Antibacterial Properties of Copper Oxide Nanoparticles (Review)
Abstract
1. Introduction
2. Literature Review
2.1. Literature Search Process
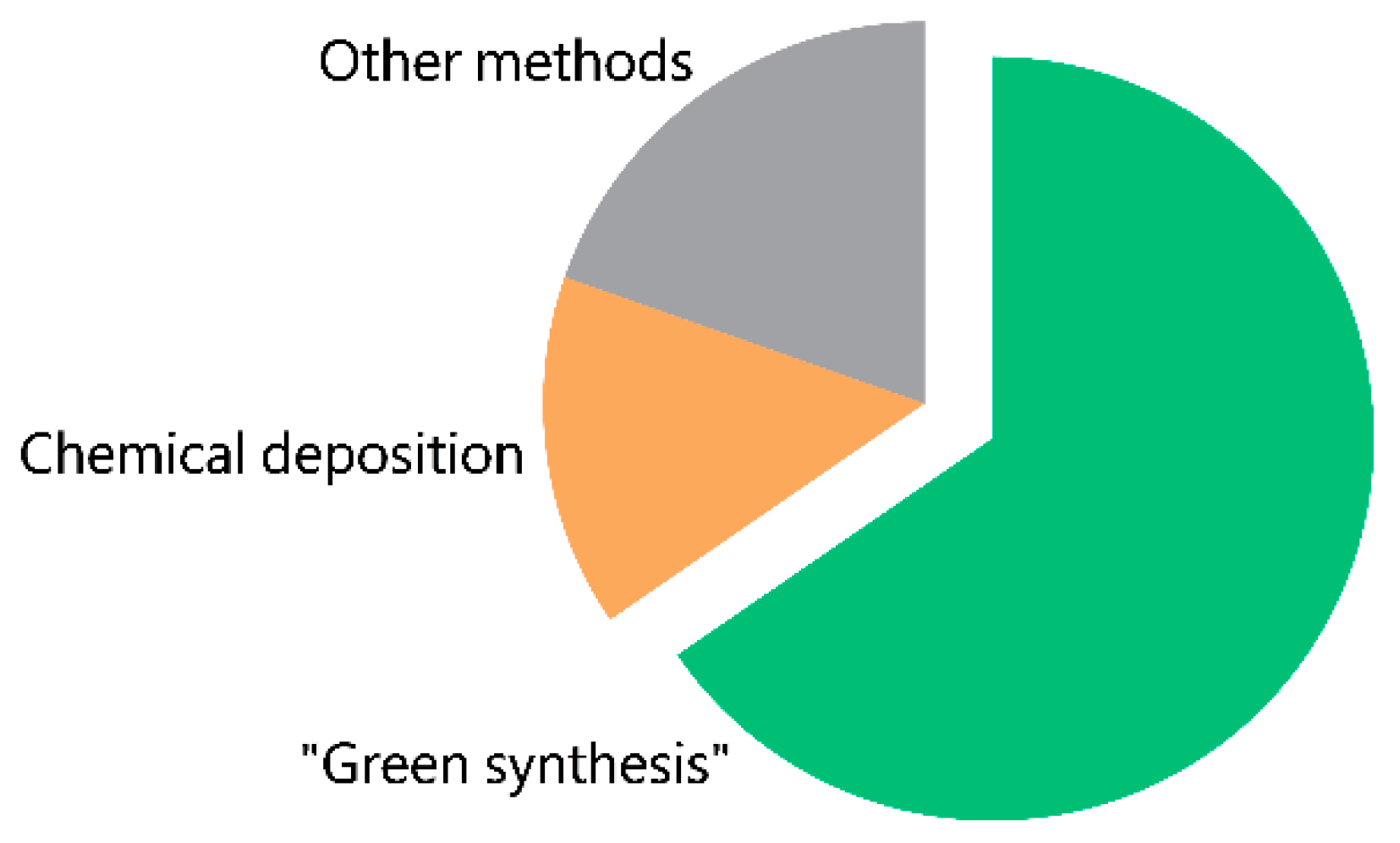
2.2. Methods of Synthesis of CopOx-NPs
2.2.1. Traditional Methods
2.2.2. “Green Methods”
3. Mechanisms of Antibacterial Activity of CopOx-NPs
3.1. Contact Interaction of CopOx-NPs with the Surface of Bacteria
3.2. ROS Release
3.3. Cation-Mediated Action and Cu-Specific Mechanisms
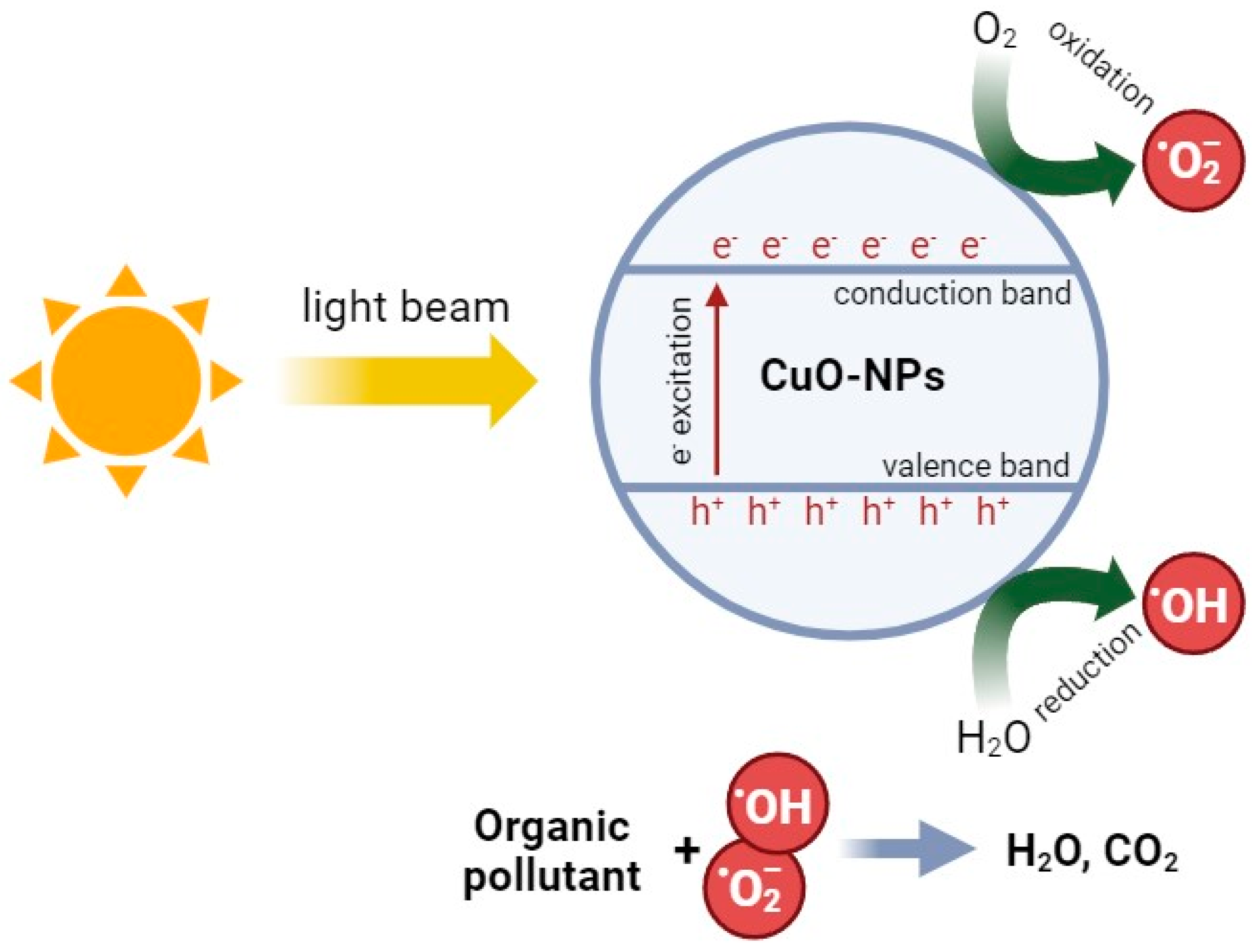
3.4. Photocatalytic Activity of CopOx-NPs
4. Ways to Improve Antibacterial Efficacy of CopOx-NPs
4.1. Functionalization of the CopOx-NPs Surface
4.2. Doping with Other NPs/Ions
5. Comparison of Antibacterial Activity of CopOx-NPs
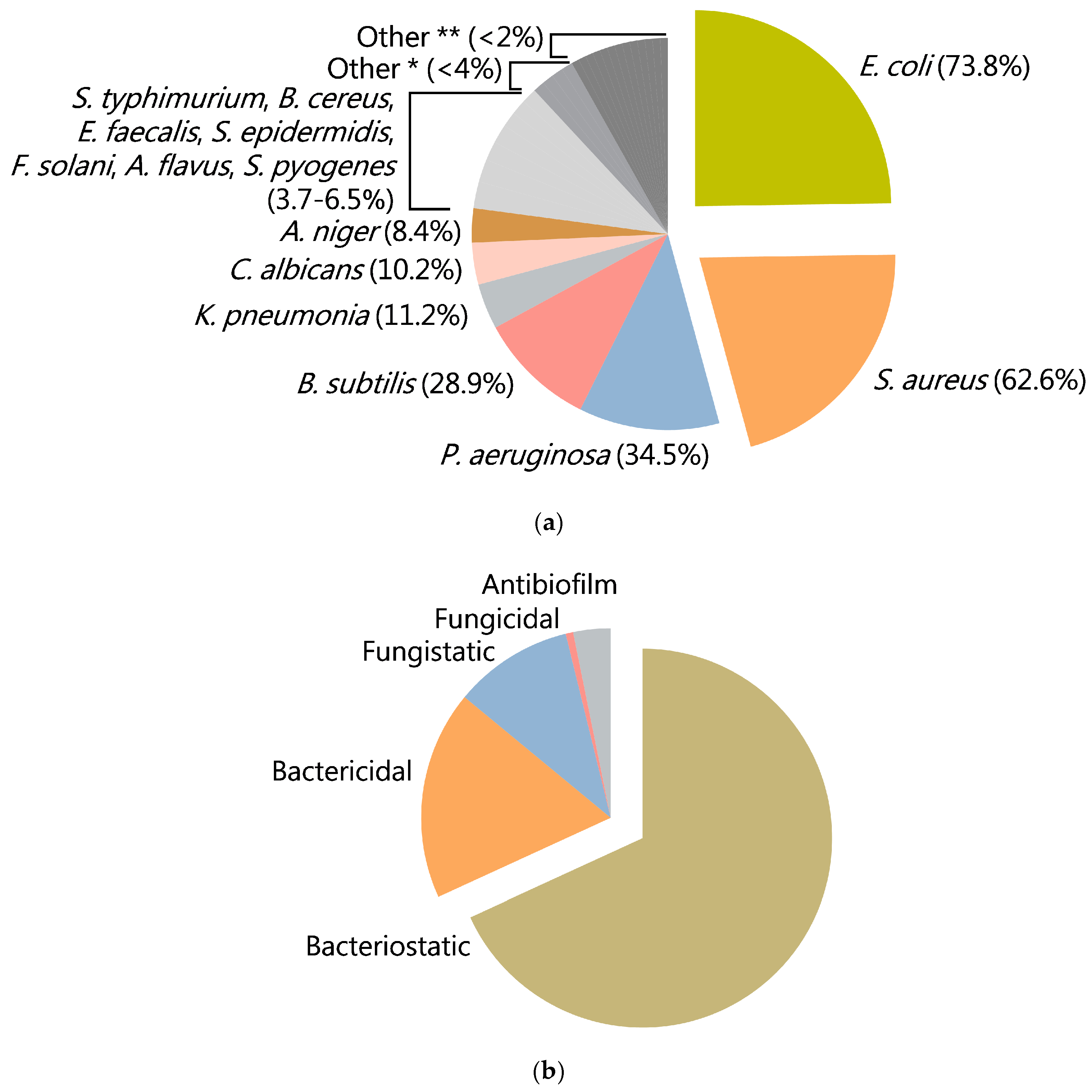
5.1. Spectrum of Microorganisms Sensitive to CuO-NPs Exposure
5.2. Gram Specificity
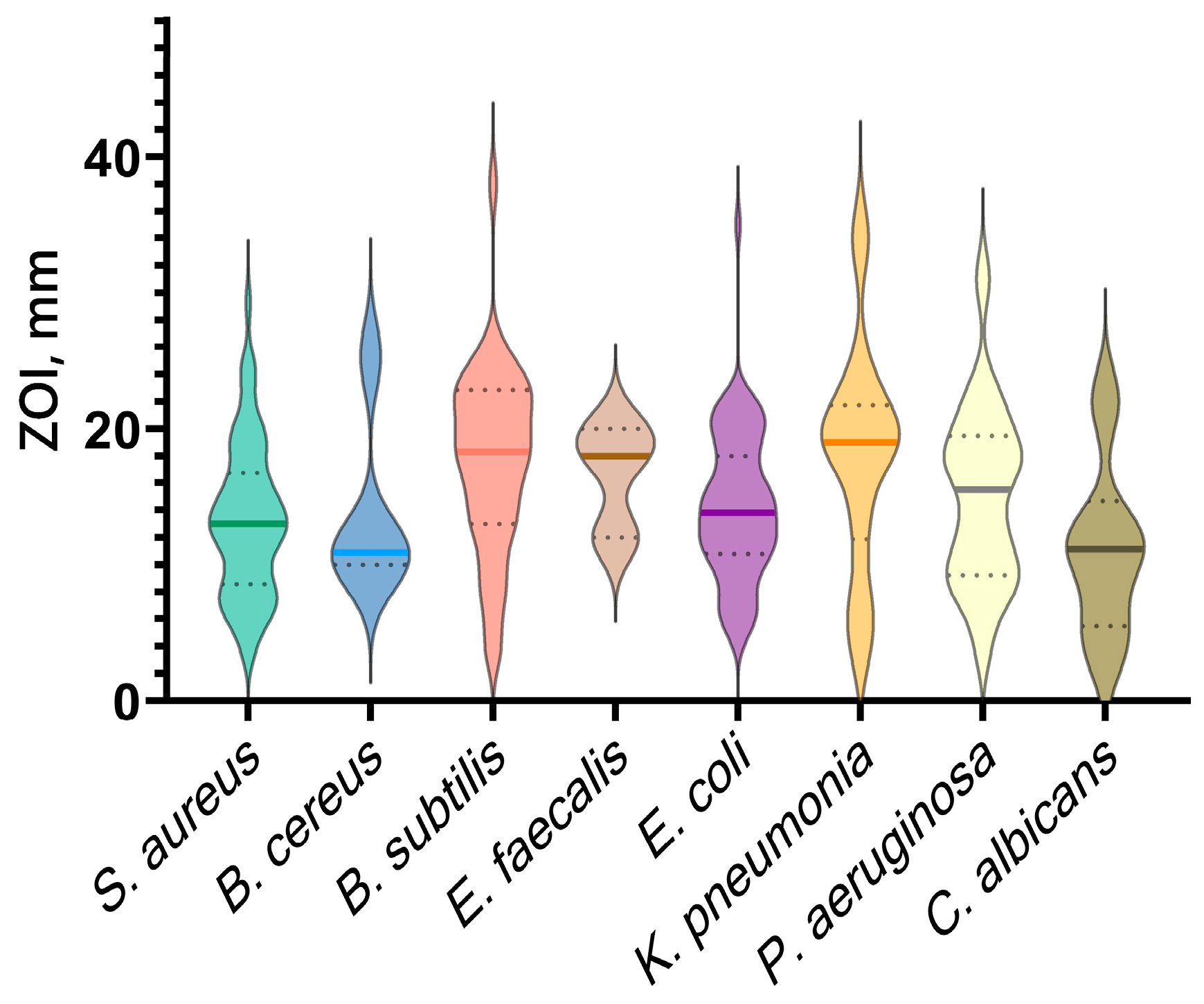
5.3. Effect of Shape and Size of CopOx-NPs on Antibacterial Properties
5.4. Comparison of Antibacterial Activity of CopOx-NPs with Other NPs
5.5. Activity of CopOx-NPs against Biofilm and Resistant Strains
6. Antifungal Activity of CopOx-NPs
7. “Antioxidant” Properties of CopOx-NPs
8. Cytotoxicity and Anticancer Properties of CopOx-NPs
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alavi, M.; Moradi, M. Different antibacterial and photocatalyst functions for herbal and bacterial synthesized silver and copper/copper oxide nanoparticles/nanocomposites: A review. Inorg. Chem. Commun. 2022, 142, 109590. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade, N.; et al. Modern physical methods and technologies in agriculture. Phys.-Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef] [PubMed]
- Leont’ev, V.K.; Pogorel’skii, I.P.; Frolov, G.A.; Karasenkov, Y.N.; Gusev, A.A.; Latuta, N.V.; Borozdkin, L.L.; Stefantsova, D.S. Antibacterial Properties of Aqueous Colloid Solutions of Metal and Metal Oxide Nanoparticles against Dental Plaque Bacteria. Nanotechnol. Russ. 2018, 13, 195–198. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef]
- Chaturvedi, K.S.; Henderson, J.P. Pathogenic adaptations to host-derived antibacterial copper. Front. Cell. Infect. Microbiol. 2014, 4, 3. [Google Scholar] [CrossRef]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance—New insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry-a narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Rall, J. Copper—A “Modern” Bioelement. Angew. Chem. Int. Ed. Engl. 2003, 35, 43–60. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.C.; Hsiao, C.-D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Troncoso, R.; Uauy, R. Biological Aspects of Copper. In Clinical and Translational Perspectives on WILSON DISEASE; Academic Press: Cambridge, MA, USA, 2019; pp. 25–31. [Google Scholar]
- Gebreslassie, Y.T.; Gebremeskel, F.G. Green and cost-effective biofabrication of copper oxide nanoparticles: Exploring antimicrobial and anticancer applications. Biotechnol. Rep. 2024, 41, e00828. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar Reddy, N.; Abhilash, P.; Rahul, M. Enhancement of heat transfer in radiator using copper oxide nano fluids. Mater. Today Proc. 2021, 39, 643–648. [Google Scholar] [CrossRef]
- Singh Sokhal, G.; Gangacharyulu, D.; Bulasara, V.K. Influence of copper oxide nanoparticles on the thermophysical properties and performance of flat tube of vehicle cooling system. Vacuum 2018, 157, 268–276. [Google Scholar] [CrossRef]
- Okoye, P.C.; Azi, S.O.; Qahtan, T.F.; Owolabi, T.O.; Saleh, T.A. Synthesis, properties, and applications of doped and undoped CuO and Cu2O nanomaterials. Mater. Today Chem. 2023, 30, 101513. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Sibhatu, A.K.; Weldegebrieal, G.K.; Sagadevan, S.; Tran, N.N.; Hessel, V. Photocatalytic activity of CuO nanoparticles for organic and inorganic pollutants removal in wastewater remediation. Chemosphere 2022, 300, 134623. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Daskalova, D.S.; Yordanova, L.P.; Pavlova, E.L. Copper and Copper Nanoparticles Applications and Their Role against Infections: A Minireview. Processes 2024, 12, 352. [Google Scholar] [CrossRef]
- ur Rehman, K.; Ullah Khan, A.; Tahir, K.; Nazir, S.; Albalawi, K.; Hassan, H.M.A.; Alabbad, E.A.; Refat, M.S.; Al-Shehri, H.S.; Mohammed Aldawsari, A. Facile synthesis of copper oxide nanoparticles (CuONPs) using green method to promote photocatalytic and biocidal applications. J. Mol. Liq. 2022, 360, 119453. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Mirza, A.A.; Suhail, M.; Jabir, N.R.; Zaidi, S.K.; Wasi, S.; Zawawi, A.; Tabrez, S.; Roy, A. Evaluation of Anticancer Potential of Biogenic Copper Oxide Nanoparticles (CuO NPs) against Breast Cancer. J. Nanomater. 2022, 2022, 5326355. [Google Scholar] [CrossRef]
- Su, Z.; Yao, C.; Tipper, J.; Yang, L.; Xu, X.; Chen, X.; Bao, G.; He, B.; Xu, X.; Zheng, Y. Nanostrategy of Targeting at Embryonic Trophoblast Cells Using CuO Nanoparticles for Female Contraception. ACS Nano 2023, 17, 25185–25204. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Arcos, M.; Dille, J.; Godet, S.; Rousse, C. Effect of the Concentration of NaBH4 and N2H4 as Reductant Agent on the Synthesis of Copper Oxide Nanoparticles and its Potential Antimicrobial Applications. Nano Biomed. Eng. 2018, 10, 392–405. [Google Scholar] [CrossRef]
- Evdokimova, O.L.; Belousova, M.E.; Evdokimova, A.V.; Kusova, T.V.; Baranchikov, A.E.; Antonets, K.S.; Nizhnikov, A.A.; Agafonov, A.V. Fast and simple approach for production of antibacterial nanocellulose/cuprous oxide hybrid films. Cellulose 2021, 28, 2931–2945. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Sagadevan, S.; Vennila, S.; Marlinda, A.R.; Al-Douri, Y.; Rafie Johan, M.; Anita Lett, J. Synthesis and evaluation of the structural, optical, and antibacterial properties of copper oxide nanoparticles. Appl. Phys. A 2019, 125, 489. [Google Scholar] [CrossRef]
- Černík, M.; Thekkae Padil, V.V. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Awwad, A.M.; Albiss, B.A.; Salem, N.M. Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract. SMU Med. J. 2015, 2, 91–101. [Google Scholar]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A.; Kim, D. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Ahmadi, S. Green synthesis and morphology dependent antibacterial activity of copper oxide nanoparticles. J. Nanostruct. 2019, 9, 163–171. [Google Scholar]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Selvaraj, S.P. Enhanced surface morphology of copper oxide (CuO) nanoparticles and its antibacterial activities. Mater. Today Proc. 2022, 50, 2865–2868. [Google Scholar] [CrossRef]
