Abstract
Carbon catabolite repression (CCR) is a common transcriptional regulatory mechanism that microorganisms use to efficiently utilize carbon nutrients, which is critical for the fitness of microorganisms and for pathogenic species to cause infection. Here, we characterized two CCR genes, VdCreA and VdCreC, in Verticillium dahliae that cause cotton Verticillium wilt disease. The VdCreA and VdCreC knockout mutants displayed slow growth with decreased conidiation and microsclerotium production and reduced virulence to cotton, suggesting that VdCreA and VdCreC are involved in growth and pathogenicity in V. dahliae. We further generated 36 highly reliable and stable ΔVdCreA and ΔVdCreC libraries to comprehensively explore the dynamic expression of genes and metabolites when grown under different carbon sources and CCR conditions. Based on the weighted gene co-expression network analysis (WGCNA) and correlation networks, VdCreA is co-expressed with a multitude of downregulated genes. These gene networks span multiple functional pathways, among which seven genes, including PYCR (pyrroline-5-carboxylate reductase), are potential target genes of VdCreA. Different carbon source conditions triggered entirely distinct gene regulatory networks, yet they exhibited similar changes in metabolic pathways. Six genes, including 6-phosphogluconolactonase and 2-ODGH (2-oxoglutarate dehydrogenase E1), may serve as hub genes in this process. Both VdCreA and VdCreC could comprehensively influence the expression of plant cell wall-degrading enzyme (PCWDE) genes, suggesting that they have a role in pathogenicity in V. dahliae. The integrated expression profiles of the genes and metabolites involved in the glycolysis/gluconeogenesis and pentose phosphate pathways showed that the two major sugar metabolism-related pathways were completely changed, and GADP (glyceraldehyde-3-phosphate) may be a pivotal factor for CCR under different carbon sources. All these results provide a more comprehensive perspective for further analyzing the role of Cre in CCR.
1. Introduction
Carbon catabolite repression (CCR) is a common mechanism used by microorganisms to coordinate the expression of the genes required for the preferential utilization of carbon sources [1]. The CCR mechanism allows microorganisms to accurately and efficiently utilize carbon nutrients under inconsistent nutritional conditions since it ensures the preferential utilization of easily metabolizable carbon sources such as D-glucose by repressing the expression of genes involved in the utilization of other alternative carbon sources [2]. In filamentous fungi, the expression of the genes encoding the polysaccharide-degrading enzymes α-amylase, cellulase, and hemicellulase, as well as other enzymes required for the utilization of carbon sources, are regulated by CCR, and as a result, the CCR system determines the hierarchy of carbon substrate utilization [2,3].
CCR has been extensively studied in the yeast Saccharomyces cerevisiae and the filamentous fungus Aspergillus nidulans. In S. cerevisiae, the DNA-binding repressor Mig1 is the main regulator of CCR and regulates the majority of the glucose-repressed genes [4]. In filamentous fungi, the CCR mechanism is regulated by a group of genes including CreA, CreB, CreC, and CreD [5]. The CreA-encoded protein contains two zinc fingers of the Cys2-His2 class, which are transcription factors that repress gene expression by binding directly to the 5′-SYGGRG-3′ motif in the promoters of the target genes [5,6]. For instance, the CreA of A. nidulans binds to the promoter region of xlnA and xlnD, which encode xylanase, and causes their direct repression; all the genes that are regulated by xlnR, which encodes the major inducer of xylanases, can be indirectly repressed by CreA [7]. In addition, CreA is a master regulator that governs diverse physiological processes including secondary metabolism, iron homeostasis, oxidative stress responses, development, N-glycan biosynthesis, the unfolded protein response, and nutrient and ion transport [8]. Ubiquitination ligases and deubiquitination enzymes interact with each other and control the transcription factors involved in the CCR mechanism. CreB encodes a deubiquitinase [9], and CreC encodes a WD40 protein, which together form a stable deubiquitination enzyme complex that eradicates ubiquitin moieties from CreA and other substrates, thereby stabilizing target proteins [10]. The CreD-encoded protein contains arrestin domains and PY motifs and has been reported to interact with the ubiquitin ligase HulA in A. nidulans; the CreD–HulA ubiquitination ligase complex helps in the ubiquitination of CreA, which opposes the CreB–CreC complex, ensuring the proper utilization of carbon sources for metabolism [11].
CCR has been widely reported to regulate fungal growth, secondary metabolism, and pathogenicity. The deletion of the CreA gene homolog Cre1 in Trichoderma reesei resulted in an altered morphology, with smaller colonies having fewer aerial hyphae and spores compared with the parental strains, as well as reduced cellulase and hemicellulase production under repressing conditions [12]. MoCreA in Magnaporthe oryzae is required for vegetative growth, conidiation, appressorium formation, and pathogenicity [13]. The CreA of A. fumigatus is not required for pulmonary infection establishment, but it is essential for infection maintenance and disease progression; the loss of CCR inhibits fungal metabolic plasticity and the ability to thrive in dynamic infection microenvironments [14]. MoCreC in M. oryzae is involved in conidiation, growth, and pathogenicity; the deletion of MoCreC resulted in a reduced vegetative growth rate, lower conidiation production, impaired appressorium formation, and significantly decreased pathogenicity [15].
Verticillium dahliae is a soil-borne hemibiotroph phytopathogen fungus that causes vascular wilt in a wide variety of plants, including the economically important crops cotton, tomato, and sunflower [16]. The infection of roots by V. dahliae in soil leads to its colonization of vascular tissues in host plants, and it then produces microsclerotia during the progression of ripeness and senescence of the diseased plants. With the progressive decomposition of plant residue, the microsclerotia are released and can survive as resting structures in the soil for several years without a host plant, which makes V. dahliae difficult to control and eradicate [17].
Carbon is one of the most important nutrients for the growth and development of fungi. For most parts of its life in the host plant, V. dahliae is restricted to the xylem vessels of the vascular system; however, the xylem fluid provides an environment with limited carbon sources [18,19]. Studies have shown that V. dahliae encodes numerous carbohydrate-active enzymes including plant cell wall-degrading enzymes (PCWDEs), which degrade cell walls into metabolizable sugars and other mono- and oligomers for the fungus to utilize, allowing them to colonize plant xylem vessels [20]. In some fungi, the genes encoding these enzymes including cellulase, amylase, and xylanase are generally transcriptionally regulated by CreA-dependent CCR, but little is known about the functions of the genes involved in CCR in V. dahliae. Here, we show that VdCreA and VdCreC function as key components of carbon catabolite repression in V. dahliae, and disturbing CCR through knockout of VdCreA or VdCreC leads to significant defects in vegetative growth, conidiation, microsclerotium production, and pathogenicity. Further comparative transcriptomic and metabolomic analysis demonstrates that VdCreA and VdCreC are the regulators of CCR, and their disruptions and different carbon source conditions result in the extensive gene expression variations, including a large number of carbon metabolism enzymes, transcription factors, and genes encoding PCWDEs, yet they exhibit similar changes in metabolic pathways.
2. Results
2.1. Identification of VdCreA and VdCreC in V. dahliae
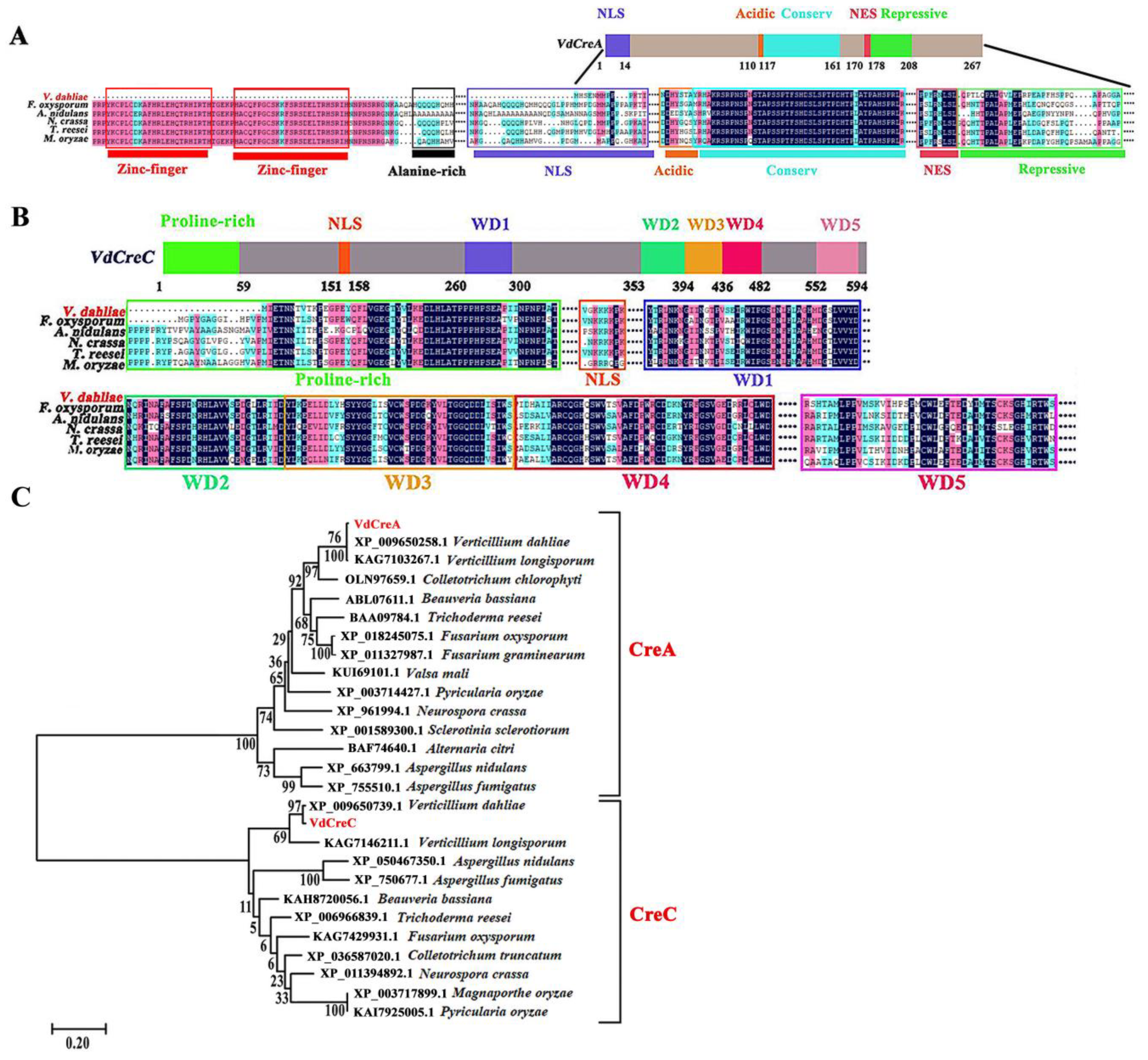
The two genes VdCreA and VdCreC were cloned and sequenced from the genomic DNA and cDNA of the V592 strain of V. dahliae, respectively. The VdCreA gene is composed of 1427 bp and contains an exon encoding 267 amino acids. The C-terminal part of the VdCreA protein has an acidic amino acid-rich region located at amino acids 110 to 116; and a highly conserved region at amino acids 117 to 161, which is identical between A. nidulans, Fusarium oxysporum, Neurospora crassa, and Trichoderma reesei; followed by a repressive region at amino acids 170 to 208 containing a nuclear export signal (NES), which has been shown to be essential for repression [21,22]. VdCreA lacks two N-terminal zinc finger domains, an alanine-rich region, and part of the nuclear location signal sequences compared to CreA proteins from other fungi (Figure 1A). The VdCreA protein shares the highest amino acid identity (99.63%) with that of V. dahliae VdLs.17, and it shares 66.33% and 51.67% amino acid identities with the CreA homologs in V. longispore and Colletotrichum chlorophyti, respectively.
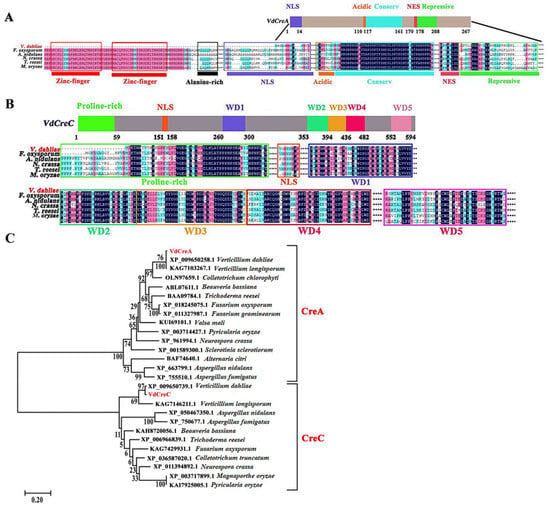
Figure 1.
Structure prediction, amino acid sequence alignment, and phylogenetic tree of VdCreA and VdCreC in V. dahliae. (A) Structure schematic diagrams of the VdCreA protein and amino acid sequence alignment between the VdCreA protein and CreA from other fungi. (B) Structure schematic diagrams of the VdCreC protein and amino acid sequence alignment between the VdCreC protein and CreC from other fungi. (C) Phylogenetic tree of VdCreA, VdCreC, and homologs from several other fungi constructed by MEGA11 using the neighbor-joining method; the bootstrap test was repeated 1000 times.
VdCreC is composed of 2357 bp containing an open reading frame (ORF) that is interrupted by seven introns and encodes 611 amino acids. VdCreC protein contains a proline-rich (20/59 residues are proline) region near the N-terminus at amino acids 1 to 59. The C-terminal part of the protein contains a series of five WD40 (β-transducin-like) repeats at amino acids 260 to 300, 353 to 394, 395 to 436, 437 to 482, and 552 to 594 (Figure 1B). The VdCreC protein shares a 98.44% amino acid identity with that of V. dahliae VdLs.17, and it shares 80.41% and 72.68% amino acid identities with the CreC homolog in V. longispore and Colletotrichum truncatum, respectively.
Phylogenetic analysis based on amino acid sequences revealed that VdCreA and VdCreC were classified into two different clades, and they clustered with homologs of CreA and CreC from other fungi, respectively, which is consistent with their amino acid sequence similarity. This suggests that CreA and CreC in filamentous fungi were highly conserved during their evolution (Figure 1C).
