In Vitro Modeling of Idiopathic Pulmonary Fibrosis: Lung-on-a-Chip Systems and Other 3D Cultures
Abstract
1. Introduction
2. 3D Cell Cultures: Modeling Pulmonary Fibrosis
2.1. 3D Hydrogels
2.2. Precision Cut Lung Slices
| 3D Model | Cellular Composition | Applicability/Main Finding | Reference |
|---|---|---|---|
| Hydrogels | Human fibroblast (CCD-19lu) and primary fibroblast | FAK/Akt signaling promoting increased collagen deposition. | [25] |
| Human lung fibroblast | Increased fibroblast activation and migration through matrix stiffening. | [27] | |
| Murine bleomycin treated lung fibroblast | PGE2 modulation of COX-2 suppression, fibroblast activation, and matrix stiffening. | [28] | |
| IPF and healthy human lung fibroblast | Matrix stiffening effects on proliferation, contraction, and resistance to PGE2. | [29] | |
| Primary human lung fibroblast | Pro-fibrotic stimuli hinder fibroblast apoptosis, altering Fas expression. | [30] | |
| Precision Cut Lung Slices | Murine bleomycin lung slices | Protein biomarker utilization in drug screening. | [38] |
| Healthy and IPF human lung slices | Modeling of early fibrosis. | [39] | |
| Human IPF lung tissue | Predictive markers of therapeutic response. | [40] | |
| Human/Murine bleomycin treated lung tissue | Differing response to Pirfenidone or Nintedanib by murine and human cultures. | [41] | |
| Lung Organoids | Human pluripotent stem cells (hPSCs) | Modeling pulmonary fibrosis; antifibrotic assessment of potential therapeutic (MGF-E8) | [44] |
| Murine mesenchymal and club cells | Mesenchymal support of bronchial organoid formation. | [45] | |
| Murine mesenchymal cells, macrophages, and bronchoalveolar stem cells | Branched bronchoalveolar organoid formation and modeling lung development. | [46] | |
| hPSC derived alveolar epithelial cells and primary human lung fibroblasts | Modeling pulmonary fibrosis: ALK5 and integrin aVb6 as therapeutic targets. | [47] | |
| Human alveolar basal cells | Bleomycin inducing honeycomb cyst formation | [48] |
2.3. Lung Organoids
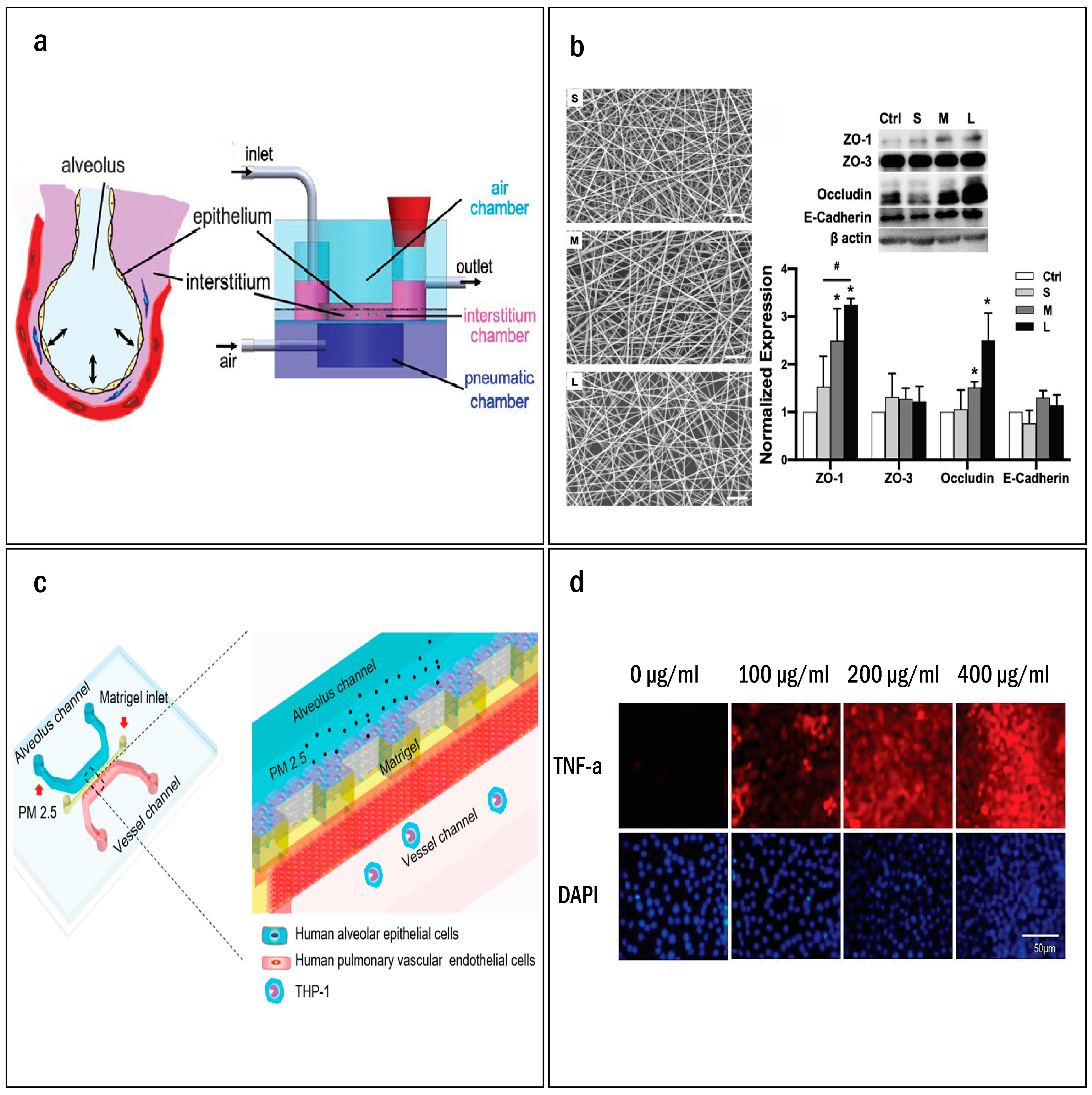
3. Lung-on-a-Chip Systems
| Modeling Approach | Cellular Composition | Key Outcome | References |
|---|---|---|---|
| Normal Physiologic State | Human alveolar epithelial and pulmonary endothelial cells | Inflammatory, immune, and stress reactions to pollutants. | [64] |
| Airway epithelial cells, lung fibroblast, and endothelial cells | Multi-layered, co-cultured replicating cellular composition of lung. | [65] | |
| Type I and II alveolar epithelial cells, endothelial cells | Medium-throughput physiologic three-dimensional stretching system | [66] | |
| Alveolar epithelial and endothelial cells | collagen-elastin membrane replicating geometric and biophysical characteristics of ECM | [62] | |
| Alveolar epithelial cells and fibroblasts | Enhanced nano spun pseudo-interstitium, improving epithelial barrier function and longevity. | [67] | |
| Alveolar Injury | Human type II A549 | Exposure to gastric contents induced cellular injury. | [68] |
| Human pulmonary alveolar epithelial and umbilical vascular endothelial cells | Nanoparticles cause dose-dependent toxicity to lung cells | [69] | |
