Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines
Abstract
1. Introduction
2. Results
2.1. The Hydroxy-Derivatives Were Synthesized Using Extracted and Purified CYP Enzymes Expressed in E.coli
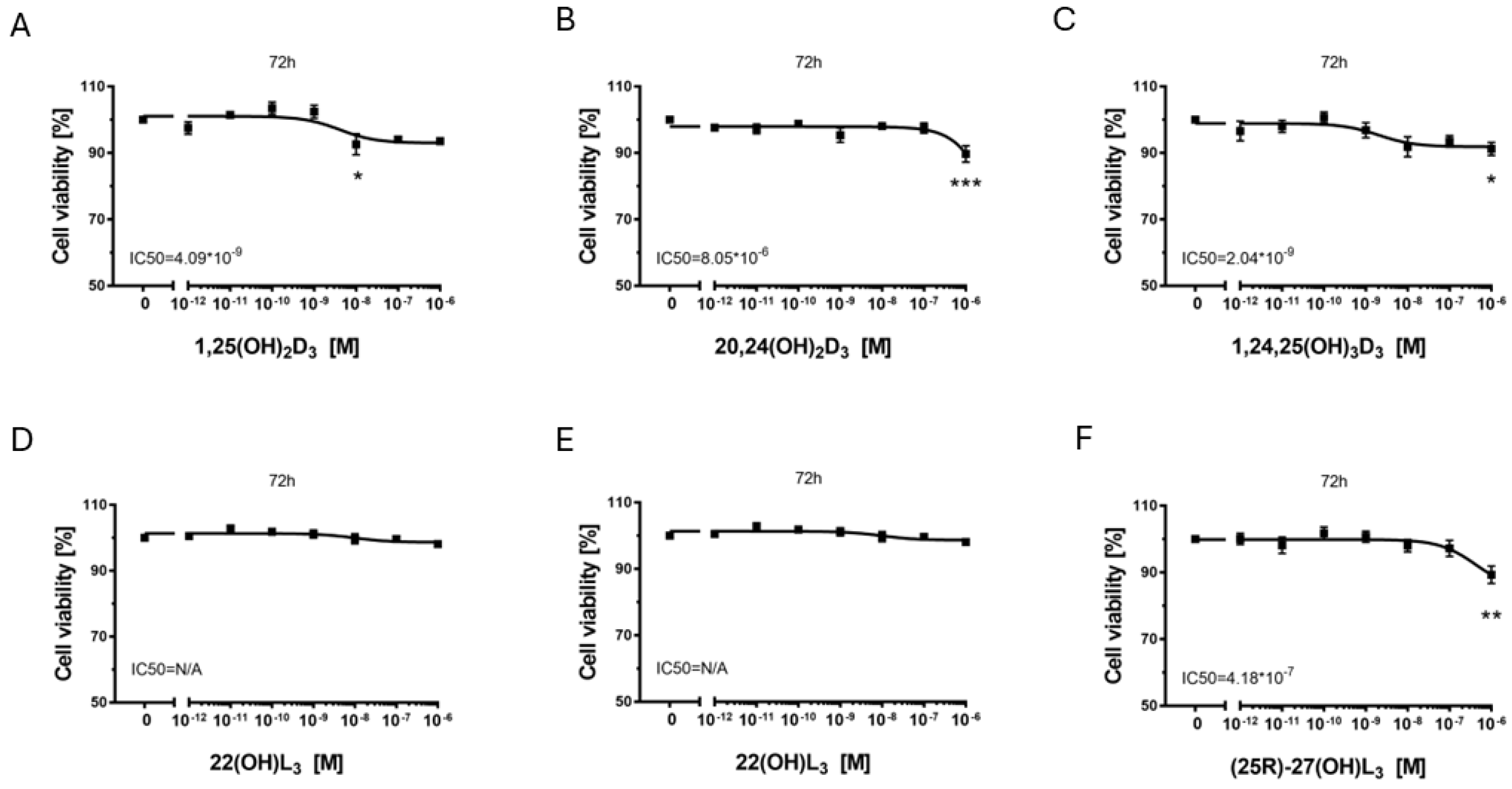
2.2. The Hydroxy-Derivatives of Vitamin D3 and L3 Affects on Proliferation of Melanoma Cells A375 and SK-MEL-28
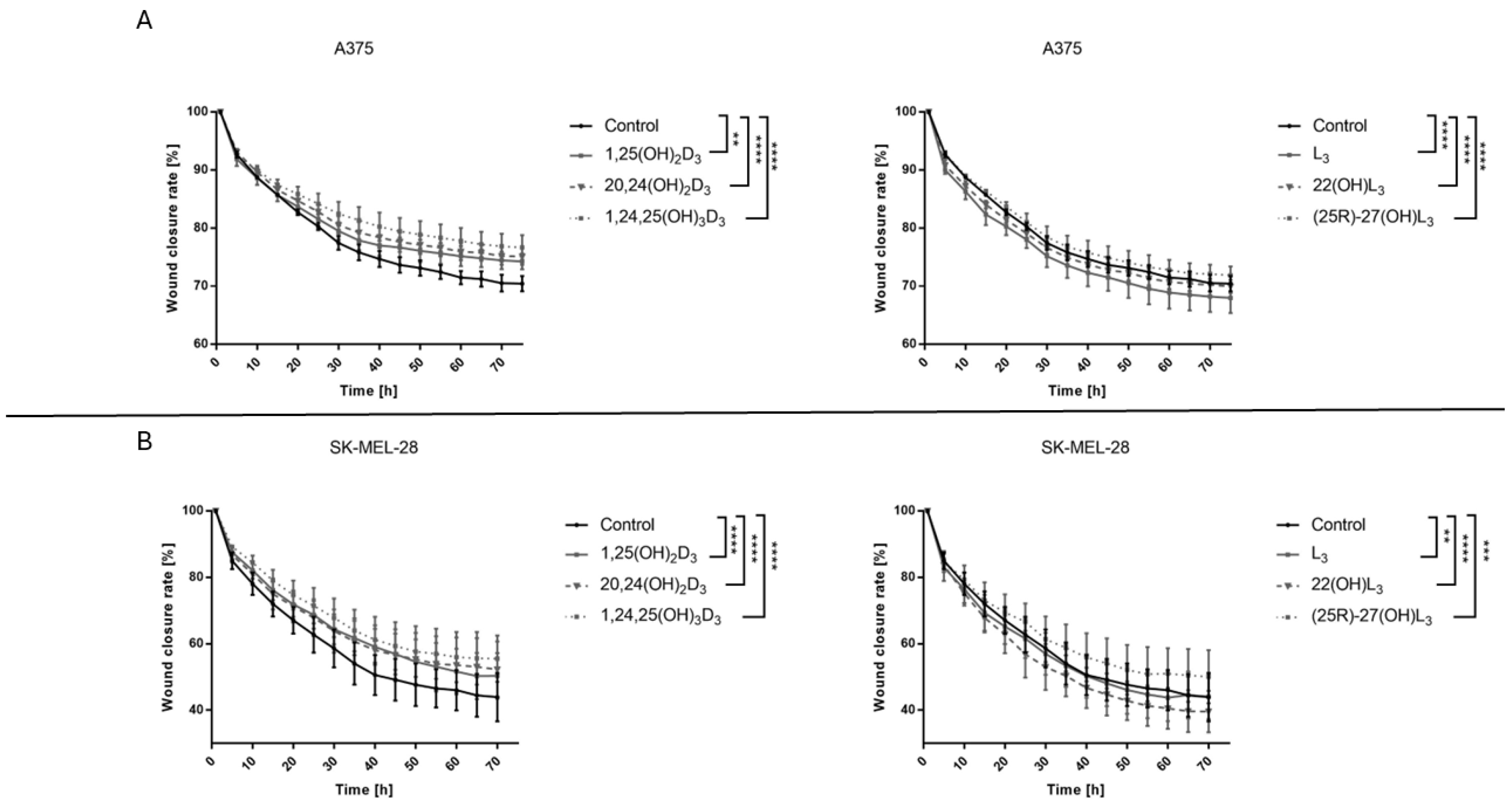
2.3. The Hydroxy-Derivatives of Vitamin D3 and L3 Affects on the Mobility of Melanoma Cells A375 and SK-MEL-28
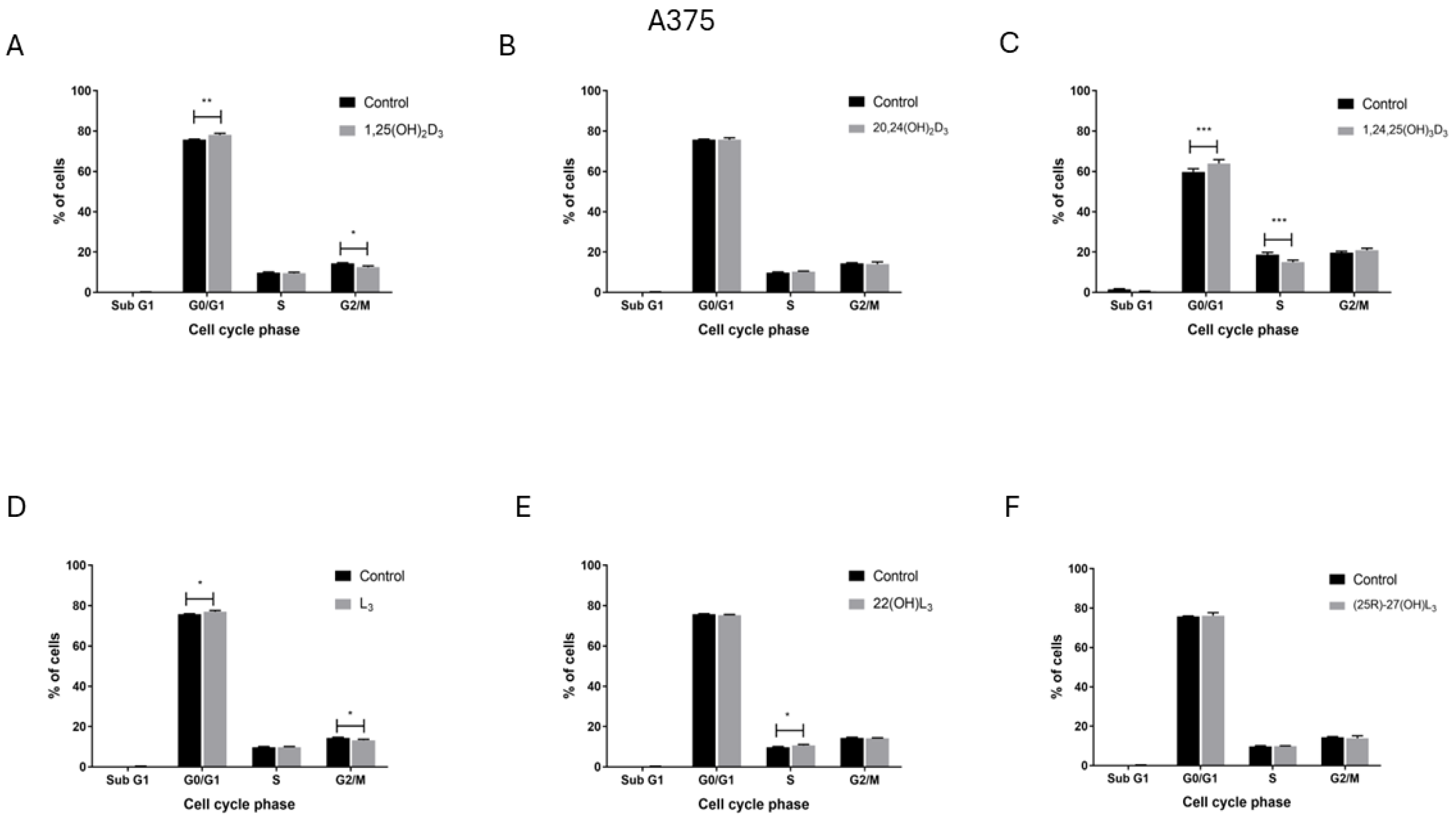
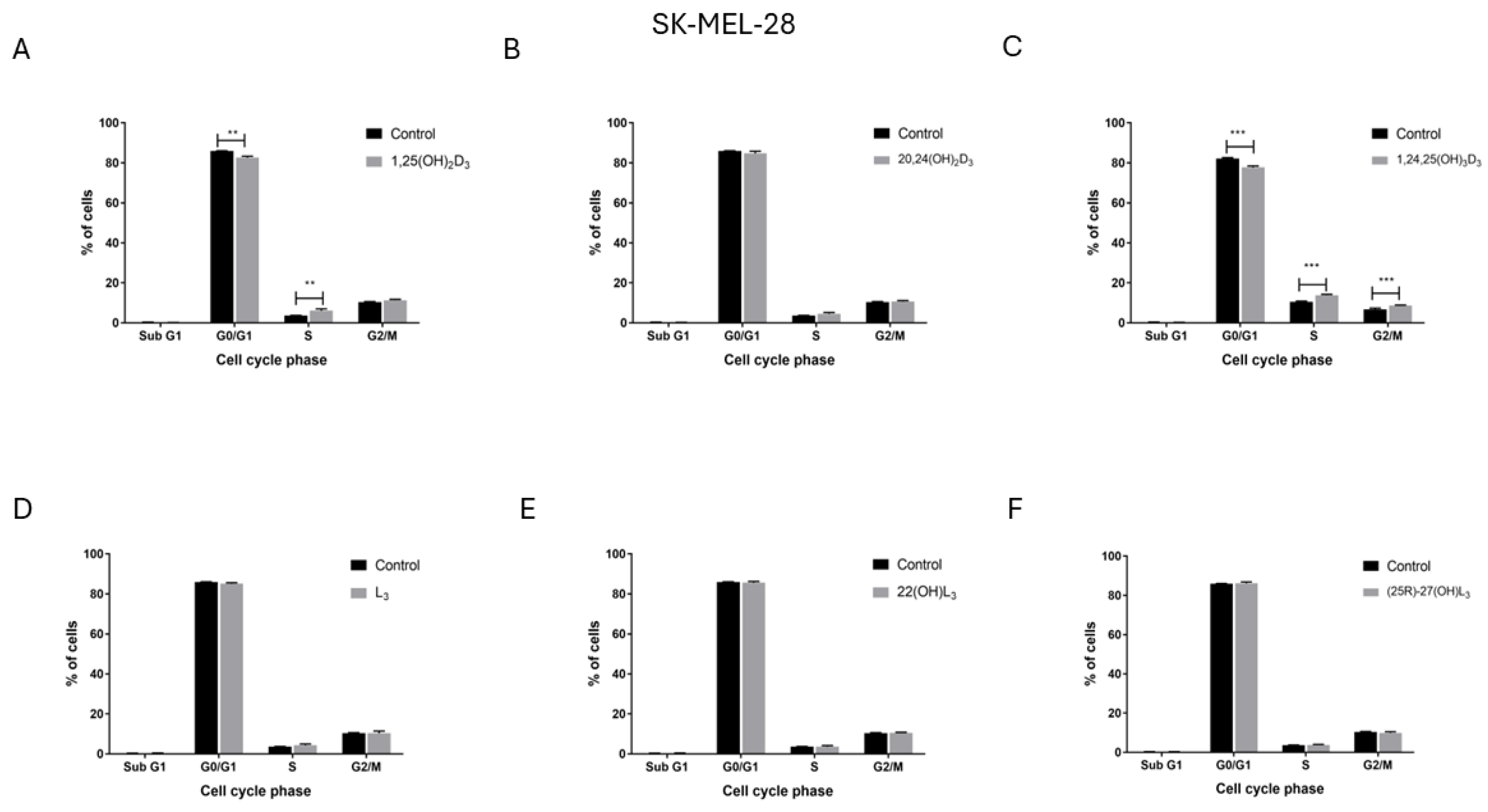
2.4. The Hydroxy-Derivatives of Vitamin D3 and L3 Influence the Distribution of Cells in the Various Phases of the Cell Cycle
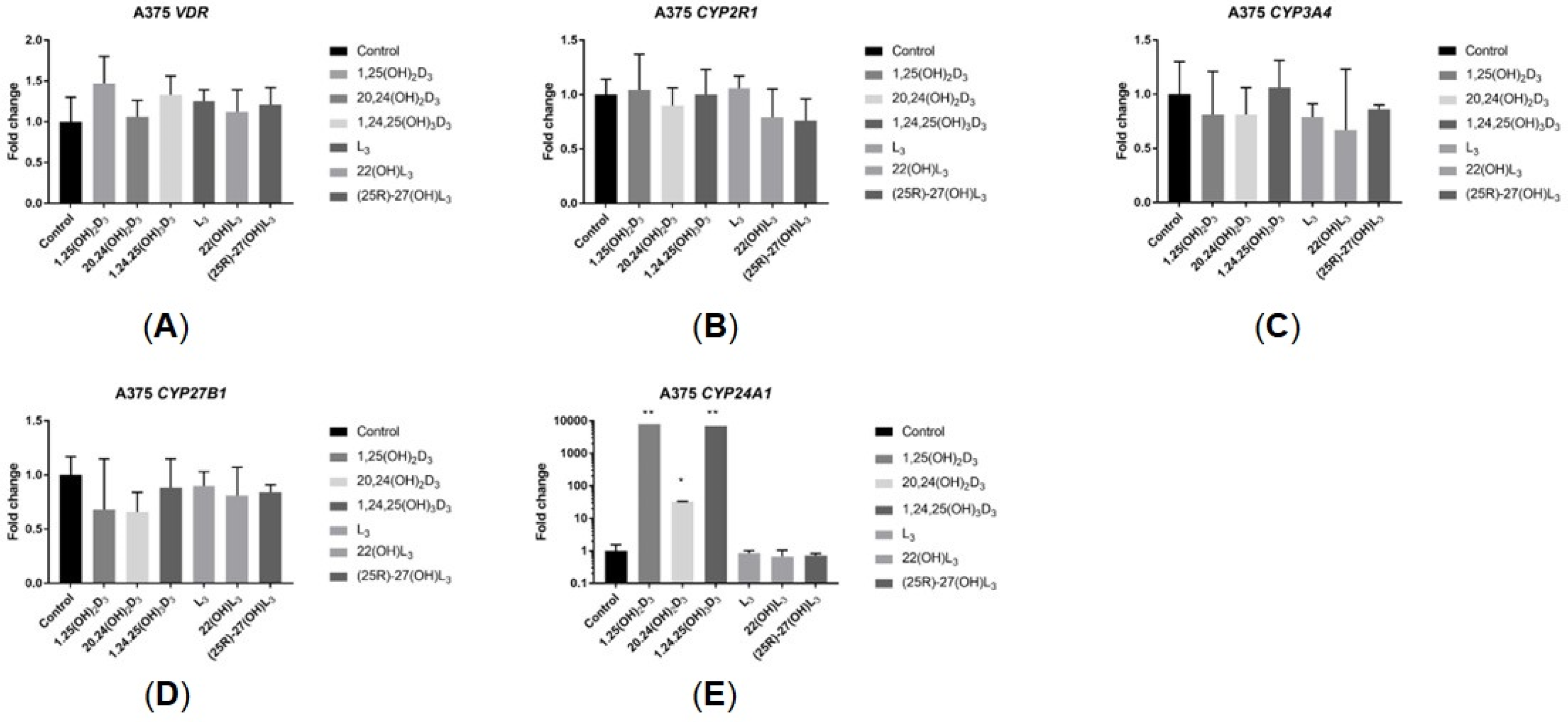
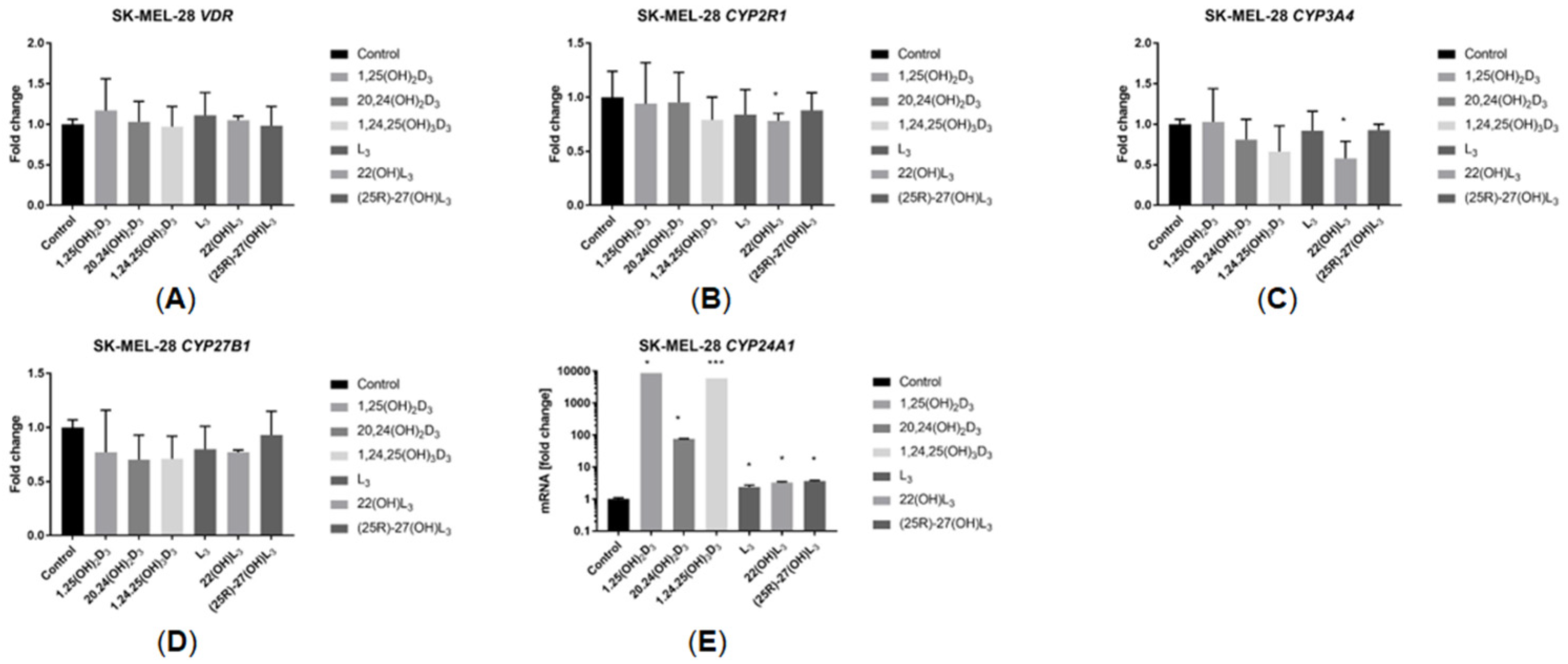
2.5. The Hydroxy-Derivatives of Vitamin D3 and L3 Impact the Expression of Genes Involved in Vitamin D Metabolism
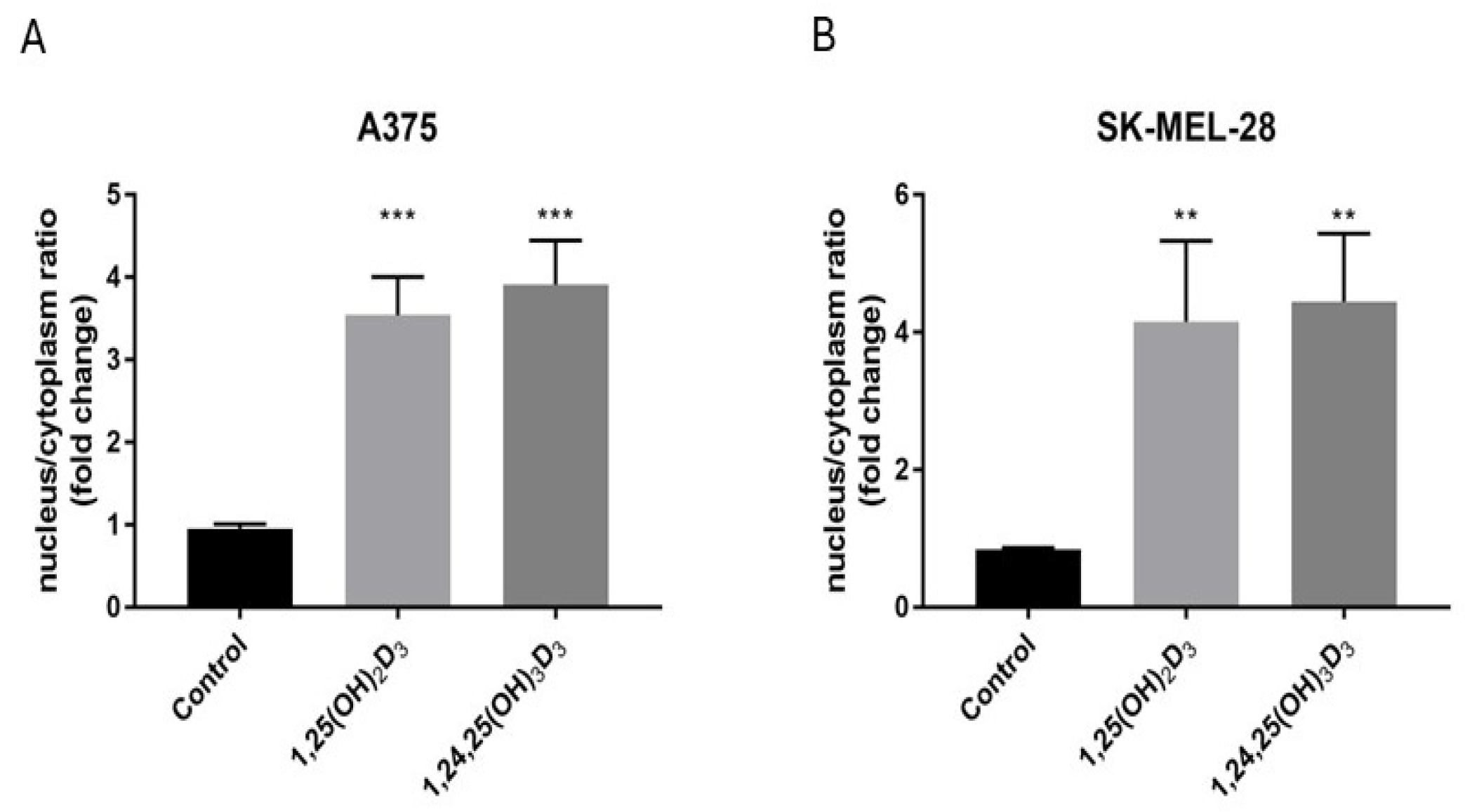
2.6. 1,25(OH)2D3 and 1,24,25(OH)3D3 Influence VDR Translocation from the Cytoplasm to the Nucleus
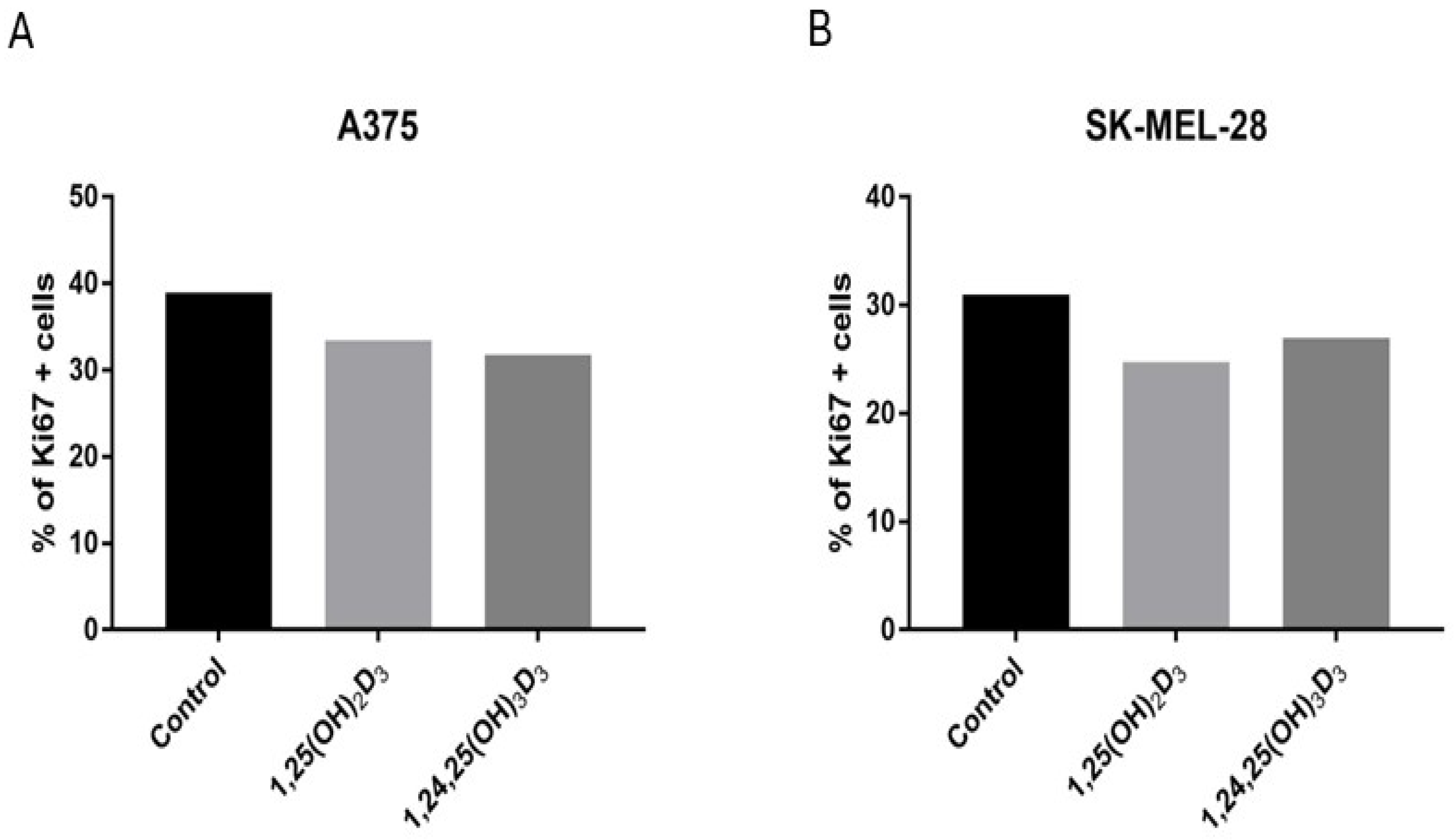
2.7. 1,25(OH)2D3 and 1,24,25(OH)3D3 Influence the Expression of the Ki67 Protein
3. Discussion
4. Materials and Methods
4.1. Synthesis of Hydroxy-Derivatives of Vitamin D3 and L3
4.2. Cell Culture
4.3. SRB Proliferation Assay
4.4. Wound Healing Assay
4.5. RT-PCR Assay of Gene Expression
4.6. Cell Cycle Analysis of Cells Treated with Hydroxy-Derivatives of Sterols and Secosteroids
4.7. VDR Translocation to the Nucleus
4.8. Ki67 Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980, 210, 203–205. [Google Scholar] [CrossRef]
