Abstract
The cytotoxic T lymphocyte-associated antigen-4 (CTLA4) gene, a member of the immunoglobulin superfamily, is crucial for maintaining immune homeostasis and preventing autoimmune diseases. Studies have shown that polymorphisms in the CTLA4 gene are linked to an increased risk of brucellosis in humans, but its association with brucellosis in goats remains unexplored. In this study, the tissue expression profile of CTLA4 in goats was investigated, and the correlation between InDel polymorphisms in the CTLA4 gene and susceptibility to brucellosis in goats was examined. The findings reveal the widespread expression of CTLA4 in goat tissues, particularly in the spleen and testes. The tested goat populations presented genotypes insertion/insertion (II), insertion/deletion (ID), and deletion/deletion (DD) at both the P1 and P2 loci, and an association analysis revealed significant differences in the distribution of genotypes and allele frequencies at the P1 and P2 loci of the CTLA4 gene between the Brucella goat case and the control groups (p < 0.05). Specifically, compared with the II genotype, the P1 and P2 loci were significantly associated with an elevated risk of brucellosis development in goats under both the codominant (ID/II) and dominant (ID + DD/II) models (P1, p = 0.042, p = 0.016; P2, p = 0.011, p = 0.014). Additionally, haplotype analysis indicated that haplotypes IP1DP2, DP1IP2, and DP1DP2 were significantly associated with an increased risk of brucellosis in goats compared to the reference haplotype IP1IP2 (p = 0.029, p = 0.012, p = 0.034). Importantly, the Lipopolysaccharide (LPS) stimulation of peripheral blood monocytes and/or macrophages from goats with the II, ID, and DD genotypes resulted in increased CTLA4 expression levels in the II genotype, leading to a robust LPS-induced inflammatory response. Through bioinformatic analysis, the observed effect of the InDel locus on Brucella pathogenesis risk in goats could be attributed to the differential binding of the transcription factors nuclear factor kappaB (NF-κB) and CCAAT/enhancer-binding protein α (C/EBPα). These findings offer potential insights for breeding strategies against brucellosis.
1. Introduction
Brucella is a Gram-negative and intracellular facultative coccidia that can infect more than 60 mammalian species, including humans [1], sheep [2], and goats [3]. Ruminants such as cattle and sheep are the primary hosts, with infection leading to late-term fetal loss, stillbirth, reduced milk production, reproductive disorders in females, and inflammatory reactions such as enlarged testes or testicular inflammation in males [4]. Among the known Brucella species, B. melitensis is recognized as the most virulent and infectious, and it can be transmitted through various routes, including the digestive tract, mucous membranes, and broken skin [5]. The period during which livestock give birth poses a significant risk for Brucella outbreaks. Additionally, high-density farming environments and interregional livestock transportation contribute to the accelerated spread of Brucella and subsequent infections. Humans can become infected through multiple routes, such as the consumption of raw meat and milk, as well as through contact with aborted animals [6]. The pathogenesis of Brucella in livestock and humans is notably similar; when Brucella enters the host, it rapidly traverses the mucosal epithelium to reach the lymph nodes and other immune organs. While the majority of these bacteria are phagocytosed and eliminated by macrophages, a small number of survivors persist, and these survivors interact with the endoplasmic reticulum to form replicative Brucella vacuoles (rBCVs), where they undergo extensive replication, subsequently infecting other tissues and cells as they circulate in the bloodstream [7]. The Shaanbei White Cashmere goat, a distinctive breed in western China known for its high-quality cashmere and meat production, exhibits strong adaptability and significant reproductive potential, contributing to its economic value [8]. However, the main obstacle to efficient reproduction in these goats is B. melitensis infection, which can lead to female infertility, miscarriage, or male orchitis [4]. While developed countries have successfully controlled livestock brucellosis, it continues to be a concern in developing regions such as Asia, the Middle East, and Latin America [9].
In China, a major livestock-farming nation, the prevalence of total brucellosis in sheep and goats has been increasing in recent years, with a national average of 2.3%; in certain provinces, the prevalence rate can reach 18.7%, and the national prevalence of brucellosis in dairy cattle stands at 1.9% [10,11]. In Tanzania, the overall national herd prevalence of brucellosis is 8.2% [12], with some herds in the northern rangelands of Kenya experiencing rates as high as 15% [13]. A high prevalence of 14.14% in livestock has been reported in the Middle East, where Brucella is endemic [14]. Brucellosis, caused by Brucella infections, is a significant global zoonotic disease that poses a serious threat to public health and results in substantial economic losses in livestock production [1]. The economic losses attributed to Brucella infection per goat have been documented to reach USD 30.80 in India and USD 162.55 in Malaysia [15,16]. Furthermore, the incidence of human cases of Brucella infection exceeds 500,000 globally each year [17], with over 90% of these cases stemming from B. melitensis [18]. Slaughter and vaccination are crucial methods for preventing and controlling the spread of brucellosis in cattle, sheep, and other populations. However, challenges have arisen, such as the difficulty in differentiating between naturally infected animals and those that have been immunized via common serological diagnostic tests like RBPT, STAT, and ELISA [19]. Additionally, vaccination may not always be effective and could lead to abortions in pregnant animals, complicating the elimination and purification of brucellosis. Although some live attenuated brucellosis vaccines, such as the Brucella M5-90Δbp26 mutant, have been developed to differentiate between vaccine immunity and natural infection [20], they require the use of the ELISA technology, increasing the costs and economic burdens on farms.
Host genetic factors have been shown to be associated with either resistance or susceptibility to Brucella [21]. Immune responses differ among individuals due to various genetic polymorphisms in immune-related genes, including insertions/deletions (InDels) and single-nucleotide polymorphisms (SNPs), which play a role in regulating inflammatory signaling pathway activities. Screening genetic markers for brucellosis resistance can be an effective strategy to combat brucellosis and minimize economic losses. Marker-assisted selection (MAS) has become a common method for identifying genetic markers related to traits such as litter size, growth, and disease resistance in goats. Single-nucleotide polymorphisms (SNPs) and insertions/deletions (InDels) are the most commonly used markers [22], allowing for accurate analyses of individual livestock genetic composition for genotypic selection. These measures can increase the efficiency of livestock breeding. Research indicates that CTLA4, a member of the immunoglobulin superfamily, plays a crucial role in negatively regulating T-cell proliferation and activation [23]. In immune homeostasis, CTLA4 functions by blocking the binding of T-cell receptors to costimulatory molecules through its interaction with B7, and this action ultimately reduces T-cell activation and expansion, thus maintaining immune tolerance [24]. Furthermore, CTLA4 also decreases the antigen-presenting capacity of dendritic cells by inhibiting the expression of costimulatory molecules [25]. Studies have indicated that overexpression or functional defects in CTLA4 can disrupt immune tolerance and increase the risk of autoimmune diseases, including systemic lupus erythematosus [26].
Inflammatory factors such as IFN-γ and TNF-α, which derive from the Th1 immune response, play crucial roles in resistance to Brucella infection and proliferation [27]. However, Brucella infection can undermine the protective immune response, resulting in the increased circulation of regulatory T-cells (Tregs) within the patient’s body, and the expression of CTLA4, a significant immune checkpoint for Tregs, is markedly elevated in individuals with chronic brucellosis, suggesting that Brucella may induce the overexpression of immune checkpoints in Tregs, potentially leading to the impaired control of Brucella infection [28]. More importantly, the T allele and CT genotype of the −318 C/T variant locus in the CTLA4 gene have been linked to an increased risk of brucellosis infection in humans [29]. Given previous research connecting CTLA4 gene variations with immune and inflammatory diseases, we hypothesized that variations in the goat CTLA4 gene could also be associated with brucellosis infections, but few reports on this have been published. Therefore, this study aimed to identify variant loci in the CTLA4 gene that may be linked to brucellosis risk in goats, offering valuable insights for breeding strategies to control brucellosis in these animals.
