Localization of Hemostasis Elements in Aspirated Coronary Thrombi at Different Stages of Evolution
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of the Study Population
2.2. Investigation of Routine Clinical, Laboratory, and Angiographic Indices
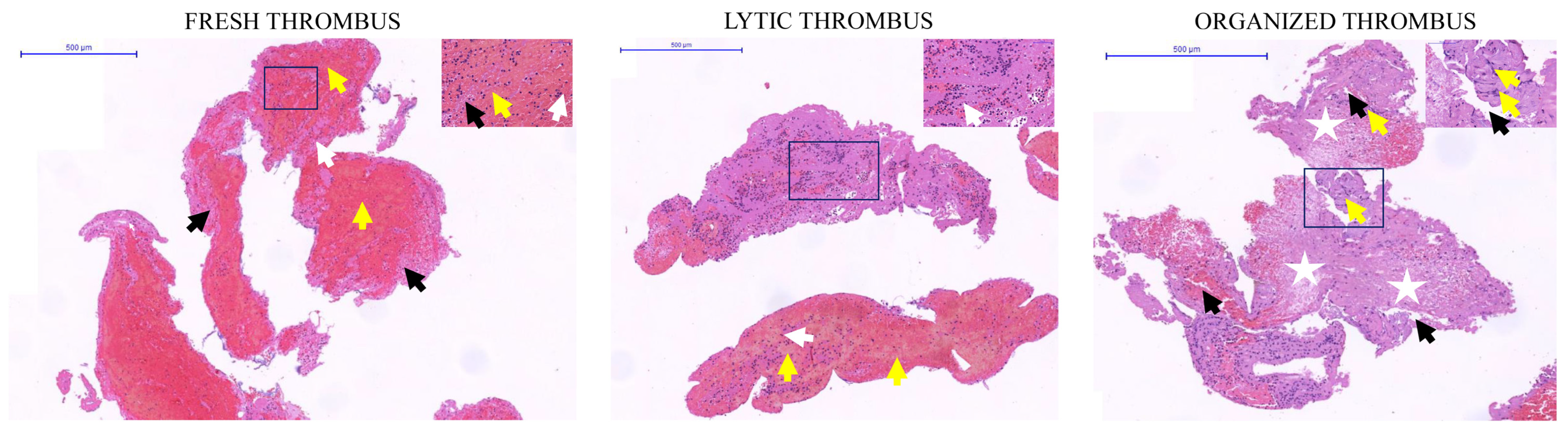
2.3. Determination of the Thrombus Age by Classical Histopathology
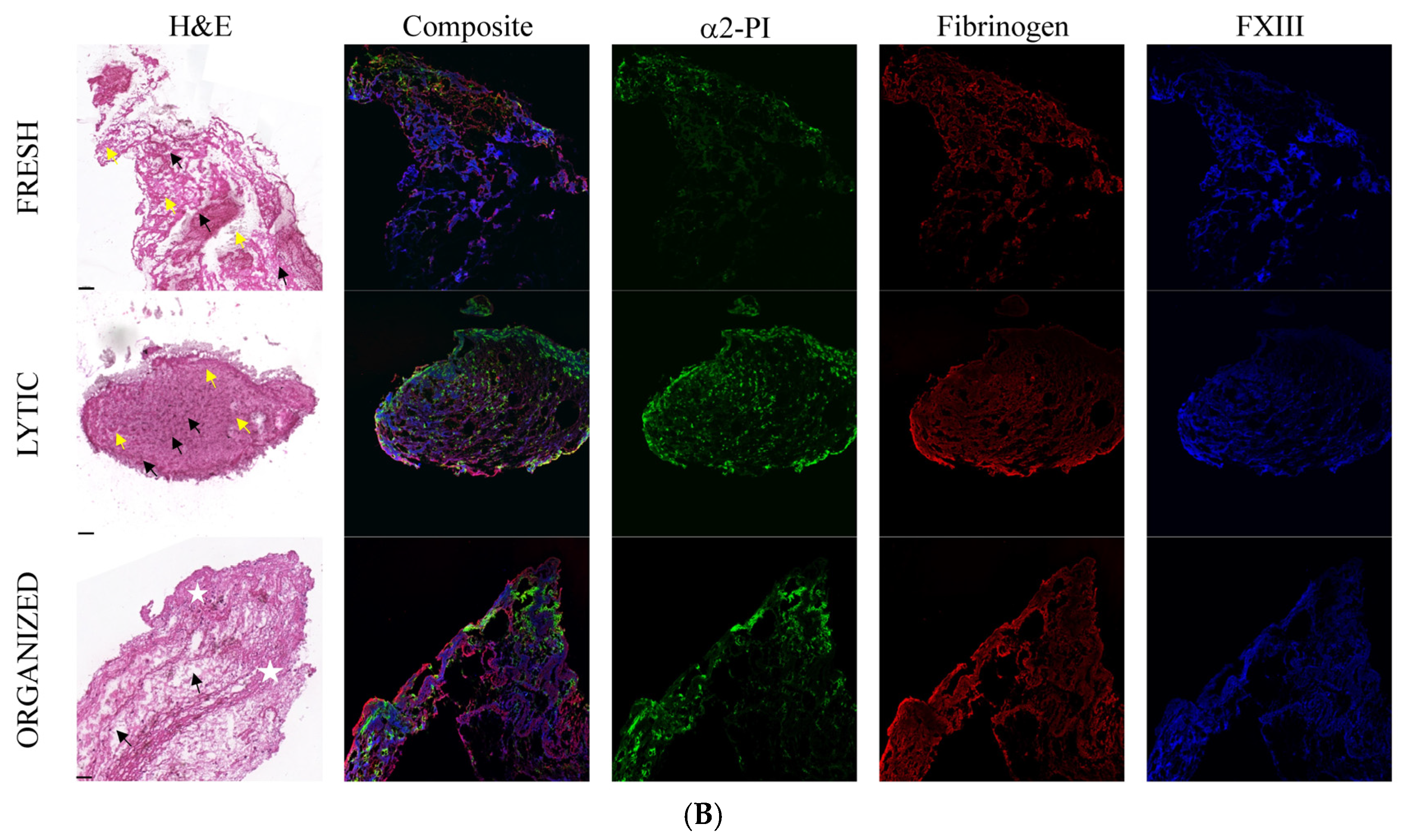
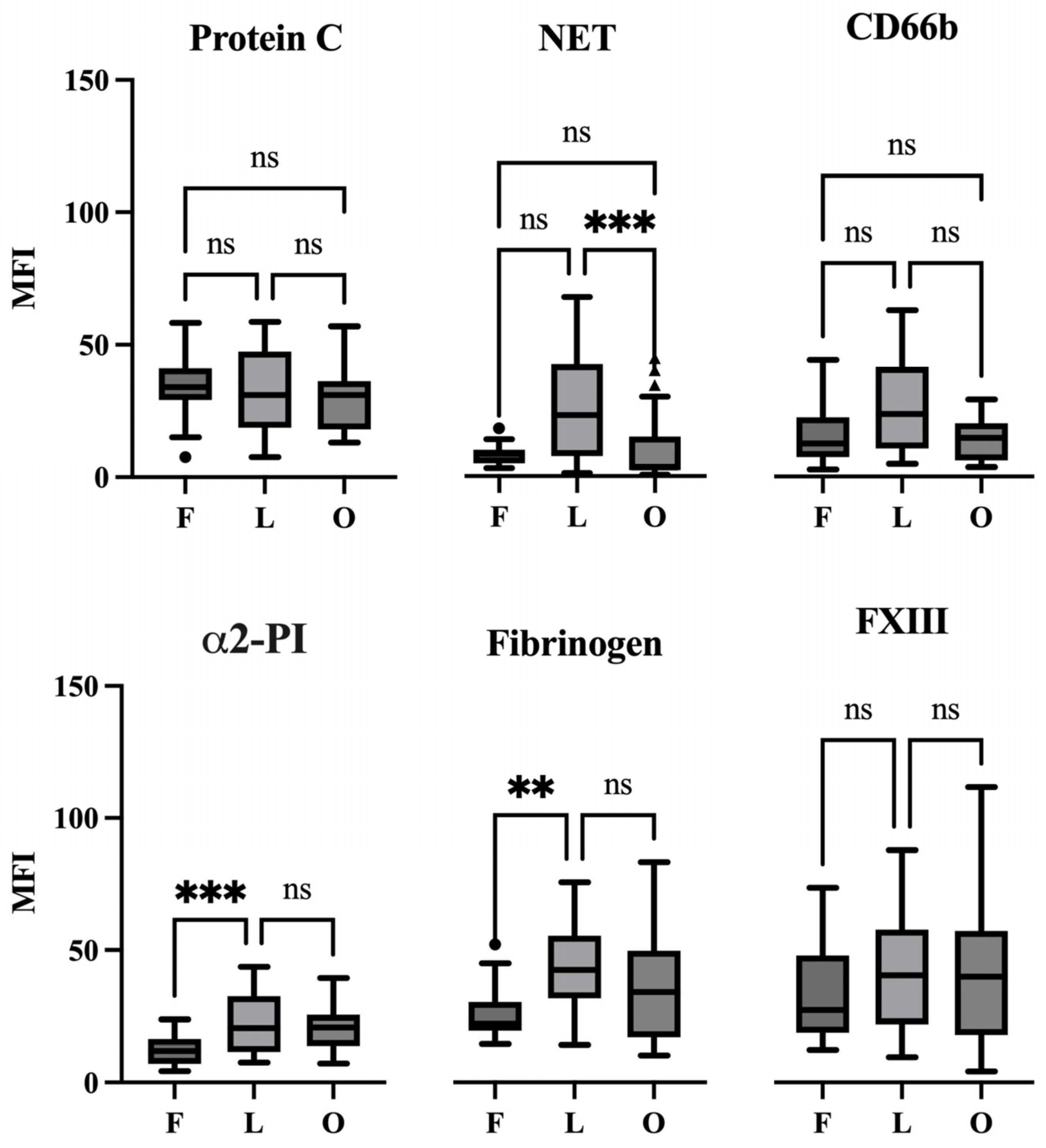
2.4. The Appearance of Hemostasis Proteins and Markers of NETs in Coronary Thrombi at Different Stages of Evolution
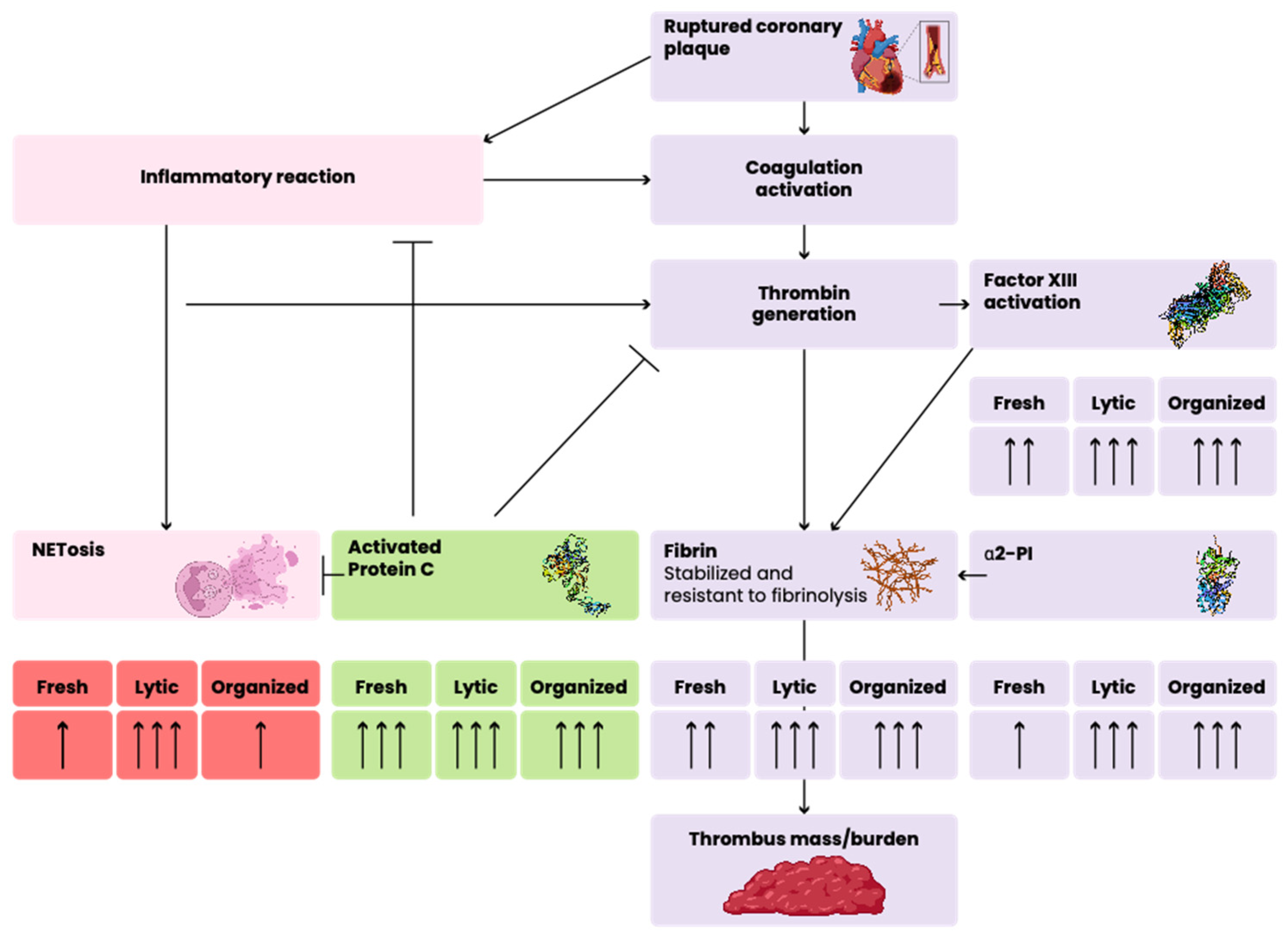
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlaar, P.J.; Svilaas, T.; van der Horst, I.C.; Diercks, G.F.; Fokkema, M.L.; de Smet, B.J.; Heuvel, A.F.v.D.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.-S.; et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): A 1-year follow-up study. Lancet 2008, 371, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Rokoss, M.J.; Gao, P.; Meeks, B.; Kedev, S.; Stankovic, G.; Moreno, R.; Gershlick, A.; et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016, 387, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Satti, Z.; Omari, M.; Bawamia, B.; Cartlidge, T.; Egred, M.; Farag, M.; Alkhalil, M. The Use of Thrombectomy during Primary Percutaneous Coronary Intervention: Resurrecting an Old Concept in Contemporary Practice. J. Clin. Med. 2024, 13, 2291. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Collet, J.-P.; Nagaswami, C.; Beygui, F.; Edmondson, K.E.; Bellemain-Appaix, A.; Cayla, G.; Pena, A.; Brugier, D.; Barthelemy, O.; et al. Composition of coronary thrombus in acute myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 1359–1367. [Google Scholar] [CrossRef]
- Cambruzzi, E.; Sebben, J.C.; David, R.B.; de Mattos, E.I.; de Melo Bernardi, G.L.; Ioppi, J.; Pêgas, K.L.; Feijó, I.P.; Gottschall, C.A.M.; de Quadros, A.S. Histopathological Evaluation of Coronary Thrombi in Patients with ST-Segment Elevation Myocardial Infarction. Rev. Bras. Cardiol. Invasiva Engl. Ed. 2012, 20, 267–273. [Google Scholar] [CrossRef]
- Sebben, J.C.; Cambruzzi, E.; Avena, L.M.; Gazeta, C.D.A.; Gottschall, C.A.M.; de Quadros, A.S. Clinical significance of histological features of thrombi in patients with myocardial infarction. Arq. Bras. Cardiol. 2013, 101, 502–510. [Google Scholar] [CrossRef]
