The Role of ACE2 in Neurological Disorders: From Underlying Mechanisms to the Neurological Impact of COVID-19
Abstract
1. Introduction
2. Biological Characteristics of ACE2 and Its Association with the Nervous System
2.1. Enzymatic Activity of ACE2
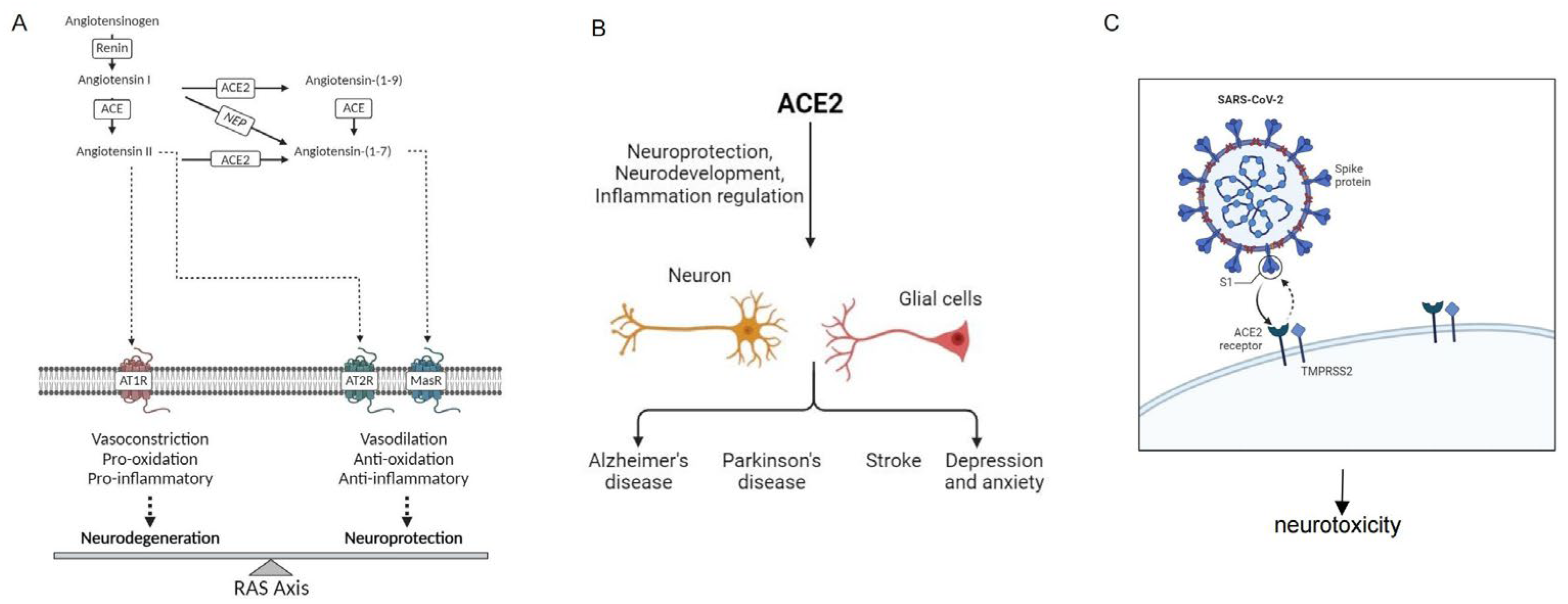
2.2. ACE2 in the Regulation of the RAS System
2.3. ACE2 as the Receptor for SARS-CoV and SARS-CoV-2
3. ACE2 and the Nervous System
3.1. Brain Expression
3.2. ACE2 and Neuroprotection
4. ACE2 and Neurological Disorders
4.1. ACE2 and AD
4.2. ACE2 and Parkinson’s Disease
4.3. ACE2 and Ischemic Stroke
4.4. ACE2 and Depression, Anxiety
5. COVID-19 and ACE2 in Neurological Diseases
6. Discussion
6.1. ACE2 as a Key Regulatory Factor in the Nervous System
6.2. ACE2 and SARS-CoV-2: A Double-Edged Sword
6.3. ACE2 and Neurological Symptoms of Long COVID
6.4. Therapeutic Potential of ACE2 in Neurological Diseases
6.4.1. Modulation of ACE2 Activity
6.4.2. Neurorestorative Therapies
6.5. Future Research Directions
6.5.1. Regulation of ACE2 Expression
6.5.2. In-Depth Study of ACE2 and COVID-19-Related Neurological Symptoms
6.5.3. Potential Therapeutic Role of ACE2 in Neurodegenerative Diseases
6.5.4. The Role of ACE2 in Neurodevelopment and Regeneration
6.5.5. The Relationship between ACE2 and Cerebrovascular Diseases
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernandez-Lopez, J.M.; Hernandez-Medina, C.; Medina-Corvalan, C.; Rodenas, M.; Francisca, A.; Perez-Garcia, C.; Echevarria, D.; Carratala, F.; Geijo-Barrientos, E.; Martinez, S. Neuronal progenitors of the dentate gyrus express the SARS-CoV-2 cell receptor during migration in the developing human hippocampus. Cell Mol. Life Sci. 2023, 80, 140. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Charnley, M.; Islam, S.; Bindra, G.K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M.D.; Han, K.; Berryman, J.T.; et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-CoV-2: Potential implications for neurological symptoms in COVID-19. Nat. Commun. 2022, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Stigliano, E.; Micera, A. COVID-19 and Alzheimer’s Disease Share Common Neurological and Ophthalmological Manifestations: A Bidirectional Risk in the Post-Pandemic Future. Cells 2023, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Harapan, B.N.; Yoo, H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J. Neurol. 2021, 268, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Wang, K.; Viveiros, A.; Kellner, M.J.; Penninger, J.M. Angiotensin-converting enzyme 2-at the heart of the COVID-19 pandemic. Cell 2023, 186, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Zech, F.; Weber, S.; Dietenberger, H.; Zhang, L.; Noettger, S.; Volcic, M.; Bergner, T.; Read, C.; Sparrer, K.M.J.; Barth, T.F.E.; et al. SARS-CoV-2 inhibition and specific targeting of infected cells by VSV particles carrying the ACE2 receptor. Signal Transduct. Target. Ther. 2023, 8, 208. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, 9. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, H.; Bao, X.; Wu, J. Angiotensin-Converting Enzyme 2 Activation Is Not a Common Feature of Angiotensin-Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2023, 71, 8867–8876. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Wysocki, J.; Pandit, J.; Batlle, D. An update on ACE2 amplification and its therapeutic potential. Acta Physiol. 2021, 231, e13513. [Google Scholar] [CrossRef] [PubMed]
