Post-Meningitic Syndrome: Pathophysiology and Consequences of Streptococcal Infections on the Central Nervous System
Abstract
:1. Introduction
2. Pathophysiology of Streptococcal Meningitis
2.1. A Brief Overview of Streptococcal Infections
2.2. Group A Streptococci
2.3. Group B Streptococci
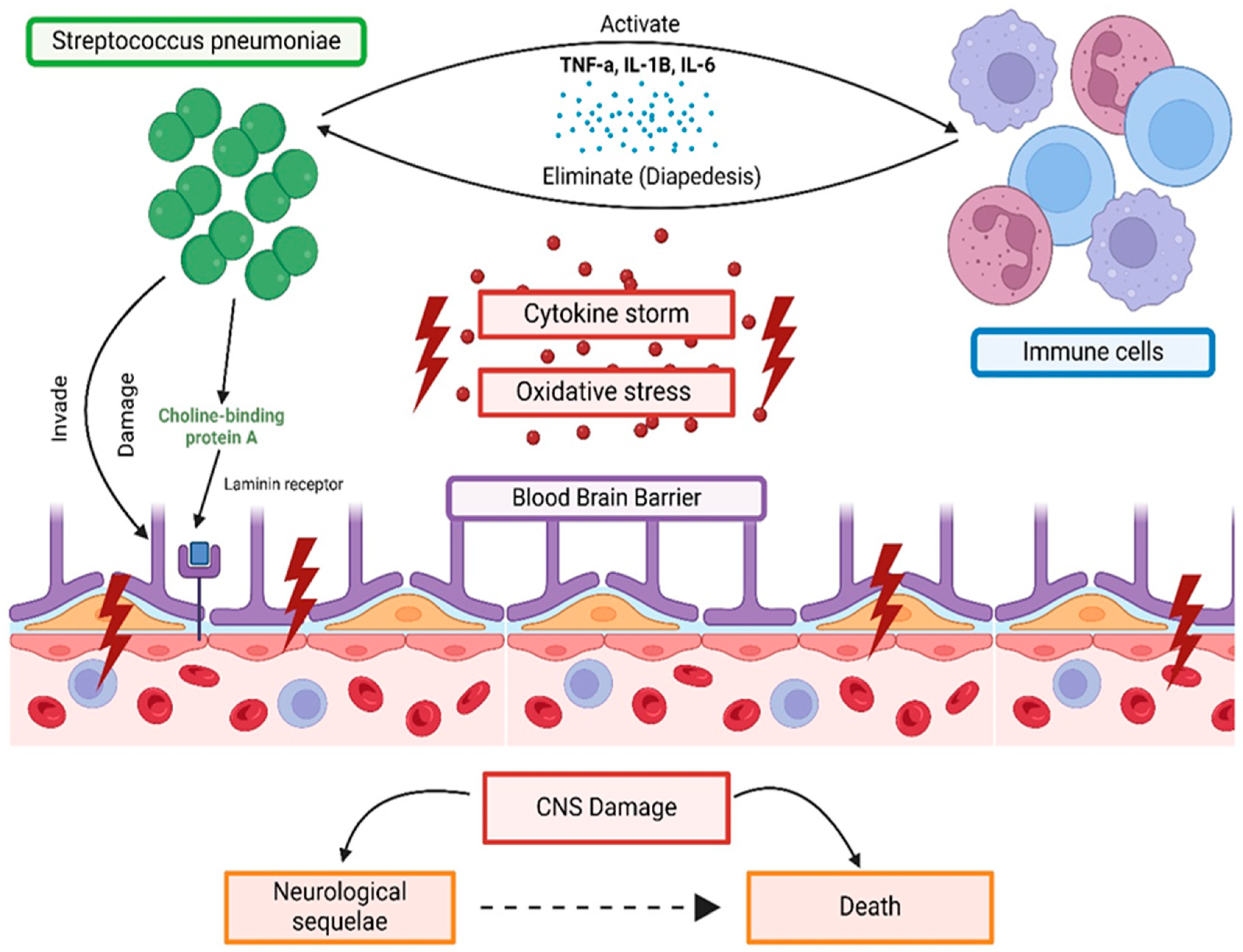
3. Mechanism of Streptococcus Species CNS Invasion and BBB Involvement
Immune Response to Streptococcal Meningitis
4. Epidemiological Evidence Showcasing Manifestations of Multisystem Dysfunction
5. Neurological Complications
5.1. Heterogeneity of Neurological Symptoms
5.2. Short-Term Neurological Complications
5.2.1. Short-Term Complications in Children
5.2.2. Short-Term Complications in Adults
5.2.3. Risk Factors for Complications
5.3. Long-Term Neurological Complications
5.3.1. Neurological Sequela: Overview
Cognitive Impairment
5.3.2. Motor and Sensory Deficits
Overview of Motor and Sensory Deficits
Motor Sequelae in Adult and Pediatric Pneumococcal Meningitis
Meningococcal Meningitis Motor Sequelae in Adults and Pediatrics
Sensory Losses and Pathophysiology of Hearing Loss
- Meningococcal meningitis and sensory loss
- Pediatric streptococcal meningitis and sensory loss
6. Psychosocial and Behavioral Implications
7. Diagnostic Challenges and Monitoring: Challenges in Identifying Long-Term Complications
8. Therapeutic Approaches and Interventions
9. Future Recommendations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sharew, A.; Bodilsen, J.; Hansen, B.R.; Nielsen, H.; Brandt, C.T. The cause of death in bacterial meningitis. BMC Infect. Dis. 2020, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Hersi, K.; Gonzalez, F.J.; Kondamudi, N.P. Meningitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459360/ (accessed on 1 August 2024).
- Hasbun, R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA 2022, 328, 2147–2154. [Google Scholar] [CrossRef]
- GBD 2019 Meningitis Antimicrobial Resistance Collaborators. Global, regional, and national burden of meningitis and its aetiologies, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 685–711. [Google Scholar] [CrossRef]