2.2. VdCreA and VdCreC Knockout Leads to Serious Defects in Vegetative Growth and Conidiation
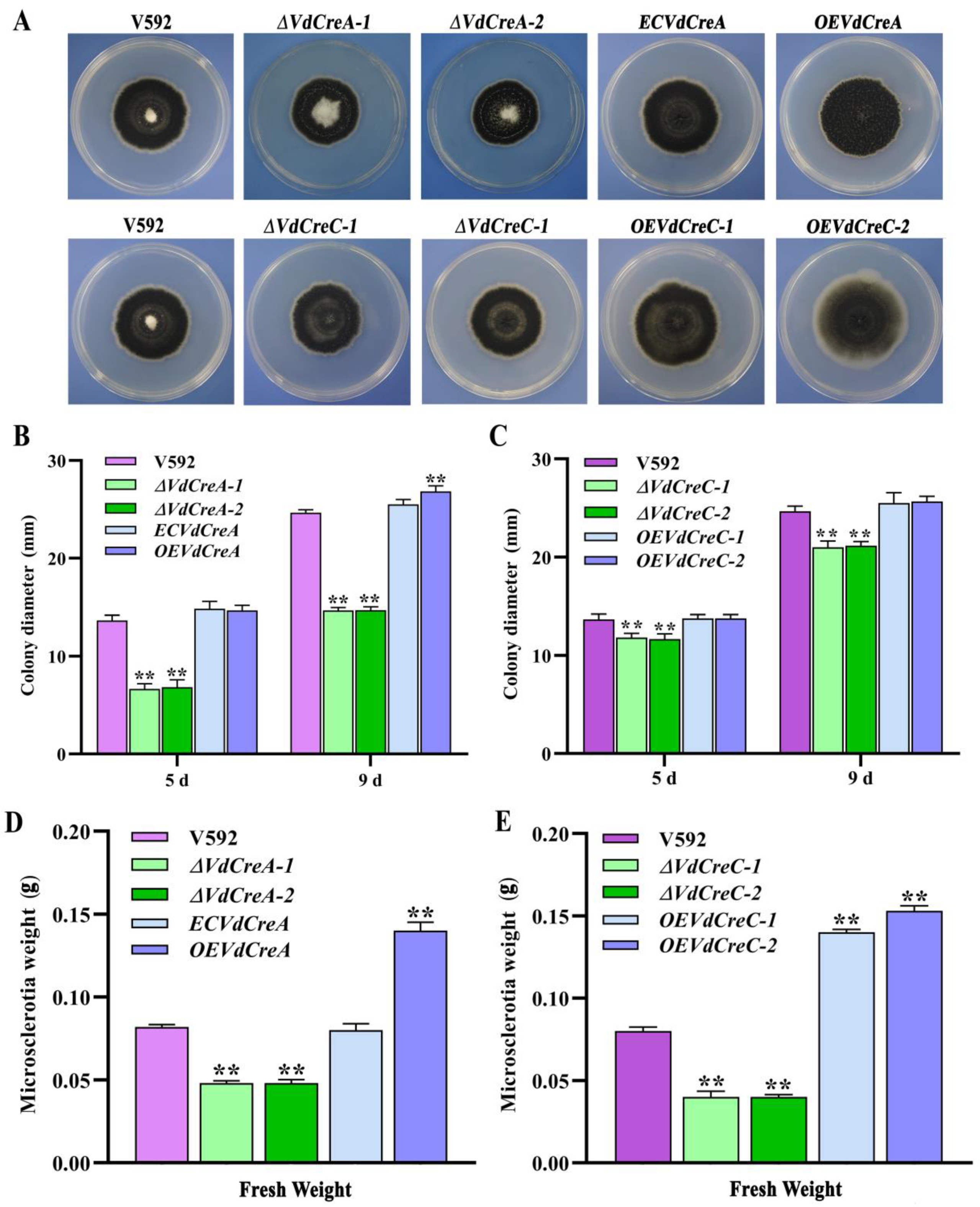
To confirm whether VdCreA and VdCreC participates in CCR and explore their biological role in the growth, development, and pathogenicity in V. dahliae, knockout mutants of VdCreA and VdCreC were generated via homologous recombination through which the targeted gene in the genome of the V592 strain was replaced with a hygromycin resistance cassette (hygromycin B phosphotransferase gene, hph), respectively (Figure S1). The two VdCreA knockout mutants (ΔVdCreA-1 and ΔVdCreA-2) and the two VdCreC knockout mutants (ΔVdCreC-1 and ΔVdCreC-2), the wild-type V592 strain, and the complementary strains or the overexpression strains were all cultured on PDA plates to compare their colony morphology and growth. ΔVdCreA displayed significantly more aerial hyphae, while ΔVdCreC exhibited decreased aerial hyphae (Figure 2A); the colony growth rate of both ΔVdCreA and ΔVdCreC were significantly lower than that of the V592 strain and their respective complementary or overexpression strains (Figure 2B,C). To further ascertain the functions of VdCreA and VdCreC in microsclerotial production, microsclerotia produced by each strain on cellophane overlaid on MM medium were weighed. The results showed that the knockout of VdCreA and VdCreC led to reduced microsclerotia in V. dahliae. Compared to the V592 strain, the microsclerotia were decreased by 1.7 times and 2.0 times in ΔVdCreA and ΔVdCreC, respectively; whereas the overexpression of VdCreA and VdCreC resulted in increased microsclerotia production by 1.7 times and 1.8 times, respectively (Figure 2D,E). The results suggested that VdCreA and VdCreC are involved in radial growth and microsclerotium production in V. dahliae.
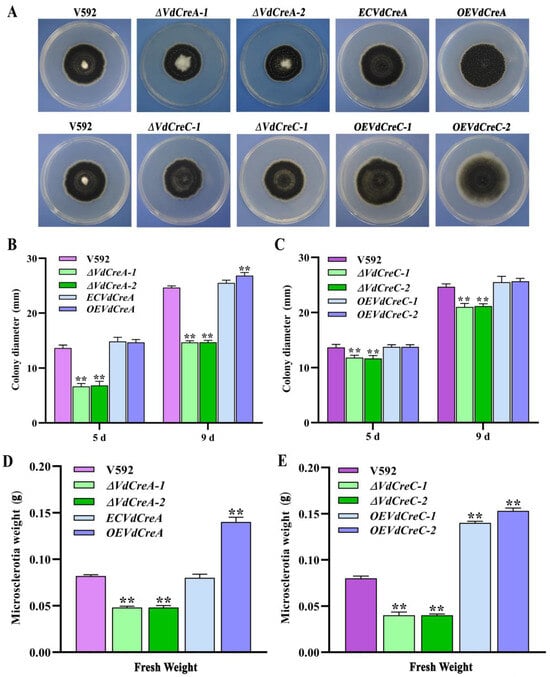
Figure 2.
Phenotype characteristics of VdCreA and VdCreC knockout mutants. (A) Colony morphologies of the V592 strain and all ΔVdCreA and ΔVdCreC mutants grown on PDA medium at 15 dpi. (B) Colony diameter of the V592 strain, ΔVdCreA, the complementary strain ECVdCreA, and the overexpression strain OEVdCreA grown on PDA medium at 5 dpi and 9 dpi. (C) Colony diameter of the V592 strain, ΔVdCreC, and the overexpression strains OEVdCreC grown on PDA medium at 5 dpi and 9 dpi. (D) The wet weight of the microsclerotia produced by the V592 strain, ΔVdCreA, ECVdCreA, and OEVdCreA. (E) The wet weight of the microsclerotia produced by the V592 strain, ΔVdCreC, and OEVdCreC. Error bars represent standard errors calculated from three replicates. ** represents a significant difference at the 0.01 probability level between the knockout mutants, the complementary strain, the overexpression strains, and the wild-type strain V592 (p < 0.01).
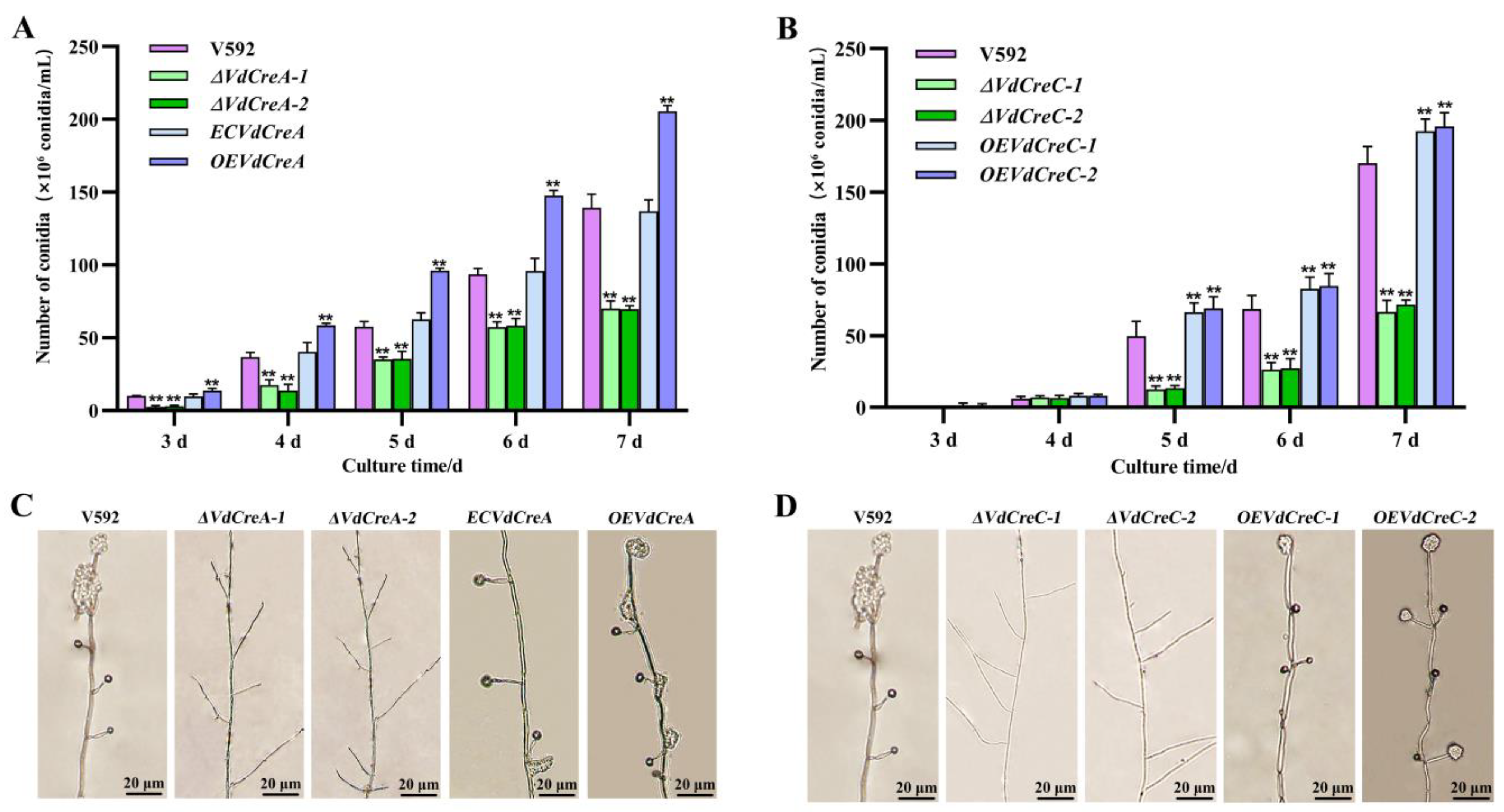
The effect of the two genes’ knockout on conidium production was also investigated. Both ΔVdCreA and ΔVdCreC showed significant reduced conidiation. At 7 days post-inoculation (dpi), ΔVdCreA and ΔVdCreC produced about 2.0 times and 2.5 times less conidia than the V592 strain, respectively; the overexpression strains OEVdCreA and OEVdCreC produced about 1.5 times and 1.1 times more conidia than the V592 strain (Figure 3A,B). Consistent with these results, under the optical microscope, ΔVdCreA and ΔVdCreC hardly differentiated into verticillate conidiophore stalks, whereas the V592 strain, the complementary strain ECVdCreA, and the overexpression strains (OEVdCreA and OEVdCreC) produced many verticillate conidiophore stalks, on which conidia were gathered (Figure 3C,D); these results indicated that VdCreA and VdCreC are critical for the conidiogenesis of V. dahliae.
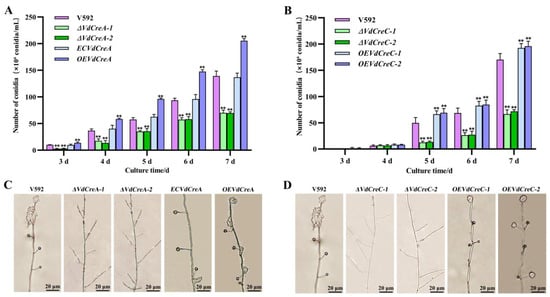
Figure 3.
The effect of VdCreA and VdCreC knockout on conidial production. (A) Conidia produced by the V592 strain, ΔVdCreA, ECVdCreA, and OEVdCreA. (B) Conidia produced by the V592 strain, ΔVdCreC, and OEVdCreC. (C) Microscopic observation of conidiophore stalks produced by the V592 strain, ΔVdCreA, ECVdCreA, and OEVdCreA. (D) Microscopic observation of conidiophore stalks produced by the V592 strain, ΔVdCreC, and OEVdCreC. Error bars represent the standard deviation. ** represents a significant difference at the 0.01 probability level between the knockout mutants, the complementary strain, the overexpression strains, and the wild-type strain V592 (p < 0.01).
2.3. VdCreA and VdCreC Are Involved in Pathogenicity
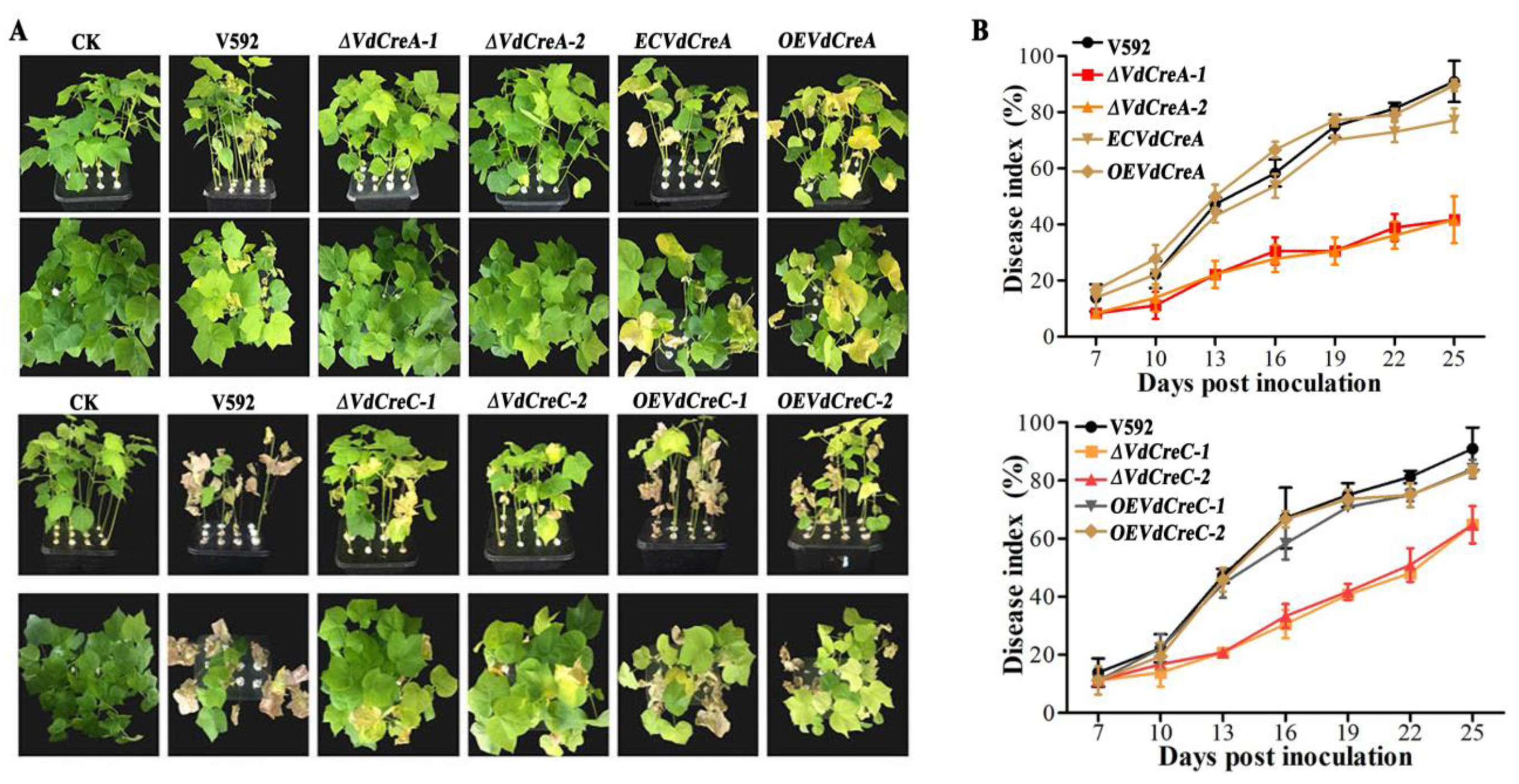
To assess the roles of VdCreA and VdCreC in virulence, the susceptible cotton cultivar Junmian No. 1 was inoculated with conidial suspensions of the wild-type strain V592, ΔVdCreA, ΔVdCreC, and their complementary strains. ΔVdCreA and ΔVdCreC exhibited markedly reduced virulence in cotton plants compared with the V592 strain, which induced severe tissue chlorosis and wilt symptoms (Figure 4A). At 25 dpi, the disease index of plants infected by ΔVdCreA and ΔVdCreC was 41.7 and 63.9, respectively; whereas the disease index of plants infected by the V592 strain reached 91.0, and the disease index caused by the complementary strains of ΔVdCreA and ΔVdCreC was restored to 83.3 and 84.0, respectively (Figure 4B). Therefore, we concluded that the VdCreA and VdCreC genes are involved in fungal virulence.
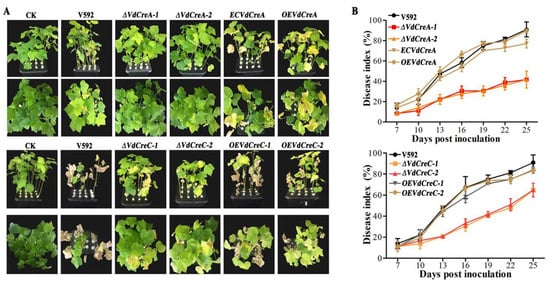
Figure 4.
The determination of virulence of different knockout mutant strains to cotton seedlings. (A) Disease symptoms of cotton plants infected with different strains at 25 days post inoculation (dpi). (B) Disease index of cotton plants infected with different strains. The error bars represent the standard errors calculated from 3 replicates.
