Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease
Abstract
1. Introduction
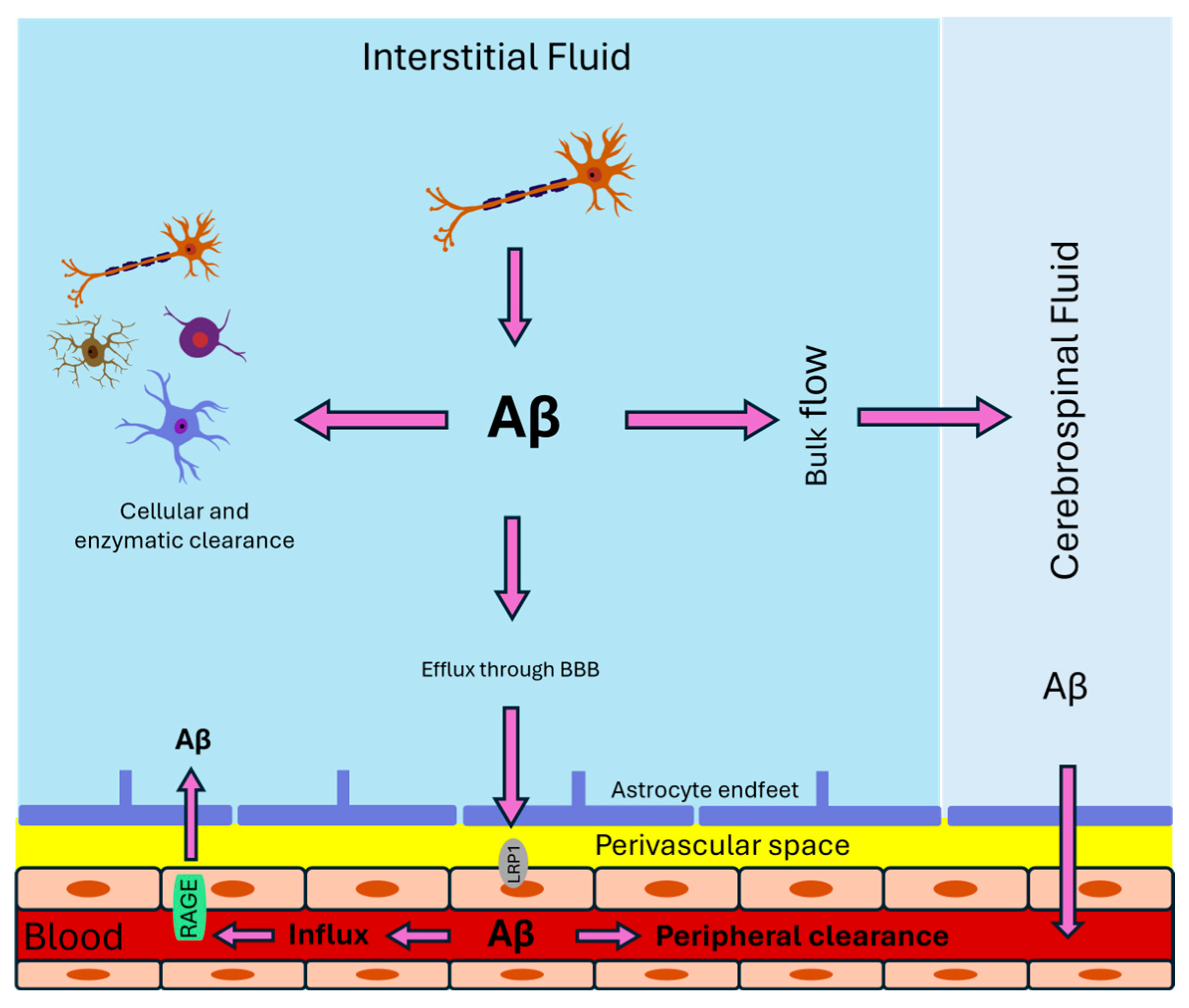
2. Aβ Transport from the Brain to the Periphery
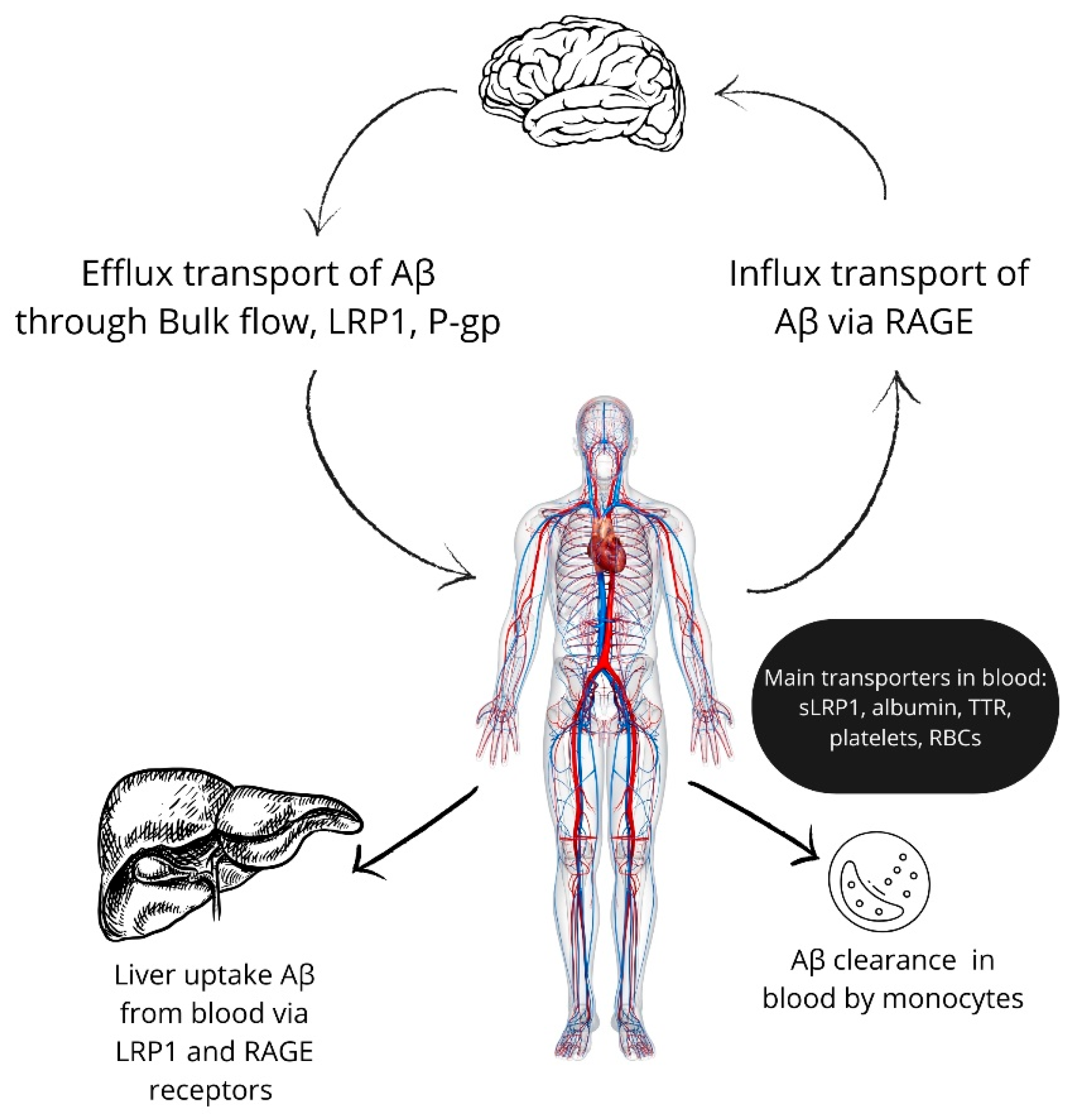
3. Aβ Transport in the Blood
4. Peripheral Clearance of Aβ
5. Alzheimer’s Disease Risk Factors and Components of Peripheral Beta-Amyloid Catabolism System
6. Aβ Degrading Enzymes
7. Targeting the Regulation of Beta-Amyloid Clearance for Clinical Use
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selkoe, D.J. Amyloid beta-peptide is produced by cultured cells during normal metabolism: A reprise. J. Alzheimer’s Dis. JAD 2006, 9, 163–168. [Google Scholar] [CrossRef]
- Dawkins, E.; Small, D.H. Insights into the physiological function of the β-amyloid precursor protein: Beyond Alzheimer’s disease. J. Neurochem. 2014, 129, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Wyttenbach, T.; Baumketner, A.; Shea, J.-E.; Bitan, G.; Teplow, D.B.; Bowers, M.T. Amyloid β-Protein: Monomer Structure and Early Aggregation States of Aβ42 and Its Pro19 Alloform. J. Am. Chem. Soc. 2005, 127, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Gervais, F.G.; Xu, D.; Robertson, G.S.; Vaillancourt, J.P.; Zhu, Y.; Huang, J.; LeBlanc, A.; Smith, D.; Rigby, M.; Shearman, M.S.; et al. Involvement of Caspases in Proteolytic Cleavage of Alzheimer’s Amyloid-β Precursor Protein and Amyloidogenic Aβ Peptide Formation. Cell 1999, 97, 395–406. [Google Scholar] [CrossRef]
- Bien, J.; Jefferson, T.; Čaušević, M.; Jumpertz, T.; Munter, L.; Multhaup, G.; Weggen, S.; Becker-Pauly, C.; Pietrzik, C.U. The Metalloprotease Meprin β Generates Amino Terminal-truncated Amyloid β Peptide Species. J. Biol. Chem. 2012, 287, 33304–33313. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.S.; Haass, C.; Lemere, C.A.; Shi, G.P.; Wong, W.S.; Teplow, D.B.; Selkoe, D.J.; Chapman, H.A. Lysosomal processing of amyloid precursor protein to A beta peptides: A distinct role for cathepsin S. Biochem. J. 1995, 311 Pt 1, 299–305. [Google Scholar] [CrossRef]
- Finder, V.H.; Glockshuber, R. Amyloid-beta aggregation. Neurodegener. Dis. 2007, 4, 13–27. [Google Scholar] [CrossRef] [PubMed]
- N’Songo, A.; Kanekiyo, T.; Bu, G. LRP1 plays a major role in the amyloid-β clearance in microglia. Mol. Neurodegener. 2013, 8, P33. [Google Scholar] [CrossRef][Green Version]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.-C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal Clearance of Amyloid-β by Endocytic Receptor LRP1. J. Neurosci. 2013, 33, 19276. [Google Scholar] [CrossRef]
- Liu, C.C.; Hu, J.; Zhao, N.; Wang, J.; Wang, N.; Cirrito, J.R.; Kanekiyo, T.; Holtzman, D.M.; Bu, G. Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition. J. Neurosci. 2017, 37, 4023–4031. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood–Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Patterson, B.W.; Lucey, B.P.; Benzinger, T.L.S.; Bateman, R.J. Importance of CSF-based Aβ clearance with age in humans increases with declining efficacy of blood-brain barrier/proteolytic pathways. Commun. Biol. 2022, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Bu, X.-L.; Liu, Y.-H.; Zhu, C.; Shen, L.-L.; Jiao, S.-S.; Zhu, X.-Y.; Giunta, B.; Tan, J.; Song, W.-H.; et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015, 130, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.Y.; Cheng, Y.; Zhuang, Z.Q.; He, C.Y.; Pan, Q.G.; Tang, M.Z.; Hu, X.L.; Shen, Y.Y.; Wang, Y.R.; Chen, S.H.; et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol. Psychiatry 2021, 26, 6074–6082. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.Y.; Tian, D.Y.; Chen, S.H.; Ren, J.R.; Sun, H.L.; Xu, M.Y.; Tan, C.R.; Fan, D.Y.; Jian, J.M.; et al. Physiological β-amyloid clearance by the liver and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2023, 145, 717–731. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, D.Y.; Wang, Y.J. Peripheral clearance of brain-derived Aβ in Alzheimer’s disease: Pathophysiology and therapeutic perspectives. Transl. Neurodegener. 2020, 9, 16. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakamura, Y.; Yamada, K.; Igarashi, H.; Kasuga, K.; Yokoyama, Y.; Ikeuchi, T.; Nishizawa, M.; Kwee, I.L.; Nakada, T. Reduced CSF Water Influx in Alzheimer’s Disease Supporting the β-Amyloid Clearance Hypothesis. PLoS ONE 2015, 10, e0123708. [Google Scholar] [CrossRef]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.E.; Meister, S.; Nahrath, J.; Meissner, J.N.; Schubert, N.; di Spiezio, A.; Baches, S.; Vandenbroucke, R.E.; Bouter, Y.; Prikulis, I.; et al. Endothelial LRP1 transports amyloid-beta(1-42) across the blood-brain barrier. J. Clin. Investig. 2016, 126, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.B.; Dohgu, S.; Hwang, M.C.; Farr, S.A.; Murphy, M.P.; Fleegal-DeMotta, M.A.; Lynch, J.L.; Robinson, S.M.; Niehoff, M.L.; Johnson, S.N.; et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J. Alzheimer’s Dis. JAD 2009, 17, 553–570. [Google Scholar] [CrossRef]
