, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Vogt, R.D.; Liu, Y.; Luo, J.; Li, T. Bioavailability and cytotoxicity of Cerium- (IV), Copper- (II), and Zinc oxide nanoparticles to human intestinal and liver cells through food. Sci. Total Environ. 2020, 702, 134700. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Z.; Cheng, B.; Xiang, K.; Chen, X.; Liu, J.; Cao, A.; Wang, Y.; Liu, Y.; Wang, H. Evaluation of the toxicity of food additive silica nanoparticles on gastrointestinal cells. J. Appl. Toxicol. 2014, 34, 424–435. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A. Influence of silica nanoparticles on cadmium-induced cytotoxicity, oxidative stress, and apoptosis in human liver HepG2 cells. Environ. Toxicol. 2020, 35, 599–608. [Google Scholar] [CrossRef]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the characteristics and cytotoxicity of titanium dioxide nanomaterials following simulated in vitro digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Ghalami, R.Z.; Kamran, M.; Van Breusegem, F.; Karpiński, S. To Be or Not to Be? Are Reactive Oxygen Species, Antioxidants, and Stress Signalling Universal Determinants of Life or Death? Cells 2022, 11, 4105. [Google Scholar] [CrossRef]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef]
- Bacova, J.; Knotek, P.; Kopecka, K.; Hromadko, L.; Capek, J.; Nyvltova, P.; Bruckova, L.; Schröterova, L.; Sestakova, B.; Palarcik, J.; et al. Evaluating the Use of TiO2 Nanoparticles for Toxicity Testing in Pulmonary A549 Cells. Int. J. Nanomed. 2022, 17, 4211–4225. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Sha, S.; Li, J.; Zhou, Z.; Cao, Y. Evaluation of in vitro toxicity of silica nanoparticles (NPs) to lung cells: Influence of cell types and pulmonary surfactant component DPPC. Ecotoxicol. Environ. Saf. 2019, 186, 109770. [Google Scholar] [CrossRef]
- Schlinkert, P.; Casals, E.; Boyles, M.; Tischler, U.; Hornig, E.; Tran, N.; Zhao, J.; Himly, M.; Riediker, M.; Oostingh, G.J.; et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J. Nanobiotechnology 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Rea, C.; O’hare, P.; Mathur, A.; Roy, S.; Dunlop, P.; Byrne, J.; Burke, G.; Meenan, B.; McLaughlin, J. Comparative in vitro cytotoxicity study of carbon nanotubes and titania nanostructures on human lung epithelial cells. J. Hazard. Mater. 