- Chen, J.; Mao, S.; Xu, Z.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborneRalstonia solanacearum. RSC Adv. 2019, 9, 3788–3799. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.; Hiremath, M.B. Biological Synthesis, Characterization and Antibacterial Activity of Novel Copper Oxide Nanoparticles (CuONPs). J. Bionanosci. 2018, 12, 92–99. [Google Scholar] [CrossRef]
- Erci, F.; Cakir-Koc, R.; Yontem, M.; Torlak, E. Synthesis of biologically active copper oxide nanoparticles as promising novel antibacterial-antibiofilm agents. Prep. Biochem. Biotechnol. 2020, 50, 538–548. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Strongly Enhanced Antibacterial Action of Copper Oxide Nanoparticles with Boronic Acid Surface Functionality. ACS Appl. Mater. Interfaces 2019, 11, 12232–12243. [Google Scholar] [CrossRef]
- Rajamohan, R.; Raorane, C.J.; Kim, S.-C.; Lee, Y.R. One Pot Synthesis of Copper Oxide Nanoparticles for Efficient Antibacterial Activity. Materials 2022, 16, 217. [Google Scholar] [CrossRef]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Faltane, M.; Rahman, A.; Dahoumane, S.A.; Kucknoor, A.; Jeffryes, C.S. Optimized production of antibacterial copper oxide nanoparticles in a microwave-assisted synthesis reaction using response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 170–178. [Google Scholar] [CrossRef]
- Andualem, W.W.; Sabir, F.K.; Mohammed, E.T.; Belay, H.H.; Gonfa, B.A.; Lázaro, M.J. Synthesis of Copper Oxide Nanoparticles Using Plant Leaf Extract of Catha edulis and Its Antibacterial Activity. J. Nanotechnol. 2020, 2020, 2932434. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendhi, A. Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Verma, A.; Bharadvaja, N. Plant-Mediated Synthesis and Characterization of Silver and Copper Oxide Nanoparticles: Antibacterial and Heavy Metal Removal Activity. J. Clust. Sci. 2021, 33, 1697–1712. [Google Scholar] [CrossRef]
- Talebian, S.; Shahnavaz, B.; Nejabat, M.; Abolhassani, Y.; Rassouli, F.B. Bacterial-mediated synthesis and characterization of copper oxide nanoparticles with antibacterial, antioxidant, and anticancer potentials. Front. Bioeng. Biotechnol. 2023, 11, 1140010. [Google Scholar] [CrossRef]
- Ontiveros-Robles, J.A.; Villanueva-Flores, F.; Juarez-Moreno, K.; Simakov, A.; Vazquez-Duhalt, R. Antibody-Functionalized Copper Oxide Nanoparticles with Targeted Antibacterial Activity. ChemistryOpen 2023, 12, e202200241. [Google Scholar] [CrossRef]
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.A.; Allahyari-Devin, M. Biosynthesis of Copper Oxide Nanoparticles Using Lactobacillus casei Subsp. Casei and its Anticancer and Antibacterial Activities. Curr. Nanosci. 2020, 16, 101–111. [Google Scholar] [CrossRef]
- Asamoah, R.B.; Yaya, A.; Mensah, B.; Nbalayim, P.; Apalangya, V.; Bensah, Y.D.; Damoah, L.N.W.; Agyei-Tuffour, B.; Dodoo-Arhin, D.; Annan, E. Synthesis and characterization of zinc and copper oxide nanoparticles and their antibacteria activity. Results Mater. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Akhter, S.M.H.; Mohammad, F.; Ahmad, S. Terminalia belerica Mediated Green Synthesis of Nanoparticles of Copper, Iron and Zinc Metal Oxides as the Alternate Antibacterial Agents Against some Common Pathogens. BioNanoScience 2019, 9, 365–372. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Hassan, S.E.-D.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Priya, M.; Venkatesan, R.; Deepa, S.; Sana, S.S.; Arumugam, S.; Karami, A.M.; Vetcher, A.A.; Kim, S.-C. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci. Rep. 2023, 13, 18838. [Google Scholar] [CrossRef] [PubMed]
- Azam, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Moniri Javadhesari, S.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C 2019, 105, 110011. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: Photocatalytic degradation and in vitro antioxidant activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Anu Thakur, N.; Kumar, K.; Sharma, K.K. Application of Co-doped copper oxide nanoparticles against different multidrug resistance bacteria. Inorg. Nano-Met. Chem. 2020, 50, 933–943. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for In Vitro Antimicrobial Activity Against Some Plant Pathogens. J. Clust. Sci. 2019, 31, 79–90. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Hamed, M.M.; Al-Agamy, M.H.; Gazwi, H.S.S.; Radwan, H.H.; Youssif, A.M.; Ali, S. Biosynthesis of Copper Oxide Nanoparticles Using Streptomyces MHM38 and Its Biological Applications. J. Nanomater. 2021, 2021, 6693302. [Google Scholar] [CrossRef]
- Tshireletso, P.; Ateba, C.N.; Fayemi, O.E. Spectroscopic and Antibacterial Properties of CuONPs from Orange, Lemon and Tangerine Peel Extracts: Potential for Combating Bacterial Resistance. Molecules 2021, 26, 586. [Google Scholar] [CrossRef]
- Prakash, S.; Elavarasan, N.; Venkatesan, A.; Subashini, K.; Sowndharya, M.; Sujatha, V. Green synthesis of copper oxide nanoparticles and its effective applications in Biginelli reaction, BTB photodegradation and antibacterial activity. Adv. Powder Technol. 2018, 29, 3315–3326. [Google Scholar] [CrossRef]
- Rajamma, R.; Gopalakrishnan Nair, S.; Abdul khadar, F.; Baskaran, B. Antibacterial and anticancer activity of biosynthesised CuO nanoparticles. IET Nanobiotechnol. 2020, 14, 833–838. [Google Scholar] [CrossRef]
- Nagaraj, E.; Karuppannan, K.; Shanmugam, P.; Venugopal, S. Exploration of Bio-synthesized Copper Oxide Nanoparticles Using Pterolobium hexapetalum Leaf Extract by Photocatalytic Activity and Biological Evaluations. J. Clust. Sci. 2019, 30, 1157–1168. [Google Scholar] [CrossRef]
- Khatami, M.; Varma, R.S.; Heydari, M.; Peydayesh, M.; Sedighi, A.; Agha Askari, H.; Rohani, M.; Baniasadi, M.; Arkia, S.; Seyedi, F.; et al. Copper Oxide Nanoparticles Greener Synthesis Using Tea and its Antifungal Efficiency on Fusarium solani. Geomicrobiol. J. 2019, 36, 777–781. [Google Scholar] [CrossRef]
- Yugandhar, P.; Vasavi, T.; Uma Maheswari Devi, P.; Savithramma, N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 2017, 7, 417–427. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Al-Maleki, A.R.; Alagiri, M.; Yahya, R.; Saravanan, S.; Vadivelu, J. In vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Adv. 2016, 6, 110986–110995. [Google Scholar] [CrossRef]
- Halder, U.; Roy, R.K.; Biswas, R.; Khan, D.; Mazumder, K.; Bandopadhyay, R. Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem. Eng. J. Adv. 2022, 11, 100294. [Google Scholar] [CrossRef]