2. Results
2.1. Gene Conservation Analysis and mRNA Expression
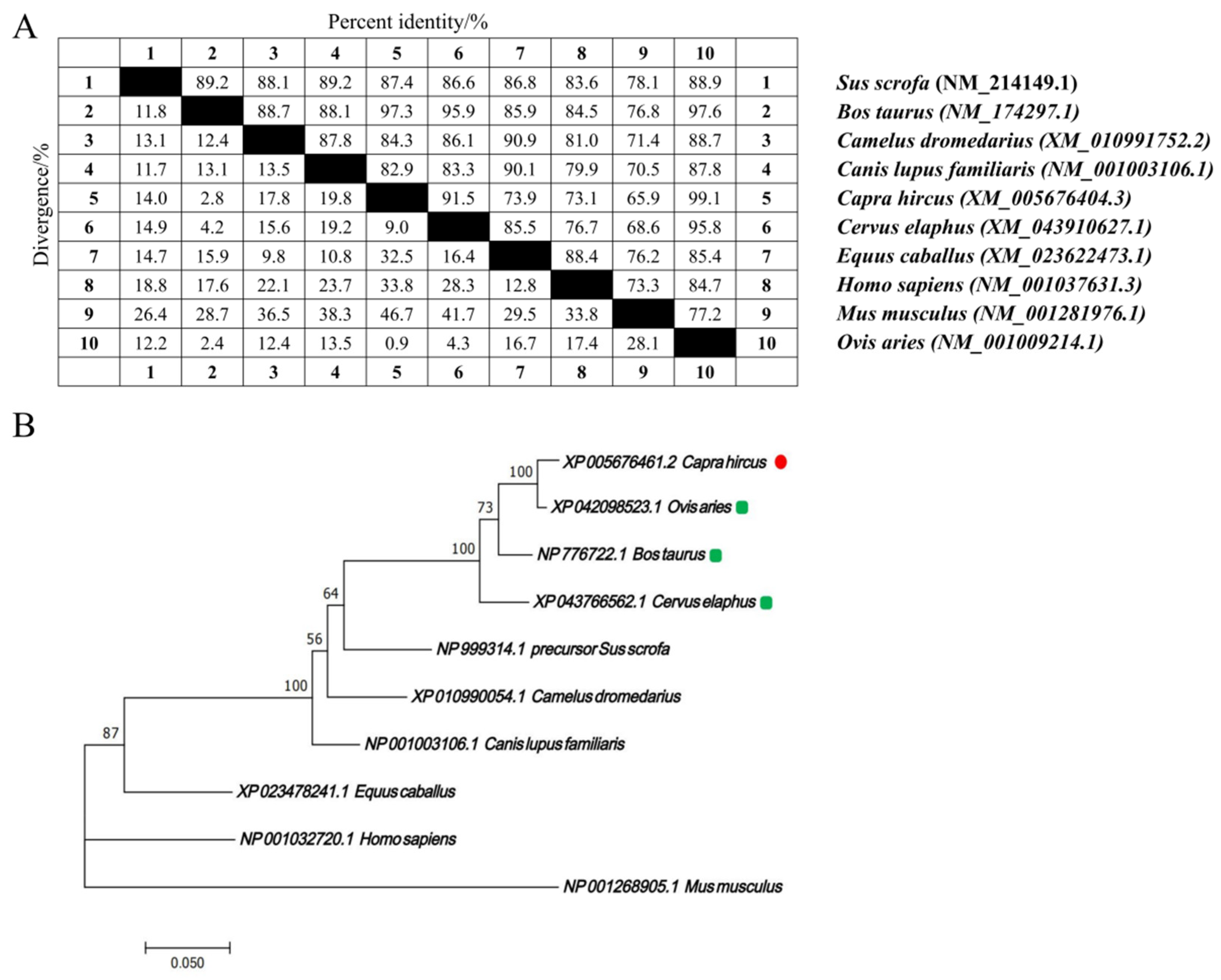
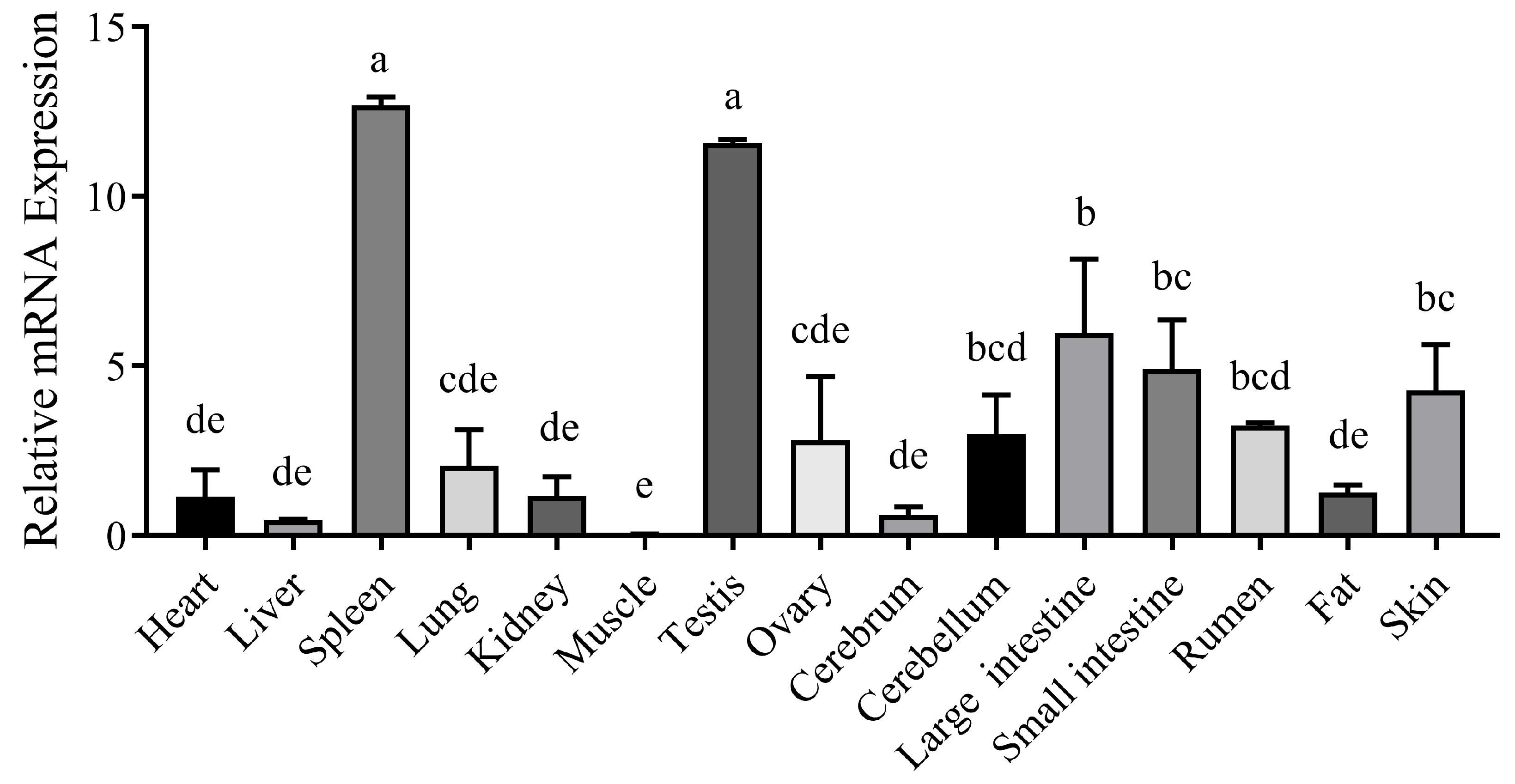
The nucleotide sequences of the CTLA4 gene from 10 different species were retrieved from GenBank and analyzed via the MegAlign software (version 7.1.0). The results indicated that the goat CTLA4 gene shared high homology with Ovis aries (99.1%), Bos taurus (97.3%), and Cervus elaphus (91.5%). Conversely, it showed lower homology with Mus musculus (65.9%) and Homo sapiens (73.1%) (Figure 1A). The phylogenetic tree constructed via the NJ joining method revealed that the genetic distance between the goat CTLA4 gene and those of Ovis aries, Bos taurus, and Cervus elaphus was the closest, suggesting a close relationship with livestock species, particularly sheep and cattle, but a more distant relationship with Homo sapiens and Mus musculus (Figure 1B). These findings were consistent with the nucleotide sequence homology analysis. Furthermore, the qRT-PCR results demonstrated that CTLA4 was broadly expressed in all the goat tissues, with the lowest expression in muscle and the highest in the spleen and testes (Figure 2), particularly the former, which is a vital immune organ.
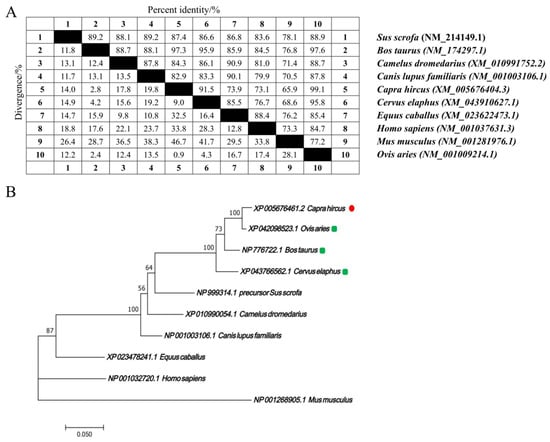
Figure 1.
CTLA4 gene bioinformatic analysis in goats: (A) nucleotide sequence homology analysis of CTLA4 gene; and (B) phylogenetic tree of CTLA4 gene in different animal species.
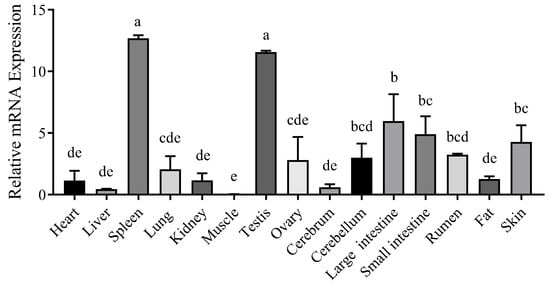
Figure 2.
Tissue expression profile of CTLA4 gene. n = 3 samples of each tissues. Columns with different letters (a–e) mean p < 0.05.