- Ramaiola, I.; Padró, T.; Peña, E.; Juan-Babot, O.; Cubedo, J.; Martin-Yuste, V.; Sabate, M.; Badimon, L. Changes in thrombus composition and profilin-1 release in acute myocardial infarction. Eur. Heart J. 2015, 36, 965–975. [Google Scholar] [CrossRef]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komáromi, I.; Katona, É. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef]
- Sakata, Y.; Aoki, N. Significance of cross-linking of alpha 2-plasmin inhibitor to fibrin in inhibition of fibrinolysis and in hemostasis. J. Clin. Investig. 1982, 69, 536–542. [Google Scholar] [CrossRef]
- Savchenko, A.S.; Martinod, K.; Seidman, M.A.; Wong, S.L.; Borissoff, J.I.; Piazza, G.; Libby, P.; Goldhaber, S.Z.; Mitchell, R.N.; Wagner, D.D.; et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 2014, 12, 860–870. [Google Scholar] [CrossRef] [PubMed]
- De Boer, O.J.; Li, X.; Teeling, P.; Mackaay, C.; Ploegmakers, H.J.; van der Loos, C.M.; Daemen, M.J.; de Winter, R.J.; van der Wal, A.C. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 2013, 109, 290–297. [Google Scholar] [PubMed]
- Farkas, Z.; Farkas, V.J.; Gubucz, I.; Szabó, L.; Bálint, K.; Tenekedjiev, K.; Nagy, A.I.; Sótonyi, P.; Hidi, L.; Nagy, Z.; et al. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb. Res. 2019, 175, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Healy, L.D.; Puy, C.; Itakura, A.; Chu, T.; Robinson, D.K.; Bylund, A.; Phillips, K.G.; Gardiner, E.E.; McCarty, O.J. Colocalization of neutrophils, extracellular DNA and coagulation factors during NETosis: Development and utility of an immunofluorescence-based microscopy platform. J. Immunol. Methods 2016, 435, 77–84. [Google Scholar] [CrossRef]
- Bereczky, Z.; Kovács, K.B.; Muszbek, L. Protein C and protein S deficiencies: Similarities and differences between two brothers playing in the same game. Clin. Chem. Lab. Med. 2010, 48 (Suppl. S1), S53–S66. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus Structural Composition in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Rakocevic, J.; Kojic, S.; Orlic, D.; Stankovic, G.; Ostojic, M.; Petrovic, O.; Zaletel, I.; Puskas, N.; Todorovic, V.; Labudovic-Borovic, M. Co-expression of vascular and lymphatic endothelial cell markers on early endothelial cells present in aspirated coronary thrombi from patients with ST-elevation myocardial infarction. Exp. Mol. Pathol. 2016, 100, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Suzuki, K.; Watanabe, Y.; Watanabe, K.; Fujioka, D.; Nakamura, T.; Obata, J.-E.; Kawabata, K.; Mishina, H.; Kugiyama, K. Phospholipase A2 expression in coronary thrombus is increased in patients with recurrent cardiac events after acute myocardial infarction. Int. J. Cardiol. 2013, 168, 4214–4221. [Google Scholar] [CrossRef] [PubMed]
- Sambola, A.; Ruiz-Meana, M.; Barba, I.; Del Blanco, B.G.; Barrabés, J.A.; Lip, G.; Vilardosa, U.; Sansaloni, S.; Rello, P.; García-Dorado, D. Glycative and oxidative stress are associated with altered thrombus composition in diabetic patients with ST-elevation myocardial infarction. Int. J. Cardiol. 2017, 243, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rittersma, S.Z.; van der Wal, A.C.; Koch, K.T.; Piek, J.J.; Henriques, J.P.; Mulder, K.J.; Ploegmakers, J.P.; Meesterman, M.; de Winter, R.J. Plaque Instability Frequently Occurs Days or Weeks Before Occlusive Coronary Thrombosis. Circulation 2005, 111, 1160–1165. [Google Scholar] [CrossRef]
- Kramer, M.C.; van der Wal, A.C.; Koch, K.T.; Ploegmakers, J.P.; van der Schaaf, R.J.; Henriques, J.P.; Baan, J.J.; Rittersma, S.Z.; Vis, M.M.; Piek, J.J.; et al. Presence of Older Thrombus Is an Independent Predictor of Long-Term Mortality in Patients with ST-Elevation Myocardial Infarction Treated with Thrombus Aspiration During Primary Percutaneous Coronary Intervention. Circulation 2008, 118, 1810–1816. [Google Scholar] [CrossRef]
- Li, X.; de Boer, O.J.; Ploegmaker, H.; Teeling, P.; Daemen, M.J.; de Winter, R.J.; van der Wal, A.C. Granulocytes in coronary thrombus evolution after myocardial infarction—Time-dependent changes in expression of matrix metalloproteinases. Cardiovasc. Pathol. 2015, 25, 40–46. [Google Scholar] [CrossRef]
- Carol, A.; Bernet, M.; Curós, A.; Rodríguez-Leor, O.; Serra, J.; Fernández-Nofrerías, E.; Mauri, J.; Bayes-Genís, A. Thrombus age, clinical presentation, and reperfusion grade in myocardial infarction. Cardiovasc. Pathol. 2014, 23, 126–130. [Google Scholar] [CrossRef]