- Rad, M.; Taran, M.; Alavi, M. Effect of Incubation Time, CuSO4 and Glucose Concentrations on Biosynthesis of Copper Oxide (CuO) Nanoparticles with Rectangular Shape and Antibacterial Activity: Taguchi Method Approach. Nano Biomed. Eng. 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Ramzan, M.; Obodo, R.M.; Mukhtar, S.; Ilyas, S.Z.; Aziz, F.; Thovhogi, N. Green synthesis of copper oxide nanoparticles using Cedrus deodara aqueous extract for antibacterial activity. Mater. Today Proc. 2021, 36, 576–581. [Google Scholar] [CrossRef]
- Rajendaran, K.; Muthuramalingam, R.; Ayyadurai, S. Green synthesis of Ag-Mo/CuO nanoparticles using Azadirachta indica leaf extracts to study its solar photocatalytic and antimicrobial activities. Mater. Sci. Semicond. Process. 2019, 91, 230–238. [Google Scholar] [CrossRef]
- Amin, F.; Fozia Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; Ahmad, R. Green Synthesis of Copper Oxide Nanoparticles Using Aerva javanica Leaf Extract and Their Characterization and Investigation of In Vitro Antimicrobial Potential and Cytotoxic Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 5589703. [Google Scholar] [CrossRef]
- Manasa, D.J.; Chandrashekar, K.R.; Madhu Kumar, D.J.; Niranjana, M.; Navada, K.M. Mussaenda frondosa L. mediated facile green synthesis of Copper oxide nanoparticles—Characterization, photocatalytic and their biological investigations. Arab. J. Chem. 2021, 14, 103184. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. Eco-friendly Ocimum tenuiflorum green route synthesis of CuO nanoparticles: Characterizations on photocatalytic and antibacterial activities. J. Environ. Chem. Eng. 2021, 9, 105395. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K. Aloe-vera leaf extract as a green agent for the synthesis of CuO nanoparticles inactivating bacterial pathogens and dye. J. Dispers. Sci. Technol. 2020, 42, 1950–1962. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Kollu, P.; Khan, S.; Pammi, S.V.N. Antibacterial activity assessment and characterization of green synthesized CuO nano rods using Asparagus racemosus roots extract. SN Appl. Sci. 2019, 1, 421. [Google Scholar] [CrossRef]
- Sabeena, G.S.R.E.P.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ.-Sci. 2022, 34, 102092. [Google Scholar] [CrossRef]
- Velsankar, K.; Vinothini, V.; Sudhahar, S.; Kumar, M.K.; Mohandoss, S. Green Synthesis of CuO nanoparticles via Plectranthus amboinicus leaves extract with its characterization on structural, morphological, and biological properties. Appl. Nanosci. 2020, 10, 3953–3971. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Nagore, P.; Ghotekar, S.; Mane, K.; Ghoti, A.; Bilal, M.; Roy, A. Structural Properties and Antimicrobial Activities of Polyalthia longifolia Leaf Extract-Mediated CuO Nanoparticles. BioNanoScience 2021, 11, 579–589. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Amin, B.H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Salem, S.S. Potential Antimicrobial and Antibiofilm Properties of Copper Oxide Nanoparticles: Time-Kill Kinetic Essay and Ultrastructure of Pathogenic Bacterial Cells. Appl. Biochem. Biotechnol. 2022, 195, 467–485. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Ghosh, R.; Dam, S.; Baskey, M. Madhuca longifolia plant mediated green synthesis of cupric oxide nanoparticles: A promising environmentally sustainable material for waste water treatment and efficient antibacterial agent. J. Photochem. Photobiol. B Biol. 2018, 189, 66–73. [Google Scholar] [CrossRef]
- Iqbal, J.; Andleeb, A.; Ashraf, H.; Meer, B.; Mehmood, A.; Jan, H.; Zaman, G.; Nadeem, M.; Drouet, S.; Fazal, H.; et al. Potential antimicrobial, antidiabetic, catalytic, antioxidant and ROS/RNS inhibitory activities of Silybum marianum mediated biosynthesized copper oxide nanoparticles. RSC Adv. 2022, 12, 14069–14083. [Google Scholar] [CrossRef]
- Alavi, M.; Dehestaniathar, S.; Mohammadi, S.; Maleki, A.; Karimi, N. Antibacterial Activities of Phytofabricated ZnO and CuO NPs by Mentha pulegium Leaf/flower Mixture Extract against Antibiotic Resistant Bacteria. Adv. Pharm. Bull. 2020, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, P.C.; Kumar, M.P.; Suresh, D.; Lingaraju, K.; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties. Mater. Sci. Semicond. Process. 2015, 33, 81–88. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Awwad, A.; Amer, M. Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem. Int. 2020. [Google Scholar]
- Saebnoori, E.; Koupaei, N.; Hassanzadeh Tabrizi, S.A. The solution plasma synthesis, characterisation, and antibacterial activities of dispersed CuO nanoparticles. Mater. Technol. 2021, 37, 1220–1229. [Google Scholar] [CrossRef]
- Sharmila, G.; Sakthi Pradeep, R.; Sandiya, K.; Santhiya, S.; Muthukumaran, C.; Jeyanthi, J.; Manoj Kumar, N.; Thirumarimurugan, M. Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: Characterization and its antibacterial application. J. Mol. Struct. 2018, 1165, 288–292. [Google Scholar] [CrossRef]
- Aziz, W.J.; Abid, M.A.; Hussein, E.H. Biosynthesis of CuO nanoparticles and synergistic antibacterial activity using mint leaf extract. Mater. Technol. 2019, 35, 447–451. [Google Scholar] [CrossRef]
- Mani, V.M.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A.J.P.G.; Ayyappa Das, M.P.; Elfasakhany, A.; Pugazhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 2021, 201, 111502. [Google Scholar] [CrossRef]
- Naseer, M.; Ramadan, R.; Xing, J.; Samak, N.A. Facile green synthesis of copper oxide nanoparticles for the eradication of multidrug resistant Klebsiella pneumonia and Helicobacter pylori biofilms. Int. Biodeterior. Biodegrad. 2021, 159, 105201. [Google Scholar] [CrossRef]
- Fazal, A.; Ara, S.; Ishaq, M.T.; Sughra, K. Green Fabrication of Copper Oxide Nanoparticles: A Comparative Antibacterial Study Against Gram-Positive and Gram-Negative Bacteria. Arab. J. Sci. Eng. 2021, 47, 523–533. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Angwe, M.K.; Wampande, E.M.; Ejobi, F.; Nxumalo, E.; Maaza, M.; Sackey, J.; Kirabira, J.B. Phyto-Mediated Copper Oxide Nanoparticles for Antibacterial, Antioxidant and Photocatalytic Performances. Front. Bioeng. Biotechnol. 2022, 10, 820218. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Mersian, H.; Alizadeh, M.; Hadi, N. Synthesis of zirconium doped copper oxide (CuO) nanoparticles by the Pechini route and investigation of their structural and antibacterial properties. Ceram. Int. 2018, 44, 20399–20408. [Google Scholar] [CrossRef]
- Rajesh, K.M.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 2018, 154, 593–600. [Google Scholar] [CrossRef]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, P.; Salam, H.A.; Venckatesh, R. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 178–181. [Google Scholar] [CrossRef]
