Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review
Abstract
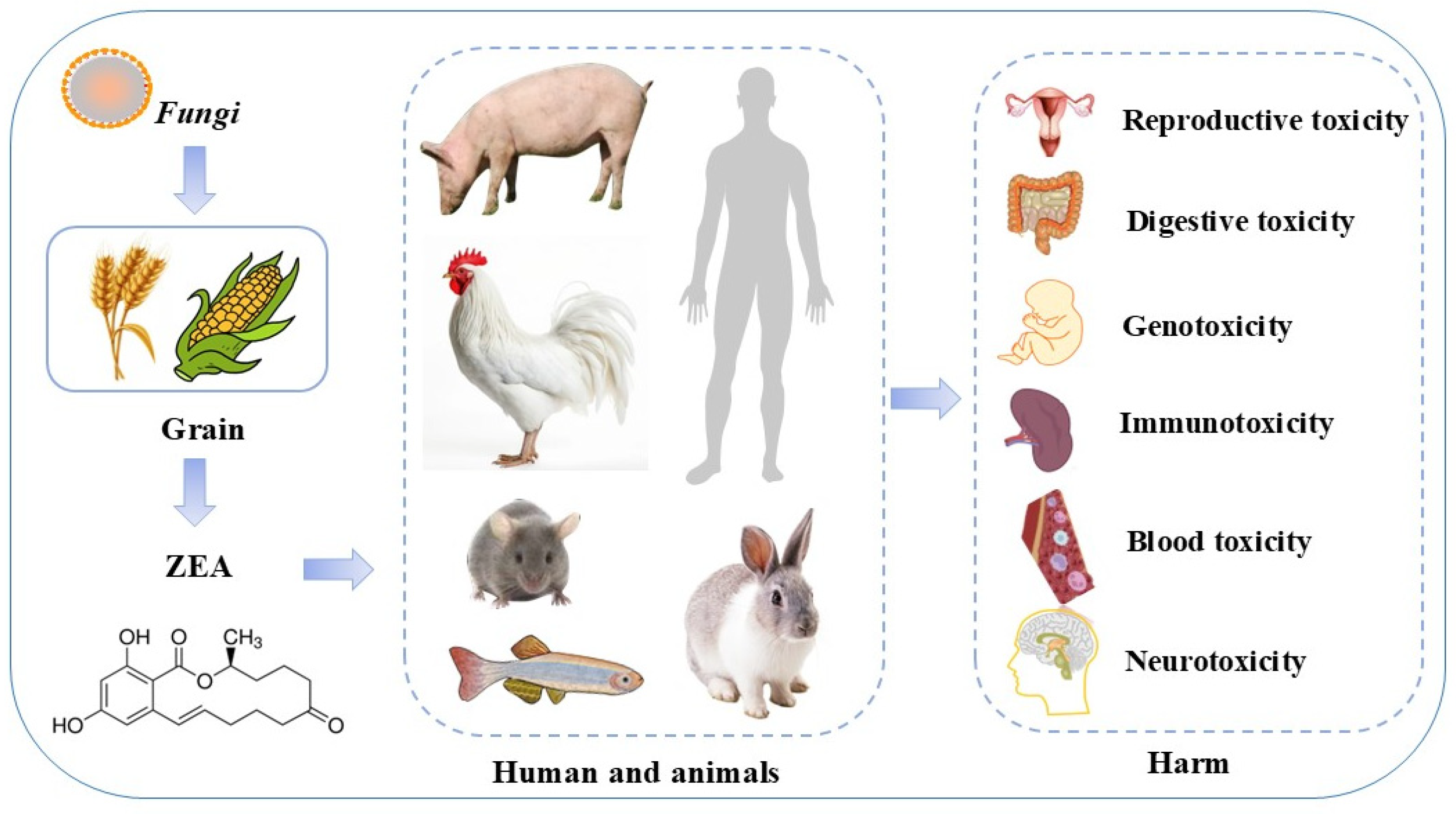
1. Introduction
2. ZEA Toxic Effects and Its Molecular Mechanism Research Progress
3. Biological Properties of RSV
4. Protective Effects of RSV against ZEA Toxicity and Its Molecular Mechanism
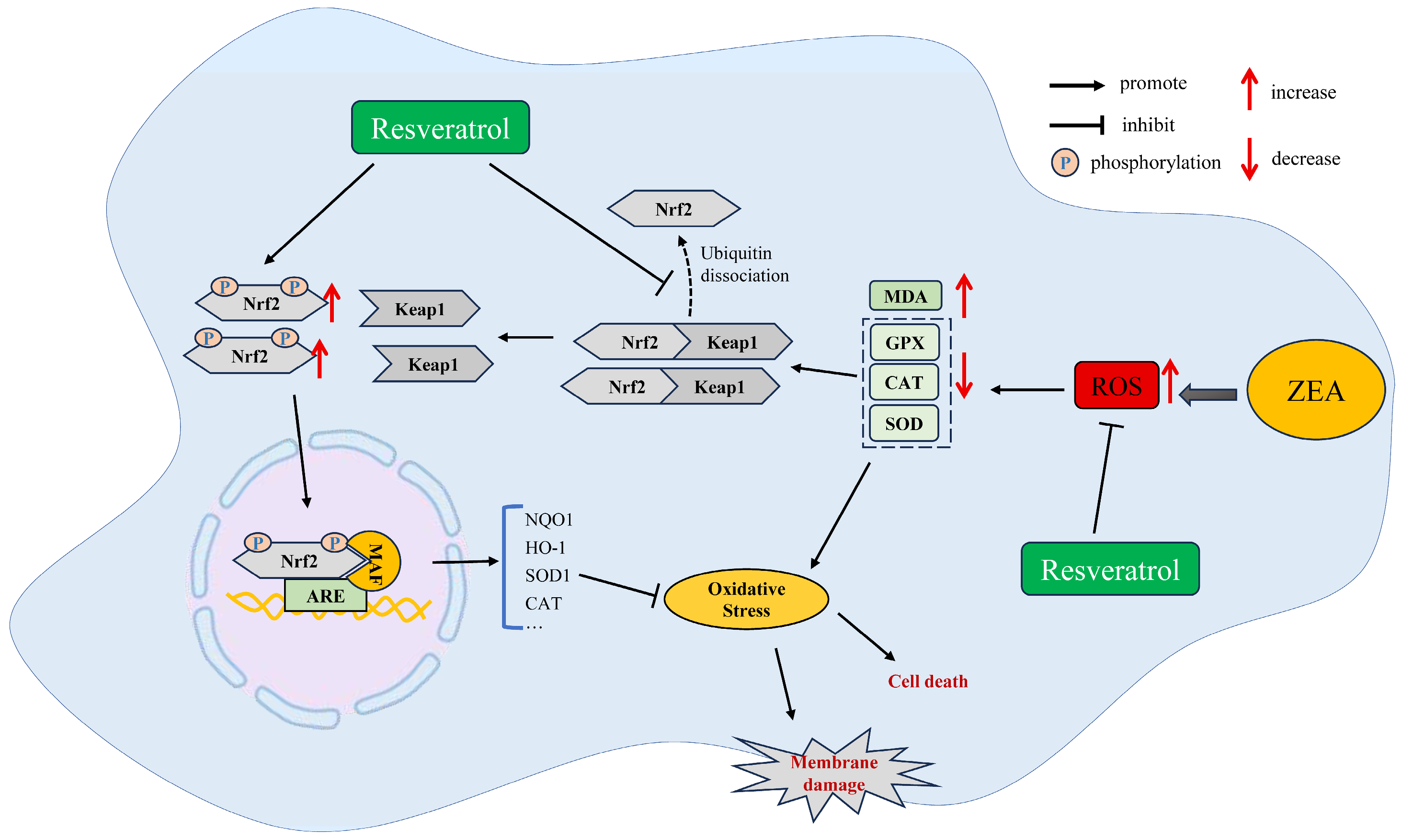
4.1. RSV Ameliorates ZEA-Induced Oxidative Stress
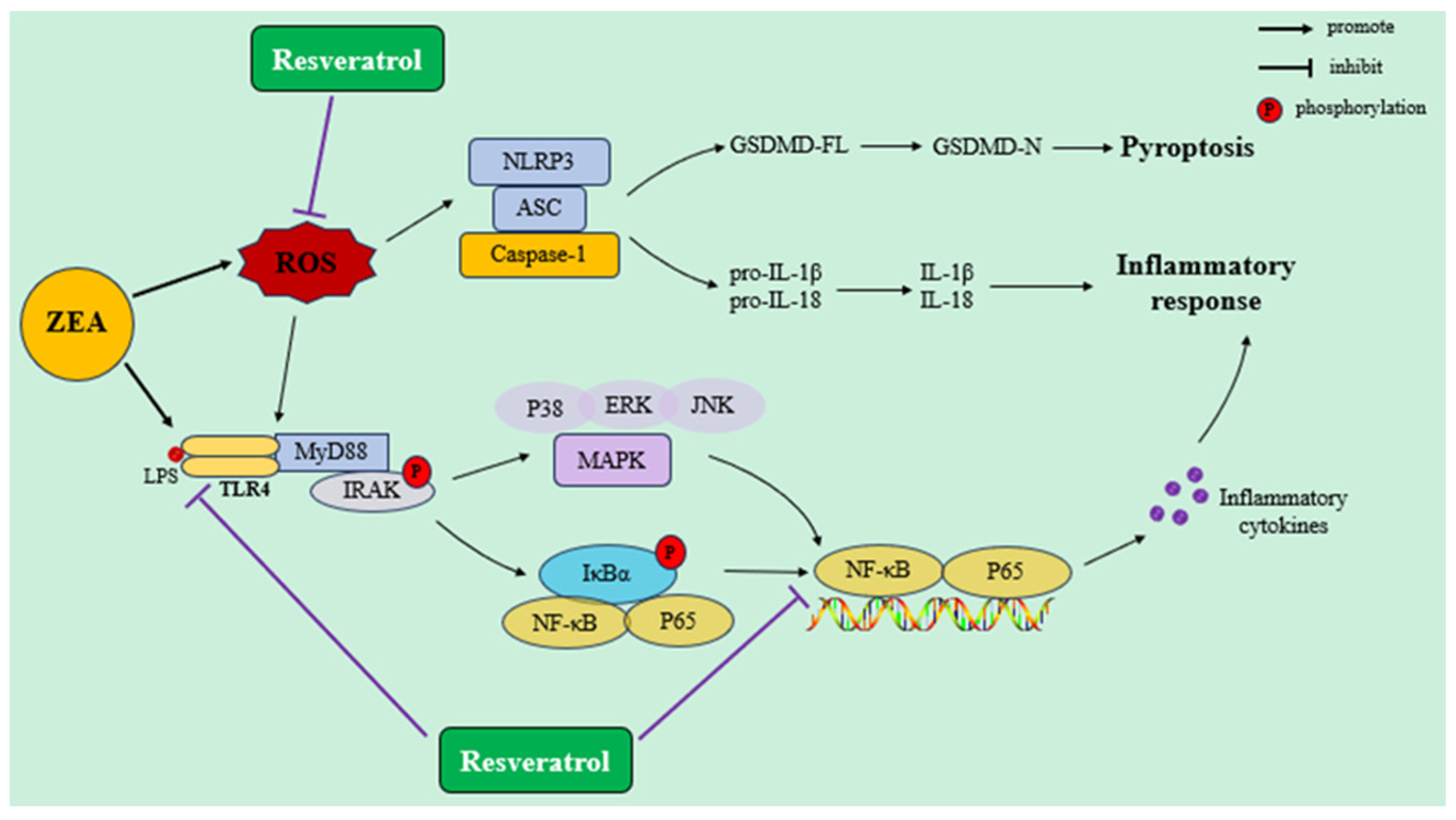
4.2. RSV Ameliorates Immunosuppression and Inflammatory Response Induced by ZEA Exposure
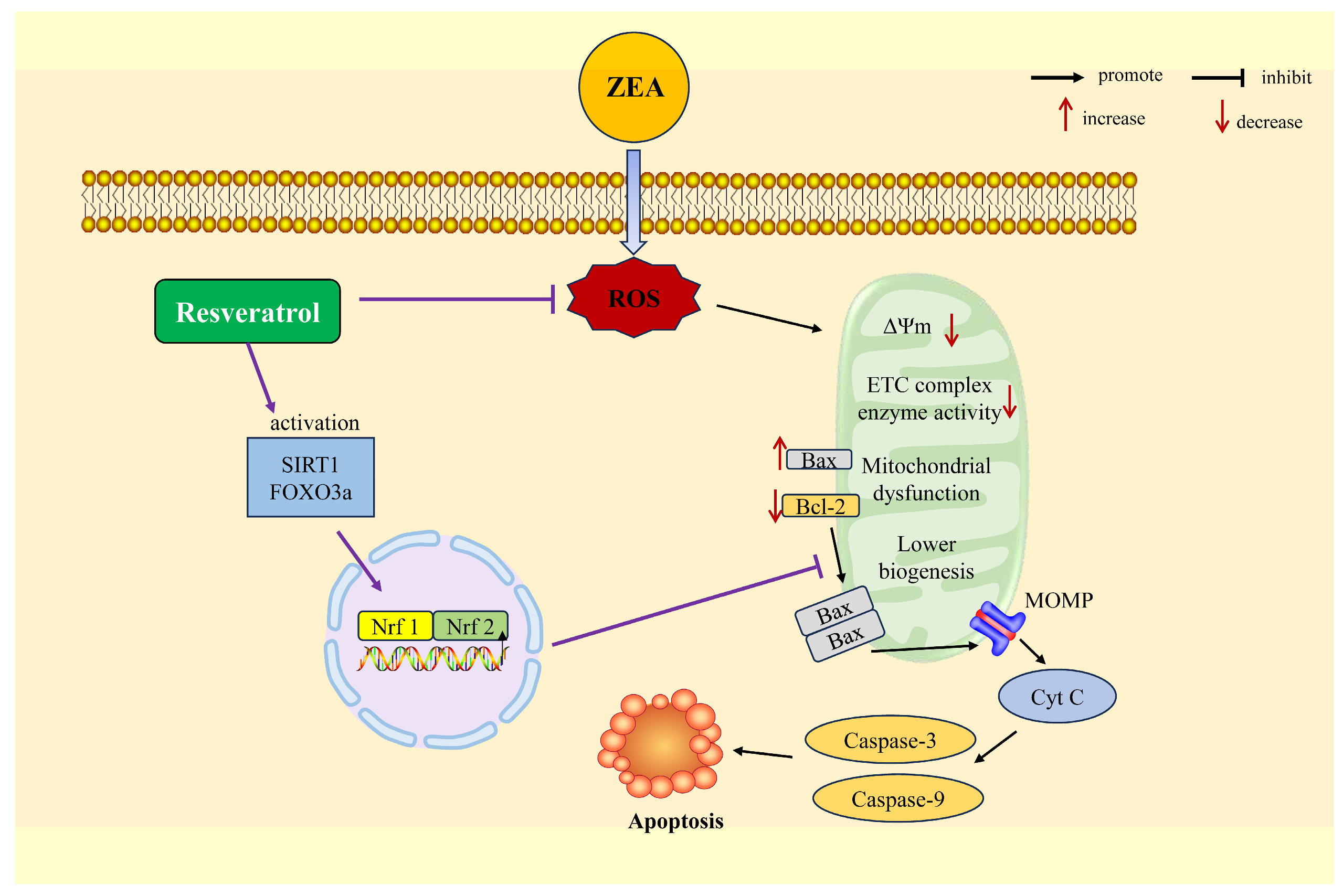
4.3. RSV Improves Mitochondrial Dysfunction and Apoptosis Induced by ZEA
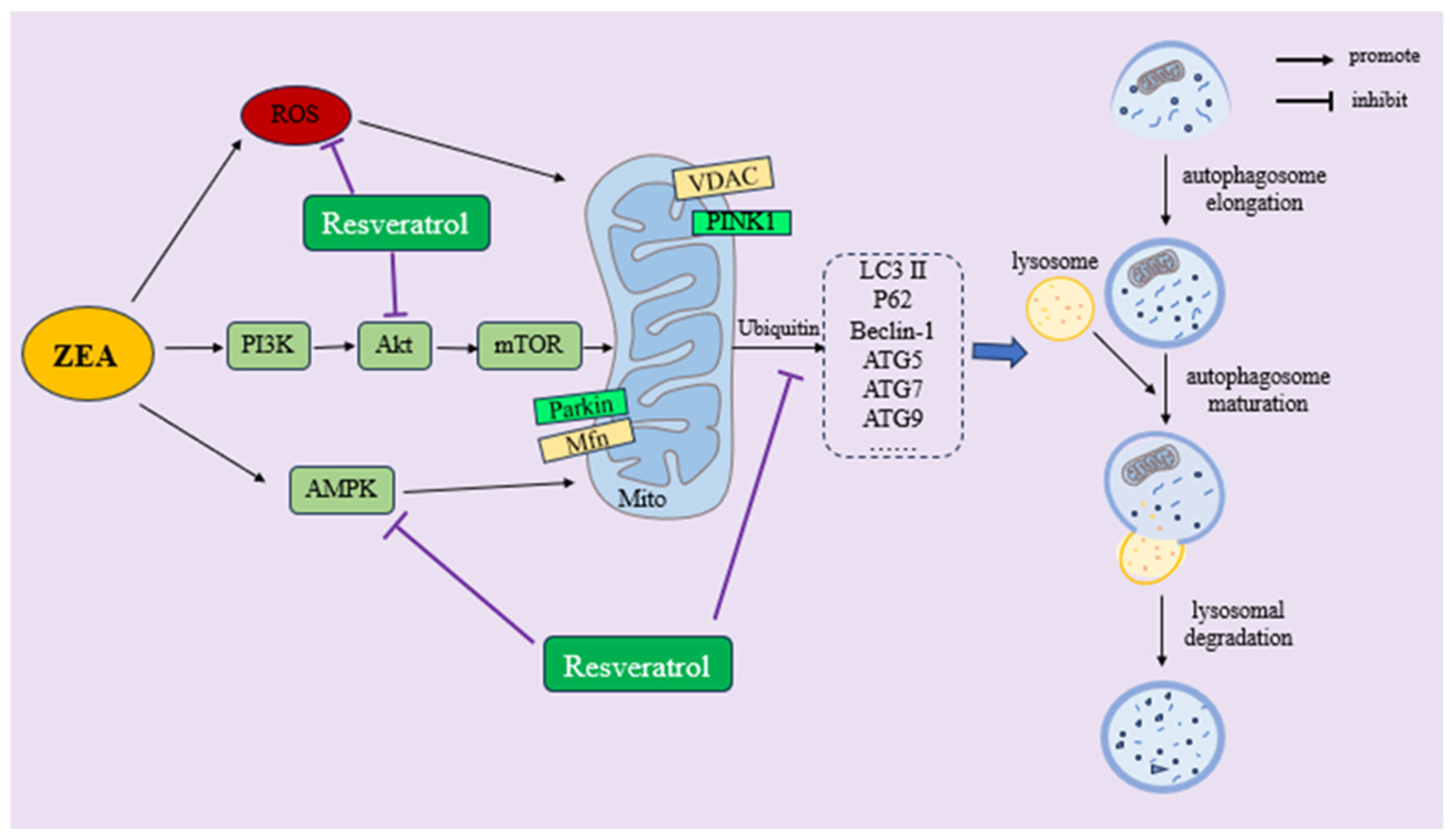
4.4. RSV Improves ZEA-Induced Autophagy and Mitochondrial Autophagy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehsanifar, M.; Rajati, R.; Gholami, A.; Reiss, J.P. Mold and Mycotoxin Exposure and Brain Disorders. J. Integr. Neurosci. 2023, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Luo, H.; Li, L.; Zhang, J.; Harvey, J.; Zhao, Y.; Zhang, G.; Liu, Y. Mycotoxin risk management in maize gluten meal. Crit. Rev. Food Sci. Nutr. 2023, 64, 7687–7706. [Google Scholar] [CrossRef] [PubMed]
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse mycotoxin threats to safe food and feed cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. Part B Surveill. 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Zhang, J.; Guo, J.; Chen, L.; Qi, D.; Zhang, N. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from central China. Food Addit. Contam. Part B Surveill. 2016, 9, 91–97. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. The toxicity mechanisms of DON to humans and animals and potential biological treatment strategies. Crit. Rev. Food Sci. Nutr. 2021, 63, 790–812. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone Promotes Cell Proliferation or Causes Cell Death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.; Xu, W.; Zhang, W.; Guang, C.; Mu, W. Zearalenone lactonase: Characteristics, modification, and application. Appl. Microbiol. Biotechnol. 2022, 106, 6877–6886. [Google Scholar] [CrossRef]
- Bao, L.; Huang, Y.; Gu, F.; Liu, W.; Guo, Y.; Chen, H.; Wang, K.; Wu, Z.; Li, J. Zearalenone induces liver injury in mice through ferroptosis pathway. Sci. Total. Environ. 2024, 952, 175875. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Zhen, H.; Hu, Y.; Xiong, K.; Li, M.; Jin, W. The occurrence and biological control of zearalenone in cereals and cereal-based feedstuffs: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2024, 41, 1344–1359. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.-H.; Chen, S.; Sun, S.-C. Zearalenone exposure impairs organelle function during porcine oocyte meiotic maturation. Theriogenology 2022, 177, 22–28. [Google Scholar] [CrossRef]
- Fushimi, Y.; Takagi, M.; Monniaux, D.; Uno, S.; Kokushi, E.; Shinya, U.; Kawashima, C.; Otoi, T.; Deguchi, E.; Fink-Gremmels, J. Effects of Dietary Contamination by Zearalenone and Its Metabolites on Serum Anti-Müllerian Hormone: Impact on the Reproductive Performance of Breeding Cows. Reprod. Domest. Anim. 2015, 50, 834–839. [Google Scholar] [CrossRef]
- Jia, S.; Ren, C.; Yang, P.; Qi, D. Effects of Intestinal Microorganisms on Metabolism and Toxicity Mitigation of Zearalenone in Broilers. Animals 2022, 12, 1962. [Google Scholar] [CrossRef]
