Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics
Abstract
1. Background
2. Methodology
3. Results/Discussion
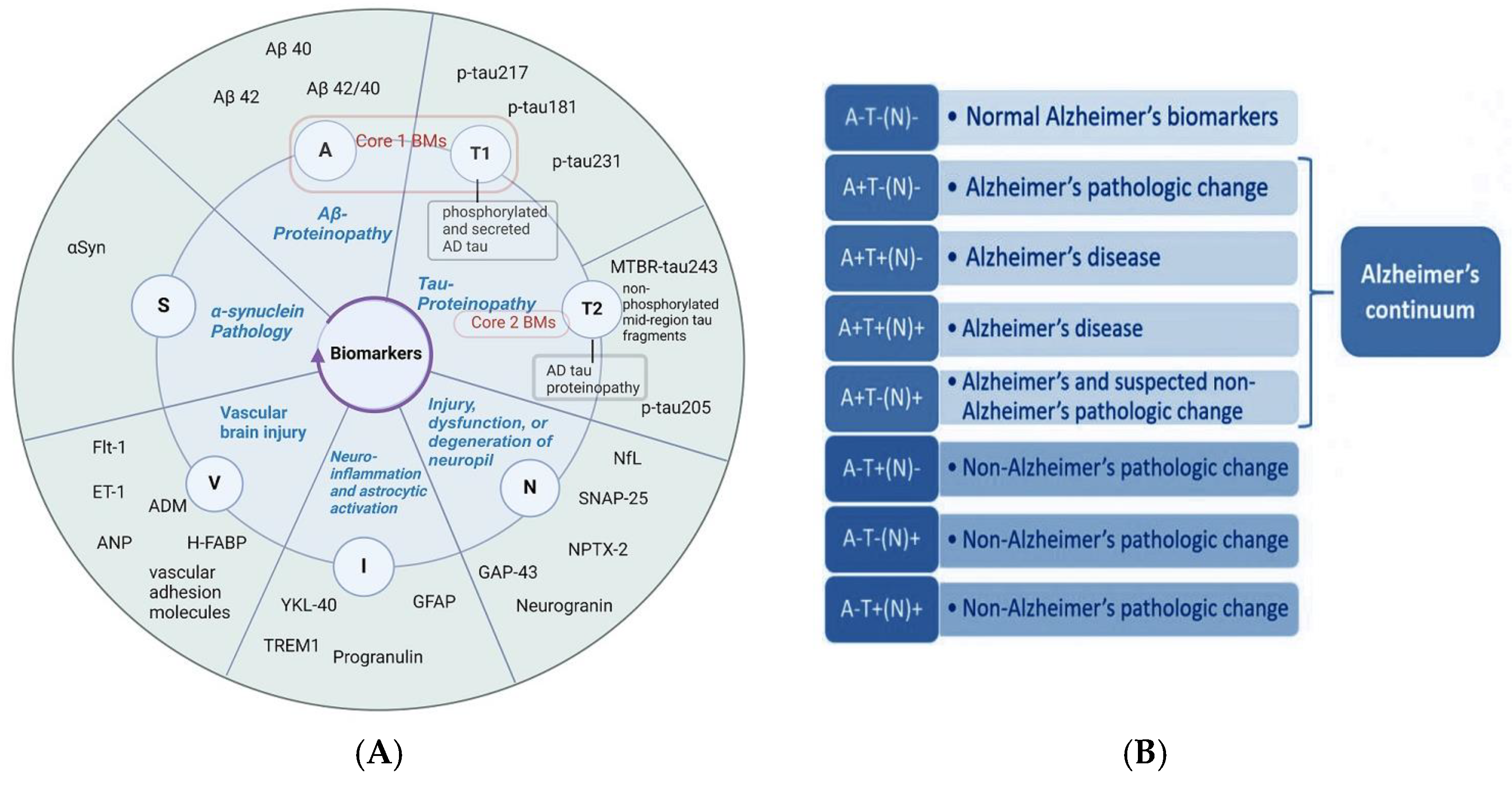
3.1. Current Insights into Different Biomarker Categorizations
- Core biomarkers of AD neuropathological changes;
- Non-specific biomarkers that are important in AD pathogenesis but are also involved in other brain diseases;
- Biomarkers of common non-AD pathologies.
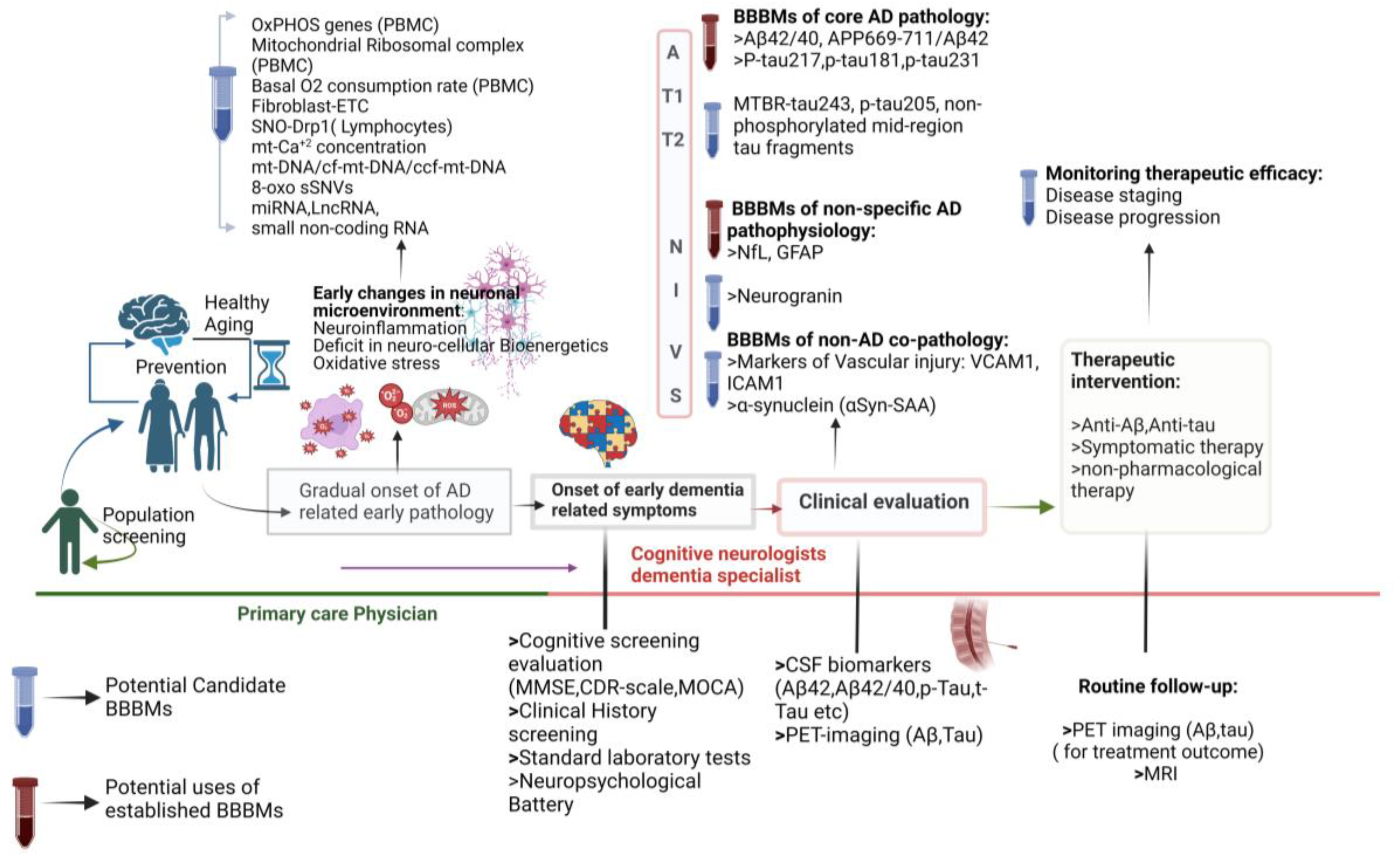
3.2. The Context for Developing BBBMs in AD
3.3. BBBMs Related to Abnormal Protein Accumulation for the Early Detection of AD
3.3.1. Plasma Biomarkers Related to Abnormal Protein Accumulation as Core Indicators of AD
Aβ and Its Variations in Plasma
Plasma p-Tau
- Nodes:
- -
- Colored nodes: Query proteins and first shell of interactors;
- White nodes: Second shell of interactors;
- Empty nodes: Proteins of unknown 3D structure;
- Filled nodes: Proteins with known or predicted 3D structure.
- Blue: Known interactions from curated databases;
- -
- Pink: Experimentally determined interactions;
- -
- Green: Predicted interactions from gene neighborhood;
- -
- Red: Predicted interactions from gene fusions;
- -
- Dark blue: Predicted interactions from gene co-occurrence;
- -
- Yellow: Interactions from text-mining;
- -
- Black: Co-expression;
- Light blue: Interactions from text-mining.