- MacLaughlin, J.A.; Anderson, R.R.; Holick, M.F. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 1982, 216, 1001–1003. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Makin, G.; Lohnes, D.; Byford, V.; Ray, R.; Jones, G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem. J. 1989, 262, 173–180. [Google Scholar] [CrossRef]
- Wierzbicka, J.; Piotrowska, A.; Żmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Tieu, E.W.; Tang, E.K.; Tuckey, R.C. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014, 281, 3280–3296. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Nonaka, Y.; Ohyama, Y.; Inouye, K. Metabolic studies using recombinant escherichia coli cells producing rat mitochondrial CYP24 CYP24 can convert 1alpha,25-dihydroxyvitamin D3 to calcitroic acid. Eur. J. Biochem. 1999, 262, 43–48. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Li, W.; Jetten, A.M.; Indra, A.K.; Mason, R.S.; Tuckey, R.C. Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin. J. Investig. Dermatol. 2024, 144, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Guryev, O.; Carvalho, R.A.; Usanov, S.; Gilep, A.; Estabrook, R.W. A pathway for the metabolism of vitamin D3: Unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc. Natl. Acad. Sci. USA 2003, 100, 14754–14759. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Li, W.; Ma, D.; Cheng, C.Y.S.; Wang, K.M.; Kim, T.K.; Jeayeng, S.; Slominski, A.T. CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J. Steroid Biochem. Mol. Biol. 2018, 181, 1–10. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Slominski, A.T.; Cheng, C.Y.; Chen, J.; Kim, T.K.; Xiao, M.; Li, W. Lumisterol is metabolized by CYP11A1: Discovery of a new pathway. Int. J. Biochem. Cell Biol. 2014, 55, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Janjetovic, Z.; Oak, A.S.W.; Postlethwaite, A.; Lin, Z.; Li, W.; Takeda, Y.; Jetten, A.M.; et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 2017, 7, 11434. [Google Scholar] [CrossRef]