- Shinohara, M.; Tachibana, M.; Kanekiyo, T.; Bu, G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: Evidence from clinical and preclinical studies: Thematic Review Series: ApoE and Lipid Homeostasis in Alzheimer’s Disease. J. Lipid Res. 2017, 58, 1267–1281. [Google Scholar] [CrossRef]
- Quinn, K.A.; Pye, V.J.; Dai, Y.P.; Chesterman, C.N.; Owensby, D.A. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp. Cell Res. 1999, 251, 433–441. [Google Scholar] [CrossRef]
- Boucher, P.; Herz, J. Signaling through LRP1: Protection from atherosclerosis and beyond. Biochem. Pharmacol. 2011, 81, 1–5. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Liu, C.C.; Shinohara, M.; Li, J.; Bu, G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 16458–16465. [Google Scholar] [CrossRef]
- Tamaki, C.; Ohtsuki, S.; Iwatsubo, T.; Hashimoto, T.; Yamada, K.; Yabuki, C.; Terasaki, T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm. Res. 2006, 23, 1407–1416. [Google Scholar] [CrossRef]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G.; et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015, 18, 978–987. [Google Scholar] [CrossRef]
- Bell, R.D.; Sagare, A.P.; Friedman, A.E.; Bedi, G.S.; Holtzman, D.M.; Deane, R.; Zlokovic, B.V. Transport Pathways for Clearance of Human Alzheimer’s Amyloid β-Peptide and Apolipoproteins E and J in the Mouse Central Nervous System. J. Cereb. Blood Flow Metab. 2007, 27, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, B.; Storck, S.E.; Reekmans, S.M.; Lechat, B.; Gordts, P.; Pradier, L.; Pietrzik, C.U.; Roebroek, A.J.M. LRP1 Has a Predominant Role in Production over Clearance of Aβ in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7234–7245. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.W.; Ammerman, L.; Chen, G.; Vogel, P.D.; Wise, J.G. Transport of Alzheimer’s associated amyloid-β catalyzed by P-glycoprotein. PLoS ONE 2021, 16, e0250371. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Callaghan, R.; Gelissen, I.C. Regulation of P-Glycoprotein in the Brain. Int. J. Mol. Sci. 2022, 23, 14667. [Google Scholar] [CrossRef]
- Wang, W.; Bodles-Brakhop, A.M.; Barger, S.W. A Role for P-Glycoprotein in Clearance of Alzheimer Amyloid β -Peptide from the Brain. Curr. Alzheimer Res. 2016, 13, 615–620. [Google Scholar] [CrossRef]
- Chiu, C.; Miller, M.C.; Monahan, R.; Osgood, D.P.; Stopa, E.G.; Silverberg, G.D. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations. Neurobiol. Aging 2015, 36, 2475–2482. [Google Scholar] [CrossRef]
- Park, R.; Kook, S.Y.; Park, J.C.; Mook-Jung, I. Aβ1–42 reduces P-glycoprotein in the blood–brain barrier through RAGE–NF-κB signaling. Cell Death Dis. 2014, 5, e1299. [Google Scholar] [CrossRef]
- Storck, S.E.; Hartz, A.M.S.; Bernard, J.; Wolf, A.; Kachlmeier, A.; Mahringer, A.; Weggen, S.; Pahnke, J.; Pietrzik, C.U. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav. Immun. 2018, 73, 21–33. [Google Scholar] [CrossRef]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-[beta] peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef]
- Arancio, O.; Zhang, H.P.; Chen, X.; Lin, C.; Trinchese, F.; Puzzo, D.; Liu, S.; Hegde, A.; Yan, S.F.; Stern, A.; et al. RAGE potentiates A[beta]-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004, 23, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Chaney, M.O.; Stine, W.B.; Kokjohn, T.A.; Kuo, Y.-M.; Esh, C.; Rahman, A.; Luehrs, D.C.; Schmidt, A.M.; Stern, D.; Yan, S.D.; et al. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1741, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Toki, S.; Chowei, H.; Saito, T.; Nakano, N.; Hayashi, Y.; Takeuchi, M.; Makita, Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001, 888, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.-F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Yan, S.D. Involvement of Microglial Receptor for Advanced Glycation Endproducts (RAGE) in Alzheimer’s Disease: Identification of a Cellular Activation Mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics 2008, 5, 409–414. [Google Scholar] [CrossRef]