3.3.2. Biomarkers Related to Abnormal Protein Accumulation in Non-Core AD Pathology
Biomarkers of TAR DNA-Binding Protein (TDP-43) Accumulation
BBBMs Associated with Synuclein Pathology
Serum Dickkopf-1(DKK1) as Candidate BBBM in AD
Plasma Visinin-like Protein-1 (VILIP-1)
3.4. BBBMs of Neuronal and Synaptic Injury
3.4.1. Plasma Neurofilaments as AD Diagnostic and Disease Progression Biomarkers
3.4.2. BBBMs Related to Pre-Synaptic Dysfunction
3.4.3. BBBMs Related to Post-Synaptic Protein Dysfunction
3.5. Blood-Based AD-Related Biomarkers Associated with Vascular Pathology
3.5.1. Fms-like Tyrosine Kinase-1 (Flt-1) in AD-Related Vascular Changes
3.5.2. Role of Endothelin 1 (ET-1) in AD-Associated Vascular Pathology
3.5.3. Alteration of Adrenomedullin (ADM) in AD
3.5.4. Role of Atrial Natriuretic Peptide (ANP) in AD-Related Vascular Alterations
3.5.5. Vascular Immune Interaction and Monokine Induced by Gamma Interferon/C-X-C Motif Chemokine Ligand 9 (MIG/CXCL9) in AD
3.5.6. Role of Heart-Type Fatty Acid-Binding Protein (H-FABP) in AD-Related Vascular Pathology
3.5.7. Alteration of Vascular Adhesion Molecule (AM) Expression and Endothelial Dysfunction in AD
3.6. BBBMs Associated with Oxidative Stress and Bioenergetics
3.6.1. BBBMs Related to Oxidative Stress
3.6.2. Blood-Based Bioenergetic Profiling
3.7. BBBMs of Neuroinflammation and Immune Dysregulation
3.8. Blood-Based Epigenetic Biomarkers Related to Early Detection and Prognosis of AD
3.8.1. DNA Methylation-Based Markers
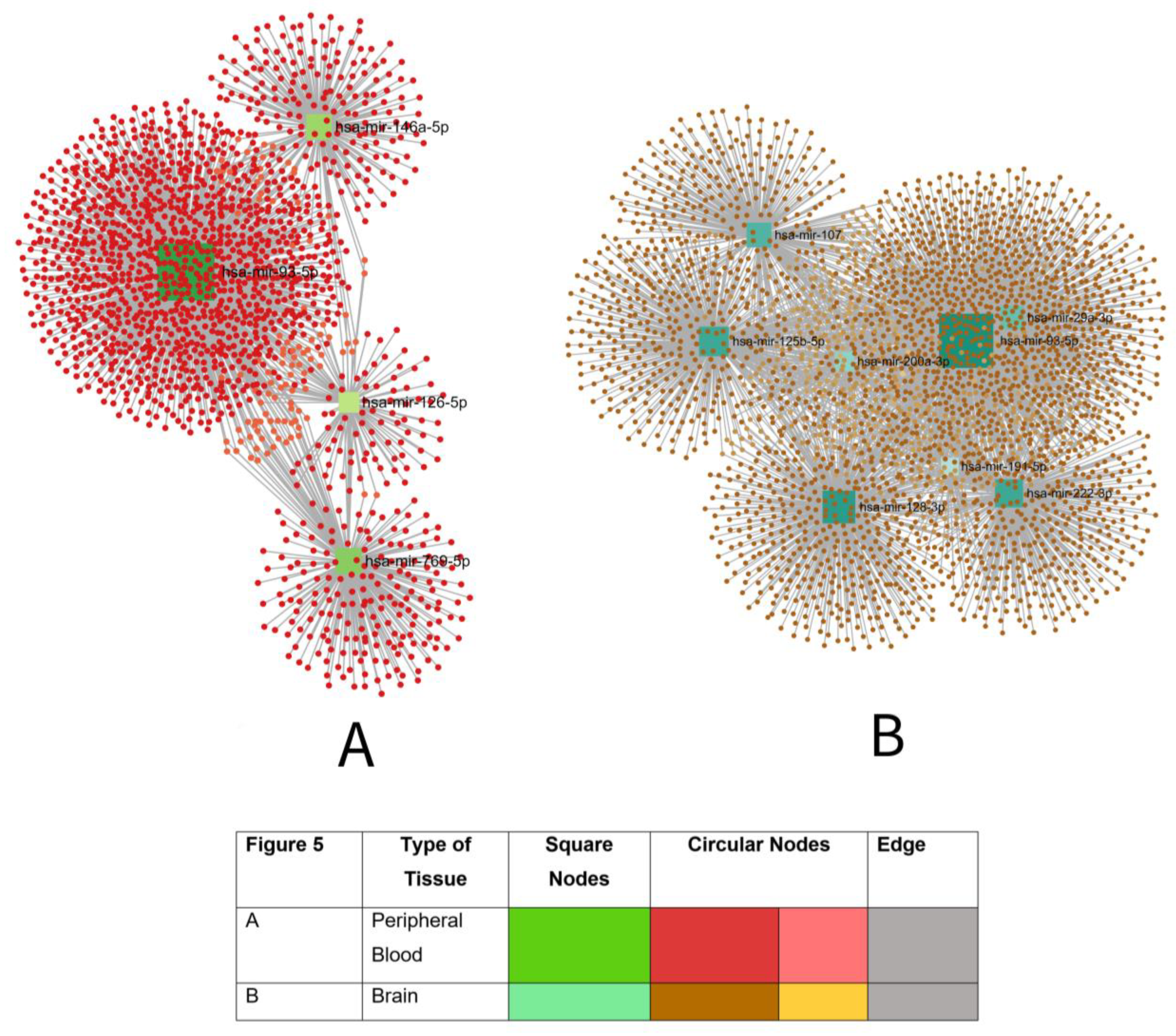
3.8.2. Potential Blood-Based microRNA Biomarkers for AD
3.8.3. Potential Blood-Based Long Non-Coding RNA (lncRNA) Biomarkers for AD
3.8.4. Markers Related to Histone Modification and DNA Alteration
3.8.5. Circular RNA (circRNA)-Related Biomarkers
3.9. Plasma Exosome-Based AD-Related Biomarkers
4. Differential Expression of BBBMs in Hereditary Subtypes of AD and Non-Alzheimer’s Dementias
5. Current Challenges and Limitations in Incorporating AD-Associated BBBMs in Clinical Practice
5.1. Lack of Standard Cut-Off Points in Plasma-Based Assay Techniques and Optimal Study Design
5.2. Changes in AD-Related BBBMs across Various Chronic Conditions
6. Future Directions and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vanessa de Jesus, R.; Guimarães, F.M.; Diniz, B.S.; Forlenza, O.V. Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dement. Neuropsychol. 2009, 3, 188. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr71. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. Early-onset Alzheimer disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Klyucherev, T.O.; Olszewski, P.; Shalimova, A.A.; Chubarev, V.N.; Tarasov, V.V.; Attwood, M.M.; Syvänen, S.; Schiöth, H.B. Advances in the development of new biomarkers for Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 25. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Angioni, D.; Delrieu, J.; Hansson, O.; Fillit, H.; Aisen, P.; Cummings, J.; Sims, J.; Braunstein, J.; Sabbagh, M.; Bittner, T. Blood biomarkers from research use to clinical practice: What must be done? A report from the EU/US CTAD Task Force. J. Prev. Alzheimer’s Dis. 2022, 9, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Croitoru, C.G.; Hodorog, D.N.; Cuciureanu, D.I. Passive Anti-Amyloid Beta Immunotherapies in Alzheimer’s Disease: From Mechanisms to Therapeutic Impact. Biomedicines 2024, 12, 1096. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H.; Ashton, N.J. Blood-based biomarkers in Alzheimer’s disease–moving towards a new era of diagnostics. Clin. Chem. Lab. Med. 2024, 62, 1063–1069. [Google Scholar] [CrossRef]
- Assfaw, A.D.; Schindler, S.E.; Morris, J.C. Advances in blood biomarkers for Alzheimer disease (AD): A review. Kaohsiung J. Med. Sci. 2024, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.C.; Kinney, J.W.; Ritter, A.; Andrews, R.D.; Toledano Strom, E.N.; Lukic, A.S.; Koenig, L.N.; Revta, C.; Fillit, H.M.; Zhong, K. Relationships between plasma biomarkers, tau PET, FDG PET, and volumetric MRI in mild to moderate Alzheimer’s disease patients. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2024, 10, e12490. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.L.; Lawler, P.E.; Bollinger, J.G.; Li, Y.; Schindler, S.E.; Li, M.; Lopez, S.; Ovod, V.; Nakamura, A.; Shaw, L.M. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: A literature review. Alzheimer’s Res. Ther. 2022, 14, 195. [Google Scholar] [CrossRef]
- Wang, M.J.; Yi, S.; Han, J.-y.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Mielke, M.M. An update on blood-based markers of Alzheimer’s disease using the SiMoA platform. Neurol. Ther. 2019, 8, 73–82. [Google Scholar] [CrossRef]
- Wang, R.; Sweeney, D.; Gandy, S.E.; Sisodia, S.S. The Profile of Soluble Amyloid β Protein in Cultured Cell Media: Detection and quantification of amyloid β protein and variants by immunoprecipitation-mass spectrometry. J. Biol. Chem. 1996, 271, 31894–31902. [Google Scholar] [CrossRef]
- Korecka, M.; Shaw, L.M. Mass spectrometry-based methods for robust measurement of Alzheimer’s disease biomarkers in biological fluids. J. Neurochem. 2021, 159, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shen, X.-N.; Wang, H.-F.; Chen, S.-D.; Zhang, Y.-R.; Chen, S.-F.; Cui, M.; Cheng, W.; Dong, Q.; Ma, T. The dynamics of plasma biomarkers across the Alzheimer’s continuum. Alzheimer’s Res. Ther. 2023, 15, 31. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.; Xiong, C. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef] [PubMed]
- Ovod, V.; Ramsey, K.N.; Mawuenyega, K.G.; Bollinger, J.G.; Hicks, T.; Schneider, T.; Sullivan, M.; Paumier, K.; Holtzman, D.M.; Morris, J.C. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s Dement. 2017, 13, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, W.; Chen, Y.; Lin, Y.; Wang, B.; Guo, Q.; Miao, Y. Plasma Aβ as a biomarker for predicting Aβ-PET status in Alzheimer’s disease: A systematic review with meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Jackson, E.N.; Harpstrite, S.E.; Verghese, P.B.; West, T.; Fogelman, I. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin. Chim. Acta 2021, 519, 267–275. [Google Scholar] [CrossRef]
- Ge, X.; Qiao, Y.; Choi, J.; Raman, R.; Ringman, J.M.; Shi, Y.; Initiative, A.s.D.N. Enhanced association of tau pathology and cognitive impairment in mild cognitive impairment subjects with behavior symptoms. J. Alzheimer’s Dis. 2022, 87, 557–568. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Aβ: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- McGrath, E.R.; Beiser, A.S.; O’Donnell, A.; Yang, Q.; Ghosh, S.; Gonzales, M.M.; Himali, J.J.; Satizabal, C.L.; Johnson, K.A.; Tracy, R.P. Blood phosphorylated tau 181 as a biomarker for amyloid burden on brain PET in cognitively healthy adults. J. Alzheimer’s Dis. 2022, 87, 1517–1526. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Ferreira, P.C.; González-Escalante, A.; Montoliu-Gaya, L.; Ortiz-Romero, P.; Kac, P.R.; Turton, M.; Kvartsberg, H.; Ashton, N.J.; Zetterberg, H. A novel ultrasensitive assay for plasma p-tau217: Performance in individuals with subjective cognitive decline and early Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 1239–1249. [Google Scholar] [CrossRef]
- Ashton, N.J.; Brum, W.S.; Di Molfetta, G.; Benedet, A.L.; Arslan, B.; Jonaitis, E.; Langhough, R.E.; Cody, K.; Wilson, R.; Carlsson, C.M.; et al. Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology. JAMA Neurol. 2024, 81, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; Initiative, A.s.D.N.; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med. 2021, 27, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Berron, D.; Smith, R.; Strandberg, O.; Proctor, N.K.; Dage, J.L.; Stomrud, E.; Palmqvist, S.; Mattsson-Carlgren, N.; Hansson, O. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021, 78, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Raho, E.M.; Di Lorenzo, F. Is blood pTau a reliable indicator of the CSF status? A narrative review. Neurol. Sci. 2024, 45, 2471–2487. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, F.; Turton, M.; Kac, P.R.; Smirnov, D.; Premi, E.; Ghidoni, R.; Benussi, L.; Cantoni, V.; Saraceno, C.; Rivolta, J. Brain-derived tau: A novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain 2023, 146, 1152–1165. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rodríguez, R.; Pérez-Yanes, S.; Montelongo, R.; Lorenzo-Salazar, J.M.; Estévez-Herrera, J.; García-Luis, J.; Íñigo-Campos, A.; Rubio-Rodríguez, L.A.; Muñoz-Barrera, A.; Trujillo-González, R. Transactive response DNA-binding protein (TARDBP/TDP-43) regulates cell permissivity to HIV-1 infection by acting on HDAC6. Int. J. Mol. Sci. 2022, 23, 6180. [Google Scholar] [CrossRef]
- Gatignol, A.; Duarte, M.; Daviet, L.; Chang, Y.-N.; Jeang, K.-T. Sequential steps in Tat trans-activation of HIV-1 mediated through cellular DNA, RNA, and protein binding factors. Gene Expr. 1996, 5, 217. [Google Scholar]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 pathology in Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 1–15. [Google Scholar] [CrossRef]
- Lopez, O.L.; Kofler, J.; Chang, Y.; Berman, S.B.; Becker, J.T.; Sweet, R.A.; Nadkarni, N.; Patira, R.; Kamboh, M.I.; Cohen, A.D. Hippocampal sclerosis, TDP-43, and the duration of the symptoms of dementia of AD patients. Ann. Clin. Transl. Neurol. 2020, 7, 1546–1556. [Google Scholar] [CrossRef]