- Danser, A.H.J.; Epstein, M.; Batlle, D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension 2020, 75, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Renin-Angiotensin System and Sex Differences in COVID-19: A Critical Assessment. Circ. Res. 2023, 132, 1320–1337. [Google Scholar] [CrossRef]
- Dang, R.; Yang, M.; Cui, C.; Wang, C.; Zhang, W.; Geng, C.; Han, W.; Jiang, P. Activation of angiotensin-converting enzyme 2/angiotensin (1-7)/mas receptor axis triggers autophagy and suppresses microglia proinflammatory polarization via forkhead box class O1 signaling. Aging Cell 2021, 20, e13480. [Google Scholar] [CrossRef]
- Joshi, S.; Chittimalli, K.; Jahan, J.; Vasam, G.; Jarajapu, Y.P. ACE2/ACE imbalance and impaired vasoreparative functions of stem/progenitor cells in aging. Geroscience 2021, 43, 1423–1436. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, F.; Gao, J.; Lei, M.; Wang, D.; Jin, X.; Guo, Y.; Shan, L.; Chen, X. Comprehensive landscape of the renin-angiotensin system in Pan-cancer: A potential downstream mediated mechanism of SARS-CoV-2. Int. J. Biol. Sci. 2021, 17, 3795–3817. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Oskotsky, B.; Butte, A.J.; Hu, Z. Systematic identification of ACE2 expression modulators reveals cardiomyopathy as a risk factor for mortality in COVID-19 patients. Genome Biol. 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.S.; Zetter, M.A.; Guerra, E.C.; Hernández-Araiza, I.; Karuzin, N.; Hernández-Pérez, O.R.; Eiden, L.E.; Zhang, L. ACE2 expression in rat brain: Implications for COVID-19 associated neurological manifestations. Exp. Neurol. 2021, 345, 113837. [Google Scholar] [CrossRef] [PubMed]
- Klempin, F.; Mosienko, V.; Matthes, S.; Villela, D.C.; Todiras, M.; Penninger, J.M.; Bader, M.; Santos, R.A.S.; Alenina, N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell Mol. Life Sci. 2018, 75, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Archie, S.R.; Ghanwatkar, Y.; Sharma, S.; Nozohouri, S.; Burks, E.; Mdzinarishvili, A.; Liu, Z.; Abbruscato, T.J. Potential role of astrocyte angiotensin converting enzyme 2 in the neural transmission of COVID-19 and a neuroinflammatory state induced by smoking and vaping. Fluids Barriers CNS 2022, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Quarleri, J.; Delpino, M.V. SARS-CoV-2 interacts with renin-angiotensin system: Impact on the central nervous system in elderly patients. Geroscience 2022, 44, 547–565. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Kaneko, N.; Satta, S.; Komuro, Y.; Muthukrishnan, S.D.; Kakarla, V.; Guo, L.; An, J.; Elahi, F.; Kornblum, H.I.; Liebeskind, D.S.; et al. Flow-Mediated Susceptibility and Molecular Response of Cerebral Endothelia to SARS-CoV-2 Infection. Stroke 2021, 52, 260–270. [Google Scholar] [CrossRef]