2.4. VdCreA and VdCreC Are Essential for Carbon Catabolite Repression
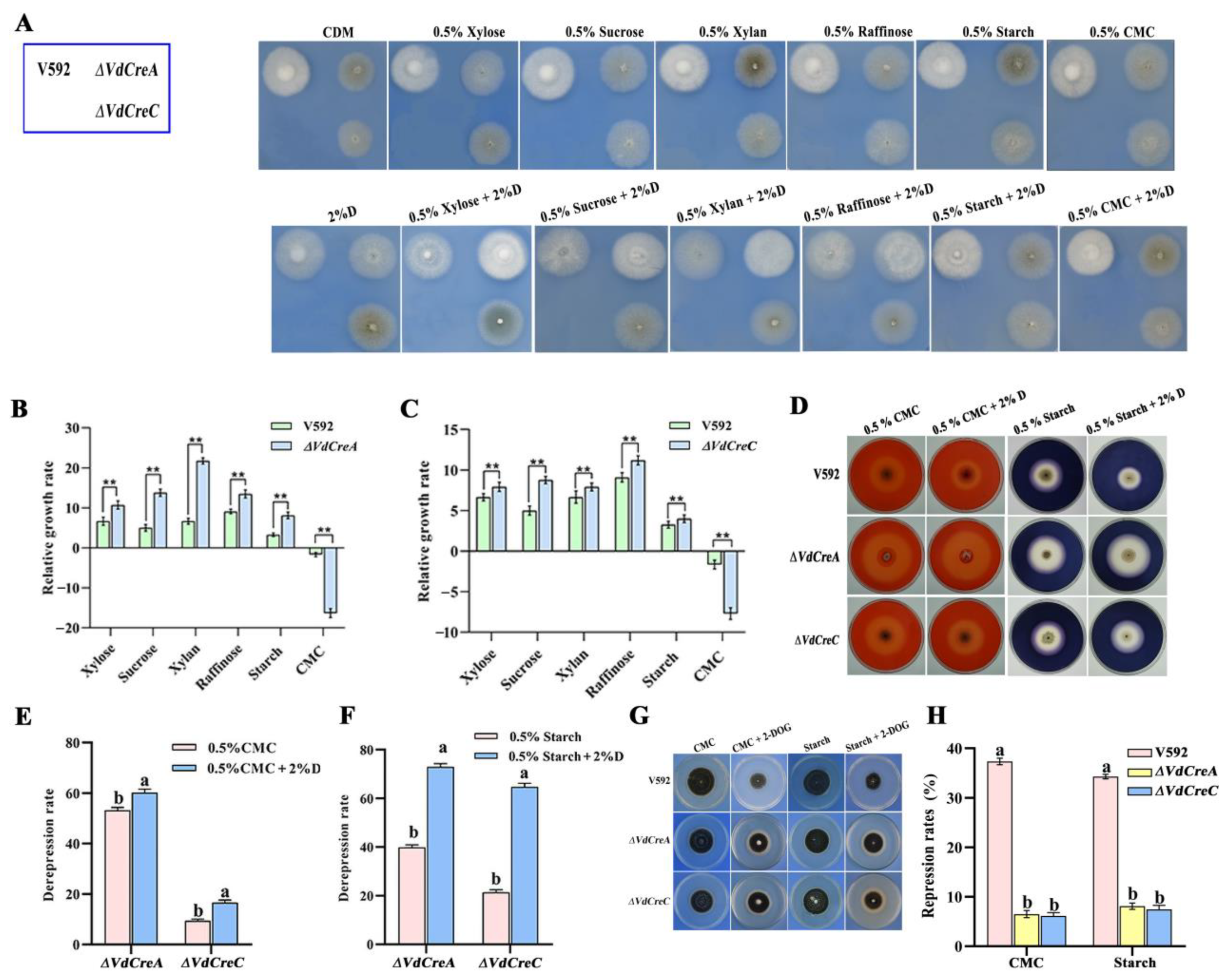
To obtain evidence of VdCreA and VdCreC being involved in CCR in V. dahliae, all strains were inoculated on Czapek–Dox agar plates containing different sole carbon sources with or without glucose. Although in most cases the growth rates of ΔVdCreA and ΔVdCreC grown on these carbon sources were slower than that of the V592 strain, the relative growth rate of each mutant was significantly higher than that of the V592 strain, except for those grown on CMC and on CMC plus glucose (Figure 5A–C). Despite each strain displaying a growth defect on CMC plus glucose compared to the growth on CMC, ΔVdCreA was found to have higher cellulase activity based on the sizes of clear zones (halos) around their colonies (Figure 5D), where the de-repression rate of cellulase activity in ΔVdCreA grown on CMC and on CMC plus glucose was 55% and 60%, respectively; similar results were also obtained in ΔVdCreC, where the de-repression rate of cellulase activity grown on CMC and CMC plus glucose was 10% and 18%, respectively (Figure 5E). The amylase activity was also measured, in which the de-repression rate of amylase activity in ΔVdCreA grown on starch and starch plus glucose was 40% and 72%, respectively; the de-repression rate of amylase activity in ΔVdCreC grown on starch and starch plus glucose was 20% and 65%, respectively (Figure 5F). Furthermore, the de-repression rates of cellulase and amylase activity were significantly increased under glucose-added conditions, especially for amylase activity. The addition of glucose caused a remarkable increase in the de-repression rate; the fold increase was 1.8 for ΔVdCreA and 3.3 for ΔVdCreC compared to no glucose addition (Figure 5F). The results suggested that VdCreA and VdCreC participated in the CCR associated with cellulase and amylase in V. dahliae.
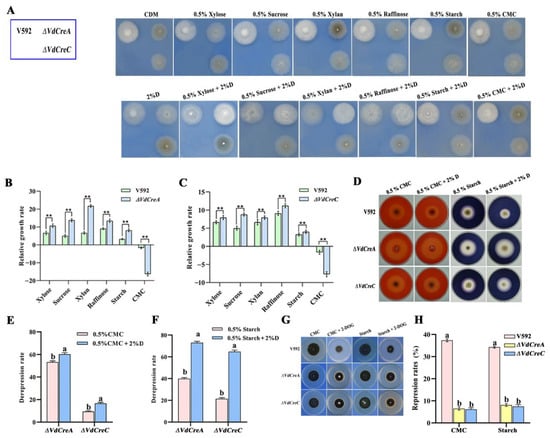
Figure 5.
VdCreA and VdCreC are essential for carbon catabolite repression. (A) Phenotypic characteristics of the all strains grown on Czapek–Dox agar plates containing different sole carbon sources with or without glucose. (B) The relative growth rate of ΔVdCreA. (C) The relative growth rate of ΔVdCreC. The relative growth rate = (the diameter on carbon sources plus glucose − the diameter on carbon sources)/(the diameter on carbon sources plus glucose) × 100%. (D) The amylase and cellulase production of ΔVdCreA and ΔVdCreC grown on Czapek–Dox agar plates containing starch or CMC with or without glucose. (E) The de-repression rate of cellulase activity in ΔVdCreA and ΔVdCreC grown on CMC and on CMC plus glucose. De-repression rate = (the sizes of clear zone of the knockout mutant − the size of clear zones of V592 strain)/(the size of clear zones of the knockout mutant) × 100%. (F) The de-repression rate of amylase activity in ΔVdCreA and ΔVdCreC grown on starch and on starch plus glucose. (G) Colony growth rates of the ΔVdCreA and ΔVdCreC grown on MM medium containing CMC and starch with or without 2-DOG. (H) The repression rates of the ΔVdCreA and ΔVdCreC mutants. Repression rate = (the diameter of untreated strain − the diameter of treated strain)/(the diameter of untreated strain) × 100%. ** represents a significant difference at the 0.01 probability level between the knockout mutants, the overexpression strains, and the wild-type strain V592 (p < 0.01). Different letters above the error bars indicate statistically significant differences at p < 0.01 from one-way ANOVA tests.
To further investigate the role of VdCreA and VdCreC in CCR, 2-deoxy-d-glucose (2-DOG) was used as a repressing carbon substrate to assess the glucose-induced repression through measuring the colony growth of the V592 strain and the two mutants grown on Czapek–Dox medium containing CMC plus 2-DOG, and starch plus 2-DOG. 2-DOG is a non-metabolizable D-glucose analog and can inhibit glucose metabolism in the cells [23]. The results showed that the growth repression rate was higher in the wild-type strain V592 than that in the ΔVdCreA and ΔVdCreC (Figure 5G,H). Thus, similar to many other filamentous fungi, VdCreA and VdCreC function as key regulators of CCR in V. dahliae.
2.5. Global Perspective of Gene and Metabolic Expression Profiles
To further gain insight into the VdCreA- and VdCreC-mediated regulation mechanisms, changes in the transcriptomics and metabolomics were assessed to compare between ΔVdCreA, ΔVdCreC, and the wild-type V592 strain under inducing conditions (starch and CMC) and repressing conditions (starch plus 2% glucose, and CMC plus 2% glucose). We constructed 36 transcriptome libraries for the V592, ΔVdCreA, and ΔVdCreC strains under different carbon sources, collecting an average of 42.17 million raw reads from each library (Table S1). The reliability of the RNA-seq data was confirmed by qRT-PCR analysis of nine randomly selected genes (Figure S2).
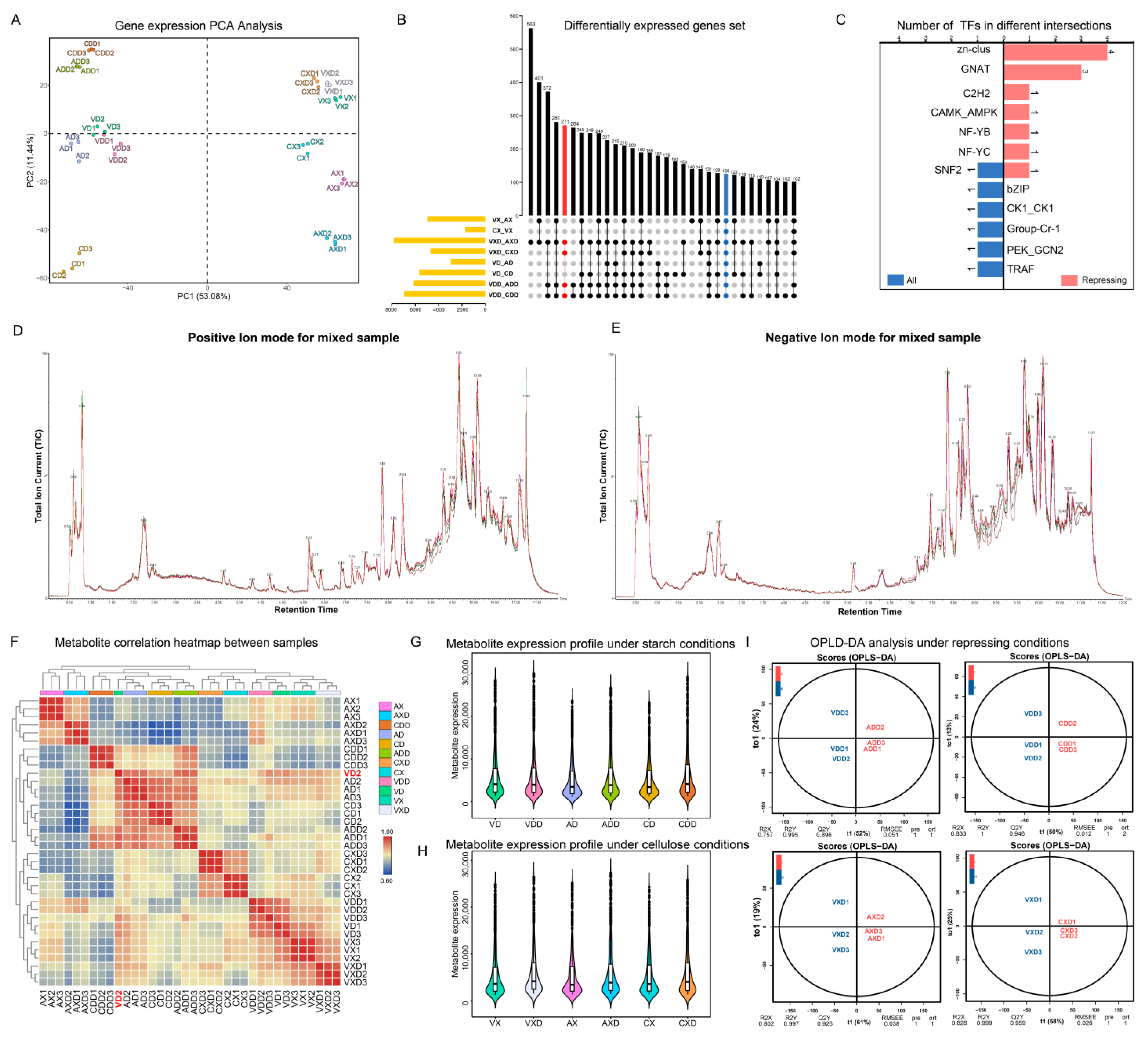
A total of 12,834 unigenes were identified, and the expression level of each gene was calculated using FPKM (fragments per kilobase of exon model per million mapped fragments) and subjected to principal component analysis (PCA) (Figure 6A). PC1 and PC2 together explained 64.52% of the variance between expression profiles. Replicates of the same treatment showed high similarity and consistency, always clustering together with no obvious outliers. Starch or cellulose conditions seemed to be the main factors affecting gene expression changes in the strains, with samples from different carbon sources consistently distributed on both sides of PC1. VdCreA gene knockout also led to gene expression changes, exhibiting an obvious separation on both sides of PC2 between VdCreA knockout mutants and wild-type strains under repression conditions. Different treatments were grouped and compared pairwise to screen for differentially expressed genes (DEGs). We used an up-set plot to display the statistical results (Figure 6B). The VXD and AXD groups had the most DEGs, with 563 genes differentially expressed only in this group. The focus was on those DEGs that were always present in each group. Under repression conditions, compared with the wild-type strain, there were the same 271 DEGs in ΔVdCreA and ΔVdCreC (marked in red), of which 126 DEGs were present in all groups. These 271 DEGs may be the main genes involved in CCR. Transcription factors are usually the hubs of gene expression regulation. We identified all transcription factors (TFs) in the 271 DEGs and grouped them (Figure 6C). In comparison, there were more TFs under repressing conditions, mainly four Zn-clus (Zn2Cys6 TF), three GNAT (Gcn5-related N-acetyltransferases) TFs, and one SNF2 TF shared by two groups.
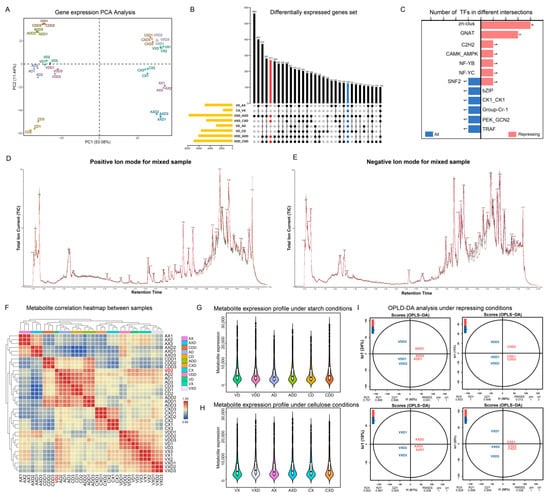
Figure 6.
Global statistics of transcriptomic and metabolomic data. (A) PCA of the expression levels of all samples. (B) Up-set diagram of DEGs in differential groups. Red annotations represent differential groups under repressing conditions, while blue annotations represent all differential groups. (C) Statistics of transcription factors in common DEGs under different groups. (D,E) The total ion current of the mixed sample. (F) Heatmap of sample correlation based on metabolite expression levels. Outlier samples are marked in red. (G) Boxplot statistics of dominant metabolites under starch conditions. (H) Boxplot statistics of dominant metabolites under cellulose conditions. (I) OPLS-DA mode diagram of different groups under repressing conditions.
The same batch of samples also underwent metabolomics analysis, detecting a total of 17,723 peaks and identifying 2986 metabolites. The TIC (total ion current) of the mixed sample was used to demonstrate the characteristics of the mass spectral peaks (Figure 6D,E). The main products in the positive ion mode were agroclavine, while in the negative ion mode, the main products were 5-δ-hydroxybutyl hydantoin. A total of 1672 (55.94%) metabolites were successfully annotated based on the public database, which can be classified into 122 classes and 214 subclasses. The classes with the highest number of identified metabolites are carboxylic acids and derivatives (254), fatty acyls (208), and organooxygen compounds (190). The subclasses with the most identified metabolites are amino acids, peptides, and analogs (231), carbohydrates and carbohydrate conjugates (119), and fatty acids and conjugates (99). We have uploaded the mass spectral characteristics, mass m/z values, and annotation results of all metabolites to facilitate the reuse of scientific data (Table S2). Based on the expression levels of metabolites, we calculated the correlation between all samples (Figure 6F). Almost all biological replicates always cluster together in the heatmap clustering results, indicating that the detection of metabolism has good repeatability. However, VD2 is an exception, it is separated from the other two replicates, and in subsequent analyses, this sample was discarded. In addition, the correlation of metabolite spectra of standard products used for quality control also proved the stability of the detection results (Figure S3). After removing the metabolites with extreme expression in each group (the top 1% and bottom 1% of expression), we counted the top 50% of metabolites in terms of expression. Overall, the range of metabolite expression changes in all groups is very large. After repression, regardless of the carbon source, the expression levels of the main metabolites in the wild-type, ΔVdCreA, and ΔVdCreC strains all increased (Figure 6G,H). We used OPLS-DA (orthogonal partial least squares discriminant analysis) to perform pairwise comparisons of groups for supervised multivariate analyses to screen for differentially expressed metabolites (DEMs). We displayed the model evaluation results between the four groups of samples after repression (Figure 6I). The predictive principal component in all four groups explained more than 50% of the variance, and samples from different groups showed clear separation in the predictive principal component. The Q2Y value of the model exceeded 89%, and the R2Y value exceeded 99%, signifying substantial shifts in metabolic dynamics within mutants, and the model could stably and reliably explain the differences in metabolites between samples.
