Metabolite Analysis of Camellia oleifera Fruit Pericarp Using UPLC-MS/MS: A Comparative Study of Three Oil Tea Varieties
Abstract
1. Introduction
2. Results
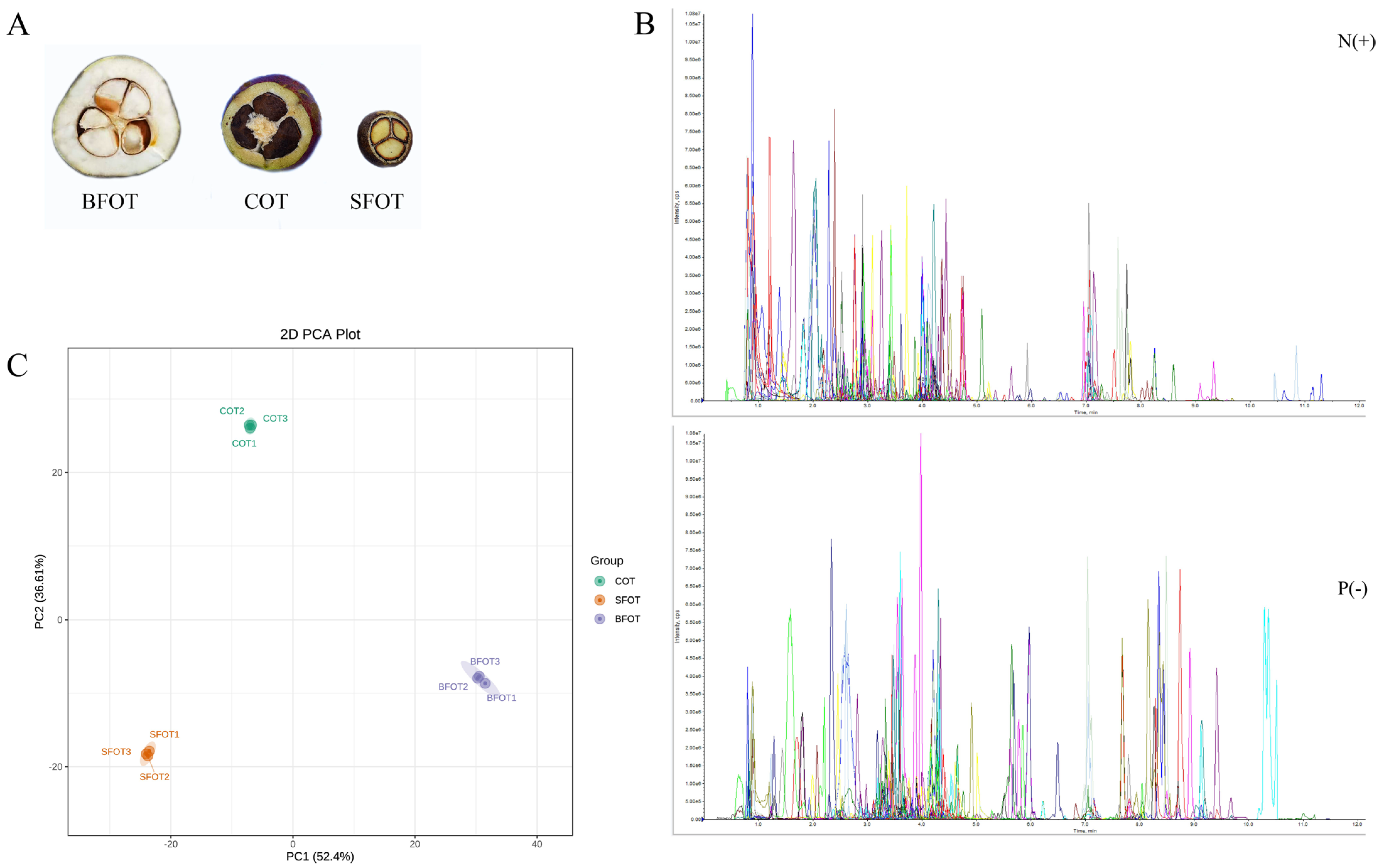
2.1. Quality Control Analysis
2.2. Partial Least-Squares Discriminant Analysis (PLS-DA)
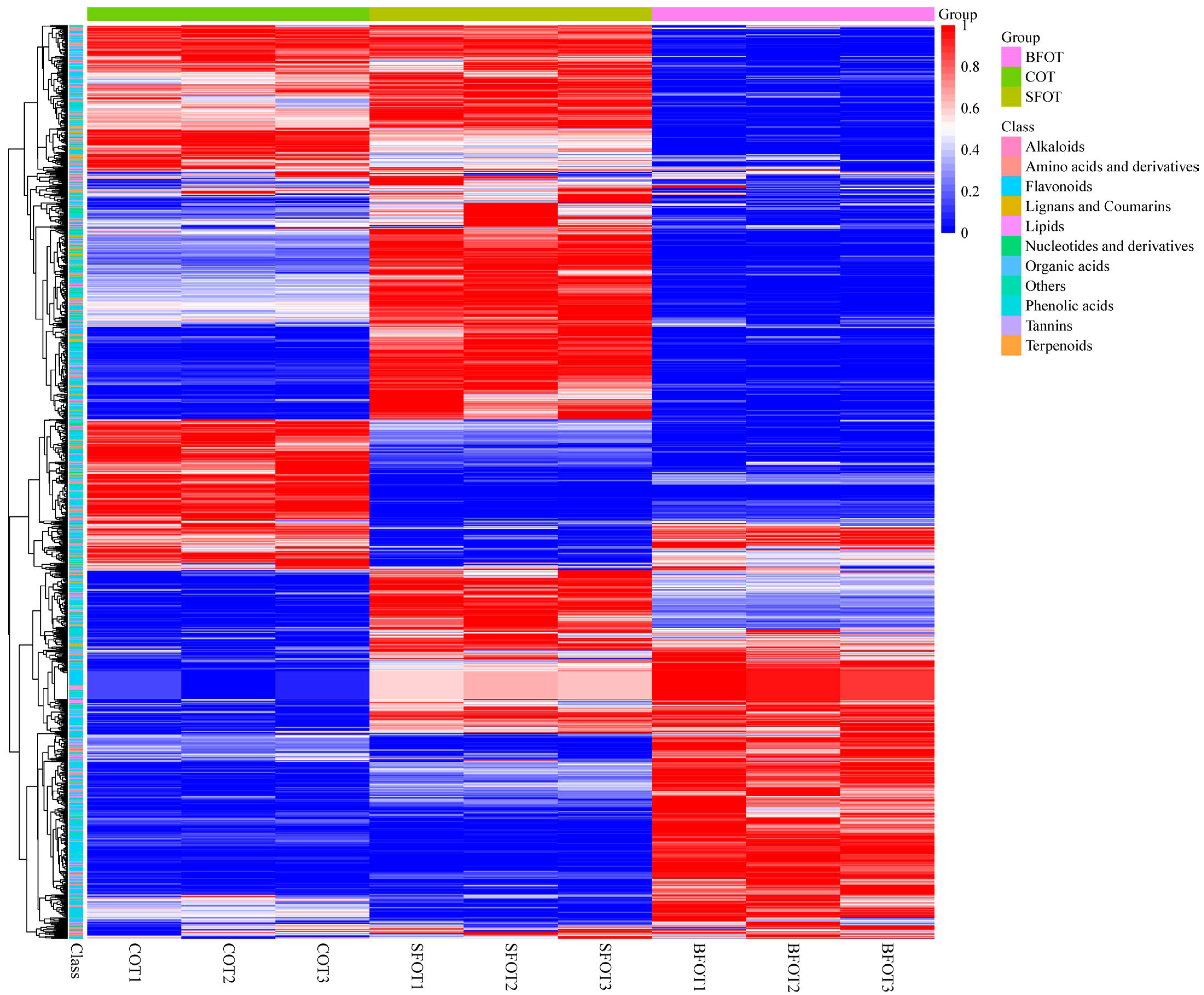
2.3. Differential Metabolite Screening
2.4. KEGG Annotation and Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Data Analysis
4.2. Plant Materials
4.3. Sample Preaparation and Extraction
4.4. UPLC-MS/MS Conditions
4.5. ESI-Q TRAP-MS/MS
4.6. Metabolite Identification and Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Chen, R.; Yang, Y.; Liang, G.; Zhang, H.; Deng, X.; Xi, R. Sugar Metabolism and Transcriptome Analysis Reveal Key Sugar Transporters during Camellia oleifera Fruit Development. Int. J. Mol. Sci. 2022, 23, 822. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, G.; Zhang, H.; Liu, J. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Cheng, Y.; Wu, S.; Ho, C. Beneficial Effects of Camellia Oil (Camellia oleifera Abel.) on Hepatoprotective and Gastroprotective Activities. J. Agric. Food Chem. 2015, 62, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2020, 75, 104242–104271. [Google Scholar]
- Li, Y.; Liao, B.; Wang, Y.; Luo, H.; Wang, S.; Li, C.; Song, W.; Zhang, K.; Yang, B.; Lu, S.; et al. Transcriptome and metabolome analyses provide insights into the relevance of pericarp thickness variations in Camellia drupifera and Camellia oleifera. Front. Plant Sci. 2022, 13, 1016475. [Google Scholar] [CrossRef]
- Tan, M.; Luo, L.; Wu, Z.; Huang, Z.; Zhang, J.; Huang, J.; Yang, Y.; Zhang, X.; Li, H. Pelletization of Camellia oleifera Abel. shell after storage: Energy consumption and pellet properties. Fuel Process. Technol. 2020, 201, 106337. [Google Scholar] [CrossRef]