2.2. Identification of Insertion/Deletion Variants in the CTLA4 Gene
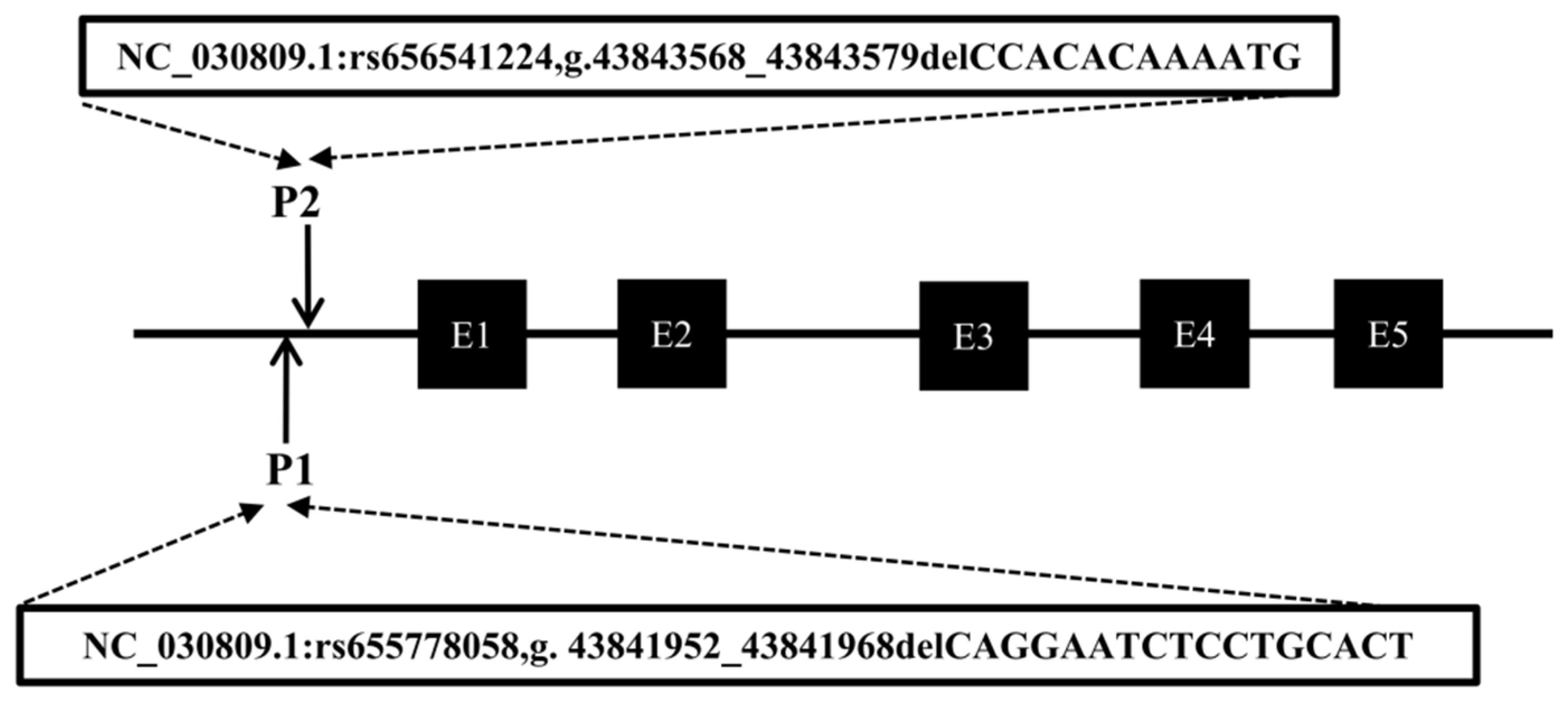
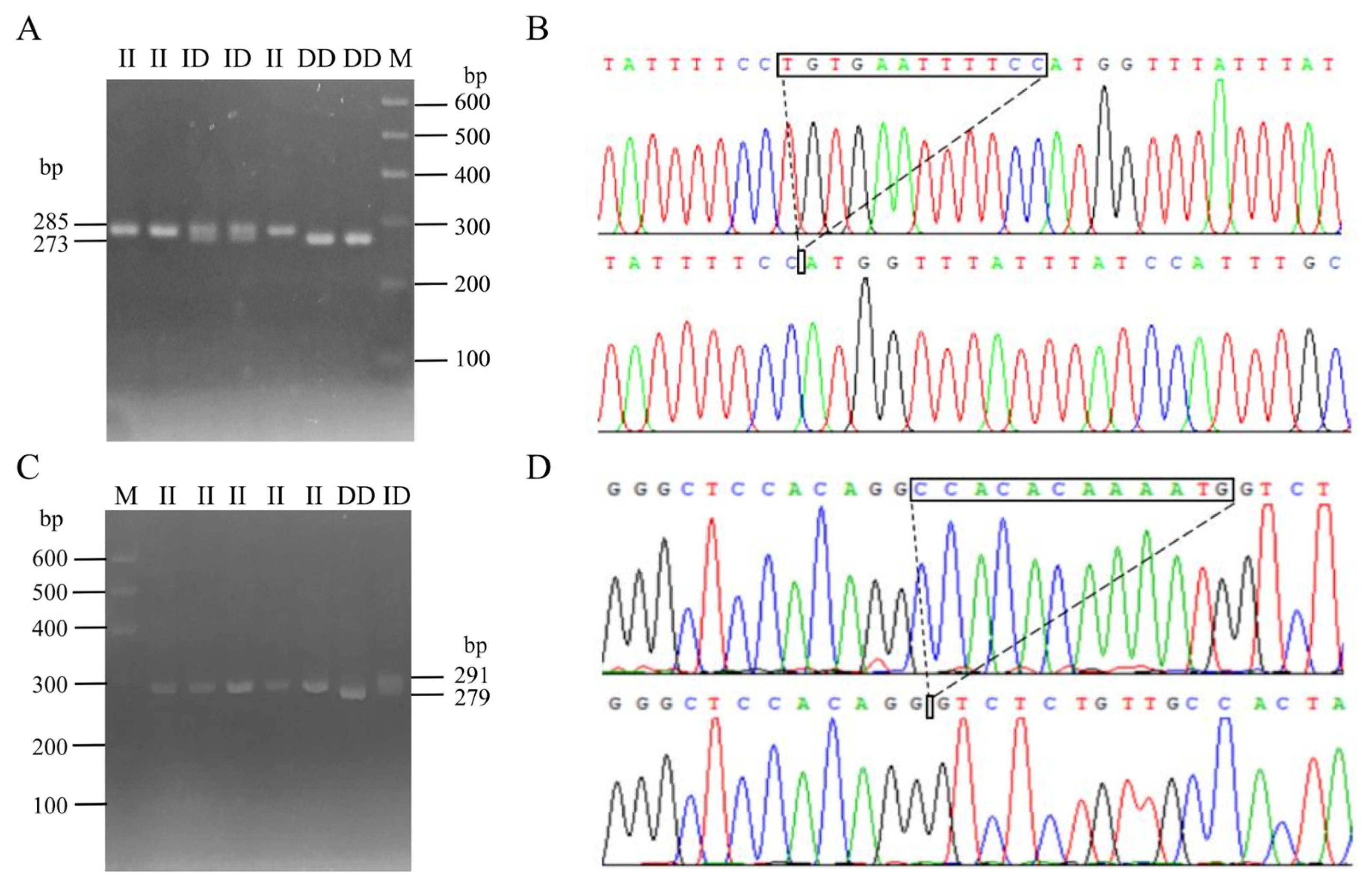
Following the method described in a previous study [30], DNA pool samples were subjected to PCR amplification to identify polymorphisms at the P1 and P2 loci of the CTLA4 gene, and the locations of these two mutations are shown in Figure 3. Gel electrophoresis of agarose (Figure 4A,C) and sequencing analysis (Figure 4B,D) revealed polymorphic loci at both P1 and P2, each of which presented three genotypes: insertion/insertion (II), insertion/deletion (ID), and deletion/deletion (DD). The II genotype displayed a single band (287 bp and 291 bp), the ID genotype presented two bands (287 bp and 273 bp; 291 bp and 279 bp), and the DD genotype presented a single band (273 bp and 279 bp). Notably, the predicted mutation sequence at the P1 locus in the Ensembl database was ACTCAGGAATCTCCTGC/-, whereas the actual sequencing result was TGTGAATTTTCC/-. The mutation sequence at the P2 locus was aligned with the predicted sequence in the Ensembl database.
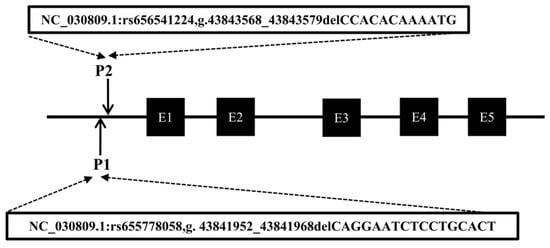
Figure 3.
Mode pattern of identified indel positions of goat CTLA4 gene. The black box represents the exons of the goat CTLA4 gene.
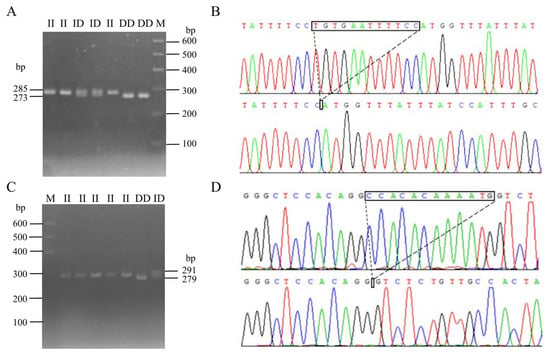
Figure 4.
InDel electrophoresis and sequencing of CTLA4 gene in goats: P1 locus electrophoresis (A) and sequencing map (B); and P2 locus electrophoresis (C) and sequencing map (D). M: 600 bp marker; II: insertion/insertion; ID: insertion/deletion; and DD: deletion/deletion.
2.3. Analysis of Genetic Parameters of CTLA4 in Goats
Table 1 displays the sample size, genotype, and allele frequency of the CTLA4 gene at the P1 and P2 loci in goats. A total of 804 and 838 goat genomes were successfully genotyped at the P1 and P2 variant sites, respectively. For both the P1 and P2 loci, the II genotype frequencies were greater across the case, control, and all goat populations (P1: 0.668, 0.586, and 0.627; P2: 0.728, 0.782, and 0.689), and the frequencies of the I alleles were consistently greater than those of the D alleles (P1: 0.804, 0.750, and 0.777; P2: 0.846, 0.806, and 0.826). The polymorphism information content (PIC) values revealed that the P1 locus presented moderate genetic diversity (0.25 < PIC < 0.50) across the case, control, and all goat populations. The genotype distributions at the P1 locus were all found to deviate from Hardy–Weinberg equilibrium (HWE) (p < 0.05). On the other hand, none of the genotype distributions at the P2 locus deviated from the HWE (p > 0.05). However, the P2 locus presented a low genetic diversity (0 < PIC < 0.25) across all goat populations.

Table 1.
Genetic parameters of polymorphic sites of the CTLA4 gene.
2.4. Association Analysis of CTLA4 Gene Polymorphisms with the Risk of Brucellosis in Goats
Previous studies have indicated a link between the risk of human brucellosis infection and a specific SNP in the CTLA4 gene [29]. As a key candidate gene potentially linked to brucellosis risk, we conducted a statistical analysis of the frequency distribution of genotypes and alleles at the P1 and P2 loci of the CTLA4 gene within the goat population under study (Table 2). The results revealed significant differences in the distribution of the three genotypes (II, ID, DD) at the P1 and P2 loci between cases and controls (p < 0.05), as well as significant disparities in the I and D alleles between the two groups (p < 0.05). These findings suggest a potential association between certain genotypes or alleles at these loci and the risk of brucellosis.

Table 2.
Genotypes and allele frequencies of polymorphic locus of CTLA4 gene in Brucella cases and controls.
Binary logistic regression analysis was used to investigate the associations between four genetic models (codominant, dominant, recessive, and allelic) and the risk of brucellosis at the P1 and P2 loci of the CTLA4 gene (Table 3). The results indicated that, at the P1 locus, compared to the II genotype, the risk of brucellosis was 1.337 and 1.422 times greater in the codominant model (ID/II) and dominant model (ID + DD/II), respectively (p = 0.042, 95% CI = 0.968–1.846; and p = 0.016, 95% CI = 1.067–1.896). Additionally, the D allele conferred a 1.303-fold greater risk of brucellosis than the I allele (p = 0.009, 95% CI = 1.028–1.652). Similarly, at the P2 locus, relative to the II genotype, the risk of brucellosis was 1.512 and 1.448 times greater in the codominant model (ID/II) and dominant model (ID + DD/II), respectively (p = 0.011, 95% CI = 1.096–2.086; and p = 0.014, 95% CI = 1.079–1.944). Furthermore, the D allele was associated with a 1.326-fold greater risk of brucellosis than the I allele (p = 0.029, 95% CI = 1.029–1.710).

Table 3.
Association between genetic modeling of polymorphic locus in the CTLA4 gene and risk of brucellosis development.
