High Blood Pressure and Impaired Brain Health: Investigating the Neuroprotective Potential of Magnesium
Abstract
:1. Introduction
2. Ageing
2.1. Ageing Mechanisms
2.2. Brain Ageing
2.2.1. Age-Related Microscopic Changes
2.2.2. Age-Related Macroscopic Changes
2.3. Cognitive Decline
2.4. Factors Contributing to Brain Ageing and Cognitive Decline
3. BP and Ageing
3.1. Definition
3.2. BP Regulation
3.2.1. Central BP Regulation
3.2.2. Peripheral BP Regulation
3.3. Effect of Sex Hormones
3.4. Measurement
3.5. Epidemiology
3.6. Risk Factors
3.7. BP-Related Health Conditions
3.8. BP Treatment
3.9. BP and Ageing Mechanisms
3.10. BP and Brain Ageing
3.10.1. BP-Related Microscopic Changes
3.10.2. BP-Related Macroscopic Changes
3.11. BP and Cognitive Decline
4. Dietary Magnesium: Underlying Mechanisms and Possible Prevention Opportunity
4.1. Magnesium and Ageing Mechanisms
4.2. Magnesium and Brain Ageing
4.3. Mechanisms Mediating Magnesium Effect on Brain Ageing/Cognitive Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| ANS | Autonomic nervous system |
| BMI | Body mass index |
| BBB | Blood–brain barrier |
| CAC | Coronary artery calcium |
| Ca | Calcium |
| CKD | Chronic kidney disease |
| CIMT | Carotid intima-media thickness |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| DBP | Diastolic blood pressure |
| ESRD | End-stage renal disease |
| ESC/ESH | European Society of Cardiology/European Society of Hypertension |
| HR | Hazard ratio |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| LDL | Low-density lipoproteins |
| MAP | Mean arterial pressure |
| Mg | Magnesium |
| MCI | Mild cognitive impairment |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| OS | Oxidative stress |
| PNS | Parasympathetic nervous system |
| RAAS | Renin-angiotensin-aldosterone system |
| RCT | Randomized controlled trials |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| TNF-α | Tumour necrosis factor-α |
| UK Biobank | United Kingdom Biobank |
| VSMCs | Vascular smooth muscle cells |
| WMLs | White matter lesions |
| WM | White matter |
References
- United Nations. World Population Ageing 2019; United Nations: New York, NY, USA, 2020; ISBN 9789211483253. [Google Scholar]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring Population Ageing: An Analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Wrigglesworth, J.; Ward, P.; Harding, I.H.; Nilaweera, D.; Wu, Z.; Woods, R.L.; Ryan, J. Factors Associated with Brain Ageing—A Systematic Review. BMC Neurol. 2021, 21, 312. [Google Scholar] [CrossRef]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-Induced Cognitive Impairment: From Pathophysiology to Public Health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Rothwell, P.M. Blood Pressure and the Brain: The Neurology of Hypertension. Pract. Neurol. 2019, 20, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Alateeq, K.; Walsh, E.I.; Cherbuin, N. Higher Blood Pressure Is Associated with Greater White Matter Lesions and Brain Atrophy: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 637. [Google Scholar] [CrossRef]
- Alateeq, K.; Walsh, E.I.; Abhayaratna, W.P.; Cherbuin, N. Effects of Higher Normal Blood Pressure on Brain Are Detectable before Middle-Age and Differ by Sex. J. Clin. Med. 2022, 11, 3127. [Google Scholar] [CrossRef]
- Forte, G.; De Pascalis, V.; Favieri, F.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J. Clin. Med. 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Ding, J.; Tang, E.Y.H.; Siervo, M.; Robinson, L.; Jagger, C.; Stephan, B.C.M. Cardiovascular Disease Risk Models and Longitudinal Changes in Cognition: A Systematic Review. PLoS ONE 2014, 9, e114431. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, J.Y.; Han, K.; Kim, D.H.; Cho, H.; Kim, K.J.; Kang, E.S.; Cha, B.-S.; Lee, Y.-H.; Park, S. Blood Pressure Levels and Risks of Dementia: A Nationwide Study of 4.5 Million People. Hypertension 2022, 79, 218–229. [Google Scholar] [CrossRef]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; DeKosky, S.T.; Kuller, L.H. Dementia and Alzheimer’s Disease Incidence in Relationship to Cardiovascular Disease in the Cardiovascular Health Study Cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.N.; Tan, C.C.; Shen, X.N.; Xu, W.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Blood Pressure and Risks of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of 209 Prospective Studies. Hypertension 2020, 76, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-Response Relationship between Dietary Magnesium Intake, Serum Magnesium Concentration and Risk of Hypertension: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The Effect of Magnesium Supplementation on Blood Pressure in Individuals with Insulin Resistance, Prediabetes, or Noncommunicable Chronic Diseases: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef]
- Boche, D.; Nicoll, J.A.R. Neuroinflammation in Ageing and in Neurodegenerative Disease. Neuropathol. Appl. Neurobiol. 2013, 39, 1–2. [Google Scholar] [CrossRef]
- Muthuraju, S.; Zakaria, R.; Karuppan, M.K.M.; Al-Rahbi, B. The Role of Neuroinflammation in Cellular Damage in Neurodegenerative Diseases. BioMed Res. Int. 2020, 2020, 9231452. [Google Scholar] [CrossRef]
- Licastro, F.; Candore, G.; Lio, D.; Porcellini, E.; Colonna-Romano, G.; Franceschi, C.; Caruso, C. Innate Immunity and Inflammation in Ageing: A Key for Understanding Age-Related Diseases. Immun. Ageing 2005, 2, 8. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Wang, D.; Li, Y.; Xing, J.; Zeng, Y.; Liu, Z.; Zhou, X.; Fan, H. Neuroinflammation of Microglial Regulation in Alzheimer’s Disease: Therapeutic Approaches. Molecules 2024, 29, 1478. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [PubMed]
- De Bont, R.; van Larebeke, N. Endogenous DNA Damage in Humans: A Review of Quantitative Data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Vijg, J.; Suh, Y. Genome Instability and Aging. Annu. Rev. Physiol. 2013, 75, 645–668. [Google Scholar] [CrossRef]
- Burkhalter, M.D.; Rudolph, K.L.; Sperka, T. Genome Instability of Ageing Stem Cells-Induction and Defence Mechanisms. Ageing Res. Rev. 2015, 23, 29–36. [Google Scholar] [CrossRef]
- Powers, E.T.; Morimoto, R.I.; Dillin, A.; Kelly, J.W.; Balch, W.E. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annu. Rev. Biochem. 2009, 78, 959–991. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between Telomere Length in Blood and Mortality in People Aged 60 Years or Older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Bernhardt, A.; Jamil, A.; Morshed, M.T.; Ponnath, P.; Gille, V.; Stephan, N.; Sauer, H.; Wartenberg, M. Oxidative Stress and Regulation of Adipogenic Differentiation Capacity by Sirtuins in Adipose Stem Cells Derived from Female Patients of Advancing Age. Sci. Rep. 2024, 14, 19885. [Google Scholar] [CrossRef]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger Signals in Stroke and Their Role on Microglia Activation after Ischemia. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774254. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Bjartmar, C.; Kidd, G.; Mörk, S.; Rudick, R.; Trapp, B.D. Neurological Disability Correlates with Spinal Cord Axonal Loss and Reduced N-Acetyl Aspartate in Chronic Multiple Sclerosis Patients. Ann. Neurol. 2000, 48, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Zirngibl, M.; Assinck, P.; Sizov, A.; Caprariello, A.V.; Plemel, J.R. Oligodendrocyte Death and Myelin Loss in the Cuprizone Model: An Updated Overview of the Intrinsic and Extrinsic Causes of Cuprizone Demyelination. Mol. Neurodegener. 2022, 17, 34. [Google Scholar] [CrossRef]
- Toth, P.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Tarantini, S.; Deak, F.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Age-Related Autoregulatory Dysfunction and Cerebromicrovascular Injury in Mice with Angiotensin II-Induced Hypertension. J. Cereb. Blood Flow Metab. 2013, 33, 1732–1742. [Google Scholar] [CrossRef]
- Rassul, S.M.; Neely, R.K.; Fulton, D. Live-Imaging in the CNS: New Insights on Oligodendrocytes, Myelination, and Their Responses to Inflammation. Neuropharmacology 2016, 110, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Conforti, L.; Gilley, J.; Coleman, M.P. Wallerian Degeneration: An Emerging Axon Death Pathway Linking Injury and Disease. Nat. Rev. Neurosci. 2014, 15, 394–409. [Google Scholar] [CrossRef]
- Park, H.T.; Kim, Y.H.; Lee, K.E.; Kim, J.K. Behind the Pathology of Macrophage-Associated Demyelination in Inflammatory Neuropathies: Demyelinating Schwann Cells. Cell. Mol. Life Sci. 2020, 77, 2497–2506. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Gackowski, D.; Rozalski, R.; Siomek, A.; Dziaman, T.; Nicpon, K.; Klimarczyk, M.; Araszkiewicz, A.; Olinski, R. Oxidative Stress and Oxidative DNA Damage Is Characteristic for Mixed Alzheimer Disease/Vascular Dementia. J. Neurol. Sci. 2008, 266, 57–62. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Salvadó, G.; Gispert, J.D.; Vilor-Tejedor, N.; Grau-Rivera, O.; Sala-Vila, A.; Sánchez-Benavides, G.; Arenaza-Urquijo, E.M.; Crous-Bou, M.; González-De-Echávarri, J.M.; et al. Amyloid Beta, Tau, Synaptic, Neurodegeneration, and Glial Biomarkers in the Preclinical Stage of the Alzheimer’s Continuum. Alzheimer’s Dement. 2020, 16, 1358–1371. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Levstek, T.; Kozjek, E.; Dolžan, V.; Trebušak Podkrajšek, K. Telomere Attrition in Neurodegenerative Disorders. Front. Cell. Neurosci. 2020, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Kuan, X.Y.; Fauzi, N.S.A.; Ng, K.Y.; Bakhtiar, A. Exploring the Causal Relationship Between Telomere Biology and Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 4169–4183. [Google Scholar] [CrossRef]
