p57Kip2 Phosphorylation Modulates Its Localization, Stability, and Interactions
Abstract
1. Introduction
2. Results
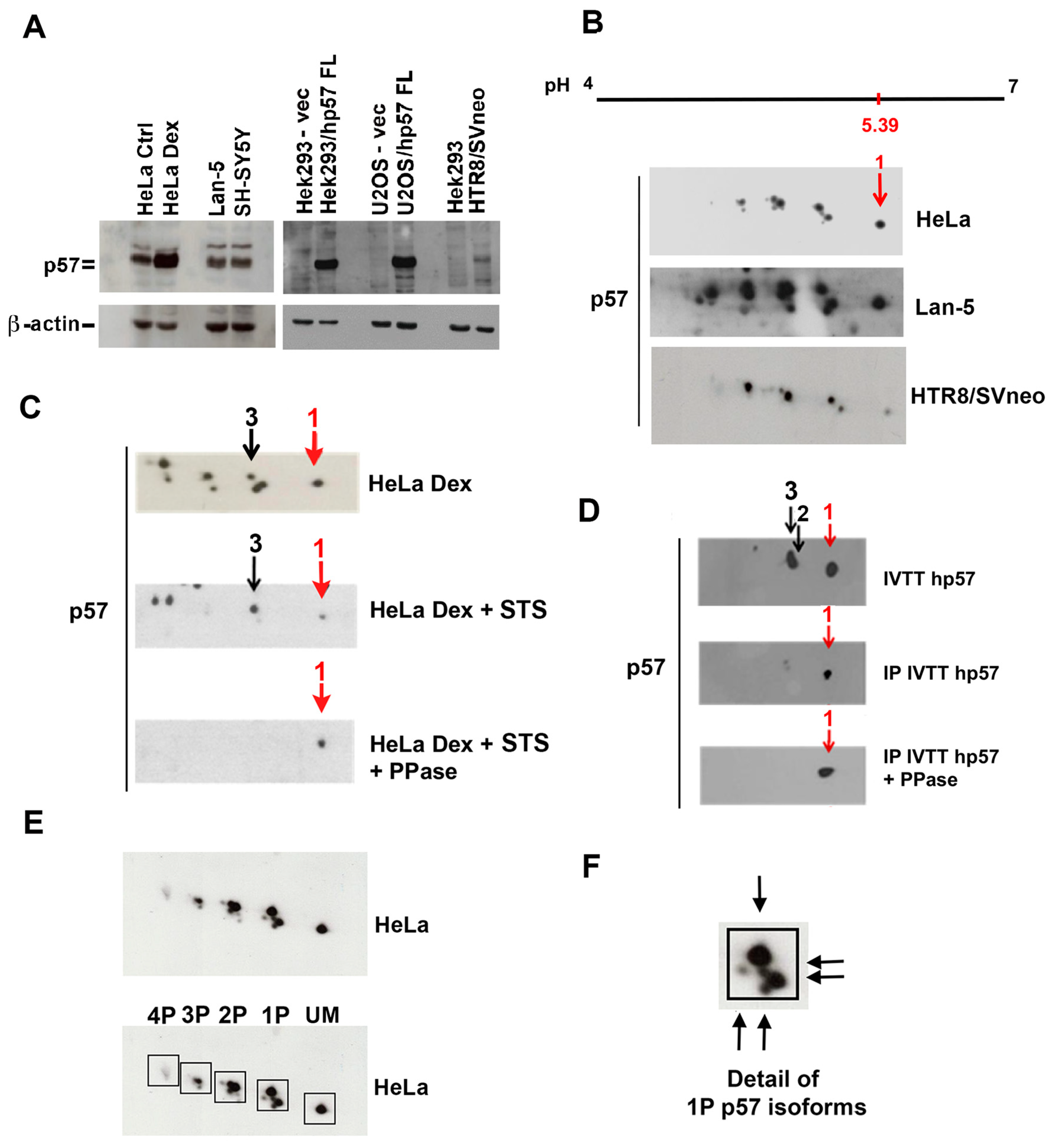
2.1. Characterization of the p57 Isoform Pattern
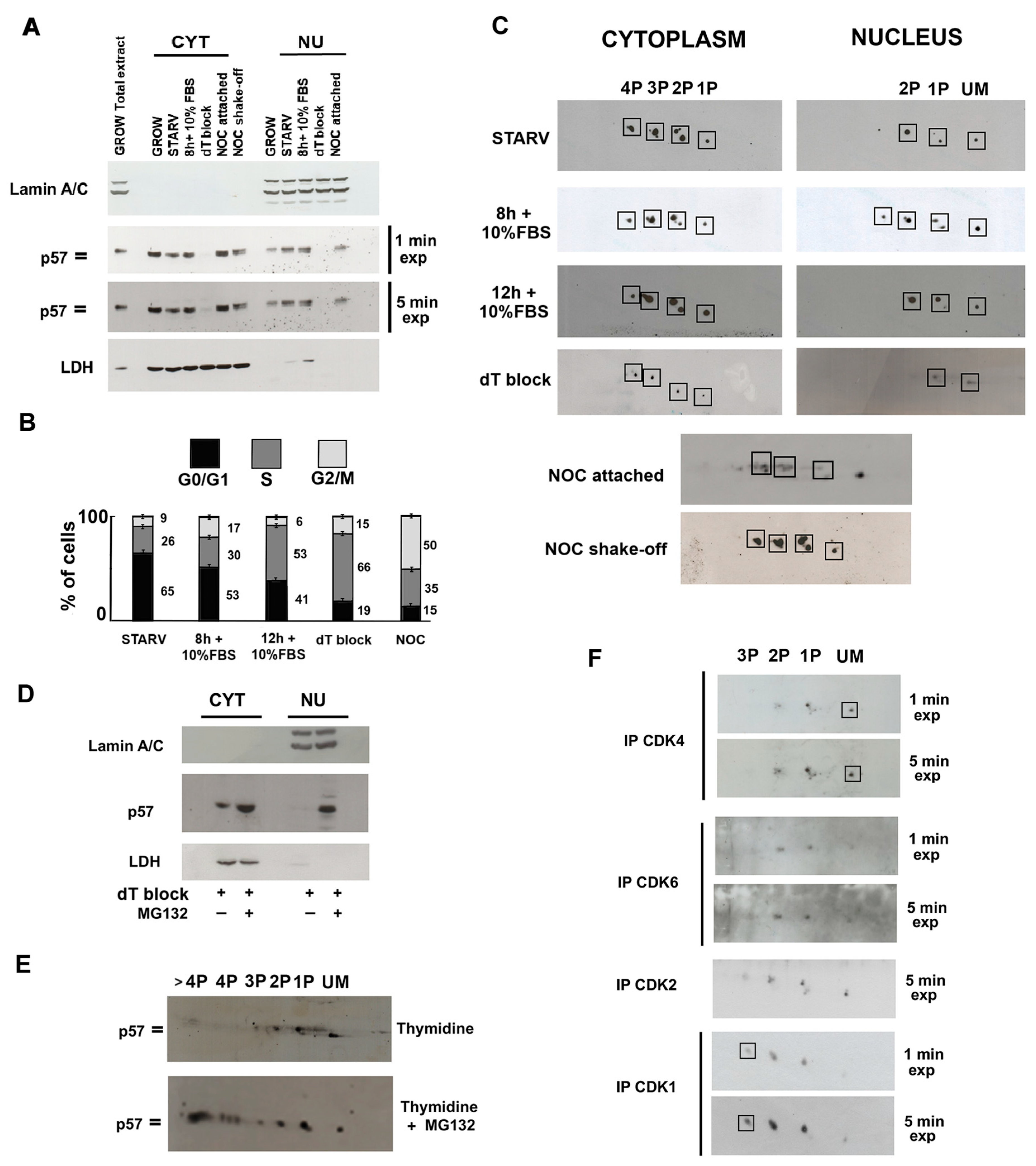
2.2. Compartmentalization of p57 Phosphoisoforms
2.3. Cell Cycle-Dependent Modulation of p57 Phosphorylation(s) and Evaluation of Phosphoisoforms Involved in the CDKs’ Interaction
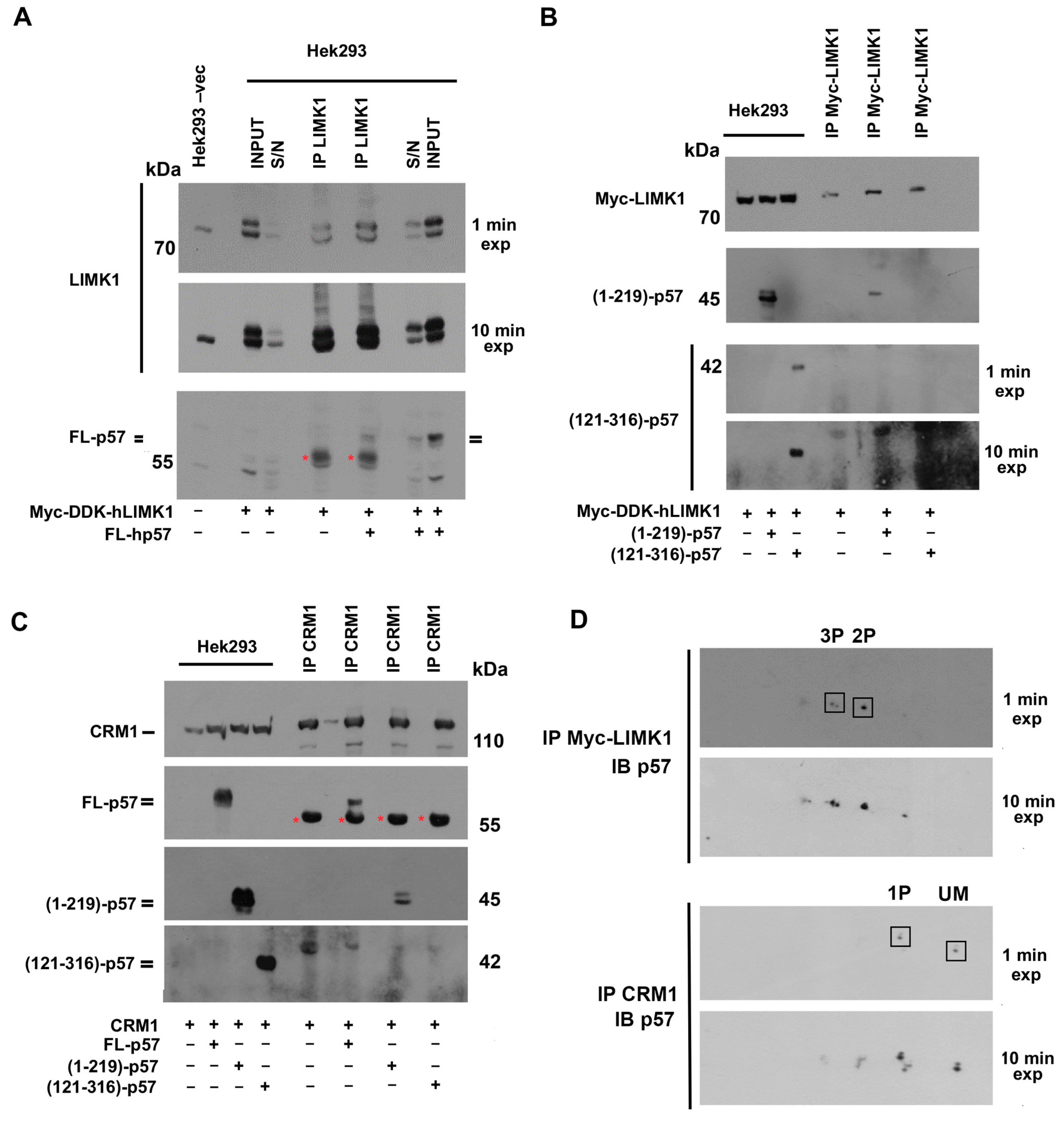
2.4. p57 Isoforms Specifically Bind Non-Canonical p57 Partners LIMK1 and CRM1
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Cell Synchronization and Verification by FACS
4.3. Cell Extract Preparation and Mono- and Two-Dimensional Western Blotting Analyses
4.4. Phosphatase Assay and IVTT Reaction
4.5. Immunoprecipitation
4.6. Plasmid Transient Transfection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, M.H.; Reynisdóttir, I.; Massagué, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995, 9, 639–649. [Google Scholar] [CrossRef]
- Matsuoka, S.; Edwards, M.C.; Bai, C.; Parker, S.; Zhang, P.; Baldini, A.; Harper, J.W.; Elledge, S.J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995, 9, 650–662. [Google Scholar] [CrossRef]
- Pateras, I.S.; Apostolopoulou, K.; Niforou, K.; Kotsinas, A.; Gorgoulis, V.G. p57KIP2: “Kip”ing the cell under control. Mol. Cancer Res. 2009, 7, 1902–1919. [Google Scholar] [CrossRef]
- Yan, Y.; Frisén, J.; Lee, M.H.; Massagué, J.; Barbacid, M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997, 11, 973–983. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakayama, K.; Nakayama, K. Mice lacking a CDK inhibitor, p57Kip2, exhibit skeletal abnormalities and growth retardation. J. Biochem. 2000, 127, 73–83. [Google Scholar] [CrossRef]
- Zhang, P.; Liégeois, N.J.; Wong, C.; Finegold, M.; Hou, H.; Thompson, J.C.; Silverman, A.; Harper, J.W.; DePinho, R.A.; Elledge, S.J. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 1997, 387, 151–158. [Google Scholar] [CrossRef]
- Romanelli, V.; Belinchón, A.; Benito-Sanz, S.; Martínez-Glez, V.; Gracia-Bouthelier, R.; Heath, K.E.; Campos-Barros, A.; García-Miñaur, S.; Fernandez, L.; Meneses, H. CDKN1C (p57Kip2) analysis in Beckwith-Wiedemann syndrome (BWS) patients: Genotype-phenotype correlations, novel mutations, and polymorphisms. Am. J. Med. Genet. 2010, 152, 1390–1397. [Google Scholar] [CrossRef]
- Brioude, F.; Netchine, I.; Praz, F.; Le Jule, M.; Calmel, C.; Lacombe, D. Mutations of the Imprinted CDKN1C Gene as a Cause of the Overgrowth Beckwith-Wiedemann Syndrome: Clinical Spectrum and Functional Characterization. Hum. Mutat. 2015, 36, 894–902. [Google Scholar] [CrossRef]
- Duquesnes, N.; Callot, C.; Jeannot, P.; Daburon, V.; Nakayama, K.I.; Manenti, S.; Davy, A.; Besson, A. p57Kip2 knock-in mouse reveals CDK-independent contribution in the development of Beckwith-Wiedemann syndrome. J. Pathol. 2016, 239, 250–261. [Google Scholar] [CrossRef]