2.5. Linkage Disequilibrium (LD) and Haplotype Association Analysis
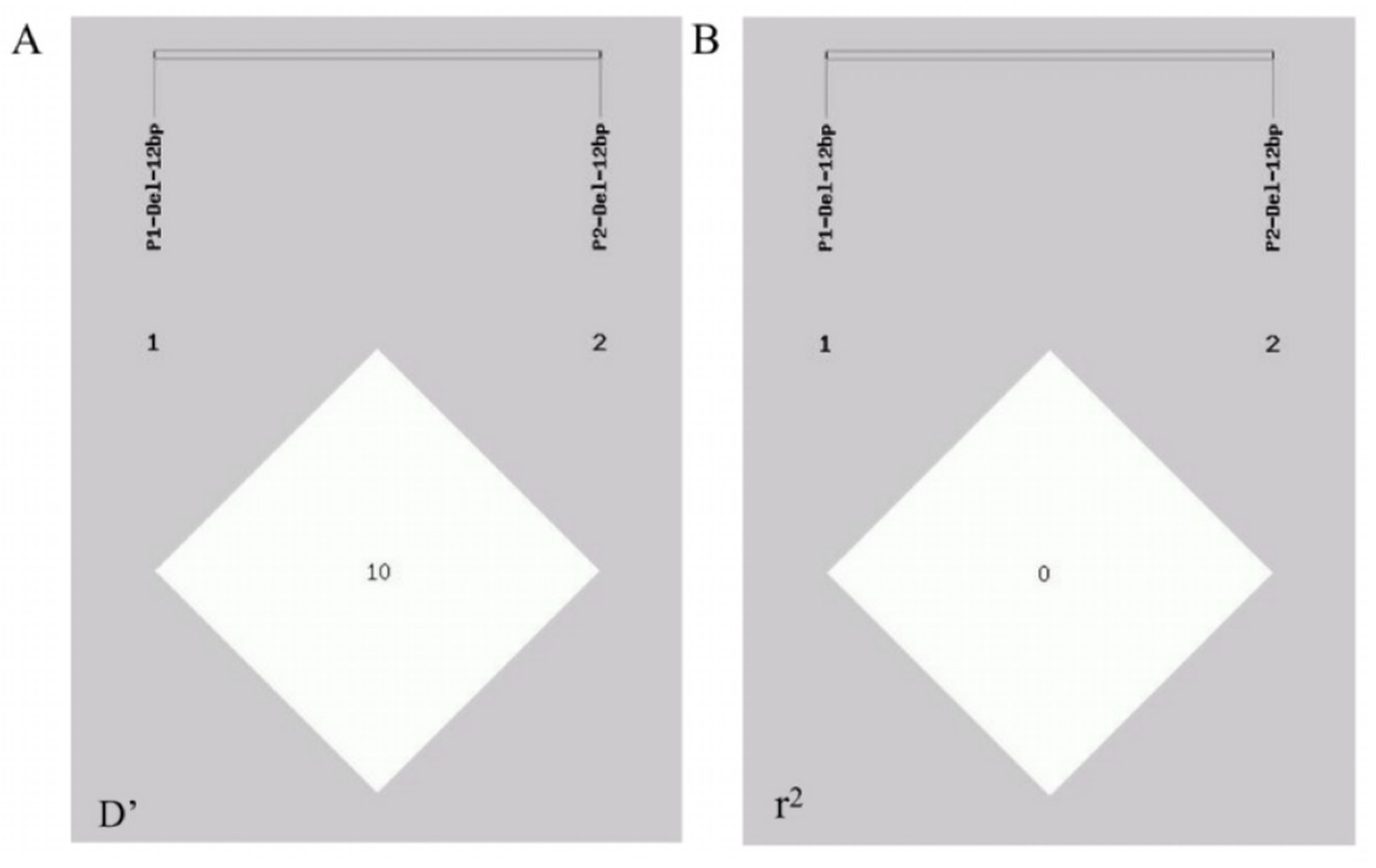
A linkage disequilibrium analysis was performed via the GDICALL website (http://www.msrcall.com/Gdicall.aspx; accessed on 12 July 2024) to explore the possibility of linkage between the two variant sites in the CTLA4 gene (Figure 5), and the D’ and r2 values were 0.108 and 0.009, respectively, indicating that there was no strong link between the two variant loci.
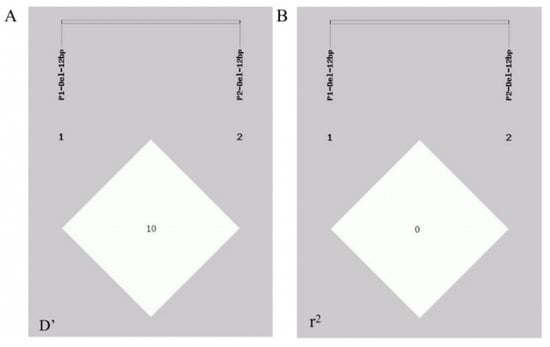
Figure 5.
Linkage disequilibrium (LD) between P1 and P2 mutation locus of CTLA4 gene in goats. (A) D’ value and (B) r2 value.
Haplotype construction was performed on the two InDel loci of P1 and P2 (Table 4), and four haplotypes, IP1IP2, DP1IP2, IP1DP2, and DP1DP2, were generated. The results revealed that, when haplotype IP1IP2 was used as a reference, the risk of brucellosis increased by 1.416, 1.415, and 1.634 times with haplotypes DP1IP2, IP1DP2, and DP1DP2, respectively (p = 0.029, 95% CI = 1.037–1.933; p = 0.012, 95% CI = 1.078–1.858; and p = 0.034, 95% CI = 1.039–2.571).

Table 4.
Relationship between CTLA4 gene haplotypes and risk of brucellosis incidence.
2.6. LPS Stimulates Changes in CTLA4 and Cytokine mRNA Expression Levels in Peripheral Blood Monocytes and/or Macrophages
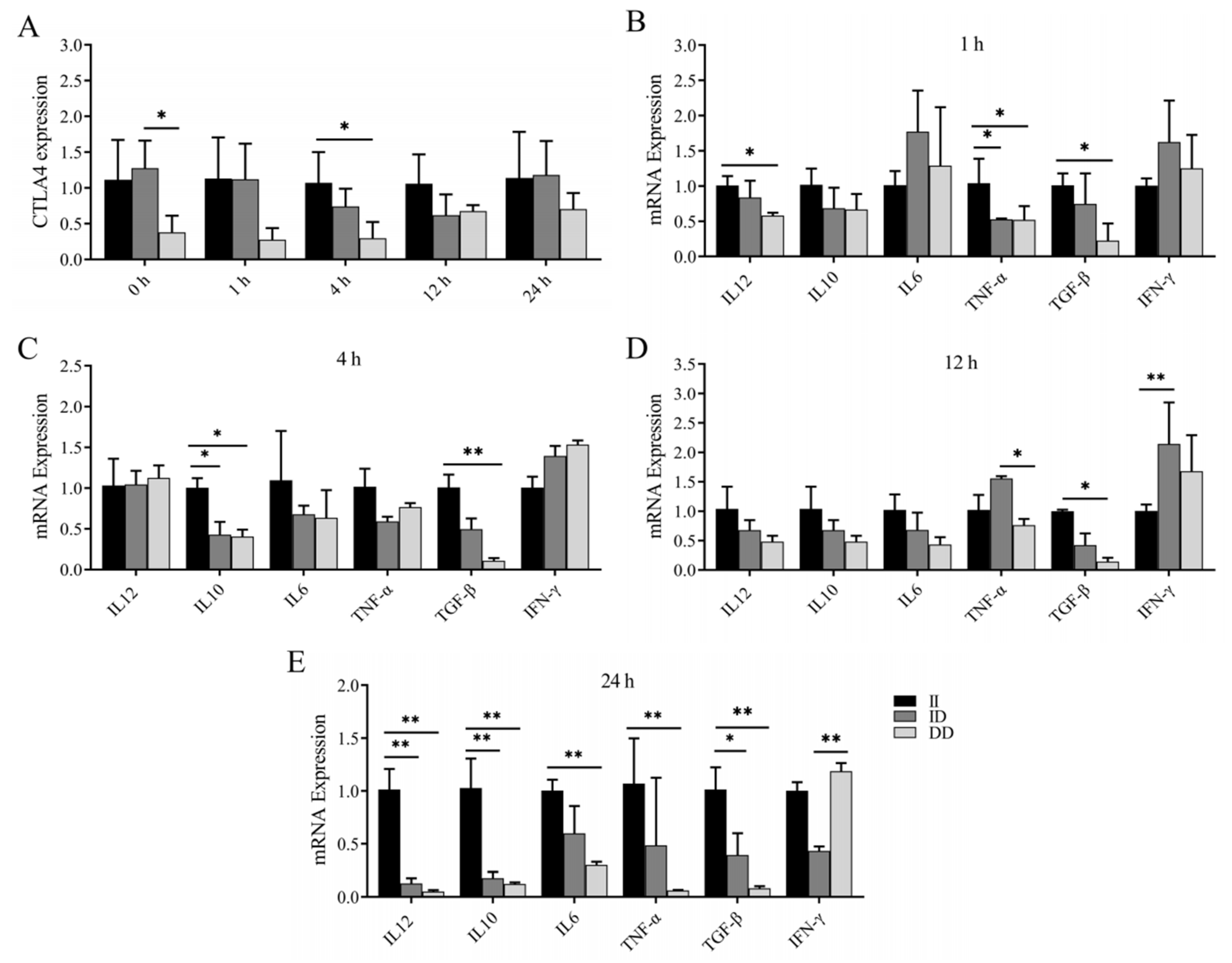
Previous studies have revealed an association between the P1 and P2 loci and susceptibility to brucellosis infection. In this study, we investigated the variations in CTLA4 and cytokine mRNA expression levels in the peripheral blood monocytes and/or macrophages of goats with different genotypes following LPS stimulation. Our findings revealed that the expression of CTLA4 in monocytes and/or macrophages of goats with the DD genotype was consistently lower than in those with the II genotype at various time points, with a significant difference observed after 4 h of LPS stimulation (p < 0.05) (Figure 6A). Additionally, at 1 h post LPS stimulation, the expression of TNF-α in goats with the II genotype was significantly greater than in goats with the ID and DD genotypes, whereas the expression of IL-12 and TGF-β was notably greater than in goats with the DD genotype (Figure 6B). Following 4 h of LPS stimulation, IL-10 expression was significantly elevated in goats with the II genotype compared with those with the ID and DD genotypes, and TGF-β expression was notably greater than in goats with the DD genotype (p < 0.05) (Figure 6C). Moreover, after 12 h of stimulation, TGF-β expression was significantly greater in goats with the II genotype than in those with the DD genotype, and IFN-γ expression was notably greater in goats with the ID genotype than in those with the II genotype (p < 0.05) (Figure 6D). Finally, following 24 h of stimulation, goats with the II genotype presented significantly higher expression levels of IL-12, IL-10, and TGF-β than those with the ID and DD genotypes, whereas the expression levels of IL-6 and TNF-α were notably greater than in goats with the DD genotype (p < 0.05) (Figure 6E).