- Johansson Kostenniemi, U.; Bazan, A.; Karlsson, L.; Silfverdal, S.A. Psychiatric Disabilities and Other Long-term Consequences of Childhood Bacterial Meningitis. Pediatr. Infect. Dis. J. 2021, 40, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schiess, N.; Groce, N.E.; Dua, T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms 2021, 9, 900. [Google Scholar] [CrossRef]
- Mohanty, S.; Johansson Kostenniemi, U.; Silfverdal, S.A.; Salomonsson, S.; Iovino, F.; Sarpong, E.M.; Bencina, G.; Bruze, G. Increased Risk of Long-Term Disabilities Following Childhood Bacterial Meningitis in Sweden. JAMA Netw. Open 2024, 7, e2352402. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Creti, R. Have group A and B streptococcal infections become neglected diseases in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1063–1064. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Fan, S.; Duan, N.; Chen, W.; Zhao, X.; Wang, L.; Du, P.; Guo, J. Genomic Epidemiology of Streptococcus Pneumoniae Isolated in a Tertiary Hospital in Beijing, China, from 2018 to 2022. Pathogens 2023, 12, 284. [Google Scholar] [CrossRef]
- Slotved, H.C.; Hoffmann, S. The Epidemiology of Invasive Group B Streptococcus in Denmark from 2005 to 2018. Front. Public Health 2020, 8, 40. [Google Scholar] [CrossRef]
- Beres, S.B.; Zhu, L.; Pruitt, L.; Olsen, R.J.; Faili, A.; Kayal, S.; Musser, J.M. Integrative Reverse Genetic Analysis Identifies Polymorphisms Contributing to Decreased Antimicrobial Agent Susceptibility in Streptococcus pyogenes. mBio 2022, 13, e0361821. [Google Scholar] [CrossRef] [PubMed]
- Caufield, P.W.; Schön, C.N.; Saraithong, P.; Li, Y.; Argimón, S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015, 94, 110s–118s. [Google Scholar] [CrossRef] [PubMed]
- Raabe Vanessa, N.; Shane Andi, L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Newberger, R.; Gupta, V. Streptococcus Group A. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559240/ (accessed on 1 August 2024).
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.D.; Daw, J.; Xu, R.; Enkel, S.; Pickering, J.; McRae, T.; Engel, M.E.; Carapetis, J.; Wyber, R.; Bowen, A.C. Modes of transmission and attack rates of group A Streptococcal infection: A protocol for a systematic review and meta-analysis. Syst. Rev. 2021, 10, 90. [Google Scholar] [CrossRef]
- Hanna, M.; Noor, A. Streptococcus Group B. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553143/ (accessed on 1 August 2024).
