Metallomic Approach to Mercury and Selenium in the Liver Tissue of Psectrogaster amazonica and Raphiodon vulpinus from the Brazilian Amazon
Abstract
1. Introduction
2. Results and Discussion
2.1. Mercury and Selenium Determination
2.2. Liver Proteome Fractionation Using 2D PAGE
2.3. Characterization of Hg/Se-Associated Protein Spots Using LC-MS/MS
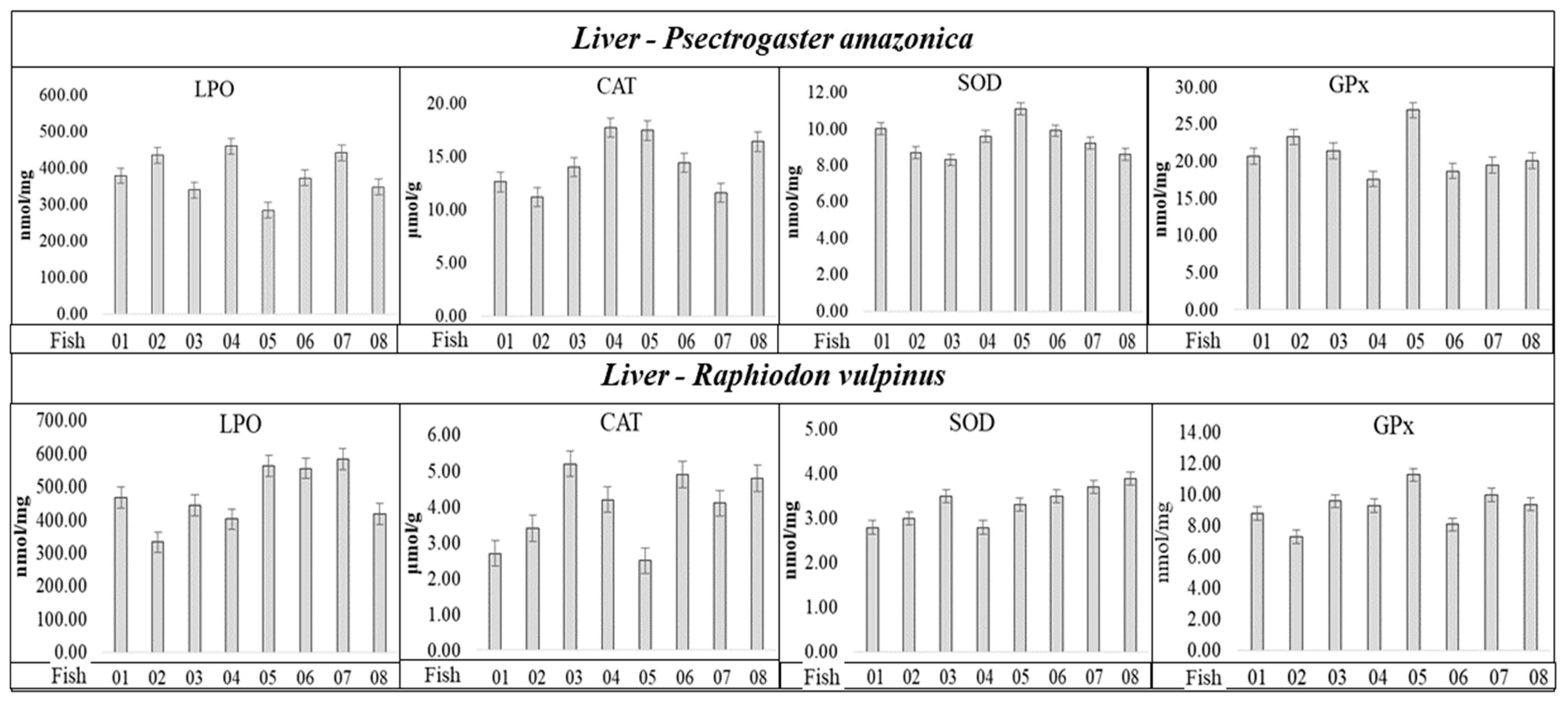
2.4. Oxidative Stress Parameters
3. Material and Methods
3.1. Samples Processing and Collection
3.2. Hg and Se Determinations
3.3. Liver Proteome Fractionation Using 2D PAGE
3.4. Characterization of Hg/Se-Linked Protein Spots Using LC-MS/MS
3.5. Analysis of Parameters Related to Oxidative Stress
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Fadini, P.S.; Jardim, W.F. Is the Negro River Basin (Amazon) impacted by naturally occurring mercury? Sci. Total Environ. 2001, 275, 71–82. [Google Scholar] [CrossRef]
- de Lacerda, L.D.; Malm, O. Contaminação por mercúrio em ecossistemas aquáticos: Uma análise das áreas críticas. Estudos Avançados 2008, 22, 173–190. [Google Scholar] [CrossRef]
- Passos, C.J.S.; Da Silva, D.S.; Lemire, M.; Fillion, M.; Guimarães, J.R.D.; Lucotte, M.; Mergler, D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 76–87. [Google Scholar] [CrossRef]
- Dórea, J.G.; Marques, R.C. Mercury levels and human health in the Amazon Basin. Ann. Hum. Biol. 2016, 43, 349–359. [Google Scholar] [CrossRef]
- Faial, K.; Deus, R.; Deus, S.; Neves, R.; Jesus, I.; Santos, E.; Alves, C.N.; Brasil, D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015, 30, 66–76. [Google Scholar] [CrossRef]
- Cunha Bataglioli, I.d.; Souza Vieira, J.C.; de Queiroz, J.V.; Fernandes, M.d.S.; Bittarello, A.C.; Braga, C.P.; Buzalaf, M.A.R.; Adamec, J.; Zara, L.F.; Padilha, P.d.M. Physiological and functional aspects of metal-binding protein associated with mercury in the liver tissue of pirarucu (Arapaima gigas) from the Brazilian Amazon. Chemosphere 2019, 236, 124320. [Google Scholar] [CrossRef] [PubMed]
- Lemire, M.; Mergler, D.; Fillion, M.; Passos, C.J.S.; Guimarães, J.R.D.; Davidson, R.; Lucotte, M. Elevated blood selenium levels in the Brazilian Amazon. Sci. Total Environ. 2006, 366, 101–111. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Ralston, N.V.C. Selenium and Mercury in Pelagic Fish in the Central North Pacific Near Hawaii. Biol. Trace Elem. Res. 2007, 119, 242–254. [Google Scholar] [CrossRef]