- Arboleda, V.A.; Lee, H.; Parnaik, R.; Fleming, A.; Banerjee, A.; Ferraz-de-Souza, B.; Délot, E.C.; Rodriguez-Fernandez, I.A.; Braslavsky, D.; Bergadá, I.; et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat. Genet. 2012, 44, 788–792. [Google Scholar] [CrossRef]
- Dias, R.P.; Maher, E.R. An imprinted IMAGe: Insights into growth regulation through genomic analysis of a rare disease. Genome Med. 2012, 4, 60. [Google Scholar] [CrossRef]
- Eggermann, K.; Bliek, J.; Brioude, F.; Algar, E.; Buiting, K.; Russo, S.; Tümer, Z.; Monk, D.; Moore, G.; Antoniadi, T.; et al. EMQN best practice guidelines for the molecular genetic testing and reporting of chromosome 11p15 imprinting disorders: Silver-Russell and Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 2016, 24, 1377–1387. [Google Scholar] [CrossRef]
- Brioude, F.; Oliver-Petit, I.; Blaise, A.; Praz, F.; Rossignol, S.; Le Jule, M.; Thibaud, N.; Faussat, A.M.; Tauber, M.; Le Bouc, Y.; et al. CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J. Med. Genet. 2013, 50, 823–830. [Google Scholar] [CrossRef]
- Stampone, E.; Caldarelli, I.; Zullo, A.; Bencivenga, D.; Mancini, F.P.; Della Ragione, F.; Borriello, A. Genetic and Epigenetic Control of CDKN1C Expression: Importance in Cell Commitment and Differentiation, Tissue Homeostasis and Human Diseases. Int. J. Mol. Sci. 2018, 19, 1055. [Google Scholar] [CrossRef]
- Della Ragione, F.; Borriello, A.; Mastropietro, S.; Della Pietra, V.; Monno, F.; Gabutti, V.; Locatelli, F.; Bonsi, L.; Bagnara, G.P.; Iolascon, A. Expression of G1-phase cell cycle genes during hematopoietic lineage. Biochem. Biophys. Res. Commun. 1997, 231, 73–76. [Google Scholar] [CrossRef]
- Cucciolla, V.; Borriello, A.; Criscuolo, M.; Sinisi, A.A.; Bencivenga, D.; Tramontano, A.; Scudieri, A.C.; Oliva, A.; Zappia, V.; Della Ragione, F. Histone deacetylase inhibitors upregulate p57Kip2 level by enhancing its expression through Sp1 transcription factor. Carcinogenesis 2008, 29, 560–567. [Google Scholar] [CrossRef][Green Version]
- Bencivenga, D.; Stampone, E.; Vastante, A.; Barahmeh, M.; Della Ragione, F.; Borriello, A. An Unanticipated Modulation of Cyclin-Dependent Kinase Inhibitors: The Role of Long Non-Coding RNAs. Cells 2022, 11, 1346. [Google Scholar] [CrossRef]
- Russo, G.L.; Stampone, E.; Cervellera, C.; Borriello, A. Regulation of p27Kip1 and p57Kip2 Functions by Natural Polyphenols. Biomolecules 2020, 10, 1316. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Cip/Kip proteins: More than just CDKs inhibitors. Genes Dev. 2004, 18, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Matsumoto, K.; Takai, Y.; Nakamura, T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J. Cell Biol. 1999, 147, 1519–1532. [Google Scholar] [CrossRef]
- Vlachos, P.; Joseph, B. The Cdk inhibitor p57Kip2 controls LIM-kinase 1 activity and regulates actin cytoskeleton dynamics. Oncogene 2009, 28, 4175–4188. [Google Scholar] [CrossRef]
- Chow, S.E.; Wang, J.S.; Lin, M.R.; Lee, C.L. Downregulation of p57kip2 promotes cell invasion via LIMK/cofilin pathway in human nasopharyngeal carcinoma cells. J. Cell. Biochem. 2011, 112, 3459–3468. [Google Scholar] [CrossRef]