- Morgan, J.A.; Zafar, N.; Cooper, D.B. Group B Streptococcus and Pregnancy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482443/ (accessed on 1 August 2024).
- Wierzbicki, I.H.; Campeau, A.; Dehaini, D.; Holay, M.; Wei, X.; Greene, T.; Ying, M.; Sands, J.S.; Lamsa, A.; Zuniga, E.; et al. Group A Streptococcal S Protein Utilizes Red Blood Cells as Immune Camouflage and Is a Critical Determinant for Immune Evasion. Cell Rep. 2019, 29, 2979–2989.e2915. [Google Scholar] [CrossRef]
- Kolar, S.L.; Kyme, P.; Tseng, C.W.; Soliman, A.; Kaplan, A.; Liang, J.; Nizet, V.; Jiang, D.; Murali, R.; Arditi, M.; et al. Group B Streptococcus Evades Host Immunity by Degrading Hyaluronan. Cell Host Microbe 2015, 18, 694–704. [Google Scholar] [CrossRef]
- Kanwal, S.; Vaitla, P. Streptococcus Pyogenes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554528/ (accessed on 1 August 2024).
- Tavares, T.; Pinho, L.; Bonifácio Andrade, E. Group B Streptococcal Neonatal Meningitis. Clin. Microbiol. Rev. 2022, 35, e0007921. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 118. [Google Scholar] [CrossRef]
- Brooks, L.R.K.; Mias, G.I. Streptococcus Pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Yau, B.; Hunt, N.H.; Mitchell, A.J.; Too, L.K. Blood–Brain Barrier Pathology and CNS Outcomes in Streptococcus PneumoniaeMeningitis. Int. J. Mol. Sci. 2018, 19, 3555. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef]
- Gil, E.; Wall, E.; Noursadeghi, M.; Brown, J.S. Streptococcus Pneumoniaemeningitis and the CNS barriers. Front. Cell Infect. Microbiol. 2022, 12, 1106596. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- van Sorge, N.M.; Doran, K.S. Defense at the border: The blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012, 7, 383–394. [Google Scholar] [CrossRef]
- Thorsdottir, S.; Henriques-Normark, B.; Iovino, F. The Role of Microglia in Bacterial Meningitis: Inflammatory Response, Experimental Models and New Neuroprotective Therapeutic Strategies. Front. Microbiol. 2019, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Herold, R.; Schroten, H.; Schwerk, C. Virulence Factors of Meningitis-Causing Bacteria: Enabling Brain Entry across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 5393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Chu, J.M.T.; Chang, R.C.C.; Wong, G.T.C. The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules 2022, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, K.H.; Wu, H.P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef]
- van de Beek, D.; Brouwer, M.; Hasbun, R.; Koedel, U.; Whitney, C.G.; Wijdicks, E. Community-acquired bacterial meningitis. Nat. Rev. Dis. Primers 2016, 2, 16074. [Google Scholar] [CrossRef]
- Ribes, S.; Nessler, S.; Heide, E.C.; Malzahn, D.; Perske, C.; Brück, W.; Nau, R. The Early Adaptive Immune Response in the Pathophysiological Process of Pneumococcal Meningitis. J. Infect. Dis. 2017, 215, 150–158. [Google Scholar] [CrossRef]
- Engelen-Lee, J.Y.; Brouwer, M.C.; Aronica, E.; van de Beek, D. Pneumococcal meningitis: Clinical-pathological correlations (MeninGene-Path). Acta Neuropathol. Commun. 2016, 4, 26. [Google Scholar] [CrossRef]
- Kastenbauer, S.; Pfister, H.W. Streptococcal meningitis in adults: Spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003, 126, 1015–1025. [Google Scholar] [CrossRef]
- Kruckow, K.L.; Zhao, K.; Bowdish, D.M.E.; Orihuela, C.J. Acute organ injury and long-term sequelae of severe pneumococcal infections. Pneumonia 2023, 15, 5. [Google Scholar] [CrossRef]
- Martín-Cerezuela, M.; Aseginolaza-Lizarazu, M.; Boronat-García, P.; Asensio-Martín, M.J.; Alamán-Laguarda, G.; Álvarez-Lerma, F.; Roa-Alonso, D.; Socias, L.; Vera-Artázcoz, P.; Ramírez-Galleymore, P. Severe community-acquired Streptococcus Pneumoniaebacterial meningitis: Clinical and prognostic picture from the intensive care unit. Crit. Care 2023, 27, 72. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Bedoui, Y.; Vagner, D.; Raffray, L.; Ah-Pine, F.; Doray, B.; Gasque, P. Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2022, 23, 9274. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.J.; Brouwer, M.C.; van der Ende, A.; van de Beek, D. Endocarditis in adults with bacterial meningitis. Circulation 2013, 127, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, Y.; Ito, T.; Nakahara, M.; Yamaguchi, K.; Yasuda, T. Sepsis-induced myocardial dysfunction: Pathophysiology and management. J. Intensive Care 2016, 4, 22. [Google Scholar] [CrossRef]
- Hirose, K.; Li, S.Z.; Gill, R.; Hartsock, J. Streptococcal meningitis Induces Hearing Loss and Cochlear Ossification Modulated by Chemokine Receptors CX3CR1 and CCR2. J. Assoc. Res. Otolaryngol. 2024, 25, 179–199. [Google Scholar] [CrossRef]
- Mańdziuk, J.; Kuchar, E.P. Streptococcal Meningitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554448/ (accessed on 1 August 2024).