- Nobre, C.; González, A.; Losoya, C.; Teixeira, J.A.; Belmares, R.; Abrunhosa, L. Detoxification of ochratoxin A and zearalenone by Pleurotus ostreatus during in vitro gastrointestinal digestion. Food Chem. 2022, 384, 132525. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, W.; Wu, H.; Zhang, W.; Mu, W. Identification of a Potent Enzyme for the Detoxification of Zearalenone. J. Agric. Food Chem. 2020, 68, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ntungwe, E.N.; Tchana, A.N.; Abia, W.A. Mycotoxin management: Exploring natural solutions for mycotoxin prevention and detoxification in food and feed. Mycotoxin Res. 2024. [Google Scholar] [CrossRef]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M.; Khan, A.M. Structural Analysis and Biological Toxicity of Aflatoxins B1 and B2 Degradation Products Following Detoxification by Ocimum basilicum and Cassia fistula Aqueous Extracts. Front. Microbiol. 2016, 7, 1105. [Google Scholar] [CrossRef]
- Chowdhury, N.N.; Islam, M.N.; Jafrin, R.; Rauf, A.; Khalil, A.A.; Bin Emran, T.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Lorenzo, J.M.; Shariati, M.A.; et al. Natural plant products as effective alternatives to synthetic chemicals for postharvest fruit storage management. Crit. Rev. Food Sci. Nutr. 2022, 63, 10332–10350. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Su, Y.; Chang, S.; Sun, Y.; Meng, X.; Shan, A. Vitamin C protects piglet liver against zearalenone-induced oxidative stress by modulating expression of nuclear receptors PXR and CAR and their target genes. Food Funct. 2017, 8, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, S.; Jiang, L.; Gao, X.; Liu, W.; Zhu, X.; Huang, W.; Zhao, H.; Wei, Z.; Wang, K.; et al. Baicalin protects against zearalenone-induced chicks liver and kidney injury by inhibiting expression of oxidative stress, inflammatory cytokines and caspase signaling pathway. Int. Immunopharmacol. 2021, 100, 108097. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Shrivastava, V.K.; Saleh, R.; Henkel, R.; Agarwal, A. Protective effects of saffron against zearalenone-induced alterations in reproductive hormones in female mice (Mus musculus). Clin. Exp. Reprod. Med. 2018, 45, 163–169. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Yang, M.; Li, J.; Wu, Y.; Fan, H.; Kong, X.; Ning, C.; Wang, S.; Xiao, W.; et al. Betulinic acid alleviates zearalenone-induced uterine injury in mice. Environ. Pollut. 2023, 316 Pt 1, 120435. [Google Scholar] [CrossRef]
- Pang, J.; Raka, F.; Heirali, A.A.; Shao, W.; Liu, D.; Gu, J.; Feng, J.N.; Mineo, C.; Shaul, P.W.; Qian, X.; et al. Resveratrol intervention attenuates chylomicron secretion via repressing intestinal FXR-induced expression of scavenger receptor SR-B1. Nat. Commun. 2023, 14, 2656. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Jung, H.J.; Hwang, I.A.; Sung, W.S.; Kang, H.; Kang, B.S.; Seu, Y.B.; Lee, D.G. Fungicidal Effect of Resveratrol on Human Infectious Fungi. Arch. Pharmacal Res. 2005, 28, 557–560. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Zhang, G. The protective effect of resveratrol against cytotoxicity induced by mycotoxin, zearalenone. Food Funct. 2016, 7, 3703–3715. [Google Scholar] [CrossRef]
- Xia, S.; Yan, C.; Gu, J.; Yuan, Y.; Zou, H.; Liu, Z.; Bian, J. Resveratrol Alleviates Zearalenone-Induced Intestinal Dysfunction in Mice through the NF-κB/Nrf2/HO-1 Signalling Pathway. Foods 2024, 13, 1217. [Google Scholar] [CrossRef]
- Cai, P.; Feng, N.; Zheng, W.; Zheng, H.; Zou, H.; Yuan, Y.; Liu, X.; Liu, Z.; Gu, J.; Bian, J. Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells. Molecules 2019, 24, 2474. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Morrissey, R.; Usborne, A.; Kapetanovic, I.; Crowell, J.; Muzzio, M.; McCormick, D. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A Mycotoxin With Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does Resveratrol Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef]
- Wu, K.; Jia, S.; Xue, D.; Rajput, S.A.; Liu, M.; Qi, D.; Wang, S. Dual effects of zearalenone on aflatoxin B1–induced liver and mammary gland toxicity in pregnant and lactating rats. Ecotoxicol. Environ. Saf. 2022, 245, 114115. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef]