- Sharma, V.; Jolly, S.S.; Hamid, T.; Sharma, D.; Chiha, J.; Chan, W.; Fuchs, F.; Bui, S.; Gao, P.; Kassam, S.; et al. Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: Insights from the TOTAL trial. Eur. Heart J. 2016, 37, 1891–1898. [Google Scholar] [CrossRef]
- Limbruno, U.; De Carlo, M.; Pistolesi, S.; Micheli, A.; Petronio, A.S.; Camacci, T.; Fontanini, G.; Balbarini, A.; Mariani, M.; De Caterina, R. Distal embolization during primary angioplasty: Histopathologic features and predictability. Am. Heart J. 2005, 150, 102–108. [Google Scholar] [CrossRef]
- Chernysh, I.N.; Nagaswami, C.; Kosolapova, S.; Peshkova, A.D.; Cuker, A.; Cines, D.B.; Cambor, C.L.; Litvinov, R.I.; Weisel, J.W. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci. Rep. 2020, 10, 5112. [Google Scholar] [CrossRef]
- Riegger, J.; Byrne, R.A.; Joner, M.; Chandraratne, S.; Gershlick, A.H.; Berg, J.M.T.; Adriaenssens, T.; Guagliumi, G.; Godschalk, T.C.; Neumann, F.-J.; et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: A report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur. Heart J. 2016, 37, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, J.; Bogaert, J.; Sadowski, M.; Woznicka, O.; Doulaptsis, K.; Ntoumpanaki, M.; Ząbczyk, M.; Nessler, J.; Undas, A. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost 2015, 113, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Ząbczyk, M.; Undas, A. Coronary thrombus composition: Links with inflammation, platelet and endothelial markers. Atherosclerosis 2014, 237, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Lee, C.S.; Tae, W.C.; Jackson, K.W.; Christiansen, V.J.; McKee, P.A. Crosslinking of alpha 2-antiplasmin to fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, A.; Aoki, N. Reversible cross-linking of α2-plasmin inhibitor to fibrinogen by fibrin-stabilizing factor. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzym. 1982, 706, 158–164. [Google Scholar] [CrossRef]
- Healy, L.D.; Rigg, R.A.; Griffin, J.H.; McCarty, O.J.T. Regulation of immune cell signaling by activated protein C. J. Leukoc. Biol. 2018, 103, 1197–1203. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C: Biased for translation. Blood 2015, 125, 2898–2907. [Google Scholar] [CrossRef]