- Giri, R.; Shen, Y.; Stins, M.; Du Yan, S.; Schmidt, A.M.; Stern, D.; Kim, K.S.; Zlokovic, B.; Kalra, V.K. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 2000, 279, C1772–C1781. [Google Scholar] [CrossRef]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N.; et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar] [CrossRef]
- Askarova, S.; Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.M. Role of Aβ-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A2 activation in astrocytes and cerebral endothelial cells. Neuroscience 2011, 199, 375–385. [Google Scholar] [CrossRef]
- Keaney, J.; Walsh, D.M.; O’Malley, T.; Hudson, N.; Crosbie, D.E.; Loftus, T.; Sheehan, F.; McDaid, J.; Humphries, M.M.; Callanan, J.J.; et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci. Adv. 2015, 1, e1500472. [Google Scholar] [CrossRef]
- Biere, A.L.; Ostaszewski, B.; Stimson, E.R.; Hyman, B.T.; Maggio, J.E.; Selkoe, D.J. Amyloid β-Peptide Is Transported on Lipoproteins and Albumin in Human Plasma. J. Biol. Chem. 1996, 271, 32916–32922. [Google Scholar] [CrossRef]
- Hone, E.; Martins, I.J.; Fonte, J.; Martins, R.N. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J. Alzheimer’s Dis. JAD 2003, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, J.; Shayo, M.; Calero, M.; Ng, D.; Tomidokoro, Y.; Gandy, S.; Rostagno, A.; Frangione, B. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J. Biol. Chem. 2004, 279, 45897–45908. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.; Deane, R.; Bell, R.D.; Johnson, B.; Hamm, K.; Pendu, R.; Marky, A.; Lenting, P.J.; Wu, Z.; Zarcone, T.; et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 2007, 13, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Murphy, R.M. Characterization of the interaction of β-amyloid with transthyretin monomers and tetramers. Biochemistry 2010, 49, 8276–8289. [Google Scholar] [CrossRef] [PubMed]
- Alemi, M.; Gaiteiro, C.; Ribeiro, C.A.; Santos, L.M.; Gomes, J.R.; Oliveira, S.M.; Couraud, P.-O.; Weksler, B.; Romero, I.; Saraiva, M.J.; et al. Transthyretin participates in beta-amyloid transport from the brain to the liver- involvement of the low-density lipoprotein receptor-related protein 1? Sci. Rep. 2016, 6, 20164. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.; Galasko, D.R.; Chevallier, N.; Koo, E.H.; Emmerling, M.R.; Roher, A.E. Amyloid-beta peptides interact with plasma proteins and erythrocytes: Implications for their quantitation in plasma. Biochem. Biophys. Res. Commun. 2000, 268, 750–756. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Lee, J.H.; Yi, D.; Jeon, S.Y.; Sohn, B.K.; Lee, J.Y.; Shin, S.A.; Kim, Y.K.; Kang, K.M.; et al. Serum albumin and beta-amyloid deposition in the human brain. Neurology 2020, 95, e815–e826. [Google Scholar] [CrossRef]
- Chen, M.; Inestrosa, N.; Ross, G.S.; Fernández, H. Platelets are the primary source of amyloid beta-peptide in human blood. Biochem. Biophys. Res. Commun. 1995, 213, 96–103. [Google Scholar] [CrossRef]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, Y.; Kucheryavykh, L. Platelet-generated amyloid beta peptides in Alzheimer’s disease and glaucoma. Histol. Histopathol. 2019, 34, 843–856. [Google Scholar] [CrossRef]
- Shen, M.; Hsiao, G.; Tsorng, H.F.; Chou, D.; Sheu, J. Expression of amyloid beta peptide in human platelets: Pivotal role of the phospholipase Cgamma2-protein kinase C pathway in platelet activation. Pharmacol. Res. 2008, 57, 151–158. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Lee, V.M.; Praticò, D. Amyloid Precursor Protein and Amyloid β Peptide in Human Platelets. J. Biol. Chem. 2001, 276, 17036–17043. [Google Scholar] [CrossRef]