- Jenkinson, C.; Desai, R.; Slominski, A.T.; Tuckey, R.C.; Hewison, M.; Handelsman, D.J. Simultaneous measurement of 13 circulating vitamin D3 and D2 mono and dihydroxy metabolites using liquid chromatography mass spectrometry. Clin. Chem. Lab. Med. 2021, 59, 1642–1652. [Google Scholar] [CrossRef]
- Trump, D.L. Calcitriol and cancer therapy: A missed opportunity. Bone Rep. 2018, 9, 110–119. [Google Scholar] [CrossRef]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Kwiatkowska, K.; Chodyński, M.; Kutner, A.; Żmijewski, M.A. Antiproliferative activity of side-chain truncated vitamin D analogs (PRI-1203 and PRI-1204) against human malignant melanoma cell lines. Eur. J. Pharmacol. 2020, 881, 173170. [Google Scholar] [CrossRef]
- Wasiewicz, T.; Piotrowska, A.; Wierzbicka, J.; Slominski, A.T.; Zmijewski, M.A. Antiproliferative Activity of Non-Calcemic Vitamin D Analogs on Human Melanoma Lines in Relation to VDR and PDIA3 Receptors. Int. J. Mol. Sci. 2018, 19, 2583. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Nadkarni, S.; Brown, G.; Kutner, A.; Żmijewski, M.A. Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D₂ Against Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2016, 17, 76. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Li, W.; Chen, J.; Kim, T.K.; Zjawiony, J.K.; Sweatman, T.W.; Miller, D.D.; Slominski, A.T. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids 2011, 76, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Li, W.; Zjawiony, J.K.; Sweatman, T.W.; Chen, J.; Miller, D.D.; Slominski, A.T. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids 2009, 74, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Janjetovic, Z.; Kim, T.K.; Sweatman, T.; Lu, Y.; Zjawiony, J.; Tuckey, R.C.; Miller, D.; Slominski, A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 2010, 75, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef]
- Cho, Y.L.; Christensen, C.; Saunders, D.E.; Lawrence, W.D.; Deppe, G.; Malviya, V.K.; Malone, J.M. Combined effects of 1,25-dihydroxyvitamin D3 and platinum drugs on the growth of MCF-7 cells. Cancer Res. 1991, 51, 2848–2853. [Google Scholar] [PubMed]
- Siwińska, A.; Opolski, A.; Chrobak, A.; Wietrzyk, J.; Wojdat, E.; Kutner, A.; Szelejewski, W.; Radzikowski, C. Potentiation of the antiproliferative effect in vitro of doxorubicin, cisplatin and genistein by new analogues of vitamin D. Anticancer. Res. 2001, 21, 1925–1929. [Google Scholar]
- Wietrzyk, J.; Pełczyńska, M.; Madej, J.; Dzimira, S.; Kuśnierczyk, H.; Kutner, A.; Szelejewski, W.; Opolski, A. Toxicity and antineoplastic effect of (24R)-1,24-dihydroxyvitamin D3 (PRI-2191). Steroids 2004, 69, 629–635. [Google Scholar] [CrossRef]
- Pelczynska, M.; Switalska, M.; Maciejewska, M.; Jaroszewicz, I.; Kutner, A.; Opolski, A. Antiproliferative activity of vitamin D compounds in combination with cytostatics. Anticancer. Res. 2006, 26, 2701–2705. [Google Scholar]
- Abe-Hashimoto, J.; Kikuchi, T.; Matsumoto, T.; Nishii, Y.; Ogata, E.; Ikeda, K. Antitumor effect of 22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic mice implanted with human breast carcinoma and its synergism with tamoxifen. Cancer Res. 1993, 53, 2534–2537. [Google Scholar] [PubMed]