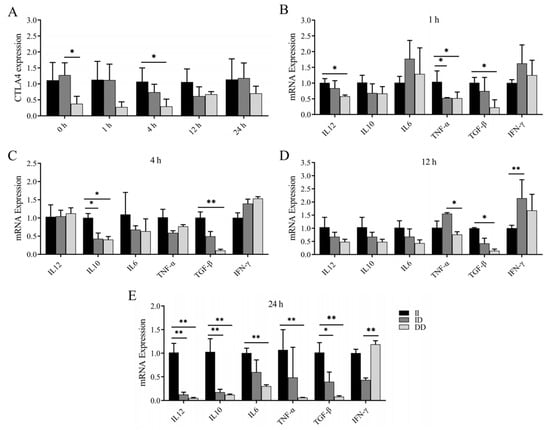
Figure 6.
Changes in CTLA4 and cytokines in peripheral blood monocytes and/or macrophages of goats of different genotypes after LPS stimulation. (A) Changes in CTLA4 expression at different time points in LPS-stimulated peripheral blood monocytes and/or macrophages; and (B–E) changes in cytokine expression of IL-6, IFN-γ, TNF-α, IL-10, IL-12, and TGF-β, respectively, at different time points after LPS stimulation. * p < 0.05 and ** p < 0.01.
2.7. Predicted Binding of Transcription Factors
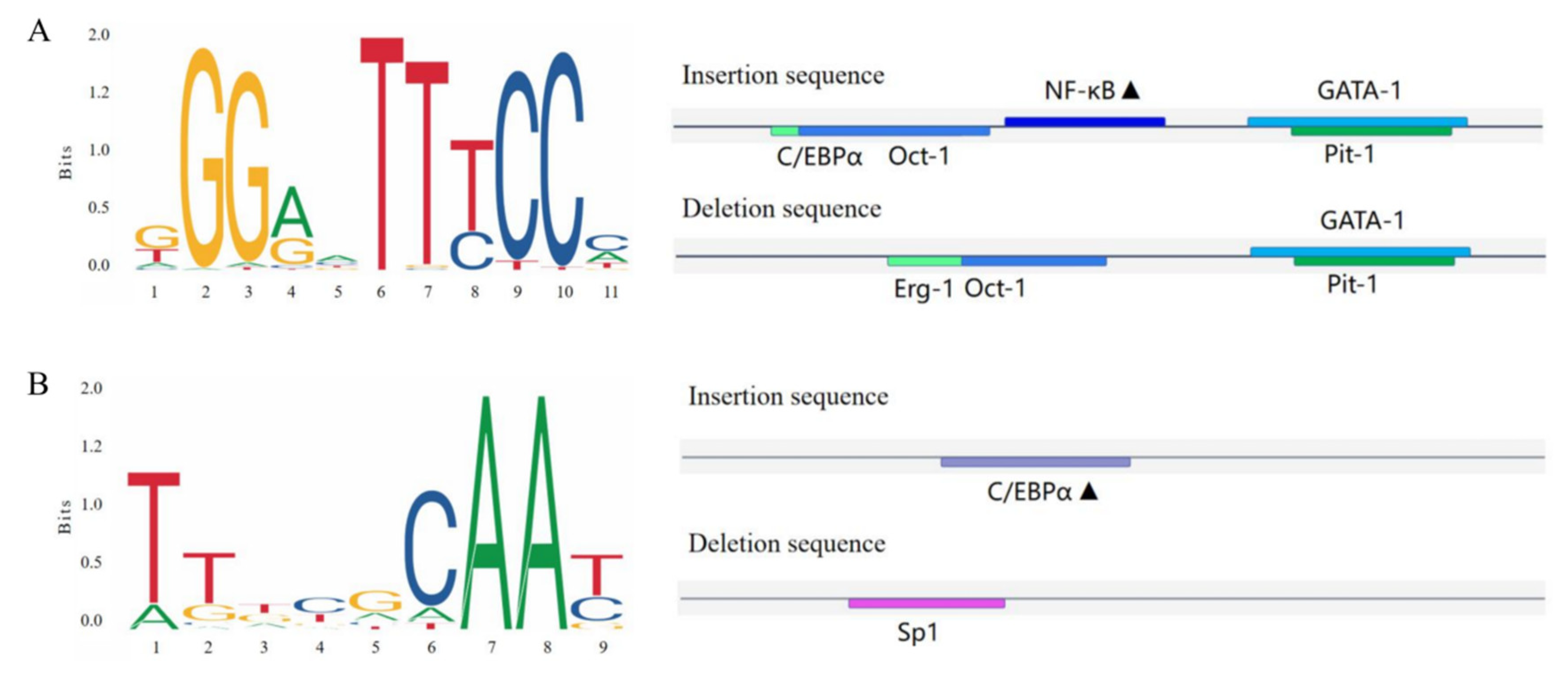
Bioinformatics was utilized to predict the transcription factor-binding sites of the CTLA4 gene variation sites in goats, as shown in Figure 7. The study revealed that the P1 (Figure 7A) and P2 (Figure 7B) insertion sequences of the CTLA4 gene had the ability to specifically bind to nuclear factor kappaB (NF-κB) and the CCAAT/enhancer-binding protein α (C/EBP-α) transcription factors, respectively.
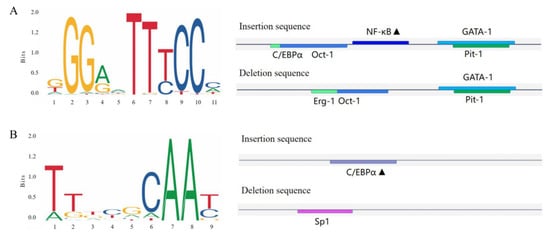
Figure 7.
Transcription factor-binding site prediction for the goat CTLA4 gene variant locus. (A,B) represent the P1 and P2 loci, and the black triangles represent potential transcription factor-binding sites.
3. Discussion
Researchers widely recognize that Brucella abortus is associated with T lymphocyte apoptosis and is able to induce the production of Th1 proinflammatory cytokines, such as IFN-g and TNF-α, in infected hosts, which are involved in the regulation of macrophage activation and bacterial proliferation [31]. Moreover, the interaction between costimulatory and coinhibitory receptors on T-cells, such as CTLA4, with ligands on antigen-presenting cells (APCs), such as CD80 and CD86, can impact the strength and duration of antigen-specific T-cell responses [32]. Studies have shown that mice lacking CTLA4 experience lymphocyte infiltration and tissue damage and ultimately develop lymphoproliferative disorders [33], highlighting the immunomodulatory role of CTLA4 in activities such as lymphocyte infiltration and the regulation of T-cell proliferation and activation [34]. Given the important role of CTLA4 in regulating T-cell activation and enhancing antigen-presenting capacity, researchers have combined the CTLA4 immunoglobulin variable region (IgV_CTLA4) with novel multiepitope vaccine proteins to develop a new vaccine against brucellosis, and this vaccine demonstrates superior immunogenicity and antigenicity both in vitro and in vivo [35]. Additionally, CTLA4 gene variants have been linked to basal cell carcinoma (BCC) [36], rectal cancer [37], acute kidney transplant rejection [38], and various inflammatory diseases [26]. While CTLA4 gene variants have been linked to human brucellosis infection [29], research on their association with brucellosis in goats is lacking. Therefore, the relationship between CTLA4 gene polymorphisms and brucellosis resistance in goats is worth exploring.
The sample sizes of existing and current studies on the relationship between genetic polymorphisms and brucellosis in goats are almost always small (N < 300) [39], which affects the accuracy and persuasiveness of the results to a certain extent; therefore, we investigated the relationship between CTLA4 gene polymorphisms and brucellosis in a large sample size of Shaanbei White Cashmere goats (N > 800) and reported that two InDel loci of the CTLA4 gene exhibited polymorphisms in these animals. Previous research has suggested that the potential mechanism behind this effect may be linked to a functional mutational polymorphism (LD) within the gene [40]; however, there is no LD between these two loci. In general, there is a direct relationship between the diversity in genetic variation and the evolutionary potential of a population, and a low genetic diversity results in the diminished adaptive capacity of individuals and the decreased reproductive capacity of the population [41]. Conversely, a higher genetic diversity enhances the evolutionary potential of the population and improves the overall immune response of the population when confronted with pathogen attacks [42]. Higher PIC values and population heterozygosity typically indicate greater genetic diversity [8], and, in this study, the PIC values and population heterozygosity of the P1 locus were found to be greater than those of the P2 locus. This finding suggests that the P2 locus has a lower level of genetic diversity, indicating that the intensity of artificial selection could be effectively intensified. In addition, the P2 locus conformed to HWE (p > 0.05), whereas the P1 locus deviated from HWE (p < 0.05), which may have been related to the fact that the tested population was subjected to high-intensity artificial selection and genetic drift, resulting in the loss of some alleles or the introduction of new mutations. In this study, when the II genotype was used as a reference, the risk of brucellosis was significantly greater in the codominant model (ID/II) and the dominant model (ID + DD/II) than in the II genotype (p < 0.05), the risk of brucellosis development was significantly greater for the D allele than for the I allele (p < 0.05), and the degree of risk of brucellosis was significantly greater for haplotypes DP1IP2, IP1DP2, and DP1DP2 than for haplotype IP1IP2 (p < 0.05). The above results suggest that the II genotype and I allele may be associated with resistance to brucellosis infection in goats; thus, individual II genotype goats may be intensively selected for brucellosis resistance for the total goat population. Notably, immunogenetic evolution in animals may be influenced by pathogen-mediated gene selection pressure, with ruminants being the primary hosts of Brucella infections [43]. Natural resistance-associated macrophage protein 1 (Nramp1) is recognized as a key gene associated with high levels of integrated disease resistance, and its genetic variation has been extensively documented in relation to Brucella resistance in livestock, including goats [43] and cattle [44]. Furthermore, the amino acid sequence of the goat Nramp1 gene is more than 96% similar to those of cattle and sheep, indicating that Brucella-mediated host selection influences the evolutionary trajectory of this gene [45]. In our research, the nucleotide sequences of goat CTLA4 presented the highest homology with those of sheep and cattle. The tree topology indicates that the CTLA4 gene is highly conserved among livestock, including goats, sheep, and cattle, and suggests that the CTLA4 gene may play a role in Brucella evolution.