- Li, T.-R.; Liu, F.-Q. β-Amyloid promotes platelet activation and activated platelets act as bridge between risk factors and Alzheimer’s disease. Mech. Ageing Dev. 2022, 207, 111725. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, L.; Zhou, L.; Xu, J.; Guo, K. Platelets transport β-amyloid from the peripheral blood into the brain by destroying the blood-brain barrier to accelerate the process of Alzheimer’s disease in mouse models. Aging 2021, 13, 7644–7659. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Begley, J.G.; Mark, R.J.; Furukawa, K. Aβ25–35 induces rapid lysis of red blood cells: Contrast with Aβ1–42 and examination of underlying mechanisms. Brain Res. 1997, 771, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Eckley, D.M.; Williamson, J.D.; Launer, L.J.; Rifkind, J.M. Do red blood cell-beta-amyloid interactions alter oxygen delivery in Alzheimer’s disease? Adv. Exp. Med. Biol. 2008, 614, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid β levels in human red blood cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef]
- Wu, C.W.; Liao, P.C.; Yu, L.; Wang, S.T.; Chen, S.T.; Wu, C.M.; Kuo, Y.M. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol. Dis. 2004, 17, 367–377. [Google Scholar] [CrossRef]
- Oyama, R.; Yamamoto, H.; Titani, K. Glutamine synthetase, hemoglobin alpha-chain, and macrophage migration inhibitory factor binding to amyloid beta-protein: Their identification in rat brain by a novel affinity chromatography and in Alzheimer’s disease brain by immunoprecipitation. Biochim. Biophys. Acta 2000, 1479, 91–102. [Google Scholar] [CrossRef]
- Ravi, L.B.; Poosala, S.; Ahn, D.; Chrest, F.J.; Spangler, E.L.; Jayakumar, R.; Nagababu, E.; Mohanty, J.G.; Talan, M.I.; Ingram, D.K.; et al. Red cell interactions with amyloid-beta(1-40) fibrils in a murine model. Neurobiol. Dis. 2005, 19, 28–37. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, Q.H.; Zhang, T.; Liu, Y.H.; Yao, X.Q.; Zeng, F.; Li, J.; Zhou, F.Y.; Wang, L.; Yan, J.C.; et al. Associations Between Hepatic Functions and Plasma Amyloid-Beta Levels-Implications for the Capacity of Liver in Peripheral Amyloid-Beta Clearance. Mol. Neurobiol. 2017, 54, 2338–2344. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, X.; Xu, Z.; Li, L.; Mo, X.; Peng, Z.; Shan, Z.; Yan, H.; Xu, J.; Liu, L. Peripheral amyloid-β clearance mediates cognitive impairment in non-alcoholic fatty liver disease. EBioMedicine 2024, 102, 105079. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.-J. A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Bu, G. The low-density lipoprotein receptor-related protein 1 and amyloid-β clearance in Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, L.A.; Kaddoumi, A. In Vitro Investigation of Amyloid-β Hepatobiliary Disposition in Sandwich-Cultured Primary Rat Hepatocytes. Drug Metab. Dispos. 2013, 41, 1787. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, C.; Mejias-Ortega, M.; Sanchez-Mejias, E.; Navarro, V.; Trujillo-Estrada, L.; Jimenez, S.; Garcia-Leon, J.A.; Fernandez-Valenzuela, J.J.; Sanchez-Mico, M.V.; Romero-Molina, C.; et al. Monocyte-derived cells invade brain parenchyma and amyloid plaques in human Alzheimer’s disease hippocampus. Acta Neuropathol. Commun. 2023, 11, 31. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef]
- Mezey, E.; Chandross, K.J.; Harta, G.; Maki, R.A.; McKercher, S.R. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290, 1779–1782. [Google Scholar] [CrossRef]
- Rangaraju, S.; Raza, S.A.; Li, N.X.A.; Betarbet, R.; Dammer, E.B.; Duong, D.; Lah, J.J.; Seyfried, N.T.; Levey, A.I. Differential Phagocytic Properties of CD45(low) Microglia and CD45(high) Brain Mononuclear Phagocytes-Activation and Age-Related Effects. Front. Immunol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 168–173. [Google Scholar] [CrossRef]
- Majumdar, A.; Chung, H.; Dolios, G.; Wang, R.; Asamoah, N.; Lobel, P.; Maxfield, F.R. Degradation of fibrillar forms of Alzheimer’s amyloid beta-peptide by macrophages. Neurobiol. Aging 2008, 29, 707–715. [Google Scholar] [CrossRef]
- Saido, T.; Leissring, M.A. Proteolytic Degradation of Amyloid ? beta-Protein. Cold Spring Harb. Perspect. Med. 2012, 2, a006379. [Google Scholar] [CrossRef] [PubMed]
- Costarelli, L.; Malavolta, M.; Giacconi, R.; Provinciali, M. Dysfunctional macrophages in Alzheimer Disease: Another piece of the “macroph-aging” puzzle? Aging 2017, 9, 1865–1866. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Lin, J.; Ringman, J.; Kermani-Arab, V.; Tsao, G.; Patel, A.; Lossinsky, A.S.; Graves, M.C.; Gustavson, A.; Sayre, J.; et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005, 7, 221–232, discussion 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Tian, D.-Y.; Shen, Y.-Y.; Cheng, Y.; Fan, D.-Y.; Sun, H.-L.; He, C.-Y.; Sun, P.-Y.; Bu, X.-L.; Zeng, F.; et al. Amyloid-beta uptake by blood monocytes is reduced with ageing and Alzheimer’s disease. Transl. Psychiatry 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fowler, C.; Li, Y.; Li, Q.-X.; Sun, J.; Pan, Y.; Jin, L.; Perez, K.A.; Dubois, C.; Lim, Y.Y.; et al. Clearance and transport of amyloid β by peripheral monocytes correlate with Alzheimer’s disease progression. Nat. Commun. 2024, 15, 7998. [Google Scholar] [CrossRef]