- Chen, P.; Liu, T.; Jiang, S.; Yang, Z.; Huang, L.; Liu, F. Effects of purified zearalenone on selected immunological and histopathologic measurements of spleen in post-weanling gilts. Anim. Nutr. 2017, 3, 212–218. [Google Scholar] [CrossRef]
- Ren, Z.; Deng, H.; Deng, Y.; Deng, J.; Zuo, Z.; Yu, S.; Shen, L.; Cui, H.; Xu, Z.; Hu, Y. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef]
- Sun, X.; Meng, H.; Xiao, J.; Liu, F.; Du, J.; Zeng, H. Pretreatment of 3-MA prevents doxorubicin-induced cardiotoxicity through inhibition of autophagy initiation. Toxicology 2023, 490, 153512. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, L.; Gao, X.; Kong, L.; Huang, Y.; Zhao, H.; Chen, Y.; Wen, L.; Li, R.; Wu, J.; et al. Ameliorative effect of betulinic acid against zearalenone exposure triggers testicular dysfunction and oxidative stress in mice via p38/ERK MAPK inhibition and Nrf2-mediated antioxidant defense activation. Ecotoxicol. Environ. Saf. 2022, 238, 113561. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Q.; Wang, J.-J.; Li, M.-H.; Tian, Y.; Zhao, A.-H.; Li, L.; De Felici, M.; Shen, W. Impaired primordial follicle assembly in offspring ovaries from zearalenone-exposed mothers involves reduced mitochondrial activity and altered epigenetics in oocytes. Cell. Mol. Life Sci. 2022, 79, 258. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Edler, L.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef]
- Wentzel, J.F.; Lombard, M.J.; Du Plessis, L.H.; Zandberg, L. Evaluation of the cytotoxic properties, gene expression profiles and secondary signalling responses of cultured cells exposed to fumonisin B1, deoxynivalenol and zearalenone mycotoxins. Arch. Toxicol. 2017, 91, 2265–2282. [Google Scholar] [CrossRef]
- He, Y.; Su, N.; Yang, H.; Yang, W.; Zhao, C.; Fu, Y.; Hu, Y.; Hu, X. ZEA mediates autophagy through the ROS-AMPK-m-TOR pathway to enhance the susceptibility of mastitis induced by Staphylococcus aureus in mice. Ecotoxicol. Environ. Saf. 2023, 266, 115548. [Google Scholar] [CrossRef]
- Sun, N.; Haseeb, A.; Sun, P.; Zhang, H.; Zhong, J.; Yin, W.; Fan, K.; Yang, H.; Zhang, Z.; Sun, Y.; et al. Scutellarin targets Wnt5a against zearalenone-induced apoptosis in mouse granulosa cells in vitro and in vivo. J. Hazard. Mater. 2024, 464, 132917. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Zhang, W.; Zhou, S.; Wen, D.; Mu, R. Chronic exposure to zearalenone induces intestinal inflammation and oxidative injury in adult Drosophila melanogaster midgut. Ecotoxicol. Environ. Saf. 2023, 251, 114555. [Google Scholar] [CrossRef]
- Wei, C.-C.; Yang, N.-C.; Huang, C.-W. Zearalenone Induces Dopaminergic Neurodegeneration via DRP-1-Involved Mitochondrial Fragmentation and Apoptosis in a Caenorhabditis elegans Parkinson’s Disease Model. J. Agric. Food Chem. 2021, 69, 12030–12038. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, S.; Huang, L.; Wang, Y.; Yang, W.; Yang, Z.; Ge, J. Effects of zearalenone-induced oxidative stress and Keap1-Nrf2 signaling pathway-related gene expression in the ileum and mesenteric lymph nodes of post-weaning gilts. Toxi-cology 2020, 429, 152337. [Google Scholar] [CrossRef]