Several studies have demonstrated correlations between gene polymorphisms, gene expression, and susceptibility to brucellosis. After Brucella infection in buffaloes, individuals with the BB genotype displayed a rapid increase in Nramp1 expression in monocytes, along with a reduced number of intracellular bacteria [46]. The GA genotype with the rs7749323 variant of the TNFAIP3 gene was linked to resistance against Brucella infection; however, the expression of the TNFAIP3 gene in the monocytes of individuals with the GA genotype was notably lower than that of the GG genotype when exposed to lipopolysaccharide (LPS) and Brucella stimulation [47]. These results suggest a connection between the mRNA expression of genes and early resistance against pathogen invasion and warrant further investigation. The inflammatory response plays a crucial role in protecting the animal body against pathogen invasion, and immunological functions are carried out through monocyte and macrophage recognition of pathogenic bacteria, phagocytosis, and the secretion of inflammatory cytokines. Therefore, in this study, we stimulated monocytes and/or macrophages from goats with different genotypes via LPS. We observed that the expression of CTLA4 in goat monocytes and/or macrophages of the DD genotype was significantly lower than that in the II genotype after 4 h of LPS stimulation. In particular, after 24 h of stimulation, the expression of IL-10, IL-12, and TGF-β in monocytes and/or macrophages of the II genotype was significantly greater than that in those of the ID and DD genotypes, and the expression of IL-6 and TNF-α was significantly greater than that in the DD genotype. Collectively, the evidence suggests that the II genotype or I allele acts as a protective factor against Brucella infection in goats. In a previous study assessing the immune response of CTLA4 to the intracellular protozoan Trypanosoma cruzi, researchers reported a significant increase in CTLA4 expression in the splenic T-cells of infected mice, and the blockade of CTLA4 was shown to increase the production of IFN-γ, TNF-α, and NO, which significantly reduced both parasitemia and mortality in mice infected with the Y strain of T. cruzi and improved host resistance to the Y strain [48]. Similarly, elevated levels of CTLA4 expression were observed in lymphocytes from patients with tuberculosis, and the blockade of CTLA4 was found to enhance the immune response to Mycobacterium tuberculosis infection [49]. In our study, we noted that CTLA4 expression levels in individual monocyte macrophages from genotype II goats increased after 4 h of LPS stimulation of the monocytes. Notably, within 24 h of LPS stimulation, TNF-α expression in individual monocyte macrophages from genotype II goats remained consistently high, thereby inducing a robust inflammatory response. Collectively, these findings indicate the involvement of CTLA4 in the regulation of the LPS-mediated inflammatory response in monocytes and/or macrophages.
Previous studies have shown that mutations in noncoding regions of genes can impact gene binding to DNA sequences, transcription factors, and splicing factors, ultimately affecting gene expression and protein translation [50]. Therefore, we propose that the specific binding of transcription factors could be responsible for this effect. Using bioinformatics to predict transcription factor-binding sites, we determined that the insertion sequences of the two variants could bind to nuclear factor Kappab (NF-κB) and CCAAT/enhancer-binding protein α (C/EBPα). NF-κB signaling is closely linked to immunoregulatory processes such as lymphoid organ development, dendritic cell antigen presentation, and innate antiviral immunity [51] and plays a role in inflammatory regulation and immune maintenance in conditions such as rheumatoid arthritis, renal inflammation, and metabolic inflammation [52]. The activation of NF-κB is frequently associated with the upregulation of antiapoptotic gene expression, and this process induces the production of cytokines and adhesion molecules that regulate the immune response, including TNF-α, and facilitates the recruitment of leukocytes to sites of inflammation. Notably, bacterial and viral infections represent significant pathways for NF-κB activation [53]. On the other hand, C/EBPα is crucial for the TLR3-mediated production of inflammatory cytokines and immune response to viral infections, and the silencing or knocking down C/EBPα has been shown to result in more severe disease [54]. It has been reported that the transcription factor C/EBPα specifically binds to mC-6, a CpG site located in the promoter region of trefoil factor 1 (TFF1), and, by constructing a C/EBPα overexpression vector, co-transfecting it with the TFF1 promoter region into porcine intestinal epithelial cells infected with porcine epidemic diarrhea virus (PEDV), a significant reduction in the rate of PEDV infestation was observed, along with the inhibition of its replication [55]. Most variants associated with complex traits, such as disease resistance, are located in noncoding regions of the genome, which makes transcription factor–DNA binding interactions a crucial method for investigating gene expression, post-transcriptional regulation, and phenotypic changes [56]. In this study, the II genotype and the I allele were found to be linked to resistance against Brucella infection in goats, and the polymorphism in the CTLA4 gene may affect gene expression and the immune response to pathogenic bacterial infection through differential binding of the transcription factors NF-κB and C/EBPα. However, it is essential to note that disease resistance is influenced by multiple genes, making it a complex trait that can be affected by various factors, including genetic background, regional environment, and individual developmental differences [57,58], and further comprehensive studies are needed to fully understand these interactions.
4. Materials and Methods
4.1. Samples and Data Collection
This study utilized 850 unrelated adult female Shaanbei White Cashmere goats from Shaanbei White Cashmere Goat Farm in Yulin city, Shaanxi province, China. Ear tissue samples were collected and stored in 75% ethanol for DNA extraction. Blood samples were obtained through jugular vein puncture and tested using the Rose Bengal Plate Agglutination Test (RBPT), as described by Rahman et al. [59]. Briefly, the RBPT involved mixing 20 μL of Rose Bengal plate antigen with goat serum on a slide, observing for 3~5 min, and comparing with positive and negative controls. The presence of agglutination indicated a positive result, while its absence indicated a negative result. Positive samples were re-examined for confirmation. All goats in this study were not vaccinated against brucellosis and were part of a high-incidence group (>30% seropositive for brucellosis), showing clinical symptoms like miscarriage and stillbirth. The goats were all raised in the same environment, resulting in an equal risk of Brucella infection. Tissue samples including heart, liver, spleen, lung, kidney, ovary, cerebrum, cerebellum, large intestine, small intestine, muscle, rumen, fat, and skin were collected from adult female goats, along with tissue samples from adult male goats (n = 3 per group) for gene expression analysis. All samples were immediately cryopreserved in liquid nitrogen for subsequent RNA extraction.
4.2. DNA Extraction, Primer Design, and Genotyping
Following the established protocol, DNA was extracted from ear tissue samples and assessed for purity and quality using a Nanodrop 2000 spectrometer (Thermo Scientific, Waltham, MA, USA) [60]. The extracted DNA was then diluted with RNA-free enzyme water to a concentration of 20 ng/μL and stored in a −20 °C refrigerator for genetic variation testing. A DNA pool was constructed using 48 randomly selected DNA samples from Shaanbei White Cashmere Goats to detect insertion/deletion mutations in the CTLA4 gene. The reference sequence and InDel variation information of the goat CTLA4 gene (GenBank accession NC_030809.1) were obtained from the NCBI (https://www.ncbi.nlm.nih.gov/; accessed on 25 April 2024) and Ensembl databases (http://asia.ensembl.org/index.html; accessed on 25 April 2024). Two pairs of primers were designed using the Primer Premier Software (version 5.0) to amplify specific fragments of the CTLA4 gene (Table 5). PCR was carried out in a 13 μL reaction mixture, with steps including predenaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 1 min, 59 °C for 20 s, and 72 °C for 20 s; the final step involved extension at 72 °C for 10 min and storage at 16 °C. Individual genotypes of the goats were determined through 3% agarose gel electrophoresis and a Fully Automated Gel Imaging System (Gel Doc EZ, BIO-RAD, Hercules, CA, USA). Genotypic sequencing was carried out by a company (Sangon Biotech, Shanghai, China).

Table 5.
InDel detection and qRT-PCR primers for CTLA4 gene in goats.
4.3. RNA Extraction, cDNA Synthesis, and qRT-PCR
Total RNA was extracted from the tissue samples using TRIzol Total RNA Extraction Reagent (Takara, Dalian, China) following the manufacturer’s protocol. First-strand cDNA was synthesized with the Prime Script™ RT kit (Takara, Dalian, China) and stored at −80 °C. qRT-PCR primers were designed using the Primer Premier5.0 software (Table 5). qRT-PCR was conducted in a 20 mL system with a reaction mixture containing 2 × ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) 10 μL, cDNA template 1 μL, and 1 μL each of the upstream and downstream primers (10 pmol/μL for each primer), along with RNase-free ddH2O 8 μL. PCR reactions were conducted following a three-step reaction procedure [30], with GAPDH serving as the internal control gene. The relative expression of each tissue was assessed using the 2−ΔΔCt method, with three repetitions for each tissue cDNA sample.
4.4. Bioinformatic Analysis
The nucleotide and protein sequences were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/; accessed on 14 July 2024). A nucleotide sequence homology comparison analysis was conducted using MegAlign (version 7.1.0) (https://www.dnastar.com/; accessed on 14 July 2024) [61], the neighbor-joining method in MEGA (version 7.0.26) (http://www.megasoftware.net/; accessed on 14 July 2024) was utilized to construct the evolutionary tree [62], the bootstrap test was performed with a repetition rate of 1000-fold and a confidence interval of 95%, and other parameters were kept at their default values. The prediction of transcription factor-binding sites for the InDel site of the CTLA4 gene was conducted through the AliBaba 2.1 website (http://gene-regulation.com/pub/programs/alibaba2/; accessed on 18 July 2024) and JASPAR (https://jaspar.genereg.net/; accessed on 18 July 2024).