- Rodhe, J.; Kavanagh, E.; Joseph, B. TAp73β-mediated suppression of cell migration requires p57Kip2 control of actin cytoskeleton dynamics. Oncotarget 2013, 4, 289–297. [Google Scholar] [CrossRef]
- Samuelsson, M.K.; Pazirandeh, A.; Okret, S. A pro-apoptotic effect of the CDK inhibitor p57Kip2 on staurosporine-induced apoptosis in HeLa cells. Biochem. Biophys. Res. Commun. 2002, 23, 702–709. [Google Scholar] [CrossRef]
- Kavanagh, E.; Vlachos, P.; Emourgeon, V.; Rodhe, J.; Joseph, B. p57KIP2 control of actin cytoskeleton dynamics is responsible for its mitochondrial pro-apoptotic effect. Cell Death Dis. 2012, 3, e311. [Google Scholar] [CrossRef]
- Russo, A.A.; Jeffrey, P.D.; Patten, A.K.; Massagué, J.; Pavletich, N.P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 1996, 25, 325–331. [Google Scholar] [CrossRef]
- Fotedar, R.; Fitzgerald, P.; Rousselle, T.; Cannella, D.; Dorée, M.; Messier, H.; Fotedar, A. p21 contains independent binding sites for cyclin and cdk2: Both sites are required to inhibit cdk2 kinase activity. Oncogene 1996, 12, 2155–2164. [Google Scholar] [PubMed]
- Watanabe, H.; Pan, Z.Q.; Schreiber-Agus, N.; DePinho, R.A.; Hurwitz, J.; Xiong, Y. Suppression of cell transformation by the cyclin-dependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. USA 1998, 95, 1392–1397. [Google Scholar] [CrossRef]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 18, 665–679. [Google Scholar] [CrossRef]
- Luo, Y.; Hurwitz, J.; Massagué, J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 1995, 375, 159–161. [Google Scholar] [CrossRef]
- Cayrol, C.; Ducommun, B. Interaction with cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene 1998, 17, 2437–2444. [Google Scholar] [CrossRef]
- Wang, B.; Liu, K.; Lin, H.Y.; Bellam, N.; Ling, S. 14-3-3 Tau regulates ubiquitin-independent proteasomal degradation of p21, a novel mechanism of p21 downregulation in breast cancer. Mol. Cell. Biol. 2010, 30, 1508–1527. [Google Scholar] [CrossRef]
- Borges, K.S.; Arboleda, V.A.; Vilain, E. Mutations in the PCNA-binding site of CDKN1C inhibit cell proliferation by impairing the entry into S phase. Cell Div. 2015, 10, 2. [Google Scholar] [CrossRef]
- Stampone, E.; Bencivenga, D.; Barone, C.; Di Finizio, M.; Della Ragione, F.; Borriello, A. A Beckwith-Wiedemann-Associated CDKN1C Mutation Allows the Identification of a Novel Nuclear Localization Signal in Human p57Kip2. Int. J. Mol. Sci. 2021, 22, 7428. [Google Scholar] [CrossRef]
- Choleva, L.; Wang, P.; Liu, H.; Wood, O.; Lambertini, L.; Scott, D.K.; Karakose, E.; Stewart, A.F. Structure-Function Analysis of p57KIP2 in the Human Pancreatic Beta Cell Reveals a Bipartite Nuclear Localization Signal. Endocrinology 2023, 165, bqad197. [Google Scholar] [CrossRef]
- Adkins, J.N.; Lumb, K.J. Intrinsic structural disorder and sequence features of the cell cycle inhibitor p57Kip2. Proteins 2002, 46, 1–7. [Google Scholar] [CrossRef]
