Tegaserod Stimulates 5-HT4 Serotonin Receptors in the Isolated Human Atrium
Abstract
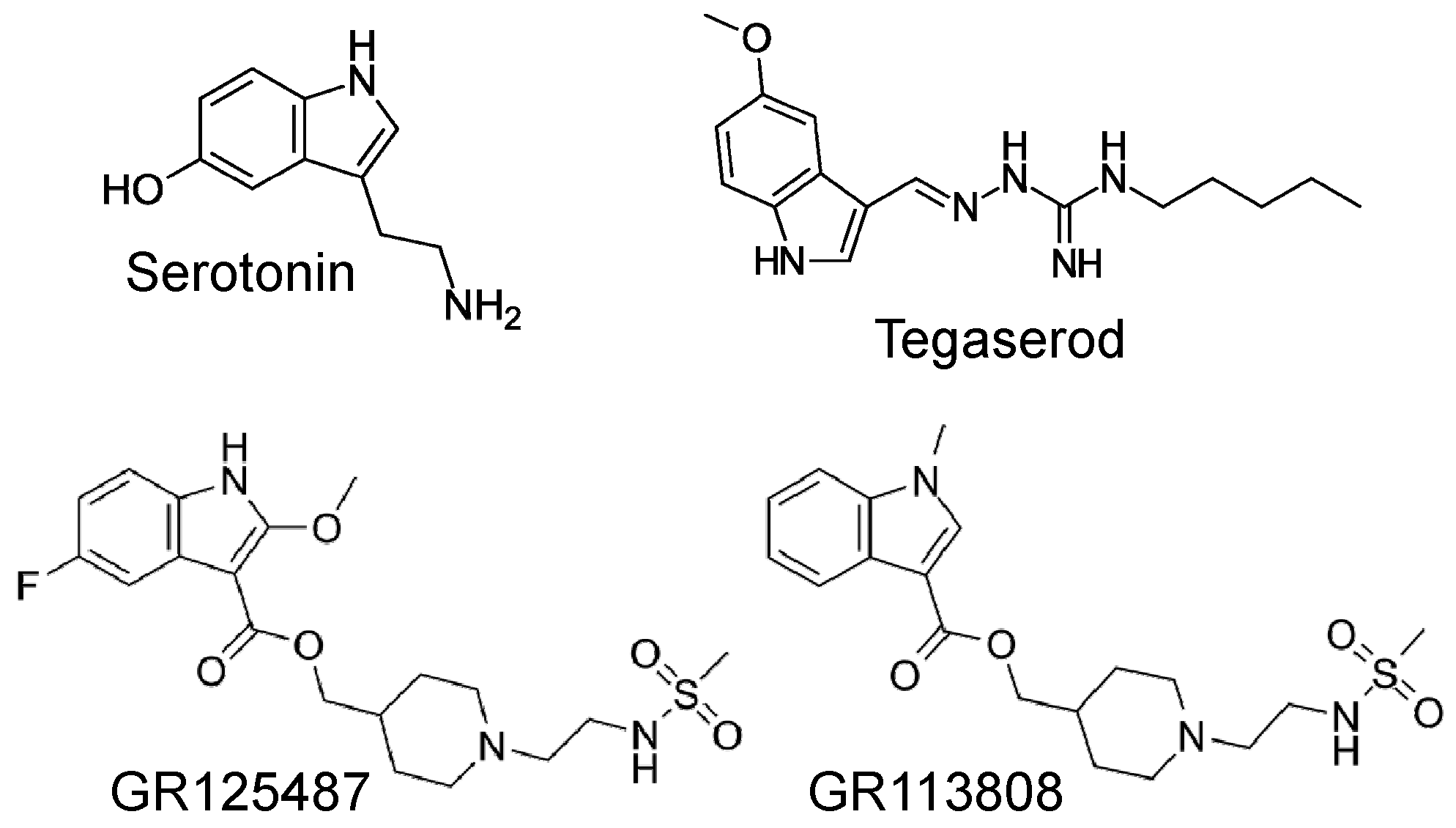
1. Introduction
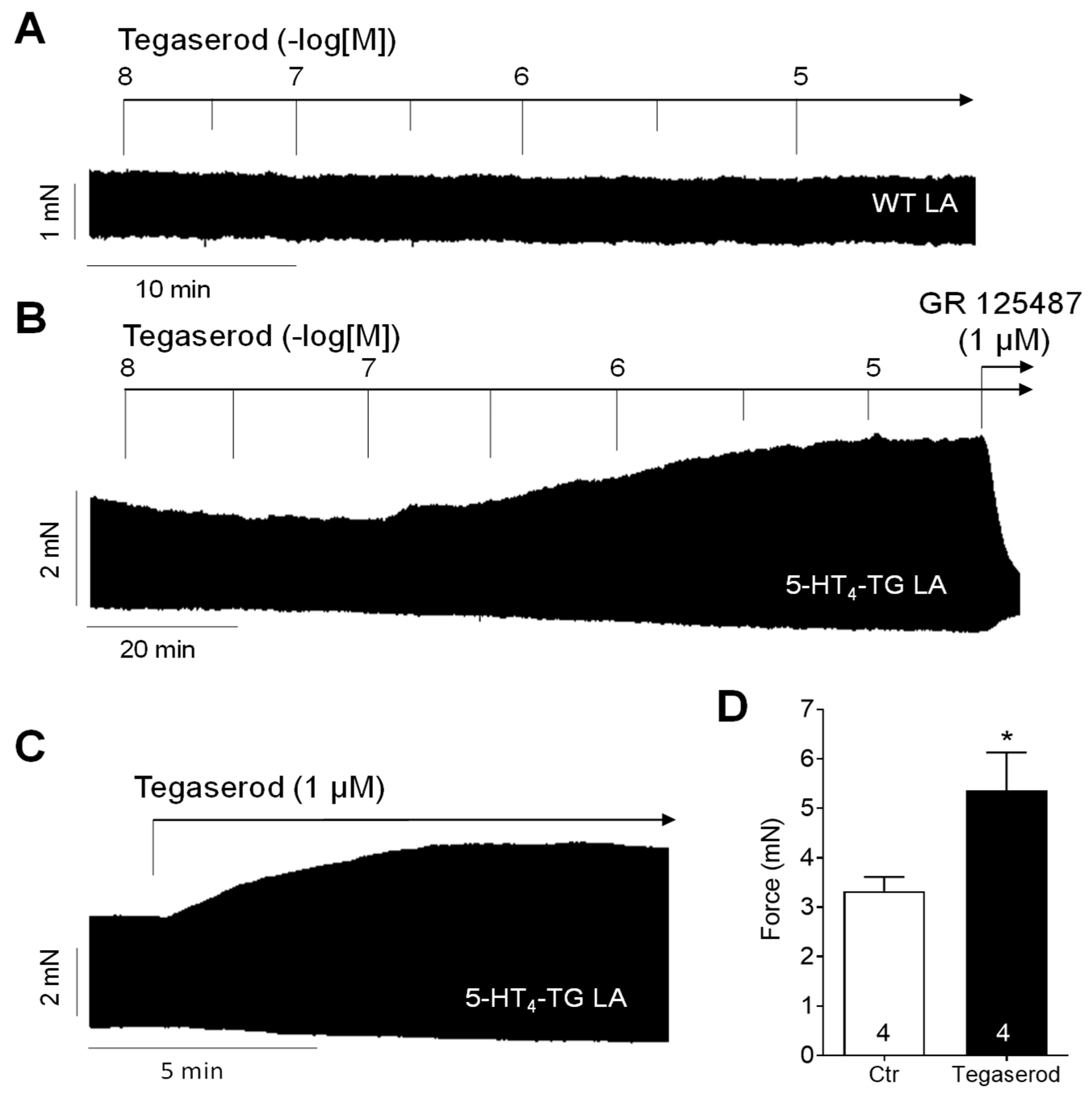
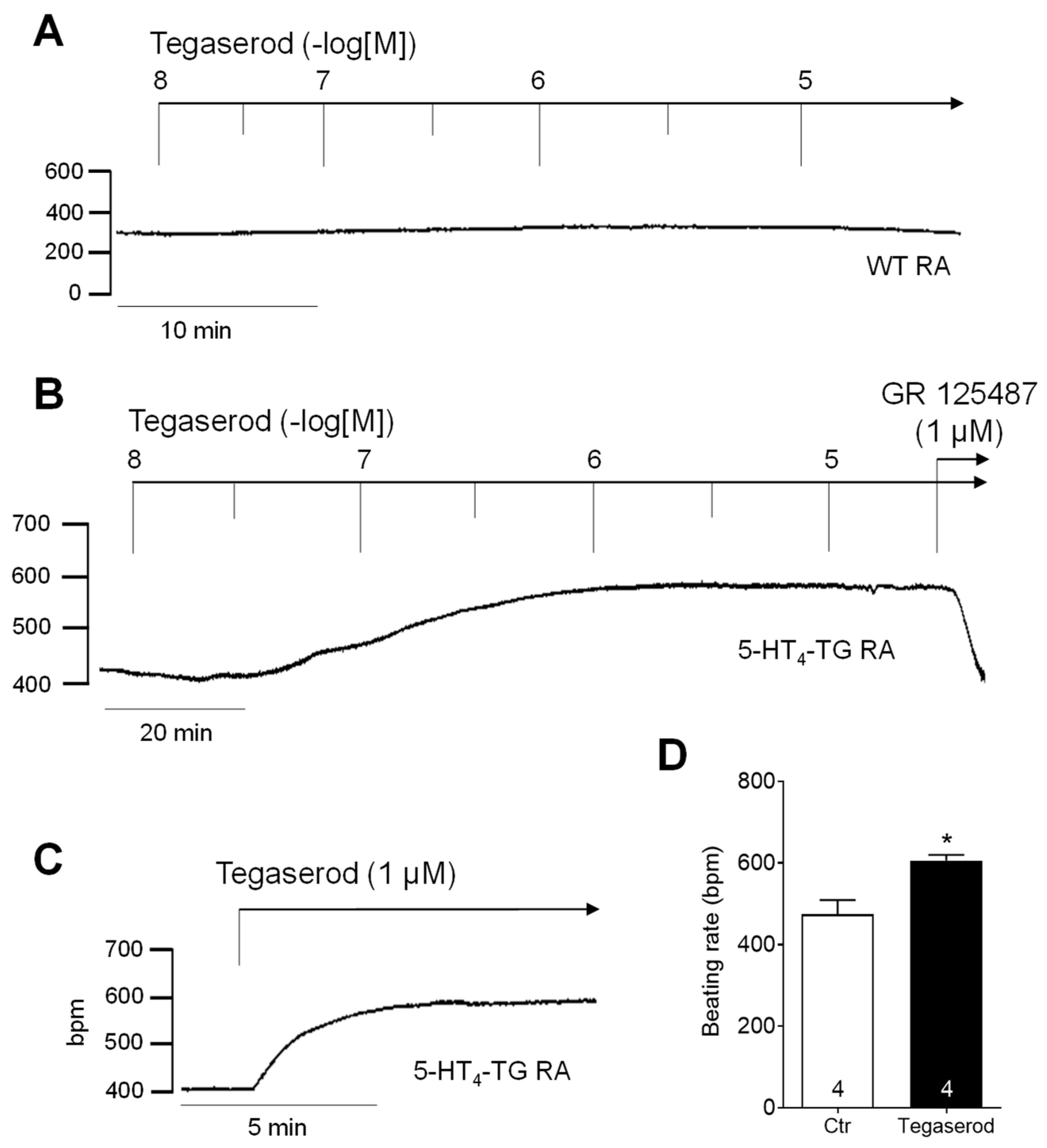
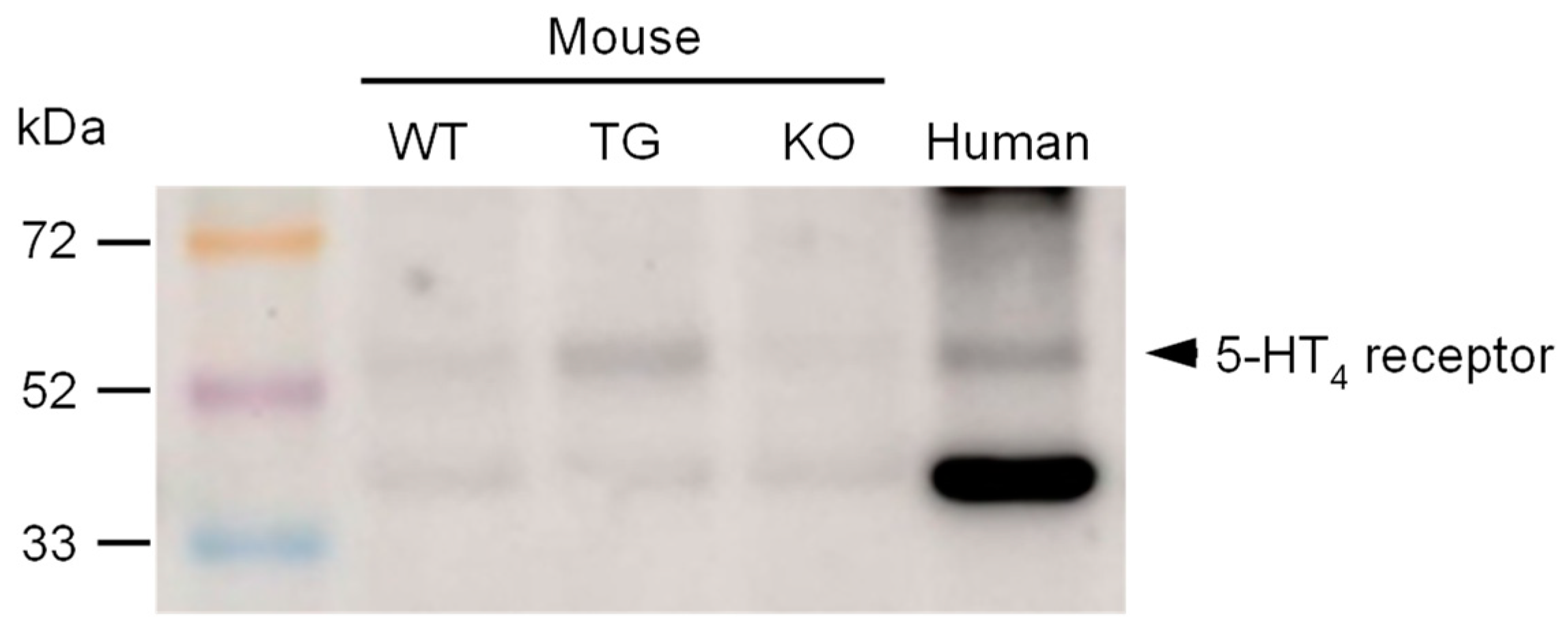
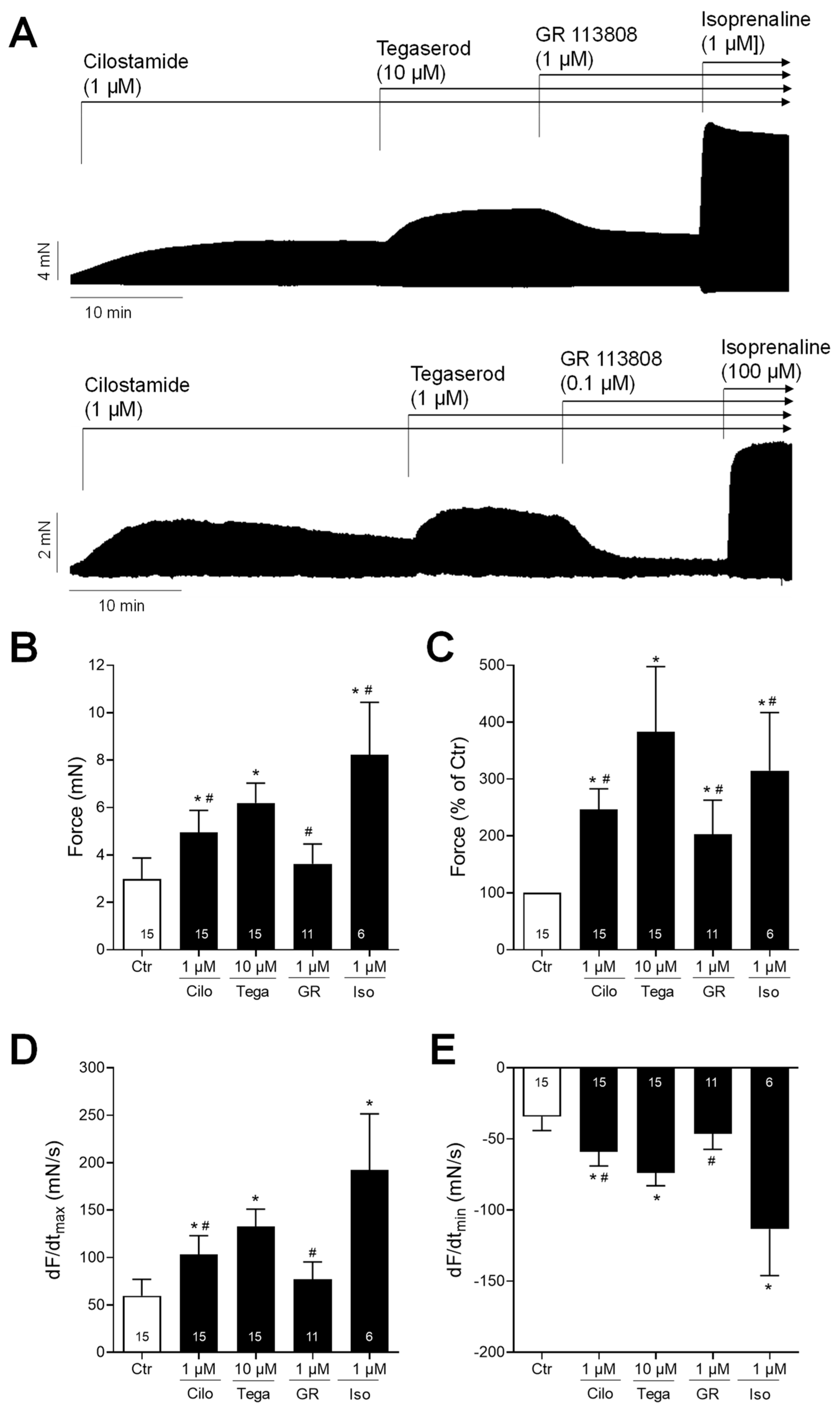
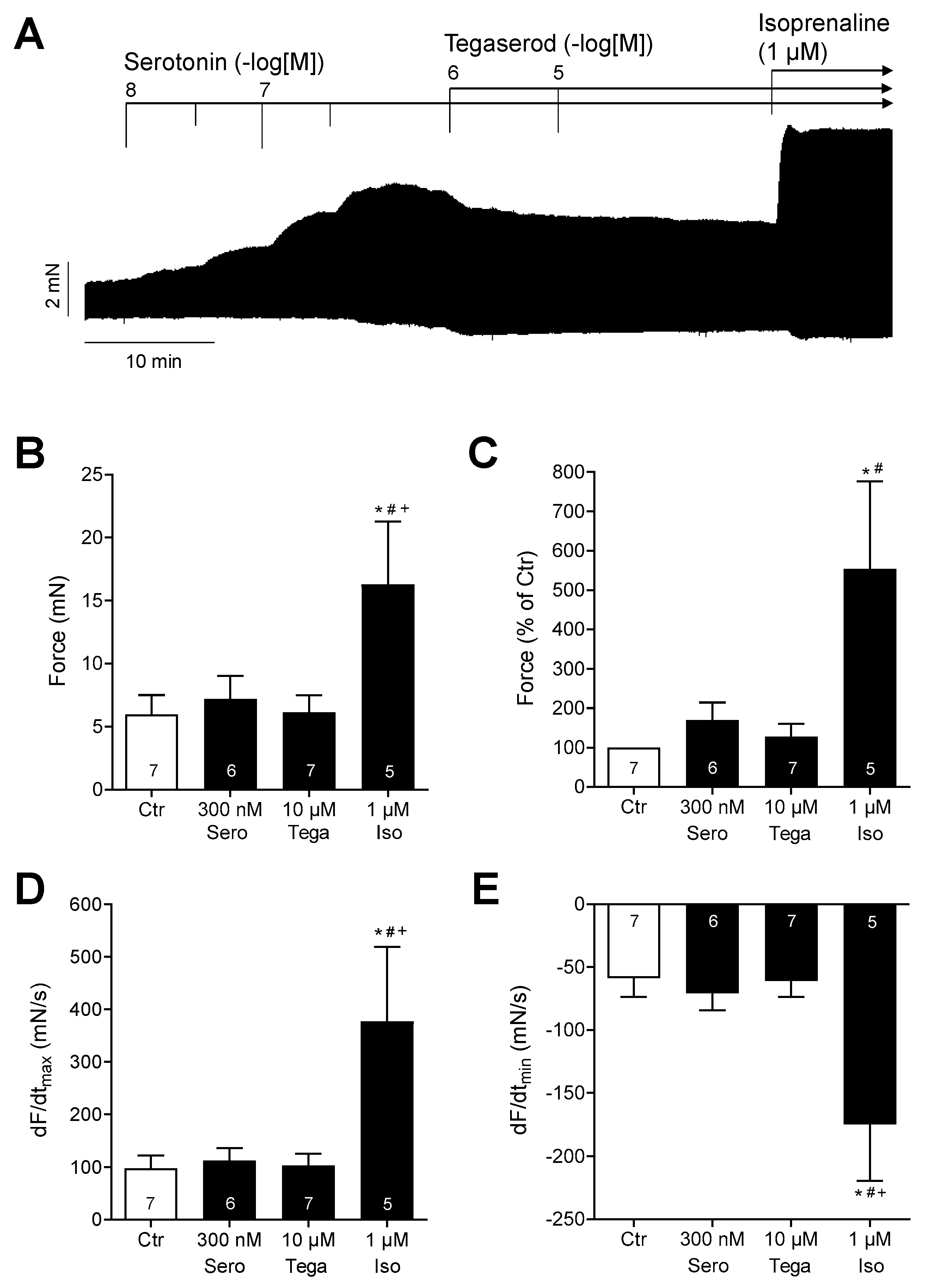
2. Results
3. Discussion
3.1. Cardiac Effects of Tegaserod in Different Models
3.2. Mechanism of Tegaserod
3.3. Role of Phosphorylation of Regulatory Proteins
3.4. Practical Aspects of Using Tegaserod
3.5. Limitations
4. Materials and Methods
4.1. Mouse Models
4.1.1. 5-HT4-TG Mice
4.1.2. 5-HT4R-KO Mice
4.2. Contractile Studies on Mouse Preparations
4.3. Contractile Studies on Human Preparations
4.4. Western Blotting
4.5. Data Analysis
4.6. Drugs and Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appel, S.; Kumle, A.; Hubert, M.; Duvauchelle, T. First pharmacokinetic-pharmacodynamic study in humans with a selective 5-hydroxytryptamine4 receptor agonist. J. Clin. Pharmacol. 1997, 37, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Beglinger, C. Tegaserod: A novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int. J. Clin. Pract. 