- Ramachandran, A.K.; Das, S.; Joseph, A. Crosstalk Between Covid-19 and Associated Neurological Disorders: A Review. Curr. Neuropharmacol. 2021, 19, 1688–1700. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, L.; Lin, Z.; Long, Q.X.; Yuan, H.; Cai, L.; Jiang, G.; Guo, X.; Yang, R.; Zhang, Z.; et al. APOE interacts with ACE2 inhibiting SARS-CoV-2 cellular entry and inflammation in COVID-19 patients. Signal Transduct. Target. Ther. 2022, 7, 261. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e11. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm. Sin. B 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme—Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.M.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef]
- Hooper, N.M.; Lambert, D.W.; Turner, A.J. Discovery and characterization of ACE2—A 20-year journey of surprises from vasopeptidase to COVID-19. Clin. Sci. 2020, 134, 2489–2501. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed. Pharmacother. 2021, 136, 111193. [Google Scholar] [CrossRef]
- Mughal, A.; O’Rourke, S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol. Ther. 2018, 190, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; McKinnie, S.M.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.A.S.; Taccone, F.S.; Annoni, F. Renin-Angiotensin System Modulation in Adults With COVID-19. JAMA 2023, 330, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Cruz-Lopez, E.O.; Tu, H.C.; Zlatev, I.; Danser, A.H.J. Targeting Angiotensinogen With N-Acetylgalactosamine-Conjugated Small Interfering RNA to Reduce Blood Pressure. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Fragoso, V.; Alexandre-Santos, B.; Salles, A.C.P.; Proença, A.B.; de Paula Alves, A.P.; Vázquez-Carrera, M.; Nóbrega, A.C.L.; Frantz, E.D.C.; Magliano, D.C. Crosstalk between the renin-angiotensin system and the endoplasmic reticulum stress in the cardiovascular system: Lessons learned so far. Life Sci. 2021, 284, 119919. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Martins, A.L.V.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. ACE2 in the renin-angiotensin system. Clin. Sci. 2020, 134, 3063–3078. [Google Scholar] [CrossRef]
- Kuriakose, J.; Montezano, A.C.; Touyz, R.M. ACE2/Ang-(1-7)/Mas1 axis and the vascular system: Vasoprotection to COVID-19-associated vascular disease. Clin. Sci. 2021, 135, 387–407. [Google Scholar] [CrossRef]
- Gross, S.; Jahn, C.; Cushman, S.; Bär, C.; Thum, T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J. Mol. Cell Cardiol. 2020, 144, 47–53. [Google Scholar] [CrossRef]
- El-Arif, G.; Farhat, A.; Khazaal, S.; Annweiler, C.; Kovacic, H.; Wu, Y.; Cao, Z.; Fajloun, Z.; Khattar, Z.A.; Sabatier, J.M. The Renin-Angiotensin System: A Key Role in SARS-CoV-2-Induced COVID-19. Molecules 2021, 26, 6945. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Bai, L.; Dong, J.; Hu, X.; Wang, J.; Paulo, J.A.; Xiong, Y.; Liang, X.; Sun, Y.; Chen, Y.; et al. USP2 inhibition prevents infection with ACE2-dependent coronaviruses in vitro and is protective against SARS-CoV-2 in mice. Sci. Transl. Med. 2023, 15, eadh7668. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, C.; Brady, M.; Deane, R. SARS-CoV-2: Is there neuroinvasion? Fluids Barriers CNS 2021, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Su, S.; Cao, Y.; Ma, C.; Qiu, W. The Altered Anatomical Distribution of ACE2 in the Brain With Alzheimer’s Disease Pathology. Front. Cell Dev. Biol. 2021, 9, 684874. [Google Scholar] [CrossRef]