- Udan, M.L.; Ajit, D.; Crouse, N.R.; Nichols, M.R. Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 2008, 104, 524–533. [Google Scholar] [CrossRef]
- Dobri, A.-M.; Dudău, M.; Enciu, A.-M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef]
- La Rosa, F.; Agostini, S.; Piancone, F.; Marventano, I.; Hernis, A.; Fenoglio, C.; Galimberti, D.; Scarpini, E.; Saresella, M.; Clerici, M. TREM2 Expression and Amyloid-Beta Phagocytosis in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8626. [Google Scholar] [CrossRef]
- Zhao, L. CD33 in Alzheimer’s Disease—Biology, Pathogenesis, and Therapeutics: A Mini-Review. Gerontology 2018, 65, 323–331. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.-Z.; Yu, J.-T.; Chi, Z.-F.; Tan, L. Increased expressions of TLR2 and TLR4 on peripheral blood mononuclear cells from patients with Alzheimer’s disease. J. Neurol. Sci. 2012, 315, 67–71. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Liu, L.; Wang, K.J.; Liu, M.Y.; Deng, Y.; Li, Z.; Zhao, W.D.; Wu, L.Y.; Chen, Y.H.; et al. Monocytes release cystatin F dimer to associate with Aβ and aggravate amyloid pathology and cognitive deficits in Alzheimer’s disease. J. Neuroinflamm. 2024, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Take, Y.; Chikai, Y.; Shimamori, K.; Kuragano, M.; Kurita, H.; Tokuraku, K. Amyloid β aggregation induces human brain microvascular endothelial cell death with abnormal actin organization. Biochem. Biophys. Rep. 2022, 29, 101189. [Google Scholar] [CrossRef] [PubMed]
- Askarova, S.; Sun, Z.; Sun, G.Y.; Meininger, G.A.; Lee, J.C. Amyloid-β peptide on sialyl-Lewis(X)-selectin-mediated membrane tether mechanics at the cerebral endothelial cell surface. PLoS ONE 2013, 8, e60972. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Actin dynamics and cofilin-actin rods in alzheimer disease. Cytoskeleton 2016, 73, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Gevorkian, G.; Gonzalez-Noriega, A.; Acero, G.; Ordoñez, J.; Michalak, C.; Munguia, M.E.; Govezensky, T.; Cribbs, D.H.; Manoutcharian, K. Amyloid-beta peptide binds to microtubule-associated protein 1B (MAP1B). Neurochem. Int. 2008, 52, 1030–1036. [Google Scholar] [CrossRef][Green Version]
- Morena, F.; Argentati, C.; Trotta, R.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; di Baldassarre, A.; Calafiore, R.; Montanucci, P.; Basta, G.; et al. A Comparison of Lysosomal Enzymes Expression Levels in Peripheral Blood of Mild- and Severe-Alzheimer’s Disease and MCI Patients: Implications for Regenerative Medicine Approaches. Int. J. Mol. Sci. 2017, 18, 1806. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, K.; Tian, Z.Y.; Wang, T.; Shang, D.S.; Li, B.; Liu, D.X.; Fang, W.G.; Wang, Z.Y.; Chen, Y.H. Decreased expression of cathepsin D in monocytes is related to the defective degradation of amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 511–520. [Google Scholar] [CrossRef]
- Kim, G.-A.; Oh, C.H.; Kim, J.W.; Jeong, S.J.; Oh, I.-H.; Lee, J.S.; Park, K.-C.; Shim, J.-J. Association between non-alcoholic fatty liver disease and the risk of dementia: A nationwide cohort study. Liver Int. 2022, 42, 1027–1036. [Google Scholar] [CrossRef]
- Jeong, S.; Oh, Y.H.; Choi, S.; Chang, J.; Kim, S.M.; Son, J.S.; Lee, G.; Ahn, J.C.; Lee, D.H.; Koo, B.K.; et al. Association of non-alcoholic fatty liver disease with incident dementia later in life among elder adults. Clin. Mol. Hepatol. 2022, 28, 510–521. [Google Scholar] [CrossRef]
- Lu, Y.; Pike, J.R.; Hoogeveen, R.C.; Walker, K.A.; Raffield, L.M.; Selvin, E.; Avery, C.L.; Engel, S.M.; Mielke, M.M.; Garcia, T.; et al. Liver integrity and the risk of Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2024, 20, 1913–1922. [Google Scholar] [CrossRef]