2011, 191, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, X.; Wang, B.; Xu, G.; Qin, Z.; Wang, J.; Wu, L.; Ju, X.; Bose, D.D.; Qiu, F.; et al. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis. 2017, 8, e2954. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R.; Anand, T.; Khanum, F. Nanosized Zinc Oxide Induces Toxicity in Human Lung Cells. ISRN Toxicol. 2013, 2013, 316075. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, J.; Yang, D.; Xu, Y.; Zhu, J.; He, D. Low Concentrations of Zinc Oxide Nanoparticles Cause Severe Cytotoxicity Through Increased Intracellular Reactive Oxygen Species. J. Biomed. Nanotechnol. 2021, 17, 2420–2432. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Lin, M.; Hu, X. ZnO nanoparticles induced cytotoxicity on human pulmonary adenocarcinoma cell line LTEP-a-2. Process Saf. Environ. Prot. 2015, 93, 265–273. [Google Scholar] [CrossRef]
- Zhuo, L.B.; Liu, Y.M.; Jiang, Y.; Yan, Z. Zinc oxide nanoparticles induce acute lung injury via oxidative stress-mediated mitochondrial damage and NLRP3 inflammasome activation: In vitro and in vivo studies. Environ. Pollut. 2024, 341, 122950. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, T.; Chen, C.; Liu, Y. Right or left: The role of nanoparticles in pulmonary diseases. Int. J. Mol. Sci. 2014, 15, 17577–17600. [Google Scholar] [CrossRef]
- Refsnes, M.; Skuland, T.; Øvrevik, J.; Låg, M. Role of scavenger receptors in silica nanoparticle-induced cytokine responses in bronchial epithelial cells. Toxicol. Lett. 2021, 353, 100–106. [Google Scholar] [CrossRef]
- Corsi, F.; Tarquini, G.D.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox? Nanomaterials 2023, 13, 2803. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Babaei, V.; Babaei, V.; Ashtarinezhad, A.; Ashtarinezhad, A.; Torshabi, M.; Torshabi, M.; Teimourian, S.; Teimourian, S.; Shahmirzaie, M.; Shahmirzaie, M.; et al. High inflammatory cytokines gene expression can be detected in workers with prolonged exposure to silver and silica nanoparticles in industries. Sci. Rep. 2024, 14, 5667. [Google Scholar] [CrossRef] [PubMed]
- NANoREG. NANoREG—A Common European approach to the Regulatory Testing of Nanomaterials; NanoRegMed: London, UK, 2013; pp. 2–3. [Google Scholar]
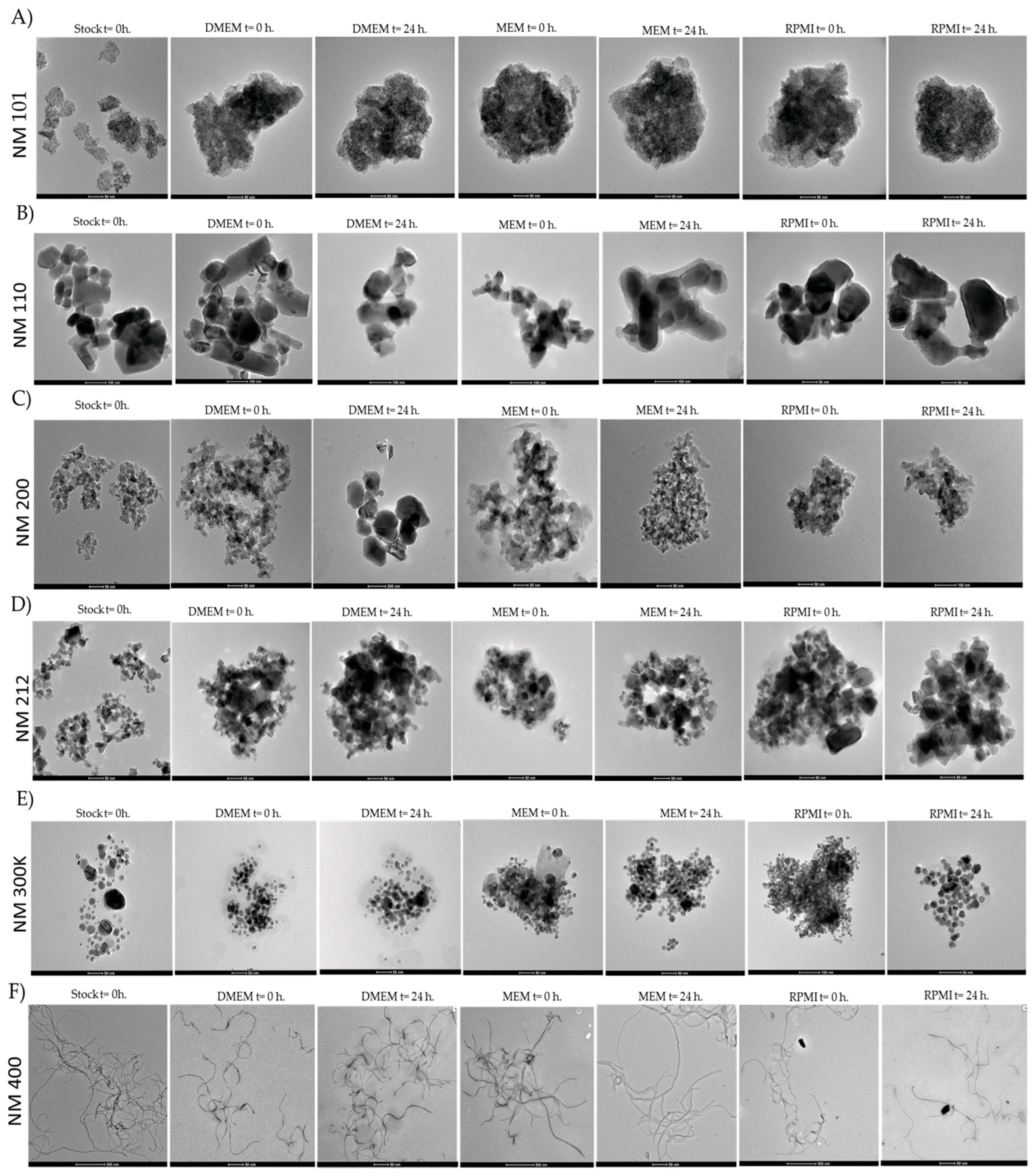