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
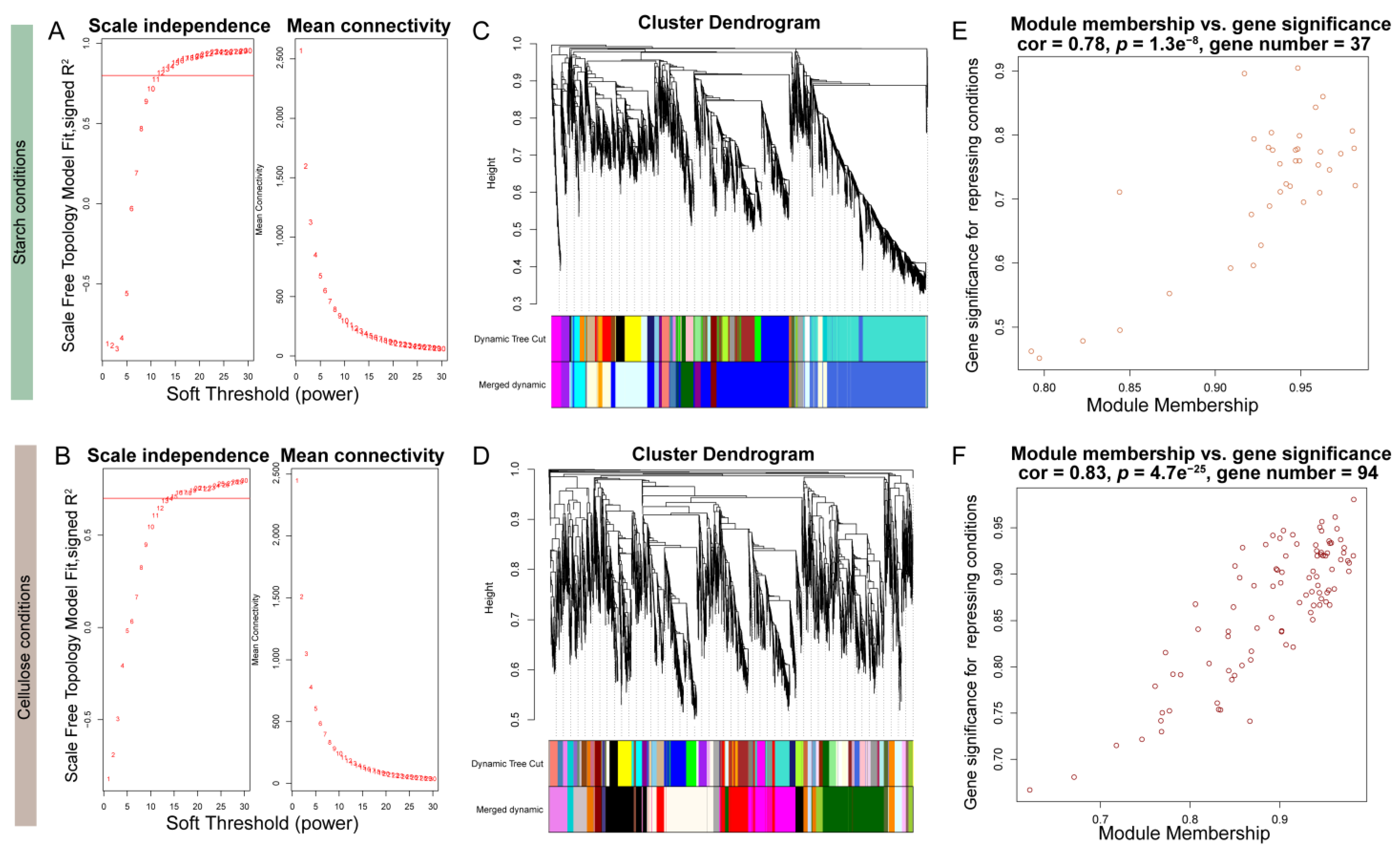
The results of gene expression profiling and metabolite correlation heatmaps both indicated that different carbon source dependencies had a significant impact on the expression of genes and metabolites in the samples. To focus on VdCreA and VdCreC, we divided the gene expression profiling results under two carbon source conditions and performed WGCNA on all DEGs separately. Specifically, DEGs with an average expression level between samples less than 1 and a missing degree greater than 50% were first filtered out. Subsequently, a sample phylogenetic tree was constructed based on expression levels (Figure S4). Under starch conditions, one sample each from the VD and VDD groups was an outlier. Under cellulose conditions, one sample each from the VX and CX groups was an outlier. These samples were removed in subsequent analyses. Then, the most suitable soft threshold was selected based on a correlation exceeding 75% (Figure 7A,B), with 12 and 15 serving as the soft thresholds under starch and cellulose conditions, respectively, to construct the adjacency matrix. The minimum module gene number was uniformly set to 30. Ultimately, under starch conditions, 5558 DEGs were divided into 18 modules, with an average of 308 genes per module. The modules containing VdCreA and VdCreC had 1478 and 1597 genes, respectively (Figure 7C). Under cellulose conditions, 5278 DEGs were divided into 21 modules, with an average of 263 genes per module. The modules containing VdCreA and VdCreC contained 679 and 211 genes, respectively (Figure 7D). The connectivity of eigengenes in different modules and the degree of association of module genes with repressing conditions were calculated separately. Under starch conditions, a module of 37 genes had a 78% correlation with repressing treatment (p = 1.3 × 10−8) (Figure 7E). Under cellulose conditions, a module of 94 genes had an 83% correlation with repressing treatment (p = 4.7 × 10−25) (Figure 7F).
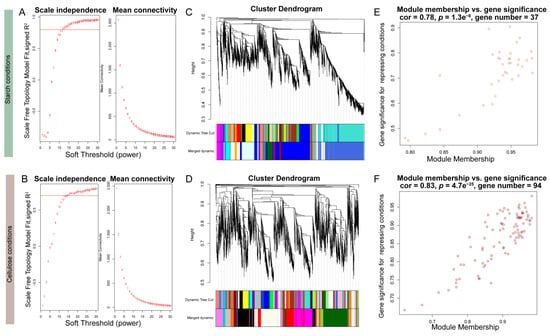
Figure 7.
WGCNA analysis in starch and cellulose conditions. (A,B) Soft-thresholding selection diagram. The red horizontal line represents a threshold of 75%. (C,D) Gene tree, module division, and merging results. (E,F) Scatter plot of module correlation with repressing conditions.
2.7. VdCreA Primarily Co-Expresses with Downregulated Genes
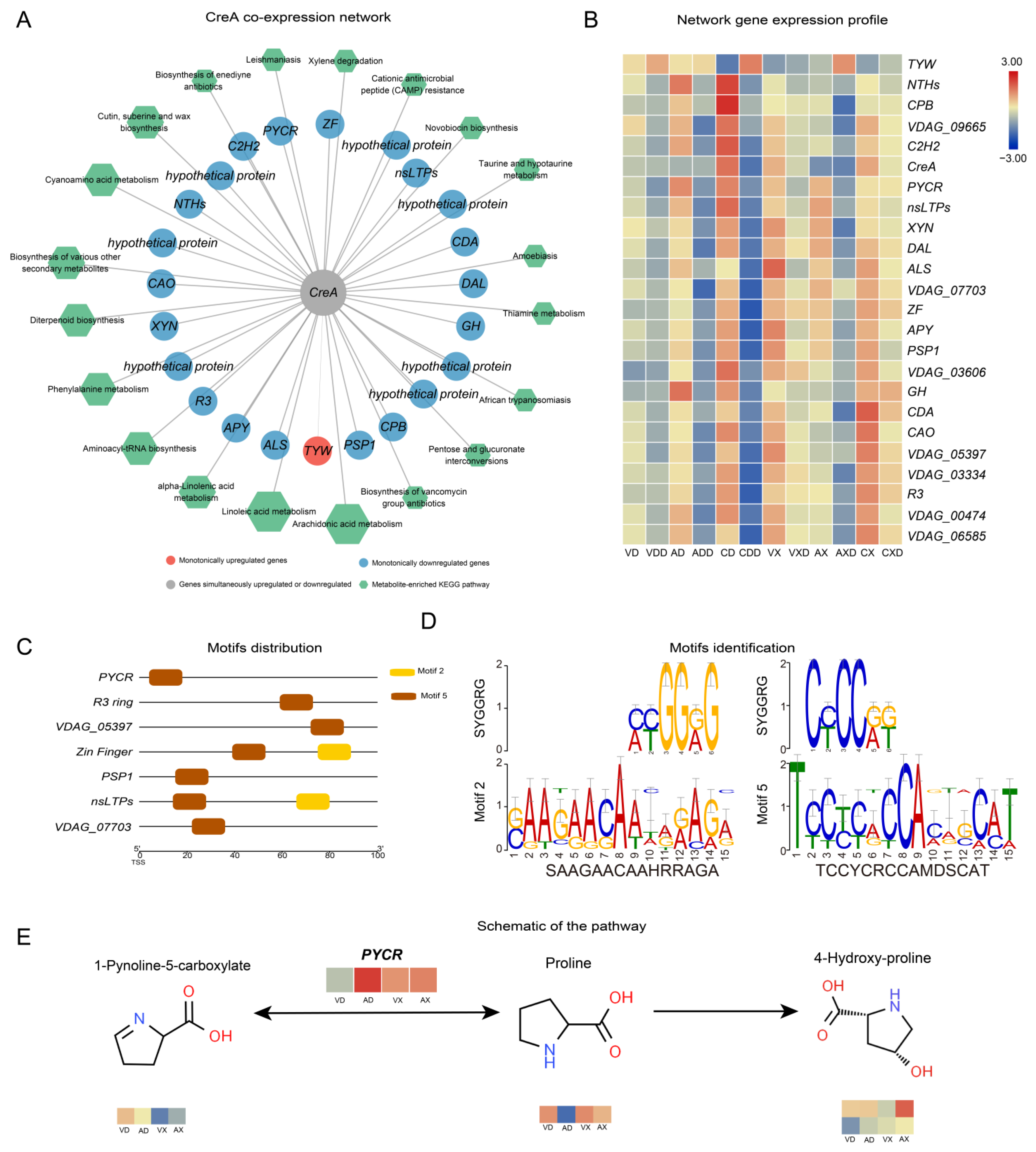
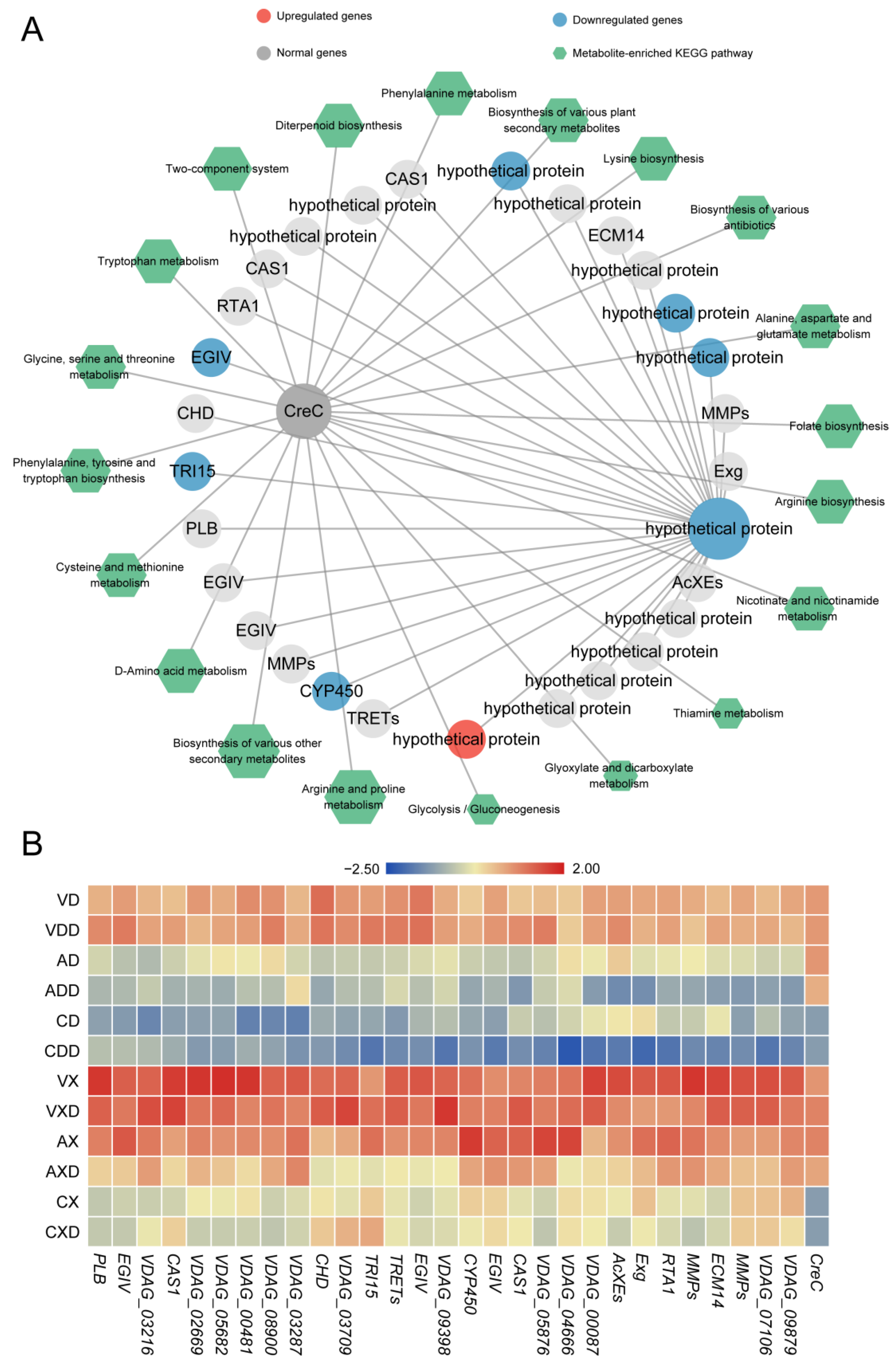
We further calculated the Spearman correlation coefficient between genes within each module and metabolites. Genes with a correlation greater than 90% that passed the significance test were considered co-expressed genes or metabolites. VdCreA co-expressed with 23 DEGs (Figure 8A), regardless of whether starch or cellulose was the carbon source. Among them, 22 were monotonically downregulated and 1 TYW (tRNA wybutosine-synthesizing protein) was monotonically upregulated. Among the monotonically downregulated genes, besides two involved in amino acid biosynthesis (PYCR, pyrroline-5-carboxylate reductase; ALS, acetolactate synthase), the functions of the remaining genes did not show obvious enrichment. Seven of these genes were hypothetical proteins, and there were also two zinc finger TFs (ZF, C2H2). At the same time, 37 DEMs were strongly correlated with VdCreA knockout. These DEMs were widely involved in various metabolic processes, mainly enriched in arachidonic acid metabolism (six DEMs), linoleic acid metabolism (five DEMs), and alpha-linolenic acid metabolism (three DEMs) pathways. The expression profiling results showed that these genes were mainly lowly expressed under repressing conditions, especially when starch was the carbon source (Figure 8B).
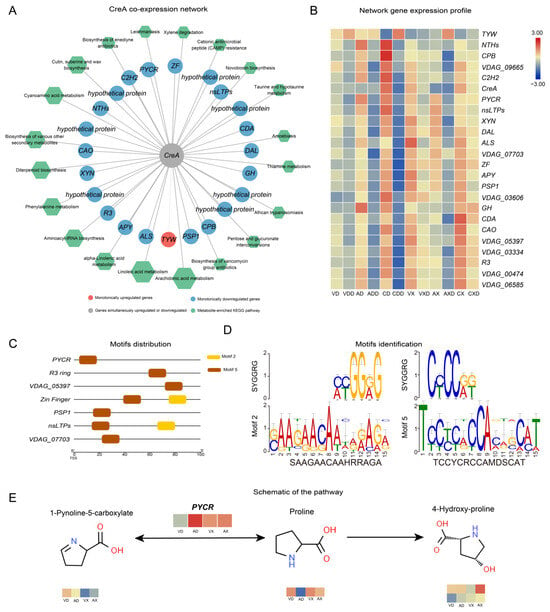
Figure 8.