- Setiadi, A.; Korim, W.S.; Elsaafien, K.; Yao, S.T. The Role of the Blood–Brain Barrier in Hypertension. Exp. Physiol. 2018, 103, 337–342. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, X.; Tao, Y.; Lan, L.; Zheng, L.; Sun, J. Tight Junction Disruption of Blood Brain Barrier in White Matter Lesions in Chronic Hypertensive Rats. Neuroreport 2015, 26, 1039–1043. [Google Scholar] [CrossRef]
- Yang, Y.; Kimura-Ohba, S.; Thompson, J.F.; Salayandia, V.M.; Cossé, M.; Raz, L.; Jalal, F.Y.; Rosenberg, G.A. Vascular Tight Junction Disruption and Angiogenesis in Spontaneously Hypertensive Rat with Neuroinflammatory White Matter Injury. Neurobiol. Dis. 2018, 114, 95–110. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory Mechanisms of Blood-Brain Barrier Damage in Ischemic Stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small Vessel Disease: Mechanisms and Clinical Implications. Lancet Neurol. 2019, 18, e684–e696. [Google Scholar] [CrossRef]
- Rajani, R.M.; Quick, S.; Ruigrok, S.R.; Graham, D.; Harris, S.E.; Verhaaren, B.F.J.; Fornage, M.; Seshadri, S.; Atanur, S.S.; Dominiczak, A.F.; et al. Reversal of Endothelial Dysfunction Reduces White Matter Vulnerability in Cerebral Small Vessel Disease in Rats. Sci. Transl. Med. 2018, 10, eaam9507. [Google Scholar] [CrossRef]
- Kassem, M.S.; Lagopoulos, J.; Stait-Gardner, T.; Price, W.S.; Chohan, T.W.; Arnold, J.C.; Hatton, S.N.; Bennett, M.R. Stress-Induced Grey Matter Loss Determined by MRI Is Primarily Due to Loss of Dendrites and Their Synapses. Mol. Neurobiol. 2013, 47, 645–661. [Google Scholar] [CrossRef]
- Fujita, S.; Mori, S.; Onda, K.; Hanaoka, S.; Nomura, Y.; Nakao, T.; Yoshikawa, T.; Takao, H.; Hayashi, N.; Abe, O. Characterization of Brain Volume Changes in Aging Individuals With Normal Cognition Using Serial Magnetic Resonance Imaging. JAMA Netw. Open 2023, 6, E2318153. [Google Scholar] [CrossRef] [PubMed]
- Fotenos, A.F.; Snyder, A.Z.; Girton, L.E.; Morris, J.C.; Buckner, R.L. Normative Estimates of Cross-Sectional and Longitudinal Brain Volume Decline in Aging and AD. Neurology 2005, 64, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Riddle, W.R.; DonLevy, S.C.; Lee, H. Modeling Brain Tissue Volumes over the Lifespan: Quantitative Analysis of Postmortem Weights and in Vivo MR Images. Magn. Reson. Imaging 2010, 28, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Uzianbaeva, L.; Neidl-Van Gorkom, K.; Zaki, N.; Charykova, I.; Al Koteesh, J.; Almansoori, T.M.; et al. Brain Morphometry and Cognitive Performance in Normal Brain Aging: Age- and Sex-Related Structural and Functional Changes. Front. Aging Neurosci. 2022, 13, 713680. [Google Scholar] [CrossRef]
- Ding, X.Q.; Maudsley, A.A.; Sabati, M.; Sheriff, S.; Schmitz, B.; Schütze, M.; Bronzlik, P.; Kahl, K.G.; Lanfermann, H. Physiological Neuronal Decline in Healthy Aging Human Brain—An in Vivo Study with MRI and Short Echo-Time Whole-Brain 1H MR Spectroscopic Imaging. Neuroimage 2016, 137, 45–51. [Google Scholar] [CrossRef]
- Farokhian, F.; Yang, C.; Beheshti, I.; Matsuda, H.; Wu, S. Age-Related Gray and White Matter Changes in Normal Adult Brains. Aging Dis. 2017, 8, 899–909. [Google Scholar] [CrossRef]
- Fraser, M.A.; Shaw, M.E.; Cherbuin, N. A Systematic Review and Meta-Analysis of Longitudinal Hippocampal Atrophy in Healthy Human Ageing. Neuroimage 2015, 112, 364–374. [Google Scholar] [CrossRef]
- Tabatabaei-Jafari, H.; Shaw, M.E.; Cherbuin, N. Cerebral Atrophy in Mild Cognitive Impairment: A Systematic Review with Meta-Analysis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 11, e487–e504. [Google Scholar] [CrossRef]
- Barnes, J.; Bartlett, J.W.; van de Pol, L.A.; Loy, C.T.; Scahill, R.I.; Frost, C.; Thompson, P.; Fox, N.C. A Meta-Analysis of Hippocampal Atrophy Rates in Alzheimer’s Disease. Neurobiol. Aging 2009, 30, 1711–1723. [Google Scholar] [CrossRef]
- Bagarinao, E.; Watanabe, H.; Maesawa, S.; Mori, D.; Hara, K.; Kawabata, K.; Yoneyama, N.; Ohdake, R.; Imai, K.; Masuda, M.; et al. An Unbiased Data-Driven Age-Related Structural Brain Parcellation for the Identification of Intrinsic Brain Volume Changes over the Adult Lifespan. Neuroimage 2018, 169, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.G.; Archer, D.; Rheault, F.; Lyu, I.; Huo, Y.; Cai, L.Y.; Bunge, S.A.; Weiner, K.S.; Gore, J.C.; Anderson, A.W.; et al. Superficial White Matter across Development, Young Adulthood, and Aging: Volume, Thickness, and Relationship with Cortical Features. Brain Struct. Funct. 2023, 228, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Pomponio, R.; Shou, H.; Doshi, J.; Mamourian, E.; Erus, G.; Nasrallah, I.; Launer, L.J.; Rashid, T.; Bilgel, M.; et al. The Brain Chart of Aging: Machine-Learning Analytics Reveals Links between Brain Aging, White Matter Disease, Amyloid Burden, and Cognition in the ISTAGING Consortium of 10,216 Harmonized MR Scans. Alzheimer’s Dement. 2021, 17, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fratiglioni, L.; Laveskog, A.; Kalpouzos, G.; Ehrenkrona, C.; Zhang, Y. Do Cardiovascular Risk Factors Explain the Link between White Matter Hyperintensities and Brain Volumes in Old Age? A Population-Based Study. Eur. J. Neurol. 2014, 21, 1076–1082. [Google Scholar] [CrossRef]
- Wang, R.Z.; Deng, Y.T.; Zhang, W.; Ning, J.; Li, H.Q.; Feng, J.F.; Cheng, W.; Yu, J.T. Associations between Adiposity and White Matter Hyperintensities: Cross-Sectional and Longitudinal Analyses of 34,653 Participants. Hum. Brain Mapp. 2024, 45, e26560. [Google Scholar] [CrossRef]
- Alber, J.; Alladi, S.; Bae, H.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White Matter Hyperintensities in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Knowledge Gaps and Opportunities. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 107–117. [Google Scholar] [CrossRef]
- Rizeq, J.; Flora, D.B.; Toplak, M.E. Changing Relations among Cognitive Abilities across Development: Implications for Measurement and Research. Clin. Neuropsychol. 2017, 31, 1353–1374. [Google Scholar] [CrossRef]
- Kuhn, L.J.; Camerota, M.; Willoughby, M.T.; Blair, C. A Comparison of Three Executive Function Batteries in a Preschool-Aged Sample. Children 2024, 11, 811. [Google Scholar] [CrossRef]
- Pronk, M.; Lissenberg-Witte, B.I.; van der Aa, H.P.A.; Comijs, H.C.; Smits, C.; Lemke, U.; Zekveld, A.A.; Kramer, S.E. Longitudinal Relationships between Decline in Speech-in-Noise Recognition Ability and Cognitive Functioning: The Longitudinal Aging Study Amsterdam. J. Speech Lang. Hear. Res. 2019, 62, 1167–1187. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Cosco, T.D.; Best, J.R.; Christie, G.J.; DiPaola, S. Predictors of the Rate of Cognitive Decline in Older Adults Using Machine Learning. PLoS ONE 2023, 18, e0280029. [Google Scholar] [CrossRef]
- Deary, I.J.; Der, G. Reaction Time, Age, and Cognitive Ability: Longitudinal Findings from Age 16 to 63 Years in Representative Population Samples. Aging Neuropsychol. Cogn. 2005, 12, 187–215. [Google Scholar] [CrossRef]
- Tucker-Drob, E.M. Global and Domain-Specific Changes in Cognition Throughout Adulthood. Dev. Psychol. 2011, 47, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Yi, D.; Byun, M.S.; Ahn, H.; Jung, J.H.; Kong, N.; Kim, M.J.; Jung, G.; Lee, J.-Y.; Lee, Y.-S.; et al. Synergistic Interaction of High Blood Pressure and Cerebral Beta-Amyloid on Tau Pathology. Alzheimer’s Res. Ther. 2022, 14, 193. [Google Scholar] [CrossRef]
- Potvin, O.; Mouiha, A.; Dieumegarde, L.; Duchesne, S. Normative Data for Subcortical Regional Volumes over the Lifetime of the Adult Human Brain. Neuroimage 2016, 137, 9–20. [Google Scholar] [CrossRef]
- Beauchet, O.; Celle, S.; Roche, F.; Bartha, R.; Montero-Odasso, M.; Allali, G.; Annweiler, C. Blood Pressure Levels and Brain Volume Reduction: A Systematic Review and Meta-Analysis. J. Hypertens. 2013, 31, 1502–1516. [Google Scholar] [CrossRef]
- Li, S.; Tan, I.; Atkins, E.; Schutte, A.E.; Gnanenthiran, S.R. The Pathophysiology, Prognosis and Treatment of Hypertension in Females from Pregnancy to Post-Menopause: A Review. Curr. Heart Fail. Rep. 2024, 21, 322–336. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guideline. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Nadar, S.; Lip, G.Y.H. Hypertension; Oxford Cardiology Library; Oxford University Press: Oxford, UK, 2015; ISBN 9780198701972. [Google Scholar]
- Sun, Z. Aging, Arterial Stiffness, and Hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef]
- Colombari, E.; Sato, M.A.; Cravo, S.L.; Bergamaschi, C.T.; Campos, R.R.; Lopes, O.U. Role of the Medulla Oblongata in Hypertension. Hypertension 2001, 38, 549–554. [Google Scholar] [CrossRef]
- Sved, A.F.; Ito, S.; Sved, J.C. Brainstem Mechanisms of Hypertension: Role of the Rostral Ventrolateral Medulla. Curr. Hypertens. Rep. 2003, 5, 262–268. [Google Scholar] [CrossRef]
- Kobuch, S.; Fatouleh, R.H.; Macefield, J.M.; Henderson, L.A.; Macefield, V.G. Differences in Regional Grey Matter Volume of the Brain Are Related to Mean Blood Pressure and Muscle Sympathetic Nerve Activity in Normotensive Humans. J. Hypertens. 2020, 38, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. Sympathetic Nervous System and Blood Pressure in Humans: Individualized Patterns of Regulation and Their Implications. Hypertension 2010, 56, 10–16. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Sympathetic Nervous System and Hypertension: New Evidences. Auton. Neurosci. 2022, 238, 102954. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [PubMed]