2002, 56, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Madia, V.N.; Messore, A.; Saccoliti, F.; Tudino, V.; De Leo, A.; De Vita, D.; Bortolami, M.; Scipione, L.; Pindinello, I.; Costi, R.; et al. Tegaserod for the Treatment of Irritable Bowel Syndrome. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 342–369. [Google Scholar] [CrossRef] [PubMed]
- Morganroth, J.; Rüegg, P.C.; Dunger-Baldauf, C.; Appel-Dingemanse, S.; Bliesath, H.; Lefkowitz, M. Tegaserod, a 5-hydroxytryptamine type 4 receptor partial agonist, is devoid of electrocardiographic effects. Am. J. Gastroenterol. 2002, 97, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.T.; Higgins, D.L.; Ero, M.P.; Amagasu, S.M.; Vickery, R.G.; Kersey, K.; Hopkins, A.; Smith, J.A.M. An in vitro investigation of the cardiovascular effects of the 5-HT(4) receptor selective agonists, velusetrag and TD-8954. Vasc. Pharmacol. 2013, 58, 150–156. [Google Scholar] [CrossRef]
- Thompson, C.A. Novartis suspends tegaserod sales at FDA’s request. Am. J. Health Syst. Pharm. 2007, 64, 1020. [Google Scholar] [CrossRef]
- Sayuk, G.S.; Tack, J. Tegaserod: What’s Old Is New Again. Clin. Gastroenterol. Hepatol. 2022, 20, 2175–2184.e19. [Google Scholar] [CrossRef]
- Kaumann, A.J. Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharmacol. Sci. 1994, 15, 451–455. [Google Scholar] [CrossRef]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4765. [Google Scholar] [CrossRef]