- Xu, J.X.; Lazartigues, E. Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus. Cell. Mol. Neurobiol. 2022, 42, 305–309. [Google Scholar] [CrossRef]
- Li, T.; Huang, H.Y.; Wang, H.D.; Gao, C.C.; Liang, H.; Deng, C.L.; Zhao, X.; Han, Y.L.; Zhou, M.L. Restoration of Brain Angiotensin-Converting Enzyme 2 Alleviates Neurological Deficits after Severe Traumatic Brain Injury via Mitigation of Pyroptosis and Apoptosis. J. Neurotrauma 2022, 39, 423–434. [Google Scholar] [CrossRef]
- Valenzuela, R.; Rodriguez-Perez, A.I.; Costa-Besada, M.A.; Rivas-Santisteban, R.; Garrido-Gil, P.; Lopez-Lopez, A.; Navarro, G.; Lanciego, J.L.; Franco, R.; Labandeira-Garcia, J.L. An ACE2/Mas-related receptor MrgE axis in dopaminergic neuron mitochondria. Redox Biol. 2021, 46, 102078. [Google Scholar] [CrossRef]
- Reveret, L.; Leclerc, M.; Emond, V.; Tremblay, C.; Loiselle, A.; Bourassa, P.; Bennett, D.A.; Hébert, S.S.; Calon, F. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Montano, M.; Corley, M.J.; Helmy, E.; Kobayashi, H.; Kinisu, M.; Suryawanshi, R.; Luo, X.; Royer, L.A.; Roan, N.R.; et al. Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons. mBio 2022, 13, e0230822. [Google Scholar] [CrossRef]
- Vitale-Cross, L.; Szalayova, I.; Scoggins, A.; Palkovits, M.; Mezey, E. SARS-CoV-2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: Clinical implications. Ebiomedicine 2022, 78, 103981. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.; Goncalves, T.L.; de Araujo, L.P.; Rosario, L.V.O.; Ferrer, V.P. Endothelial cells and SARS-CoV-2: An intimate relationship. Vascul. Pharmacol. 2021, 137, 106829. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, T.T.; Li, J.X.; Xiao, X.; Liu, L.; Li, H.H.; Guo, S.B. ACE2 Rescues Sepsis-Associated Encephalopathy by Reducing Inflammation, Oxidative Stress, and Neuronal Apoptosis via the Nrf2/Sestrin2 Signaling Pathway. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Sun, L.; Zhu, C.; Wei, J. The role of ACE2 in RAS axis on microglia activation in Parkinson’s disease. Neuroscience 2024, 553, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, Z.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Ali, N.H.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. The potential role of brain renin-angiotensin system in the neuropathology of Parkinson disease: Friend, foe or turncoat? J. Cell. Mol. Med. 2024, 28, e18495. [Google Scholar] [CrossRef] [PubMed]
- Achar, A.; Ghosh, C. COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Relevance. Cells 2020, 9, 2360. [Google Scholar] [CrossRef]
- Evans, C.E.; Miners, J.S.; Piva, G.; Willis, C.L.; Heard, D.M.; Kidd, E.J.; Good, M.A.; Kehoe, P.G. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020, 139, 485–502. [Google Scholar] [CrossRef]