- Ouerd, S.; Frenette, A.J.; Williamson, D.; Serri, K.; D’Aragon, F.; Bichet, D.G.; Charbonney, E. Vasopressin Use in the Support of Organ Donors: Physiological Rationale and Review of the Literature. Crit. Care Explor. 2023, 5, E0907. [Google Scholar] [CrossRef]
- Kougias, P.; Weakley, S.M.; Yao, Q.; Lin, P.H.; Chen, C. Arterial Baroreceptors in the Management of Systemic Hypertension. Med. Sci. Monit. 2010, 16, RA1. [Google Scholar]
- Heusser, K.; Tank, J.; Engeli, S.; Diedrich, A.; Menne, J.; Eckert, S.; Peters, T.; Sweep, F.C.; Haller, H.; Pichlmaier, A.M.; et al. Carotid Baroreceptor Stimulation, Sympathetic Activity, Baroreflex Function, and Blood Pressure in Hypertensive Patients. Hypertension 2010, 55, 619–626. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Masi, S.; Taddei, S. The Renin-Angiotensin-Aldosterone System: A Crossroad from Arterial Hypertension to Heart Failure. Heart Fail. Rev. 2020, 25, 31–42. [Google Scholar] [CrossRef]
- Moiz, A.; Zolotarova, T.; Eisenberg, M.J. Outpatient Management of Essential Hypertension: A Review Based on the Latest Clinical Guidelines. Ann. Med. 2024, 56, 2338242. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Thum, T. Critical Role of the Nitric Oxide/Reactive Oxygen Species Balance in Endothelial Progenitor Dysfunction. Antioxidants Redox Signal. 2011, 15, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; Van Esch, J.H.M.; Roks, A.J.M.; Van Den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Ram, C.V.S. Direct Inhibition of Renin: A Physiological Approach to Treat Hypertension and Cardiovascular Disease. Future Cardiol. 2009, 5, 453–465. [Google Scholar] [CrossRef]
- Becher, U.M.; Endtmann, C.; Tiyerili, V.; Nickenig, G.; Werner, N. Endothelial Damage and Regeneration: The Role of the Renin-Angiotensin- Aldosterone System. Curr. Hypertens. Rep. 2011, 13, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial Dysfunction and Cardiovascular Disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291. [Google Scholar] [CrossRef]
- Costantino Iadecola, R.L.D. Hypertension and Cerebrovascular Dysfunction Costantino. Bone 2008, 23, 2971–3010. [Google Scholar] [CrossRef]
- Clarke, C.; Flores-Muñoz, M.; McKinney, C.A.; Milligan, G.; Nicklin, S.A. Regulation of Cardiovascular Remodeling by the Counter-Regulatory Axis of the Renin-Angiotensin System. Future Cardiol. 2013, 9, 23–38. [Google Scholar] [CrossRef]
- Sadoshima, S.; Busija, D.W.; Heistad, D.D. Mechanisms of Protection against Stroke in Stroke-Prone Spontaneously Hypertensive Rats. Am. J. Physiol. Heart Circ. Physiol. 1983, 13, H406–H412. [Google Scholar] [CrossRef]
- Baumbach, G.L.; Heistad, D.D.; Siems, J.E. Effect of Sympathetic Nerves on Composition and Distensibility of Cerebral Arterioles in Rats. J. Physiol. 1989, 416, 123–140. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional Vascular Contributions to Cognitive Impairment and Dementia: Mechanisms and Consequences of Cerebral Autoregulatory Dysfunction, Endothelial Impairment, and Neurovascular Uncoupling in Aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Camarda, N.D.; Ibarrola, J.; Biwer, L.A.; Jaffe, I.Z. Mineralocorticoid Receptors in Vascular Smooth Muscle: Blood Pressure and Beyond. Hypertension 2024, 81, 1008–1020. [Google Scholar] [CrossRef]
- Iadecola, C.; Park, L.; Capone, C. Threats to the Mind: Aging, Amyloid, and Hypertension. Stroke 2009, 40, S40–S44. [Google Scholar] [CrossRef]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr. Hypertens. Rep. 2022, 24, 185–192. [Google Scholar] [CrossRef]
- Ghazi, L.; Bello, N.A. Hypertension in Women Across the Lifespan. Curr. Atheroscler. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Bagdey, P.S.; Ansari, J.A.; Barnwal, R.K. Prevalence and Epidemiological Factors Associated with Hypertension among Post-Menopausal Women in an Urban Area of Central India. Clin. Epidemiol. Glob. Health 2019, 7, 111–114. [Google Scholar] [CrossRef]
- Kishore, J.; Gupta, N.; Kohli, C.; Kumar, N. Prevalence of Hypertension and Determination of Its Risk Factors in Rural Delhi. Int. J. Hypertens. 2016, 2016, 7962595. [Google Scholar] [CrossRef]
- Tyagi, R.; Dhall, M.; Kapoor, S. Bio-Social Predictors of Hypertension among Premenopausal and Postmenopausal Women. SAGE Open 2015, 5, 2158244015574227. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- NICE. Hypertension in Adults: Diagnosis and Management; NICE National Institute For Health and Care Excellence: Manchester, UK, 2011. [Google Scholar]
- Ryuzaki, M.; Morimoto, S.; Niiyama, M.; Seki, Y.; Yoshida, N.; Oshima, Y.; Mizuguchi, Y.; Watanabe, D.; Ando, T.; Ichihara, A. The Relationships between the Differences in the Central Blood Pressure and Brachial Blood Pressure and Other Factors in Patients with Essential Hypertension. Intern. Med. 2017, 56, 587–596. [Google Scholar] [CrossRef]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central Blood Pressure: Current Evidence and Clinical Importance. Eur. Heart J. 2014, 35, 1719. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.C.; Mak, M.; Kuyper, L.M.; Bittman, J.; Mangat, B.; Lindsay, H.; Kim Sing, C.; Xu, L.; Wong, H.; Dawes, M.; et al. Home Blood Pressure Telemonitoring Technology for Patients with Asymptomatic Elevated Blood Pressure Discharged From the Emergency Department: Pilot Study. JMIR Form. Res. 2024, 8, e49592. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, T.; Intan-Goey, V.; Scherrenberg, M.; Falter, M.; Kario, K.; Akashi, Y.; Dendale, P. Automatic Transmission of Home Blood Pressure Data Can Be Effective in Managing Hypertension: A Systematic Review and Meta-Analysis. Eur. Heart J.-Digit. Health 2022, 3, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, D.; Artinian, N.T.; Basile, J.N.; Krakoff, L.R.; Margolis, K.L.; Rakotz, M.K.; Wozniak, G. Self-Measured Blood Pressure Monitoring at Home: A Joint Policy Statement from the American Heart Association and American Medical Association. Circulation 2020, 142, E42–E63. [Google Scholar] [CrossRef]
- Nessler, K.; Krztoń-Królewiecka, A.; Suska, A.; Mann, M.R.; Nessler, M.B.; Windak, A. The Reliability of Patient Blood Pressure Self-Assessments—A Cross-Sectional Study. BMC Prim. Care 2023, 24, 2. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Gustin IV, W.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic Patterns of Age-Related Changes in Blood Pressure: The Framingham Heart Study. Circulation 1997, 96, 308–315. [Google Scholar] [CrossRef]
- Pinto, E. Blood Pressure and Ageing. Postgrad. Med. J. 2007, 83, 109–114. [Google Scholar] [CrossRef]
- Safar, M.E.; Lange, C.; Tichet, J.; Blacher, J.; Eschwège, E.; Balkau, B. The Data from an Epidemiologic Study on the Insulin Resistance Syndrome Study: The Change and the Rate of Change of the Age-Blood Pressure Relationship. J. Hypertens. 2008, 26, 1903–1911. [Google Scholar] [CrossRef]
- Safar, M.E.; Lange, C.; Blacher, J.; Eschwège, E.; Tichet, J.; Balkau, B. Mean and Yearly Changes in Blood Pressure with Age in the Metabolic Syndrome: The DESIR Study. Hypertens. Res. 2011, 34, 91–97. [Google Scholar] [CrossRef]
- Cheng, S.; Xanthakis, V.; Sullivan, L.M.; Vasan, R.S. Blood Pressure Tracking over the Adult Life Course: Patterns and Correlates in the Framingham Heart Study. Hypertension 2012, 60, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.N.; Nguyen, T.; Dhamoon, A.S. Physiology, Blood Pressure Age Related Changes; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Oberman, A.; Lane, N.E.; Harlan, W.R.; Graybiel, A.; Mitchell, R.E. Trends in Systolic Blood Pressure in the Thousand Aviator Cohort over a Twenty-Four-Year Period. Circulation 1967, 36, 812–822. [Google Scholar] [CrossRef]
- Schaare, H.L.; Kharabian Masouleh, S.; Beyer, F.; Kumral, D.; Uhlig, M.; Reinelt, J.D.; Reiter, A.M.; Lampe, L.; Babayan, A.; Erbey, M.; et al. Association of Peripheral Blood Pressure with Gray Matter Volume in 19- to 40-Year-Old Adults. Neurology 2019, 92, e758–e773. [Google Scholar] [CrossRef] [PubMed]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Jensen, L.J. Functional, Structural and Proteomic Effects of Ageing in Resistance Arteries. Int. J. Mol. Sci. 2024, 25, 2601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Rivara, M.B. Hypertension in Older Adolescents and Young Adults. In Pediatric Hypertension, 5th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 723–738. ISBN 9783031062315. [Google Scholar]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories over the Life Course. JAMA Cardiol. 2020, 5, 255–262. [Google Scholar] [CrossRef]
- Gurven, M.; Blackwell, A.D.; Rodríguez, D.E.; Stieglitz, J.; Kaplan, H. Does Blood Pressure Inevitably Rise with Age?: Longitudinal Evidence among Forager-Horticulturalists. Hypertension 2012, 60, 25–33. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics-2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e39. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Guo, X.; Sun, G.; Li, Z.; Zheng, L.; Yang, H.; Yu, S.; Li, W.; Zou, L.; et al. Prevalence and Risk Factors of Hypertension among Pre- and Post-Menopausal Women: A Cross-Sectional Study in a Rural Area of Northeast China. Maturitas 2015, 80, 282–287. [Google Scholar] [CrossRef]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D.; et al. A Call to Action and a Lifecourse Strategy to Address the Global Burden of Raised Blood Pressure on Current and Future Generations: The Lancet Commission on Hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef] [PubMed]
- Kupper, N.; Ge, D.; Treiber, F.A.; Snieder, H. Emergence of Novel Genetic Effects on Blood Pressure and Hemodynamics in Adolescence: The Georgia Cardiovascular Twin Study. Hypertension 2006, 47, 948–954. [Google Scholar] [CrossRef]