- Katisko, K.; Huber, N.; Kokkola, T.; Hartikainen, P.; Krüger, J.; Heikkinen, A.-L.; Paananen, V.; Leinonen, V.; Korhonen, V.E.; Helisalmi, S. Serum total TDP-43 levels are decreased in frontotemporal dementia patients with C9orf72 repeat expansion or concomitant motoneuron disease phenotype. Alzheimer’s Res. Ther. 2022, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Cordts, I.; Wachinger, A.; Scialo, C.; Lingor, P.; Polymenidou, M.; Buratti, E.; Feneberg, E. TDP-43 proteinopathy specific biomarker development. Cells 2023, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Sephton, C.F.; Cenik, B.; Cenik, B.K.; Herz, J.; Yu, G. TDP-43 in central nervous system development and function: Clues to TDP-43-associated neurodegeneration. Biol. Chem. 2012, 393, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Wilhite, R.; Sage, J.M.; Bouzid, A.; Primavera, T.; Agbas, A. Platelet phosphorylated TDP-43: An exploratory study for a peripheral surrogate biomarker development for Alzheimer’s disease. Future Sci. OA 2017, 3, FSO238. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Barbour, R.; Kling, K.; Anderson, J.P.; Banducci, K.; Cole, T.; Diep, L.; Fox, M.; Goldstein, J.M.; Soriano, F.; Seubert, P. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008, 5, 55–59. [Google Scholar] [CrossRef]
- Kasuga, K.; Tokutake, T.; Ishikawa, A.; Uchiyama, T.; Tokuda, T.; Onodera, O.; Nishizawa, M.; Ikeuchi, T. Differential levels of α-synuclein, β-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, K.; Nishizawa, M.; Ikeuchi, T. α-Synuclein as CSF and Blood Biomarker of Dementia with Lewy Bodies. Int. J. Alzheimer’s Dis. 2012, 2012, 437025. [Google Scholar] [CrossRef][Green Version]
- Daniele, S.; Baldacci, F.; Piccarducci, R.; Palermo, G.; Giampietri, L.; Manca, M.L.; Pietrobono, D.; Frosini, D.; Nicoletti, V.; Tognoni, G. α-Synuclein heteromers in red blood cells of Alzheimer’s disease and Lewy body dementia patients. J. Alzheimer’s Dis. 2021, 80, 885–893. [Google Scholar] [CrossRef]
- Laske, C.; Fallgatter, A.J.; Stransky, E.; Hagen, K.; Berg, D.; Maetzler, W. Decreased α-synuclein serum levels in patients with Lewy body dementia compared to Alzheimer’s disease patients and control subjects. Dement. Geriatr. Cogn. Disord. 2011, 31, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic interactions between Aβ, tau, and α-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Daniele, S.; Piccarducci, R.; Giampietri, L.; Pietrobono, D.; Giorgi, F.S.; Nicoletti, V.; Frosini, D.; Libertini, P.; Lo Gerfo, A. Potential diagnostic value of red blood cells α-synuclein heteroaggregates in Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 6451–6459. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gu, X.; Li, H.; Lei, S.; Wang, Z.; Wang, J.; Yin, P.; Zhang, C.; Wang, F.; Liu, C. The role of DKK1 in Alzheimer’s disease: A potential intervention point of brain damage prevention? Pharmacol. Res. 2019, 144, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Storto, M.; Gaviraghi, G.; Terstappen, G.C.; Nicoletti, F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J. Neurosci. 2004, 24, 6021–6027. [Google Scholar] [CrossRef]
- Purro, S.A.; Galli, S.; Salinas, P.C. Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. J. Mol. Cell Biol. 2014, 6, 75–80. [Google Scholar] [CrossRef]
- Seib, D.R.; Corsini, N.S.; Ellwanger, K.; Plaas, C.; Mateos, A.; Pitzer, C.; Niehrs, C.; Celikel, T.; Martin-Villalba, A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 2013, 12, 204–214. [Google Scholar] [CrossRef]
- Marzo, A.; Galli, S.; Lopes, D.; McLeod, F.; Podpolny, M.; Segovia-Roldan, M.; Ciani, L.; Purro, S.; Cacucci, F.; Gibb, A. Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Curr. Biol. 2016, 26, 2551–2561. [Google Scholar] [CrossRef]
- Tay, L.; Leung, B.; Yeo, A.; Chan, M.; Lim, W.S. Elevations in Serum Dickkopf-1 and disease progression in community-dwelling older adults with mild cognitive impairment and mild-to-moderate Alzheimer’s disease. Front. Aging Neurosci. 2019, 11, 278. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Macy, E.; Xiong, C.; Carter, D.; Cairns, N.J.; Fagan, A.M.; Head, D.; Mintun, M.A.; Ladenson, J.H. Visinin-like protein-1: Diagnostic and prognostic biomarker in Alzheimer disease. Ann. Neurol. 2011, 70, 274–285. [Google Scholar] [CrossRef]
- Halbgebauer, S.; Steinacker, P.; Riedel, D.; Oeckl, P.; Anderl-Straub, S.; Lombardi, J.; von Arnim, C.A.; Nagl, M.; Giese, A.; Ludolph, A.C. Visinin-like protein 1 levels in blood and CSF as emerging markers for Alzheimer’s and other neurodegenerative diseases. Alzheimer’s Res. Ther. 2022, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Karantali, E.; Kazis, D. A meta-analysis on the levels of VILIP-1 in the CSF of Alzheimer’s disease compared to normal controls and other neurodegenerative conditions. Aging Clin. Exp. Res. 2021, 33, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shi, M.; Li, Y.; Zhao, X. Elevated plasma neurofilament light was associated with multi-modal neuroimaging features in Alzheimer’s Disease signature regions and predicted future tau deposition. Res. Sq. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017, 74, 557–566. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, D.; Linder, J.; Jakobson Mo, S.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L.; Lenfeldt, N. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 2020, 95, e827–e838. [Google Scholar] [CrossRef] [PubMed]
- Kivisäkk, P.; Carlyle, B.C.; Sweeney, T.; Quinn, J.P.; Ramirez, C.E.; Trombetta, B.A.; Mendes, M.; Brock, M.; Rubel, C.; Czerkowicz, J. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 58. [Google Scholar] [CrossRef]
- Halbgebauer, S.; Steinacker, P.; Hengge, S.; Oeckl, P.; Rumeileh, S.A.; Anderl-Straub, S.; Lombardi, J.; Von Arnim, C.A.; Giese, A.; Ludolph, A.C. CSF levels of SNAP-25 are increased early in Creutzfeldt-Jakob and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1059–1065. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bianchi, A.; Nemni, R.; Clerici, M. SNAP-25 in serum is carried by exosomes of neuronal origin and is a potential biomarker of Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 5792–5798. [Google Scholar] [CrossRef]
- Libiger, O.; Shaw, L.M.; Watson, M.H.; Nairn, A.C.; Umaña, K.L.; Biarnes, M.C.; Canet-Avilés, R.M.; Jack Jr, C.R.; Breton, Y.A.; Cortes, L. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Saunders, T.S.; Gadd, D.A.; Spires-Jones, T.L.; King, D.; Ritchie, C.; Muniz-Terrera, G. Associations between cerebrospinal fluid markers and cognition in ageing and dementia: A systematic review. Eur. J. Neurosci. 2022, 56, 5650–5713. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Q.; Skudder-Hill, L.; Toyota, T.; Wei, W.; Adachi, H. CSF GAP-43 as a biomarker of synaptic dysfunction is associated with tau pathology in Alzheimer’s disease. Sci. Rep. 2022, 12, 17392. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimer’s Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, T.; Blandino, V.; Maniscalco, L.; Matranga, D.; Graziano, F.; Guajana, F.; Agnello, L.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V. Biomarkers related to synaptic dysfunction to discriminate alzheimer’s disease from other neurological disorders. Int. J. Mol. Sci. 2022, 23, 10831. [Google Scholar] [CrossRef]
- Zhong, L.; Gerges, N.Z. Neurogranin and synaptic plasticity balance. Commun. Integr. Biol. 2010, 3, 340–342. [Google Scholar] [CrossRef]
- Kvartsberg, H.; Portelius, E.; Andreasson, U.; Brinkmalm, G.; Hellwig, K.; Lelental, N.; Kornhuber, J.; Hansson, O.; Minthon, L.; Spitzer, P. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimer’s Res. Ther. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Wellington, H.; Paterson, R.W.; Portelius, E.; Törnqvist, U.; Magdalinou, N.; Fox, N.C.; Blennow, K.; Schott, J.M.; Zetterberg, H. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 2016, 86, 829–835. [Google Scholar] [CrossRef]
- He, M.; Sun, L.; Cao, W.; Yin, C.; Sun, W.; Liu, P.; Tan, L.; Xu, Z.; Zhao, W. Association between plasma exosome neurogranin and brain structure in patients with Alzheimer’s disease: A protocol study. BMJ Open 2020, 10, e036990. [Google Scholar] [CrossRef]
- Shibuya, M.J.G. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti-and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Barbaccia, M.L.; Mercuri, N.B.; Graziani, G.; Ledonne, A. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: Pathophysiological roles and therapeutic implications. J Pharmacol. Res. 2024, 201, 107101. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.-F.; Cao, H.; Fu, A.K.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25800–25809. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Ashby, E.L.; Wellington, D.; Barrow, V.M.; Palmer, J.C.; Kehoe, P.G.; Esiri, M.M.; Love, S. Pathophysiology of white matter perfusion in Alzheimer’s disease and vascular dementia. Brain 2014, 137, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Tayler, H.M.; Love, S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 36, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front. Immunol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Inflammation and white matter damage in vascular cognitive impairment. Stroke 2009, 40, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, H.; Larrayoz, I.M.; Martisova, E.; Solas, M.; Howlett, D.R.; Francis, P.T.; Gil-Bea, F.J.; Martínez, A.; Ramírez, M. Increased levels of brain adrenomedullin in the neuropathology of Alzheimer’s disease. J Mol. Neurobiol. 2018, 55, 5177–5183. [Google Scholar] [CrossRef]
- Noda, M.; Matsuda, T. Central regulation of body fluid homeostasis. Proc. Jpn. Acad. Ser. B 2022, 98, 283–324. [Google Scholar] [CrossRef]