- Yang, D.Y.; Chen, Y.W.; Gunn, J.M.; Belzile, N. Selenium and mercury in organisms: Interactions and mechanisms. Environ. Rev. 2008, 16, 71–92. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Sørmo, E.G.; Ciesielski, T.M.; Øverjordet, I.B.; Lierhagen, S.; Eggen, G.S.; Berg, T.; Jenssen, B.M. Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ. Sci. Technol. 2011, 45, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Siscar, R.; Koenig, S.; Torreblanca, A.; Solé, M. The role of metallothionein and selenium in metal detoxification in the liver of deep-sea fish from the NW Mediterranean Sea. Sci. Total Environ. 2014, 466–467, 898–905. [Google Scholar] [CrossRef]
- Tomza-Marciniak, A.; Pilarczyk, B.; Drozd, R.; Pilarczyk, R.; Juszczak-Czasnojć, M.; Havryliak, V.; Podlasińska, J.; Udała, J. Selenium and mercury concentrations, Se:Hg molar ratios and their effect on the antioxidant system in wild mammals. Environ. Pollut. 2023, 322, 121234. [Google Scholar] [CrossRef]
- Bittarello, A.C.; Vieira, J.C.S.; Braga, C.P.; Bataglioli, I.d.C.; de Oliveira, G.; Rocha, L.C.; Zara, L.F.; Buzalaf, M.A.R.; de Oliveira, L.C.S.; Adamec, J.; et al. Metalloproteomic approach of mercury-binding proteins in liver and kidney tissues of Plagioscion squamosissimus (corvina) and Colossoma macropomum (tambaqui) from Amazon region: Possible identification of mercury contamination biomarkers. Sci. Total Environ. 2020, 711, 134547. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.C.S.; Braga, C.P.; De Queiroz, J.V.; Cavecci-Mendonça, B.; de Oliveira, G.; de Freitas, N.G.; Fernandes, A.A.H.; Fernandes, M.d.S.; Buzalaf, M.A.R.; Adamec, J.; et al. The effects of mercury exposure on Amazonian fishes: An investigation of potential biomarkers. Chemosphere 2023, 316, 137779. [Google Scholar] [CrossRef]
- de Almeida, E.C.; Faria, V.D.; Cirinêu, F.D.; Santiago, M.G.A.; Miotto, B.; Vieira, J.C.S.; Braga, C.P.; Adamec, J.; Fernandes, A.A.H.; Buzalaf, M.A.R.; et al. Metalloproteomic Investigation of Hg-Binding Proteins in Renal Tissue of Rats Exposed to Mercury Chloride. Int. J. Mol. Sci. 2024, 25, 164. [Google Scholar] [CrossRef]
- Mellingen, R.M.; Myrmel, L.S.; Rasinger, J.D.; Lie, K.K.; Bernhard, A.; Madsen, L.; Nøstbakken, O.J. Dietary Selenomethionine Reduce Mercury Tissue Levels and Modulate Methylmercury Induced Proteomic and Transcriptomic Alterations in Hippocampi of Adolescent BALB/c Mice. Int. J. Mol. Sci. 2022, 23, 12242. [Google Scholar] [CrossRef]