- Bah, A.; Forman-Kay, J.D. Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications. J. Biol. Chem. 2016, 291, 6696–6705. [Google Scholar] [CrossRef]
- Child, E.S.; Mann, D.J. The intricacies of p21 phosphorylation: Protein/protein interactions, subcellular localization and stability. Cell Cycle 2006, 5, 1313–1319. [Google Scholar] [CrossRef]
- Borriello, A.; Cucciolla, V.; Oliva, A.; Zappia, V.; Della Ragione, F. p27Kip1 metabolism: A fascinating labyrinth. Cell Cycle 2007, 6, 1053–1061. [Google Scholar] [CrossRef]
- Bencivenga, D.; Stampone, E.; Roberti, D.; Della Ragione, F.; Borriello, A. p27Kip1, an Intrinsically Unstructured Protein with Scaffold Properties. Cells 2021, 10, 2254. [Google Scholar] [CrossRef]
- Creff, J.; Besson, A. Functional Versatility of the CDK Inhibitor p57Kip2. Front. Cell Dev. Biol. 2020, 7, 584590. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Wu, C.; Ba, Q.; Lu, D.; Li, W.; Salovska, B.; Hou, P.; Mueller, T.; Rosenberger, G.; Gao, E.; Di, Y. Global and Site-Specific Effect of Phosphorylation on Protein Turnover. Dev. Cell 2021, 11, 111–124.e6. [Google Scholar] [CrossRef]
- Leibovitch, M.P.; Kannengiesser, C.; Leibovitch, S.A. Signal-induced ubiquitination of p57Kip2 is independent of the C-terminal consensus Cdk phosphorylation site. FEBS Lett. 2003, 543, 125–128. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Nakamoto, T.; Nishimori, S.; Tanaka, K.; Chiba, T. A new ubiquitin ligase involved in p57KIP2 proteolysis regulates osteoblast cell differentiation. EMBO Rep. 2008, 9, 878–884. [Google Scholar] [CrossRef]
- Kamura, T.; Hara, T.; Kotoshiba, S.; Yada, M.; Ishida, N.; Imaki, H.; Hatakeyama, S.; Nakayama, K.; Nakayama, K.I. Degradation of p57Kip2 mediated by SCFSkp2dependent ubiquitylation. Proc. Natl. Acad. Sci. USA 2003, 100, 10231–10236. [Google Scholar] [CrossRef]
- Pomella, S.; Cassandri, M.; D’Archivio, L.; Porrazzo, A.; Cossetti, C.; Phelps, D. MYOD-SKP2 axis boosts tumorigenesis in fusion negative rhabdomyosarcoma by preventing differentiation through p57Kip2 targeting. Nat. Commun. 2023, 14, 8373. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, H.Y.; Shin, J.; Phan, L.; Fang, L.; Che, T.F.; Su, C.H.; Yeung, S.C.; Lee, M.H. CDK inhibitor p57Kip2 is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle 2013, 12, 935–943. [Google Scholar] [CrossRef]
- Urano, T.; Yashiroda, H.; Muraoka, M.; Tanaka, K.; Hosoi, T.; Inoue, S.; Ouchi, Y.; Toyoshima, H. p57Kip2 is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor beta1. J. Biol. Chem. 1999, 274, 12197–12200. [Google Scholar] [CrossRef]
- Iolascon, A.; Giordani, L.; Borriello, A.; Carbone, R.; Izzo, A.; Tonini, G.P.; Gambini, C.; Della Ragione, F. Reduced expression of transforming growth factor-beta receptor type III in high stage neuroblastomas. Br. J. Cancer 2000, 82, 1171–1176. [Google Scholar] [CrossRef]
- Nishimori, S.; Tanaka, Y.; Chiba, T.; Fujii, M.; Imamura, T.; Miyazono, K. Smad-mediated transcription is required for transforming growth factor-beta1-induced p57Kip2 proteolysis in osteoblastic cells. J. Biol. Chem. 2001, 276, 10700–10705. [Google Scholar] [CrossRef]