- Gowri, M.; Latha, N.; Rajan, M. Copper Oxide Nanoparticles Synthesized Using Eupatorium odoratum, Acanthospermum hispidum Leaf Extracts, and Its Antibacterial Effects Against Pathogens: A Comparative Study. BioNanoScience 2019, 9, 545–552. [Google Scholar] [CrossRef]
- Shehab, W.S.; Elsayed, D.A.; Abdel Hamid, A.M.; Assy, M.G.; Mouneir, S.M.; Hamed, E.O.; Mousa, S.M.; El-Bassyouni, G.T. CuO nanoparticles for green synthesis of significant anti-Helicobacter pylori compounds with in silico studies. Sci. Rep. 2024, 14, 1608. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Abbas, H.S. Antimicrobial activity of biosynthesized Cuo/Se nanocomposite against Helicobacter pylori. Arab. J. Chem. 2023, 16, 105095. [Google Scholar] [CrossRef]
- Sonia, S.; Jayram, N.D.; Suresh Kumar, P.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Effect of NaOH concentration on structural, surface and antibacterial activity of CuO nanorods synthesized by direct sonochemical method. Superlattices Microstruct. 2014, 66, 1–9. [Google Scholar] [CrossRef]
- Teklu, B.; Kadiri, S.K.; Vidavalur, S. Green synthesis of copper oxide nanoparticles using Balanites aegyptiaca stem bark extract and investigation of antibacterial activity. Results Chem. 2023, 6, 101152. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K.; Ighalo, J.O. Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain. Environ. Res. 2022, 32, 2. [Google Scholar] [CrossRef]
- Pagar, T.; Ghotekar, S.; Pansambal, S.; Pagar, K.; Oza, R. Biomimetic synthesis of CuO nanoparticle using Capparis decidua and their antibacterial activity. Adv. J. Sci. Eng. 2020, 1, 133–137. [Google Scholar]
- Gopalakrishnan, R.; Ashokkumar, M. Rare earth metals (Ce and Nd) induced modifications on structural, morphological, and photoluminescence properties of CuO nanoparticles and antibacterial application. J. Mol. Struct. 2021, 1244, 131207. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, K. Effect of (Ag, Co) co-doping on the structural and antibacterial efficiency of CuO nanoparticles: A rapid microwave assisted method. J. Environ. Chem. Eng. 2020, 8, 104011. [Google Scholar] [CrossRef]
- Hesabizadeh, T.; Sung, K.; Park, M.; Foley, S.; Paredes, A.; Blissett, S.; Guisbiers, G. Synthesis of Antibacterial Copper Oxide Nanoparticles by Pulsed Laser Ablation in Liquids: Potential Application against Foodborne Pathogens. Nanomaterials 2023, 13, 2206. [Google Scholar] [CrossRef]
- Shafiq, A.; Jeong, U.; Han, Y.; Kim, Y.; Lee, J.; Kim, B.S. Green Synthesis of Copper Oxide Nanoparticles from Waste Solar Panels Using Piper nigrum Fruit Extract and Their Antibacterial Activity. Catalysts 2024, 14, 472. [Google Scholar] [CrossRef]
- Oetiker, N.; Salinas, D.; Lucero-Mora, J.; Orellana, R.; Quiroz-Muñoz, M.; Bravo, D.; Pérez-Donoso, J.M. Antimicrobial Effect of Copper Nanoparticles on Relevant Supragingival Oral Bacteria. Microorganisms 2024, 12, 624. [Google Scholar] [CrossRef]
- Rehman, F.U.; Mahmood, R.; Haq, S.; Ahmad, P.; Din, S.U.; Khandaker, M.U.; Idris, A.M.; Zekker, I. Phytogenic Fabrication of Copper Oxide Nanoparticles for Antibacterial and Antioxidant Screening: Physico-Chemical Study. Crystals 2022, 12, 1796. [Google Scholar] [CrossRef]
- Aien, J.; Khan, A.A.; Haq, S.; Khan, A.R.; Elmnasri, K.; Ben Ali, M.; Al-Harbi, M.S.; Alghonaim, M.I.; Alsalamah, S.A.; Qurtam, A.A.; et al. Antibacterial, Antioxidant and Physicochemical Properties of Pipper nigram Aided Copper Oxide Nanoparticles. Crystals 2023, 13, 330. [Google Scholar] [CrossRef]
- Rivera-Mendoza, D.; Quiñones, B.; Huerta-Saquero, A.; Castro-Longoria, E. Antimicrobial Activity of Green Synthesized Silver and Copper Oxide Nanoparticles against the Foodborne Pathogen Campylobacter jejuni. Antibiotics 2024, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Haseen, U.; Jalal, M.; Khan, R.A.; Alsalme, A.; Ahmad, H.; Khan, H.M. Green Synthesis of Copper Oxide Nanoparticles from the Leaves of Aegle marmelos and Their Antimicrobial Activity and Photocatalytic Activities. Molecules 2023, 28, 7499. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Raorane, C.J.; Kim, S.-C.; Ashokkumar, S.; Lee, Y.R. Novel Microwave Synthesis of Copper Oxide Nanoparticles and Appraisal of the Antibacterial Application. Micromachines 2023, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Jayakumar, M.N.; Ahmad, H.; Gokhale, T. Antimicrobial Activity of Biogenic Metal Oxide Nanoparticles and Their Synergistic Effect on Clinical Pathogens. Int. J. Mol. Sci. 2023, 24, 9998. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2020, 199, 2788–2799. [Google Scholar] [CrossRef]
- Dadi, R.; Azouani, R.; Traore, M.; Mielcarek, C.; Kanaev, A. Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater. Sci. Eng. C 2019, 104, 109968. [Google Scholar] [CrossRef]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Chen, N.-F.; Liao, Y.-H.; Lin, P.-Y.; Chen, W.-F.; Wen, Z.-H.; Hsieh, S. Investigation of the Characteristics and Antibacterial Activity of Polymer-Modified Copper Oxide Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12913. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.; Ghozlan, H.A.; Sabry, S.A.; Abouelkheir, S.S. Copper oxide nanoparticles (CuO-NPs) as a key player in the production of oil-based paint against biofilm and other activities. Heliyon 2024, 10, e29758. [Google Scholar] [CrossRef]
- Saha, T.; Bin Mobarak, M.; Uddin, M.N.; Quddus, M.S.; Naim, M.R.; Pinky, N.S. Biogenic synthesis of copper oxide (CuO) NPs exploiting Averrhoa carambola leaf extract and its potential antibacterial activity. Mater. Chem. Phys. 2023, 305, 127979. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Gusev, A.A.; Altabaeva, Y.V.; Perova, S.Y. Biological Effects of Freshly Prepared and 24-h Aqueous Dispersions of Copper and Copper Oxide Nanoparticles on E. coli Bacteria. Nanotechnol. Russ. 2018, 13, 173–181. [Google Scholar] [CrossRef]
- Rani, A.; Asha, S.; Mini, M.; Rajan, P.P.; Tomy, M.; Jose, A.; Ts, X.; Kumar, P. Exploring the antibacterial and antibiofilm potential of copper oxide nanoparticles biosynthesized using Centratherum punctatum leaf extract. S. Afr. J. Bot. 2024, 164, 1–8. [Google Scholar] [CrossRef]
- Khatami, M.; Heli, H.; Mohammadzadeh Jahani, P.; Azizi, H.; Lima Nobre, M.A. Copper/copper oxide nanoparticles synthesis usingStachys lavandulifoliaand its antibacterial activity. IET Nanobiotechnol. 2017, 11, 709–713. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Du, B.D.; Phu, D.V.; Quoc, L.A.; Hien, N.Q. Synthesis and Investigation of Antimicrobial Activity of Cu2O Nanoparticles/Zeolite. J. Nanopart. 2017, 2017, 7056864. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; del Val, J.; Riveiro, A.; Rodríguez, D.; Arias-González, F.; Gil, J.; Pou, J. Fabrication and Deposition of Copper and Copper Oxide Nanoparticles by Laser Ablation in Open Air. Nanomaterials 2020, 10, 300. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, M.; Wu, M.; Zhang, H.; Song, Q.; Yu, S. Synthesis of biochar-based Cu2O nanoparticles and their antibacterial activity against Escherichia coli. Inorg. Nano-Met. Chem. 2019, 49, 12–16. [Google Scholar] [CrossRef]