- Santiago, M.G.A.; Faria, V.D.; Cirinêu, F.D.; Silva, L.L.d.L.Q.d.; de Almeida, E.C.; Cavallini, N.G.; Vieira, J.C.S.; Fernandes, A.A.H.; Braga, C.P.; Zara, L.F.; et al. Metalloproteomic approach to liver tissue of rats exposed to mercury. Chemosphere 2023, 312, 137222. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3 Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Universal Protein Resource (UniProt). 2024. Available online: http://www.uniprot.org/ (accessed on 28 April 2024).
- Vieira, J.C.S.; Camila, V.; Braga, P.; Oliveira, G.D. Identification of protein biomarkers of mercury toxicity in fish. Env. Chem. Lett. 2017, 15, 717–724. [Google Scholar] [CrossRef]
- Soonhee, K. Participative Management and Job Satisfaction: Lessons for Management Leadership. Public Adm. Rev. 2002, 62, 231. [Google Scholar]
- Vieira, J.C.S.; Cavecci, B.; Queiroz, J.V.; Braga, C.P.; Padilha, C.C.F.; Leite, A.L.; Figueiredo, W.S.; Buzalaf, M.A.R.; Zara, L.F.; Padilha, P.M. Determination of the Mercury Fraction Linked to Protein of Muscle and Liver Tissue of Tucunaré (Cichla spp.) from the Amazon Region of Brazil. Arch. Environ. Contam. Toxicol. 2015, 69, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.J.; Knight, J.; Murray, M.S.; Milliner, D.S.; Holmes, R.P.; Lowther, W.T. 4-Hydroxy-2-oxoglutarate aldolase inactivity in primary hyperoxaluria type 3 and glyoxylate reductase inhibition. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1544–1552. [Google Scholar] [CrossRef]
- Leube, R.E.; Schwarz, N. Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 569–578. [Google Scholar]
- Silva, F.A.; Neves, R.C.F.; Quintero-Pinto, L.G.; Padilha, C.C.; Jorge, S.M.; Barros, M.M.; Pezzato, L.E.; Padilha, P.M. Determination of selenium by GFAAS in slurries of fish feces to estimate the bioavailability of this micronutrient in feed used in pisciculture. Chemosphere 2007, 68, 1542–1547. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Woollard, A.C.S.; Wolff, S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Erden, M.; Bor, N.M. Changes of reduced glutathion, glutathion reductase, and glutathione peroxidase after radiation in guinea pigs. Biochem. Med. 1984, 31, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Crouch, R.K.; Gandy, S.E.; Kimsey, G.; Galbraith, R.A.; Galbraith, G.M.P.; Buse, M.G. The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes 1981, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical a Methods; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]

| P. amazonica/NRC | Hgtotal Concentration (µg kg−1) | Setotal Concentration (µg kg−1) |