4.5. LPS In Vitro Stimulation of Peripheral Blood Monocytes and/or Macrophages
Peripheral blood monocytes and/or macrophages were isolated from adult goats that tested negative in the Rose Bengal Plate Agglutination Test. The cells were obtained through density gradient centrifugation following the manufacturer’s instructions (TBD, Tianjin, China) and cultured in RM-1640 medium (Gibco, New York, NY, USA) supplemented with 10% fetal bovine serum (Gibco, New York, NY, USA), 1% non-essential amino acids (Gibco, New York, NY, USA), 1% β-Mercaptoethanol (Gibco, New York, NY, USA), and 1% dual antibodies (Solarbio, Beijing, China) for 48 h. Subsequently, the cells were stimulated with 1 μg/mL LPS (GENE-LAB, Hangzhou, China) for 0, 4, 12, and 24 h [63]. TRIzol total RNA extraction reagent was used to extract RNA from the monocytes and/or macrophages, and cDNA was synthesized to analyze the expression levels of cytokines such as CTLA4, IL-6, IFN-γ, TNF-α, IL-10, IL-12, and TGF-β. GAPDH was used as an internal control gene, the relative expression levels were quantified using the 2−ΔΔCt method, and three replicates were performed for each cDNA sample.
4.6. Statistical Analysis
Population heterozygosity (He), homozygosity (Ho), and polymorphic information content (PIC), along with other genetic indicators, were calculated using the Nei method for the CTLA4 gene variant locus [64]. Hardy–Weinberg equilibrium (HWE), linkage disequilibrium (LD) structures, and haplotype construction were assessed using the SHEsis platform (http://analysis.bio-x.cn; accessed on 12 July 2024) and the GDICALL website (http://www.msrcall.com/Gdicall.aspx; accessed on 12 July 2024), and the LD was considered strong if R2 > 0.33. The distribution frequencies of different genotypes and alleles were analyzed using the Chi-squared test in the SPSS 23.0 software. Additionally, a logistic regression model was utilized to calculate the odds ratio (OR) values and 95% confidence intervals (95% CIs) to evaluate the impact of various genetic models, alleles, or haplotypes on the risk of brucellosis [65].
5. Conclusions
This study reports the identification of two novel InDel mutations in the CTLA4 gene within a population of Shaanbei White Cashmere Goats. Individuals possessing the II genotype or I allele of these InDels exhibited a reduced susceptibility to brucellosis. Stimulation of monocytes and/or macrophages with LPS revealed that those with the protective I allele demonstrated an increased secretion of anti-inflammatory cytokines, including IL-10, IL-12, and TGF-β. These findings underscore the role of the CTLA4 gene in the immune response to brucellosis infections and may serve as a significant genetic marker for breeding goats with enhanced resistance to brucellosis.
Author Contributions
C.W.: data curation, methodology, formal analysis, and writing—original draft. X.L.: data curation, methodology, formal analysis, and writing—original draft. Z.R.: methodology and formal analysis. X.D.: methodology and formal analysis. N.L.: formal analysis. X.S.: methodology and formal analysis. W.W.: methodology and formal analysis. L.Q.: methodology and resources. H.Z.: conceptualization, and writing—review and editing. J.H.: conceptualization and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2023YFF1000904, 2022YFD1302201), the National Natural Science Foundation of China (32072806, 32372970), the Key Technologies Demonstration of Animal Husbandry in the Shaanxi province (2024NYGG005-6, 20221086, 20230978), the Inner Mongolia Autonomous Region Open Competition Projects (2022JBGS0025), the Major Project of the Shaanxi Provincial Department of Education (22JY075), the Horizontal Project of Northwest Agriculture and Forestry University (2022HX144, H2023060246), and the Xinjiang Uygur Autonomous Region Scientific Research and Innovation Platform Construction Project “State Key Laboratory of Genetic Improvement and Germplasm Innovation of Grass-fed Livestock Co-construction by the Province and Ministry”.
Institutional Review Board Statement
All animal procedures were approved by the Northwest A&F University Animal Policy and Welfare Committee, and the animal experiments, such as sample collection, were conducted under the guidance of the Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data/models supporting the findings of this study are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, T.; Zhu, Y.; Shi, J.; Shang, K.; Yin, Z.; Shi, H.; He, Y.; Ding, J.; Zhang, F. The development of a human Brucella mucosal vaccine: What should be considered? Life Sci. 2024, 355, 122986. [Google Scholar] [CrossRef]
- Li, Y.; Tan, D.; Xue, S.; Shen, C.; Ning, H.; Cai, C.; Liu, Z. Prevalence, distribution and risk factors for brucellosis infection in goat farms in Ningxiang, China. BMC Vet. Res. 2021, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Siengsanan-Lamont, J.; Kong, L.; Heng, T.; Khoeun, S.; Tum, S.; Selleck, P.W.; Gleeson, L.J.; Blacksell, S.D. Risk mapping using serologic surveillance for selected One Health and transboundary diseases in Cambodian goats. PLoS Neglected Trop. Dis. 2023, 17, e0011244. [Google Scholar] [CrossRef]
- Rossetti, C.A.; Arenas-Gamboa, A.M.; Maurizio, E. Caprine brucellosis, A historically neglected disease with significant impact on public health. PLoS Neglected Trop. Dis. 2017, 11, e0005692. [Google Scholar] [CrossRef]
- De Massis, F.; Di Girolamo, A.; Petrini, A.; Pizzigallo, E.; Giovannini, A. Correlation between animal and human brucellosis in Italy during the period 1997–2002. Clin. Microbiol. Infect. 2005, 11, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Sibhat, B.; Tessema, T.S.; Nile, E.; Asmare, K. Brucellosis in Ethiopia: A comprehensive review of literature from the year 2000–2020 and the way forward. Transbound. Emerg. Dis. 2022, 69, e1231–e1252. [Google Scholar] [CrossRef]
- Miller, C.N.; Smith, E.P.; Cundiff, J.A.; Knodler, L.A.; Bailey Blackburn, J.; Lupashin, V.; Celli, J. A Brucella Type IV Effector Targets the COG Tethering Complex to Remodel Host Secretory Traffic and Promote Intracellular Replication. Cell Host Microbe 2017, 22, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, H.; Wang, K.; Cui, Y.; Chen, R.; Liu, J.; Zhu, H.; Qu, L.; Pan, C. Goat SPEF2, Expression profile, indel variants identification and association analysis with litter size. Theriogenology 2019, 139, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Bari, M.S.; Hossain, M.A.; Muhammad, N.; Ahmed, S.; Rahman, A.; Hoque, S.M.; Islam, A. An overview of Brucellosis. Mymensingh Med. J. 2011, 20, 742–747. [Google Scholar]
- Ran, X.; Chen, X.; Wang, M.; Cheng, J.; Ni, H.; Zhang, X.X.; Wen, X. Brucellosis seroprevalence in ovine and caprine flocks in China during 2000–2018: A systematic review and meta-analysis. BMC Vet. Res. 2018, 14, 393. [Google Scholar] [CrossRef]
- Ran, X.; Cheng, J.; Wang, M.; Chen, X.; Wang, H.; Ge, Y.; Ni, H.; Zhang, X.X.; Wen, X. Brucellosis seroprevalence in dairy cattle in China during 2008–2018: A systematic review and meta-analysis. Acta Trop. 2019, 189, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Dohoo, I.; Lindahl, J.; Verdugo, C.; Akuku, I.; Grace, D. Prevalence of tuberculosis, brucellosis and trypanosomiasis in cattle in Tanzania: A systematic review and meta-analysis. Anim. Health Res. Rev. 2016, 17, 16–27. [Google Scholar] [CrossRef]
- Njeru, J.; Wareth, G.; Melzer, F.; Henning, K.; Pletz, M.W.; Heller, R.; Neubauer, H. Systematic review of brucellosis in Kenya: Disease frequency in humans and animals and risk factors for human infection. BMC Public Health 2016, 16, 853. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.O. Brucellosis in food-producing animals in Mosul, Iraq: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235862. [Google Scholar] [CrossRef] [PubMed]
- Bamaiyi, P.H.; Hassan, L.; Khairani-Bejo, S.; Zainal Abidin, M. The economic impact attributable to brucellosis among goat farms in Peninsula Malaysia and cost benefit analysis. Res. Opin. Anim. Vet. Sci. 2015, 5, 57–64. [Google Scholar]
- Sulima, M.; Venkataraman, K. Economic losses due to Brucella melitensis infection in sheep and goats. Tamilnadu J. Vet. Anim. Sci. 2010, 6, 191–192. [Google Scholar]
- Godfroid, J.; Al Dahouk, S.; Pappas, G.; Roth, F.; Matope, G.; Muma, J.; Marcotty, T.; Pfeiffer, D.; Skjerve, E. A “One Health” surveillance and control of brucellosis in developing countries, moving away from improvisation. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 241–248. [Google Scholar] [CrossRef]
- Hou, Q.; Sun, X.; Zhang, J.; Liu, Y.; Wang, Y.; Jin, Z. Modeling the transmission dynamics of sheep brucellosis in Inner Mongolia Autonomous Region, China. Math. Biosci. 2013, 242, 51–58. [Google Scholar] [CrossRef]
- Blasco, J.M.; Molina-Flores, B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 95–104. [Google Scholar] [CrossRef]
- Li, T.; Tong, Z.; Huang, M.; Tang, L.; Zhang, H.; Chen, C. Brucella melitensis M5-90Δbp26 as a potential live vaccine that allows for the distinction between natural infection and immunization. Can. J. Microbiol. 2017, 63, 719–729. [Google Scholar] [CrossRef]