| Time Point (Hours) | Medium | NM 101 (TiO2 NM) | NM 110 (ZnO NM) | NM 200 (SiO2 NM) | NM 212 (CeO2 NM) | NM 300 K (Ag NM) | NM 400 (MWCNT NM) |
|---|---|---|---|---|---|---|---|
| Z-Ave (d. nm) ± SD | Z-Ave (d. nm) ± SD | Z-Ave (d. nm) ± SD | Z-Ave (d. nm) ± SD | Z-Ave (d. nm) ± SD | Z-Ave (d. nm) ± SD | ||
| 0 h | Batch | 15.49 ± 0.22 | 23.63 ± 0.23 | 337.23 ± 9.83 | 415.6 ± 134.32 | 3.38 ± 3.35 | 1.08 ± 0.94 |
| DMEM | 490.49 ± 27.49 * | 240.77 ± 4.08 * | 276.05 ± 6.96 | 311.57 ± 5.05 | 67.95 ± 0.92 * | 611.58 ± 18.09 * | |
| 24 h | DMEM | 472.02 ± 15.90 * | 293.94 ± 8.11 * | 258.26 ± 14.86 | 247.13 ± 3.53 | 74.12 ± 0.91 * | 428.16 ± 25.65 * |
| 0 h | Batch | 16.97 ± 0.15 | 26.27 ± 0.28 | 350.45 ± 15.23 | 438.66 ± 79.13 | 1.3 ± 1.95 | 1.86 ± 1.06 |
| MEM | 452.92 ± 8.34 * | 267 ± 7.78 * | 288.69 ± 12.07 | 337.51 ± 8.6 | 63.63 ± 0.81 * | 518.8 ± 23.51 * | |
| 24 h | MEM | 279.71 ± 5.34 * | 224.85 ± 5.45 * | 259.74 ± 32.50 | 274.24 ± 6.06 | 66.83 ± 1.53 * | 312.88 ± 10.15 * |
| 0 h | Batch | 11.68 ± 3.23 | 23.66 ± 1.12 | 344.83 ± 24.46 | 431.07 ± 59.83 | 14.75 ± 7.95 | 1.86 ± 1.06 |
| RPMI | 587.11 ± 16.31 * | 289.09 ± 6.49 * | 282.40 ± 11.25 | 291.54 ± 5.86 | 64.94 ± 2.65 * | 546.78 ± 17.51 * | |
| 24 h | RPMI | 278.88 ± 5.92 * | 213.90 ± 4.93 * | 223.69 ± 9.87 | 241.95 ± 5.46 | 53.55 ± 1.01 * | 406.54 ± 61.07 * |
| MTS Assay | Alamar Blue Assay | Neutral Red Assay | |||||
|---|---|---|---|---|---|---|---|
| NM Code | Cell Type | IC₅₀ (µg/mL) | IC₈₀ (µg/mL) | IC₅₀ (µg/mL) | IC₈₀ (µg/mL) | IC₅₀ (µg/mL) | IC₈₀ (µg/mL) |
| NM 101 (TiO2 NM) | 3T3 | >100 | >100 | >100 | >100 | >100 | >100 |
| Caco-2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| A549 | >100 | >100 | >100 | >100 | >100 | >100 | |
| HepG2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| THP-1 | >100 | >100 | >100 | >100 | >100 | >100 | |
| NM 110 (ZnO NM) | 3T3 | 2.69 ± 0.32 * | 6.06 ± 0.93 * | 5.55 ± 0.39 * | 20.24 ± 1.58 * | 3.74 ± 0.35 * | 17.30 ± 1.92 * |
| Caco-2 | 15.04 ± 2.05 * | 20.78 ± 1.66 * | 14.93 ± 1.95 * | 21.73 ± 2.17 * | 14.23 ± 1.84 * | 20.09 ± 2.65 * | |
| A549 | 16.39 ± 1.53 * | 25.87 ± 2.35 * | 64.65 ± 3.96 * | >100 | 14.55 ± 1.23 * | 28.16 ± 2.42 * | |
| HepG2 | 19.48 ± 2.42 * | 25.26 ± 2.19 * | 17.24 ± 1.05 * | 32.66 ± 1.97 * | 17.10 ± 1.52 * | 21.97 ± 1.04 * | |
| THP-1 | 50.55 ± 4.02 * | >100 | >100 | >100 | >100 | >100 | |
| NM 200 (SiO2 NM) | 3T3 | >100 | >100 | >100 | >100 | >100 | >100 |