|---|---|---|
| 1 | 84.50 ± 2.500 | 126.6 ± 2.500 |
| 2 | 122.4 ± 3.040 | 149.3 ± 2.700 |
| 3 | 81.10 ± 0.9000 | 121.8 ± 2.200 |
| 4 | 123.5 ± 1.700 | 152.1 ± 2.800 |
| 5 | 120.9 ± 2.830 | 147.5 ± 2.600 |
| 6 | 86.70 ± 3.250 | 128.1 ± 2.300 |
| 7 | 87.80 ± 4.24 | 129.4 ± 2.400 |
| 8 | 125.5 ± 2.620 | 153.7 ± 2.800 |
| DOLT-4 | 2.530 ± 0.04430 * | 8.110 ± 0.1403 * |
| R. vulpinus/NRC | Hgtotal Concentration (µg kg−1) | Setotal Concentration (µg kg−1) |
|---|---|---|
| 1 | 143.2 ± 2.600 | 244.3 ± 4.400 |
| 2 | 586.6 ± 10.70 | 726.6 ± 12.80 |
| 3 | 136.4 ± 2.200 | 231.2 ± 3.900 |
| 4 | 146.5 ± 2.700 | 249.1 ± 4.600 |
| 5 | 555.6 ± 9.900 | 666.8 ± 11.30 |
| 6 | 645.9 ± 11.60 | 775.1 ± 13.20 |
| 7 | 156.8 ± 2.800 | 265.2 ± 4.700 |
| 8 | 528.3 ± 9.400 | 644.2 ± 10.90 |
| DOLT-4 | 2.519 ± 0.04836 * | 8.122 ± 0.1535 * |
| Groups Identification | Hgtotal Concentration (µg kg−1) | Setotal Concentration (µg kg−1) |
|---|---|---|
| Pellet-Pa-HgSe 1 | 81.44 ± 1.341 | 121.6 ± 2.100 |
| Pellet-Pa-HgSe 2 | 118.2 ± 1.824 | 148.5 ± 2.314 |
| Pellet-Rv-HgSe 1 | 137.8 ± 2.243 | 237.6 ± 3.971 |
| Pellet-Rv-HgSe 2 | 564.6 ± 8.522 | 679.4 ± 9.342 |
| DOLT-4 | 2.514 ± 0.04091 * | 8.115 ± 0.1393 * |
| ID. Spots | Protein Accession | Protein Description | Protein Score | CHg (mg kg−1) | CSe (mg kg−1) |
|---|---|---|---|---|---|
| P. amazonica | |||||
| 59 | P02140 P02018 | Hemoglobin subunit beta Hemoglobin subunit alpha | 2527.965 188.4514 | 9.700 ± 0.1400 | 16.15 ± 0.2100 |
| 60 | P02017 P02140 P80856 | Hemoglobin subunit alpha Hemoglobin subunit beta Fatty acid-binding protein_ liver | 2305.963 1368.896 1131.812 | 17.24 ± 0.2300 | 33.41 ± 0.3900 |
| 61 | P80856 Q9I8L5 | Fatty acid-binding protein_ liver Fatty acid-binding protein 10-A_ liver basic | 1522.318 1214.747 | 19.72 ± 0.2500 | 35.44 ± 0.4300 |
| 69 | P83751 | Actin_ cytoplasmic 1 | 3731.136 | 14.20 ± 0.1900 | 25.32 ± 0.3000 |
| R. vulpinus | |||||
| 1 | O73872 | Superoxide dismutase [Cu-Zn] | 615.6831 | 22.11 ± 0.3100 | 34.82 ± 0.4600 |
| 4 | Q90XG0 | Triosephosphate isomerase B | 1373.102 | 24.20 ± 0.3600 | 39.20 ± 0.5100 |
| 6 | Q90XG0 | Triosephosphate isomerase B | 4885.841 | 23.12 ± 0.3300 | 37.60 ± 0.4900 |
| 8 | Q6NY77 | 4-hydroxy-2-oxoglutarate aldolase_ mitochondrial | 126.5413 | 21.43 ± 0.2800 | 33.73 ± 0.4300 |
| 10 | P83751 P49055 | Actin_ cytoplasmic 1 Actin_ alpha skeletal muscle | 8121.286 3804.837 | 28.44 ± 0.3100 | 43.12 ± 0.5100 |
| 17 | Q9PTY0 | ATP synthase subunit beta_ mitochondrial | 128.7956 | 17.32 ± 0.2300 | 31.25 ± 0.4100 |