- AlYahya, S.; Rani, B.J.; Ravi, G.; Yuvakkumar, R.; Arun, A.; Ameen, F.; AlNadhary, S. Size dependent magnetic and antibacterial properties of solvothermally synthesized cuprous oxide (Cu2O) nanocubes. J. Mater. Sci. Mater. Electron. 2018, 29, 17622–17629. [Google Scholar] [CrossRef]
- Thanuja, J.; Udayabhanu; Nagaraju, G.; Naika, H.R. Biosynthesis of Cu4O3 nanoparticles using Razma seeds: Application to antibacterial and cytotoxicity activities. SN Appl. Sci. 2019, 1, 1646. [Google Scholar] [CrossRef]
- Tarangini, K.; Sherlin, S.N.; Jakkala, S.; Borah, P.; Wacławek, S.; Sabarinath, S.; Rao, K.J.; Padil, V.V.T. Optimising Biosynthesis of Antimicrobial Copper Nanoparticles Using Aqueous Aegle marmelos Leaf Extract-Based Medium. Ecol. Chem. Eng. S 2024, 31, 7–17. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Bhat, A.H.; Kavitha, K.; Ramanan, A.; Rajagopal, K.; Srinivasan, R.; Manikandan, E. Enhanced Biosynthesis Synthesis of Copper Oxide Nanoparticles (CuO-NPs) for their Antifungal Activity Toxicity against Major Phyto-Pathogens of Apple Orchards. Pharm. Res. 2020, 37, 246. [Google Scholar] [CrossRef] [PubMed]
- Simakin, A.V.; Voronov, V.V.; Shafeev, G.A. Nanoparticle formation during laser ablation of solids in liquids. Phys. Wave Phenom. 2007, 15, 218–240. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Ren, E.; Zhang, C.; Li, D.; Pang, X.; Liu, G. Leveraging metal oxide nanoparticles for bacteria tracing and eradicating. View 2020, 1, 20200052. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Cioffi, N.; Ditaranto, N.; Torsi, L.; Picca, R.A.; Sabbatini, L.; Valentini, A.; Novello, L.; Tantillo, G.; Bleve-Zacheo, T.; Zambonin, P.G. Analytical characterization of bioactive fluoropolymer ultra-thin coatings modified by copper nanoparticles. Anal. Bioanal. Chem. 2004, 381, 607–616. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2013, 114, 538–556. [Google Scholar] [CrossRef]
- Kirakosyan, G.; Trchounian, K.; Vardanyan, Z.; Trchounian, A. Copper (II) Ions Affect Escherichia coli Membrane Vesicles’ SH-Groups and a Disulfide-Dithiol Interchange Between Membrane Proteins. Cell Biochem. Biophys. 2008, 51, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, H.O.S.; Bandyopadhyay, P. Understanding Cu+2 binding with DNA: A molecular dynamics study comparing Cu2+ and Mg2+ binding to the Dickerson DNA. bioRxiv 2024. [Google Scholar] [CrossRef]
- Glazkova, E.A.; Bakina, O.V.; Rodkevich, N.G.; Mosunov, A.A.; Vornakova, E.A.; Chzhou, V.R.; Lerner, M.I. Copper ferrite/copper oxides (I, II) nanoparticles synthesized by electric explosion of wires for high performance photocatalytic and antibacterial applications. Mater. Sci. Eng. B 2022, 283, 115845. [Google Scholar] [CrossRef]
- Aaga, G.F.; Anshebo, S.T. Green synthesis of highly efficient and stable copper oxide nanoparticles using an aqueous seed extract of Moringa stenopetala for sunlight-assisted catalytic degradation of Congo red and alizarin red s. Heliyon 2023, 9, e16067. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Gao, Y.; Feng, C. Photocatalytic degradation of methylene blue by magnetically recoverable Fe3O4/Ag6Si2O7 under simulated visible light. Powder Technol. 2018, 326, 247–254. [Google Scholar] [CrossRef]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Venkata Rao, J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef]
- Shinde, V.V.; Dalavi, D.S.; Mali, S.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Surfactant free microwave assisted synthesis of ZnO microspheres: Study of their antibacterial activity. Appl. Surf. Sci. 2014, 307, 495–502. [Google Scholar] [CrossRef]
- Ahmadipour, S.; Field, R.A.; Miller, G.J. Prospects for anti-Candida therapy through targeting the cell wall: A mini-review. Cell Surf. 2021, 7, 100063. [Google Scholar] [CrossRef]
- Garcia-Marin, L.E.; Juarez-Moreno, K.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Highly Antifungal Activity of Biosynthesized Copper Oxide Nanoparticles against Candida albicans. Nanomaterials 2022, 12, 3856. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Vakhrusheva, T.V.; Sokolov, A.V.; Moroz, G.D.; Kostevich, V.A.; Gorbunov, N.P.; Smirnov, I.P.; Grafskaia, E.N.; Latsis, I.A.; Panasenko, O.M.; Lazarev, V.N. Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity. Antioxidants 2022, 11, 2419. [Google Scholar] [CrossRef]
- Konovalova, G.G.; Sharapov, M.G.; Tikhaze, A.K.; Lankin, V.Z. The Role of Natural Low Molecular Weight Dicarbonyls in Atherogenesis and Diabetogenesis. Rev. Cardiovasc. Med. 2024, 25, 295. [Google Scholar] [CrossRef]
- Mikhailova, D.V.; Shevchenko, O.G.; Golubev, D.A.; Platonova, E.Y.; Zemskaya, N.V.; Shoeva, O.Y.; Gordeeva, E.I.; Patov, S.A.; Shaposhnikov, M.V.; Khlestkina, E.K.; et al. Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants 2023, 12, 2010. [Google Scholar] [CrossRef]
- Eltayeb, L.M.H.; Yagi, S.; Mohamed, H.M.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential Oils Composition and Biological Activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia Grown Wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef]


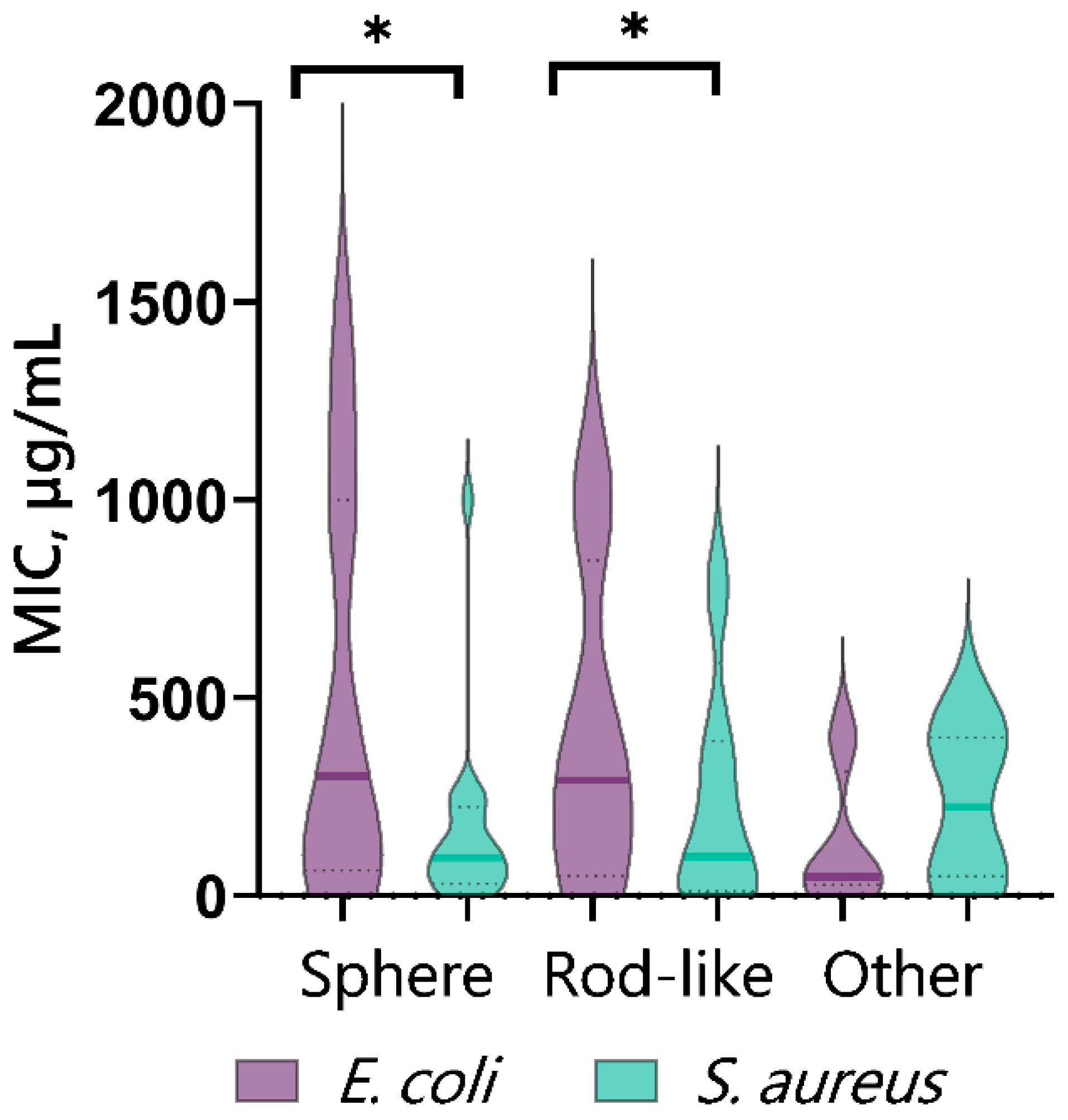
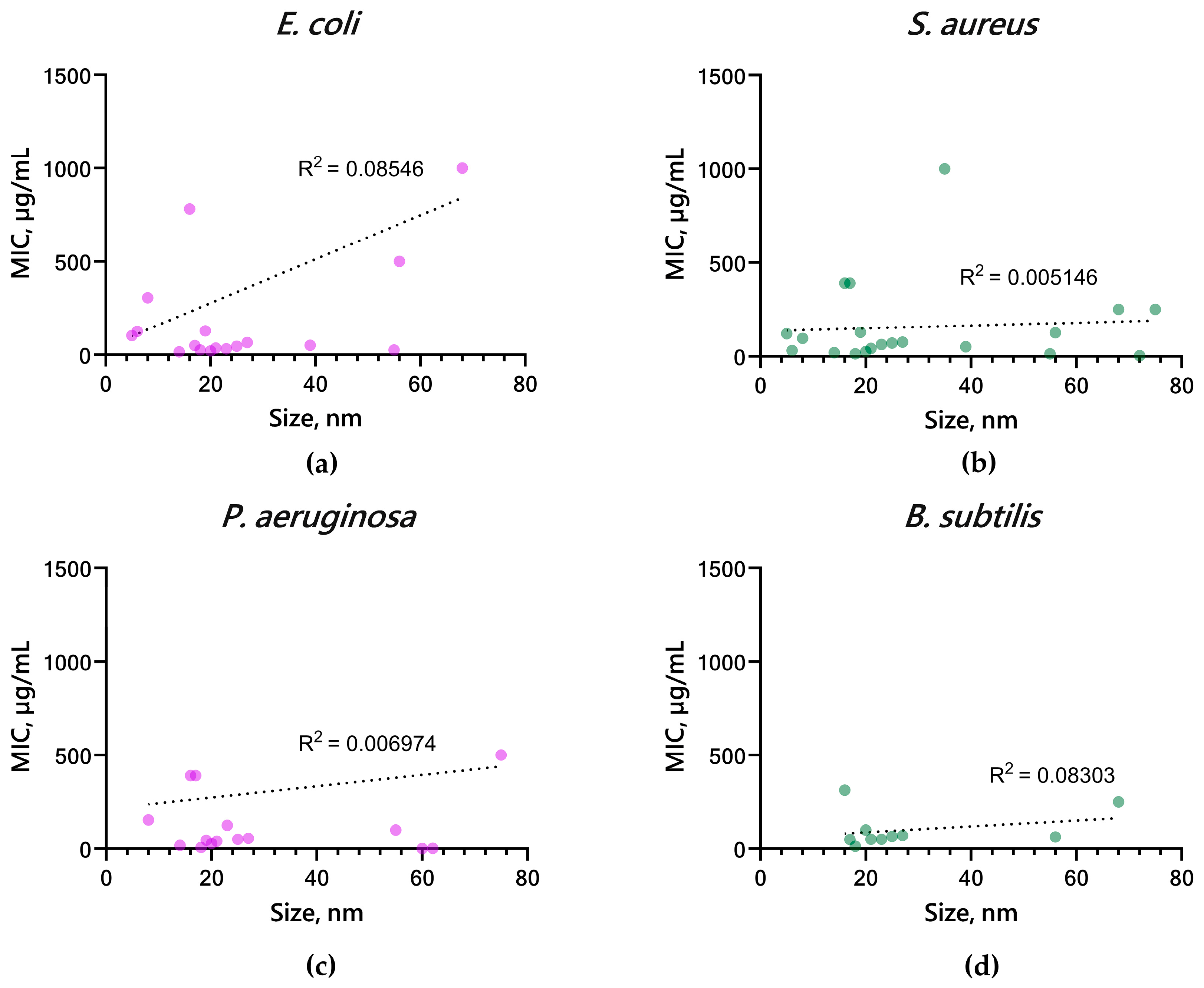
| No. | Synthesis Method | Composition | Size, nm | Shape | Concentration | Medium, Conditions | Microorganism | Biol Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Electrochemical reduction using tetrabutylammonium bromide | CuO | 5–10 | Sph. | 50–100 µg/mL | NB, 24 h, 37 °C, NA, 1 h | E. coli, S. aureus | BS | [27] |
| 2 | Chemical precipitation followed by drying (for Cu2O, the reducing agent hydrazine is added) | Tryptophan-coated CuO, Cu2O | 30 for CuO, 40 for Cu2O | Sph. | 0.1 mM, 0.05 mM (MIC); 0.25 mM, 0.1 mM (MBC) | NA, 18 h | E. coli | BS, BC | [28] |
| 3 | Combustion using ascorbic acid as a coating agent | CuO | 36, 45 | Sph. | 100 µg/mL | NA, 24 h, 37 °C | E. coli, S. typhi, M. luteus, P. fluorescens, S. flexneri, V. cholerae | BS | [29] |
| 4 | Colloidal–thermal synthesis (using caraya gum) | CuO | 2–10 | Sph. | 10–100 µg/mL | NB, 18–24 h, 35 °C | E. coli, S. aureus | BS, BC | [30] |
| 5 | Actinomycete-mediated “green synthesis” | CuO | 61.7 | Sph. | 5–100 µg/mL | Agar for the isolation of Actinomycetes and starch-casein agar, 7–14 days, 27 °C | S. aureus, B. cereus, P. mirabilis, E. tarda, A. caviae, A. hydrophila, V. anguillarum | BS | [31] |