- Tack, J.; Camilleri, M.; Chang, L.; Chey, W.D.; Galligan, J.J.; Lacy, B.E.; Müller-Lissner, S.; Quigley, E.M.M.; Schuurkes, J.; de Maeyer, J.H.; et al. Systematic review: Cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment. Pharmacol. Ther. 2012, 35, 745–767. [Google Scholar] [CrossRef]
- Smith, J.A.M.; Beattie, D.T.; Marquess, D.; Shaw, J.P.; Vickery, R.G.; Humphrey, P.P.A. The in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kaumann, A.J. Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn Schmiedebergs. Arch. Pharmacol. 1990, 342, 619–622. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Sanders, L.; Brown, A.M.; Murray, K.J.; Brown, M.J. A 5-HT4-like receptor in human right atrium. Naunyn Schmiedebergs. Arch. Pharmacol. 1991, 344, 150–159. [Google Scholar] [CrossRef]
- Sanders, L.; Kaumann, A.J. A 5-HT4-like receptor in human left atrium. Naunyn Schmiedebergs. Arch. Pharmacol. 1992, 345, 382–386. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Levy, F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef]
- Afzal, F.; Andressen, K.W.; Mørk, H.K.; Aronsen, J.M.; Sjaastad, I.; Dahl, C.P.; Skomedal, T.; Levy, F.O.; Osnes, J.-B.; Qvigstad, E. 5-HT4-elicited positive inotropic response is mediated by cAMP and regulated by PDE3 in failing rat and human cardiac ventricles. Br. J. Pharmacol. 