| A549 | >100 | >100 | >100 | >100 | >100 | >100 | |
| Caco-2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| HepG2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| THP-1 | >100 | >100 | 35.91 ± 2.83 * | >100 | 66.64 ± 5.02 * | >100 | |
| NM 212 (CeO2 NM) | 3T3 | >100 | >100 | >100 | >100 | >100 | >100 |
| Caco-2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| THP-1 | >100 | >100 | >100 | >100 | >100 | >100 | |
| A549 | >100 | >100 | >100 | >100 | >100 | >100 | |
| HepG2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| NM 300 K (Ag NM) | 3T3 | 91.21 ± 5.67 | >100 | >100 | >100 | >100 | >100 |
| Caco-2 | 4.45 ± 0.53 * | 60.39 ± 4.95 * | 18.32 ± 2.05 * | >100 | 98.06 ± 6.53 | >100 | |
| A549 | >100 | >100 | >100 | >100 | >100 | >100 | |
| HepG2 | 25.77 ± 2.05 * | >100 | 24.19 ± 2.23 * | 43.98 ± 4.07 * | 1.02 ± 0.13 * | >100 | |
| THP-1 | 95.73 ± 5.93 | >100 | >100 | >100 | 5.15 ± 0.98 * | >100 | |
| NM 400 (MWCNT NM) | 3T3 | >100 | >100 | 69.38 ± 3.87 * | >100 | >100 | >100 |
| Caco-2 | >100 | >100 | 64.86 ± 5.62 * | >100 | >100 | >100 | |
| A549 | >100 | >100 | >100 | >100 | >100 | >100 | |
| HepG2 | >100 | >100 | >100 | >100 | >100 | >100 | |
| THP-1 | >100 | >100 | 93.40 ± 6.85 | >100 | >100 | >100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Romero, J.; Mentxaka, G.; García-Salvador, A.; Katsumiti, A.; Carregal-Romero, S.; Goñi-de-Cerio, F. Assessing the Toxicity of Metal- and Carbon-Based Nanomaterials In Vitro: Impact on Respiratory, Intestinal, Skin, and Immune Cell Lines. Int. J. Mol. Sci. 2024, 25, 10910. https://doi.org/10.3390/ijms252010910
Carrillo-Romero J, Mentxaka G, García-Salvador A, Katsumiti A, Carregal-Romero S, Goñi-de-Cerio F. Assessing the Toxicity of Metal- and Carbon-Based Nanomaterials In Vitro: Impact on Respiratory, Intestinal, Skin, and Immune Cell Lines. International Journal of Molecular Sciences. 2024; 25(20):10910. https://doi.org/10.3390/ijms252010910
Chicago/Turabian StyleCarrillo-Romero, Juliana, Gartze Mentxaka, Adrián García-Salvador, Alberto Katsumiti, Susana Carregal-Romero, and Felipe Goñi-de-Cerio. 2024. "Assessing the Toxicity of Metal- and Carbon-Based Nanomaterials In Vitro: Impact on Respiratory, Intestinal, Skin, and Immune Cell Lines" International Journal of Molecular Sciences 25, no. 20: 10910. https://doi.org/10.3390/ijms252010910