| Human alveolar epithelial cells, pulmonary vascular endothelial cells, and human acute leukemia monocytic cell lines (THP-1) | Air pollutants disrupt alveolar-capillary interface, induce inflammation, and trigger immune cell recruitment. | [70] | |
| Immortalized human alveolar epithelial cell lines (iAECs), primary human lung microvascular endothelial cells, and peripheral blood mononuclear cells (PBMCs). | Bacterial endotoxin exposure causing alveolar barrier disruption and inflammation. | [71] | |
| Human primary alveolar epithelial cells and human lung microvascular endothelial cells (HMVEC-L) | Radiation induced lung injury. Therapeutic response to lovastatin and prednisolone. | [72] | |
| Induction of Fibrosis | Pulmonary Fibroblast | Simulate fibrotic events, predicts antifibrotic effects of pirfenidone and nintedanib | [73] |
| Primary human lung microvascular endothelial cells iAECs, and PBMCs | TGFβ1 induced epithelial to mesenchymal transition. | [71] | |
| Human-induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) and primary human lung fibroblast. | Stromal-endothelial interactions modulate changes in vessel density, expression levels, and tissue stiffness. | [74] | |
| Therapeutic Testing and Drug Delivery | Primary human lung fibroblasts | Antifibrotic drug efficacy. | [75] |
| Primary cell-derived immortalized alveolar epithelial cells (AXiAECs), macrophages (THP-1), and endothelial (HLMVEC) cells | Aerosol delivery system and inhaled steroid efficacy. | [76] |

3.1. Physiologic Memetic Models
3.1.1. Essential Cell Types and Physiologic Stretch
3.1.2. Modeling the Interstitial Space
3.2. Modeling Alveolar Injury
3.2.1. Endogenous Cellular Injury
3.2.2. Exogenous Pollutants Causing Cellular Injury
3.2.3. Infectious Agents
3.2.4. Radiation Effects
3.2.5. Inducing a Fibrotic State
3.3. Therapeutic Testing and Drug Delivery
4. 3D Model Applications Beyond Pulmonary Fibrosis: Insights into Lung Injuries
5. Outlooks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Swigris, J.J.; Lezotte, D.C.; Norris, J.M.; Wilson, C.G.; Brown, K.K. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 2007, 176, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71, 112–127. [Google Scholar] [CrossRef]
- Cameli, P.; Carleo, A.; Bergantini, L.; Landi, C.; Prasse, A.; Bargagli, E. Oxidant/Antioxidant Disequilibrium in Idiopathic Pulmonary Fibrosis Pathogenesis. Inflammation 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Zhu, K.; Xu, A.; Xia, W.; Li, P.; Han, R.; Wang, E.; Zhou, S.; Wang, R. Integrated analysis of the molecular mechanisms in idiopathic pulmonary fibrosis. Int. J. Med. Sci. 2021, 18, 3412–3424. [Google Scholar] [CrossRef]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- The Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu, G.; Anstrom, K.J.; King, T.E., Jr.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- King, C.S.; Nathan, S.D. Idiopathic pulmonary fibrosis: Effects and optimal management of comorbidities. Lancet Respir. Med. 2017, 5, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ Clin. Res. Ed. 2007, 334, 197. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Vazquez-Armendariz, A.I.; Barroso, M.M.; El Agha, E.; Herold, S. 3D In Vitro Models: Novel Insights into Idiopathic Pulmonary Fibrosis Pathophysiology and Drug Screening. Cells 2022, 11, 1526. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Chen, Y.; Black, L.D.; Aldridge, B.B.; Kaplan, D.L. Engineered cell and tissue models of pulmonary fibrosis. Adv. Drug Deliv. Rev. 2018, 129, 78–94. [Google Scholar] [CrossRef]