- Rodier, G.; Montagnoli, A.; Di Marcotullio, L.; Coulombe, P.; Draetta, G.F.; Pagano, M.; Meloche, S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001, 20, 6672–6682. [Google Scholar] [CrossRef]
- Ishida, N.; Hara, T.; Kamura, T.; Yoshida, M.; Nakayama, K.; Nakayama, K.I. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 2002, 277, 14355–14358. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.K.; Kotchetkov, R.; Cariou, S.; Resch, A.; Lupetti, R.; Beniston, R.G.; Melchior, F.; Hengst, L.; Slingerland, J.M. CRM1/Ran-mediated nuclear export of p27Kip1 involves a nuclear export signal and links p27 export and proteolysis. Mol. Biol. Cell 2003, 14, 201–213. [Google Scholar] [CrossRef]
- Göttle, P.; Sabo, J.K.; Heinen, A.; Venables, G.; Torres, K.; Tzekova, N.; Parras, C.M.; Kremer, D.; Hartung, H.P.; Cate, H.S.; et al. Oligodendroglial maturation is dependent on intracellular protein shuttling. J. Neurosci. 2015, 35, 906–919. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, Z.; Mao, H.; Schnellmann, R.G.; Zhuang, S. Src regulates cell cycle protein expression and renal epithelial cell proliferation via PI3K/Akt signaling-dependent and -independent mechanisms. Am. J. Physiol. Ren. Physiol. 2008, 295, F145–F152. [Google Scholar] [CrossRef][Green Version]
- Swadling, J.B.; Warnecke, T.; Morris, K.L.; Barr, A.R. Conserved Cdk inhibitors show unique structural responses to tyrosine phosphorylation. Biophys. J. 2022, 121, 2312–2329. [Google Scholar] [CrossRef]
- Jäkel, H.; Peschel, I.; Kunze, C.; Weinl, C.; Hengst, L. Regulation of p27Kip1 by mitogen-induced tyrosine phosphorylation. Cell Cycle 2012, 11, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Guiley, K.Z.; Stevenson, J.W.; Lou, K.; Barkovich, K.J.; Kumarasamy, V.; Wijeratne, T.U.; Bunch, K.L.; Tripathi, S.; Knudsen, E.S.; Witkiewicz, A.K.; et al. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 2019, 366, eaaw2106. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Bencivenga, D.; Cucciolla, V.; Oliva, A.; Usala, E.; Danise, P.; Ronzoni, L.; Perrotta, S.; Della Ragione, F. p57Kip2 is a downstream effector of BCR-ABL kinase inhibitors in chronic myelogenous leukemia cells. Carcinogenesis 2011, 1, 10–18. [Google Scholar] [CrossRef]
- Joseph, B.; Andersson, E.R.; Vlachos, P.; Södersten, E.; Liu, L.; Teixeira, A.I.; Hermanson, O. p57Kip2 is a repressor of Mash1 activity and neuronal differentiation in neural stem cells. Cell Death Differ. 2009, 16, 1256–1265. [Google Scholar] [CrossRef]
- Kullmann, M.K.; Podmirseg, S.R.; Roilo, M.; Hengst, L. The CDK inhibitor p57Kip2 enhances the activity of the transcriptional coactivator FHL2. Sci. Rep. 2020, 10, 7140. [Google Scholar] [CrossRef]
- Kullmann, M.K.; Pegka, F.; Ploner, C.; Hengst, L. Stimulation of c-Jun/AP-1-Activity by the Cell Cycle Inhibitor p57Kip2. Front. Cell Dev. Biol. 2021, 9, 664609. [Google Scholar] [CrossRef]