VdCreA may target the regulation of PYCR expression, affecting the normal growth of the strain. (A) Co-expression network related to the VdCreA gene. The circular nodes represent genes, and the color of the circles represents the expression trend of the gene. Hexagonal nodes represent the pathways enriched with metabolites in the network. (B) Expression heatmap of genes in the co-expression network: the redder the color, the higher the expression level, and the bluer the color, the lower the expression level. (C) Motif 2 and motif 5 are distributed in the proximal promoters of seven co-expressed genes. (D) The identification of motif 2 and motif 5. (E) The changes in metabolites in the PYCR pathway.
Further, we identified the conserved motifs in the promoters of 23 genes co-expressed with VdCreA, and a total of 10 motifs were identified across all genes (Figure S5). Two of these motifs (Motif 2 and Motif 5) were identified as SYGGRG-like motifs (Figure 8D). SYGGRG is considered the consensus sequence for CreA binding target genes [5], these two motifs are distributed in the proximal promoters of seven co-expressed genes (Figure 8C), including genes encoding PYCR, PSP1 (PSP1 domain-containing protein), nsLTPs (non-specific lipid-transfer protein), two zinc fingers (RING-3 and zinc finger), and two hypothetical proteins (VDAG_05397 and VDAG_07703). These genes may be regulated by VdCreA, functioning downstream. We focused on the changes in metabolites in each gene’s respective pathway (Figure 8D). PYCR, which participates in the arginine and proline metabolism pathway and catalyzes the interconversion between proline and 1-Pynoline-5-carboxylate, is highly expressed in ΔVdCreA, while the proline content in ΔVdCreA is extremely low. This suggests that VdCreA may target the regulation of PYCR expression, thereby mediating the conversion between proline and arginine and affecting the normal growth of the strain.
2.8. VdCreC Indirectly Engages in Multiple Metabolic Dynamics
In contrast, VdCreC was only strongly directly correlated with one hypothetical protein (VDAG_09398). We further screened 28 secondary network genes co-expressed with this gene (Figure 9A). After VdCreC knockout, under different carbon source conditions, 7 genes were monotonically downregulated, 1 gene was monotonically upregulated, and 22 genes exhibited no differential expression. These genes include Exg (exoglucanase) involved in cellulose hydrolysis, CHD (choline dehydrogenase) involved in choline metabolism, PLB (pectate lyase B) involved in pectin degradation, etc. In addition, 13 genes were annotated as hypothetical proteins, and their functions are largely unknown. In total, 84 metabolites were strongly correlated with VdCreC expression. Most of these metabolites were involved in the synthesis of various amino acids such as arginine, proline, and phenylalanine. The rest were enriched in secondary metabolite synthesis, glycolysis/gluconeogenesis, folate biosynthesis, and other pathways. The expression profiling showed that although the expression trends of these genes were not completely consistent, compared with the wild type, the overall expression level of these genes decreased after VdCreC knockout, regardless of the carbon source; when cellulose was the carbon source, the expression level of these genes was higher (Figure 9B). The findings imply that, despite the absence of co-expression between VdCreC and a multitude of functional genes at the gene expression level, and the lack of significant differential expression in a large number of genes within the co-expression network, it is nevertheless indirectly implicated in alterations in a multitude of pathways related to carbon metabolism.
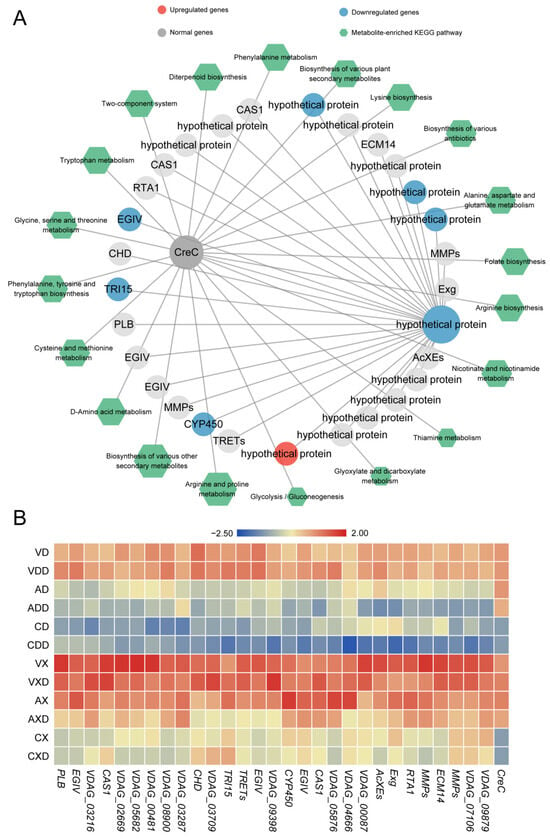
Figure 9.
Co-expression network related to repressing conditions. (A) Co-expression network related to the VdCreC gene. The circular nodes represent genes, and the color of the circles represents the expression trend of the gene. Hexagonal nodes represent the pathways enriched with metabolites in the network. (B) Expression heatmap of genes in the co-expression network: the redder the color, the higher the expression level, and the bluer the color, the lower the expression level.
2.9. Distinct Carbon Sources Induce Entirely Different Expression Networks
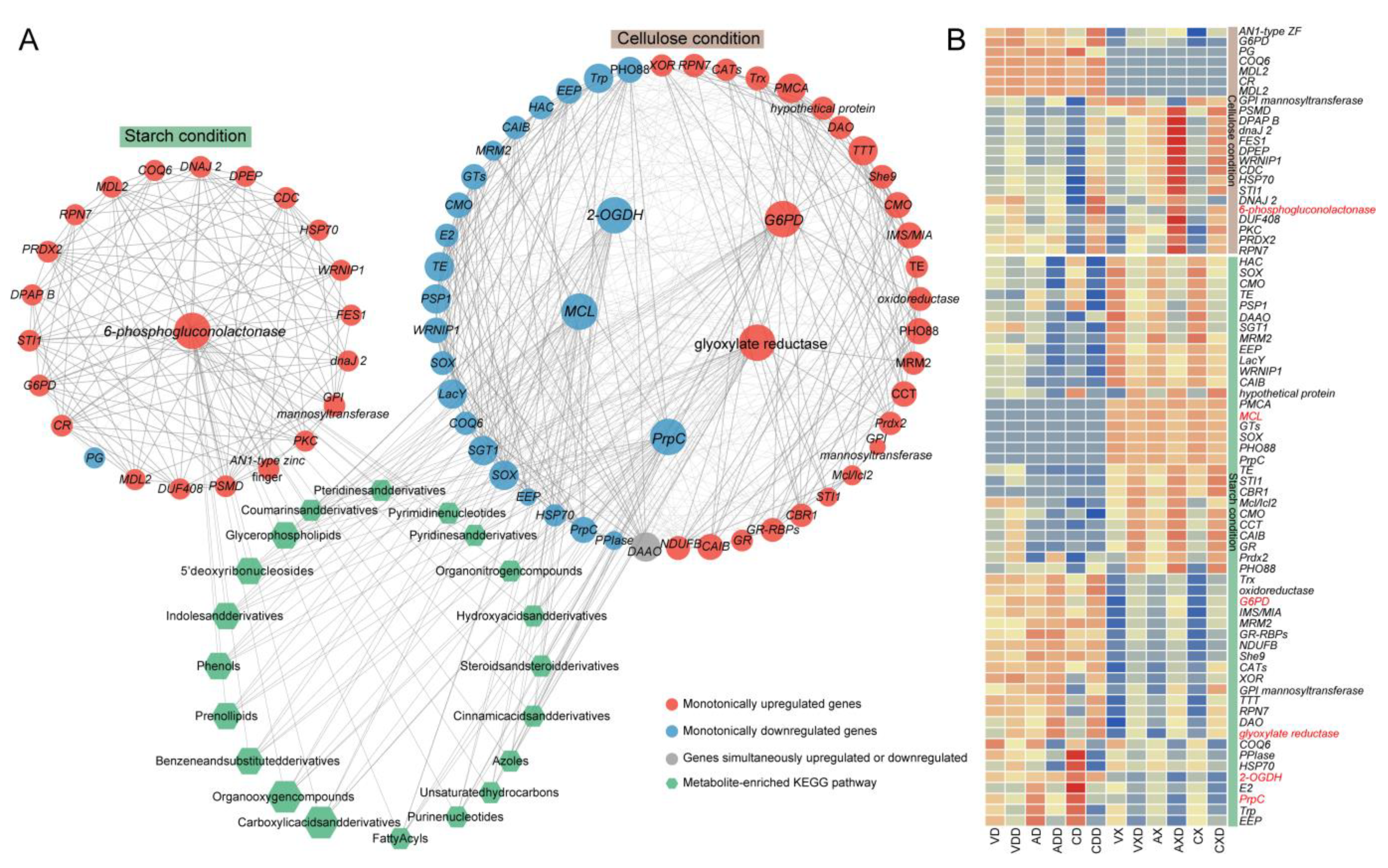
We extracted two modules related to repressing from the WGCNA results and performed KEGG enrichment on the modules’ genes (Figure S6). Among the top 20 enriched pathways, 4 were consistent: carbon metabolism, pentose phosphate pathway, arachidonic acid metabolism, and glutathione metabolism. Further, a similar process was used to construct the co-expression network of genes and metabolites within the module, with genes involved in carbon metabolism serving as hub genes (Figure 10A). Under starch conditions, the hub gene encoded a 6-phosphogluconolactonase, which is one of the core roles in the pentose phosphate pathway, and it was always upregulated after repression in all samples. There were 22 co-expressed genes with the hub gene, of which 21 were only upregulated, and 1 PG (polygalacturonase)-encoding gene was only downregulated. Under cellulose conditions, among the five hub genes, the genes encoding G6PD (glucose-6-phosphate 1-dehydrogenase) and glyoxylate reductase were always upregulated. G6PD is the rate-limiting enzyme of the pentose phosphate pathway, and glyoxylate reductase is involved in the synthesis of succinate in the glyoxylate cycle. One PrpC (2-methylcitrate synthase), one MCL (mitochondrial 2-methylisocitrate lyase), and one 2-OGDH (2-oxoglutarate dehydrogenase E1) were always downregulated under repressing conditions. These three genes encode key enzymes in the citric acid cycle. There were 47 genes co-expressed with the hub genes, of which 25 genes were always upregulated, 21 genes were always downregulated, and 1 DAAO (D-amino acid oxidase) gene had opposite expression trends in different groups. Surprisingly, although the two gene expression networks were markedly distinct, the gene functions in the two modules overlapped and their strongly correlated metabolites were basically the same, suggesting that the strain may complete CCR through similar pathways. The genes in the two co-expression networks were strongly correlated with as many as 685 metabolites. Enrichment analysis showed that these metabolites were widely involved in lipid metabolism, nucleotide metabolism, amino acid metabolism, carbohydrate metabolism, etc., indicating that CCR could systematically affect the activity and efficiency of various biochemical processes. These metabolites were mainly enriched in carboxylic acids and derivatives (14 metabolites), organooxygen compounds (12 metabolites), prenol lipids (10 metabolites), and benzene and substituted derivatives (10 metabolites). Regardless of which module’s co-expressed genes had different preferences for carbon sources, according to the expression profile, they can generally be divided into two categories (Figure 10B). One category contains most genes, such as 6-phosphogluconolactonase, PrpC, G6PD, 2-OGDH, and glyoxylate reductase, etc. Once the strain was in repressing conditions, regardless of the carbon source or strain, its expression level would stably increase or decrease. The difference was that some of them had higher expression in starch, and others had higher expression in cellulose. These genes may participate in the CCR process regardless of the carbon source. However, some other genes were only induced in one carbon source, such as MCL, which had almost no expression under starch conditions and was downregulated by repressing induction under cellulose conditions. PG was only downregulated under starch conditions. Whether they participated in CCR was related to the carbon source faced by the strain. The subtle differences in expression patterns between these co-expressed genes may be the handle for the strain to fine-tune CCR to efficiently utilize carbon sources. The core genes functioned as hub nodes within the expression network, offering a leverage point for further analysis of CCR.
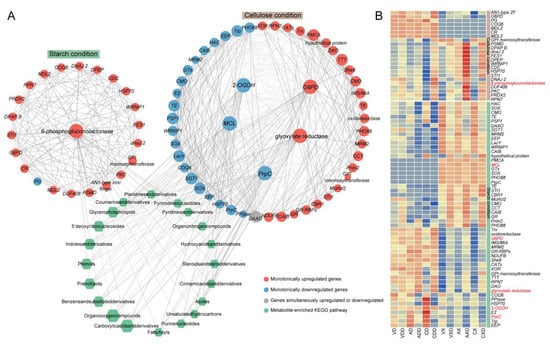
Figure 10.
Distinct carbon sources induce entirely different expression networks. (A) The co-expression network of genes and metabolites within the module, with genes involved in carbon metabolism serving as hub genes. The circular nodes represent genes, and the color of the circles represents the expression trend of the gene. Hexagonal nodes represent the pathways enriched with metabolites in the network. (B) Expression heatmap of genes in the co-expression network and metabolites: the redder the color, the higher the expression level, and the bluer the color, the lower the expression level. The labels of hub genes are highlighted in red.
2.10. The Glycolysis/Gluconeogenesis Pathway Is Entirely Altered
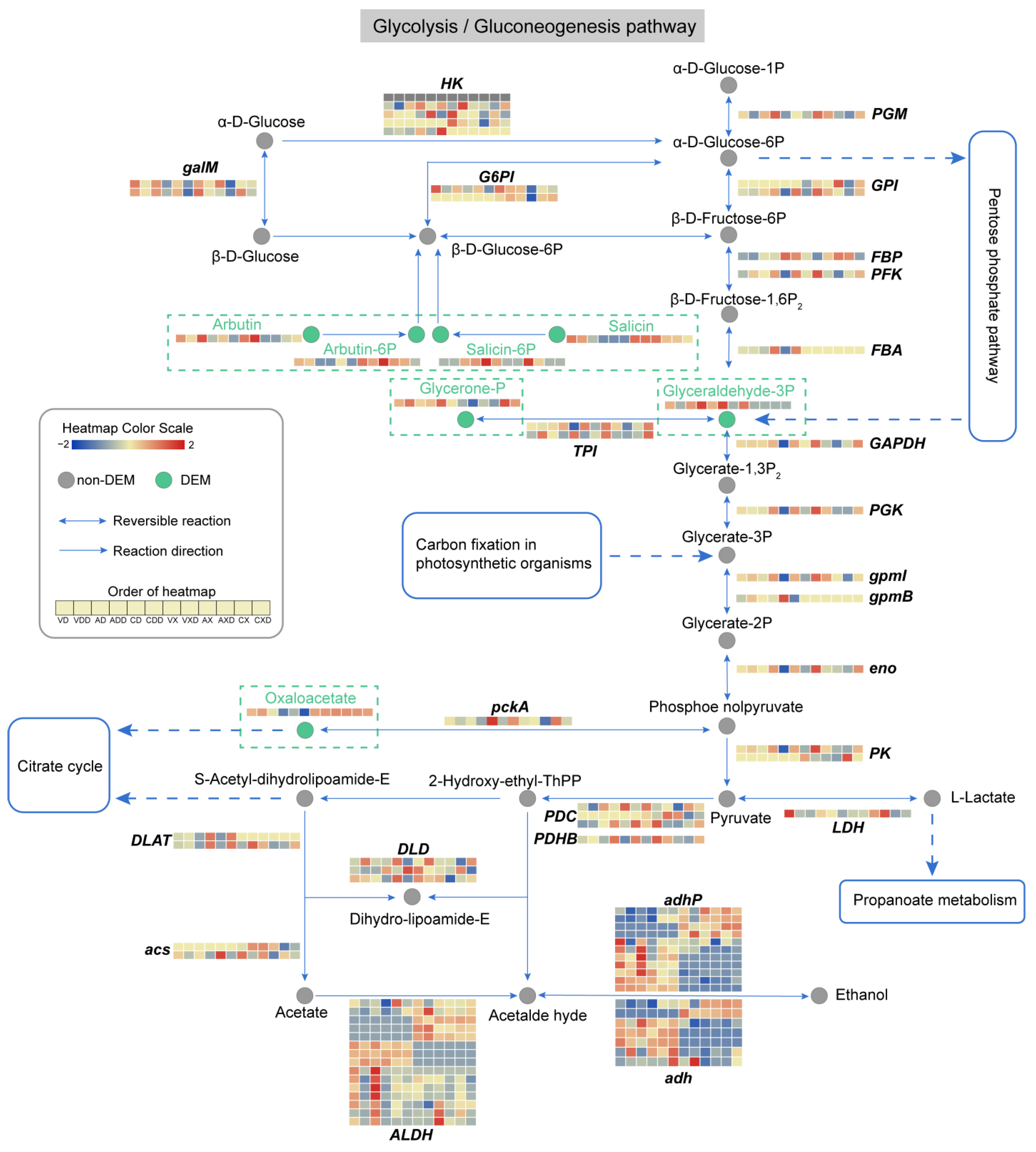
We integrated the expression profiles of genes and metabolites, focusing on the glycolysis/gluconeogenesis and the pentose phosphate pathway, which are considered to be the main pathways in the CCR process. The changes in the glycolysis/gluconeogenesis pathway were thorough (Figure 11), with 91 genes and 7 metabolites in this pathway detected to have differential changes. The core change was GADP (glyceraldehyde-3P), an intermediate product of glycolysis and one of the sugar products of the Calvin cycle. When starch was the carbon source and was subject to glucose repression, the content of GADP in the wild-type strain decreased, while the content of this substance greatly increased in ΔVdCreA and ΔVdCreC. In contrast, there was no obvious change in this substance when cellulose was the carbon source. One reason may be the upregulation of FBA (fructose-1,6-diphosphate aldolase) after VdCreA or VdCreC knockout. This enzyme catalyzes the cleavage of β-D-Fructose-1,6P2 into DHAP (Glycerone-P) and GADP, and was also only induced under starch conditions. The content of DHAP was also significantly different under different carbon sources. The difference was that the content of this substance in ΔVdCreC was higher. Two TPI (Triose phosphate isomerase) genes, which encode enzymes that catalyze the interconversion of DHAP and GADP, had significant differential expression in different groups and inconsistent expression trends. Another important metabolite that changed was oxaloacetate, a key intermediate in glycolysis and gluconeogenesis. In the samples with VdCreA or VdCreC knockout, under starch conditions, the content of oxaloacetate significantly decreased under CCR status, while its content was higher throughout when cellulose was the carbon source. The differential effects of the two carbon sources on pathway-related genes were common. Some G6PI (glucose-6-phosphate isomerase), GPI (glucose phosphate isomerase), PK (pyruvate kinase), and acs (acetyl-coenzyme A synthetase) genes only responded to cellulose carbon sources, while some FBA, gpmB (probable phosphoglycerate mutase GpmB), and DLAT (dihydrolipoamide S-acetyltransferase) genes only responded to starch carbon sources. In addition, a large number of genes encoding the ALDH (aldehyde dehydrogenase), adhP (10-acetyl-3,7-dihydroxyphenoxazine), and adh (alcohol dehydrogenase) enzymes had differential expression and large differences in expression patterns. These enzymes catalyze the conversion between acetate, acetaldehyde, and ethanol, although their contents had not been detected to have differential changes. As anticipated, the impact of CCR on glycolysis/gluconeogenesis was significant, exhibiting variances between the two carbon sources. Irrespective of the carbon source, GADP and oxaloacetate seemed to be of particular importance, potentially serving as pivotal elements of CCR, attributable to coordinated alterations in their metabolic and genetic strata.