- Ben Ammar, R.; Abu Zahra, H.; Abu Zahra, A.M.; Alfwuaires, M.; Alamer, S.A.; Metwally, A.M.; Althnaian, T.A.; Al-Ramadan, S.Y. Protective Effect of Fucoxanthin on Zearalenone-Induced Hepatic Damage through Nrf2 Mediated by PI3K/AKT Signaling. Mar. Drugs 2023, 21, 391. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liao, W.; Dong, J.; Chen, X.; Huang, L.; Yang, W.; Jiang, S. Zearalenone Promotes Uterine Hypertrophy through AMPK/mTOR Mediated Autophagy. Toxins 2024, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.K.M.; Lo, E.K.K.; Chen, C.; Zhang, F.; Felicianna; Ismaiah, M.J.; El-Nezami, H. Zearalenone attenuates colitis associated colorectal tumorigenesis through Ras/Raf/ERK pathway suppression and SCFA-producing bacteria promotion. Biomed. Pharmacother. 2023, 164, 114973. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Wang, J.; Han, S.; Zhang, Y.; Ma, M.; Zhu, Q.; Zhang, K.; Yin, H. Zearalenone Induces Apoptosis and Cytoprotective Autophagy in Chicken Granulosa Cells by PI3K-AKT-mTOR and MAPK Signaling Pathways. Toxins 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. ERβ and NFκB—Modulators of Zearalenone-Induced Oxidative Stress in Human Prostate Cancer Cells. Toxins 2020, 12, 199. [Google Scholar] [CrossRef]
- Yang, L.-J.; Zhou, M.; Huang, L.-B.; Yang, W.-R.; Yang, Z.-B.; Jiang, S.-Z.; Ge, J.-S. Zearalenone-Promoted Follicle Growth through Modulation of Wnt-1/β-Catenin Signaling Pathway and Expression of Estrogen Receptor Genes in Ovaries of Postweaning Piglets. J. Agric. Food Chem. 2018, 66, 7899–7906. [Google Scholar] [CrossRef]
- Wu, S.-X.; Xiong, R.-G.; Huang, S.-Y.; Zhou, D.-D.; Saimaiti, A.; Zhao, C.-N.; Shang, A.; Zhang, Y.-J.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of resveratrol for prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 63, 12422–12440. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy: Preventive and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 17573. [Google Scholar] [CrossRef]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sajeev, A.; Koklesova, L.; Samuel, S.M.; Kubatka, P.; Büsselberg, D.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol as sensitizer in colorectal cancer plasticity. Cancer Metastasis Rev. 2023, 43, 55–85. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Pan, S.; Feng, Y.; He, H.; Cheng, S.; Wang, L.; Wang, L.; Pathak, J.L. Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients 2023, 15, 2115. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β induces proliferation in colorectal cancer cells and resveratrol can down-modulate it. Exp. Biol. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Tseng, P.-C.; Hou, S.-M.; Chen, R.-J.; Peng, H.-W.; Hsieh, C.-F.; Kuo, M.-L.; Yen, M.-L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef]
- Tang, S.; Botchway, B.O.; Zhang, Y.; Wang, X.; Huang, M.; Liu, X. Resveratrol can improve spinal cord injury by activating Nrf2/HO-1 signaling pathway. Ann. Anat. Anat. Anz. 2024, 251, 152180. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of Resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef]
- Perrone, G.; Nicoletti, I.; Pascale, M.; De Rossi, A.; De Girolamo, A.; Visconti, A. Positive Correlation between High Levels of Ochratoxin A and Resveratrol-Related Compounds in Red Wines. J. Agric. Food Chem. 2007, 55, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.-W.; Talukder, M.; Guo, K.; Li, Y.-H.; Li, J.-L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, B.; Li, J.; Lv, Z.; Jiang, H.; Liu, Y.; Yang, X.; Lu, J.; Zhang, Z. Resveratrol Alleviates Liver Fibrosis Induced by Long-Term Inorganic Mercury Exposure through Activating the Sirt1/PGC-1α Signaling Pathway. J. Agric. Food Chem. 2024, 72, 15985–15997. [Google Scholar] [CrossRef]
- Lee, I.-T.; Lin, C.-C.; Hsu, C.-K.; Wu, M.-Y.; Cho, R.-L.; Yang, C.-M. Resveratrol inhibits Staphylococcus aureus-induced TLR2/MyD88/NF-κB-dependent VCAM-1 expression in human lung epithelial cells. Clin. Sci. 2014, 127, 375–390. [Google Scholar] [CrossRef]
- Luo, X.; Wu, S.; Jia, H.; Si, X.; Song, Z.; Zhai, Z.; Bai, J.; Li, J.; Yang, Y.; Wu, Z. Resveratrol alleviates enterotoxigenic Escherichia coli K88-induced damage by regulating SIRT-1 signaling in intestinal porcine epithelial cells. Food Funct. 2022, 13, 7346–7360. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef]