- Østergaard, C.; Konradsen, H.B.; Samuelsson, S. Clinical presentation and prognostic factors of Streptococcus Pneumoniaemeningitis according to the focus of infection. BMC Infect. Dis. 2005, 5, 93. [Google Scholar] [CrossRef]
- Klobassa, D.S.; Zoehrer, B.; Paulke-Korinek, M.; Gruber-Sedlmayr, U.; Pfurtscheller, K.; Strenger, V.; Sonnleitner, A.; Kerbl, R.; Ausserer, B.; Arocker, W.; et al. The burden of streptococcal meningitis in Austrian children between 2001 and 2008. Eur. J. Pediatr. 2014, 173, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.W.; Feiden, W.; Einhäupl, K.M. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch. Neurol. 1993, 50, 575–581. [Google Scholar] [CrossRef]
- Bor, M.; Çokuğraş, H. Factors associated with early complications in inpatients who were treated in our clinic between 1992 and 2011 with a diagnosis of acute bacterial meningitis. Turk. Pediatri. Ars. 2020, 55, 149–156. [Google Scholar] [CrossRef]
- Kloek, A.T.; Brouwer, M.C.; Schmand, B.; Tanck, M.W.T.; van de Beek, D. Long-term neurologic and cognitive outcome and quality of life in adults after pneumococcal meningitis. Clin. Microbiol. Infect. 2020, 26, 1361–1367. [Google Scholar] [CrossRef]
- Jit, M. The risk of sequelae due to streptococcal meningitis in high-income countries: A systematic review and meta-analysis. J. Infect. 2010, 61, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Weisfelt, M.; van de Beek, D.; Spanjaard, L.; Reitsma, J.B.; de Gans, J. Clinical features, complications, and outcome in adults with pneumococcal meningitis: A prospective case series. Lancet Neurol. 2006, 5, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.J.; Brouwer, M.C.; van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 2016, 73, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Heckenberg, S.G.B.; de Gans, J.; Brouwer, M.C.; Weisfelt, M.; Piet, J.R.; Spanjaard, L.; van der Ende, A.; van de Beek, D. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: A prospective cohort study. Medicine 2008, 87, 185–192. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Generoso, J.S.; Collodel, A.; Dominguini, D.; Faller, C.J.; Tardin, F.; Bhatti, G.S.; Petronilho, F.; Dal-Pizzol, F.; Barichello, T. Receptor for Advanced Glycation End Products (RAGE) Mediates Cognitive Impairment Triggered by Pneumococcal Meningitis. Neurotherapeutics 2021, 18, 640–653. [Google Scholar] [CrossRef]
- Hoogman, M.; van de Beek, D.; Weisfelt, M.; de Gans, J.; Schmand, B. Cognitive outcome in adults after bacterial meningitis. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1092–1096. [Google Scholar] [CrossRef]
- Free, S.L.; Li, L.M.; Fish, D.R.; Shorvon, S.D.; Stevens, J.M. Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 1996, 37, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Schmidt, H.; Heimann, B.; Djukic, M.; Mazurek, C.; Fels, C.; Wallesch, C.W.; Nau, R. Neuropsychological sequelae of bacterial and viral meningitis. Brain 2006, 129, 333–345. [Google Scholar] [CrossRef]
- Tabusi, M.; Thorsdottir, S.; Lysandrou, M.; Narciso, A.R.; Minoia, M.; Srambickal, C.V.; Widengren, J.; Henriques-Normark, B.; Iovino, F. Neuronal death in streptococcal meningitis is triggered by pneumolysin and RrgA interactions with β-actin. PLoS Pathog. 2021, 17, e1009432. [Google Scholar] [CrossRef]