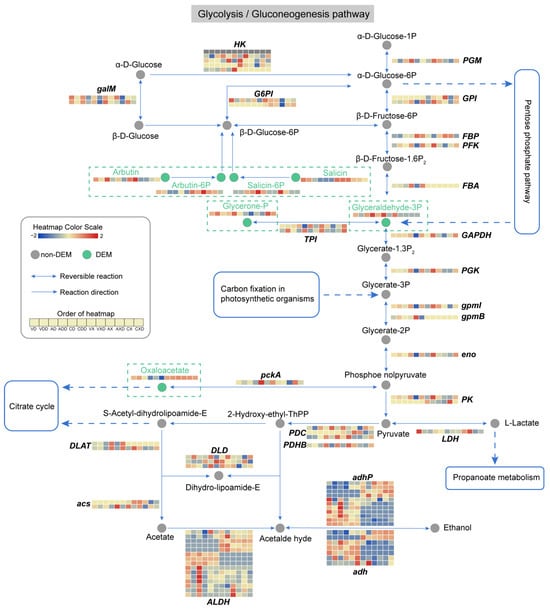
Figure 11.
Analysis of the glycolysis/gluconeogenesis metabolic pathway. Circles represent metabolites, gray circles represent non-DEMs, green circles represent DEMs, which are highlighted in the dashed rectangular box for emphasis. Arrows indicate the direction of reaction, and the genes encoding the enzymes catalyzing the reaction are displayed at the corresponding positions. The dashed arrows indicate connections to other related metabolic pathways. The heatmap represents the expression level of genes or metabolites.
2.11. The Pentose Phosphate Pathway Experiences Significant Impact
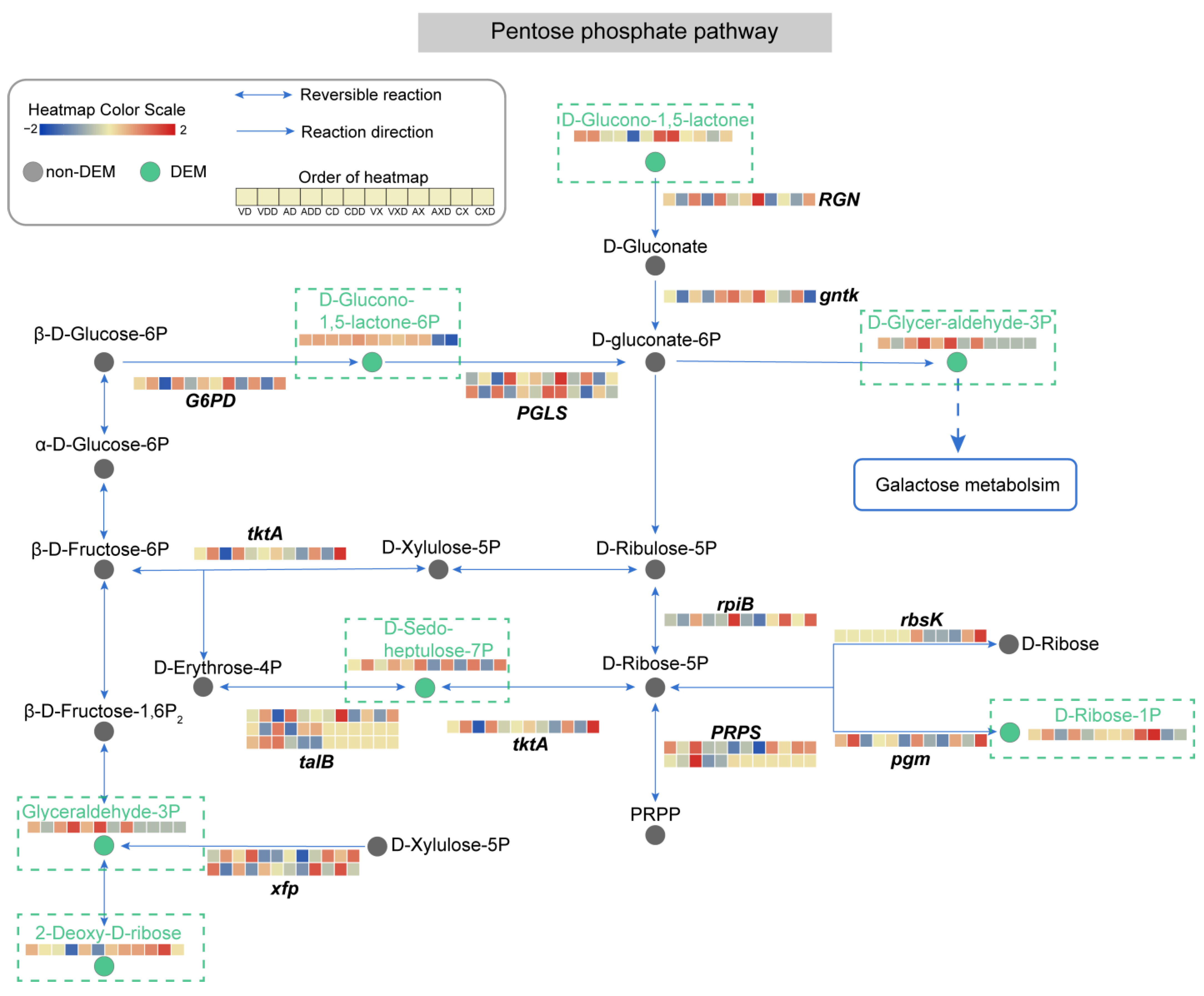
Differential expression was detected in 37 genes and 6 metabolites in the pentose phosphate pathway (Figure 12). Under starch conditions, the content of 2-Deoxy-D-ribose was lower, and the knockout of VdCreA or VdCreC and repression conditions further caused its content to decrease. However, under cellulose conditions, this substance had a higher content in all types of strains and was hardly affected by repression. Ribokinase (rbsK) catalyzes the phosphorylation of 2-Deoxy-D-ribose to form DR5P (D-Ribose-5P), and its expression only changed under cellulose conditions. Although DR5P did not change differentially in our results, the difference in the content of 2-Deoxy-D-ribose and the expression of rbsK under different carbon sources may be another reason for the change in GADP. The strain adjusted the substrate content and enzyme activity, etc., to adjust the specific reaction rate, thereby adapting to different carbon sources. D-glucono-1,5-lactone (GDL) is hydrolyzed by RGN (gluconolactonase) to produce D-Gluconate, which is an important intermediate in sugar metabolism. Then, D-Gluconate is phosphorylated by gluconokinase (gntk) to G6P (D-gluconate-6P), and G6P can be further converted to GAP (D-glycer-aldehyde-3P), which is one of the products of galactose metabolism. In our results, the content of GDL in ΔVdCreA and ΔVdCreC was lower than that in wild-type strains. Before and after glucose repression, the gene encoding RGN was downregulated under starch conditions and upregulated under cellulose conditions. GAP only accumulated in ΔVdCreA and ΔVdCreC when starch was the carbon source. When cellulose was the carbon source, the content of GAP in the mutant was lower. Although content changes in D-Gluconate and G6P were not detected, the gene encoding gntk that catalyzes its phosphorylation was essentially downregulated after repression. The content of D-sedo-heptulose-7P (S7P) increased in all types of strains after repression. Among the genes encoding enzymes involved in the change in S7P content, the expression of tktA (transketolase) and one talB (transaldolase) changed synchronously. In addition, in the pentose phosphate pathway, there were also some PRPS, rbsK, and talB genes that were only induced by one type of carbon source. These results reflected the changes in the main pathway activity and key metabolite content of V. dahliae during the CCR process. The pentose phosphate pathway underwent significant modification. GDL may be noteworthy as a progenitor to vital intermediates of glucose metabolism, which is markedly transformed in both the genetic and metabolic strata in reaction to both carbon sources.
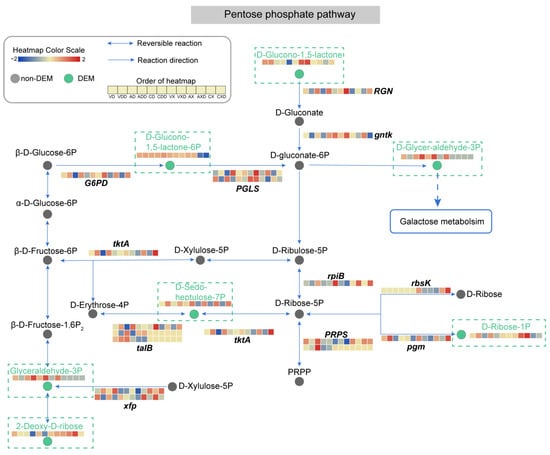
Figure 12.
Analysis of the pentose phosphate metabolic pathway. Circles represent metabolites, gray circles represent non-DEMs, green circles represent DEMs, which are highlighted in the dashed rectangular box for emphasis. Arrows indicate the direction of reaction, and the genes encoding the enzymes catalyzing the reaction are displayed at the corresponding positions. The dashed arrows indicate connections to other related metabolic pathways. The heatmap represents the expression level of genes or metabolites.
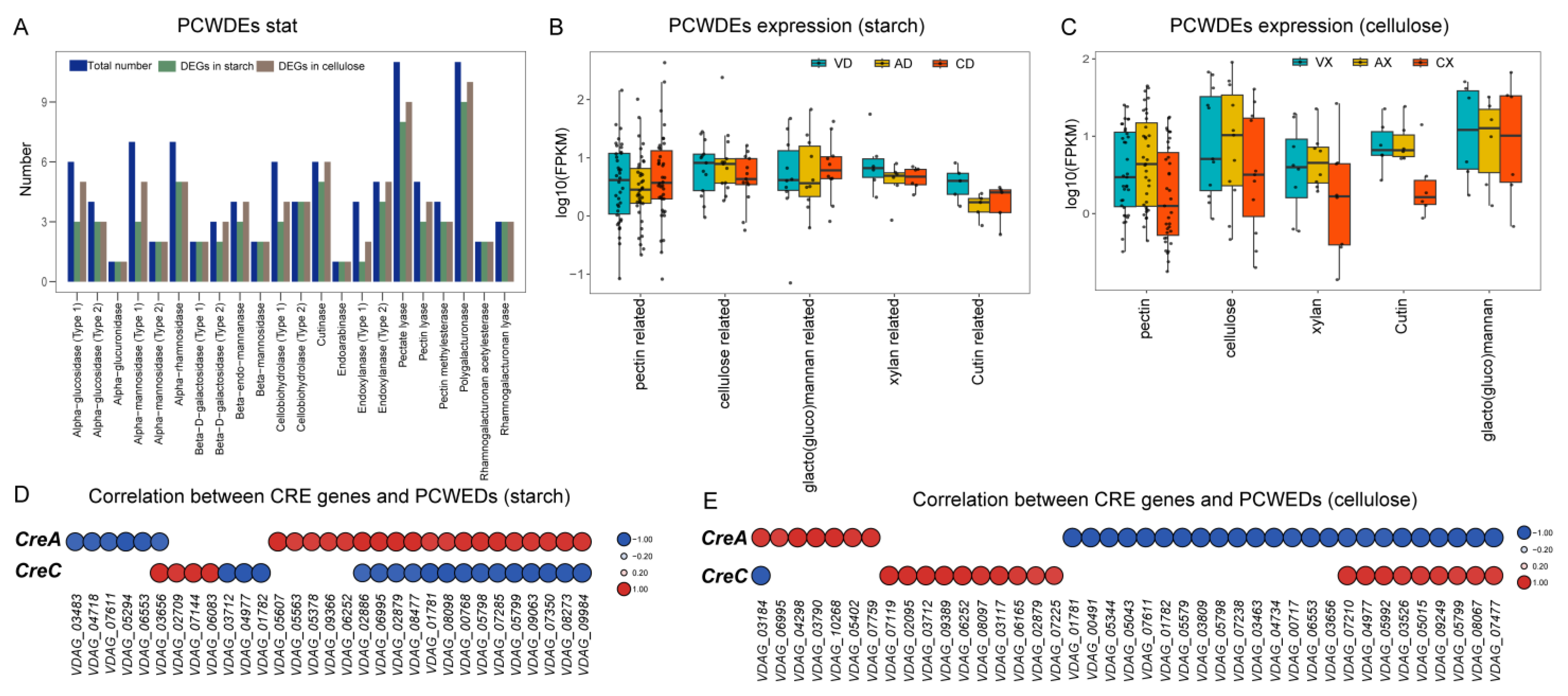
2.12. VdCreA and VdCreC Influence the Expression of Genes Encoding PCWDE
Plant cell wall-degrading enzymes (PCWDE) are commonly associated with fungal pathogenicity. We collected all PCWDE genes from V. dahliae based on published data, totaling 100 genes across 22 gene families. We screened the sets of DEGs from different comparative groups (Figure 13A). Regardless of the carbon source, almost all PCWDE genes showed significant differential expression between at least two groups. Under starch conditions, 72 out of 100 PCWDE genes (72%) were identified as DEGs, while under cellulose conditions, 85 (85%) PCWDE genes exhibited differential expression. Among the two largest PCWDE gene families responsible for degrading the pectin main chain, namely polygalacturonase and pectate lyase, 17 and 19 out of the total 22 members, respectively, showed differential expression under starch and cellulose conditions. Further grouping of PCWDEs based on their respective functions and statistical analysis of expression level distribution and median expression within each group focused on those unaffected by glucose repression. Under starch conditions (Figure 13B), despite varying degrees, ΔVdCreA led to a decrease in the expression levels of all PCWDE genes, while ΔVdCreC resulted in a decrease in the expression levels of PCWDE genes associated with pectin, cellulose, xylan, and cutin, with an increase in the expression levels of PCWDE genes related to galactomannan. Conversely, under cellulose as the carbon source (Figure 13C), there was a noticeable overall increase in the PCWDE expression trend in ΔVdCreA, particularly in terms of pectin and cellulose, while a significant decrease was observed in PCWDE expression in ΔVdCreC. To elucidate the specific roles of VdCreA and VdCreC, we selected PCWDE genes that showed a significant strong correlation with the expression of VdCre (Pearson correlation coefficient > 0.8, p value < 0.05). Under starch conditions (Figure 13D), 19 PCWDE genes were significantly positively correlated with VdCreA, while 6 were significantly negatively correlated. Among all 25 significantly correlated genes, 16 (64%) were involved in pectin degradation on the plant cell wall. In total, 17 PCWDE genes were significantly negatively correlated with VdCreC, while 4 were significantly positively correlated. Out of all 21 PCWDE genes, 17 (80.95%) were associated with pectin degradation. Significant correlations were found between VdCreA and VdCreC and a large number of PCWDE genes involved in pectin degradation, with VdCreA primarily showing positive correlations and VdCreC showing negative correlations. Conversely, under cellulose as the carbon source (Figure 13E), 24 out of 41 PCWDE genes were significantly negatively correlated with VdCreA, while 19 were significantly positively correlated with VdCreC. Moreover, among the 31 and 20 PCWDE genes correlated with VdCreA and VdCreC, respectively, 19 (61.29%) and 10 (50%) were associated with pectin degradation. Despite a decrease in the number of PCWDE genes associated with pectin degradation, it still approached or exceeded half of the total genes correlated with VdCreA and VdCreC. All these results collectively suggest that VdCre could influence the expression changes in fungal cell wall-degrading enzyme genes, thereby leading to alterations in the pathogenicity of the strain. This regulatory effect varies under different carbon sources, but consistently exerts a significant impact on pectin degradation-related activities.