- Van Rijn, M.J.E.; Schut, A.F.C.; Aulchenko, Y.S.; Deinum, J.; Sayed-Tabatabaei, F.A.; Yazdanpanah, M.; Isaacs, A.; Axenovich, T.I.; Zorkoltseva, I.V.; Zillikens, M.C.; et al. Heritability of Blood Pressure Traits and the Genetic Contribution to Blood Pressure Variance Explained by Four Blood-Pressure-Related Genes. J. Hypertens. 2007, 25, 565–570. [Google Scholar] [CrossRef]
- Kaufman, J.S.; Dolman, L.; Rushani, D.; Cooper, R.S. The Contribution of Genomic Research to Explaining Racial Disparities in Cardiovascular Disease: A Systematic Review. Am. J. Epidemiol. 2015, 181, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Morgado, J.; Sanches, B.; Anjos, R.; Coelho, C. Programming of Essential Hypertension: What Pediatric Cardiologists Need to Know. Pediatr. Cardiol. 2015, 36, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Malta, D.; Petersen, K.S.; Johnson, C.; Trieu, K.; Rae, S.; Jefferson, K.; Santos, J.A.; Wong, M.M.Y.; Raj, T.S.; Webster, J.; et al. High Sodium Intake Increases Blood Pressure and Risk of Kidney Disease. From the Science of Salt: A Regularly Updated Systematic Review of Salt and Health Outcomes (August 2016 to March 2017). J. Clin. Hypertens. 2018, 20, 1654–1665. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Forman, J.P.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. Dietary Fatty Acids and the Risk of Hypertension in Middle-Aged and Older Women. Hypertension 2010, 56, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of Increased Potassium Intake on Cardiovascular Risk Factors and Disease: Systematic Review and Meta-Analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef]
- Behers, B.J.; Melchor, J.; Behers, B.M.; Meng, Z.; Swanson, P.J.; Paterson, H.I.; Mendez Araque, S.J.; Davis, J.L.; Gerhold, C.J.; Shah, R.S.; et al. Vitamins and Minerals for Blood Pressure Reduction in the General, Normotensive Population: A Systematic Review and Meta-Analysis of Six Supplements. Nutrients 2023, 15, 4223. [Google Scholar] [CrossRef]
- Tasnim, S.; Tang, C.; Musini, V.M.; Wright, J.M. Effect of Alcohol on Blood Pressure. Cochrane Database Syst. Rev. 2020, 2020, CD012787. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum Iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef]
- Santangeli, E.; Abbati, C.; Chen, R.; Di Carlo, A.; Leoni, S.; Piscaglia, F.; Ferri, S. Pathophysiological-Based Nutritional Interventions in Cirrhotic Patients with Sarcopenic Obesity: A State-of-the-Art Narrative Review. Nutrients 2024, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Stampfer, M.J.; Curhan, G.C. Diet and Lifestyle Risk Factors Associated with Incident Hypertension in Women. JAMA 2009, 302, 401–411. [Google Scholar] [CrossRef]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of Weight Reduction on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef]
- Hayashi, T.; Tsumura, K.; Suematsu, C.; Okada, K.; Fujii, S.; Endo, G. Walking to Work and the Risk for Hypertension in Men: The Osaka Health Survey. Ann. Intern. Med. 1999, 131, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shariful Islam, M.; Fardousi, A.; Sizear, M.I.; Rabbani, M.G.; Islam, R.; Saif-Ur-Rahman, K.M. Effect of Leisure-Time Physical Activity on Blood Pressure in People with Hypertension: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 10639. [Google Scholar] [CrossRef] [PubMed]
- Halperin, R.O.; Michael Gaziano, J.; Sesso, H.D. Smoking and the Risk of Incident Hypertension in Middle-Aged and Older Men. Am. J. Hypertens. 2008, 21, 148–152. [Google Scholar] [CrossRef]
- Bowman, T.S.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. A Prospective Study of Cigarette Smoking and Risk of Incident Hypertension in Women. J. Am. Coll. Cardiol. 2007, 50, 2085–2092. [Google Scholar] [CrossRef]
- Gress, T.W.; Nieto, F.J.; Shahar, E.; Wofford, M.R.; Brancati, F.L. Hypertension and Antihypertensive Therapy as Risk Factors for Type 2 Diabetes Mellitus. Atherosclerosis Risk in Communities Study. N. Engl. J. Med. 2000, 342, 905–912. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-Specific Relevance of Usual Blood Pressure to Vascular Mortality: A Meta-Analysis of Individual Data for One Million Adults in 61 Prospective Studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Anderson, A.H.; Yang, W.; Townsend, R.R.; Pan, Q.; Chertow, G.M.; Kusek, J.W.; Charleston, J.; He, J.; Kallem, R.; Lash, J.P.; et al. Time-Updated Systolic Blood Pressure and the Progression of Chronic Kidney Disease: A Cohort Study. Ann. Intern. Med. 2015, 162, 258–265. [Google Scholar] [CrossRef]
- Reynolds, K.; Gu, D.; Muntner, P.; Kusek, J.W.; Chen, J.; Wu, X.; Duan, X.; Chen, C.-S.; Klag, M.J.; Whelton, P.K.; et al. A Population-Based, Prospective Study of Blood Pressure and Risk for End-Stage Renal Disease in China. J. Am. Soc. Nephrol. 2007, 18, 1928–1935. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Cai, X.; Mai, W.; Hu, Y.; Tang, H.; Xu, D. Prehypertension and Incidence of Cardiovascular Disease: A Meta-Analysis. BMC Med. 2013, 11, 177. [Google Scholar] [CrossRef]
- Talari, H.R.; Zakizade, M.; Soleimani, A.; Bahmani, F.; Ghaderi, A.; Mirhosseini, N.; Eslahi, M.; Babadi, M.; Mansournia, M.A.; Asemi, Z.; et al. Effects of Magnesium Supplementation on Carotid Intima-Media Thickness and Metabolic Profiles in Diabetic Haemodialysis Patients: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2019, 121, 809–817. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational and Intervention Studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef]
- Walsh, E.I.; Burns, R.; Abhayaratna, W.P.; Anstey, K.J.; Cherbuin, N. Physical Activity and Blood Glucose Effects on Weight Gain Over 12 Years in Middle-Aged Adults. J. Obes. Chronic Dis. 2018, 2, 20–25. [Google Scholar] [CrossRef]
- Kruger, J.; Blanck, H.M.; Gillespie, C. Dietary and Physical Activity Behaviors among Adults Successful at Weight Loss Maintenance. Int. J. Behav. Nutr. Phys. Act. 2006, 3, 17. [Google Scholar] [CrossRef]
- Nelson, L.; Esler, M.D.; Jennings, G.L.; Korner, P.I. Effect of Changing Levels of Physical Activity on Blood-Pressure and Haemodynamics in Essential Hypertension. Lancet 1986, 328, 473–476. [Google Scholar] [CrossRef]
- Abegaz, T.M.; Shehab, A.; Gebreyohannes, E.A.; Bhagavathula, A.S.; Elnour, A.A. Nonadherence to Antihypertensive Drugs a Systematic Review and Meta-Analysis. Medicine 2017, 96, e5641. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shao, F.; Chen, K.; Wang, Y.; Wu, Z.; Wang, Y.; Wu, Z.; Wang, Y.; Gao, Y.; Cornelius, V.; et al. Time to Clinical Benefit of Intensive Blood Pressure Lowering in Patients 60 Years and Older with Hypertension: A Secondary Analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood Pressure Lowering for Prevention of Cardiovascular Disease and Death: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef]
- Cohuet, G.; Struijker-Boudier, H. Mechanisms of Target Organ Damage Caused by Hypertension: Therapeutic Potential. Pharmacol. Ther. 2006, 111, 81–98. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. A High-Fat, Refined-Carbohydrate Diet Induces Endothelial Dysfunction and Oxidant/Antioxidant Imbalance and Depresses NOS Protein Expression. J. Appl. Physiol. 2005, 98, 203–210. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.S.; Chien, S. Shear Stress-Initiated Signaling and Its Regulation of Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- He, M.; Martin, M.; Marin, T.; Chen, Z.; Gongol, B. Endothelial Mechanobiology. APL Bioeng. 2020, 4, 010904. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Cushman, M.; Siscovick, D.S.; Blumenthal, R.S.; Palmas, W.; Burke, G.; Herrington, D.M. The Relationship between Inflammation, Obesity and Risk for Hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Hum. Hypertens. 2011, 25, 73–79. [Google Scholar] [CrossRef]
- Shi, P.; Diez-Freire, C.; Jun, J.Y.; Qi, Y.; Katovich, M.J.; Li, Q.; Sriramula, S.; Francis, J.; Sumners, C.; Raizada, M.K. Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension 2010, 56, 297–303. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ding, X.; Duan, B.; Li, L.; Wang, X. Serum Levels of TNF-α and IL-6 Are Associated with Pregnancy-Induced Hypertension. Reprod. Sci. 2016, 23, 1402–1408. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of Inflammation, Immunity, and Oxidative Stress in Hypertension: New Insights and Potential Therapeutic Targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Segiet, A.; Smykiewicz, P.; Kwiatkowski, P.; Żera, T. Tumour Necrosis Factor and Interleukin 10 in Blood Pressure Regulation in Spontaneously Hypertensive and Normotensive Rats. Cytokine 2019, 113, 185–194. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Yu, H.; Zhang, Y.; Dai, Y.; Ning, C.; Tao, L.; Sun, H.; Kellems, R.E.; Blackburn, M.R.; et al. Interleukin 6 Underlies Angiotensin II-Induced Hypertension and Chronic Renal Damage. Hypertension 2012, 59, 136–144. [Google Scholar] [CrossRef]
- Majid, D.S.A.; Prieto, M.C.; Castillo, A.; Chamberlain, C.; Navar, L.G. Augmentation of Nitric Oxide Deficient Hypertension by High Salt Diet Is Associated With Reduced TNF-α Receptor Type 1 Expression in the Kidneys. Am. J. Hypertens. 2024, 37, 717–725. [Google Scholar] [CrossRef]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 Promotes Angiotensin II-Induced Hypertension and Vascular Dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Torpy, D.J.; Papanicolaou, D.A.; Lotsikas, A.J.; Wilder, R.L.; Chrousos, G.P.; Pillemer, S.R. Responses of the Sympathetic Nervous System and the Hypothalamic-Pituitary-Adrenal Axis to Interleukin-6: A Pilot Study in Fibromyalgia. Arthritis Rheum. 2000, 43, 872–880. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Zinman, B.; Hanley, A.J.G.; Harris, S.B.; Kwan, J.; Fantus, I.G. Circulating Tumor Necrosis Factor-α Concentrations in a Native Canadian Population with High Rates of Type 2 Diabetes Mellitus1. J. Clin. Endocrinol. Metab. 1999, 84, 272–278. [Google Scholar] [CrossRef]