- Gadd, V.L.; Patel, P.J.; Jose, S.; Horsfall, L.; Powell, E.E.; Irvine, K.M. Altered Peripheral Blood Monocyte Phenotype and Function in Chronic Liver Disease: Implications for Hepatic Recruitment and Systemic Inflammation. PLoS ONE 2016, 11, e0157771. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.V.; Steinberg, R.A.; Han, D.; Sumbria, R.K. Alcohol as a Modifiable Risk Factor for Alzheimer’s Disease-Evidence from Experimental Studies. Int. J. Mol. Sci. 2023, 24, 9492. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Chang, R.; Steinberg, R.A.; Arce, A.; Yang, J.; van der Eb, P.; Abdullah, T.; Chandrashekar, D.V.; Eck, S.M.; Meza, P.; et al. Modulation of hepatic amyloid precursor protein and lipoprotein receptor-related protein 1 by chronic alcohol intake: Potential link between liver steatosis and amyloid-β. Front. Physiol. 2022, 13, 930402. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.V.; Roules, G.C.; Jagadeesan, N.; Panchal, U.R.; Oyegbesan, A.; Imiruaye, O.E.; Zhang, H.; Garcia, J.; Kaur, K.; Win, S.; et al. Hepatic LRP-1 plays an important role in amyloidosis in Alzheimer’s disease mice: Potential role in chronic heavy alcohol feeding. Neurobiol. Dis. 2024, 199, 106570. [Google Scholar] [CrossRef] [PubMed]
- Haag, F.; Janicova, A.; Xu, B.; Powerski, M.; Fachet, M.; Bundkirchen, K.; Neunaber, C.; Marzi, I.; Relja, B.; Sturm, R. Reduced phagocytosis, ROS production and enhanced apoptosis of leukocytes upon alcohol drinking in healthy volunteers. Eur. J. Trauma Emerg. Surg. 2022, 48, 2689–2699. [Google Scholar] [CrossRef]
- Mørland, H.; Johnsen, J.; Bjørneboe, A.; Bjørneboe, G.-E.A.; Drevon, C.A.; Mørland, J.; Mørland, B. Reduced IgG Fc-Receptor-mediated Phagocytosis in Human Monocytes Isolated from Alcoholics. Alcohol. Clin. Exp. Res. 1988, 12, 755–759. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, H.J.; Kim, H.M.; Yang, A.H.; Lee, B.W.; Kang, E.S.; Lee, H.C.; Cha, B.S. Upregulation of hepatic LRP1 by rosiglitazone: A possible novel mechanism of the beneficial effect of thiazolidinediones on atherogenic dyslipidemia. J. Mol. Endocrinol. 2012, 49, 165–174. [Google Scholar] [CrossRef]
- Laatsch, A.; Merkel, M.; Talmud, P.J.; Grewal, T.; Beisiegel, U.; Heeren, J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis 2009, 204, 105–111. [Google Scholar] [CrossRef]
- Lecube, A.; Pachón, G.; Petriz, J.; Hernández, C.; Simó, R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS ONE 2011, 6, e23366. [Google Scholar] [CrossRef]
- Moraes, D.; Mousovich-Neto, F.; Cury, S.S.; Oliveira, J.; Souza, J.D.S.; Freire, P.P.; Dal-Pai-Silva, M.; Mori, M.; Fernandez, G.J.; Carvalho, R.F. The Transcriptomic Landscape of Age-Induced Changes in Human Visceral Fat and the Predicted Omentum-Liver Connectome in Males. Biomedicines 2023, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, C.; Zhou, X.; Zhou, W.; Hornburg, D.; Wu, S.; Snyder, M.P. Nonlinear dynamics of multi-omics profiles during human aging. Nat. Aging, 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Bland, N.D.; Pinney, J.W.; Thomas, J.E.; Turner, A.J.; Isaac, R.E. Bioinformatic analysis of the neprilysin (M13) family of peptidases reveals complex evolutionary and functional relationships. BMC Evol. Biol. 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Hashiguchi, A.; Yuan, J.; Yoshimura, A.; Mitsui, J.; Ishiura, H.; Tanaka, M.; Ishihara, S.; Tanabe, H.; Nozuma, S.; et al. Mutations in MME cause an autosomal-recessive Charcot-Marie-Tooth disease type 2. Ann. Neurol. 2016, 79, 659–672. [Google Scholar] [CrossRef] [PubMed]
- El-Amouri, S.S.; Zhu, H.; Yu, J.; Marr, R.; Verma, I.M.; Kindy, M.S. Neprilysin: An enzyme candidate to slow the progression of Alzheimer’s disease. Am. J. Pathol. 2008, 172, 1342–1354. [Google Scholar] [CrossRef]
- Whyteside, A.R.; Turner, A.J. Human neprilysin-2 (NEP2) and NEP display distinct subcellular localisations and substrate preferences. FEBS Lett. 2008, 582, 2382–2386. [Google Scholar] [CrossRef][Green Version]
- Campbell, D.J. Long-term neprilysin inhibition—Implications for ARNIs. Nat. Rev. Cardiol. 2017, 14, 171–186. [Google Scholar] [CrossRef]
- Carty, N.C.; Nash, K.; Lee, D.; Mercer, M.; Gottschall, P.E.; Meyers, C.; Muzyczka, N.; Gordon, M.N.; Morgan, D. Adeno-associated Viral (AAV) Serotype 5 Vector Mediated Gene Delivery of Endothelin-converting Enzyme Reduces Aβ Deposits in APP + PS1 Transgenic Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 1580–1586. [Google Scholar] [CrossRef]
- Eckman, E.A.; Reed, D.K.; Eckman, C.B. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. J. Biol. Chem. 2001, 276, 24540–24548. [Google Scholar] [CrossRef]