- Hershberger, P.A.; Yu, W.D.; Modzelewski, R.A.; Rueger, R.M.; Johnson, C.S.; Trump, D.L. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin. Cancer Res. 2001, 7, 1043–1051. [Google Scholar] [PubMed]
- Elmaci, I.; Ozpinar, A.; Perez, J.L.; Altinoz, M.A. From epidemiology and neurometabolism to treatment: Vitamin D in pathogenesis of glioblastoma Multiforme (GBM) and a proposal for Vitamin D+ all-trans retinoic acid + Temozolomide combination in treatment of GBM. Metab. Brain Dis. 2019, 34, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Rybarczyk, A.; Tuckey, R.C.; Slominski, A.T.; Żmijewski, M.A. Vitamin D and its low calcemic analogs modulate the anticancer properties of cisplatin and dacarbazine in the human melanoma A375 cell line. Int. J. Oncol. 2019, 54, 1481–1495. [Google Scholar] [CrossRef]
- Piotrowska, A.; Beserra, F.P.; Wierzbicka, J.M.; Nowak, J.I.; Żmijewski, M.A. Vitamin D Enhances Anticancer Properties of Cediranib, a VEGFR Inhibitor, by Modulation of VEGFR2 Expression in Melanoma Cells. Front. Oncol. 2021, 11, 763895. [Google Scholar] [CrossRef]
- Piotrowska, A.; Zaucha, R.; Król, O.; Żmijewski, M.A. Vitamin D Modulates the Response of Patient-Derived Metastatic Melanoma Cells to Anticancer Drugs. Int. J. Mol. Sci. 2023, 24, 8037. [Google Scholar] [CrossRef]
- Podgorska, E.; Drzal, A.; Matuszak, Z.; Swakon, J.; Slominski, A.; Elas, M.; Urbanska, K. Calcitriol and Calcidiol Can Sensitize Melanoma Cells to Low⁻LET Proton Beam Irradiation. Int. J. Mol. Sci. 2018, 19, 2236. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid Biochem. Mol. Biol. 2018, 177, 159–170. [Google Scholar] [CrossRef]