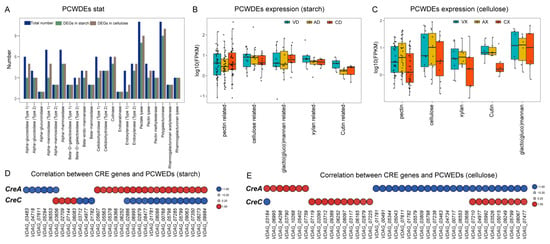
Figure 13.
Both VdCreA and VdCreC influence the expression of fungal cell wall-degrading enzyme genes depending on different carbon sources. (A) Bar graph illustrating the statistics of 22 PCWDE gene families. The x-axis represents the gene family names, and the corresponding bars indicate the total number of PCWDE genes in each family and the number of differentially expressed genes under starch conditions and under cellulose conditions. (B) Box plots depicting the expression levels of PCWDE genes under starch conditions. All expression levels were normalized by taking logarithms. Different colors represent samples from different groups, and the median expression level is depicted by a black solid line within each box plot. (C) Box plots illustrating the expression levels of PCWDE genes under cellulose conditions, with normalization by logarithm. Similar to (B), different colors denote samples from different groups, and the median expression level is indicated by a black solid line within each box plot. (D) Heat map depicting the correlation between VdCre genes and PCWDE genes under starch conditions. All correlation coefficients are greater than 80% with a p value less than 0.05. Negative correlations are represented in blue, while positive correlations are shown in red. Larger circles in the heat map indicate higher correlation values. (E) Heat map representing the correlation between VdCre genes and PCWDE genes under cellulose conditions. Similar to (D), all correlation coefficients exceed 80% with a p value less than 0.05. Negative correlations are depicted in blue, and positive correlations are displayed in red. Larger circles in the heat map signify higher correlation values.
3. Discussion
Studies have found that CreA has two conserved zinc finger domains, which allow it to repress gene expression by binding directly to the 5′-SYGGRG-3′ motif in the promoters of the target genes [5]. It is therefore proposed that CreA regulates CCR by a double-lock control; that is, CreA physically competes with upstream regulators for DNA binding at the promoter of downstream genes to repress their expression (i.e., block transcriptional activation) [24,25]. However, we found that VdCreA in V. dahliae lacks two N-terminal zinc finger domains and part of the nuclear location signal sequences. Transcriptomics revealed VdCreA co-expressed with 23 genes (Figure 8A) regardless of whether starch or cellulose was used as the carbon source, and SYGGRG-like motifs were identified in the proximal promoters of the seven co-expressed genes including genes encoding PYCR, PSP1, nsLTPs, two zinc fingers, and two hypothetical proteins. These results suggested that VdCreA could directly regulate the expression of these target genes, and it is speculated that VdCreA may co-regulate the expression of these target genes with other proteins due to VdCreA lacking zinc finger domains.
A recent study revealed that only a small number of genes are actually subjected to the double-lock control, while the expression of the majority of the downstream pathway genes is indirectly regulated by CreA [7]. Our study showed that there were 271 DEGs in ΔVdCreA compared with the wild-type strain under repression conditions, indicating these 271 DEGs may be the main genes involved in CCR and VdCreA may indirectly regulate most of these genes. Moreover, there were more TFs in the 271 DEGs under repressing conditions, mainly four Zn-clus (Zn2Cys6 TF) and three GNAT (Gcn5-related N-acetyltransferases) TFs. Zn-clus is a class of fungus-specific TFs, widely involved in various metabolic processes [26]. GNAT belongs to the lysine acetyltransferases enzymes, and it is thought to be mainly responsible for histone acetylation modification [27]. The two groups shared one SNF2 TF, which is a well-known part of the ATP-dependent chromatin remodelers [28]. This suggests that VdCreA may indirectly regulate the expression of more genes by affecting the expression of these TF genes.
Compared to the wild-type strain V592, the VdCreA knockout mutant tends to grow slowly with reduced conidiation and microsclerial production, which is in line with previous studies [13,29,30]. This could be due to the downregulation of some important cellular processes like protein translation or amino acid metabolism by the knockout of VdCreA. We found that PYCR, participating in the arginine and proline metabolism pathway, was highly expressed in ΔVdCreA, while the proline content in ΔVdCreA was extremely low. Similar results were observed in Staphylococcus aureus, where PYCR (ProC) under CCR interferes with the synthesis from proline to arginine [31]. This suggests that VdCreA may target the regulation of PYCR expression, thereby mediating the conversion between proline and arginine and affecting the normal growth of the strain.
In this study, we identified and functionally characterized VdCreC, a protein containing a WD40-repeat domain in V. dahliae. Our results showed that VdCreC is a key regulator of CCR and also closely involved in conidiation, microsclerial production, and pathogenicity in this fungus. This is consistent with MoCreC in M. oryzae [15]. Transcriptomic analyses revealed that VdCreC was only strongly directly correlated with 1 gene (VDAG_09398) and indirectly correlated with 28 secondary network genes. However, metabolic analysis showed that 84 metabolites were strongly correlated with VdCreC expression. These metabolites were involved in the synthesis of various amino acids such as arginine, proline, and phenylalanine or enriched in secondary metabolite synthesis, glycolysis/gluconeogenesis, and folate biosynthesis. This suggests that VdCreC is indirectly implicated in alterations in a multitude of pathways related to carbon metabolism, and it may affect the normal growth of the strain by participating in the synthesis of various amino acids and some secondary metabolites. This is congruent with the role of VdCreC in preserving protein stability.
We demonstrated that distinct carbon source conditions induced entirely different gene expression networks in V. dahliae. One gene encoding a 6-phosphogluconolactonase served as a hub gene under starch conditions, and five genes encoding G6PD, 2-ODGH, PrpC, MCL, and glyoxylate reductase served as hub genes under cellulose conditions. There was no overlap of genes in the two co-expression networks when starch and cellulose were used as the carbon source; however, the resultant gene functions in the two networks overlapped and their strongly correlated metabolites exhibited similarities. This suggests that V. dahliae may complete CCR through similar pathways with different gene expressions. Enrichment analysis showed that these metabolites were widely involved in lipid metabolism, nucleotide metabolism, amino acid metabolism, carbohydrate metabolism, etc., indicating that CCR could systematically affect the activity and efficiency of various biochemical processes, thereby widely mediating the growth and development and physiological metabolism dynamics of V. dahliae. This is consistent with previous findings of intricate connections between the regulation of carbon metabolism and diverse cellular functions [8,30].
We have further analyzed the integrated expression profiles of genes and metabolites in the glycolysis/gluconeogenesis and pentose phosphate pathway under different carbon sources and CCR conditions. The results showed that the two major sugar metabolism-related pathways were completely changed. There were 91 DEGs in the glycolysis/gluconeogenesis pathway and 37 DEGs in the pentose phosphate pathway; however, only the contents of 7 and 6 metabolites in the two pathways have been detected to have differential changes, respectively. Among them, GADP changes significantly and is an important metabolite associated with the two pathways, indicating GADP may be a pivotal factor of CCR under different carbon sources. These results imply that the metabolites with significant changes in content may be directly participating in carbon metabolism, thereby affect fungal growth and development. However, the contents of most metabolites in the two pathways were not altered, while the genes encoding the enzymes that catalyze these metabolites had significant differential expression. This disparity in expression networks is potentially instrumental to fungal adaptation to intricate carbon sources. Through the modulation of core genes or nuanced adjustment of peripheral gene expression, fungi can exhibit sufficient flexibility in modulating the CCR process.
It was reported that the V. dahliae genome encodes a large number of PCWDEs, and characterization of the exoproteome revealed that at least 52 proteins participate in the pectin and cellulose degradation pathways [32]. All these PCDWEs are responsible for fungal pathogenicity. In this study, we found that both VdCreA and VdCreC influenced the expression of genes encoding PCDWEs, and their influence depended on different carbon sources. Under starch conditions, VdCreA knockout led to a decrease in the expression levels of almost all PCWDE genes (Figure 13B), 19 out of 25 PCWDE genes were significantly positively correlated with VdCreA, and 64% were involved in pectin degradation; while under cellulose conditions, there was a noticeable overall increase in the PCWDE expression trend (Figure 13C), 24 out of 41 PCWDE genes were significantly negatively correlated with VdCreA, and 61.29% were associated with pectin degradation. Under starch conditions, VdCreC knockout resulted in an overall decrease in the expression levels of PCWDE genes, except for an increase in the expression levels of PCWDE genes related to galactomannan (Figure 13B); 17 out of 21 PCWDE genes were significantly negatively correlated with VdCreC, and 80.95% were associated with pectin degradation; while under cellulose conditions, there was a significant decrease in PCWDE expression (Figure 13C), 19 out of 41 PCWDE genes were significantly positively correlated with VdCreC, and 50% were associated with pectin degradation. In addition, irrespective of the carbon source, VdCreA and VdCreC either had opposite regulatory effects on the expression of the same PCWDE genes or regulated the expression of different CWDE genes, respectively (Figure 13D,E). These results collectively suggest that VdCreA and VdCreC may use different regulatory mechanisms to regulate PCWDE genes’ expression, thereby leading to alterations in the pathogenicity of the strain.
4. Materials and Methods
4.1. Fungal Strains and Growth Conditions
The virulent defoliating strain V592 of V. dahliae isolated from an infected cotton plant was used as the wild-type strain [33], and mutants were generated from V592 in this study. To assess their phenotypic characteristics, fungal strains were cultured on potato dextrose agar (PDA: 200 g potato, 15 g glucose, 15 g agar powder) plates at 25 °C in the dark for 15 days or cultured on Czapek–Dox media (CDM: 2 g NaNO3, 1 g K2HPO4, 0.5 g KCl, 0.24 g MgSO4, 0.01 g FeSO4·7H2O) containing different sole carbon sources with or without D-glucose at 25 °C in the dark for 7 days (Constant-Temperature Incubator, MMM Company, Stadlern, Germany). To collect conidia for spore count and infection assays, fungal strains were cultured in liquid Czapek–Dox medium for 7 days at 25 °C with shaking at 150 rpm/min (Intelligent shaker, Tianjin Honour Instrument Co., Ltd., Tianjin, China).
4.2. Identification and Phylogenetic Analysis of Target Genes
The mycelia of the V592 strain were collected from PDA medium cultured at 25 °C in the dark for 7 days to extract genomic DNA and total RNA using a fungal DNA Extraction Kit (BIOER, Hangzhou, China) and fungal RNA Kit (Omega Inc., Norwalk, CT, USA) according to the manufacturer’s procedures, respectively. cDNA synthesis was performed with the PrimeScript™ RT reagent kit (TaKaRa, Dalian, China). The genomic DNA and cDNA were used as templates to amplify the full lengths of VdCreA and VdCreC. All of the primers used in this study are listed in Table S3. The conserved motifs and protein structure of VdCreA and VdCreC were analyzed by the MEME program (https://meme-suite.org/meme/tools/meme (accessed on 23 October 2023)). The sequences of homologous proteins of CreA and CreC from other fungi were downloaded from the NCBI database (http://www.ncbi.nlm.nih.gov/ (accessed on 23 October 2023)). The phylogenetic analysis was accomplished using MEGA 7.0 via the neighbor-joining method and bootstrap tests replicated 1000 times. The phylogenetic tree was visualized by the Interactive Tree of Life online tool (https://itol.embl.de/ (accessed on 23 October 2023)).
4.3. Vector Construction and Fungal Transformation
The VdCreA and VdCreC single-gene knockout mutants and their complementary strains were obtained by Agrobacterium tumefaciens-mediated transformation (ATMT) [34]. To generate the knockout plasmids, upstream and downstream fragments of each target gene were amplified from the genomic DNA of the V592 strain using corresponding primers. Then, the resultant PCR products were fused with linearized pGKO-HPT by using the ClonExpress MultiS One Step Cloning Kit (Vazyme, Nanjing, China) to construct a knockout plasmid of each target gene. To generate the complementary and overexpression plasmids, each intact target gene including the encoding region, native promoter, and terminator was amplified from amplified from the V592 strain, then fused with an XbaI/BamHI-linearized p1300-Neo-oLiC-Cas9-TtrpC vector, and then reintroduced to the corresponding gene-knockout strain to generate complementation strains or reintroduced to the V592 strain to generate overexpression strains by ATMT. The primers used for the plasmid constructions are listed in Table S3.
4.4. Phenotype Assays
To determine the effects of each single-gene (VdCreA and VdCreC) knockout on fungal growth and the utilization of different carbon sources, the fungal mycelium of each strain cultured on PDA medium at 25 °C in the dark for 7 days was inoculated using a sterilized toothpick into the center of Czapek–Dox agar containing different carbon sources (0.5% glucose, 0.5% xylose, 0.5% sucrose, 0.5% xylan, 0.5% raffinose, 0.5% carboxymethyl cellulose, 0.5% starch, 15 g agar) with or without 2% glucose. The growth rate was measured by checking the diameter of each colony. Czapek–Dox agar with 100 g CMC plus 20 mg/mL 2-DOG, and 100 g starch plus 20 mg/mL 2-DOG were used to test the CCR. For assessment of conidial production, 100 µL aliquots of a 107 conidia/mL suspension of each strain were inoculated into Czapek–Dox liquid medium and incubated for 7 days at 25 °C with 150 rpm/min shaking, then the number of conidia were counted under a microscope with a hemocytometer. To observe the hyphal morphology, each fungal strain was inoculated in the center of a PDA plate, sterilized cover glass slides were inserted into the media, and the sample maintained at 25 °C in the dark for 3 days; then, the hyphal morphology on the cover glass slides was observed under a microscope. For assessments of microsclerotial accumulation, 100 µL aliquots of a 107 conidia/mL suspension of each strain were dropped onto minimal medium (MM: 2 g NaNO3, 1 g KH2PO4, 0.5 g KCl, 0.5 g MgSO4·7H2O, 2 g glucose, 10 mg citric acid, 10 mg ZnSO4·7H2O, 10 mg FeSO4·7H2O, 2.6 mg NH4Fe(SO4)3·12H2O, 0.5 mg CuSO4·5H2O, 0.1 mg MnSO4·H2O, 0.1 mg H3BO3, 0.1 mg Na2MoO4·2H2O, 15 g agar) plates overlaid with cellophane and incubated in the dark for 15 days at 25 °C (Constant-Temperature Incubator, MMM company, Stadlern, Germany); then, the resulting microsclerotia was scraped off from the cellophane to weigh the wet weight.
4.5. Plate Assay of Amylase and Cellulase Activity
The fungal mycelium of each strain cultured on PDA medium at 25 °C in the dark for 7 days was inoculated in the center of Czapek–Dox agar plates containing 0.5% starch, 0.5% carboxymethyl cellulose (CMC), and 0.5% starch plus 2% glucose, and 0.5% CMC plus 2% glucose, respectively. After incubation at 25 °C in the dark for 10 days, amylase activity was visualized using an iodine test, and cellulase activity was visualized by staining with 0.1% Congo red followed by de-staining with 0.7 M NaCl.