- Mahinrad, S.; Sabayan, B.; Garner, C.R.; Lloyd-Jones, D.M.; Sorond, F.A. N-terminal pro brain, N-terminal pro atrial natriuretic peptides, and dynamic cerebral autoregulation. J. Am. Heart Assoc. 2020, 9, e018203. [Google Scholar] [CrossRef]
- Qi, X.M.; Ma, J.F. The role of amyloid beta clearance in cerebral amyloid angiopathy: More potential therapeutic targets. Transl. Neurodegener. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Mahinrad, S.; Bulk, M.; Van Der Velpen, I.; Mahfouz, A.; van Roon-Mom, W.; Fedarko, N.; Yasar, S.; Sabayan, B.; Van Heemst, D.; Van Der Weerd, L. Natriuretic peptides in post-mortem brain tissue and cerebrospinal fluid of non-demented humans and Alzheimer’s disease patients. Front. Neurosci. 2018, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Cheng, H.; Wang, P.; Wu, Y.; Lu, S.; Zhou, Y.; Wang, X.B.; Zhu, X. CXCL9 may serve as a potential biomarker for primary Sjögren’s syndrome with extra-glandular manifestations. Arthritis Res. Ther. 2024, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, T.K.; Dunachie, S.J.; Todryk, S.; Hill, A.V.; Fletcher, H.A. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J. Immunol. Methods 2009, 340, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Sun, Y.; Xie, X.; Zhao, Y. Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Alzheimer’s Res. Ther. 2023, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Hertze, J.; Ohlsson, M.; Nägga, K.; Höglund, K.; Basun, H.; Annas, P.; Lannfelt, L.; Andreasen, N.; Minthon, L. Cerebrospinal fluid levels of heart fatty acid binding protein are elevated prodromally in Alzheimer’s disease and vascular dementia. J. Alzheimer’s Dis. 2013, 34, 673–679. [Google Scholar] [CrossRef]
- Desikan, R.S.; Thompson, W.K.; Holland, D.; Hess, C.P.; Brewer, J.B.; Zetterberg, H.; Blennow, K.; Andreassen, O.A.; McEvoy, L.K.; Hyman, B.T. Heart fatty acid binding protein and Aβ-associated Alzheimer’s neurodegeneration. Mol. Neurodegener. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Sashindranath, M.; Nandurkar, H.H. Endothelial dysfunction in the brain: Setting the stage for stroke and other cerebrovascular complications of COVID-19. Stroke 2021, 52, 1895–1904. [Google Scholar] [CrossRef]
- Jickling, G.C.; Ander, B.P.; Zhan, X.; Stamova, B.; Hull, H.; DeCarli, C.; Sharp, F.R. Progression of cerebral white matter hyperintensities is related to leucocyte gene expression. Brain 2022, 145, 3179–3186. [Google Scholar] [CrossRef]
- Chen, J.; Dai, A.-X.; Tang, H.-L.; Lu, C.-H.; Liu, H.-X.; Hou, T.; Lu, Z.-J.; Kong, N.; Peng, X.-Y.; Lin, K.-X. Increase of ALCAM and VCAM-1 in the Plasma Predicts the Alzheimer’s Disease. Front. Immunol. 2023, 13, 1097409. [Google Scholar] [CrossRef]
- Papasavvas, E.; Azzoni, L.; Pistilli, M.; Hancock, A.; Reynolds, G.; Gallo, C.; Ondercin, J.; Kostman, J.R.; Mounzer, K.; Shull, J. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. Aids 2008, 22, 1153–1161. [Google Scholar] [CrossRef]
- Austin, S.A.; Katusic, Z.S. Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid. J. Cereb. Blood Flow. Metab. Metab. 2020, 40, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, G.; Cavalieri, M.; Galvani, M.; Passaro, A.; Munari, M.; Bosi, C.; Zurlo, A.; Fellin, R. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J. Neurol. Sci. 2008, 272, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.D.; Chambers, A.B.; Ott, B.R.; Daiello, L.A.; Initiative, A.s.D.N. Peripheral markers of vascular endothelial dysfunction show independent but additive relationships with brain-based biomarkers in association with functional impairment in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 80, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: Ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Popescu, B.O.; Toescu, E.C.; Popescu, L.M.; Bajenaru, O.; Muresanu, D.F.; Schultzberg, M.; Bogdanovic, N. Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci. 2009, 283, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Fan, L.M.; Liu, F.; Smith, C.; Li, J.-M. Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Kanski, J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid β-peptide 1–42. Peptides 2002, 23, 1299–1309. [Google Scholar] [CrossRef]
- Lovell, M.; Robertson, J.; Teesdale, W.; Campbell, J.; Markesbery, W. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Bartzokis, G.; Sultzer, D.; Cummings, J.; Holt, L.E.; Hance, D.B.; Henderson, V.W.; Mintz, J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry 2000, 57, 47–53. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.; Lovell, M. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 1998, 19, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.; Close, D.; Stern, S. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiol. Aging 2002, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Greilberger, J.; Koidl, C.; Greilberger, M.; Lamprecht, M.; Schroecksnadel, K.; Leblhuber, F.; Fuchs, D.; Oettl, K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic. Res. 2008, 42, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Clark, C.M.; Lee, V.M.Y.; Trojanowski, J.Q.; Rokach, J.; FitzGerald, G.A. Increased 8, 12-iso-iPF2α-VI in Alzheimer’s disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000, 48, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.; Yao, Y.; Hyman, B.; Growdon, J.; Pratico, D. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener. Dis. 2007, 4, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, C.; Fiorillo, C.; Sorbi, S.; Latorraca, S.; Nacmias, B.; Bagnoli, S.; Nassi, P.; Liguri, G. Oxidative stress and reduced antioxidant defenses in peripheral cells from familial Alzheimer’s patients. Free Radic. Biol. Med. 2002, 33, 1372–1379. [Google Scholar] [CrossRef]
- Rao, A.; Bharani, M.; Pallavi, V. Role of antioxidants and free radicals in health and disease. Adv Pharmacol Toxicol 2006, 7, 29–38. [Google Scholar]
- Butterfield, D.A.; Reed, T.T.; Perluigi, M.; De Marco, C.; Coccia, R.; Keller, J.N.; Markesbery, W.R.; Sultana, R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: Implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007, 1148, 243–248. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Yu, H.L.; Chertkow, H.M.; Bergman, H.; Schipper, H.M. Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics 2003, 3, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Mattioli, P.; Aldred, S.; Cecchetti, R.; Stahl, W.; Griffiths, H.; Senin, U.; Sies, H.; Mecocci, P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: Relevance to Alzheimer disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 2004, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C.; Cherubini, A.; Ingegni, T.; Mattioli, P.; Catani, M.; Rinaldi, P.; Cecchetti, R.; Stahl, W.; Senin, U. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 2002, 59, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, E.; Sardas, S.; Aslan, S.; Isik, E.; Karakaya, A.E. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Birlouez-Aragon, I.; Nourhashémi, F.; Vellas, B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int. J. Geriatr. Psychiatry 1998, 13, 749–754. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Bayer, A.J.; Johnston, J.; Warner, C.; Maxwell, S.R. Altered plasma antioxidant status in subjects with Alzheimer’s disease and vascular dementia. Int. J. Geriatr. Psychiatry 1998, 13, 840–845. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Gao, R.; Ma, S.L. Is mitochondria DNA variation a biomarker for AD? Genes 2022, 13, 1789. [Google Scholar] [CrossRef]
- Mahapatra, G.; Gao, Z.; Bateman III, J.R.; Lockhart, S.N.; Bergstrom, J.; DeWitt, A.R.; Piloso, J.E.; Kramer, P.A.; Gonzalez-Armenta, J.L.; Amick, K.A. Blood-based bioenergetic profiling reveals differences in mitochondrial function associated with cognitive performance and Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 1466–1478. [Google Scholar] [CrossRef]
- Bhatia, S.; Rawal, R.; Sharma, P.; Singh, T.; Singh, M.; Singh, V. Mitochondrial dysfunction in Alzheimer’s disease: Opportunities for drug development. Curr. Neuropharmacol. 2022, 20, 675. [Google Scholar] [CrossRef]
- Maynard, S.; Hejl, A.-M.; Dinh, T.-S.T.; Keijzers, G.; Hansen, Å.M.; Desler, C.; Moreno-Villanueva, M.; Bürkle, A.; Rasmussen, L.J.; Waldemar, G. Defective mitochondrial respiration, altered dNTP pools and reduced AP endonuclease 1 activity in peripheral blood mononuclear cells of Alzheimer’s disease patients. Aging 2015, 7, 793. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.; Helguera, P.; Nemati, Z.; Bohannan, R.C.; Thomas, J.; Samuel, S.E.; Argueta, J.; Doran, E.; Wallace, D.C.; Lott, I.T. Metabolic and growth rate alterations in lymphoblastic cell lines discriminate between Down syndrome and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Veitinger, M.; Varga, B.; Guterres, S.B.; Zellner, M. Platelets, a reliable source for peripheral Alzheimer’s disease biomarkers? Acta Neuropathol. Commun. 2014, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Toledo, G.; Silva-Lucero, M.-d.-C.; Herrera-Díaz, J.; García, D.-E.; Arias-Montaño, J.-A.; Cardenas-Aguayo, M.-D.-C. Patient-derived fibroblasts with presenilin-1 mutations, that model aspects of Alzheimer’s disease pathology, constitute a potential object for early diagnosis. Front. Aging Neurosci. 2022, 14, 921573. [Google Scholar] [CrossRef]
- Bell, S.M.; Burgess, T.; Lee, J.; Blackburn, D.J.; Allen, S.P.; Mortiboys, H. Peripheral glycolysis in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8924. [Google Scholar] [CrossRef]