- Yao, S.; Wei, W.; Cao, R.; Lu, L.; Liang, S.; Xiong, M.; Zhang, C.; Liang, X.; Ma, Y. Resveratrol alleviates zea-induced decidualization disturbance in human endometrial stromal cells. Ecotoxicol. Environ. Saf. 2021, 207, 111511. [Google Scholar] [CrossRef]
- Virk, P.; Al-Mukhaizeem, N.A.R.; Bin Morebah, S.H.; Fouad, D.; Elobeid, M. Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. Food Chem. Toxicol. 2020, 146, 111840. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Fu, Y.; Gu, Y.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells. Toxins 2022, 14, 733. [Google Scholar] [CrossRef]
- Xu, J.; Sun, L.; He, M.; Zhang, S.; Gao, J.; Wu, C.; Zhang, D.; Dai, J. Resveratrol Protects against Zearalenone-Induced Mitochondrial Defects during Porcine Oocyte Maturation via PINK1/Parkin-Mediated Mitophagy. Toxins 2022, 14, 641. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Murakami, S.; Kusano, Y.; Okazaki, K.; Akaike, T.; Motohashi, H. NRF2 signalling in cytoprotection and metabolism. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Lin, L.; Wu, Q.; Lu, F.; Lei, J.; Zhou, Y.; Liu, Y.; Zhu, N.; Yu, Y.; Ning, Z.; She, T.; et al. Nrf2 signaling pathway: Current status and potential therapeutic targetable role in human cancers. Front. Oncol. 2023, 13, 1184079. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Rajendran, P.; Ammar, R.B.; Al-Saeedi, F.J.; Mohamed, M.E.; ElNaggar, M.A.; Al-Ramadan, S.Y.; Bekhet, G.M.; Soliman, A.M. Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the PI3K/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 217. [Google Scholar] [CrossRef]
- Wu, F.; Wang, F.; Tang, Z.; Yang, X.; Liu, Y.; Zhao, M.; Liu, S.; Han, S.; Zhang, Z.; Chen, B. Quercetagetin alleviates zearalenone-induced liver injury in rabbits through Keap1/Nrf2/ARE signaling pathway. Front. Pharmacol. 2023, 14, 1271384. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, S.; Huang, L.; Wang, Y.; Yang, W. Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells. Toxins 2022, 14, 793. [Google Scholar] [CrossRef] [PubMed]
- Gheraibia, S.; Belattar, N.; Diab, K.A.; Hassan, M.E.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Costus speciosus extract protects against the oxidative damage of zearalenone via modulation of inflammatory cytokines, Nrf2 and iNOS gene expression in rats. Toxicon 2022, 214, 62–73. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, P.; Xie, H.; Wei, C.; Wang, Q.; Yang, B.; Sun, Y.; Li, Y.; Luo, Y.; Song, Y.; et al. Resveratrol improves meat quality traits by activating the lncRNAs-KEAP1-NRF2 axis in pigs. Meat Sci. 2024, 209, 109411. [Google Scholar] [CrossRef]
- Ding, K.-N.; Lu, M.-H.; Guo, Y.-N.; Liang, S.-S.; Mou, R.-W.; He, Y.-M.; Tang, L.-P. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114411. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, G.; Lin, L.; Zhang, Y.; Luo, X.; Ma, X.; Aiyisake, A.; Cheng, Q. Resveratrol Attenuates Sepsis-Induced Cardiomyopathy in Rats through Anti-Ferroptosis via the Sirt1/Nrf2 Pathway. J. Investig. Surg. 2023, 36, 2157521. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, C.; Xu, C.; Xie, D.; Wang, Q.; Liu, W.; Zhao, H.; He, F.; Chen, B.; Xi, Y.; et al. Activation of p62-NRF2 Axis Protects against Doxorubicin-Induced Ferroptosis in Cardiomyocytes: A Novel Role and Molecular Mechanism of Resveratrol. Am. J. Chin. Med. 2022, 50, 2103–2123. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Obremski, K.; Gajęcka, M.; Gajęcki, M.T.; Zielonka, Ł. Changes in the Subpopulations of Porcine Peripheral Blood Lymphocytes Induced by Exposure to Low Doses of Zearalenone (ZEN) and Deoxynivalenol (DON). Molecules 2016, 21, 557. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Su, Y.; Sun, Y.; Meng, X.; Shi, B.; Shan, A. Response of the nuclear receptors PXR and CAR and their target gene mRNA expression in female piglets exposed to zearalenone. Toxicon 2018, 151, 111–118. [Google Scholar] [CrossRef]