| 6 | “Green synthesis” using Malva sylvestris extract | CuO | 14 | Sph. | – | – | Shigella, Listeria | BS | [32] |
| 7 | Chemical deposition | CuO | 23 | Sph. | 31.25–250 µg/mL (MIC) | NA, 24 h, 37 °C, MHB, 12 h, 37 °C | E. coli, P. aeruginosa, K. pneumonia, E. faecalis, S. flexneri, S. typhimurium, P. vulgaris, S. aureus | BS | [33] |
| 8 | “Green Synthesis” using Aloe vera extract | CuO | 9–23 | Rod, Sph. | 10 µg/disk | MHA, 24 h, 37 °C | E. coli, S. aureus | BS | [34] |
| 9 | Thermoplasmic technology | CuO, CuO/Ag | 20–95 | Sph. | 100–5000 µg/mL | TSB, 24 h, 37 °C | S. aureus, S. epidermidis, P. aeruginosa, E. coli, Proteus sp. | BC | [35] |
| 10 | Chemical deposition | CuO | 70–90 | Rope-shaped | 50–200 µg/disk | NA, 24 h, 37 °C | P. aeruginosa, S. aureus, B. subtilis, E. coli | BS | [36] |
| 11 | “Green synthesis” using C. papaya extract | CuO | 40–80 | Sph. | 50–250 µg/mL | NB, 12, 24 and 72 h, 30 °C | R. solanacearum | BS, BC | [37] |
| 12 | “Green synthesis” using dried goat and sheep fecal matter | CuO | 29.20 ± 15.9 (32.30 ± 32.9) | Sph. | 100 µg/mL | NA, 24 h | B. subtilis, S. typhimurium | BS | [38] |
| 13 | “Green synthesis” using Thymbra spicata leaf extract | CuO-NPs + Thymbra spicata extract | 26.8, 21 | Sph. | 0.78–200 µg/mL | MHA, 24 h, 35 °C, MHB, 24 h, 35 °C | B. cereus, S. aureus, E. coli, S. typhimurium | BS, AB | [39] |
| 14 | Chemical deposition | CuO, CuONPs/GLYMO, CuONPs/GLYMO/4-HPBA (CuO + BA) | 13, 106 ± 6 (for CuONPs/GLYMO), 121 ± 4 (for CuONPs/GLYMO/4-HPBA) | – | 5–250 µg/mL | LB broth, 10 min., 1 h, 6 h, 37 °C | S. aureus, S. choleraesuis, C. albicans, P. aeruginosa, B. subtilis | BS | [40] |
| 15 | “Green synthesis” using apple peel extract | CuO | 25–55 | Square | 100–300 µg/mL | LB agar, 24 h, 37 °C | E. coli, S. aureus | BS, BC, AB | [41] |
| 16 | Microwave synthesis from starch, glucose, and CuCl2 | CuO | 1.36 ± 0.6 | Sph. | 0.0113, 0.00113, 0.000113 M/L | NA, 24 h, 37 °C | E. coli, S. epidermidis, S. aureus, B. megatarium | BS | [42] |
| 17 | “Green synthesis” using Catha edulis extract | CuO | 28.1, 25.3, 18.2 | Sph. | 20, 40 mg/mL | MHA, 24 h, 37 °C | S. aureus, S. pyogenes, K. pneumonia, E. coli | BS | [43] |
| 18 | “Green synthesis” using Psidium guajava extract | CuO | 40–150 | Sph. | 20–80 µg/mL | NB, MHA, 24 h, 37 °C | E. coli, P. aeruginosa, S. pneumoniae, S. epidermidis | BC | [44] |
| 19 | “Green synthesis” using Catharanthus roseus extract | CuO | <100 | Sph. | 10, 30, 50 µL/disk | NA, during night, 37 °C | S. aureus | BS | [45] |
| 20 | “Green synthesis” using Stenotrophomonas sp. strain BS95 | CuO | 55.92 (crude), 68.35 (calcined) | Sph. | 31.25–2000 µg/mL | MHB, 18 h, 37 °C, MHA, 24 h, 37 °C | B. subtilis, S. aureus, P. putida, E. coli | BS, BC | [46] |
| 21 | Commercially available NPs (Sigma-Aldrich) functionalized with specific antibodies | CuO, CuO-NP-AbGram, CuO-NP-AbGram+ | 242 ± 96 (CuO), 40 ± 42 (CuO-NP-AbGram+), 707 ± 243 (CuO-NP-AbGram-) | – | MIC: 1250/2250 µg/mL (CuO), 850/2900 µg/mL (CuO-NP-AbGram-), 1300/1600 µg/mL (CuO-NP-AbGram+) | LB broth, 24 h, 37 °C | E. coli, B. subtilis | BS | [47] |
| 22 | “Green synthesis” using cultures of Lactobacillus casei | CuO | 40–110 | Sph. | 0.075–5 mg/mL | MHA, 24 h, 37 °C | S. aureus, P. aeruginosa | BS, BC | [48] |
| 23 | Chemical deposition | CuO | 100 | Rod. | 0.01–5 mg/mL | NHA, MHB, overnight, 37 °C | E. coli, S. aureus | BS | [49] |
| 24 | “Green synthesis” using Terminalia belerica extract | CuO | 9–14 | Sph. | 1–10 mg/mL | NA, 24 h, 35 ± 2 °C | S. aureus, B. subtilis, E. coli, K. pneumoniae, S. enterica | BS | [50] |
| 25 | “Green synthesis” using aqueous extract of Portulaca oleracea | CuO | 5–30 | Sph. | 3.12–200 µg/mL | MHA, 24 h, 35 ± 2 °C | S. aureus, B. subtilis, P. aeruginosa, E. coli, C. albicans | BS, FS | [51] |
| 26 | “Green synthesis” using Morinda citrifolia extract | CuO | 20–50 | Sph. | 15–25 µL/disk | MHA, 24 h, 37 °C (bacteria); Dextrose agar, 24 h, 37 °C (fungi) | E. coli, B. subtilis, S. aureus; A. flavus, A. niger, P. frequentans | BS, FS | [52] |
| 27 | Gel combustion method | CuO | 20–27 | Sph. | 10–100 µg/mL | NA, 24 h, 35 ± 2 °C | E. coli, P. aeruginosa, B. subtilis, S. aureus | BS, BC | [53] |
| 28 | Mechanochemical processing | CuO | 7, 14 | Sph. | 1.25–10 mg/mL | NB, 24–72 h | E. coli, S. aureus | BS, BC | [54] |
| 29 | Chemical sol–gel method/“Green synthesis” using Solanum nigrum extract | CuO | 32, 25 | Sph. | 50, 100 µL/disk | NA, 24 h, 35 °C | B. subtilis, S. saprophyticus, E. coli, P. aeruginosa | BS | [55] |
| 30 | Microwave fusion | CuO, Cu0.96Ag0.02Ni0.02O, Cu0.94Ag0.02Ni0.04O, Cu0.92Ag0.02Ni0.06O, Cu0.90Ag0.02Ni0.08O | 16.53–29.81 | Rod, Needle, Sph. | 10, 25, 50 mg/mL | MHA, 24 h, 37 °C | B. subtilis, S. aureus, E. coli, P. aeruginosa | BS, BC | [56] |
| 31 | “Green fusion” using Penicillium chrysogenum | CuO | 9.7–10.7 | Sph. | 250 µg/mL | NA, 24 h, 37 °C; PDA, 5 days, 28 °C | E. amylovora, R. solanacearum, F. oxysporum, A. niger, P. citrinum, E. cichoracearum, A. solani | BS, FS | [57] |
| 32 | “Green synthesis” using Streptomyces strain MHM38 | CuO | 1.72–13.49 | Sph. | 200 mg/mL | MHA, 24 h, 37 °C, potato D-glucose agar/Sabouraud dextrose agar, 120 h, 28 °C | E. faecalis, S. typhimurium, P. aeruginosa, E. coli, R. solani, F. solani, A. niger, C. albicans | BS, BS | [58] |
| 33 | “Green synthesis” using orange, lemon, and tangerine peel extracts | CuO | 48–76 | Sph. | 25, 50 mg/mL | MHA, during night, 37 °C | E. faecalis, S. aureus, L. monocytogenes, S. pneumoniae, C. perfringens, E. coli, M. catarrhalis, S. diarizonae, C. coli, P. aeruginosa | BS | [59] |
| 34 | “Green synthesis” using Cordia sebestena extract | CuO | 20–35 | Sph. | 1000 µg/mL | NA, 24 h, 37 °C | B. subtilis, S. aureus, E. coli, K. pneumoniae | BS | [60] |
| 35 | “Green synthesis” using an extract of Nilgirianthus ciliatus | CuO | 20 | Sph. | 250, 500, 1000 µg/mL | MHA, 24 h, 37 °C | E. coli, P. aeruginosa, S. aureus, S. mutans | BS | [61] |
| 36 | “Green synthesis” using Pterolobium hexapetalum extract | CuO | 10–50 | Sph. | 10–50 µg/mL | NA, 24 h, 37 °C | S. aureus, B. subtilis, E. coli | BS | [62] |
| 37 | “Green synthesis” using Stachys lavandulifolia extract | CuO | <80 | – | 80 µg/mL | PDA, 24–72 h, 28 °C | F. solani | FS | [63] |
| 38 | “Green synthesis” using Syzygium alternifolium extract | CuO | 5–13 | Sph. | 5–80 µg/mL | NB, PDA, 24–48 h, 37 °C | B. subtilis, E. coli, S. aureus, K. pneumonia, P. vulgaris, P. aeruginosa, S. typhimurium, A. solani, A. flavus, A. niger, P. chrysogenum, T. harzianum | BS, FS | [64] |