- Bossy, B.; Petrilli, A.; Klinglmayr, E.; Chen, J.; Lütz-Meindl, U.; Knott, A.B.; Masliah, E.; Schwarzenbacher, R.; Bossy-Wetzel, E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S513–S526. [Google Scholar] [CrossRef]
- Huang, D.-X.; Yu, X.; Yu, W.-J.; Zhang, X.-M.; Liu, C.; Liu, H.-P.; Sun, Y.; Jiang, Z.-P. Calcium signaling regulated by cellular membrane systems and calcium homeostasis perturbed in Alzheimer’s disease. Front. Cell. Dev. Dev. Biol. Biol. 2022, 10, 834962. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.M.; Barber, R.C.; Jones, H.P.; Thorpe Jr, R.J.; Sun, J.; Zhou, Z.; Phillips, N.R. Integrative blood-based characterization of oxidative mitochondrial DNA damage variants implicates Mexican American’s metabolic risk for developing Alzheimer’s disease. Sci. Rep. 2023, 13, 14765. [Google Scholar] [CrossRef]
- Moya, G.E.; Rivera, P.D.; Dittenhafer-Reed, K.E. Evidence for the role of mitochondrial DNA release in the inflammatory response in neurological disorders. Int. J. Mol. Sci. 2021, 22, 7030. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.R.; Li, Y.-M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of microglia and astrocytes in Alzheimer’s disease: From neuroinflammation to Ca2+ homeostasis dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. GFAP as a potential biomarker for Alzheimer’s disease: A systematic review and meta-analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Parvizi, T.; König, T.; Wurm, R.; Silvaieh, S.; Altmann, P.; Klotz, S.; Rommer, P.S.; Furtner, J.; Regelsberger, G.; Lehrner, J. Real-world applicability of glial fibrillary acidic protein and neurofilament light chain in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 887498. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.; AB da Cruz e Silva, O.; Henriques, A. Impact of cytokines and chemokines on Alzheimer’s disease neuropathological hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Perea, J.R.; Lleó, A.; Alcolea, D.; Fortea, J.; Ávila, J.; Bolós, M. Decreased CX3CL1 levels in the cerebrospinal fluid of patients with Alzheimer’s disease. Front. Neurosci. 2018, 12, 609. [Google Scholar] [CrossRef]
- Vacínová, G.; Vejražkova, D.; Rusina, R.; Holmerová, I.; Vaňková, H.; Jarolímová, E.; Včelák, J.; Bendlová, B.; Vaňková, M. Regulated upon activation, normal T cell expressed and secreted (RANTES) levels in the peripheral blood of patients with Alzheimer’s disease. Neural Regen. Res. 2021, 16, 796–800. [Google Scholar] [CrossRef]
- Martens, L.H.; Zhang, J.; Barmada, S.J.; Zhou, P.; Kamiya, S.; Sun, B.; Min, S.-W.; Gan, L.; Finkbeiner, S.; Huang, E.J. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Investig. 2012, 122, 3955–3959. [Google Scholar] [CrossRef]
- Mendsaikhan, A.; Tooyama, I.; Walker, D.G. Microglial progranulin: Involvement in Alzheimer’s disease and neurodegenerative diseases. Cells 2019, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, A.; Lista, S.; Lemercier, P.; Chiesa, P.A.; Zetterberg, H.; Blennow, K.; Potier, M.-C.; Habert, M.-O.; Baldacci, F.; Cavedo, E. Association of plasma YKL-40 with brain amyloid-β levels, memory performance, and sex in subjective memory complainers. Neurobiol. Aging 2020, 96, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hua, F.; Zhang, L.; Lin, Y.; Fang, P.; Chen, S.; Ying, J.; Wang, X. Dual roles of interleukin-33 in cognitive function by regulating central nervous system inflammation. J. Transl. Med. 2022, 20, 369. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Piancone, F.; La Rosa, F.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Clerici, M. IL-33 and its decoy sST2 in patients with Alzheimer’s disease and mild cognitive impairment. J. Neuroinflammation 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Casati, M.; Ferri, E.; Gussago, C.; Mazzola, P.; Abbate, C.; Bellelli, G.; Mari, D.; Cesari, M.; Arosio, B. Increased expression of TREM 2 in peripheral cells from mild cognitive impairment patients who progress into Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Tan, M.-S.; Yu, J.-T.; Sun, L.; Tan, L.; Wang, Y.-L.; Jiang, T.; Tan, L. Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2014, 38, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xie, Y.; Qi, X.; Yang, S. TREM1 as a novel prognostic biomarker and tumor immune microenvironment evaluator in glioma. Medicine 2023, 102, e36410. [Google Scholar] [CrossRef] [PubMed]
- Španić Popovački, E.; Babić Leko, M.; Langer Horvat, L.; Brgić, K.; Vogrinc, Ž.; Boban, M.; Klepac, N.; Borovečki, F.; Šimić, G. Soluble TREM2 concentrations in the cerebrospinal fluid correlate with the severity of neurofibrillary degeneration, cognitive impairment, and inflammasome activation in Alzheimer’s disease. Neurol. Int. 2023, 15, 842–856. [Google Scholar] [CrossRef] [PubMed]
- AlMansoori, M.E.; Jemimah, S.; Abuhantash, F.; AlShehhi, A. Predicting early Alzheimer’s with blood biomarkers and clinical features. Sci. Rep. 2024, 14, 6039. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, J.; Yang, Y.; Zhang, Z.; Zhong, H.; Zeng, G.; Zhou, D.; Nowakowski, R.S.; Long, J.; Wu, C. Identification of candidate DNA methylation biomarkers related to Alzheimer’s disease risk by integrating genome and blood methylome data. Transl. Psychiatry 2023, 13, 387. [Google Scholar] [CrossRef]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing Res. Rev. 2019, 49, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Tarazona-Sánchez, A.; Braza-Boils, A.; Balaguer, A.; Ferré-González, L.; Cañada-Martínez, A.J.; Baquero, M.; Cháfer-Pericás, C. Plasma microRNAs as potential biomarkers in early Alzheimer disease expression. Sci. Rep. 2022, 12, 15589. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Tordera, L.; Papandreou, C.; Novau-Ferré, N.; García-González, P.; Rojas, M.; Marquié, M.; Chapado, L.A.; Papagiannopoulos, C.; Fernàndez-Castillo, N.; Valero, S. Exploring small non-coding RNAs as blood-based biomarkers to predict Alzheimer’s disease. Cell Biosci. 2024, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, A.; Takousis, P.; Evangelou, E.; Prokopenko, I.; Alexopoulos, P.; Udeh-Momoh, C.-M.; Price, G.; Middleton, L.; Perneczky, R.; Initiative, A.s.D.N. Association of Blood MicroRNA Expression and Polymorphisms with Cognitive and Biomarker Changes in Older Adults. J. Prev. Alzheimer’s Dis. 2024, 11, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Yuen, S.C.; Liang, X.; Zhu, H.; Jia, Y.; Leung, S.-w. Prediction of differentially expressed microRNAs in blood as potential biomarkers for Alzheimer’s disease by meta-analysis and adaptive boosting ensemble learning. Alzheimer’s Res. Ther. 2021, 13, 1–30. [Google Scholar] [CrossRef]
- Khodayi, M.; Khalaj-Kondori, M.; Feizi, M.A.H.; Bonyadi, M.J.; Talebi, M. Plasma lncRNA profiling identified BC200 and NEAT1 lncRNAs as potential blood-based biomarkers for late-onset Alzheimer’s disease. EXCLI J. 2022, 21, 772. [Google Scholar] [CrossRef]
- Feng, L.; Liao, Y.-T.; He, J.-C.; Xie, C.-L.; Chen, S.-Y.; Fan, H.-H.; Su, Z.-P.; Wang, Z. Plasma long non-coding RNA BACE1 as a novel biomarker for diagnosis of Alzheimer disease. BMC Neurol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.A.; Smith, M.d.A.C.; Chen, E.S. Histone modifications in Alzheimer’s disease. Genes 2023, 14, 347. [Google Scholar] [CrossRef]
- De Plano, L.M.; Saitta, A.; Oddo, S.; Caccamo, A. Epigenetic Changes in Alzheimer’s Disease: DNA Methylation and Histone Modification. Cells 2024, 13, 719. [Google Scholar] [CrossRef]
- Salameh, Y.; Bejaoui, Y.; El Hajj, N. DNA methylation biomarkers in aging and age-related diseases. Front. Genet. 2020, 11, 480672. [Google Scholar] [CrossRef]
- Thrush, K.L.; Bennett, D.A.; Gaiteri, C.; Horvath, S.; van Dyck, C.H.; Higgins-Chen, A.T.; Levine, M.E. Aging the brain: Multi-region methylation principal component based clock in the context of Alzheimer’s disease. Aging 2022, 14, 5641. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chu, C.; Pang, Y.; Cai, H.; Jia, L. A circular RNA blood panel that differentiates Alzheimer’s disease from other dementia types. Biomark. Res. 2022, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Chen, Y.-H.; Zhang, K. Identification of circular RNA hsa_Circ_0003391 in peripheral blood is potentially associated with Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 601965. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Morikawa, S.; Nakashima, M.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Suga, N.; Tsuji, A.; Matsuda, S. CircRNAs and RNA-binding proteins involved in the pathogenesis of cancers or central nervous system disorders. Non-Coding RNA 2023, 9, 23. [Google Scholar] [CrossRef]
- Yang, Q.; Li, F.; He, A.T.; Yang, B.B. Circular RNAs: Expression, localization, and therapeutic potentials. Mol. Ther. 2021, 29, 1683–1702. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Tatebe, H.; DeLeo, A.M.; Ikezu, S.; Tokuda, T.; Gygi, S.P.; Stern, R.A.; Ikezu, T. Proteomic profiling of extracellular vesicles isolated from cerebrospinal fluid of former national football league players at risk for chronic traumatic encephalopathy. Front. Neurosci. 2019, 13, 1059. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic profiling of extracellular vesicles derived from cerebrospinal fluid of Alzheimer’s disease patients: A pilot study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: Novel biomarkers for neurological disorders. Front. Physiol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607. e601. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J. 2019, 33, 231. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, D.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Biragyn, A.; Masharani, U.; Frassetto, L.; Petersen, R.C.; Miller, B.L.; Goetzl, E.J. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015, 29, 589. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J. Advancing medicine for Alzheimer’s disease: A plasma neural exosome platform. FASEB J. 2020, 34, 13079–13084. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Möbius, W.; Goebbels, S.; Nave, K.-A. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Lanoiselée, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef]