- Shahzad, K.; Kohli, S.; Al-Dabet, M.M.; Isermann, B. Cell biology of activated protein C. Curr. Opin. Hematol. 2019, 26, 41–50. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood 2018, 132, 159–169. [Google Scholar] [CrossRef]
- Wildhagen, K.C.A.A.; Lutgens, E.; Loubele, S.T.G.B.; Cate, H.T.; Nicolaes, G.A.F. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb. Haemost. 2011, 106, 1034–1045. [Google Scholar] [CrossRef]
- Healy, L.D.; Puy, C.; Fernández, J.A.; Mitrugno, A.; Keshari, R.S.; Taku, N.A.; Chu, T.T.; Xu, X.; Gruber, A.; Lupu, F.; et al. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J. Biol. Chem. 2017, 292, 8616–8629. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; van der Wal, A.C.; Pabittei, D.R.; Mackaaij, C.; van Leeuwen, M.B.; Li, X.; de Boer, O.J. Neutrophil Extracellular Traps Participate in All Different Types of Thrombotic and Haemorrhagic Complications of Coronary Atherosclerosis. Thromb. Haemost. 2018, 118, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.M.; Jakowitsch, J.; Panzenböck, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Neutrophils and Neutrophil Extracellular Traps in Cardiovascular Disease: An Overview and Potential Therapeutic Approaches. Biomedicines 2022, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, R.; Liu, C.; Zhou, P.; Li, J.; Wang, Y.; Zhao, X.; Zhao, H.; Song, L.; Yan, H. Associations of NETs with inflammatory risk and atherosclerotic severity in ST-segment elevation myocardial infarction. Thromb. Res. 2021, 203, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Fender, A.C.; Dobrev, D. Coronary clot composition after myocardial infarction: Thrombus age matters. Int. J. Cardiol. Heart Vasc. 2020, 26, 100450. [Google Scholar] [CrossRef]
- Pertiwi, K.R.; de Boer, O.J.; Gabriels, P.A.; van der Wal, A.C. Etosis, rather than apoptosis or cell proliferation, typifies thrombus progression—An immunohistochemical study of coronary aspirates. IJC Heart Vasc. 2020, 26, 100439. [Google Scholar] [CrossRef]
- Mozzini, C.; Garbin, U.; Pasini, A.M.F.; Cominacini, L. An exploratory look at NETosis in atherosclerosis. Intern. Emerg. Med. 2017, 12, 13–22. [Google Scholar] [CrossRef]