2008, 155, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Brattelid, T.; Qvigstad, E.; Lynham, J.A.; Molenaar, P.; Aass, H.; Geiran, O.; Skomedal, T.; Osnes, J.-B.; Levy, F.O.; Kaumann, A.J. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn Schmiedebergs. Arch. Pharmacol. 2004, 370, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Brattelid, T.; Qvigstad, E.; Moltzau, L.R.; Bekkevold, S.V.S.; Sandnes, D.L.; Birkeland, J.A.K.; Skomedal, T.; Osnes, J.-B.; Sjaastad, I.; Levy, F.O. The cardiac ventricular 5-HT4 receptor is functional in late foetal development and is reactivated in heart failure. PLoS ONE 2012, 7, e45489. [Google Scholar] [CrossRef]
- Medhurst, A.D.; Kaumann, A.J. Characterization of the 5-HT4 receptor mediating tachycardia in piglet isolated right atrium. Br. J. Pharmacol. 1993, 110, 1023–1030. [Google Scholar] [CrossRef]
- Gergs, U.; Baumann, M.; Böckler, A.; Buchwalow, I.B.; Ebelt, H.; Fabritz, L.; Hauptmann, S.; Keller, N.; Kirchhof, P.; Klöckner, U.; et al. Cardiac overexpression of the human 5-HT4 receptor in mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H788–H798. [Google Scholar] [CrossRef]