- Weston, P.S.J.; Poole, T.; Ryan, N.S.; Nair, A.; Liang, Y.; Macpherson, K.; Druyeh, R.; Malone, I.B.; Ahsan, R.L.; Pemberton, H.; et al. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 2017, 89, 2167–2175. [Google Scholar] [CrossRef]
- Preische, O.; Schultz, S.A.; Apel, A.; Kuhle, J.; Kaeser, S.A.; Barro, C.; Gräber, S.; Kuder-Buletta, E.; LaFougere, C.; Laske, C.; et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019, 25, 277–283. [Google Scholar] [CrossRef]
- Johansson, C.; Thordardottir, S.; Laffita-Mesa, J.; Rodriguez-Vieitez, E.; Zetterberg, H.; Blennow, K.; Graff, C. Plasma biomarker profiles in autosomal dominant Alzheimer’s disease. Brain 2023, 146, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.A.; Liu, L.; Schultz, A.P.; Fitzpatrick, C.D.; Levin, R.; Bellier, J.P.; Shirzadi, Z.; Joseph-Mathurin, N.; Chen, C.D.; Benzinger, T.L.S.; et al. γ-Secretase activity, clinical features, and biomarkers of autosomal dominant Alzheimer’s disease: Cross-sectional and longitudinal analysis of the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet. Neurol. 2024, 23, 913–924. [Google Scholar] [CrossRef]
- Morenas-Rodríguez, E.; Li, Y.; Nuscher, B.; Franzmeier, N.; Xiong, C.; Suárez-Calvet, M.; Fagan, A.M.; Schultz, S.; Gordon, B.A.; Benzinger, T.L.S.; et al. Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: A longitudinal observational study. Lancet. Neurol. 2022, 21, 329–341. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Qiu, Y.; Sun, D.; Zhou, H. Peripheral GFAP and NfL as early biomarkers for dementia: Longitudinal insights from the UK Biobank. BMC Med. 2024, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front Neurosci 2021, 15, 689938. [Google Scholar] [CrossRef] [PubMed]
- Katisko, K.; Cajanus, A.; Huber, N.; Jääskeläinen, O.; Kokkola, T.; Kärkkäinen, V.; Rostalski, H.; Hartikainen, P.; Koivisto, A.M.; Hannonen, S.; et al. GFAP as a biomarker in frontotemporal dementia and primary psychiatric disorders: Diagnostic and prognostic performance. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1305–1312. [Google Scholar] [CrossRef]
- Donaghy, P.C.; Firbank, M.; Petrides, G.; Lloyd, J.; Barnett, N.; Olsen, K.; Heslegrave, A.; Zetterberg, H.; Thomas, A.J.; O’Brien, J.T. The relationship between plasma biomarkers and amyloid PET in dementia with Lewy bodies. Park. Relat. Disord. 2022, 101, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Suridjan, I.; van der Flier, W.M.; Monsch, A.U.; Burnie, N.; Baldor, R.; Sabbagh, M.; Vilaseca, J.; Cai, D.; Carboni, M.; Lah, J.J. Blood-based biomarkers in Alzheimer’s disease: Future directions for implementation. Alzheimer’s Dement. 2023, 15, e12508. [Google Scholar] [CrossRef]
- Syrjanen, J.A.; Campbell, M.R.; Algeciras-Schimnich, A.; Vemuri, P.; Graff-Radford, J.; Machulda, M.M.; Bu, G.; Knopman, D.S.; Jack, C.R.; Petersen, R.C., Jr.; et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Barthélemy, N.R.; He, Y.; Bateman, R.J.; Hansson, O. Mitigating the Associations of Kidney Dysfunction With Blood Biomarkers of Alzheimer Disease by Using Phosphorylated Tau to Total Tau Ratios. JAMA Neurol. 2023, 80, 516–522. [Google Scholar] [CrossRef]
- Manouchehrinia, A.; Piehl, F.; Hillert, J.; Kuhle, J.; Alfredsson, L.; Olsson, T.; Kockum, I. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 2020, 7, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.A.; Wijesekara, N.; Fraser, P.E.; De Felice, F.G. The Link Between Tau and Insulin Signaling: Implications for Alzheimer’s Disease and Other Tauopathies. Front. Cell. Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef]
- Baldacci, F.; Lista, S.; Manca, M.L.; Chiesa, P.A.; Cavedo, E.; Lemercier, P.; Zetterberg, H.; Blennow, K.; Habert, M.O.; Potier, M.C.; et al. Age and sex impact plasma NFL and t-Tau trajectories in individuals with subjective memory complaints: A 3-year follow-up study. Alzheimer’s Res. Ther. 2020, 12, 147. [Google Scholar] [CrossRef]
- Mondal, R.; Deb, S.; Shome, G.; Sarkar, V.; Lahiri, D.; Datta, S.S.; Benito-León, J. Molecular dynamics of amyloid-β transport in Alzheimer’s disease: Exploring therapeutic plasma exchange with albumin replacement—Current insights and future perspectives. Neurología 2024, in press. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J. Cholesterol-dependent amyloid β production: Space for multifarious interactions between amyloid precursor protein, secretases, and cholesterol. Cell Biosci. 2023, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, A.; Pannee, J.; Karikari, T.K.; Rodriguez, J.L.; Ashton, N.J.; Nicholas, J.M.; Cash, D.M.; Coath, W.; Lane, C.A.; Parker, T.D.; et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 2021, 144, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Brum, W.S.; Docherty, K.F.; Ashton, N.J.; Zetterberg, H.; Hansson, O.; McMurray, J.J.V.; Blennow, K. Effect of Neprilysin Inhibition on Alzheimer Disease Plasma Biomarkers: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2024, 81, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hedna, R.; Kovacic, H.; Pagano, A.; Peyrot, V.; Robin, M.; Devred, F.; Breuzard, G. Tau Protein as Therapeutic Target for Cancer? Focus on Glioblastoma. Cancers 2022, 14, 5386. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y. Challenges associated with using extracellular vesicles as biomarkers in neurodegenerative disease. Expert Rev. Mol. Diagn. 2023, 23, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Mondragón-Llorca, H.; Pascual-Leone, A. Noninvasive brain stimulation in Alzheimer’s disease: Systematic review and perspectives for the future. Exp. Gerontol. 2011, 46, 611–627. [Google Scholar] [CrossRef]
- Hall, J.D.; Green, J.M.; Chen, Y.A.; Liu, Y.; Zhang, H.; Sundman, M.H.; Chou, Y.H. Exploring the potential of combining transcranial magnetic stimulation and electroencephalography to investigate mild cognitive impairment and Alzheimer’s disease: A systematic review. GeroScience 2024, 46, 3659–3693. [Google Scholar] [CrossRef]
- Koch, G.; Spampinato, D. Alzheimer disease and neuroplasticity. Handb. Clin. Neurol. 2022, 184, 473–479. [Google Scholar] [CrossRef]


| Name of the Blood-Based Biomarkers | Underlying Pathophysiology | Categorization (NIA-AA 2024) | Relevance | Trend of the Biomarker in Plasma | Assessment Techniques | |
|---|---|---|---|---|---|---|
| Amyloid β (Aβ) | Aβ42 | Plasma biomarkers related to amyloid accumulation in AD | Core 1 biomarker (A) | Early detection of AD in asymptomatic individuals can facilitate the transition from normal cognition to mild cognitive impairment or AD | Decreased in AD and mild cognitive impairment compared to controls | Enzyme-linked immunosorbent assay (ELISA), Luminex xMAP Technology, single-molecule array (SIMOA), liquid chromatography-mass spectrometry, and immunoprecipitation mass spectrometry |
| Aβ40 | Plasma biomarkers related to amyloid accumulation in AD | Core 1 biomarker (A) | Early detection of AD in asymptomatic individuals can indicate progression from normal cognition to mild cognitive impairment or AD | Decreased in AD and mild cognitive impairment compared to controls | ||
| Aβ42/40 | Plasma biomarkers related to amyloid accumulation in AD | Core 1 biomarker (A) | It can identify the early stages of AD and predict cognitive decline in accordance with cerebrospinal fluid and neuroimaging biomarkers | Decreased Aβ42/Aβ40 ratio in AD and mild cognitive impairment compared to controls | ||
| Tau | p-tau217 | Plasma biomarkers of phosphorylated and secreted tau in AD | Core 1 biomarker (T1) | Early detection of AD in asymptomatic individuals can accurately predict the progression from subjective cognitive decline and mild cognitive impairment to dementia when combined with other risk factors | Increased in AD and mild cognitive impairment compared to controls | ELISA, Luminex xMAP Technology, SIMOA, liquid chromatography-mass spectrometry, and immunoprecipitation mass spectrometry |
| p-tau181 | Plasma biomarkers of phosphorylated and secreted tau in AD | Core 1 biomarker (T1) | Early detection of AD in asymptomatic individuals distinguishes between Aβ-positron emission tomography (PET) positive and Aβ-PET negative individuals, as well as correlates with disease progression to dementia. This detection is associated with tau-burdened brain areas exhibiting AD-related atrophic changes | Increased in AD and mild cognitive impairment compared to controls | ||
| p-tau231 | Plasma biomarkers of phosphorylated and secreted tau in AD | Core 1 biomarker (T1) | Early detection of AD) in asymptomatic individuals differentiates between patients with and without AD pathology during post-mortem assessment | Increased in AD and mild cognitive impairment compared to controls | ||
| MTBR-tau243 | Plasma biomarkers related to tau accumulation in AD | Core 2 biomarker (T2) | Elevated in the later stages of AD, this biomarker, along with the Core 1 biomarker, is strongly associated with tau-PET imaging and disease progression, reflecting the staging of biological disease severity | Increased in AD and mild cognitive impairment compared to controls | ||