- Lin, Z.; Marepally, S.R.; Ma, D.; Myers, L.K.; Postlethwaite, A.E.; Tuckey, R.C.; Cheng, C.Y.; Kim, T.K.; Yue, J.; Slominski, A.T.; et al. Chemical Synthesis and Biological Activities of 20S,24S/R-Dihydroxyvitamin D3 Epimers and Their 1α-Hydroxyl Derivatives. J. Med. Chem. 2015, 58, 7881–7887. [Google Scholar] [CrossRef]
- Tieu, E.W.; Tang, E.K.; Chen, J.; Li, W.; Nguyen, M.N.; Janjetovic, Z.; Slominski, A.; Tuckey, R.C. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem. Pharmacol. 2012, 84, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Fraher, L.J.; Sandler, L.M.; O’Riordan, J.L. Demonstration of circulating 1,24,25-trihydroxyvitamin D3 in man by radioimmunoassay. Clin. Endocrinol. 1982, 16, 337–343. [Google Scholar] [CrossRef]
- Cuylen, S.; Blaukopf, C.; Politi, A.Z.; Müller-Reichert, T.; Neumann, B.; Poser, I.; Ellenberg, J.; Hyman, A.A.; Gerlich, D.W. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 2016, 535, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- Zanrè, V.; Campagnari, R.; Cerulli, A.; Masullo, M.; Cardile, A.; Piacente, S.; Menegazzi, M. Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation. Int. J. Mol. Sci. 2022, 23, 1121. [Google Scholar] [CrossRef]
- Gambichler, T.; Bindsteiner, M.; Höxtermann, S.; Kreuter, A. Serum 25-hydroxyvitamin D serum levels in a large German cohort of patients with melanoma. Br. J. Dermatol. 2013, 168, 625–628. [Google Scholar] [CrossRef]
- Saiag, P.; Aegerter, P.; Vitoux, D.; Lebbé, C.; Wolkenstein, P.; Dupin, N.; Descamps, V.; Aractingi, S.; Funck-Brentano, E.; Autier, P.; et al. Prognostic Value of 25-hydroxyvitamin D3 Levels at Diagnosis and During Follow-up in Melanoma Patients. J. Natl. Cancer Inst. 2015, 107, djv264. [Google Scholar] [CrossRef]