- Fan, Y. Study on the Decarboxylation of Fatty Acids in Vegetable Oil by Biochar-Based Photocatalytic Materials of Camellia shell. Master’s Thesis, Hunan University, Changsha, China, 2022. [Google Scholar]
- Ma, L. The comprehensive utilization of Camellia oleifera seed. China Food Addit. 2007, 7, 126–129. [Google Scholar]
- Xu, H.; Kuai, Y.; Zhan, Z.; Dong, J. Henolic Contents and Antioxidant Activity of Fruit Peels and Changes in Phenolic Composition during in Vitro Simulated Digestion. Food Sci. 2019, 40, 23–30. [Google Scholar]
- Frenich, A.G.; Vidal, J.L.M.; Romero-González, R.; Aguilera-Luiz, M.d.M. Simple and high-throughput method for the multimycotoxin analysis in cereals and related foods by ultra-high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2009, 117, 705–712. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Lv, J.; Chne, Y. Determination of 13 halobenzoquinone disinfection by-products in drinking water using solid phase extraction-ultra performance liquid chromatography-triple quadrupole mass spectrometry. Chin. J. Chromatogr. 2023, 41, 482–489. [Google Scholar] [CrossRef]
- Farag, M.A.; Shakour, Z.T.A. Metabolomics driven analysis of 11 Portulaca leaf taxa as analysed via UPLC-ESI-MS/MS and chemometrics. Phytochemistry 2019, 161, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chen, C.; Yang, L.; Wang, Y.; Duan, A.; Wang, D. Analysis of metabolites in young and mature Docynia delavayi (Franch.) Schneid leaves using UPLC-ESI-MS/MS. PeerJ 2022, 10, e12844. [Google Scholar] [CrossRef] [PubMed]
- Abdel Ghani, A.E.; Al-Saleem, M.S.M.; Abdel-Mageed, W.M.; AbouZeid, E.M.; Mahmoud, M.Y.; Abdallah, R.H. UPLC-ESI-MS/MS Profiling and Cytotoxic, Antioxidant, Anti-Inflammatory, Antidiabetic, and Antiobesity Activities of the Non-Polar Fractions of Salvia hispanica L. Aerial Parts. Plants 2023, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC-MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Vijayanand, P. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Sci. Technol. 2010, 43, 1026–1031. [Google Scholar] [CrossRef]