- Kamel, A.S.; Abdelkader, N.F.; Abd El-Rahman, S.S.; Emara, M.; Zaki, H.F.; Khattab, M.M. Stimulation of ACE2/ANG(1-7)/Mas Axis by Diminazene Ameliorates Alzheimer’s Disease in the D-Galactose-Ovariectomized Rat Model: Role of PI3K/Akt Pathway. Mol. Neurobiol. 2018, 55, 8188–8202. [Google Scholar] [CrossRef]
- McMahon, C.L.; Hurley, E.M.; Muniz Perez, A.; Estrada, M.; Lodge, D.J.; Hsieh, J. Prenatal SARS-CoV-2 infection results in neurodevelopmental and behavioral outcomes in mice. JCI Insight 2024, 9, e179068. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.A.; Cruz, G.; Syty, M.D.; Wang, F.; Wang, X.; Duan, A.; Halterman, M.; Xiong, Q.; Palop, J.J.; Ge, S. Neural circuit mechanisms underlying aberrantly prolonged functional hyperemia in young Alzheimer’s disease mice. Mol. Psychiatry 2024. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.J.; Ma, X.T.; Wang, W.; Zhao, B.; Chen, Y.; Chen, C.; Bihl, J.C. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J. Cell. Mol. Med. 2018, 22, 1873–1882. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Wong, S.; Al Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimers Res. Ther. 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, R.; Kehoe, P.G.; Miners, J.S. Dysregulation of ACE-1 in Normal Aging and the Early Stages of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xue, X.; Zhang, Q.Q.; Wang, S.Y.; Gong, P.Y.; E, Y.; Jiang, T.; Zhang, Y.D. ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging 2020, 12, 14819–14829. [Google Scholar] [CrossRef]
- Sun, X.; Deng, Y.; Ge, P.; Peng, Q.; Soufiany, I.; Zhu, L.; Duan, R. Diminazene Ameliorates Neuroinflammation by Suppression of Astrocytic miRNA-224-5p/NLRP3 Axis in Alzheimer’s Disease Model. J. Inflamm. Res. 2023, 16, 1639–1652. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, L.; Qin, C. Overexpression of ACE2 ameliorates Aβ-induced blood-brain barrier damage and angiogenesis by inhibiting NF-κB/VEGF/VEGFR2 pathway. Animal Model. Exp. Med. 2023, 6, 237–244. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 2023, 18, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.M.; Pedrosa, M.A.; Quijano, A.; Valenzuela, R.; Garrido-Gil, P.; Sanchez-Andrade, M.; Suarez-Quintanilla, J.A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, H.; Sun, L.; Wei, J. Role of Inflammation in the Development of COVID-19 to Parkinson’s Disease. J. Inflamm. Res. 2024, 17, 3259–3282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tiwari, V.; Tiwari, P.; Parul; Mishra, A.; Hanif, K.; Shukla, S. Angiotensin-Converting Enzyme 2 Activation Mitigates Behavioral Deficits and Neuroinflammatory Burden in 6-OHDA Induced Experimental Models of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 2021, 225, 107848. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Kaushik, M.; Parveen, S.; Tabassum, H.; Parvez, S. Cross-Talk Between Key Players in Patients with COVID-19 and Ischemic Stroke: A Review on Neurobiological Insight of the Pandemic. Mol. Neurobiol. 2020, 57, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ren, X.S.; Zhao, M.X.; Zhou, B.; Han, Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016, 6, 34621. [Google Scholar] [CrossRef]