- Correction to: Islam et al., Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 241. [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone Mycotoxin Affects Immune Mediators, MAPK Signalling Molecules, Nuclear Receptors and Genome-Wide Gene Expression in Pig Spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef]
- Cai, G.; Sun, K.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Mechanism and effects of Zearalenone on mouse T lymphocytes activation in vitro. Ecotoxicol. Environ. Saf. 2018, 162, 208–217. [Google Scholar] [CrossRef]
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Liu, G.; Lu, Y.; Shi, L.; Ren, Y.; Kong, J.; Zhang, M.; Chen, M.; Liu, W. TLR4-MyD88 signaling pathway is responsible for acute lung inflammation induced by reclaimed water. J. Hazard. Mater. 2020, 396, 122586. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, S.; Duncan, G.S.; Millar, D.G.; Wada, T.; Mirtsos, C.; Takada, H.; Wakeham, A.; Itie, A.; Li, S.; et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 2002, 416, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Liu, M.; Qu, Z.; Zhang, Y.; Yin, S.; Shan, A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ. Toxicol. Pharmacol. 2014, 37, 580–591. [Google Scholar] [CrossRef]
- Ma, E.; Wu, C.; Chen, J.; Wo, D.; Ren, D.-N.; Yan, H.; Peng, L.; Zhu, W. Resveratrol prevents Ang II-induced cardiac hypertrophy by inhibition of NF-κB signaling. Biomed. Pharmacother. 2023, 165, 115275. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Lv, N.; Wang, L.; Gan, J.; Jiang, X.; Wang, Y. Therapeutic Role and Potential Mechanism of Resveratrol in Atherosclerosis: TLR4/NF-κB/HIF-1α. Mediat. Inflamm. 2023, 2023, 1097706. [Google Scholar] [CrossRef]
- Pan, W.; Yu, H.; Huang, S.; Zhu, P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS ONE 2016, 11, e0147034. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Fan, J.; Chen, Y.; Wang, H.; Ge, Y.; Liang, H.; Li, W.; Liu, H.; Lv, Z.; et al. The role of ROS/p38 MAPK/NLRP3 inflammasome cascade in arsenic-induced depression-/anxiety-like behaviors of mice. Ecotoxicol. Environ. Saf. 2023, 261, 115111. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hai, S.; Wang, C.; Chen, C.; Rahman, S.U.; Zhao, C.; Bazai, M.A.; Feng, S.; Wang, X. Zearalenone induces mitochondria-associated endoplasmic reticulum membranes dysfunction in piglet Sertoli cells based on endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2023, 254, 114710. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Garrido, M.; Frangiamone, M.; Font, G.; Cimbalo, A.; Manyes, L. In vitro blood brain barrier exposure to mycotoxins and carotenoids pumpkin extract alters mitochondrial gene expression and oxidative stress. Food Chem. Toxicol. 2021, 153, 112261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zeng, C.; Sun, X.; Zhang, Q.; Wang, Y.; Xia, R.; Mai, Q.; Xue, G.; Huang, H.; Wang, F. Zearalenone modulates the function of goat endometrial cells via the mitochondrial quality control system. FASEB J. 2024, 38, e23701. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Gao, K.; Zhang, L.; Lin, P.; Wang, A.; Jin, Y. Zearalenone Induces MLKL-Dependent Necroptosis in Goat Endometrial Stromal Cells via the Calcium Overload/ROS Pathway. Int. J. Mol. Sci. 2022, 23, 10170. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Tan, X.; Zhao, M.; Ma, Y.; Li, Y. Proteomic analysis of zearalenone toxicity on mouse thymic epithelial cells. J. Appl. Toxicol. 2022, 42, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Hai, S.; Chen, J.; Ma, L.; Wang, C.; Chen, C.; Rahman, S.U.; Zhao, C.; Feng, S.; Wu, J.; Wang, X. Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress. Toxins 2023, 15, 471. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zou, J.; Zhong, X.; Li, J.; Kang, R.; Tang, D. HMGB1 in the interplay between autophagy and apoptosis in cancer. Cancer Lett. 2024, 581, 216494. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Ordóñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W.G. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2019, 34, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-L.; Wang, B.-J.; Wang, L.; Shan, Y.-P.; Zou, H.; Song, R.-L.; Wang, T.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Hao, Z.; Sun, P.; Fan, K.; Yin, W.; Guo, J.; Zheng, X.; Sun, N.; Li, H. Study on the mechanism of scutellarin’s protective effect against ZEA-induced mouse ovarian granulosa cells injury. Food Chem. Toxicol. 2022, 170, 113481. [Google Scholar] [CrossRef]
- Lee, H.-J.; Oh, S.-Y.; Jo, I. Zearalenone Induces Endothelial Cell Apoptosis through Activation of a Cytosolic Ca2+/ERK1/2/p53/Caspase 3 Signaling Pathway. Toxins 2021, 13, 187. [Google Scholar] [CrossRef]
- Shaito, A.; Al-Mansoob, M.; Ahmad, S.M.; Haider, M.Z.; Eid, A.H.; Posadino, A.M.; Pintus, G.; Giordo, R. Resveratrol-Mediated Regulation of Mitochondria Biogenesis-associated Pathways in Neurodegenerative Diseases: Molecular Insights and Potential Therapeutic Applications. Curr. Neuropharmacol. 2023, 21, 1184–1201. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Xu, T.; Lei, Y.; Gao, M.; Lin, H. Resveratrol alleviates imidacloprid-induced mitochondrial apoptosis, necroptosis, and immune dysfunction in chicken lymphocyte lines by inhibiting the ROS/MAPK signaling pathway. Environ. Toxicol. 2024, 39, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Cozzi, M.F.; Allegra, A.; Gangemi, S. Oxidative stress, mitochondrial dysfunction, and respiratory chain enzyme defects in inflammatory myopathies. Autoimmun. Rev. 2023, 22, 103308. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Miller, D.R.; Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell 2021, 56, 906–918. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, H.; Han, S.; Zhang, H.; Hu, J. Zearalenone induces oxidative stress and autophagy in goat Sertoli cells. Ecotoxicol. Environ. Saf. 2023, 252, 114571. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Feng, N.; Zheng, W.; Zheng, H.; Cai, P.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Zearalenone Exposure Disrupts Blood–Testis Barrier Integrity through Excessive Ca2+-Mediated Autophagy. Toxins 2021, 13, 875. [Google Scholar] [CrossRef]
- Kou, X.; Chen, N. Resveratrol as a Natural Autophagy Regulator for Prevention and Treatment of Alzheimer’s Disease. Nutrients 2017, 9, 927. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qin, S.; Zhou, Z.; Zeng, Q.; Long, R.; Mao, Z.; Dong, X.; Zhao, R.; Zhang, R.; et al. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem. Pharmacol. 2022, 202, 115139. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Sun, H.; Huang, Y.; Yao, H.; Zhao, C.; Wang, J.; Zhu, H. Resveratrol Protects Rat Ovarian Luteinized Granulosa Cells from H2O2-Induced Dysfunction by Activating Autophagy. Int. J. Mol. Sci. 2023, 24, 10914. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Liu, S.; Zhao, X.; Liu, H.; Zhou, T.; Wang, X.; Liu, H.; Tang, L.; Chen, H. Resveratrol inhibits autophagy against myocardial ischemia-reperfusion injury through the DJ-1/MEKK1/JNK pathway. Eur. J. Pharmacol. 2023, 951, 175748. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.-W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef] [PubMed]

| Cell/Animals | Treatments | Regulated Effects of RSV | References |
|---|---|---|---|