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431. [Google Scholar] [CrossRef]
- Asano, S.; Ito, S.; Takahashi, K.; Furuya, K.; Kondo, M.; Sokabe, M.; Hasegawa, Y. Matrix stiffness regulates migration of human lung fibroblasts. Physiol. Rep. 2017, 5, e13281. [Google Scholar] [CrossRef]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef]
- Marinković, A.; Liu, F.; Tschumperlin, D.J. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am. J. Respir. Cell Mol. Biol. 2013, 48, 422–430. [Google Scholar] [CrossRef]
- Dodi, A.E.; Ajayi, I.O.; Chang, C.; Beard, M.; Ashley, S.L.; Huang, S.K.; Thannickal, V.J.; Tschumperlin, D.J.; Sisson, T.H.; Horowitz, J.C. Regulation of fibroblast Fas expression by soluble and mechanical pro-fibrotic stimuli. Respir. Res. 2018, 19, 91. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Linthicum, W.; Man, K.; He, X.; Wen, Q.; Rojanasakul, L.W.; Rojanasakul, Y.; Yang, Y. Substrate Stiffness-Dependent Carbon Nanotube-Induced Lung Fibrogenesis. Nano Lett. 2019, 19, 5443–5451. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Uhl, F.E.; Pineda, R.H.; Bailey, K.E.; Rojas, M.; Wagner, D.E.; Königshoff, M. Applications and Approaches for Three-Dimensional Precision-Cut Lung Slices. Disease Modeling and Drug Discovery. Am. J. Respir. Crit. Care Med. 2020, 62, 681–691. [Google Scholar] [CrossRef]
- Liu, G.; Betts, C.; Cunoosamy, D.M.; Åberg, P.M.; Hornberg, J.J.; Sivars, K.B.; Cohen, T.S. Use of precision cut lung slices as a translational model for the study of lung biology. Respir. Res. 2019, 20, 162. [Google Scholar] [CrossRef]
- Hansen, N.U.B.; Karsdal, M.A.; Brockbank, S.; Cruwys, S.; Rønnow, S.; Leeming, D.J. Tissue turnover of collagen type I, III and elastin is elevated in the PCLS model of IPF and can be restored back to vehicle levels using a phosphodiesterase inhibitor. Respir. Res. 2016, 17, 76. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Staab-Weijnitz, C.A.; Lehmann, M.; Lindner, M.; Peschel, B.; Königshoff, M.; Wagner, D.E. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L896–L902. [Google Scholar] [CrossRef]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Liu, G.; Zhou, Y.; Luckhardt, T.; Bae, S.; Liu, R.-m.; Rangarajan, S.; et al. 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. JCI Insight 2017, 2, e91377. [Google Scholar] [CrossRef]
- Lehmann, M.; Buhl, L.; Alsafadi, H.N.; Klee, S.; Hermann, S.; Mutze, K.; Ota, C.; Lindner, M.; Behr, J.; Hilgendorff, A.; et al. Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir. Res. 2018, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Preuß, E.B.; Schubert, S.; Werlein, C.; Stark, H.; Braubach, P.; Höfer, A.; Plucinski, E.K.J.; Shah, H.R.; Geffers, R.; Sewald, K.; et al. The Challenge of Long-Term Cultivation of Human Precision-Cut Lung Slices. Am. J. Pathol. 2022, 192, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; An, G.H.; Kim, J.-Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.-H.; Han, C.; Yang, S.-R.; Kim, J.-H.; et al. Human pluripotent stem cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, A.; Vazquez-Armendariz, A.I.; Kheirollahi, V.; Chu, X.; Tata, A.; Rivetti, S.; Günther, S.; Lebrigand, K.; Herold, S.; Braun, T.; et al. Identification of a Repair-Supportive Mesenchymal Cell Population during Airway Epithelial Regeneration. Cell Rep. 2020, 33, 108549. [Google Scholar] [CrossRef]