- Keller, N.; Dhein, S.; Neumann, J.; Gergs, U. Cardiovascular effects of cisapride and prucalopride on human 5-HT4 receptors in transgenic mice. Naunyn Schmiedebergs. Arch. Pharmacol. 2018, 391, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Seidler, T.; Fehse, C.; Marušáková, M.; Hofmann, B.; Gergs, U. Cardiovascular effects of metoclopramide and domperidone on human 5-HT4-serotonin-receptors in transgenic mice and in human atrial preparations. Eur. J. Pharmacol. 2021, 901, 174074. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Schulz, N.; Fehse, C.; Azatsian, K.; Čináková, A.; Marušáková, M.; Hofmann, B.; Gergs, U. Cardiovascular effects of bufotenin on human 5-HT4 serotonin receptors in cardiac preparations of transgenic mice and in human atrial preparations. Naunyn Schmiedebergs. Arch. Pharmacol. 2023, 396, 1471–1485. [Google Scholar] [CrossRef]
- De Maeyer, J.H.; Prins, N.H.; Schuurkes, J.A.J.; Lefebvre, R.A. Differential effects of 5-hydroxytryptamine4 receptor agonists at gastric versus cardiac receptors: An operational framework to explain and quantify organ-specific behavior. J. Pharmacol. Exp. Ther. 2006, 317, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Chan, K.Y.; de Vries, R.; van den Bogeardt, A.J.; de Maeyer, J.H.; Schuurkes, J.A.J.; Villalón, C.M.; Saxena, P.R.; Danser, A.H.J.; MaassenVanDenBrink, A. Inotropic effects of prokinetic agents with 5-HT(4) receptor agonist actions on human isolated myocardial trabeculae. Life Sci. 2012, 90, 538–544. [Google Scholar] [CrossRef]