- Creff, J.; Nowosad, A.; Prel, A.; Pizzoccaro, A.; Aguirrebengoa, M.; Duquesnes, N.; Callot, C.; Jungas, T.; Dozier, C.; Besson, A. p57Kip2 acts as a transcriptional corepressor to regulate intestinal stem cell fate and proliferation. Cell Rep. 2023, 42, 112659. [Google Scholar] [CrossRef]
- Jia, H.; Cong, Q.; Chua, J.F.; Liu, H.; Xia, X.; Zhang, X.; Lin, J.; Habib, S.L.; Ao, J.; Zuo, Q.; et al. p57Kip2 is an unrecognized DNA damage response effector molecule that functions in tumor suppression and chemoresistance. Oncogene 2015, 34, 3568–3581. [Google Scholar] [CrossRef]
- Rossi, M.N.; Antonangeli, F. Cellular Response upon Stress: p57 Contribution to the Final Outcome. Mediat. Inflamm. 2015, 2015, 259325. [Google Scholar] [CrossRef]
- Stampone, E.; Bencivenga, D.; Barone, C.; Aulitto, A.; Verace, F.; Della Ragione, F.; Borriello, A. High Dosage Lithium Treatment Induces DNA Damage and p57Kip2 Decrease. Int. J. Mol. Sci. 2020, 21, 1169. [Google Scholar] [CrossRef]
- Chang, T.S.; Kim, M.J.; Ryoo, K.; Park, J.; Eom, S.J.; Shim, J.; Nakayama, K.I.; Nakayama, K.; Tomita, M.; Takahashi, K.; et al. p57KIP2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein Kinase. J. Biol. Chem. 2003, 278, 48092–48098. [Google Scholar] [CrossRef]
- Samuelsson, M.K.; Pazirandeh, A.; Davani, B.; Okret, S. p57Kip2, a glucocorticoid-induced inhibitor of cell cycle progression in HeLa cells. Mol. Endocrinol. 1999, 13, 1811–1822. [Google Scholar] [CrossRef]
- Avrahami, D.; Li, C.; Yu, M.; Jiao, Y.; Zhang, J.; Naji, A.; Ziaie, S.; Glaser, B.; Kaestner, K.H. Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication. J. Clin. Investig. 2014, 124, 670–674. [Google Scholar] [CrossRef]
- Wang, L.; Jin, S.; Dai, P.; Zhang, T.; Shi, Y.; Ai, G.; Shao, X.; Xie, Y.; Xu, J.; Chen, Z.; et al. p57Kip2 is a master regulator of human adipose derived stem cell quiescence and senescence. Stem Cell Res. 2020, 44, 101759. [Google Scholar] [CrossRef]
- Tury, A.; Mairet-Coello, G.; DiCicco-Bloom, E. The cyclin-dependent kinase inhibitor p57Kip2 regulates cell cycle exit, differentiation, and migration of embryonic cerebral cortical precursors. Cereb. Cortex 2011, 21, 1840–1856. [Google Scholar] [CrossRef]
- Tesio, M.; Trumpp, A. Breaking the cell cycle of HSCs by p57 and friends. Cell Stem Cell 2011, 9, 187–192. [Google Scholar] [CrossRef]
- Jadasz, J.J.; Rivera, F.J.; Taubert, A.; Kandasamy, M.; Sandner, B.; Weidner, N.; Aktas, O.; Hartung, H.P.; Aigner, L.; Küry, P. p57kip2 regulates glial fate decision in adult neural stem cells. Development 2012, 139, 3306–3315. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Bencivenga, D.; Criscuolo, M.; Cucciolla, V.; Tramontano, A.; Oliva, A.; Perrotta, S.; Della Ragione, F. p57Kip2 and cancer: Time for a critical appraisal. Mol. Cancer Res. 2011, 9, 1269–1284. [Google Scholar] [CrossRef]
- Kavanagh, E.; Joseph, B. The hallmarks of CDKN1C (p57, KIP2) in cancer. Biochim. Biophys. Acta 2011, 1816, 50–56. [Google Scholar] [CrossRef]