- Puszkarska, A.; Niklas, A.; Gluszek, J.; Lipski, D.; Niklas, K. The Concentration of Tumor Necrosis Factor in the Blood Serum and in the Urine and Selected Early Organ Damages in Patients with Primary Systemic Arterial Hypertension. Medicine 2019, 98, e15773. [Google Scholar] [CrossRef]
- Brasier, A.R.; Li, J.; Wimbish, K.A. Tumor Necrosis Factor Activates Angiotensinogen Gene Expression by the Rel A Transactivator. Hypertension 1996, 27, 1009–1017. [Google Scholar] [CrossRef]
- Chen, P.Y.; Schwartz, M.A.; Simons, M. Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front. Cardiovasc. Med. 2020, 7, 53. [Google Scholar] [CrossRef]
- Schwartz, C.J.; Valente, A.J.; Sprague, E.A.; Kelley, J.L.; Nerem, R.M. The Pathogenesis of Atherosclerosis: An Overview. Clin. Cardiol. 1991, 14, 1–16. [Google Scholar] [CrossRef]
- Schmederer, Z.; Rolim, N.; Bowen, T.S.; Linke, A.; Wisloff, U.; Adams, V. Endothelial Function Is Disturbed in a Hypertensive Diabetic Animal Model of HFpEF: Moderate Continuous vs. High Intensity Interval Training. Int. J. Cardiol. 2018, 273, 147–154. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Wang, J.C.; Bennett, M. Aging and Atherosclerosis: Mechanisms, Functional Consequences, and Potential Therapeutics for Cellular Senescence. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef]
- Wen, Y.D.; Wang, H.; Zhu, Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell. Longev. 2018, 2018, 4010395. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Zhang, L.; Zang, G.; Sun, Z.; Wang, Z. Role of Endothelial Cells in Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 895005. [Google Scholar] [CrossRef]
- Buendía, P.; De Oca, A.M.; Madueño, J.A.; Merino, A.; Martín-Malo, A.; Aljama, P.; Ramírez, R.; Rodríguez, M.; Carracedo, J. Endothelial Microparticles Mediate Inflammation-Induced Vascular Calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Skenteris, N.T.; Seime, T.; Witasp, A.; Karlöf, E.; Wasilewski, G.B.; Heuschkel, M.A.; Jaminon, A.M.; Oduor, L.; Dzhanaev, R.; Kronqvist, M.; et al. Osteomodulin Attenuates Smooth Muscle Cell Osteogenic Transition in Vascular Calcification. Clin. Transl. Med. 2022, 12, e682. [Google Scholar] [CrossRef]
- Youwakim, J.; Girouard, H. Inflammation: A Mediator between Hypertension and Neurodegenerative Diseases. Am. J. Hypertens. 2021, 34, 1014–1030. [Google Scholar] [CrossRef]
- Iadecola, C. Hypertension and Dementia. Hypertension 2014, 64, 3–5. [Google Scholar] [CrossRef]
- Tomassoni, D.; Avola, R.; Di Tullio, M.A.; Sabbatini, M.; Vitaioli, L.; Amenta, F. Increased Expression of Glial Fibrillary Acidic Protein in the Brain of Spontaneously Hypertensive Rats. Clin. Exp. Hypertens. 2004, 26, 335–350. [Google Scholar] [CrossRef]
- Gelderblom, M.; Weymar, A.; Bernreuther, C.; Velden, J.; Arunachalam, P.; Steinbach, K.; Orthey, E.; Arumugam, T.V.; Leypoldt, F.; Simova, O.; et al. Neutralization of the IL-17 Axis Diminishes Neutrophil Invasion and Protects from Ischemic Stroke. Blood 2012, 120, 3793–3802. [Google Scholar] [CrossRef]
- Park, H.S.; You, M.J.; Yang, B.; Jang, K.B.; Yoo, J.; Choi, H.J.; Lee, S.-H.; Bang, M.; Kwon, M.-S. Chronically Infused Angiotensin II Induces Depressive-like Behavior via Microglia Activation. Sci. Rep. 2020, 10, 22082. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Gao, D.; Wei, J.; Yuan, H.; Niu, X.; Zhang, Q. Age-Related Changes in Hypertensive Brain Damage in the Hippocampi of Spontaneously Hypertensive Rats. Mol. Med. Rep. 2016, 13, 2552–2560. [Google Scholar] [CrossRef]
- Laporte, J.P.; Faulkner, M.E.; Gong, Z.; Akhonda, M.A.B.S.; Ferrucci, L.; Egan, J.M.; Bouhrara, M. Hypertensive Adults Exhibit Lower Myelin Content: A Multicomponent Relaxometry and Diffusion Magnetic Resonance Imaging Study. Hypertension 2023, 80, 1728–1738. [Google Scholar] [CrossRef]
- Tucsek, Z.; Noa Valcarcel-Ares, M.; Tarantini, S.; Yabluchanskiy, A.; Fülöp, G.; Gautam, T.; Orock, A.; Csiszar, A.; Deak, F.; Ungvari, Z. Hypertension-Induced Synapse Loss and Impairment in Synaptic Plasticity in the Mouse Hippocampus Mimics the Aging Phenotype: Implications for the Pathogenesis of Vascular Cognitive Impairment. GeroScience 2017, 39, 385–406. [Google Scholar] [CrossRef]
- Carnevale, D.; Mascio, G.; D’Andrea, I.; Fardella, V.; Bell, R.D.; Branchi, I.; Pallante, F.; Zlokovic, B.; Yan, S.S.; Lembo, G. Hypertension Induces Brain β-Amyloid Accumulation, Cognitive Impairment, and Memory Deterioration through Activation of Receptor for Advanced Glycation End Products in Brain Vasculature. Hypertension 2012, 60, 188–197. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Cho, H.; Jang, Y.K.; Lee, J.S.; Jang, H.; Kim, Y.; Kim, K.W.; Ryu, Y.H.; Choi, J.Y.; et al. Assessment of Extent and Role of Tau in Subcortical Vascular Cognitive Impairment Using 18F-AV1451 Positron Emission Tomography Imaging. JAMA Neurol. 2018, 75, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Anrather, J.; Zhou, P.; Girouard, H.; Frys, K.; Milner, T.A.; Iadecola, C. Angiotensin II Impairs Neurovascular Coupling in Neocortex through NADPH Oxidase-Derived Radicals. Circ. Res. 2004, 95, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.; Lonterman-Monasch, S.; van der Flier, W.M.; Kramer, M.H.; Maier, A.B.; Muller, M. Malnutrition and Risk of Structural Brain Changes Seen on Magnetic Resonance Imaging in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 2457–2463. [Google Scholar] [CrossRef]
- Jalal, F.Y.; Yang, Y.; Thompson, J.; Lopez, A.C.; Rosenberg, G.A. Myelin Loss Associated with Neuroinflammation in Hypertensive Rats. Stroke 2012, 43, 1115–1122. [Google Scholar] [CrossRef]
- Naessens, D.M.P.; Coolen, B.F.; De Vos, J.; Vanbavel, E.; Strijkers, G.J.; Bakker, E.N.T.P. Altered Brain Fluid Management in a Rat Model of Arterial Hypertension. Fluids Barriers CNS 2020, 17, 41. [Google Scholar] [CrossRef]
- Bedussi, B.; Naessens, D.M.P.; De Vos, J.; Olde Engberink, R.; Wilhelmus, M.M.M.; Richard, E.; Hove, M.T.; Vanbavel, E.; Bakker, E.N.T.P. Enhanced Interstitial Fluid Drainage in the Hippocampus of Spontaneously Hypertensive Rats. Sci. Rep. 2017, 7, 744. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Dziadkowiak, E.; Zimny, A.; Paradowski, B. Cerebral Small Vessel Disease: A Review. Adv. Clin. Exp. Med. 2021, 30, 349–356. [Google Scholar] [CrossRef]
- Du, R.; Xue, Y.; Lu, C. A Quantitative MRI Comparative Study of Imaging Markers for Cerebral Small Vessel Disease in the Middle-Aged and Elderly Patients with and without Hypertension. Natl. Med. J. China 2024, 104, 2619–2625. [Google Scholar] [CrossRef]
- Power, M.C.; Schneider, A.L.C.; Wruck, L.; Griswold, M.; Coker, L.H.; Alonso, A.; Jack, C.R.; Knopman, D.; Mosley, T.H.; Gottesman, R.F. Life-Course Blood Pressure in Relation to Brain Volumes. Alzheimer’s Dement. 2016, 12, 890–899. [Google Scholar] [CrossRef]
- Lane, C.A.; Barnes, J.; Nicholas, J.M.; Sudre, C.H.; Cash, D.M.; Parker, T.D.; Malone, I.B.; Lu, K.; James, S.-N.; Keshavan, A.; et al. Associations between Blood Pressure across Adulthood and Late-Life Brain Structure and Pathology in the Neuroscience Substudy of the 1946 British Birth Cohort (Insight 46): An Epidemiological Study. Lancet Neurol. 2019, 18, e942–e952. [Google Scholar] [CrossRef]
- Wilkinson, I.; Webb, A.J.S. Consistency of Associations of Systolic and Diastolic Blood Pressure with White Matter Hyperintensities: A Meta-Analysis. Int. J. Stroke 2022, 17, 291–298. [Google Scholar] [CrossRef]
- Peters, R.; Xu, Y.; Cherbuin, N.; Sachdev, P.S.; Anstey, K.J. Higher Diastolic Blood Pressure Aged 40–44 Is Associated with Declining Cognition and Increasing White Matter Lesions over 8–12 Year Follow Up. Alzheimer’s Dement. 2020, 16, e45569. [Google Scholar] [CrossRef]
- Foster-Dingley, J.C.; Van Der Grond, J.; Moonen, J.E.F.; Van Den Berg-Huijsmans, A.A.; De Ruijter, W.; Van Buchem, M.A.; de Craen, A.J.; van der Mast, R.C. Lower Blood Pressure Is Associated With Smaller Subcortical Brain Volumes in Older Persons. Am. J. Hypertens. 2015, 28, 1127–1133. [Google Scholar] [CrossRef]
- Volgman, A.S.; Bairey Merz, C.N.; Aggarwal, N.T.; Bittner, V.; Bunch, T.J.; Gorelick, P.B.; Maki, P.; Patel, H.N.; Poppas, A.; Ruskin, J.; et al. Sex Differences in Cardiovascular Disease and Cognitive Impairment: Another Health Disparity for Women? J. Am. Heart Assoc. 2019, 8, 013154. [Google Scholar] [CrossRef] [PubMed]
- George, K.M.; Maillard, P.; Gilsanz, P.; Fletcher, E.; Peterson, R.L.; Fong, J.; Mayeda, E.R.; Mungas, D.M.; Barnes, L.L.; Glymour, M.M.; et al. Association of Early Adulthood Hypertension and Blood Pressure Change With Late-Life Neuroimaging Biomarkers. JAMA Netw. Open 2023, 6, e236431. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Mortby, M.E.; Janke, A.L.; Sachdev, P.S.; Abhayaratna, W.P.; Anstey, K.J. Blood Pressure, Brain Structure, and Cognition: Opposite Associations in Men and Women. Am. J. Hypertens. 2015, 28, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Nguyen Duc, N.; Bui Van, N.; Vo, H.L.; Nam, K.D.; Si Anh, H.N.; Minh, H.T.; Tuan, N.T.; Duy, T.M.; Thu, H.T.T.; Chu-Dinh, T.; et al. Impact of Body Mass Index and Waist Circumference on Blood Pressure: A Cross-Sectional Survey in a Population Living in the Vietnam Northern Mountainous. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1399–1404. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Tabatabaei-Jafari, H.; Hornberger, M.; Cherbuin, N. Age, Menstruation History, and the Brain. Menopause 2020, 28, e167–e174. [Google Scholar] [CrossRef]