- Suffredini, S.; Cerbai, E.; Giunti, G.; El Mouelhi, M.; Pfannkuche, H.-J.; Mugelli, A. Electrophysiological characterization of isolated human atrial myocytes exposed to tegaserod. Basic Clin. Pharmacol. Toxicol. 2010, 106, 416–421. [Google Scholar] [CrossRef]
- Conlon, K.; De Maeyer, J.H.; Bruce, C.; Schuurkes, J.A.J.; Christie, L.; McRedmond, J.; Derakhchan, K.; Wade, P.R. Nonclinical Cardiovascular Studies of Prucalopride, a Highly Selective 5-Hydroxytryptamine 4 Receptor Agonist. J. Pharmacol. Exp. Ther. 2018, 364, 156–169. [Google Scholar] [CrossRef]
- Ismair, M.G.; Kullak-Ublick, G.A.; Blakely, R.D.; Fried, M.; Vavricka, S.R. Tegaserod inhibits the serotonin transporter SERT. Digestion 2007, 75, 90–95. [Google Scholar] [CrossRef]
- Gergs, U.; Neumann, J.; Simm, A.; Silber, R.-E.; Remmers, F.O.; Läer, S. Phosphorylation of phospholamban and troponin I through 5-HT4 receptors in the isolated human atrium. Naunyn Schmiedebergs. Arch. Pharmacol. 2009, 379, 349–359. [Google Scholar] [CrossRef]
- Funk, F.; Kronenbitter, A.; Hackert, K.; Oebbeke, M.; Klebe, G.; Barth, M.; Koch, D.; Schmitt, J.P. Phospholamban pentamerization increases sensitivity and dynamic range of cardiac relaxation. Cardiovasc. Res. 2023, 119, 1568–1582. [Google Scholar] [CrossRef]