| 39 | “Green synthesis” using Tribulus terrestris extract | CuO | 5–22 | Sph. | 2.5–50 µg/mL | MHA, MHB, 24 h, 37 °C | E. coli, P. aeruginosa, S. aureus, B. cereus | BS, BC | [65] |
| 40 | Co-precipitation | CuO | 108 ± 14 | Sph. | 1–10 µg/mL | MHA, MHB, during night, 30 °C | P. aeruginosa | BS, BC | [66] |
| 41 | “Green synthesis” using Halomonas elongata extract | CuO | 57–79 | Rectang. | 0.28, 0.39, 0.56 mM | NA, 48 h, 37 °C | E. coli, S. aureus | BS | [67] |
| 42 | “Green Synthesis” using Cedrus deodara extract | CuO | – | Sph. | 25–150 µg/mL | 1 day, 37 °C | S. aureus, E. coli | BS | [68] |
| 43 | “Green fusion” using Azardirachta indica extract | Ag and Mo-dopped CuO-NPs | 25, 19.8, 17.20, 14.57, 11.23 | – | – | NA, 24 h, 37 °C | P. aeruginosa, S. aureus, S. marcescens, C. albicans, A. niger | BS, FS | [69] |
| 44 | “Green synthesis” using Aerva javanica extract | CuO | 15–23 | Sph. | 50–200 µg/mL | MHA, 24 h, 37 °C; MHA, 48–72 h, 27 °C | P. aeruginosa, E. coli, S. aureus, A. baumannii, C. albicans, C. krusei, C. tropicalis | BS, FS, BC, FC | [70] |
| 45 | “Green synthesis” using Mussaenda frondosa extract | CuO | 2–10 | Sph. | 96–593 µg/mL (MIC) | NA, 24 h, 37 °C | S. aureus, B. subtilis, E. coli, P. aeruginosa, P. vulgaris | BS | [71] |
| 46 | “Green synthesis” using Ocimum tenuiflorum extract | CuO | 6–18; 4–8 × 12–44 | Sph., Rod | 10–50 mg/mL | NA | B. subtilis, S. aureus, E. coli | BS, BC | [72] |
| 47 | “Green Synthesis” using Aloe vera extract | CuO | 5–20 | Sph., Rod, Capsule | 10–50 mg/mL | NA | B. subtilis, S. aureus, E. coli | BS, BC | [73] |
| 48 | “Green synthesis” using Asparagus racemosus extract | CuO | 50–100 × 400–500 | Rod | 50 µg/mL | MHA, 24 h, 37 °C | E. coli, B. subtilus, K. pneumonia, A. hydrophila, P. fluorescens, Y. ruckeri, F. branchiophilum, E. tarda | BS | [74] |
| 49 | “Green synthesis” using Salacia reticulate extract/Chemical precipitation | CuO | 42.2/84 | – | 20–80 µg/mL | MHA, 37 °C | E. coli, S. aureus, Enterobacter, B. subtilis, P. aeruginosa | BS | [75] |
| 50 | “Green synthesis” using Plectranthus amboinicus extract | CuO | 5–30 | Sph. | 30–150 µg/mL | NA, 24 h, 37 °C | E. coli, S. aureus, B. subtilis, S. pyogenes, P. aeruginosa, K. pheumoniae, C. albicans, C. tropicalis, A. niger, A. flavus | BS, FS | [76] |
| 51 | “Green synthesis” using Caesalpinia bonducella extract | CuO | 13.07 | “Rice grain” | 30/well | NA, 24 h, 37 °C | S. aureus, Aeromonas sp. | BS | [77] |
| 52 | “Green synthesis” using Polyalthia longifolia extract | CuO | 50–60 | Quasi-sph. | 12.5–125 µg/mL | – | P. aeruginosa, S. aureus, E. coli, S. pyogenes; A. niger, E. floccosum, A. clavatus, C. albicans | BS, FS | [78] |
| 53 | “Green synthesis” using Penicillium chrysogenum | CuO | 4–15, 11–53.8 | Rod, Sph. | 25–100 µg/mL/1.56–50 µg/mL (MIC) | LB broth, during night, 37 °C | K. oxytoca, E. coli, S. aureus, B. cereus | BS, BC | [79] |
| 54 | “Green synthesis” using Madhuca longifolia extract | CuO | 30, 120 | Unreg., Sph. | 20–30 mg/mL | MHA, 24 h, 37 °C | E. coli, S. aureus, B. subtilis | BS | [80] |
| 55 | “Green synthesis” using Silybum marianum extract | CuO | 15 | Sph. | 4, 20 mg/mL | NA, 24 h, 37 °C | E. aerogenes, S. typhi | BS | [81] |
| 56 | “Green synthesis” using Mentha pulegium extract | CuO | 26.92 ± 4.7 | Sph. | 0.625–10 mg/mL | MHA, 24 h, 37 °C | E. coli, S. aureus | BS, BC | [82] |
| 57 | “Green synthesis” using Tinospora cordifolia extract | CuO | 6–8 | Spongy | 500, 1000 µg/disk | NA, 48 h, 37 °C | K. aerogenes, P. aeruginosa, E. coli, S. aureus | BS | [83] |
| 58 | “Green synthesis” using Gloriosa superba extract | CuO | 5–10 | Sph. | 500, 1000 µg/disk | NA, 24–36 h, 37 °C | K. aerogenes, P. desmolyticum, E. coli, S. aureus | BS | [84] |
| 59 | Commercially available NPs (Sigma-Aldrich) | CuO | 48 ± 7 | – | 0.049–100 µg/mL | LB broth, 24 h, 37 °C/MHB, 48 h, 42 °C | Salmonella sp., Campylobacter sp. | BS, BC | [85] |
| 60 | “Green synthesis” using Ailanthus altissima extract | CuO | 20 | Sph. | 20–120 µg/mL | - | S. aureus, E. coli | BS | [86] |
| 61 | Synthesis using plasma | CuO | 25–160 | Unreg., Sph. | 25–100 µg/mL | NA, 24 h, 37 °C | S. aureus, P. aeruginosa | BS, BC | [87] |
| 62 | “Green synthesis” using Bauhinia tomentosa extract | CuO | 22–40 | Sph. | 1 mg/mL | NA, 24 h, 35 °C | E. coli, P. aeruginosa | BS | [88] |
| 63 | “Green synthesis” using mint leaf extract | CuO | 22–25 | Cube | 250 µg/mL | – | E. coli, B. subtilis | BS | [89] |
| 64 | “Green synthesis” using the endophytic fungus Aspergillus terreus | CuO | <100 | – | 1 mg/mL | MHA, 24 h, 37 °C/48 h, 28 °C | S. typhi, S. aureus, P. mirabilis, P. aeruginosa, K. pneuemoniae, E. coli, V. cholerae, S. epidermidis, C. albicans, A. niger | BS | [90] |
| 65 | “Green synthesis” using Cassia fistula and Melia azedarach extracts | CuO | 43.8/28.2 | Sph./Hemisph. | 0.06–2 µg/mL | Blood agar, TSB 72 h, 37 °C | K. pneumonia, H. pylori | BS, AB | [91] |
| 66 | “Green synthesis” using Brassica oleracea/Solanum tuberosum/Pisum sativum extracts | CuO | 32.5/40.7/47.2 | Unreg. | 35–45 µg/mL | LB agar, 24 h, 37 °C | P. aeruginosa, S. aureus, E. coli, B. subtilis | BS | [92] |
| 67 | “Green Synthesis” using Camellia sinensis/Prunus africana extracts | CuO | 6/8 | Sph. | 30–250 µg/mL (MIC) | MHA, 24 h, 37 °C | E. coli, K. pneumonia, S. aureus | BS, BC | [93] |
| 68 | “Green synthesis” using Cissus vitiginea extract | CuO | 20 | Sph. | 25–75 µL/disk | LB agar, 24 h, 37 °C | E. coli, Enterococcus sp., Proteus sp., Klebsiella sp. | BS | [94] |
| 69 | Pechini method | CuO, CuO/Zr (1, 3, 5, 7%) | 60, 50, 40, 30, 20 | – | – | MHA, 18 h, 37 °C | E. faecalis, S. mutans, E. coli, S. maltophilia | BS | [95] |
| 70 | “Green synthesis” using Syzygium aromaticum extract | CuO | 20 | Sph. | 4–16 µL/disk | NA, 24 h, 37 °C; Potato dextrose agar, 72 h, 37 °C | Bacillus sp., Penicillium sp. Pseudomonas spp., E. coli; A. niger, A. flavus, Penicillium spp. | BS, FS | [96] |
| 71 | “Green synthesis” using Tabernaemontana divaricate extract | CuO | 48 ± 4 | Sph. | 25, 50 µg/mL | MHA, 24 h, 37 °C | E. coli | BS | [97] |
| 72 | “Green synthesis” using Eupatorium odoratum/Acanthospermum hispidum extracts | CuO | – | Sph. | 100 µL/disk | MHA, 24 h, 37 °C | S. aureus, B. cereus, E. coli | BS | [98] |
| 73 | Chemical deposition | CuO | – | Sheet-like, Flower-like | 1.95–62.5 μg/mL | MHA with 10% blood, 72 h, 37 °C | H. pylori | BS, BC | [99] |
| 74 | “Green synthesis” using Punica granatum peel aqueous extract | CuO/Se | 92.18 | Sph. | 8 μg/mL (MIC) | MHA with 10% blood, 72 h, 37 °C | H. pylori | BS | [100] |
| 75 | One-step sonochemical synthesis | CuO | 50–100 | Rod | 25, 100 μL/L | LB agar, 24 h, 35 °C | S. typhimurium, S.aureus | BS | [101] |