- Angoff, R.; Himali, J.J.; Maillard, P.; Aparicio, H.J.; Vasan, R.S.; Seshadri, S.; Beiser, A.S.; Tsao, C.W. Relations of Metabolic Health and Obesity to Brain Aging in Young to Middle-Aged Adults. J. Am. Heart Assoc. 2022, 11, e022107. [Google Scholar] [CrossRef]
- Henn, R.E.; Elzinga, S.E.; Glass, E.; Parent, R.; Guo, K.; Allouch, A.A.; Mendelson, F.E.; Hayes, J.; Webber-Davis, I.; Murphy, G.G.; et al. Obesity-Induced Neuroinflammation and Cognitive Impairment in Young Adult versus Middle-Aged Mice. Immun. Ageing 2022, 19, 67. [Google Scholar] [CrossRef]
- Su, C.; Wu, H.; Yang, X.; Zhao, B.; Zhao, R. The Relation between Antihypertensive Treatment and Progression of Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medecine 2021, 100, E26749. [Google Scholar] [CrossRef]
- Carnovale, C.; Perrotta, C.; Baldelli, S.; Cattaneo, D.; Montrasio, C.; Barbieri, S.S.; Pompilio, G.; Vantaggiato, C.; Clementi, E.; Pozzi, M. Antihypertensive Drugs and Brain Function: Mechanisms Underlying Therapeutically Beneficial and Harmful Neuropsychiatric Effects. Cardiovasc. Res. 2023, 119, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Naessens, D.M.P.; de Vos, J.; Richard, E.; Wilhelmus, M.M.M.; Jongenelen, C.A.M.; Scholl, E.R.; van der Wel, N.N.; Heijst, J.A.; Teunissen, C.E.; Strijkers, G.J.; et al. Effect of Long-Term Antihypertensive Treatment on Cerebrovascular Structure and Function in Hypertensive Rats. Sci. Rep. 2023, 13, 3481. [Google Scholar] [CrossRef]
- Forte, G.; Casagrande, M. Brain Sciences Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sci. 2020, 10, 919. [Google Scholar] [CrossRef]
- Rossi, R.; Chiurlia, E.; Nuzzo, A.; Cioni, E.; Origliani, G.; Modena, M.G. Flow-Mediated Vasodilation and the Risk of Developing Hypertension in Healthy Postmenopausal Women. J. Am. Coll. Cardiol. 2004, 44, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Tsukamoto, T.; Morita, K.; Katoh, C.; Furumoto, T.; Fujii, S.; Tamaki, N.; Tsutsui, H. Plasma Interleukin-6 and Tumor Necrosis Factor-α Can Predict Coronary Endothelial Dysfunction in Hypertensive Patients. Hypertens. Res. 2007, 30, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M. Profiles and Predictive Values of Interleukin-6 in Aortic Dissection: A Review. Braz. J. Cardiovasc. Surg. 2019, 34, 596–604. [Google Scholar] [CrossRef]
- Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Shah, B.; Bohra, M.; Singh, U.; Jagtap, P.; Baidya, F.; Kalia, K.; et al. The Role of Inflammasomes in Atherosclerosis and Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4234–4245. [Google Scholar] [CrossRef]
- Gaba, P.; O’Donoghue, M.L.; Park, J.G.; Wiviott, S.D.; Atar, D.; Kuder, J.F.; Im, K.; Murphy, S.A.; De Ferrari, G.M.; Gaciong, Z.A.; et al. Association between Achieved Low-Density Lipoprotein Cholesterol Levels and Long-Term Cardiovascular and Safety Outcomes: An Analysis of FOURIER-OLE. Circulation 2023, 147, 1192–1203. [Google Scholar] [CrossRef]
- Jensky, N.E.; Criqui, M.H.; Wright, M.C.; Wassel, C.L.; Brody, S.A.; Allison, M.A. Blood Pressure and Vascular Calcification. Hypertension 2010, 55, 990–997. [Google Scholar] [CrossRef]
- Ritz, M.-F.; Grond-Ginsbach, C.; Engelter, S.; Lyrer, P. Gene Expression Suggests Spontaneously Hypertensive Rats May Have Altered Metabolism and Reduced Hypoxic Tolerance. Curr. Neurovasc. Res. 2012, 9, 10–19. [Google Scholar] [CrossRef]
- Espinoza Oyarce, D.A.; Shaw, M.E.; Alateeq, K.; Cherbuin, N. Volumetric Brain Differences in Clinical Depression in Association with Anxiety: A Systematic Review with Meta-Analysis. J. Psychiatry Neurosci. 2020, 45, 406–429. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Miller, J.E.; Cipolla, M.J. Memory Impairment in Spontaneously Hypertensive Rats Is Associated with Hippocampal Hypoperfusion and Hippocampal Vascular Dysfunction. J. Cereb. Blood Flow Metab. 2020, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Petrovic, M.; Strandberg, T. Hypertension Management in Older and Frail Older Patients. Circ. Res. 2019, 124, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hayden, K.M.; May, N.S.; Haring, B.; Liu, Z.; Henderson, V.W.; Chen, J.-C.; Gracely, E.J.; Wassertheil-Smoller, S.; Rapp, S.R. Association between Blood Pressure Levels and Cognitive Impairment in Older Women: A Prospective Analysis of the Women’s Health Initiative Memory Study. Lancet Healthy Longev. 2022, 3, e42–e53. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Deal, J.A.; McKhann, G.M.; Mosley, T.H.; Sharrett, A.R.; et al. Associations between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Abell, J.G.; Kivimäki, M.; Dugravot, A.; Tabak, A.G.; Fayosse, A.; Shipley, M.; Sabia, S.; Singh-Manoux, A. Association between Systolic Blood Pressure and Dementia in TheWhitehall II Cohort Study: Role of Age, Duration, and Threshold Used to Define Hypertension. Eur. Heart J. 2018, 39, 3119–3125. [Google Scholar] [CrossRef]
- Launer, L.J.; Masaki, K.; Petrovitch, H.; Foley, D.; Havlik, R.J. The Association Between Midlife Blood Pressure Levels and Late-Life Cognitive Function: The Honolulu-Asia Aging Study. JAMA J. Am. Med. Assoc. 1995, 274, 1846–1851. [Google Scholar] [CrossRef]
- Carnevale, D.; Lembo, G. “Alzheimer-like” Pathology in a Murine Model of Arterial Hypertension. Biochem. Soc. Trans. 2011, 39, 939–944. [Google Scholar] [CrossRef]
- Qiu, C.; Winblad, B.; Fratiglioni, L. The Age-Dependent Relation of Blood Pressure to Cognitive Function and Dementia. Lancet Neurol. 2005, 4, 487–499. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for Primary Prevention of Alzheimer’s Disease: An Analysis of Population-Based Data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Joas, E.; Bäckman, K.; Gustafson, D.; Östling, S.; Waern, M.; Guo, X.; Skoog, I. Blood Pressure Trajectories from Midlife to Late Life in Relation to Dementia in Women Followed for 37 Years. Hypertension 2012, 59, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Yasar, S.; Anderson, C.S.; Andrews, S.; Antikainen, R.; Arima, H.; Beckett, N.; Beer, J.C.; Bertens, A.S.; Booth, A.; et al. Investigation of Antihypertensive Class, Dementia, and Cognitive Decline: A Meta-Analysis. Neurology 2020, 94, e267–e281. [Google Scholar] [CrossRef]
- Ding, J.; Davis-Plourde, K.L.; Sedaghat, S.; Tully, P.J.; Wang, W.; Phillips, C.; Pase, M.P.; Himali, J.J.; Windham, B.G.; Griswold, M.; et al. Antihypertensive Medications and Risk for Incident Dementia and Alzheimer’s Disease: A Meta-Analysis of Individual Participant Data from Prospective Cohort Studies. Lancet Neurol. 2020, 19, 61–70. [Google Scholar] [CrossRef]
- Dallaire-Théroux, C.; Quesnel-Olivo, M.H.; Brochu, K.; Bergeron, F.; O’Connor, S.; Turgeon, A.F.; Laforce, R.J.; Verreault, S.; Camden, M.-C.; Duchesne, S. Evaluation of Intensive vs Standard Blood Pressure Reduction and Association with Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2134553. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. J. Am. Med. Assoc. 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joosten, M.M.; de Borst, M.H.; Bakker, S.J.L. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Li, L.; Bao, J.; Wang, Z.H.; Zeng, J.; Liu, E.J.; Li, X.G.; Huang, R.X.; Gao, D.; Li, M.Z.; et al. Magnesium Protects Cognitive Functions and Synaptic Plasticity in Streptozotocin-Induced Sporadic Alzheimer’s Model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef]
- Lo, K.; Liu, Q.; Madsen, T.; Rapp, S.; Chen, J.C.; Neuhouser, M.; Shadyab, A.; Pal, L.; Lin, X.; Shumaker, S.; et al. Relations of Magnesium Intake to Cognitive Impairment and Dementia among Participants in the Women’s Health Initiative Memory Study: A Prospective Cohort Study. BMJ Open 2019, 9, 030052. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Tao, M.; Liu, J.; Cervantes, D. Association between Magnesium Intake and Cognition in US Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12250. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef]
- Laurant, P.; Hayoz, D.; Brunner, H.; Berthelot, A. Dietary Magnesium Intake Can Affect Mechanical Properties of Rat Carotid Artery. Br. J. Nutr. 2000, 84, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral Magnesium Supplementation Improves Endothelial Function and Attenuates Subclinical Atherosclerosis in Thiazide-Treated Hypertensive Women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef]
- Amighi, J.; Sabeti, S.; Schlager, O.; Mlekusch, W.; Exner, M.; Lalouschek, W.; Ahmadi, R.; Minar, E.; Schillinger, M. Low Serum Magnesium Predicts Neurological Events in Patients with Advanced Atherosclerosis. Stroke 2004, 35, 22–27. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Gao, J.; Yang, Q.; Qu, H.; Shi, J. Association between Dietary Magnesium and 10-Year Risk of a First Hard Atherosclerotic Cardiovascular Disease Event. Am. J. Med. Sci. 2024, 2024, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Moradi, S.; Nezamoleslami, S.; Moosavian, S.P.; Kermani, M.a.H.; Lazaridi, A.V.; Miraghajani, M. The Effects of Magnesium Supplementation on Lipid Profile Among Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Trace Elem. Res. 2021, 199, 861–873. [Google Scholar] [CrossRef]