- Eckman, E.A.; Watson, M.; Marlow, L.; Sambamurti, K.; Eckman, C.B. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J. Biol. Chem. 2003, 278, 2081–2084. [Google Scholar] [CrossRef]
- Llovera, R.E.; de Tullio, M.; Alonso, L.G.; Leissring, M.A.; Kaufman, S.B.; Roher, A.E.; de Prat Gay, G.; Morelli, L.; Castaño, E.M. The catalytic domain of insulin-degrading enzyme forms a denaturant-resistant complex with amyloid beta peptide: Implications for Alzheimer disease pathogenesis. J. Biol. Chem. 2008, 283, 17039–17048. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.E.; Cottrell, G.S.; Roosterman, D.; Pikios, S.; Muller, L.; Steinhoff, M.; Bunnett, N.W. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J. Cell Biol. 2007, 179, 981–997. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.D.; Stevenson, T.; Ahn, K. Hydrolysis of Peptide Hormones by Endothelin-converting Enzyme-1: A Comparison with Neprilysin. J. Biol. Chem. 1999, 274, 4053–4058. [Google Scholar] [CrossRef]
- Palmer, J.; Love, S. Endothelin receptor antagonists: Potential in Alzheimer’s disease. Pharmacol. Res. 2011, 63, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, R.; Gambina, G.; Broggio, E.; Ruggeri, M.; Corbo, R.M. C-338A polymorphism of the endothelin-converting enzyme (ECE-1) gene and the susceptibility to sporadic late-onset Alzheimer’s disease and coronary artery disease. Dis. Markers 2008, 24, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Luxiang, C.; Huadong, Z.; Yanjiang, W.; Zhiqiang, X.; Hongyuan, C.; Lihua, H.; Xu, Y. Endothelin-converting enzyme-1 promoter polymorphisms and susceptibility to sporadic late-onset Alzheimer’s disease in a Chinese population. Dis. Markers 2009, 27, 211–215. [Google Scholar] [CrossRef]
- Russell, F.D.; Davenport, A.P. Evidence for Intracellular Endothelin-Converting Enzyme-2 Expression in Cultured Human Vascular Endothelial Cells. Circ. Res. 1999, 84, 891–896. [Google Scholar] [CrossRef]
- Liao, X.; Cai, F.; Sun, Z.; Zhang, Y.; Wang, J.; Jiao, B.; Guo, J.; Li, J.; Liu, X.; Guo, L.; et al. Identification of Alzheimer’s disease-associated rare coding variants in the ECE2 gene. JCI Insight 2020, 5, e135119. [Google Scholar] [CrossRef]
- Wei, L.; Alhenc-Gelas, F.; Corvol, P.; Clauser, E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991, 266, 9002–9008. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Elkins, J.S.; Douglas, V.C.; Johnston, S.C. Alzheimer disease risk and genetic variation in ACE. Neurology 2004, 62, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Hu, X.; Song, H.; Yin, K.; Bateman, R.J.; Cirrito, J.R.; Xiao, Q.; Hsu, F.F.; Turk, J.W.; Xu, J.; et al. Matrix Metalloproteinase-9 Degrades Amyloid-β Fibrils in Vitro and Compact Plaques in Situ. J. Biol. Chem. 2006, 281, 24566–24574. [Google Scholar] [CrossRef] [PubMed]
- Buss, A.; Pech, K.; Roelver, S.; Bloemeke, B.; Klotzsch, C.; Breuer, S. Functional polymorphisms in matrix metalloproteinases -1, -3, -9 and -12 in relation to cervical artery dissection. BMC Neurol. 2009, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef]
- Deb, S.; Wenjun Zhang, J.; Gottschall, P.E. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003, 970, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Weiss, A.; Fritzius, T.; Heinrich, J.; Moelling, K. The PDZ protein MPP2 interacts with c-Src in epithelial cells. Exp. Cell Res. 2009, 315, 2888–2898. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Lu, Y.; Zhang, T.; Zhao, W. The Genetic Association of MMP-2 Gene Polymorphisms with the Susceptibility to Alzheimer’s Disease. J. Integr. Neurosci. 2024, 23, 52. [Google Scholar] [CrossRef]
- Biswas, C.; Zhang, Y.; DeCastro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995, 55, 434–439. [Google Scholar]
- Kaushik, D.K.; Hahn, J.N.; Yong, V.W. EMMPRIN, an upstream regulator of MMPs, in CNS biology. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 44–46, 138–146. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, H.; Walian, P.J.; Jap, B.K. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer’s disease amyloid beta-peptide production. Proc. Natl. Acad. Sci. USA 2005, 102, 7499–7504. [Google Scholar] [CrossRef]
- Vetrivel, K.S.; Zhang, X.; Meckler, X.; Cheng, H.; Lee, S.; Gong, P.; Lopes, K.O.; Chen, Y.; Iwata, N.; Yin, K.J.; et al. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J. Biol. Chem. 2008, 283, 19489–19498. [Google Scholar] [CrossRef] [PubMed]