| 76 | “Green synthesis” using Balanites aegyptiaca extract | CuO | 9.79–30.8 (10–30) | Sph. | 3.125–100 μg/mL | MHB, 12 h, 37 °C | B. substilis, E. faecalis, E. coli, V. cholerae | BS | [102] |
| 77 | “Green synthesis” using Bergenia ciliata extract | CuO | 20 | Sph., Hexag. | 6.25, 25 μg/mL (MIC) | NA., 24 h, 37 °C | B. subtilis, S. aureus, E. coli, S. typhi | BS | [103] |
| 78 | “Green synthesis” using Capparis decidua extract | CuO | 5–40 | Sph. | 50, 100 μg/mL (MIC) | – | B. subtilis, S. aureus, E. coli | BS | [104] |
| 79 | Co-precipitation | CuO, CuO/Ce, CuO/Nd | 25.23, 27.27, 30.93 | Rod., Flake, Nano-spam | 20 mg/mL | MHA, 24 h | E. aerogenes, B. subtilis | BS | [105] |
| 80 | Microwave fusion | CuO, Cu0.96Ag0.02Zn0.02O, Cu0.94Ag0.02Zn0.04O, Cu0.92Ag0.02Zn0.06O, Cu0.90Ag0.02Zn0.08O | 16.53, 22.17, 22.994, 24.94, 25.047 | Rod, Sph. (for 0.02 Ag) | 10–50 μg/mL | MHA, 24 h, 37 °C | S. aureus, B. subtilis, E. coli, P. aeruginosa | BS, BC | [106] |
| 81 | Laser ablation | CuO/Cu2O | <100 | Sph. | – | BHI, 18 h, 37 °C | S. enterica subsp. enterica ser. Typhimurium, E. coli, S. sonnei, Y. enterocolitica, V. parahaemolyticus, B. cereus, L. monocytogenes | BS | [107] |
| 82 | “Green synthesis” using Piper nigrum fruit extract | CuO | 60 | Sph. | 50, 100 μg/mL | LB, 24 h, 37 °C | E. coli, S. aureus | BS | [108] |
| 83 | Commercially available NPs from NANOTEC S.A. (Santiago, Chile) | Cu2O-NPs, CuO-NPs | 40–70 | – | 100–500 μg/mL | BHI with bacitracin (0.2 units/mL), MRS-agar, 48 h, 37 °C | S. mutans, S. salivarius, S. sanguinis, L. rhamnosus | BS | [109] |
| 84 | “Green synthesis” using Bergenia ciliata leaf extract | CuO | 50 | Differ. | 50–1000 μg/mL | NA, 24 h, 37 °C | S. aureus, E. coli | BS | [110] |
| 85 | “Green synthesis” using Piper nigrum fruit extract | CuO | 37–54 | Sph. | 5–100 µg/mL | NA, 24 h, 37 °C | S. aureus, S. epidermidis, S. pyogenes, E. coli, S. marcescom, K. pneumonia | BS | [111] |
| 86 | Chemical deposition | CuO | 2.9 ± 0.9 | Quasispher. | 10 μg/mL (MIC) | PDA, 4 days, 30 °C | C. jejuni | BS | [112] |
| 87 | “Green synthesis” using Aegle marmelos leaf extract | CuO | 32 | Differ., Rectang. | 400, 800 μg/mL (MIC) | MHA, SDA, 2–18 h, 37/28 °C | E. coli, S. aureus, C. albicans, C. dubliniensis | BS, FS | [113] |
| 88 | Microwave fusion, “Green synthesis” apple peel extract | CuO | 25–40 | Square | 25, 50 μg/mL (MIC) | MHA | E. coli, S. aureus | BS | [114] |
| 89 | “Green synthesis”, Chemical deposition | CuO | 81.23 | Sph. | 250, 125, 31.25 μg/mL (MIC) | MHA | E. coli, S. aureus, C. albicans | BS, FS | [115] |
| 90 | “Green synthesis” using Penicillium chrysogenum | CuO | 10.5–59.7 | Sph., Hexag. | 9–5000; 10 mg/mL | MHB, 24 h, 37 °C; PDA, 5 days, 30 °C | S. aureus, P. aeruginosa, B. subtilis, S. typhimurium, E. coli, F. solani, F. oxysporum, S. sclerotia, A. terreus | BS, FS | [116] |
| 91 | Chemical sol–gel method | CuO | 3 | – | 0.5 M, 0.75 M, 1 M, 1.5 M | MHA, 24 h, 35 ± 1 °C | P. aeruginosa, Staphylococcus sp., E. coli | BS | [117] |
| 92 | “Green synthesis” using Ganoderma sessile mushroom extract | CuO | 1–15 | Quasisph. | 0.62–19.9 μg/mL | LB agar, MHB, 24 h, 37 °C | S. aureus, E. coli, P. aeruginosa | BS | [118] |
| 93 | Chemical deposition | CuO, gelatin | 18 ± 6, 370 ± 131 | Sph. | 2.5 × 10−3–2.5 × 10−8 M/L | NA, 24 h, 30 ± 1 °C | G. candidum, P. digitatum, M. racemosus | FS | [119] |
| 94 | Hydrothermal fusion | CuO-NPs coated with PD, PVP, PVA, PEG | 834.8, 504.4, 417.9, 87.7, 266.5 | Rectang., Rod-like, Brick-like | 50–500 μg/mL | LB medium, 16 h, 37 °C | E. coli | BS | [120] |
| 95 | “Green synthesis” using cell-free supernatant of Bacillus siamensis HS | CuO | 2–41 | Sph. | 50–600 μg/mL | NA, 24 h, 37 °C | S. aureus, B. subtilis, E. faecalis, C. albicans, E. coli, P. aeruginosa, K. pneumoniae, V. damsela, Pseudoalteromonas spp. | BS, AB | [121] |
| 96 | “Green synthesis” using Averrhoa carambola leaf extract | CuO | 98 ± 26 | Sph. | 6.25–100 μg/mL | MHA, MHB, 24 h, 37 °C | B. megaterium, S. aureus, E. coli, S. typhi, P. aeruginosa | BS, BC | [122] |
| 97 | Chemical deposition | CuO | 100 | Sph. | 0.01–1 g/L | – | E. coli | BS, BC | [123] |
| 98 | “Green synthesis” using Centratherum punctatum leaf extract | CuO | 20–30 | Sph. | 9.3 mg/mL (MIC) | MHA, MHB, PDA, PDB, 16 h, 37 °C | S. aureus, B. cereus, K. pneumonia, P. aeruginosa, E. coli, A. baumannii S. mutans, E. faecium, C. albicans | FS, BS, AB | [124] |
| 99 | “Green synthesis” using Stachys lavandulifolia extract | Cu/Cu2O composite | 80 | Sph. | – | MHA, 24 h, 37 °C | P. aeruginosa | BS | [125] |
| 100 | Synthesis from copper sulfate pentahydrate using Bacillus cereus | Cu2O | 30 ± 2 | Sph. | 0–500 μg/mL, 1–2 mg/mL | TSB, 24 h, 37 °C, NA, 24 h, 37 °C | B. subtilis, P. aeruginosa | BS | [126] |
| 101 | Chemical deposition | Cu2O/zeolite | 5–30 | Worm-like | 150, 500 mg/L | LB agar | E. coli | BC | [127] |
| 102 | Laser ablation | Cu2O (with an admixture of Cu, CuO) | <5 | Sph. | – | BHI broth | S. aureus | BC | [128] |
| 103 | Chemical deposition usingNaBH4 and N2H4 | Cu2O/Cu2O + CuO/CuO | 2–20 | Hemisph. | – | MHA, 24 h, 35 °C | S. aureus, E. coli, P. aeruginosa | BS, BC | [25] |
| 104 | Chemical deposition | Cu2O | 25.54, 25.83 | Cube | 10–100 μg/mL | PDA | F. solani | FS | [129] |
| 105 | Chemical deposition | Cu2O, Cu2O/biochar | – | – | 56, 40 μg/mL (MIC) | LB agar, 24 h, 37 °C | E. coli | BS | [130] |
| 106 | Solvothermal synthesis | Cu2O | 2000–6000 | Cube | 2 mg/mL | LB agar, 24 h, 36 °C | B. thuringiensis, P. aeruginosa | BS | [131] |
| 107 | “Green synthesis” using Razma seeds | Cu4O3 | 27 | Spongy | 200–600 μg/disk | NA, 36 h, 37 °C | S. aureus, E. coli | BS | [132] |
| 108 | “Green synthesis” using Aegle marmelos seeds | Cu4O3 | 200 | Sph. | – | NA | E. coli | BS | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. https://doi.org/10.3390/ijms252111563
Gudkov SV, Burmistrov DE, Fomina PA, Validov SZ, Kozlov VA. Antibacterial Properties of Copper Oxide Nanoparticles (Review). International Journal of Molecular Sciences. 2024; 25(21):11563. https://doi.org/10.3390/ijms252111563
Chicago/Turabian StyleGudkov, Sergey V., Dmitry E. Burmistrov, Polina A. Fomina, Shamil Z. Validov, and Valery A. Kozlov. 2024. "Antibacterial Properties of Copper Oxide Nanoparticles (Review)" International Journal of Molecular Sciences 25, no. 21: 11563. https://doi.org/10.3390/ijms252111563
APA StyleGudkov, S. V., Burmistrov, D. E., Fomina, P. A., Validov, S. Z., & Kozlov, V. A. (2024). Antibacterial Properties of Copper Oxide Nanoparticles (Review). International Journal of Molecular Sciences, 25(21), 11563. https://doi.org/10.3390/ijms252111563