- Stockmann, C.; Ampofo, K.; Byington, C.L.; Filloux, F.; Hersh, A.L.; Blaschke, A.J.; Cowan, P.; Korgenski, K.; Mason, E.O.; Pavia, A.T. Streptococcal meningitis in children: Epidemiology, serotypes, and outcomes from 1997–2010 in Utah. Pediatrics 2013, 132, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef]
- Heckenberg, S.G.; Brouwer, M.C.; van der Ende, A.; van de Beek, D. Adjunctive dexamethasone in adults with meningococcal meningitis. Neurology 2012, 79, 1563–1569. [Google Scholar] [CrossRef]
- Rasmussen, N.; Johnsen, N.J.; Bohr, V.A. Otologic sequelae after pneumococcal meningitis: A survey of 164 consecutive cases with a follow-up of 94 survivors. Laryngoscope 1991, 101, 876–882. [Google Scholar] [CrossRef]
- Weightman, N.C.; Sajith, J. Incidence and outcome of streptococcal meningitis in northern England. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Duval, X.; Taha, M.K.; Lamaury, I.; Escaut, L.; Gueit, I.; Manchon, P.; Tubiana, S.; Hoen, B. One-Year Sequelae and Quality of Life in Adults with Meningococcal Meningitis: Lessons from the COMBAT Multicentre Prospective Study. Adv. Ther. 2022, 39, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef]
- Al-Janabi, H.; Van Exel, J.; Brouwer, W.; Trotter, C.; Glennie, L.; Hannigan, L.; Coast, J. Measuring Health Spillovers for Economic Evaluation: A Case Study in Meningitis. Health Econ. 2016, 25, 1529–1544. [Google Scholar] [CrossRef]
- Zeevat, F.; Simons, J.J.M.; Westra, T.A.; Wilschut, J.C.; van Sorge, N.M.; Boersma, C.; Postma, M.J. Cost of Illness Analysis of Invasive Meningococcal Disease Caused by Neisseria Meningitidis Serogroup B in the Netherlands-a Holistic Approach. Infect. Dis. Ther. 2024, 13, 481–499. [Google Scholar] [CrossRef]
- Scanferla, E.; Fasse, L.; Gorwood, P. Subjective experience of meningitis survivors: A transversal qualitative study using interpretative phenomenological analysis. BMJ Open 2020, 10, e037168. [Google Scholar] [CrossRef]
- Schmidt, H.; Cohrs, S.; Heinemann, T.; Goerdt, C.; Djukic, M.; Heimann, B.; Wallesch, C.W.; Nau, R. Sleep disorders are long-term sequelae of both bacterial and viral meningitis. J. Neurol. Neurosurg. Psychiatry 2006, 77, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.J.; Müller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic Review of Invasive Meningococcal Disease: Sequelae and Quality of Life Impact on Patients and Their Caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Confederation of Meningitis Organisations. Available online: https://www.comomeningitis.org/ (accessed on 1 June 2024).
- Ocampo, F.F.; Espiritu, A.I.; Jamora, R.D.G. Current status and challenges in the care of patients with bacterial meningitis in the Philippines: A scoping review. Trop. Med. Int. Health 2022, 27, 38–48. [Google Scholar] [CrossRef]
- Seitz, D.P.; Chan, C.C.; Newton, H.T.; Gill, S.S.; Herrmann, N.; Smailagic, N.; Nikolaou, V.; Fage, B.A. Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a primary care setting. Cochrane Database Syst. Rev. 2018, 2, Cd011415. [Google Scholar] [CrossRef] [PubMed]
- Rayi, A.; Murr, N.I. Electroencephalogram. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563295/ (accessed on 1 August 2024).