- Iolascon, A.; Giordani, L.; Moretti, A.; Basso, G.; Borriello, A.; Della Ragione, F. Analysis of CDKN2A, CDKN2B, CDKN2C, and cyclin Ds gene status in hepatoblastoma. Hepatology 1998, 4, 989–995. [Google Scholar] [CrossRef]
- Sale, M.J.; Balmanno, K.; Saxena, J.; Ozono, E.; Wojdyla, K.; McIntyre, R.E.; Gilley, R.; Woroniuk, A.; Howarth, K.D.; Hughes, G.; et al. MEK1/2 inhibitor withdrawal reverses acquired resistance driven by BRAFV600E amplification whereas KRASG13D amplification promotes EMT-chemoresistance. Nat. Commun. 2019, 10, 2030. [Google Scholar] [CrossRef]
- Monti, B. p57kip2 nuclear export as a marker of oligodendrocytes differentiation: Towards an innovative phenotyping screening for the identification of myelin repair drugs. EBioMedicine 2021, 66, 103298. [Google Scholar] [CrossRef]
- Reynaud, E.G.; Guillier, M.; Leibovitch, M.P.; Leibovitch, S.A. Dimerization of the amino terminal domain of p57Kip2 inhibits cyclin D1-cdk4 kinase activity. Oncogene 2000, 19, 1147–1152. [Google Scholar] [CrossRef][Green Version]
- Hengst, L.; Göpfert, U.; Lashuel, H.A.; Reed, S.I. Complete inhibition of Cdk/cyclin by one molecule of p21Cip1. Genes Dev. 1998, 12, 3882–3888. [Google Scholar] [CrossRef]
- LaBaer, J.; Garrett, M.D.; Stevenson, L.F.; Slingerland, J.M.; Sandhu, C.; Chou, H.S.; Fattaey, A.; Harlow, E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997, 11, 847–862. [Google Scholar] [CrossRef]
- Blain, S.W.; Montalvo, E.; Massagué, J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 1997, 272, 25863–25872. [Google Scholar] [CrossRef]
- Martínez-Jañez, N.; Ezquerra, M.B.; Manso Sanchez, L.M. First-line therapy with palbociclib in patients with advanced HR+/HER2− breast cancer: The real-life study PALBOSPAIN. Breast Cancer Res. Treat. 2024, 206, 317–328. [Google Scholar] [CrossRef]
- Ullah, Z.; Kohn, M.J.; Yagi, R.; Vassilev, L.T.; DePamphilis, M.L. Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev. 2008, 22, 3024–3036. [Google Scholar] [CrossRef]
- MacLean, H.E.; Guo, J.; Knight, M.C.; Zhang, P.; Cobrinik, D.; Kronenberg, H.M. The cyclin-dependent kinase inhibitor p57Kip2 mediates proliferative actions of PTHrP in chondrocytes. J. Clin. Investig. 2004, 113, 1334–1343. [Google Scholar] [CrossRef]
- Saito, M.; Mulati, M.; Talib, S.Z.; Kaldis, P.; Takeda, S.; Okawa, A.; Inose, H. The Indispensable Role of Cyclin-Dependent Kinase 1 in Skeletal Development. Sci. Rep. 2016, 6, 20622. [Google Scholar] [CrossRef]
- Yokoo, T.; Toyoshima, H.; Miura, M.; Wang, Y.; Iida, K.T.; Suzuki, H.; Sone, H.; Shimano, H.; Gotoda, T.; Nishimori, S. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J. Biol. Chem. 2003, 278, 52919–52923. [Google Scholar] [CrossRef]
- Kremer, D.; Heinen, A.; Jadasz, J.; Göttle, P.; Zimmermann, K.; Zickler, P.; Jander, S.; Hartung, H.P.; Küry, P. p57kip2 is dynamically regulated in experimental autoimmune encephalomyelitis and interferes with oligodendroglial maturation. Proc. Natl. Acad. Sci. USA 2009, 106, 9087–9092. [Google Scholar] [CrossRef]