- Vazquez-Armendariz, A.I.; Heiner, M.; El Agha, E.; Salwig, I.; Hoek, A.; Hessler, M.C.; Shalashova, I.; Shrestha, A.; Carraro, G.; Mengel, J.P.; et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020, 39, e103476. [Google Scholar] [CrossRef]
- Suezawa, T.; Kanagaki, S.; Moriguchi, K.; Masui, A.; Nakao, K.; Toyomoto, M.; Tamai, K.; Mikawa, R.; Hirai, T.; Murakami, K.; et al. Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 2021, 16, 2973–2987. [Google Scholar] [CrossRef]
- Blumer, S.; Khan, P.; Artysh, N.; Plappert, L.; Savic, S.; Knudsen, L.; Jonigk, D.; Kuehnel, M.P.; Prasse, A.; Hostettler, K.E. The use of cultured human alveolar basal cells to mimic honeycomb formation in idiopathic pulmonary fibrosis. Respir. Res. 2024, 25, 26. [Google Scholar] [CrossRef]
- Bosáková, V.; De Zuani, M.; Sládková, L.; Garlíková, Z.; Jose, S.S.; Zelante, T.; Hortová Kohoutková, M.; Frič, J. Lung Organoids—The Ultimate Tool to Dissect Pulmonary Diseases? Front. Cell Dev. Biol. 2022, 10, 899368. [Google Scholar] [CrossRef]
- Razian, G.; Yu, Y.; Ungrin, M. Production of Large Numbers of Size-controlled Tumor Spheroids Using Microwell Plates. J. Vis. Exp. JoVE 2013, 81, e50665. [Google Scholar] [CrossRef]
- Ungrin, M.D.; Joshi, C.; Nica, A.; Bauwens, C.; Zandstra, P.W. Reproducible, Ultra High-Throughput Formation of Multicellular Organization from Single Cell Suspension-Derived Human Embryonic Stem Cell Aggregates. PLoS ONE 2008, 3, e1565. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kimura, M.; Shrestha, S.; Lewis, P.; Lee, S.; Cai, Y.; Joshi, P.; Acharya, P.; Liu, J.; Yang, Y.; et al. A Pillar and Perfusion Plate Platform for Robust Human Organoid Culture and Analysis. Adv. Healthc. Mater. 2023, 13, e2302502. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.S.; Costa, A.; Sarmento, B. Building three-dimensional lung models for studying pharmacokinetics of inhaled drugs. Adv. Drug Deliv. Rev. 2021, 170, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Rahman, Z.; ten Dijke, P.; Boukany, P.E. Microfluidics meets 3D cancer cell migration. Trends Cancer 2022, 8, 683–697. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef]
- Anyaduba, T.D.; Otoo, J.A.; Schlappi, T.S. Picoliter Droplet Generation and Dense Bead-in-Droplet Encapsulation via Microfluidic Devices Fabricated via 3D Printed Molds. Micromachines 2022, 13, 1946. [Google Scholar] [CrossRef]
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, Y.; Yan, J.; Xiong, Y.; Wang, W.; Lei, J.; Jiang, T. Recapitulating essential pathophysiological characteristics in lung-on-a-chip for disease studies. Front. Immunol. 2023, 14, 1093460. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; Schmid, R.A.; et al. Second-generation lung-on-a-chip array with a stretchable biological membrane. bioRxiv 2019. [Google Scholar] [CrossRef]