- Affholter, J.A.; Hsieh, C.L.; Francke, U.; Roth, R.A. Insulin-degrading enzyme: Stable expression of the human complementary DNA, characterization of its protein product, and chromosomal mapping of the human and mouse genes. Mol. Endocrinol. 1990, 4, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Hubin, E.; Cioffi, F.; Rozenski, J.; van Nuland, N.A.; Broersen, K. Characterization of insulin-degrading enzyme-mediated cleavage of Aβ in distinct aggregation states. Biochim. Biophys. Acta 2016, 1860, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- McCord, L.A.; Liang, W.G.; Dowdell, E.; Kalas, V.; Hoey, R.J.; Koide, A.; Koide, S.; Tang, W.J. Conformational states and recognition of amyloidogenic peptides of human insulin-degrading enzyme. Proc. Natl. Acad. Sci. USA 2013, 110, 13827–13832. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Walsh, D.M.; Ye, Z.; Vekrellis, K.; Zhang, J.; Podlisny, M.B.; Rosner, M.R.; Safavi, A.; Hersh, L.B.; Selkoe, D.J. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J. Biol. Chem. 1998, 273, 32730–32738. [Google Scholar] [CrossRef]
- Schupf, N.; Lee, A.; Park, N.; Dang, L.H.; Pang, D.; Yale, A.; Oh, D.K.; Krinsky-McHale, S.J.; Jenkins, E.C.; Luchsinger, J.A.; et al. Candidate genes for Alzheimer’s disease are associated with individual differences in plasma levels of beta amyloid peptides in adults with Down syndrome. Neurobiol. Aging 2015, 36, e2901–e2910. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Helisalmi, S.; Mannermaa, A.; Pirttilä, T.; Soininen, H.; Hiltunen, M. Combined risk effects of IDE and NEP gene variants on Alzheimer disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1268–1270. [Google Scholar] [CrossRef]
- Yadollahikhales, G.; Rojas, J.C. Anti-Amyloid Immunotherapies for Alzheimer’s Disease: A 2023 Clinical Update. Neurotherapeutics 2023, 20, 914–931. [Google Scholar] [CrossRef]
- Terao, I.; Kodama, W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 2024, 94, 102203. [Google Scholar] [CrossRef]
- Moon, J.H.; Kang, S.B.; Park, J.S.; Lee, B.W.; Kang, E.S.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Up-regulation of hepatic low-density lipoprotein receptor–related protein 1: A possible novel mechanism of antiatherogenic activity of hydroxymethylglutaryl–coenzyme A reductase inhibitor: Atorvastatin and hepatic LRP1 expression. Metabolism 2011, 60, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhu, Q.; Miao, T.; Tao, J.; Ju, X.; Sun, Z.; Li, H.; Xu, G.; Chen, H.; Han, L. LRP1-upregulated nanoparticles for efficiently conquering the blood-brain barrier and targetedly suppressing multifocal and infiltrative brain metastases. J. Control. Release 2019, 303, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Pfeifer, J.A.; Hickey, J.P.; Collins, A.E.; Kalisch, B.E. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Sarathlal, K.C.; Kakoty, V.; Krishna, K.V.; Dubey, S.K.; Chitkara, D.; Taliyan, R. Neuroprotective Efficacy of Co-Encapsulated Rosiglitazone and Vorinostat Nanoparticle on Streptozotocin Induced Mice Model of Alzheimer Disease. ACS Chem. Neurosci. 2021, 12, 1528–1541. [Google Scholar] [CrossRef]
- Olmastroni, E.; Molari, G.; de Beni, N.; Colpani, O.; Galimberti, F.; Gazzotti, M.; Zambon, A.; Catapano, A.L.; Casula, M. Statin use and risk of dementia or Alzheimer’s disease: A systematic review and meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2022, 29, 804–814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoy, A.; Umbayev, B.; Kassenova, A.; Kaupbayeva, B.; Askarova, S. Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 10964. https://doi.org/10.3390/ijms252010964
Tsoy A, Umbayev B, Kassenova A, Kaupbayeva B, Askarova S. Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(20):10964. https://doi.org/10.3390/ijms252010964
Chicago/Turabian StyleTsoy, Andrey, Bauyrzhan Umbayev, Aliya Kassenova, Bibifatima Kaupbayeva, and Sholpan Askarova. 2024. "Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 20: 10964. https://doi.org/10.3390/ijms252010964
APA StyleTsoy, A., Umbayev, B., Kassenova, A., Kaupbayeva, B., & Askarova, S. (2024). Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease. International Journal of Molecular Sciences, 25(20), 10964. https://doi.org/10.3390/ijms252010964