- Brvar, M.; Chan, M.Y.; Dawson, A.H.; Ribchester, R.R.; Eddleston, M. Magnesium Sulfate and Calcium Channel Blocking Drugs as Antidotes for Acute Organophosphorus Insecticide Poisoning–a Systematic Review and Meta-Analysis. Clin. Toxicol. 2018, 56, 725–736. [Google Scholar] [CrossRef]
- Agus, Z.S.; Kelepouris, E.; Dukes, I.; Morad, M. Cytosolic Magnesium Modulates Calcium Channel Activity in Mammalian Ventricular Cells. Am. J. Physiol.-Cell Physiol. 1989, 256, C452–C455. [Google Scholar] [CrossRef]
- Villa-Bellosta, R. Impact of Magnesium:Calcium Ratio on Calcification of the Aortic Wall. PLoS ONE 2017, 12, e0178872. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s Physiologic Calcium Blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; O’Donnell, C.J.; Jacques, P.F.; Meigs, J.B.; Hoffmann, U.; McKeown, N.M. Magnesium Intake Is Inversely Associated with Coronary Artery Calcification: The Framingham Heart Study. JACC Cardiovasc. Imaging 2014, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Milne, F.J. Magnesium Supplementation Attenuates, but Does Not Prevent, Development of Hypertension in Spontaneously Hypertensive Rats. Am. J. Hypertens. 1999, 12, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Sado, T.; Oi, H.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; Ueda, S.; et al. Aging Impairs the Protective Effect of Magnesium Supplementation on Hypertension in Spontaneously Hypertensive Rats. Magnes. Res. 2007, 20, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Furberg, C.D.; Wright, J.T.; Davis, B.R.; Cutler, J.A.; Alderman, M.; Black, H.; Cushman, W.; Grimm, R.; Haywood, L.J.; Leenen, F.; et al. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J. Am. Med. Assoc. 2002, 288, 2981–2997. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Vaqar, S. Hypermagnesemia. Nutr. Rev. 1968, 26, 12–15. [Google Scholar] [CrossRef]
- Zhang, X.; Del Gobbo, L.C.; Hruby, A.; Rosanoff, A.; He, K.; Dai, Q.; Costello, R.B.; Zhang, W.; Song, Y. The Circulating Concentration and 24-h Urine Excretion of Magnesium Dose- and Time-Dependently Respond to Oral Magnesium Supplementation in a Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2016, 146, 595–602. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 223. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and Inflammation: Advances and Perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the Inflammatory Response: Potential Physiopathological Implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.Y.; van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium Intake and Plasma Concentrations of Markers of Systemic Inflammation and Endothelial Dysfunction in Women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.N.; Demler, O.V.; Liu, Y.; Farukhi, Z.; Adelman, S.J.; Collins, H.L.; Ridker, P.M.; Rader, D.J.; Glynn, R.J.; Mora, S. Anti-Inflammatory HDL Function, Incident Cardiovascular Events, and Mortality: A Secondary Analysis of the JUPITER Randomized Clinical Trial. J. Am. Heart Assoc. 2020, 9, e016507. [Google Scholar] [CrossRef]
- Romeo, V.; Cazzaniga, A.; Maier, J.A.M. Magnesium and the Blood-Brain Barrier in Vitro: Effects on Permeability and Magnesium Transport. Magnes. Res. 2019, 32, 16–24. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High Concentrations of Magnesium Modulate Vascular Endothelial Cell Behaviour in Vitro. Biochim. Biophys. Acta-Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Barria, A.; Malinow, R. NMDA Receptor Subunit Composition Controls Synaptic Plasticity by Regulating Binding to CaMKII. Neuron 2005, 48, 289–301. [Google Scholar] [CrossRef]
- Deutschenbaur, L.; Beck, J.; Kiyhankhadiv, A.; Muhlhauser, M.; Borgwardt, S.; Walter, M.; Hasler, G.; Sollberger, D.; Lang, U.E. Role of Calcium, Glutamate and NMDA in Major Depression and Therapeutic Application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 325–333. [Google Scholar] [CrossRef]
- Campo-Soria, C.; Chang, Y.; Weiss, D.S. Mechanism of Action of Benzodiazepines on GABA A Receptors. Br. J. Pharmacol. 2006, 148, 984–990. [Google Scholar] [CrossRef]
- Chou, M.-H.; Yang, Y.K.; Wang, J.-D.; Lin, C.-Y.; Lin, S.-H. The Association of Serum and Dietary Magnesium with Depressive Symptoms. Nutrients 2023, 15, 774. [Google Scholar] [CrossRef]
- Glick, J.L. Dementias: The Role of Magnesium Deficiency and an Hypothesis Concerning the Pathogenesis of Alzheimer’s Disease. Med. Hypotheses 1990, 31, 211–225. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.-G.; et al. Elevation of Brain Magnesium Prevents Synaptic Loss and Reverses Cognitive Deficits in Alzheimer’s Disease Mouse Model. Mol. Brain 2014, 7, 65. [Google Scholar] [CrossRef]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of Structural and Functional Synapse Density by L-Threonate through Modulation of Intraneuronal Magnesium Concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Rui, W.; Wu, S. Magnesium Lithospermate B Promotes Proliferation and Differentiation of Neural Stem Cells in Vitro and Enhances Neurogenesis in Vivo. Tissue Cell 2018, 53, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, Y.; Shi, Y.; Ma, Y.; Hu, Y.; Wang, M.; Li, X. Elevation of Brain Magnesium Potentiates Neural Stem Cell Proliferation in the Hippocampus of Young and Aged Mice. J. Cell. Physiol. 2016, 231, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guan, P.P.; Guo, J.W.; Wang, Y.; Cao, L.L.; Xu, G.B.; Konstantopoulos, K.; Wang, Z.Y.; Wang, P. By Suppressing the Expression of Anterior Pharynx-Defective-1α and -1β and Inhibiting the Aggregation of β-Amyloid Protein, Magnesium Ions Inhibit the Cognitive Decline of Amyloid Precursor Protein/Presenilin 1 Transgenic Mice. FASEB J. 2015, 29, 5044–5058. [Google Scholar] [CrossRef]
- Yu, X.; Guan, P.-P.; Zhu, D.; Liang, Y.-Y.; Wang, T.; Wang, Z.-Y.; Wang, P. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor α and the Activity of γ-Secretase in a β-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 172. [Google Scholar] [CrossRef]
- Wang, P.; Yu, X.; Guan, P.P.; Guo, J.W.; Wang, Y.; Zhang, Y.; Zhao, H.; Wang, Z.Y. Magnesium Ion Influx Reduces Neuroinflammation in Aβ Precursor Protein/Presenilin 1 Transgenic Mice by Suppressing the Expression of Interleukin-1β. Cell. Mol. Immunol. 2017, 14, 451–464. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Lin, W.; Zhang, S. Magnesium Promotes the Regeneration of the Peripheral Nerve. Front. Cell Dev. Biol. 2021, 9, 717854. [Google Scholar] [CrossRef]
- Pan, H.C.; Chin, C.S.; Yang, D.Y.; Ho, S.P.; Chen, C.J.; Hwang, S.M.; Chang, M.H.; Cheng, F.C. Human Amniotic Fluid Mesenchymal Stem Cells in Combination with Hyperbaric Oxygen Augment Peripheral Nerve Regeneration. Neurochem. Res. 2009, 34, 1304–1316. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Zhang, L.; Han, F.; Pang, K.L.; Li, X.; Shen, J.Y. Magnesium Boosts the Memory Restorative Effect of Environmental Enrichment in Alzheimer’s Disease Mice. CNS Neurosci. Ther. 2018, 24, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, M.; Chen, Z.; Lu, J.; Liu, Y.; Zhou, L.; Xu, X.; Fan, D.; Chui, D. Magnesium Modulates Amyloid-β Protein Precursor Trafficking and Processing. J. Alzheimer’s Dis. 2010, 20, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zheng, X.; Ma, Z.-T.; Lv, J.-Y.; Jiang, W.-J.; Liu, M.-Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13, 799824. [Google Scholar] [CrossRef] [PubMed]
- Borella, P.; Giardino, A.; Neri, M.; Andermarker, E. Magnesium and Potassium Status in Elderly Subjects with and without Dementia of the Alzheimer Type. Magnes. Res. 1990, 3, 283–289. [Google Scholar] [PubMed]
- Basun, H.; Forssell, L.G.; Wetterberg, L.; Winblad, B. Metals and Trace Elements in Plasma and Cerebrospinal Fluid in Normal Aging and Alzheimer’s Disease. J. Neural. Transm. Park. Dis. Dement. Sect. 1991, 3, 231–258. [Google Scholar]
- Boström, F.; Hansson, O.; Gerhardsson, L.; Lundh, T.; Minthon, L.; Stomrud, E.; Zetterberg, H.; Londos, E. CSF Mg and Ca as Diagnostic Markers for Dementia with Lewy Bodies. Neurobiol. Aging 2009, 30, 1265–1271. [Google Scholar] [CrossRef]
- Shore, D.; Henkin, R.I.; Nelson, N.R.; Agarwal, R.P.; Wyatt, R.J. Hair and Serum Copper, Zinc, Calcium, and Magnesium Concentrations in Alzheimer-type Dementia. J. Am. Geriatr. Soc. 1984, 32, 892–895. [Google Scholar] [CrossRef]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers. Dis. Other Demen. 2016, 31, 208–213. [Google Scholar] [CrossRef]
- Andrási, E.; Igaz, S.; Molnár, Z.; Makó, S. Disturbances of Magnesium Concentrations in Various Brain Areas in Alzheimer’s Disease. Magnes. Res. 2000, 13, 189–196. [Google Scholar]
- Alateeq, K.; Walsh, E.I.; Cherbuin, N. Dietary Magnesium Intake Is Related to Larger Brain Volumes and Lower White Matter Lesions with Notable Sex Differences. Eur. J. Nutr. 2023, 62, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, J.; Cheng, Y.; Li, R.; Wang, Y.; Chen, Y.; Scott, T.; Tucker, K.L. Magnesium and Cognitive Health in Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100272. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; De Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of Dietary Magnesium Intake to Biomarkers of Inflammation and Endothelial Dysfunction in an Ethnically Diverse Cohort of Postmenopausal Women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Keshavarz, M.; Dehpour, A.R. Effect of Oral Magnesium Sulfate Administration on Blood Pressure and Lipid Profile in Streptozocin Diabetic Rat. Eur. J. Pharmacol. 2007, 560, 201–205. [Google Scholar] [CrossRef]