- Wittmann, T.; Lohse, M.J.; Schmitt, J.P. Phospholamban pentamers attenuate PKA-dependent phosphorylation of monomers. J. Mol. Cell. Cardiol. 2015, 80, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rouet, R.; Worou, M.E.; Puddu, P.E.; Lemoine, S.; Plaud, B.; Salle, L.; Gerard, J.-L.; Hanouz, J.-L. Nifedipine blocks ondansetron electrophysiological effects in rabbit purkinje fibers and decreases early after depolarization incidence. Curr. Clin. Pharmacol. 2012, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ouadid, H.; Seguin, J.; Dumuis, A.; Bockaert, J.; Nargeot, J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol. Pharmacol. 1992, 41, 346–351. [Google Scholar] [PubMed]
- Appel-Dingemanse, S. Clinical pharmacokinetics of tegaserod, a serotonin 5-HT(4) receptor partial agonist with promotile activity. Clin. Pharmacokinet. 2002, 41, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.E.; Zollinger, M.; Dannecker, R.; Tynes, R.; Heitz, F.; Fischer, V. In vitro metabolism of tegaserod in human liver and intestine: Assessment of drug interactions. Drug Metab. Dispos. 2001, 29, 1269–1276. [Google Scholar]
- Compan, V.; Zhou, M.; Grailhe, R.; Gazzara, R.A.; Martin, R.; Gingrich, J.; Dumuis, A.; Brunner, D.; Bockaert, J.; Hen, R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J. Neurosci. 2004, 24, 412–419. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesse, C.; Neumann, J.; Compan, V.; Ponimaskin, E.; Müller, F.E.; Hofmann, B.; Gergs, U. Tegaserod Stimulates 5-HT4 Serotonin Receptors in the Isolated Human Atrium. Int. J. Mol. Sci. 2024, 25, 11133. https://doi.org/10.3390/ijms252011133
Hesse C, Neumann J, Compan V, Ponimaskin E, Müller FE, Hofmann B, Gergs U. Tegaserod Stimulates 5-HT4 Serotonin Receptors in the Isolated Human Atrium. International Journal of Molecular Sciences. 2024; 25(20):11133. https://doi.org/10.3390/ijms252011133
Chicago/Turabian StyleHesse, Christin, Joachim Neumann, Valerie Compan, Evgeni Ponimaskin, Franziska E. Müller, Britt Hofmann, and Ulrich Gergs. 2024. "Tegaserod Stimulates 5-HT4 Serotonin Receptors in the Isolated Human Atrium" International Journal of Molecular Sciences 25, no. 20: 11133. https://doi.org/10.3390/ijms252011133
APA StyleHesse, C., Neumann, J., Compan, V., Ponimaskin, E., Müller, F. E., Hofmann, B., & Gergs, U. (2024). Tegaserod Stimulates 5-HT4 Serotonin Receptors in the Isolated Human Atrium. International Journal of Molecular Sciences, 25(20), 11133. https://doi.org/10.3390/ijms252011133