- Wang, K.; Man, K.; Liu, J.; Liu, Y.; Chen, Q.; Zhou, Y.; Yang, Y. Microphysiological Systems: Design, Fabrication, and Applications. ACS Biomater. Sci. Eng. 2020, 6, 3231–3257. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Stucki, J.D.; Hobi, N.; Galimov, A.; Stucki, A.O.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Frick, M.; Funke-Chambour, M.; Geiser, T.; et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci. Rep. 2018, 8, 14359. [Google Scholar] [CrossRef]
- Man, K.; Liu, J.; Liang, C.; Corona, C.; Story, M.D.; Meckes, B.; Yang, Y. Biomimetic Human Lung Alveolar Interstitium Chip with Extended Longevity. ACS Appl. Mater. Interfaces 2023, 15, 36888–36898. [Google Scholar] [CrossRef]
- Felder, M.; Stucki, A.O.; Stucki, J.D.; Geiser, T.; Guenat, O.T. The potential of microfluidic lung epithelial wounding: Towards in vivo-like alveolar microinjuries. Integr. Biol. 2014, 6, 1132–1140. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Jiang, L.; Qin, J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol. Res. 2018, 7, 1048–1060. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Chen, W.; Jiang, L.; Chen, C.; Qin, J. Assessment of Air Pollutant PM2.5 Pulmonary Exposure Using a 3D Lung-on-Chip Model. ACS Biomater. Sci. Eng. 2020, 6, 3081–3090. [Google Scholar] [CrossRef]
- Sengupta, A.; Roldan, N.; Kiener, M.; Froment, L.; Raggi, G.; Imler, T.; de Maddalena, L.; Rapet, A.; May, T.; Carius, P.; et al. A New Immortalized Human Alveolar Epithelial Cell Model to Study Lung Injury and Toxicity on a Breathing Lung-On-Chip System. Front. Toxicol. 2022, 4, 840606. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, Q.; Jiang, A.; Wen, A.M.; Mannix, R.J.; Man, Y.; Hall, S.; Javorsky, E.; Ingber, D.E. A human lung alveolus-on-a-chip model of acute radiation-induced lung injury. Nat. Commun. 2023, 14, 6506. [Google Scholar] [CrossRef] [PubMed]
- Asmani, M.; Velumani, S.; Li, Y.; Wawrzyniak, N.; Hsia, I.; Chen, Z.; Hinz, B.; Zhao, R. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat. Commun. 2018, 9, 2066. [Google Scholar] [CrossRef] [PubMed]
- Akinbote, A.; Beltran-Sastre, V.; Cherubini, M.; Visone, R.; Hajal, C.; Cobanoglu, D.; Haase, K. Classical and Non-classical Fibrosis Phenotypes Are Revealed by Lung and Cardiac Like Microvascular Tissues On-Chip. Front. Physiol. 2021, 12, 735915. [Google Scholar] [CrossRef] [PubMed]
- Hsia, I.; Asmani, M.; Zhao, R. Predicting the preclinical efficacy of anti-fibrosis agents using a force-sensing fibrosis on chip system. Biosens. Bioelectron. 2023, 228, 115194. [Google Scholar] [CrossRef]
- Sengupta, A.; Dorn, A.; Jamshidi, M.; Schwob, M.; Hassan, W.; De Maddalena, L.L.; Hugi, A.; Stucki, A.O.; Dorn, P.; Marti, T.M.; et al. A multiplex inhalation platform to model in situ like aerosol delivery in a breathing lung-on-chip. Front. Pharmacol. 2023, 14, 1114739. [Google Scholar] [CrossRef]
- Si, L.; Prantil-Baun, R.; Benam, K.H.; Bai, H.; Rodas, M.; Burt, M.; Ingber, D.E. Discovery of influenza drug resistance mutations and host therapeutic targets using a human airway chip. bioRxiv 2019. [Google Scholar] [CrossRef]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. Human organ chip-enabled pipeline to rapidly repurpose therapeutics during viral pandemics. bioRxiv 2020. [Google Scholar] [CrossRef]