- Yunoki, K.; Naruko, T.; Inoue, T.; Sugioka, K.; Inaba, M.; Iwasa, Y.; Komatsu, R.; Itoh, A.; Haze, K.; Yoshiyama, M.; et al. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc. Interv. 2013, 6, 377–385. [Google Scholar] [CrossRef]
- D’Alessandro, E.; Becker, C.; Bergmeier, W.; Bode, C.; Bourne, J.H.; Brown, H.; Buller, H.R.; Cate-Hoek, A.J.T.; Cate, V.T.; van Cauteren, Y.J.M.; et al. Thrombo-Inflammation in Cardiovascular Disease: An Expert Consensus Document from the Third Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2020, 120, 538–564. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; Shahzad, K.; Jouppila, A.; Holthöfer, H.; Isermann, B.; Lassila, R. Thrombosis and Inflammation—A Dynamic Interplay and the Role of Glycosaminoglycans and Activated Protein C. Front. Cardiovasc. Med. 2022, 9, 866751. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]

| Acute (<12 h), n = 69 | Subacute (12–24 h), n = 28 | Late (>24 h), n = 28 | p Acute vs. Subacute | p Acute vs. Late | p Subacute vs. Late | |
|---|---|---|---|---|---|---|
| Age (years), mean, SD | 60.9 (11.4) | 56.8 (10.3) | 57.5 (12.1) | 0.107 | 0.186 | 0.805 |
| Gender (male/female), n | 48/21 | 21/7 | 18/10 | 0.592 | 0.613 | 0.383 |
| CK (U/L), median, IQR | 180 (191) | 1178 (682) | 539.5 (1050) | <0.001 | <0.001 | 0.069 |
| One-year survival (frequency), % | 87.9 | 85.7 | 81.5 | 0.754 | 0.403 | 0.671 |
| Ejection fraction, median, IQR | 47 (10) | 42 (14) | 48 (16) | 0.120 | 0.767 | 0.205 |
| TIMI flow pre-procedure (0–3 grade), n | 47/10/7/5 | 20/3/3/2 | 22/3/2/1 | 0.969 | 0.766 | 0.890 |
| TIMI flow post-procedure (0–3 grade), n | 0/0/7/62 | 0/1/6/21 | 0/2/5/21 | 0.088 | 0.041 | 0.809 |
| MBG (0–3 grade), n | 3/12/17/37 | 4/5/11/8 | 5/5/5/13 | 0.075 | 0.171 | 0.314 |
| Distal embolization yes/no, n | 19/50 | 6/22 | 11/17 | 0.533 | 0.257 | 0.146 |
| Thrombus mass (mg) median, IQR | 17.1 (16.2) | 18.7 (35.8) | 35.0 (32.9) | 0.136 | <0.001 | 0.195 |
| WBC (G/L) median, IQR | 12.69 (6.16) | 14.71 (4.80) | 12.89 (4.56) | 0.206 | 0.561 | 0.350 |
| Neutrophil cell count (G/L) median, IQR | 9.96 (6.04) | 11.27 (5.62) | 10.11 (4.48) | 0.193 | 0.735 | 0.350 |
| Monocyte cell count (G/L) median, IQR | 0.63 (0.33) | 0.81 (0.55) | 1.06 (0.76) | 0.010 | <0.001 | 0.189 |