- Fang, S.; Sui, D.; Wang, Y.; Liu, H.; Chiang, Y.J.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; et al. Association of Vitamin D Levels With Outcome in Patients With Melanoma After Adjustment For C-Reactive Protein. J. Clin. Oncol. 2016, 34, 1741–1747. [Google Scholar] [CrossRef]
- Muralidhar, S.; Filia, A.; Nsengimana, J.; Poźniak, J.; O’Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D-VDR Signaling Inhibits Wnt/β-Catenin-Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019, 79, 5986–5998. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer. Res. 2020, 40, 473–489. [Google Scholar] [CrossRef] [PubMed]
- La Marra, F.; Stinco, G.; Buligan, C.; Chiriacò, G.; Serraino, D.; Di Loreto, C.; Cauci, S. Immunohistochemical evaluation of vitamin D receptor (VDR) expression in cutaneous melanoma tissues and four VDR gene polymorphisms. Cancer Biol. Med. 2017, 14, 162–175. [Google Scholar] [CrossRef]
- Becker, A.L.; Carpenter, E.L.; Slominski, A.T.; Indra, A.K. The Role of the Vitamin D Receptor in the Pathogenesis, Prognosis, and Treatment of Cutaneous Melanoma. Front. Oncol. 2021, 11, 743667. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Evans, S.R.; Houghton, A.M.; Schumaker, L.; Brenner, R.V.; Buras, R.R.; Davoodi, F.; Nauta, R.J.; Shabahang, M. Vitamin D receptor and growth inhibition by 1,25-dihydroxyvitamin D3 in human malignant melanoma cell lines. J. Surg. Res. 1996, 61, 127–133. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011, 42, 618–631. [Google Scholar] [CrossRef]
- Rao, D.S.; Campbell, M.J.; Koeffler, H.P.; Ishizuka, S.; Uskokovic, M.R.; Spagnuolo, P.; Reddy, G.S. Metabolism of 1alpha,25-dihydroxyvitamin D(3) in human promyelocytic leukemia (HL-60) cells: In vitro biological activities of the natural metabolites of 1alpha,25-dihydroxyvitamin D(3) produced in HL-60 cells. Steroids 2001, 66, 423–431. [Google Scholar] [CrossRef]