- Bansal, M.; Shah, A.; Gosai, B.; Shah, P. A Novel 3-Step Tuning Fork Hearing Test; Preliminary Report on Its Clinical Utility. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74, 234–241. [Google Scholar] [CrossRef]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M.P.; Gonzenbach, R.; Kesselring, J. Neurorehabilitation: Applied neuroplasticity. J. Neurol. 2017, 264, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.F.; Seth, N.H.; Deodhe, N.P. Scope of an Integrative Neurophysiotherapy Approach in Achieving Gross Motor Milestones in a Child with Meningitis: A Case Report. Cureus 2023, 15, e49540. [Google Scholar] [CrossRef]
- Rehabilitation. World Health Organization 22 April 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 1 July 2024).
- Barman, A.; Chatterjee, A.; Bhide, R. Cognitive Impairment and Rehabilitation Strategies After Traumatic Brain Injury. Indian J. Psychol. Med. 2016, 38, 172–181. [Google Scholar] [CrossRef]
- Greenwood, B.; Sow, S.; Preziosi, M.-P. Defeating meningitis by 2030—An ambitious target. Trans. R. Soc. Trop. Med. Hygiene 2021, 115, 1099–1101. [Google Scholar] [CrossRef]


| Therapeutic Approach | Focus | Interventions |
|---|---|---|
| Physical Therapy | Muscle strength and mobility | Exercises targeting muscle recovery, mobility improvement, and prevention of secondary complications. Early integrative neurophysiotherapy is especially successful in children. |
| Occupational Therapy | Daily living skills | Training patients in self-care activities, fine motor skills, and adaptive techniques to support independence. |
| Speech Therapy | Communication and swallowing | Exercises for improving speech production, articulation, language comprehension, and swallowing challenges. |
| Cognitive Rehabilitation | Memory, attention, and problem solving | Cognitive tasks and exercises to restore memory, attention, problem solving, and executive functioning skills. |
| Psychological Support | Coping with emotional issues | Psychological counseling, emotional support, and education to assist patients in managing stress, anxiety, or depression following neurological complications. |
| Neuroplasticity-focused Therapy | Brain reorganization and motor recovery | Targeted exercises, sensory stimulation, and cognitive tasks designed to promote brain rewiring and restoration of motor and cognitive skills. |
| Early Intervention (Children) | Motor and developmental skills | Goal-oriented approaches to improve mobility and coordination in children through early physiotherapy management and stimulation techniques. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaddoura, R.; Abdalbari, K.; Kadom, M.; Badla, B.A.; Hijleh, A.A.; Hanifa, M.; AlAshkar, M.; Asbaita, M.; Othman, D.; Faraji, H.; et al. Post-Meningitic Syndrome: Pathophysiology and Consequences of Streptococcal Infections on the Central Nervous System. Int. J. Mol. Sci. 2024, 25, 11053. https://doi.org/10.3390/ijms252011053
Kaddoura R, Abdalbari K, Kadom M, Badla BA, Hijleh AA, Hanifa M, AlAshkar M, Asbaita M, Othman D, Faraji H, et al. Post-Meningitic Syndrome: Pathophysiology and Consequences of Streptococcal Infections on the Central Nervous System. International Journal of Molecular Sciences. 2024; 25(20):11053. https://doi.org/10.3390/ijms252011053
Chicago/Turabian StyleKaddoura, Rachid, Karim Abdalbari, Mhmod Kadom, Beshr Abdulaziz Badla, Amin Abu Hijleh, Mohamed Hanifa, Masa AlAshkar, Mohamed Asbaita, Deema Othman, Hanan Faraji, and et al. 2024. "Post-Meningitic Syndrome: Pathophysiology and Consequences of Streptococcal Infections on the Central Nervous System" International Journal of Molecular Sciences 25, no. 20: 11053. https://doi.org/10.3390/ijms252011053
APA StyleKaddoura, R., Abdalbari, K., Kadom, M., Badla, B. A., Hijleh, A. A., Hanifa, M., AlAshkar, M., Asbaita, M., Othman, D., Faraji, H., AlBakri, O., Tahlak, S., Hijleh, A. A., Kabbani, R., Resen, M., Abdalbari, H., Du Plessis, S. S., & Omolaoye, T. S. (2024). Post-Meningitic Syndrome: Pathophysiology and Consequences of Streptococcal Infections on the Central Nervous System. International Journal of Molecular Sciences, 25(20), 11053. https://doi.org/10.3390/ijms252011053