| Eosinophil cell count (G/L) median, IQR | 0.09 (0.17) | 0.03 (0.21) | 0.04 (0.07) | 0.141 | 0.014 | 0.774 |
| Platelet count (G/L) median, IQR | 242 (96) | 243 (82) | 235 (76) | 0.524 | 0.564 | 0.946 |
| eGFR (mL/min/1.73 m2) median, IQR | 84.5 (22) | 90 (30) | 88.5 (17) | 0.146 | 0.626 | 0.323 |
| CRP (mg/L) median, IQR | 3.18 (5.46) | 7.41 (27.41) | 40.60 (106.1) | 0.021 | <0.001 | 0.003 |
| Coronary occlusion site, IRA (LAD/CX/RCA), n | 29/26/14 | 11/10/7 | 17/10/1 | 0.878 | 0.079 | 0.055 |
| Smoking (frequency), % | 66 | 80 | 77 | 0.216 | 0.339 | 0.789 |
| Hypertension (frequency), % | 65 | 71 | 75 | 0.555 | 0.349 | 0.763 |
| Diabetes mellitus (frequency), % | 33 | 32 | 25 | 0.814 | 0.631 | 0.554 |
| Hyperlipidemia (frequency), % | 48 | 43 | 41 | 0.617 | 0.497 | 0.874 |
| BMI (kg/m2) median, IQR | 28 (7) | 30 (10) | 29 (7) | 0.396 | 0.305 | 0.791 |
| Survivals, n = 104 | Non-Survivals, n = 17 | p | |
|---|---|---|---|
| Age (years) mean, SD | 58.1 (11.1) | 64.3 (11.4) | 0.034 |
| Gender (male/female), n | 75/29 | 9/8 | 0.112 |
| Smoking (frequency), % | 73 | 50 | 0.091 |
| Hypertension (frequency), % | 69.2 | 70.6 | 0.910 |
| Diabetes mellitus (frequency), % | 26.9 | 58.8 | 0.024 |
| Hyperlipidemia (frequency), % | 48.0 | 33.3 | 0.286 |
| BMI (kg/m2) median, IQR | 29 (7) | 27 (9) | 0.624 |
| Time of occlusion (acute–subacute–late), n | 58/24/22 | 8/4/5 | 0.722 |
| CK (U/L) median, IQR | 264.5 (805) | 823 (2596) | 0.010 |
| Ejection fraction, median, IQR | 48 (10) | 35 (18) | <0.001 |
| TIMI flow pre-procedure (0–3 grade), n | 72/14/11/7 | 14/2/1/0 | 0.603 |
| TIMI flow post-procedure (0–3 grade), n | 0/1/12/91 | 0/2/6/9 | <0.001 |
| MBG (0–3 grade), n | 4/18/29/53 | 8/3/2/4 | <0.001 |
| Coronary occlusion site, IRA (LAD/CX/RCA), n | 28/8/68 | 7/1/9 | 0.485 |
| WBC (G/L) median, IQR | 12.89 (5.40) | 16.95 (9.09) | 0.001 |
| Neutrophil cell count (G/L) median, IQR | 9.73 (5.47) | 13.73 (7.24) | <0.001 |
| Monocyte cell count (G/L) median, IQR | 0.68 (0.54) | 0.88 (0.74) | 0.199 |
| Eosinophil cell count (G/L) median, IQR | 0.05 (0.16) | 0.015 (0.14) | 0.097 |
| Platelet count (G/L) median, IQR | 237 (89) | 261.5 (84) | 0.050 |