- Pascual, D.W.; Yang, X.; Wang, H.; Goodwin, Z.; Hoffman, C.; Clapp, B. Alternative strategies for vaccination to brucellosis. Microbes Infect. 2018, 20, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, D.; Zhang, S.; Yang, Y.; Bai, Y.; Akhatayeva, Z.; Pan, C.; Zhu, H.; Qu, L.; Lan, X. Goat SMAD family member 1 (SMAD1): mRNA expression, genetic variants, and their associations with litter size. Theriogenology 2022, 193, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Agusti, A.; Gulsvik, A.; Bakke, P.; Coxson, H.; Lomas, D.A.; Silverman, E.K.; Pillai, S.G.; ICGN investigators. CTLA4 gene polymorphisms are associated with chronic bronchitis. Eur. Respir. J. 2009, 34, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.K. The link between circulating follicular helper T cells and autoimmunity. Nat. Rev. Immunol. 2022, 22, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninezhad, F.; Masoumi, J.; Bakhshivand, M.; Baghbanzadeh, A.; Mokhtarzadeh, A.; Kazemi, T.; Aghebati-Maleki, L.; Shotorbani, S.S.; Jafarlou, M.; Brunetti, O.; et al. CTLA-4 silencing in dendritic cells loaded with colorectal cancer cell lysate improves autologous T cell responses in vitro. Front. Immunol. 2022, 13, 931316. [Google Scholar] [CrossRef]
- Louthrenoo, W.; Kasitanon, N.; Wongthanee, A.; Kuwata, S.; Takeuchi, F. CTLA-4 polymorphisms in Thai patients with rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. Int. J. Rheum. Dis. 2021, 24, 1378–1385. [Google Scholar] [CrossRef]
- Skendros, P.; Pappas, G.; Boura, P. Cell-mediated immunity in human brucellosis. Microbes Infect. 2011, 13, 134–142. [Google Scholar] [CrossRef]
- Sun, H.L.; Du, X.F.; Tang, Y.X.; Li, G.Q.; Yang, S.Y.; Wang, L.H.; Li, X.W.; Ma, C.J.; Jiang, R.M. Impact of immune checkpoint molecules on FoxP3+ Treg cells and related cytokines in patients with acute and chronic brucellosis. BMC Infect. Dis. 2021, 21, 1025. [Google Scholar] [CrossRef]
- Eskandari-Nasab, E.; Moghadampour, M.; Najibi, H.; Hadadi-Fishani, M. Investigation of CTLA-4 and CD86 gene polymorphisms in Iranian patients with brucellosis infection. Microbiol. Immunol. 2014, 58, 135–141. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, H.; Wang, K.; Xu, H.; Zhang, X.; Zhu, H.; Liu, J.; Qu, L.; Lan, X.; Pan, C. Insertion/Deletion Within the KDM6A Gene Is Significantly Associated With Litter Size in Goat. Front. Genet. 2018, 9, 91. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Goenka, R. Host immune responses to the intracellular bacteria Brucella, does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 2011, 26, 407–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Khattri, R.; Auger, J.A.; Griffin, M.D.; Sharpe, A.H.; Bluestone, J.A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 1999, 162, 5784–5791. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Quezada, S.A.; Chambers, C.A.; Korman, A.J.; Allison, J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009, 206, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, Y.; Yin, Z.; Shi, J.; Shang, K.; Tian, T.; Shi, H.; Ding, J.; Zhang, F. Design a novel of Brucellosis preventive vaccine based on IgV_CTLA-4 and multiple epitopes via immunoinformatics approach. Microb. Pathog. 2024, 195, 106909. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Diaz, J.M.; Zambrano-Román, M.; Padilla-Gutiérrez, J.R.; Valle, Y.; Muñoz-Valle, J.F.; Valdés-Alvarado, E. Association of CTLA-4 (AT)n Variants in Basal Cell Carcinoma and Squamous Cell Carcinoma Patients from Western Mexico. Curr. Issues Mol. Biol. 2024, 46, 8368–8375. [Google Scholar] [CrossRef]
- Akhtar, M.S. The Variant Allele Frequency of CTLA-4 rs11571317 (−658 C/T) Polymorphism With Colorectal Cancer Susceptibility in the Saudi Population and Other Ethnic Groups. Cureus 2023, 15, e50091. [Google Scholar] [CrossRef]
- Ghoneim, M.E.; Sheashaa, H.; Wafa, E.; Awadalla, A.; Ahmed, A.E.; Sobh, M.; Shokeir, A.A. Impact of CD 28, CD86, CTLA-4 and PD-1 genes polymorphisms on acute renal allograft rejection and graft survival among Egyptian recipients. Sci. Rep. 2024, 14, 2047. [Google Scholar] [CrossRef]
- Hasenauer, F.C.; Rossi, U.A.; Caffaro, M.E.; Raschia, M.A.; Maurizio, E.; Poli, M.A.; Rossetti, C.A. Association of TNF rs668920841 and INRA111 polymorphisms with caprine brucellosis: A case-control study of candidate genes involved in innate immunity. Genomics 2020, 112, 3925–3932. [Google Scholar] [CrossRef]
- Omar, A.I.; Alam, M.B.B.; Notter, D.R.; Zhao, S.; Faruque, M.O.; Thi, T.N.T.; Yin, L.; Li, J.; Azmal, S.A.; Du, X. Association of single nucleotide polymorphism in NLRC3, NLRC5, HIP1, and LRP8 genes with fecal egg counts in goats naturally infected with Haemonchus contortus. Trop. Anim. Health Prod. 2020, 52, 1583–1598. [Google Scholar] [CrossRef]
- DiLeo, M.F.; Nair, A.; Kardos, M.; Husby, A.; Saastamoinen, M. Demography and environment modulate the effects of genetic diversity on extinction risk in a butterfly metapopulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2309455121. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Quéméré, E.; Rossi, S.; Petit, E.; Marchand, P.; Merlet, J.; Game, Y.; Galan, M.; Gilot-Fromont, E. Genetic epidemiology of the Alpine ibex reservoir of persistent and virulent brucellosis outbreak. Sci. Rep. 2020, 10, 4400. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, I.; Sharma, A.; Singh, R.; Deb, S.M.; Singh, D.K.; Mitra, A. Association of microsatellite (GT)n polymorphism at 3′UTR of NRAMP1 with the macrophage function following challenge with Brucella LPS in buffalo (Bubalus bubalis). Vet. Microbiol. 2008, 129, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.M.; Pazzola, M.; Pisano, C.; Carcangiu, V.; Diaz, M.L.; Nieddu, M.; Robledo, R.; Mezzanotte, R.; Dettori, M.L. Chromosomal localisation and genetic variation of the SLC11A1 gene in goats (Capra hircus). Vet. J. 2011, 190, 60–65. [Google Scholar] [CrossRef]
- Capparelli, R.; Alfano, F.; Amoroso, M.G.; Borriello, G.; Fenizia, D.; Bianco, A.; Roperto, S.; Roperto, F.; Iannelli, D. Protective effect of the Nramp1 BB genotype against Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 2007, 75, 988–996. [Google Scholar] [CrossRef]
- Lou, L.; Bao, W.; Liu, X.; Song, H.; Wang, Y.; Zhang, K.; Gao, W.; Li, H.; Tu, Z.; Wang, S. An Autoimmune Disease-Associated Risk Variant in the TNFAIP3 Gene Plays a Protective Role in Brucellosis That Is Mediated by the NF-κB Signaling Pathway. J. Clin. Microbiol. 2018, 56, e01363. [Google Scholar] [CrossRef]
- Martins, G.A.; Tadokoro, C.E.; Silva, R.B.; Silva, J.S.; Rizzo, L.V. CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. J. Immunol. 2004, 172, 4893–4901. [Google Scholar] [CrossRef]
- Wang, P.H.; Wu, M.F.; Hsu, C.Y.; Lin, S.Y.; Chang, Y.N.; Lee, H.S.; Wei, Y.F.; Shu, C.C. The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection. Biomedicines 2021, 9, 1479. [Google Scholar] [CrossRef]
- Huang, D.W.; Wang, J.X.; Liu, Q.Y.; Chu, M.X.; Di, R.; He, J.N.; Cao, G.L.; Fang, L.; Feng, T.; Li, N. Analysis on DNA sequence of TSHB gene and its association with reproductive seasonality in goats. Mol. Biol. Rep. 2013, 40, 1893–1904. [Google Scholar] [CrossRef]
- Jin, J.; Hu, H.; Li, H.S.; Yu, J.; Xiao, Y.; Brittain, G.C.; Zou, Q.; Cheng, X.; Mallette, F.A.; Watowich, S.S.; et al. Noncanonical NF-κB pathway controls the production of type I interferons in antiviral innate immunity. Immunity 2014, 40, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.K.; Zammit, N.W.; Walters, S.N.; Koay, Y.C.; Wu, J.; Tan, B.M.; Villanueva, J.E.; Brink, R.; Loudovaris, T.; Cantley, J.; et al. Nuclear factor κB-inducing kinase activation as a mechanism of pancreatic β cell failure in obesity. J. Exp. Med. 2015, 212, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Bhargavan, B.; Woollard, S.M.; Kanmogne, G.D. Toll-like receptor-3 mediates HIV-1 transactivation via NFκB and JNK pathways and histone acetylation, but prolonged activation suppresses Tat and HIV-1 replication. Cell Signal. 2016, 28, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Zong, Q.; Wang, H.; Wu, S.; Cai, D.; Bao, W. C/EBPα Epigenetically Modulates TFF1 Expression via mC-6 Methylation in the Jejunum Inflammation Induced by a Porcine Coronavirus. Front. Immunol. 2022, 13, 881289. [Google Scholar] [CrossRef]
- Peña-Martínez, E.G.; Rodríguez-Martínez, J.A. Decoding Non-coding Variants: Recent Approaches to Studying Their Role in Gene Regulation and Human Diseases. Front. Biosci. (Sch. Ed.) 2024, 16, 4. [Google Scholar] [CrossRef]
- Schokker, D.; Peters, T.H.; Hoekman, A.J.; Rebel, J.M.; Smits, M.A. Differences in the early response of hatchlings of different chicken breeding lines to Salmonella enterica serovar Enteritidis infection. Poult. Sci. 2012, 91, 346–353. [Google Scholar] [CrossRef]
- Kim, Y.C.; Won, S.Y.; Jeong, B.H. Absence of single nucleotide polymorphisms (SNPs) in the open reading frame (ORF) of the prion protein gene (PRNP) in a large sampling of various chicken breeds. BMC Genom. 2019, 20, 922. [Google Scholar] [CrossRef]
- Rahman, S.U.; Zhu, L.; Cao, L.; Zhang, Y.; Chu, X.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Prevalence of Caprine brucellosis in Anhui province, China. Vet. World 2019, 12, 558–564. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4703. [Google Scholar] [CrossRef]
- Shrivastava, K.; Kumar, P.; Sahoo, N.R.; Kumar, A.; Khan, M.F.; Kumar, A.; Prasad, A.; Patel, B.H.; Nasir, A.; Bhushan, B.; et al. Genotyping of major histocompatibility complex Class II DRB gene in Rohilkhandi goats by polymerase chain reaction-restriction fragment length polymorphism and DNA sequencing. Vet. World 2015, 8, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7, Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, G.; Zhang, J.; Zhang, X.; Cui, M.; Guo, Y.; Liu, G.; Li, G.; Feng, J.; Lian, Z. Transgenic cloned sheep overexpressing ovine toll-like receptor 4. Theriogenology 2013, 80, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3333. [Google Scholar] [CrossRef]
- Sepanjnia, A.; Eskandari-Nasab, E.; Moghadampour, M.; Tahmasebi, A.; Dahmardeh, F. TGFβ1 genetic variants are associated with an increased risk of acute brucellosis. Infect. Dis. 2015, 47, 458–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).