| 26 | P18520 Q6NWF6 | Intermediate filament protein ON3 Keratin_ type II cytoskeletal 8 | 144.8645 144.8645 | 14.35 ± 0.2100 | 26.82 ± 0.3100 |
| Variables | LPO Sp.CC | CAT Sp.CC | SOD Sp.CC | GPx Sp.CC |
|---|---|---|---|---|
| P. amazonica | - | - | - | - |
| Group with <CHgtotal | 0.8425 * | −0.4597 NS | 0.6156 * | −0.8815 * |
| Group with >CHgtotal | 0.2697 NS | 0.1018 NS | −0.7555 * | −0.7767 * |
| Group with <CSetotal | 0.8427 * | −0.5095 NS | 0.6962 * | −0.8348 * |
| Group with >CSetotal | 0.3634 NS | 0.2220 NS | −0.6800 * | −0.8749 * |
| R. vulpinus | − | − | − | − |
| Group with <CHgtotal | 0.7342 * | −0.2536 NS | 0.2877 NS | 0.4822 NS |
| Group with >CHgtotal | 0.3017 NS | 0.2864 NS | −0.3387 NS | −0.6000 * |
| Group with <CSetotal | 0.7135 * | −0.3071 NS | 0.2328 NS | 0.4282 NS |
| Group with >CSetotal | 0.1301 NS | 0.2822 NS | −0.4178 NS | −0.6826 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataglioli, I.; Vieira, J.; Siva, J.d.; Andrade, L.; Faria, V.; Corcoba, R.; Almeida, R.d.; Zara, L.; Buzalaf, M.; Adamec, J.; et al. Metallomic Approach to Mercury and Selenium in the Liver Tissue of Psectrogaster amazonica and Raphiodon vulpinus from the Brazilian Amazon. Int. J. Mol. Sci. 2024, 25, 11946. https://doi.org/10.3390/ijms252211946
Bataglioli I, Vieira J, Siva Jd, Andrade L, Faria V, Corcoba R, Almeida Rd, Zara L, Buzalaf M, Adamec J, et al. Metallomic Approach to Mercury and Selenium in the Liver Tissue of Psectrogaster amazonica and Raphiodon vulpinus from the Brazilian Amazon. International Journal of Molecular Sciences. 2024; 25(22):11946. https://doi.org/10.3390/ijms252211946
Chicago/Turabian StyleBataglioli, Izabela, José Vieira, Joyce da Siva, Luane Andrade, Victor Faria, Rebeca Corcoba, Ronaldo de Almeida, Luiz Zara, Marília Buzalaf, Jiri Adamec, and et al. 2024. "Metallomic Approach to Mercury and Selenium in the Liver Tissue of Psectrogaster amazonica and Raphiodon vulpinus from the Brazilian Amazon" International Journal of Molecular Sciences 25, no. 22: 11946. https://doi.org/10.3390/ijms252211946
APA StyleBataglioli, I., Vieira, J., Siva, J. d., Andrade, L., Faria, V., Corcoba, R., Almeida, R. d., Zara, L., Buzalaf, M., Adamec, J., & Padilha, P. (2024). Metallomic Approach to Mercury and Selenium in the Liver Tissue of Psectrogaster amazonica and Raphiodon vulpinus from the Brazilian Amazon. International Journal of Molecular Sciences, 25(22), 11946. https://doi.org/10.3390/ijms252211946