- De Araújo Esteves Duarte, I.; Milenkovic, D.; Borges, T.K.; de Lacerda de Oliveira, L.; Costa, A.M. Brazilian passion fruit as a new healthy food: From its composition to health properties and mechanisms of action. Food Funct. 2021, 12, 11106–11120. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Mokhtari, I.; Mokhtari, C.; Moumou, M.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Hakmaoui, A.; Harnafi, H. Polyphenol-rich extract from loquat fruit peel prevents hyperlipidemia and hepato-nephrotoxicity in mice: In vivo study and in silico prediction of possible mechanisms involving identified polyphenols and/or their circulating metabolites. Food Funct. 2023, 14, 7489–7505. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules 2011, 16, 10104–10122. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Wang, W.; Zhang, G. Leaf metabolites profiling between red and green phenotypes of Suaeda salsa by widely targeted metabolomics. Funct. Plant Biol. FPB 2019, 46, 845–856. [Google Scholar] [CrossRef]
- Rose, J.K.; Lee, S.J. Straying off the highway: Trafficking of secreted plant proteins and complexity in the plant cell wall proteome. Plant Physiol. 2010, 153, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ren, R.; Wei, Y.; Jin, J.; Ahmad, S.; Lu, C.; Wu, J.; Zheng, C.; Yang, F.; Zhu, G. Comparative Metabolomic Analysis Reveals Distinct Flavonoid Biosynthesis Regulation for Leaf Color Development of Cymbidium sinense ‘Red Sun’. Int. J. Mol. Sci. 2020, 21, 1869. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Song, X.; Zhao, X.K.; Wei, M.X.; Gao, S.G.; Zhou, F.Y.; Han, X.N.; Xu, R.H.; Wang, R.; Fan, Z.M.; et al. Serum Metabolomic Profiling Reveals Biomarkers for Early Detection and Prognosis of Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 790933. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Shen, X.; Liu, J.; Zhao, D.; Sun, Y.; Wang, L.; Liu, Y.; Gong, X.; Liu, Y.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]



| Metabolite Class I | Quantity | Percentage |
|---|---|---|
| Flavonoids | 277 | 24.80% |
| Phenolic acids | 221 | 19.79% |
| Lipids | 108 | 9.67% |
| Others | 100 | 8.95% |
| Amino acids and their derivatives | 93 | 8.33% |
| Organic acids | 83 | 7.43% |
| Nucleotides and their derivatives | 59 | 5.28% |
| Alkaloids | 57 | 5.10% |
| Lignans and coumarins | 52 | 4.66% |
| Tannins | 44 | 3.94% |
| Terpenoids | 23 | 2.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xu, J.; Qu, S.; Jiang, X.; Wang, G. Metabolite Analysis of Camellia oleifera Fruit Pericarp Using UPLC-MS/MS: A Comparative Study of Three Oil Tea Varieties. Int. J. Mol. Sci. 2024, 25, 11973. https://doi.org/10.3390/ijms252211973
Chen S, Xu J, Qu S, Jiang X, Wang G. Metabolite Analysis of Camellia oleifera Fruit Pericarp Using UPLC-MS/MS: A Comparative Study of Three Oil Tea Varieties. International Journal of Molecular Sciences. 2024; 25(22):11973. https://doi.org/10.3390/ijms252211973
Chicago/Turabian StyleChen, Shengqun, Jiajuan Xu, Shuang Qu, Xia Jiang, and Gang Wang. 2024. "Metabolite Analysis of Camellia oleifera Fruit Pericarp Using UPLC-MS/MS: A Comparative Study of Three Oil Tea Varieties" International Journal of Molecular Sciences 25, no. 22: 11973. https://doi.org/10.3390/ijms252211973
APA StyleChen, S., Xu, J., Qu, S., Jiang, X., & Wang, G. (2024). Metabolite Analysis of Camellia oleifera Fruit Pericarp Using UPLC-MS/MS: A Comparative Study of Three Oil Tea Varieties. International Journal of Molecular Sciences, 25(22), 11973. https://doi.org/10.3390/ijms252211973