- Guo, H.; Lv, Y.; Tian, T.; Hu, T.H.; Wang, W.J.; Sui, X.; Jiang, L.; Ruan, Z.P.; Nan, K.J. Downregulation of p57 accelerates the growth and invasion of hepatocellular carcinoma. Carcinogenesis 2011, 32, 1897–1904. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Tian, T.; Han, L.; Ruan, Z.; Liang, X.; Wang, W.; Nan, K. The role of cytoplasmic p57 in invasion of hepatocellular carcinoma. BMC Gastroenterol. 2015, 15, 104. [Google Scholar] [CrossRef][Green Version]
- Bencivenga, D.; Tramontano, A.; Borgia, A.; Negri, A.; Caldarelli, I.; Oliva, A.; Perrotta, S.; Della Ragione, F.; Borriello, A. P27Kip1 serine 10 phosphorylation determines its metabolism and interaction with cyclin-dependent kinases. Cell Cycle. 2014, 13, 3768–3782. [Google Scholar] [CrossRef][Green Version]
- Bencivenga, D.; Stampone, E.; Aulitto, A.; Tramontano, A.; Barone, C.; Negri, A.; Roberti, D.; Perrotta, S.; Della Ragione, F.; Borriello, A. A cancer-associated CDKN1B mutation induces p27 phosphorylation on a novel residue: A new mechanism for tumor suppressor loss-of-function. Mol. Oncol. 2021, 15, 915–941. [Google Scholar] [CrossRef]
- Borriello, A.; Naviglio, S.; Bencivenga, D.; Caldarelli, I.; Tramontano, A.; Speranza, M.C.; Stampone, E.; Sapio, L.; Negri, A.; Oliva, A. Histone Deacetylase Inhibitors Increase p27Kip1 by Affecting Its Ubiquitin-Dependent Degradation through Skp2 Downregulation. Oxid. Med. Cell. Longev. 2016, 2016, 2481865. [Google Scholar] [CrossRef]
- Natalicchio, M.I.; Improta, G.; Zupa, A.; Cursio, O.E.; Stampone, E.; Possidente, L.; Teresa Gerardi, A.M.; Vita, G.; Martini, M.; Cassano, A. Pyrosequencing evaluation of low-frequency KRAS mutant alleles for EGF receptor therapy selection in metastatic colorectal carcinoma. Future Oncol. 2014, 10, 713–723. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stampone, E.; Bencivenga, D.; Dassi, L.; Sarnelli, S.; Campagnolo, L.; Lacconi, V.; Della Ragione, F.; Borriello, A. p57Kip2 Phosphorylation Modulates Its Localization, Stability, and Interactions. Int. J. Mol. Sci. 2024, 25, 11176. https://doi.org/10.3390/ijms252011176
Stampone E, Bencivenga D, Dassi L, Sarnelli S, Campagnolo L, Lacconi V, Della Ragione F, Borriello A. p57Kip2 Phosphorylation Modulates Its Localization, Stability, and Interactions. International Journal of Molecular Sciences. 2024; 25(20):11176. https://doi.org/10.3390/ijms252011176
Chicago/Turabian StyleStampone, Emanuela, Debora Bencivenga, Luisa Dassi, Sara Sarnelli, Luisa Campagnolo, Valentina Lacconi, Fulvio Della Ragione, and Adriana Borriello. 2024. "p57Kip2 Phosphorylation Modulates Its Localization, Stability, and Interactions" International Journal of Molecular Sciences 25, no. 20: 11176. https://doi.org/10.3390/ijms252011176
APA StyleStampone, E., Bencivenga, D., Dassi, L., Sarnelli, S., Campagnolo, L., Lacconi, V., Della Ragione, F., & Borriello, A. (2024). p57Kip2 Phosphorylation Modulates Its Localization, Stability, and Interactions. International Journal of Molecular Sciences, 25(20), 11176. https://doi.org/10.3390/ijms252011176