| Non-phosphorylated mid-region tau fragments | Plasma tau-related biomarkers associated with tau accumulation in AD | Core 2 biomarker (T2) | Elevated in the later stages of AD, the staging of biological disease severity is associated with the Core 1 biomarker | Increased in AD and mild cognitive impairment compared to controls | ||
| α-Synuclein | α-Synuclein/tau | Biomarkers related to abnormal protein accumulation in non-core AD pathology, specifically synuclein pathology | Biomarkers of non-AD co-pathology (S) | Total α-synuclein levels in the blood may not differ significantly between patients with neurodegenerative diseases. However, the oligomeric or phosphorylated forms of α-synuclein are associated with accelerated cognitive dysfunction | Decreased in AD and mild cognitive impairment vs. controls | Seed amplification assays: Protein Misfolding Cyclic Amplification and Real-Time Quaking-Induced Conversion Detection techniques: ELISA, Western blotting, Quantitative Mass Spectrometry, Luminex xMAP Technology, Surface Plasmon Resonance–Dynamic Light Scattering, and Immuno-Polymerase Chain Reaction |
| α-Synuclein/Aβ 42 | Biomarkers of non-AD co-pathology | Increased in AD and mild cognitive impairment compared to controls | ||||
| Dickkopf-1 | Biomarkers related to abnormal protein accumulation in non-core AD pathology | Research biomarker | Elevated levels correlate with disease severity, particularly cognitive decline, and synaptic loss, and help differentiate AD from other neurodegenerative conditions | Increased in AD | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Visinin-like protein-1 (VILIP-1) | Biomarkers related to abnormal protein accumulation in non-core AD pathology | Research biomarker | Increased levels are observed in AD; however, no significant differences in concentrations are found between AD-mild cognitive impairment patients and other neurodegenerative groups | Increased in AD | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Plasma neurofilament light chain (NfL) | Injury, dysfunction, or degeneration of neuropil | Biomarkers of non-specific processes involved in AD pathophysiology (N) | Increased levels in Aβ-positive patients with AD and mild cognitive impairment are associated with the degree of cognitive impairment and are utilized as monitoring biomarkers to indicate the severity of neurodegeneration | Increased in AD and mild cognitive impairment compared to controls | ELISA, Luminex xMAP Technology, electrochemiluminescence immunoassay, mass spectrometry, and SIMOA | |
| Synaptosome-associated protein of 25 kDa (SNAP-25) | Neuronal and synaptic injury related to presynaptic dysfunction. | Biomarkers of non-specific processes involved in AD pathophysiology (N) | Cerebrospinal concentrations can differentiate between various neurodegenerative diseases such as AD, Parkinson’s disease, and amyotrophic lateral sclerosis | Decreased in AD compared to controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Neuronal pentraxin 2 (NPTX-2) | Neuronal and synaptic injury related to presynaptic dysfunction. | Biomarkers of non-specific processes involved in AD pathophysiology (N) | It has the potential as a biomarker for the early detection of AD | Decreased in AD vs. controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Growth-associated protein 43 (GAP-43) | Neuronal and synaptic injury related to presynaptic dysfunction. | Biomarkers of non-specific processes involved in AD pathophysiology (N) | It has the potential as a biomarker for the early detection of AD | Increased in AD compared to controls | ELISA, Western blotting Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Neurogranin (NG) | Neuronal and synaptic injury related to postsynaptic protein dysfunction | Biomarkers of non-specific processes involved in AD pathophysiology (N) | It has the potential as a biomarker for the early detection of AD | Decreased in AD vs. controls | ELISA, Luminex xMAP Technology, electrochemiluminescence immunoassay, mass spectrometry, and SIMOA | |
| Fms-like tyrosine kinase-1 (Flt-1) | Vascular damage related to AD | Research biomarker (V) | It assesses total vascular involvement and aids in the early detection of vascular changes associated with AD | Increased in AD compared to controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Endothelin 1 (ET-1) | Vascular damage related to AD | Research biomarker (V) | It reflects vascular impairment in AD | Increased in AD compared to controls | ELISA, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Atrial natriuretic peptide (ANP) | Vascular damage related to AD | Research biomarker (V) | It leads to reduced cerebral blood flow and impairment of neurovascular health | Increased in AD compared to controls | ELISA, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Monokine induced by gamma interferon (MIG/CXCL9) | Vascular damage related to AD | Research biomarker (V) | It indicates the presence of ongoing chronic neuroinflammatory processes | Increased in AD compared to controls | ELISA, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Heart-type fatty acid-binding protein (H-FABP) | Vascular damage related to AD | Research biomarker (V) | It shows potential as a probable biomarker for the early detection of AD, as elevated levels have been found in the preclinical phase of AD dementia | Increased in AD compared to controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Vascular Adhesion Molecule-1 (VCAM-1) | Soluble vascular cell adhesion molecule-1 (sVCAM-1) | Vascular damage related to AD | Research biomarker (V) | Elevated sVCAM levels indicate the burden of atherosclerosis in AD, showing a significant correlation between age and the severity of cognitive decline | Increased in AD compared to controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno-Polymerase Chain Reaction, and mass spectrometry |
| Soluble intercellular adhesion molecule-1 (sICAM-1) | Vascular damage related to AD | Research biomarker (V) | Elevated levels of sICAM-1 indicate the burden of atherosclerosis in AD | Increased in AD compared to controls | ELISA, Western blotting, Luminex xMAP Technology, Immuno- Immuno-Polymerase Chain Reaction, and mass spectrometry | |
| Metabolic products secondary to lipid peroxidation | Malondialdehyde (MDA) | Oxidative stress | Research biomarker | Increased levels are observed in familial AD associated with mutations in the amyloid precursor protein and presenilin-1 genes | Increased in AD compared to controls | High-performance liquid chromatography, liquid chromatography-mass spectrometry, ELISA, and gas chromatography-mass spectrometry |
| 4-hydroxynonenal (HNE) | Oxidative stress | Research biomarker | Increased levels are observed in familial AD associated with mutations in the amyloid precursor protein and presenilin-1 genes | Increased in AD compared to controls | ||
| Increased F2-isoprostanes | Oxidative stress | Research biomarker | As a potential marker of oxidative stress during the mild cognitive impairment phase of AD, its levels correlate with the disease continuum, ranging from subjective cognitive decline to mild cognitive impairment and eventually to AD | Increased in AD compared to controls | ||
| Free radicals | Reactive oxygen species | Oxidative damage | Research biomarker | Reactive oxygen species modify neuronal macromolecules and induce hyperphosphorylation of tau protein during the prodromal phases of AD | Increased in AD | Dichloro-fluorescein Diacetate Assay, Electron Spin Resonance Spectroscopy, Nitroblue Tetrazolium Assay, and Flow Cytometry with reactive oxygen species-sensitive dyes |
| Reactive nitrogen species | Oxidative damage | Research biomarker | Nitrosylation of critical proteins in neurons impairs their function, promoting neurodegenerative processes | Increased in AD | Nitrotyrosine ELISA, Electron Spin Resonance Spectroscopy, and Western blot for 3-Nitrotyrosine-modified proteins | |
| Nucleoside 8-hydroxyguanosine (8-OHG) | Oxidative damage | Research biomarker | It is significant for assessing the gradient of DNA oxidative damage in patients with AD, enabling the early detection of oxidative damage to plasma DNA | Increased in lymphocytes of AD patients compared to control | ELISA, high-performance liquid chromatography with electrochemical detection, liquid chromatography-mass spectrometry, Western Blot using specific anti-5.8-OHG antibodies, immunoprecipitation, and gas chromatography-mass spectrometry | |
| Mitochondrial respiratory complex I-V genes (OxPHOS genes) | Bioenergetic abnormality | Research biomarker | An imbalance between nuclear and mitochondrial genome-encoded OXPHOS transcripts may trigger a negative feedback loop, reducing mitochondrial translation and compromising OXPHOS efficiency. This imbalance is likely to result in the increased generation of harmful reactive oxygen species | Reduced expression in early AD patients | Quantitative polymerase chain reaction, Western blot, Immunohistochemistry, and Blue Native Gel Electrophoresis | |
| S-nitrosylated dynamin-related protein 1 (SNO-Drp1) | Bioenergetic abnormality | Research biomarker | SNO-Drp1 can lead to increased mitochondrial fission, synapse loss, and neuronal damage in mouse models, primary neuronal cultures, and post-mortem tissue | Increased levels of SNO-Drp1 are observed in peripheral blood lymphocytes of patients with AD. However, there are contradictory findings indicating that SNO-Drp1 levels do not differ significantly between AD patients and controls | Biotin Switch Assay, mass spectrometry, Nitroso-Proteome Profiling, immunoprecipitation, and Western blot | |