- Aydin, M.; Naumova, E.A.; Paulsen, F.; Zhang, W.; Gopon, F.; Theis, C.; Lutz, S.; Ehrke-Schulz, E.; Arnold, W.H.; Wirth, S.; et al. House Dust Mite Exposure Causes Increased Susceptibility of Nasal Epithelial Cells to Adenovirus Infection. Viruses 2020, 12, 1151. [Google Scholar] [CrossRef]
- Kwak, T.J.; Lee, E. In vitro modeling of solid tumor interactions with perfused blood vessels—PubMed. Sci. Rep. 2020, 10, 20142. [Google Scholar] [CrossRef]
- Temples, M.N.; Adjei, I.M.; Nimocks, P.M.; Djeu, J.; Sharma, B. Engineered Three-Dimensional Tumor Models to Study Natural Killer Cell Suppression—PubMed. ACS Biomater. Sci. Eng. 2020, 6, 4179–4199. [Google Scholar] [CrossRef] [PubMed]
- Veith, I.; Mencattini, A.; Picant, V.; Serra, M.; Leclerc, M.; Comes, M.C.; Mami-Chouaib, F.; Camonis, J.; Descroix, S.; Shirvani, H.; et al. Apoptosis mapping in space and time of 3D tumor ecosystems reveals transmissibility of cytotoxic cancer death. PLoS Comput. Biol. 2021, 17, e1008870. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, J.; Chung, J.J.; Jung, Y.; Kim, S.H. Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation. Trends Biotechnol. 2020, 38, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Koceva, H.; Amiratashani, M.; Rennert, K.; Mosig, A.S. Immunocompetent Alveolus-on-Chip Model for Studying Alveolar Mucosal Immune Responses. J. Vis. Exp. 2024, 207, e66602. [Google Scholar] [CrossRef] [PubMed]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Zhang, K.; Xi, J.; Wang, Y.; Xue, J.; Li, B.; Huang, Z.; Zheng, Z.; Liang, N.; Wei, Z. A Microfluidic Chip-Based Automated System for Whole-Course Monitoring the Drug Responses of Organoids. Anal. Chem. 2024, 96, 10092–10101. [Google Scholar] [CrossRef]
- Allen, R.J.; Guillen-Guio, B.; Oldham, J.M.; Ma, S.-F.; Dressen, A.; Paynton, M.L.; Kraven, L.M.; Obeidat, M.e.; Li, X.; Ng, M.; et al. Genome-Wide Association Study of Susceptibility to Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 564–574. [Google Scholar] [CrossRef]
- Zhang, K.; Xi, J.; Zhao, H.; Wang, Y.; Xue, J.; Liang, N.; Wei, Z. A dual-functional microfluidic chip for guiding personalized lung cancer medicine: Combining EGFR mutation detection and organoid-based drug response test. Lab Chip 2024, 24, 1762–1774. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona, C.; Man, K.; Newton, C.A.; Nguyen, K.T.; Yang, Y. In Vitro Modeling of Idiopathic Pulmonary Fibrosis: Lung-on-a-Chip Systems and Other 3D Cultures. Int. J. Mol. Sci. 2024, 25, 11751. https://doi.org/10.3390/ijms252111751
Corona C, Man K, Newton CA, Nguyen KT, Yang Y. In Vitro Modeling of Idiopathic Pulmonary Fibrosis: Lung-on-a-Chip Systems and Other 3D Cultures. International Journal of Molecular Sciences. 2024; 25(21):11751. https://doi.org/10.3390/ijms252111751
Chicago/Turabian StyleCorona, Christopher, Kun Man, Chad A. Newton, Kytai T. Nguyen, and Yong Yang. 2024. "In Vitro Modeling of Idiopathic Pulmonary Fibrosis: Lung-on-a-Chip Systems and Other 3D Cultures" International Journal of Molecular Sciences 25, no. 21: 11751. https://doi.org/10.3390/ijms252111751
APA StyleCorona, C., Man, K., Newton, C. A., Nguyen, K. T., & Yang, Y. (2024). In Vitro Modeling of Idiopathic Pulmonary Fibrosis: Lung-on-a-Chip Systems and Other 3D Cultures. International Journal of Molecular Sciences, 25(21), 11751. https://doi.org/10.3390/ijms252111751