- Zheng, J.L.; Li, G.Z.; Chen, S.Z.; Wang, J.J.; Olson, J.E.; Xia, H.J.; Lazartigues, E.; Zhu, Y.L.; Chen, Y.F. Angiotensin converting enzyme 2/Ang-(1-7)/mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci. Ther. 2014, 20, 452–459. [Google Scholar] [CrossRef]
- Schwabenland, M.; Salie, H.; Tanevski, J.; Killmer, S.; Lago, M.S.; Schlaak, A.E.; Mayer, L.; Matschke, J.; Püschel, K.; Fitzek, A.; et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 2021, 54, 1594–1610 e1511. [Google Scholar] [CrossRef]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef]
- Bennion, D.M.; Jones, C.H.; Donnangelo, L.L.; Graham, J.T.; Isenberg, J.D.; Dang, A.N.; Rodriguez, V.; Sinisterra, R.D.M.; Sousa, F.B.; Santos, R.A.S.; et al. Neuroprotection by post-stroke administration of an oral formulation of angiotensin-(1-7) in ischaemic stroke. Exp. Physiol. 2018, 103, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; de Kloet, A.D.; Pati, D.; Hiller, H.; Smith, J.A.; Pioquinto, D.J.; Ludin, J.A.; Oh, S.P.; Katovich, M.J.; Frazier, C.J.; et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology 2016, 105, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.A.; de Kloet, A.D.; Smeltzer, M.D.; Cahill, K.M.; Hiller, H.; Bruce, E.B.; Pioquinto, D.J.; Ludin, J.A.; Katovich, M.J.; Raizada, M.K.; et al. Coupling corticotropin-releasing-hormone and angiotensin converting enzyme 2 dampens stress responsiveness in male mice. Neuropharmacology 2018, 133, 85–93. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, A.D.; Cahill, K.M.; Scott, K.A.; Krause, E.G. Overexpression of angiotensin converting enzyme 2 reduces anxiety-like behavior in female mice. Physiol. Behav. 2020, 224, 113002. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.W.; Han, Y.; Nair, M.S.; Harschnitz, O.; Zhu, J.; Wang, P.; Koo, S.Y.; Lacko, L.A.; Chandar, V.; et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Cell Stem Cell 2024, 31, 196–211 e196. [Google Scholar] [CrossRef]
- Li, Z.; Lin, D.; Xu, X.; Liu, X.; Zhang, J.; Huang, K.; Wang, F.; Liu, J.; Zhang, Z.; Tao, E. Central nervous system complications in SARS-CoV-2-infected patients. J. Neurol. 2023, 270, 4617–4631. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef]
- Martínez-Mámol, R.; Giordano-Santini, R.; Kaulich, E.; Cho, A.N.; Przybyla, M.; Riyadh, M.A.; Robinson, E.; Chew, K.Y.; Amor, R.; Meunier, F.A.; et al. SARS-CoV-2 infection and viral fusogens cause neuronal and glial fusion that compromises neuronal activity. Sci. Adv. 2023, 9, 23. [Google Scholar]
- Adesse, D.; Gladulich, L.; Alvarez-Rosa, L.; Siqueira, M.; Marcos, A.C.; Heider, M.; Motta, C.S.; Torices, S.; Toborek, M.; Stipursky, J. Role of aging in Blood-Brain Barrier dysfunction and susceptibility to SARS-CoV-2 infection: Impacts on neurological symptoms of COVID-19. Fluids Barriers Cns 2022, 19, 63. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e955. [Google Scholar] [CrossRef] [PubMed]