| Mitochondrial DNA | Bioenergetic abnormality | Research biomarker | Mitochondrial DNA copy number serves as an indirect indicator of mitochondrial function, providing valuable information about bioenergetics as a contributing factor in the progression of AD | Decreased in patients with AD | Quantitative polymerase chain reaction, digital droplet polymerase chain reaction, and Southern blotting | |
| 8-oxo-7,8-dihydroguanine somatic single nucleotide variants (8oxoG sSNVs) | Bioenergetic abnormality | Research biomarker | Due to its inflammatory endophenotype, the circulating cell-free mtDNA 8oxoG variant can be utilized as an enhanced biomarker | Increased in AD patients | 8-oxoG DNA Glycosylase Assay, Comet Assay with Formamidopyrimidine-DNA Glycosylase, ELISA, and high-performance liquid chromatography with electrochemical detection | |
| Circulating cell-free mtDNA | Bioenergetic abnormality | Research biomarker | Cellular mitochondrial DNA copy number can serve as a potential biomarker of mitochondrial biogenesis and cellular energetics, reflecting mitochondrial health in AD | Increased in AD patients | Quantitative polymerase chain reaction, digital droplet quantitative polymerase chain reaction, and Southern blotting | |
| Intermediate filament glial fibrillary acidic protein (GFAP) | Neuroinflammation and immune dysregulation | Research biomarker (I) | Marker of astrogliosis observed in chronic inflammatory processes, such as in progressing AD | Increased in AD patients | ELISA, electrochemiluminescence immunoassay, and Mesoscale Discovery Immunoassay V-PLEX | |
| CX3CL1 (Fractalkine) | Neuroinflammation and immune dysregulation | Research biomarker (I) | Significantly elevated in the plasma of patients with mild cognitive impairment and AD compared to other neuroinflammatory disease processes | Increased in AD and MCI | ELISA, Western blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| C-C motif chemokine ligand 23 (CCL23) | Neuroinflammation and immune dysregulation | Research biomarker (I) | Their plasma concentration has also been found to have a predictive value for the progression from MCI to AD | Increased in AD | ELISA, Western blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| C-C chemokine ligands or regulated upon activation, normal T cell expressed and secreted (RANTES/CCL5) | Neuroinflammation and immune dysregulation | Research biomarker (I) | Elevated in AD and correlated with the neuroinflammatory burden | Increased in AD | ELISA, Western blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| YKL-40 | Neuroinflammation and immune dysregulation | Research biomarker (I) | Increasingly expressed in astrocytes during neuroinflammatory changes, plasma YKL-40 levels have been shown to positively correlate with the results of the sensitive Free and Cued Selective Reminding Test | Increased in AD | ELISA, Western blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| Progranulin | Neuroinflammation and immune dysregulation | Research biomarker (I) | Increased expression of the progranulin gene is found in the blood of patients with mild cognitive impairment and AD | Increased in AD | ELISA, Western blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| Triggering receptor expressed on myeloid cells 2 (TREM2) | Neuroinflammation and immune dysregulation | Research biomarker (I) | Messenger RNA levels in peripheral mononuclear cells have been found to distinguish between amnestic mild cognitive impairment, AD, and healthy control individuals and are dependent on the apolipoprotein E genotype | Increased in AD | ELISA, Western Blot, Immunohistochemistry, Flow Cytometry, and Luminex | |
| Neuronal-derived exosomes | P-S396-tau | Tauopathy | Research biomarker | It can predict the development of AD up to 10 years before the clinical onset of sporadic AD | Increased in AD | Proteomic analysis of extracellular vesicles, such as through ELISA |
| p-tau181 | Tauopathy | Research biomarker | It has the potential to predict the development of AD up to 10 years before the clinical onset of sporadic AD | Increased in AD and mild cognitive patients compared to controls | ELISA and ultra-sensitive inhouse SIMOA | |
| Synaptotagmin | Synaptopathy | Research biomarker | Its impairment leads to decreased neurotransmission, neuroplasticity, and long-term potentiation, thus hampering memory formation | Reduced in AD | ELISA, liquid chromatography-mass spectrometry, and SIMOA | |
| Synaptophysin | Synaptic loss and dysfunction | Research biomarker | Loss of proper functioning synapse leads to impaired signal transmission and, thus, cognitive impairment | Reduced in AD | ELISA, liquid chromatography-mass spectrometry, and SIMOA | |
| Phosphorylation of insulin receptor substrate-1 (IRS-1) at serine 312 (P-S312-IRS-1) | Neuroinflammation and insulin resistance | Research biomarker | Its increment promotes insulin resistance, leading to progressive neurodegeneration | Increased in AD vs. controls | ELISA, liquid chromatography-mass spectrometry, and SIMOA | |
| Phosphorylation at multiple tyrosine residues of insulin receptor substrate-1 (P-panY-IRS-1) | Insulin resistance and synaptic dysfunction | Research biomarker | Its reduction promotes insulin resistance, leading to progressive neurodegeneration | Downregulated in AD | ELISA, liquid chromatography-mass spectrometry, and SIMOA | |
| N-(1-carboxymethyl)-L-lysine | Reactive oxygen species-mediated damage | Research biomarker | It can differentiate between the early and moderate stages of AD | Downregulated in AD | ELISA, liquid chromatography-mass spectrometry, and SIMOA | |
| Malondialdehyde | Tauopathy | Research biomarker | When neurons absorb microglia-derived exosomes containing tau, it triggers additional abnormal tau aggregation | Increases in AD | ELISA and liquid chromatography-mass spectrometry | |
| Astrocyte-derived exosomes | Neuroinflammation | Research biomarker | Plasma levels of various complement components, such as C1q, C3b, and factor D, could serve as predictive biomarkers for the progression of mild cognitive impairment to AD | Increases in AD | ELISA and liquid chromatography-mass spectrometry | |
| Source of the microRNA | Names of microRNA | Reference |
|---|---|---|
| Whole blood | hsa-miR-107 | [161,162,163,165] |
| Plasma | hsa-miR-92a-3p, hsa-miR-486-5p, hsa-miR-29a-3p, hsa-miR-107, hsa-miR-128-3p, hsa-miR-132-3p, hsa-miR-34c-5p, hsa-let-7d-5p, hsa-miR-191-5p, hsa-miR-200a-3p, hsa-miR-483-5p, hsa-miR-486-5p, hsa-miR-502-3p, hsa-miR-548k, hsa-miR-339-5p, hsa-miR-221-5p, hsa-miR-144-5p, hsa-miR-382-5p, hsa-miR-146b-5p, hsa-miR-224-5p, hsa-miR-625-5p, hsa-miR-769-5p, hsa-miR-454-5p, hsa-miR-548d-5p, hsa-miR-877-5p, hsa-miR-146a, hsa-miR-125b, | |
| Serum | hsa-miR-106-b-3p, hsa-miR-22-3p, hsa-miR-126-5p, hsa-miR148b-5p, hsa-miR-181c-3p, hsa-miR-93-5p, hsa-miR-29c-3p, hsa-miR-132-3p, hsa-miR-222-3p, hsa-let-7d-5p, hsa-miR-191-5p, hsa-miR-146a, hsa-miR-125b, hsa-miR-135a | |
| Peripheral blood mononuclear cells | hsa-miR-128-3p, hsa-miR-34c-5p |
| Biomarker | Hereditary AD (Early Onset) | Sporadic AD (Late Onset) |
|---|---|---|
| Aβ42/Aβ40 Ratio | Decreased earlier, often in the preclinical phase | Decreased later, closer to symptom onset |
| P-tau181 | Increases 6–10 years before symptoms; correlates with Aβ pathology | Rises later but remains a strong marker of tau pathology |
| T-tau | Poor discriminatory power; less significant changes | Variable changes; lacks a strong association with AD progression |
| NfL | Increases closer to symptom onset; higher variability | Rises later; more consistent correlation with neurodegeneration |
| GFAP | Rises ten years before symptoms, indicating early astrocytic activation | Early rise, but slightly later than in hereditary forms |
| Dementia Type | GFAP | p-Tau181 | NfL | T-Tau | Correlating Features |
|---|---|---|---|---|---|
| AD | Elevated early | Elevated | Elevated | No significant alteration | Correlates with Aβ, tau pathology |
| FTD | Elevated | Mildly elevated | Elevated | Elevated | p-Tau181 demonstrates higher diagnostic accuracy in distinguishing FTD from AD |
| LBD | Elevated | Mildly elevated | Elevated | Elevated | All biomarkers overlap with AD pathology |
| VaD | No significant alteration | No significant alteration | Elevated | No significant alteration | Correlates with the extent of neurovascular damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhauria, M.; Mondal, R.; Deb, S.; Shome, G.; Chowdhury, D.; Sarkar, S.; Benito-León, J. Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics. Int. J. Mol. Sci. 2024, 25, 10911. https://doi.org/10.3390/ijms252010911
Dhauria M, Mondal R, Deb S, Shome G, Chowdhury D, Sarkar S, Benito-León J. Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics. International Journal of Molecular Sciences. 2024; 25(20):10911. https://doi.org/10.3390/ijms252010911
Chicago/Turabian StyleDhauria, Mrinmay, Ritwick Mondal, Shramana Deb, Gourav Shome, Dipanjan Chowdhury, Shramana Sarkar, and Julián Benito-León. 2024. "Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics" International Journal of Molecular Sciences 25, no. 20: 10911. https://doi.org/10.3390/ijms252010911
APA StyleDhauria, M., Mondal, R., Deb, S., Shome, G., Chowdhury, D., Sarkar, S., & Benito-León, J. (2024). Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics. International Journal of Molecular Sciences, 25(20), 10911. https://doi.org/10.3390/ijms252010911