- Fazlali, M.; Kharazmi, F.; Kamran, M.; Malekzadeh, K.; Talebi, A.; Khosravi, F.; Soltani, N. Effect of Oral Magnesium Sulfate Administration on Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 Gene Expression to Prevent Atherosclerosis in Diabetic Rat Vessels. J. Diabetes Investig. 2019, 10, 650–658. [Google Scholar] [CrossRef]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary Magnesium Supplementation Prevents and Reverses Vascular and Soft Tissue Calcifications in Uremic Rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef]
- Gao, F.; Ding, B.; Zhou, L.; Gao, X.; Guo, H.; Xu, H. Magnesium Sulfate Provides Neuroprotection in Lipopolysaccharide-Activated Primary Microglia by Inhibiting NF-ΚB Pathway. J. Surg. Res. 2013, 184, 944–950. [Google Scholar] [CrossRef]
- Tai, Y.; Qiu, Y.; Bao, Z. Magnesium Lithospermate B Suppresses Lipopolysaccharide-Induced Neuroinflammation in BV2 Microglial Cells and Attenuates Neurodegeneration in Lipopolysaccharide-Injected Mice. J. Mol. Neurosci. 2018, 64, 80–92. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, M.E.; Gómez-Delgado, F.; Arenas de Larriva, A.P.; Canalejo, A.; Gómez-Luna, P.; Herencia, C.; López-Moreno, J.; Rodríguez, M.; López-Miranda, J.; Almadén, Y. Serum Magnesium Is Associated with Carotid Atherosclerosis in Patients with High Cardiovascular Risk (CORDIOPREV Study). Sci. Rep. 2019, 9, 8013. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hara, A.; Ohkubo, T.; Kikuya, M.; Shintani, Y.; Metoki, H.; Inoue, R.; Asayama, K.; Kanno, A.; Nakashita, M.; et al. Serum Magnesium, Ambulatory Blood Pressure, and Carotid Artery Alteration: The Ohasama Study. Am. J. Hypertens. 2010, 23, 1292–1298. [Google Scholar] [CrossRef]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.R.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of Serum and Dietary Magnesium with Cardiovascular Disease, Hypertension, Diabetes, Insulin, and Carotid Arterial Wall Thickness: The Aric Study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Randell, E.W.; Mathews, M.; Gadag, V.; Zhang, H.; Sun, G. Relationship between Serum Magnesium Values, Lipids and Anthropometric Risk Factors. Atherosclerosis 2008, 196, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, J.; Wang, L.; Li, S.; Zhang, Q.; Xiao, P.; Wang, K.; Zhuang, M.; Jiang, Y. Association between Serum Magnesium and Blood Lipids: Influence of Type 2 Diabetes and Central Obesity. Br. J. Nutr. 2018, 120, 250–258. [Google Scholar] [CrossRef]
- Altura, B.M.; Shah, N.C.; Jiang, X.-C.; Li, Z.; Perez-Albela, J.L.; Sica, A.C.; Altura, B.T. Short-Term Magnesium Deficiency Results in Decreased Levels of Serum Sphingomyelin, Lipid Peroxidation, and Apoptosis in Cardiovascular Tissues. Am. J. Physiol. Circ. Physiol. 2009, 297, H86–H92. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Perticone, F.; Ientile, R.; Barbagallo, M.; Corica, F. Serum Magnesium and Lipids: More Clarity Is Needed. Atherosclerosis 2007, 192, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhang, R.; Li, G. Effect of Magnesium on Vascular Calcification in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Ren. Fail. 2023, 45, 2182603. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary Magnesium Intake and the Risk of Cardiovascular Disease, Type 2 Diabetes, and All-Cause Mortality: A Dose-Response Meta-Analysis of Prospective Cohort Studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of Magnesium Supplements on Serum C-Reactive Protein: A Systematic Review and Meta-Analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef]
- Al-Ghazali, K.; Eltayeb, S.; Musleh, A.; Al-Abdi, T.; Ganji, V.; Shi, Z. Serum Magnesium and Cognitive Function Among Qatari Adults. Front. Aging Neurosci. 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, J.Q.; Tolstrup, J.S.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma Concentrations of Magnesium and Risk of Dementia: A General Population Study of 102 648 Individuals. Clin. Chem. 2021, 67, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Walsh, E.I.; Shaw, M.; Luders, E.; Anstey, K.J.; Sachdev, P.S.; Abhayaratna, W.P.; Gaser, C. Optimal Blood Pressure Keeps Our Brains Younger. Front. Aging Neurosci. 2021, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Alateeq, K.; Walsh, E.I.; Ambikairajah, A.; Cherbuin, N. Association between Dietary Magnesium Intake, Inflammation, and Neurodegeneration. Eur. J. Nutr. 2024, 63, 1807–1818. [Google Scholar] [CrossRef]
- Appannah, G.; Murray, K.; Trapp, G.; Dymock, M.; Oddy, W.H.; Ambrosini, G.L. Dietary Pattern Trajectories across Adolescence and Early Adulthood and Their Associations with Childhood and Parental Factors. Am. J. Clin. Nutr. 2021, 113, 36–46. [Google Scholar] [CrossRef]
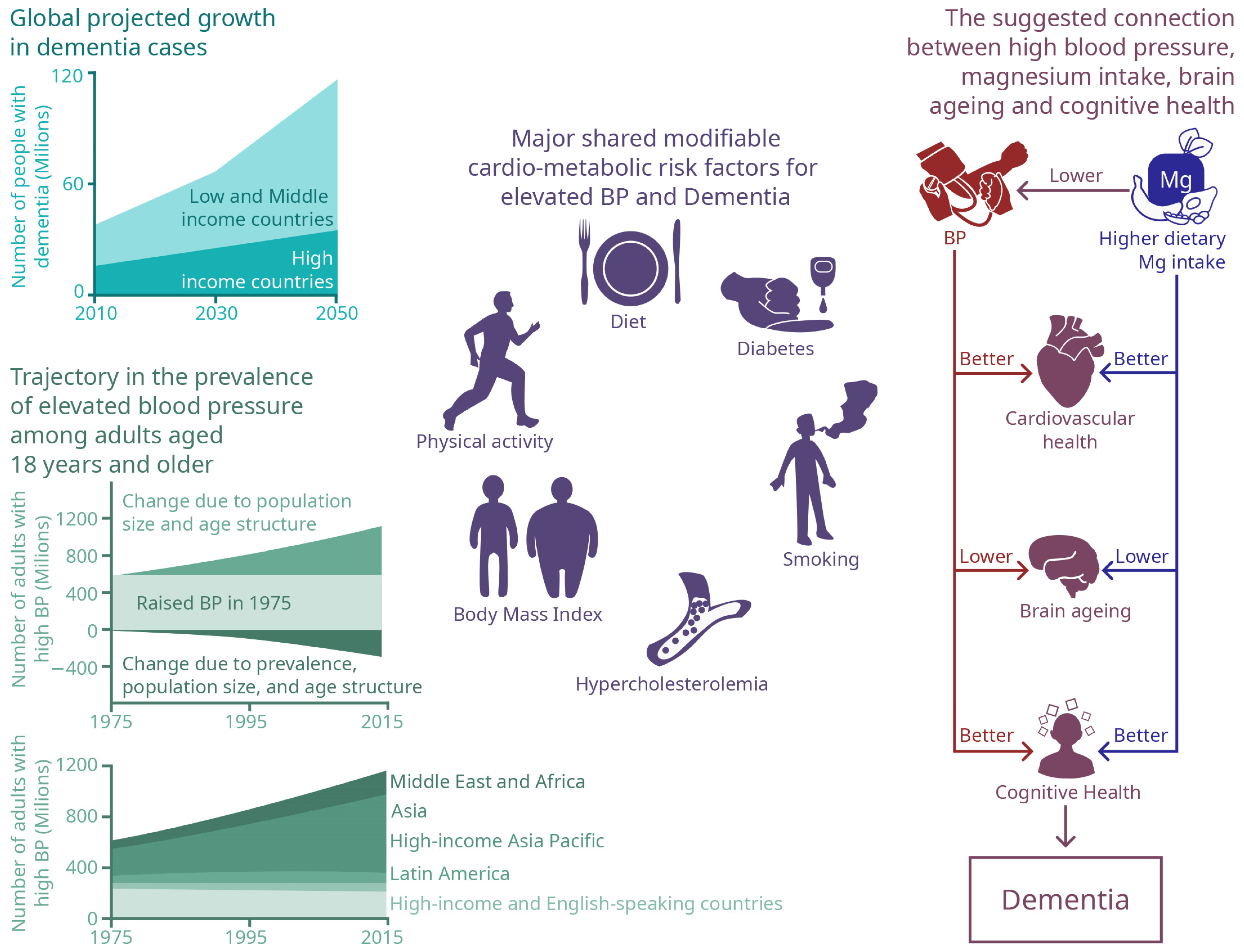

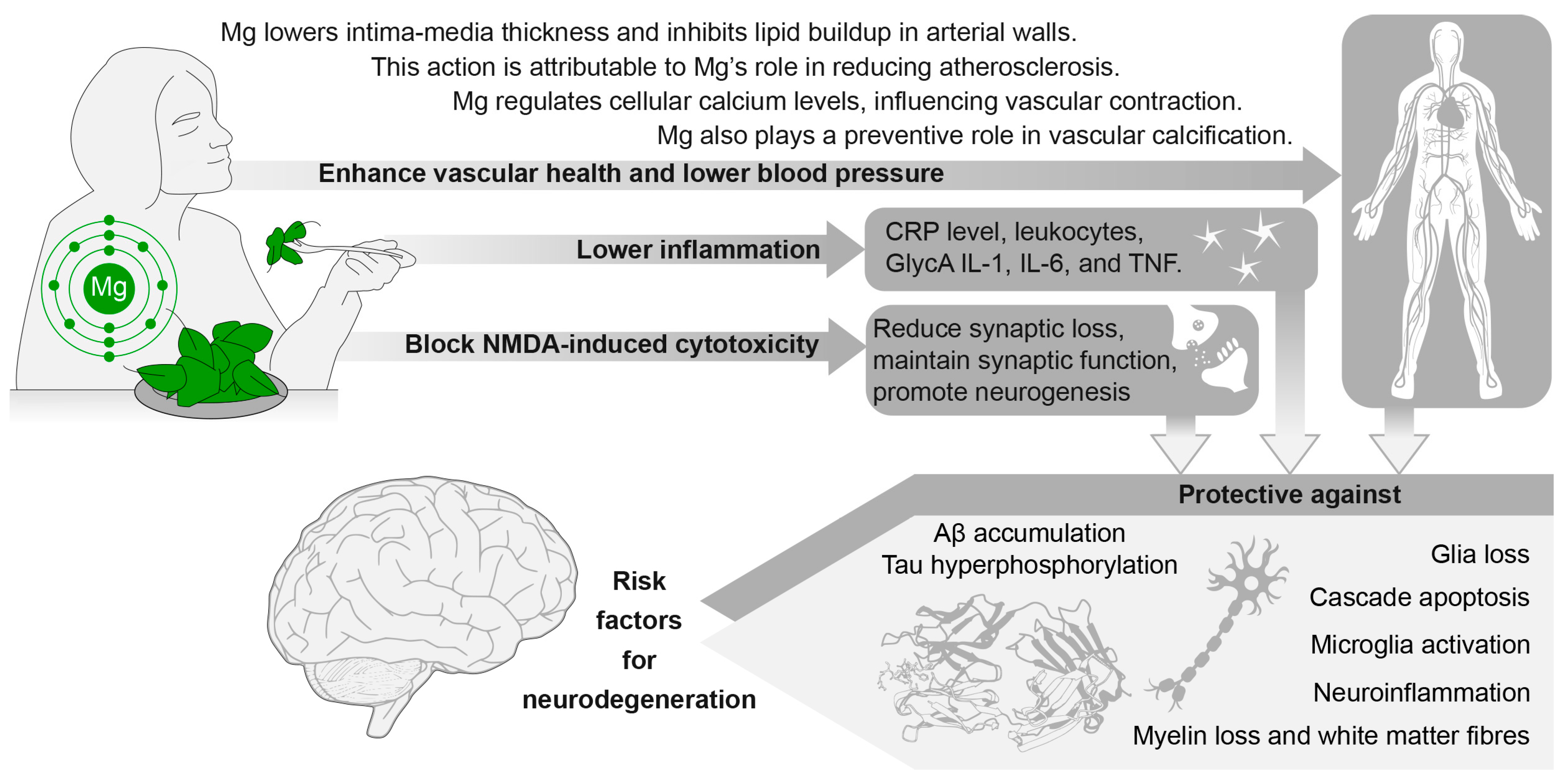
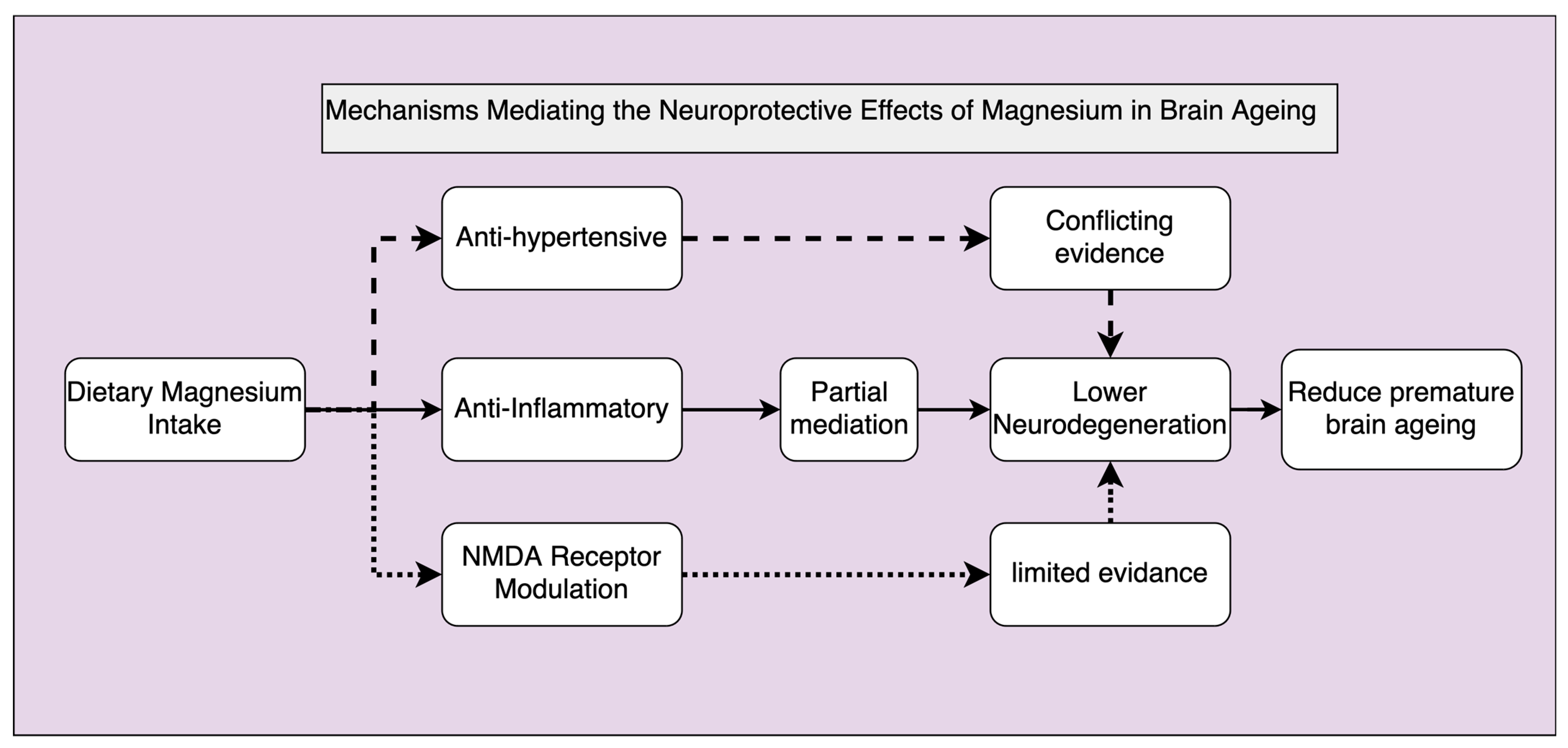
| Domain | Type of Research | Effects of ↑ BP | Effects of ↑ SBP | Effects of ↑ DBP | Effects of ↑ MAP |
|---|---|---|---|---|---|
| Ageing mechanisms | Animal | ↑ Endothelium dysfunction [168,169,170]. | |||
| ↑ Proinflammatory cytokines, including IL-1β [174], TNF-α, IL-6 [175,176], and IFN-γ [177], IL-17 [180]. | |||||
| ↑ Plaque formation and atherosclerosis [186,187] | |||||
| ↑ Calcium deposits and vessel stiffness [188,191,192]. | |||||
| Human | ↑ Endothelial function assessed by ↑ FMD [230]. | ||||
| ↑ Pro-inflammatory cytokines including IL-6 and CRP [173,182,231,232]. | |||||
| ↑ Atherosclerosis [233,234]. | |||||
| ↑ Calcium deposits and vessel stiffness [114]. | ↑ Calcium deposits and vessel stiffness [235]. | ||||
| Neurodegeneration | Animal | ↑ Glial and dendritic loss [18,19,236]. | |||
| ↑ Myelin disruption [38]. | |||||
| ↑ Vascular impairment including ischemia [207,208,209]. | |||||
| ↓ Synaptic density [202]. | |||||
| ↑ Aβ-plaque deposition [204]. ↑ Tauopathy [204]. | |||||
| Human | ↓ Brain volume, hippocampal volume [212,213]. ↑ WML volume [7,214]. | ↓ GM, WM, hippocampal volume [7,212,213,237], and amygdala volume [237]. ↑ WML volume [7,214]. | ↓ GM, WM, hippocampal volume [7,212,213,237] and amygdala [237]. ↑ WML volume [7,214]. | ↓ GM, WM, hippocampal volume [7,237], and amygdala [237]. ↑ WML volume [7]. | |
| Cognitive decline | Animal | ↑ Cognitive decline [238]. | |||
| Human | ↓ Executive function, memory, Motor speed, and attention [9,239]. ↑ Cognitive decline [240,240,241,242]. | ↑ Cognitive decline [242,243]. | |||
| Dementia | Animal | ↑ AD like pathology [244]. | |||
| Human | ↑ Vascular dementia risk [245]. ↑ AD [246]. | ↑ Dementia risk [241,247]. ↑ AD [247]. |
| Domain | Type of Research | Effects of ↑ Mg (Serum) | Effects of ↑ Mg (Dietary) | Effects of ↑ Mg (Brain Levels) |
|---|---|---|---|---|
| Ageing mechanisms | Animal | ↓ Thickness of intima-media layers in blood vessels [258]. | ||
| ↓ Atherosclerosis [311,312]. | ||||
| ↓ Plasma oxLDL | ||||
| ↓ Vascular calcification [313] | ||||
| ↓ BP levels [269,270]. | ↓ BP levels [269]. | |||
| ↓ Expression of proinflammatory cytokines including TNF-a and IL-1B [294]. | ↓ Microglia activation inhibition [314,315]. ↓ Expression of pro-inflammatory cytokines, including TNF-α, IL-1α, IL-1β, and IL-6 [295,314,315]. | |||
| ↓ Blocking of cytotoxic effects of NMDA [283]. | ||||
| Human | ↓ Carotid intima-media thickness [316]. | ↓ Carotid intima-media thickness [160]. | ||
| ↓ Atherosclerotic plaques [317,318]. | ↓ Atherosclerotic plaques [259,261]. | |||
| ↓ LDL levels [319,320,321,322]. | ↓ Serum LDL levels [263]. ↑ HDL levels [263]. | |||
| ↓ Coronary artery calcium [268]. No effect on vascular calcification [323]. | ||||
| No link between serum Mg concentration and reduced risk of hypertension [16]. | ↓ BP (e.g., SBP, DBP) levels [16,17,324]. | |||
| ↓ Chronic inflammation [276]. No link between serum Mg and inflammation markers including CRP and ESR [316]. | ↓ CRP levels [278,279,325] ↓ Blood levels of pro-inflammatory cytokines, including IL-6 and TNF-α [278,325]. | |||
| Neurodegeneration | Animal | ↑ NSC [292]. | ||
| ↓ Synaptic loss [289,298,326]. | ↑ Synaptic plasticity [253] | |||
| ↓ Aβ-plaque deposition [289,293]. | ↓ Aβ-plaque deposition [289,295]. ↓ Tau hyperphosphorylation [253]. | |||
| Human | ↑ Brain volumes including GM, WM, and hippocampal volume [307]. ↓ WMLs [307]. | |||
| Cognitive decline | Animal | ↑ Learning and memory abilities [289,298]. | ↑ Learning and memory abilities [253]. | |
| Human | ↑ Cognitive function [327]. | ↓ Cognitive decline [256]. ↓ Transition to MCI [254,257]. | ||
| Dementia | Animal | ↑ Cognitive function in AD mic [298]. | ||
| Human | ↓ Plasma Mg in AD [300]. No association between plasma Mg concentrations and AD [328]. | ↓ Dementia [254]. ↓ Vascular dementia and AD and all Dementia type [255]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alateeq, K.; Walsh, E.I.; Cherbuin, N. High Blood Pressure and Impaired Brain Health: Investigating the Neuroprotective Potential of Magnesium. Int. J. Mol. Sci. 2024, 25, 11859. https://doi.org/10.3390/ijms252211859
Alateeq K, Walsh EI, Cherbuin N. High Blood Pressure and Impaired Brain Health: Investigating the Neuroprotective Potential of Magnesium. International Journal of Molecular Sciences. 2024; 25(22):11859. https://doi.org/10.3390/ijms252211859
Chicago/Turabian StyleAlateeq, Khawlah, Erin I. Walsh, and Nicolas Cherbuin. 2024. "High Blood Pressure and Impaired Brain Health: Investigating the Neuroprotective Potential of Magnesium" International Journal of Molecular Sciences 25, no. 22: 11859. https://doi.org/10.3390/ijms252211859
APA StyleAlateeq, K., Walsh, E. I., & Cherbuin, N. (2024). High Blood Pressure and Impaired Brain Health: Investigating the Neuroprotective Potential of Magnesium. International Journal of Molecular Sciences, 25(22), 11859. https://doi.org/10.3390/ijms252211859