- Hersh, E.V.; Wolff, M.; Moore, P.A.; Theken, K.N.; Daniell, H. A Pair of “ACEs”. J. Dent. Res. 2022, 101, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Mur, I.; Pomar, V.; Casademont, J.; de Benito, N. The four horsemen of a viral Apocalypse: The pathogenesis of SARS-CoV-2 infection (COVID-19). EBioMedicine 2020, 58, 102887. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 789180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Wang, C.W.; Chuang, H.C.; Tan, T.H. ACE2 in chronic disease and COVID-19: Gene regulation and post-translational modification. J. Biomed. Sci. 2023, 30, 71. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e907. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Bao, L.L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct. Target. Ther. 2021, 6, 337. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Neuropathophysiology of coronavirus disease 2019: Neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology 2021, 29, 939–963. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2023, 401, e21–e33. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; GuO, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Martin, E.M.; Reuken, P.A.; Scholcz, A.; Ganse-Dumrath, A.; Srowig, A.; Utech, I.; Kozik, V.; Radscheidt, M.; Brodoehl, S.; et al. Long COVID is associated with severe cognitive slowing: A multicentre cross-sectional study. Eclinicalmedicine 2024, 68, 102434. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Azor, A.; Atchison, C.; render, W.; Hellyer, P.J.; Giunchiglia, V.; Husain, M.; Cooke, G.S.; Cooper, E.; Lound, A.; et al. Cognition and Memory after COVID-19 in a Large Community Sample. N. Engl. J. Med. 2024, 390, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The immunology of long, COVID. Nat. Rev. Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salazar, B.; Holwerda, M.; Studle, C.; Piragyte, I.; Mercader, N.; Engelhardt, B.; Rieben, R.; Döring, Y. COVID-19 and the Vasculature: Current Aspects and Long-Term Consequences. Front. Cell Dev. Biol. 2022, 10, 824851. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef]
- Garcia-Escobar, A.; Jimenez-Valero, S.; Galeote, G.; Jurado-Román, A.; García-Rodríguez, J.; Moreno, R. The soluble catalytic ectodomain of ACE2 a biomarker of cardiac remodelling: New insights for heart failure and COVID-19. Heart Fail. Rev. 2021, 26, 961–971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Kong, X.; Liu, T.; Xian, M.; Wei, J. The Role of ACE2 in Neurological Disorders: From Underlying Mechanisms to the Neurological Impact of COVID-19. Int. J. Mol. Sci. 2024, 25, 9960. https://doi.org/10.3390/ijms25189960
Li J, Kong X, Liu T, Xian M, Wei J. The Role of ACE2 in Neurological Disorders: From Underlying Mechanisms to the Neurological Impact of COVID-19. International Journal of Molecular Sciences. 2024; 25(18):9960. https://doi.org/10.3390/ijms25189960
Chicago/Turabian StyleLi, Jingwen, Xiangrui Kong, Tingting Liu, Meiyan Xian, and Jianshe Wei. 2024. "The Role of ACE2 in Neurological Disorders: From Underlying Mechanisms to the Neurological Impact of COVID-19" International Journal of Molecular Sciences 25, no. 18: 9960. https://doi.org/10.3390/ijms25189960
APA StyleLi, J., Kong, X., Liu, T., Xian, M., & Wei, J. (2024). The Role of ACE2 in Neurological Disorders: From Underlying Mechanisms to the Neurological Impact of COVID-19. International Journal of Molecular Sciences, 25(18), 9960. https://doi.org/10.3390/ijms25189960