| eGFR (mL/min/1.73 m2) median, IQR | 87.5 (14) | 67.5 (39) | 0.007 |
| CRP (mg/L) median, IQR | 4.99 (11.66) | 11.12 (35.34) | 0.585 |
| No. of affected coronary arteries (1–3 vessel disease), n | 50/36/18 | 7/6/4 | 0.794 |
| Distal embolization frequency (% of pts) Stent length (mm) median, IQR Stent diameter (mm) median, IQR Length of LAD (1–3 categories), n Proximal/mid/distal thrombus localization, n | 25.0 28.0 (26.0) 3.00 (0.5) 5/51/48 42/47/15 | 47.1 28.0 (26.0) 3.5 (0.5) 3/7/7 9/6/2 | 0.061 0.649 0.634 0.141 0.623 |
| Thrombus mass (mg) Thrombus mass below and above 20 mg, n | 18.2 (16.1) 58/46 | 41.7 (30.8) 3/14 | 0.002 0.004 |
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| Thrombus mass | 6.890 | 1.210–39.234 | 0.030 |
| Diabetes mellitus | 11.685 | 2.027–67.374 | 0.006 |
| Ejection fraction | 0.858 | 0.782–0.941 | 0.001 |
| Neutrophil cell count | 1.289 | 1.065–1.560 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pituk, D.; Balogh, L.; Horváth, E.; Hegyi, Z.; Baráth, B.; Bogáti, R.; Szűcs, P.; Papp, Z.; Katona, É.; Bereczky, Z. Localization of Hemostasis Elements in Aspirated Coronary Thrombi at Different Stages of Evolution. Int. J. Mol. Sci. 2024, 25, 11746. https://doi.org/10.3390/ijms252111746
Pituk D, Balogh L, Horváth E, Hegyi Z, Baráth B, Bogáti R, Szűcs P, Papp Z, Katona É, Bereczky Z. Localization of Hemostasis Elements in Aspirated Coronary Thrombi at Different Stages of Evolution. International Journal of Molecular Sciences. 2024; 25(21):11746. https://doi.org/10.3390/ijms252111746
Chicago/Turabian StylePituk, Dóra, László Balogh, Emőke Horváth, Zoltán Hegyi, Barbara Baráth, Réka Bogáti, Péter Szűcs, Zoltán Papp, Éva Katona, and Zsuzsanna Bereczky. 2024. "Localization of Hemostasis Elements in Aspirated Coronary Thrombi at Different Stages of Evolution" International Journal of Molecular Sciences 25, no. 21: 11746. https://doi.org/10.3390/ijms252111746
APA StylePituk, D., Balogh, L., Horváth, E., Hegyi, Z., Baráth, B., Bogáti, R., Szűcs, P., Papp, Z., Katona, É., & Bereczky, Z. (2024). Localization of Hemostasis Elements in Aspirated Coronary Thrombi at Different Stages of Evolution. International Journal of Molecular Sciences, 25(21), 11746. https://doi.org/10.3390/ijms252111746