| Human endometrial stromal cells | Cells were treated with 50 μM ZEA for 48 h and then with RSV for 24 h. | ZEA exhibited its inhibitory action through nuclear translocation of ERα. ZEA exposure led to dampened progress of decidualization. RSV administration restored impaired decidualization process by induction of antioxidative gene glutathione peroxidase 3 (GPX3). | [80] |
| HEK293cells | HEK293 cells were first pretreated with 2 μM RSV for 24 h, and then the cells were treated with 80 μM ZEA for 24 h. | RSV alleviated ZEA-induced OS and apoptosis by recovering the activity of manganese superoxide dismutase (MnSOD), increased MMP and cell viability, decreased ROS, decreased the expression of pro-apoptotic genes, and activated the SIRT1/FOXO3a pathway. | [29] |
| Rat Sertoli cells | Sertoli cells were treated with 0, 5, 10, 20 μM ZEA and 5 μM RSV for 24 h. | The ZEA-induced cytotoxicity and decline in lactic acid production in SCs were alleviated by the use of RSV. | [31] |
| Porcine Oocyte | Porcine oocytes were exposed to 20 μM ZEA with or without 2 mM RSV. | RSV redressed ZEA-induced mitochondrial depolarization, OS, and apoptosis, and accelerated mitochondrial DNA copy during maturation, which improved embryonic development. | [83] |
| TM4 cells | TM4 cells were treated with 5 μM RSV and 20 μM ZEA for 24 h. | RSV pretreatment significantly reduced the expression of apoptosis-related proteins, increased cell viability, and reduced MDA and ROS levels by increasing Nrf2 nuclear translocation and HO-1 expression. RSV protected TM4 cells from ZEA-induced OS and apoptosis via PI3K/Akt-mediated activation of the Nrf2/HO-1 signaling pathway. | [82] |
| 5-week-old male BALB/c mice | Mice were orally administrated with RSV at 50,100, or 200 mg/kg BW with ZEA at 40 mg/kg BW for 12 days. After treatment, jejunum tissue and serum were collected for assessment. | RSV pretreatment significantly reduced serum DAO, and D-lactate levels altered intestinal morphology and markedly restored TJ protein levels, intestinal goblet cell number, and MUC-2 gene expression after ZEA challenge. RSV supplementation attenuates intestinal OS, inflammation, and intestinal mucosal barrier damage induced by ZEA exposure by modulating NF-κB and Nrf2/HO-1 signaling pathways. | [30] |
| Male Wistar rats (BW is in the range of 100–150 g) | Rats were injected intraperitoneally with 2 mg/kg ZEA once a week and gavaged with 5 mg/kg RSV daily for 3 weeks. Serum and liver and kidney tissues were collected. | RSV improves ZEA-induced alterations in serum biochemical markers (HGB, PLT, MCV, MPV, RSV), markers of OS (MDA, SOD), immunotoxicity (IgG, IgM), and biomarkers of DNA damage, prevents DNA damage, and regulates DNA repair. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Q.; Xu, W.; Yang, F.; Li, J.; Wei, W.; Chen, X.; Liu, Y.; Zhang, Z. Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review. Int. J. Mol. Sci. 2024, 25, 11003. https://doi.org/10.3390/ijms252011003
Lv Q, Xu W, Yang F, Li J, Wei W, Chen X, Liu Y, Zhang Z. Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review. International Journal of Molecular Sciences. 2024; 25(20):11003. https://doi.org/10.3390/ijms252011003
Chicago/Turabian StyleLv, Qiongxia, Wenjing Xu, Fan Yang, Jiahui Li, Wenjuan Wei, Xiaoguang Chen, Yumei Liu, and Ziqiang Zhang. 2024. "Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review" International Journal of Molecular Sciences 25, no. 20: 11003. https://doi.org/10.3390/ijms252011003
APA StyleLv, Q., Xu, W., Yang, F., Li, J., Wei, W., Chen, X., Liu, Y., & Zhang, Z. (2024). Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review. International Journal of Molecular Sciences, 25(20), 11003. https://doi.org/10.3390/ijms252011003