- Mayer, E.; Bishop, J.E.; Ohnuma, N.; Norman, A.W. Biological activity assessment of the vitamin D metabolites 1,25-dihydroxy-24-oxo-vitamin D3 and 1,23,25-trihydroxy-24-oxo-vitamin D3. Arch. Biochem. Biophys. 1983, 224, 671–676. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef]
- Wyatt, C.; Lucas, R.M.; Hurst, C.; Kimlin, M.G. Vitamin D deficiency at melanoma diagnosis is associated with higher Breslow thickness. PLoS ONE 2015, 10, e0126394. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Kim, T.K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Tieu, E.W.; Li, W.; Chen, J.; Kim, T.K.; Ma, D.; Slominski, A.T.; Tuckey, R.C. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J. Steroid Biochem. Mol. Biol. 2015, 149, 153–165. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domżalski, P.; Piotrowska, A.; Tuckey, R.C.; Zmijewski, M.A. Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2024, 25, 10914. https://doi.org/10.3390/ijms252010914
Domżalski P, Piotrowska A, Tuckey RC, Zmijewski MA. Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines. International Journal of Molecular Sciences. 2024; 25(20):10914. https://doi.org/10.3390/ijms252010914
Chicago/Turabian StyleDomżalski, Paweł, Anna Piotrowska, Robert C. Tuckey, and Michał A. Zmijewski. 2024. "Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines" International Journal of Molecular Sciences 25, no. 20: 10914. https://doi.org/10.3390/ijms252010914
APA StyleDomżalski, P., Piotrowska, A., Tuckey, R. C., & Zmijewski, M. A. (2024). Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines. International Journal of Molecular Sciences, 25(20), 10914. https://doi.org/10.3390/ijms252010914