4.6. Gene Expression Analysis by RT-qPCR
The fungal strains were cultured on PDA medium with different carbon source at 25 °C in the dark for 7 days. The extraction of total RNA of each fungal strain and cDNA synthesis was as described above. qPCR amplification was performed using the PowerUp SYBR Green Master Mix kit (Thermo Fisher, Vilniaus, Lithuania) with the 7500 real-time PCR system. The obtained results were normalized against the expression of the β-tubulin (DQ266153) of V. dahliae. The relative expression level was calculated using the 2−∆∆Ct method. The primers used for gene expression are listed in Table S3.
4.7. Pathogenicity Assays
Pathogenicity assays were performed at the three or fourth true leaf stage of upland cotton cultivar Junmian No. 1 using the unimpaired root-dip inoculation method as previously described [33]. The cotton seedlings were inoculated by immersing their root into 200 mL of 107 conidia/mL suspension of each strain for 30 min, then planted in 1/10 MS liquid medium (Coolaber, Beijing, China) and placed in an environmentally controlled chamber with a photoperiod of 16 h of light and 8 h of darkness at 28 °C. Each fungal strain was tested on three pots of cotton seedlings, with 12 seedlings per pot. The disease severity was evaluated by the percent of leaves showing wilt symptoms [35]. The formula of the disease index (DI) value = [Σ(the seedlings of every grade × relative grade)/(total seedlings × 4)] × 100. The infection assays were performed at least three times.
4.8. Transcriptomic Determination and Analysis
RNA-seq was performed on the wild-type V592, ΔVdCreA, and ΔVdCreC strains before and after glucose repression, using starch and cellulose as carbon sources, respectively. The correspondence between labels and the samples were as follows: VD: wild-type strain using starch as a carbon source, VX: wild-type strain using cellulose as a carbon source, VDD: wild-type strain using starch as a carbon source under glucose inhibition, VXD: wild-type strain using cellulose as a carbon source under glucose inhibition, AD: ΔVdCreA strain using starch as a carbon source, AX: ΔVdCreA strain using cellulose as a carbon source, ADD: ΔVdCreA strain using starch as a carbon source under glucose inhibition, AXD: ΔVdCreA strain using cellulose as a carbon source under glucose inhibition, CD: ΔVdCreC strain using starch as a carbon source, CX: ΔVdCreC strain using cellulose as a carbon source, CDD: ΔVdCreC strain using starch as a carbon source under glucose inhibition, CXD: ΔVdCreC strain using cellulose as a carbon source under glucose inhibition. Three biological replicates were set for each sample group (added suffix labels 1, 2, 3 for distinction). The extraction of total RNA from each sample was identical to the aforementioned method, total RNA was detected for purity, concentration, and integrity using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) and Agient2100 (Agilent Technologies, Santa Clara, CA, USA). RNA that passed the test underwent PE150 library construction and second-generation sequencing on the Illumina NovaSeq platform, with library construction and sequencing work commissioned to Beijing Biomarker Biotechnology Co., Ltd., Beijing, China.
The raw reads after sequencing were first filtered for low quality and adapter sequences, and then the filtered sequences were aligned to the V. dahliae reference genome (ASM15067v2) using HISAT2. The expression of unigenes was calculated and normalized by FPKM, and differential screening was performed through EBseq2. Those with a fold-change greater than 2 and a false discovery rate (FDR) less than 0.01 were considered differentially expressed genes (DEGs). De novo annotation of unigenes was performed through eggNOG-Mapper (v5), and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed through the R package clusterProfiler (p value < 0.05). PCA analysis were performed using the R package (PCAtools). In addition, Tbtools (V2) and R (V4) were used for the visualization of all heat maps, box plots, violin plots, and up-set diagrams. All DEGs were first filtered based on expression and missing degree, and then WGCNA was performed through the R package (WGCNA), calculating the scale-free topological fit index and mean connectivity within 1–30 and selecting the optimal soft threshold. Hierarchical clustering of DEGs was performed through the topological overlap measure (TOM), and the similarity module merge threshold was set to 0.25. The Spearman correlation coefficient between genes and metabolites was calculated through R, and the correlation network was visualized by Cytoscape (V3). The identification of consistent sequences in the promoter region was accomplished through MEME-chip, and the sequences of SYGGRG and CGGG motif were compared with the identified motif.
4.9. Untargeted Metabolite Detection
The samples used for RNA-seq were also used for untargeted metabolite detection, which was completed by Beijing Biomarker Biotechnology Co., Ltd. Specifically, the samples were dissolved in 0.1% formic acid aqueous solution (mobile phase A) and 0.1% formic acid acetonitrile (mobile phase B). Based on the LC/MS system of a Waters Acquity I-Class PLUS ultra-high performance liquid in tandem with a Waters Xevo G2-XS QT high-resolution mass spectrometer (Waters, Corporation, Milford, MA, USA), dual-channel data collection was performed for low collision energy and high collision energy. The mobile phase A used a gradient of 98%–2%–2%–98%, running for 0.25 min, 10 min, 13 min, and 13.1 min, while maintaining a flow rate of 400 μL/min throughout. The low collision energy was 2 V, and the high collision energy range was 10–40 V. The scanning frequency of the mass spectrometer was 0.2 s. The parameters of the ESI ion source were as follows: capillary voltage: 2000 V (positive ion mode) or −1500 V (negative ion mode); cone voltage: 30 V; ion source temperature: 150 °C; desolvent gas temperature: 500 °C; backflush gas flow rate: 50 L/h; desolventizing gas flow rate: 800 L/h. The chromatographic column was a Waters Acquity UPLC HSS T3 (1.8 μm, 2.1 × 100 mm) (Waters, Corporation, MA, USA). The original data were collected through MassLynx (v4.2) and then peak extraction and alignment by Progenesis QI (V1). Metabolite identification was completed through the METLIN database, with mass deviation controlled within 100 ppm. The functions of all metabolites were annotated using the KEGG, HDBM (human metabolome database), and Lipidmaps (lipid metabolites and pathways strategy) databases. OPLS-DA modeling was applied through the R package ropls, and a permutation test (n = 200) was used to verify the stability of the model. The VIP value of metabolites was calculated through multiple cross-validation, and the differential multiples (greater than 1), p value (less than 0.05), and VIP (greater than 1) of the OPLS-DA model were used to screen differentially expressed metabolites (DEMs). The Spearman correlation and KEGG enrichment were consistent with RNA-seq.
4.10. Data Statistical Analysis
SPSS 26.0 software was used for statistical analysis of the data. Duncan’s new multiple range method was used to test the significance of the difference. ** represents a significant difference at p < 0.01 from Student’s t-test.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms252111575/s1.
Author Contributions
Conceptualization, Y.W. and J.H.; methodology and data curation, Y.W. and D.X.; software, Q.L.; validation, Y.W., D.X. and B.Y.; investigation, Y.W.; writing—original draft preparation, Y.W. and J.H.; writing—review and editing, Y.W. and J.H.; visualization, Y.W. and B.Y.; supervision, Q.L. and J.H.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32060590), Guiding Science and Technology Planning Project of BINGTUAN (No. 2022ZD009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw sequencing data can be accessed through the Gene Expression Omnibus with the accession number (the data are being audited).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernandez, J.; Wright, J.D.; Hartline, D.; Quispe, C.F.; Madayiputhiya, N.; Wilson, R.A. Principles of carbon catabolite repression in the rice blast fungus: tps1, nmr1-3, and a mate-family pump regulate glucose metabolism during infection. PLoS Genet. 2012, 8, e1002673. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, E.; Uchida, R.; Asano, K.; Kanamaru, K.; Kimura, M.; Kimura, T.; Kobayashi, T. cAMP signaling factors regulate carbon catabolite repression of hemicellulase genes in Aspergillus nidulans. AMB Express 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, E.; Li, Y.; Uchida, R.; Nohara, T.; Asano, K.; Hattori, A.; Kimura, T.; Kanamaru, K.; Kimura, M.; Kobayashi, T. CreA-independent carbon catabolite repression of cellulase genes by trimeric G-protein and protein kinase A in Aspergillus nidulans. Curr. Genet. 2019, 65, 941–952. [Google Scholar] [CrossRef] [PubMed]
- De Vit, M.J.; Waddle, J.A.; Johnston, M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol. Biol. Cell 1997, 8, 1603–1618. [Google Scholar] [CrossRef]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon Catabolite Repression in Filamentous Fungi. Int. J. Mol. Sci. 2017, 19, 48. [Google Scholar] [CrossRef]
- Cupertino, F.B.; Virgilio, S.; Freitas, F.Z.; de Souza Candido, T.; Bertolini, M.C. Regulation of glycogen metabolism by the CRE-1, RCO-1 and RCM-1 proteins in Neurospora crassa. The role of CRE-1 as the central transcriptional regulator. Fungal Genet. Biol. 2015, 77, 82–94. [Google Scholar] [CrossRef]
- Tamayo, E.N.; Villanueva, A.; Hasper, A.A.; de Graaff, L.H.; Ramón, D.; Orejas, M. CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes in Aspergillus nidulans. Fungal Genet. Biol. 2008, 45, 984–993. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, L.; Alam, M.A.; Pardeshi, L.; Miao, Z.; Wang, F.; Tan, K.; Hynes, M.J.; Kelly, J.M.; Wong, K.H. Carbon Catabolite Repression Governs Diverse Physiological Processes and Development in Aspergillus nidulans. mBio 2022, 13, e0373421. [Google Scholar] [CrossRef]
- Lockington, R.A.; Kelly, J.M. Carbon catabolite repression in Aspergillus nidulans involves deubiquitination. Mol. Microbiol. 2001, 40, 1311–1321. [Google Scholar] [CrossRef]
- Lockington, R.A.; Kelly, J.M. The WD40-repeat protein CreC interacts with and stabilizes the deubiquitinating enzyme CreB in vivo in Aspergillus nidulans. Mol. Microbiol. 2002, 43, 1173–1182. [Google Scholar] [CrossRef]
- Boase, N.A.; Kelly, J.M. A role for creD, a carbon catabolite repression gene from Aspergillus nidulans, in ubiquitination. Mol. Microbiol. 2004, 53, 929–940. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Ma, W.; Jiang, Y.; Hou, S.; Tan, Y.; Yuan, Q.; Niu, K.; Fang, X. Redesigning transcription factor Cre1 for alleviating carbon catabolite repression in Trichoderma reesei. Synth. Syst. Biotechnol. 2020, 5, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Cai, R.; Guo, J.; Zhong, Z.; Bao, J.; Wang, Z.; Chen, X.; Zhou, J.; Lu, G.-D. Carbon catabolite repressor MoCreA is required for the asexual development and pathogenicity of the rice blast fungus. Fungal Genet. Biol. 2020, 146, 103496. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.R.; Mark, K.M.K.; Thammahong, A.; Ries, L.N.A.; Dhingra, S.; Caffrey-Carr, A.K.; Cheng, C.; Black, C.C.; Bowyer, P.; Bromley, M.J.; et al. Filamentous fungal carbon catabolite repression supports metabolic plasticity and stress responses essential for disease progression. PLoS Pathog. 2017, 13, e1006340. [Google Scholar] [CrossRef] [PubMed]
- Matar, K.A.O.; Chen, X.; Chen, D.; Anjago, W.M.; Norvienyeku, J.; Lin, Y.; Chen, M.; Wang, Z.; Ebbole, D.J.; Lu, G.-D. WD40-repeat protein MoCreC is essential for carbon repression and is involved in conidiation, growth and pathogenicity of Magnaporthe oryzae. Curr. Genet. 2016, 63, 685–696. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Dong, J.; Yang, X.; Sui, A. Interactions between Verticillium dahliae and its host: Vegetative growth, pathogenicity, plant immunity. Appl. Microbiol. Biotechnol. 2014, 98, 6921–6932. [Google Scholar] [CrossRef]
- Timpner, C.; Braus-Stromeyer, S.A.; Tran, V.T.; Braus, G.H. The Cpc1 Regulator of the Cross-Pathway Control of Amino Acid Biosynthesis Is Required for Pathogenicity of the Vascular Pathogen Verticillium longisporum. Mol. Plant. Microbe Interact. 2013, 26, 1312–1324. [Google Scholar] [CrossRef]
- Singh, S.; Braus-Stromeyer, S.A.; Timpner, C.; Tran, V.T.; Lohaus, G.; Reusche, M.; Knüfer, J.; Teichmann, T.; von Tiedemann, A.; Braus, G.H. Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross-pathway control in the xylem. Appl. Microbiol. Biotechnol. 2010, 85, 1961–1976. [Google Scholar] [CrossRef]
- Yang, X.; Ben, S.; Sun, Y.; Fan, X.; Tian, C.; Wang, Y. Genome-Wide Identification, Phylogeny and Expression Profile of Vesicle Fusion Components in Verticillium dahliae. PLoS ONE 2013, 8, e68681. [Google Scholar] [CrossRef]
- Shroff, R.A.; Lockington, R.A.; Kelly, J.M. Analysis of mutations in the creA gene involved in carbon catabolite repression in Aspergillus nidulans. Can. J. Microbiol. 1996, 42, 950–959. [Google Scholar] [CrossRef]
- Ries, L.N.; Beattie, S.R.; Espeso, E.A.; Cramer, R.A.; Goldman, G.H. Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics 2016, 203, 335–352. [Google Scholar] [CrossRef] [PubMed]
- McCartney, R.R.; Chandrashekarappa, D.G.; Zhang, B.B.; Schmidt, M.C. Genetic Analysis of Resistance and Sensitivity to 2-Deoxyglucose in Saccharomyces cerevisiae. Genetics 2014, 198, 635–646. [Google Scholar] [CrossRef]
- Felenbok, B.; Flipphi, M.; Nikolaev, I. Ethanol catabolism in Aspergillus nidulans: A model system for studying gene regulation. Prog. Nucleic Acid Res. 2001, 69, 149–204. [Google Scholar] [CrossRef]
- Mathieu, M.; Felenbok, B. The Aspergillus nidulans CREA protein mediates glucose repression of the ethanol regulon at various levels through competition with the ALCR-specific transactivator. EMBO J. 1994, 13, 4022–4027. [Google Scholar] [CrossRef]
- Liao, D.; Chen, Y.; Qi, J.; Zhang, H.; Sun, P.; Chen, C.; Li, X. Temporal transcriptomics reveal the molecular mechanism of dormancy and germination regulated by temperature in Paris polyphylla seed. Hortic. Plant J. 2022, 9, 848–866. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Chen, Y.; Zhao, Y.; Ma, Z. Protein acetylation and deacetylation in plant-pathogen interactions. Environ. Microbiol. 2021, 23, 4841–4855. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Diego-Martin, B.; Kuo, P.H.; Jami-Alahmadi, Y.; Vashisht, A.A.; Wohlschlegel, J.; Jacobsen, S.E.; Blázquez, M.A.; Gallego-Bartolomé, J. Comprehensive identification of SWI/SNF complex subunits underpins deep eukaryotic ancestry and reveals new plant components. Commun. Biol. 2022, 5, 549. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, B.; Zhang, C.; Zhang, H.; Xing, F.; Liu, Y. Carbon Catabolite Repression Gene AoCreA Regulates Morphological Development and Ochratoxin A Biosynthesis Responding to Carbon Sources in Aspergillus ochraceus. Toxins 2020, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Khosravi, C.; Lubbers, R.J.M.; Kun, R.S.; Pontes, M.V.A.; Battaglia, E.; Chen, C.; Dalhuijsen, S.; Daly, P.; Lipzen, A.; et al. CreA-mediated repression of gene expression occurs at low monosaccharide levels during fungal plant biomass conversion in a time and substrate dependent manner. Cell Surf. 2021, 7, 100050. [Google Scholar] [CrossRef]
- Jeong, B.; Shah, M.A.; Roh, E.; Kim, K.; Park, I.; Bae, T. Staphylococcus aureus Does Not Synthesize Arginine from Proline under Physiological Conditions. J. Bacteriol. 2022, 204, e0001822. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Sun, T.; Zhu, Q.-H.; Feng, H.; Li, Y.; Liu, F.; Zhang, X.; Sun, J.; Li, Y. VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen. Int. J. Mol. Sci. 2023, 25, 294. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhou, B.-J.; Li, G.-Y.; Jia, P.-S.; Li, H.; Zhao, Y.-L.; Zhao, P.; Xia, G.-X.; Guo, H.-S. A Glutamic Acid-Rich Protein Identified in Verticillium dahliae from an Insertional Mutagenesis Affects Microsclerotial Formation and Pathogenicity. PLoS ONE 2010, 5, e15319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, H.; Hua, C.; Guo, H.S.; Zhang, J. An improved single-step cloning strategy simplifies the Agrobacterium tumefaciens-mediated transformation (ATMT)-based gene-disruption method for Verticillium dahliae. Phytopathology 2016, 106, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, B.; Hua, C.; Meng, P.; Wang, S.; Chen, Z.; Du, Y.; Gao, F.; Huang, J. VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae. Sci. China Life Sci. 2017, 60, 868–879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).