A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome
Abstract
1. Introduction
2. Results
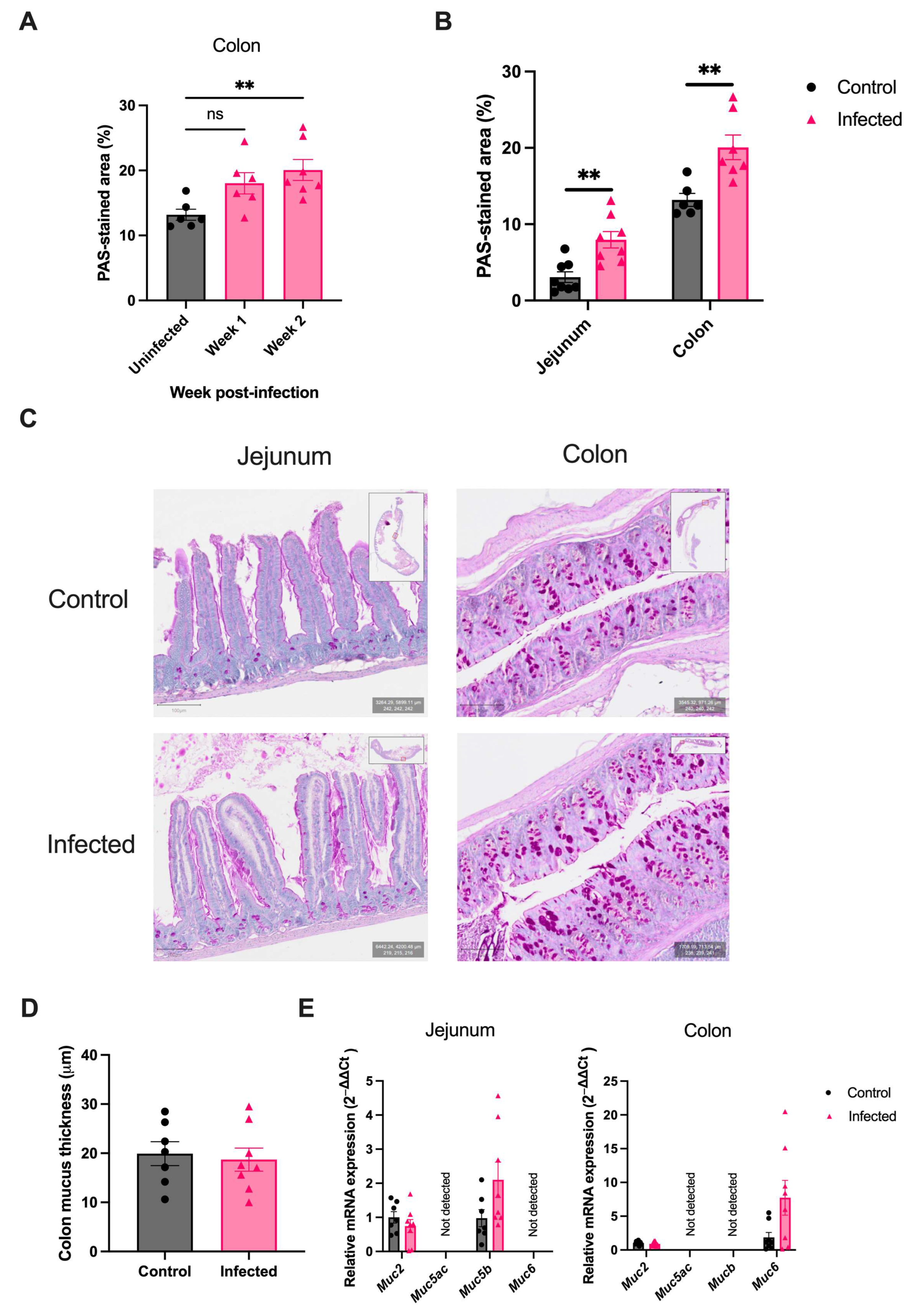
2.1. H. polygyrus Induces Colonic Goblet Cell Hyperplasia
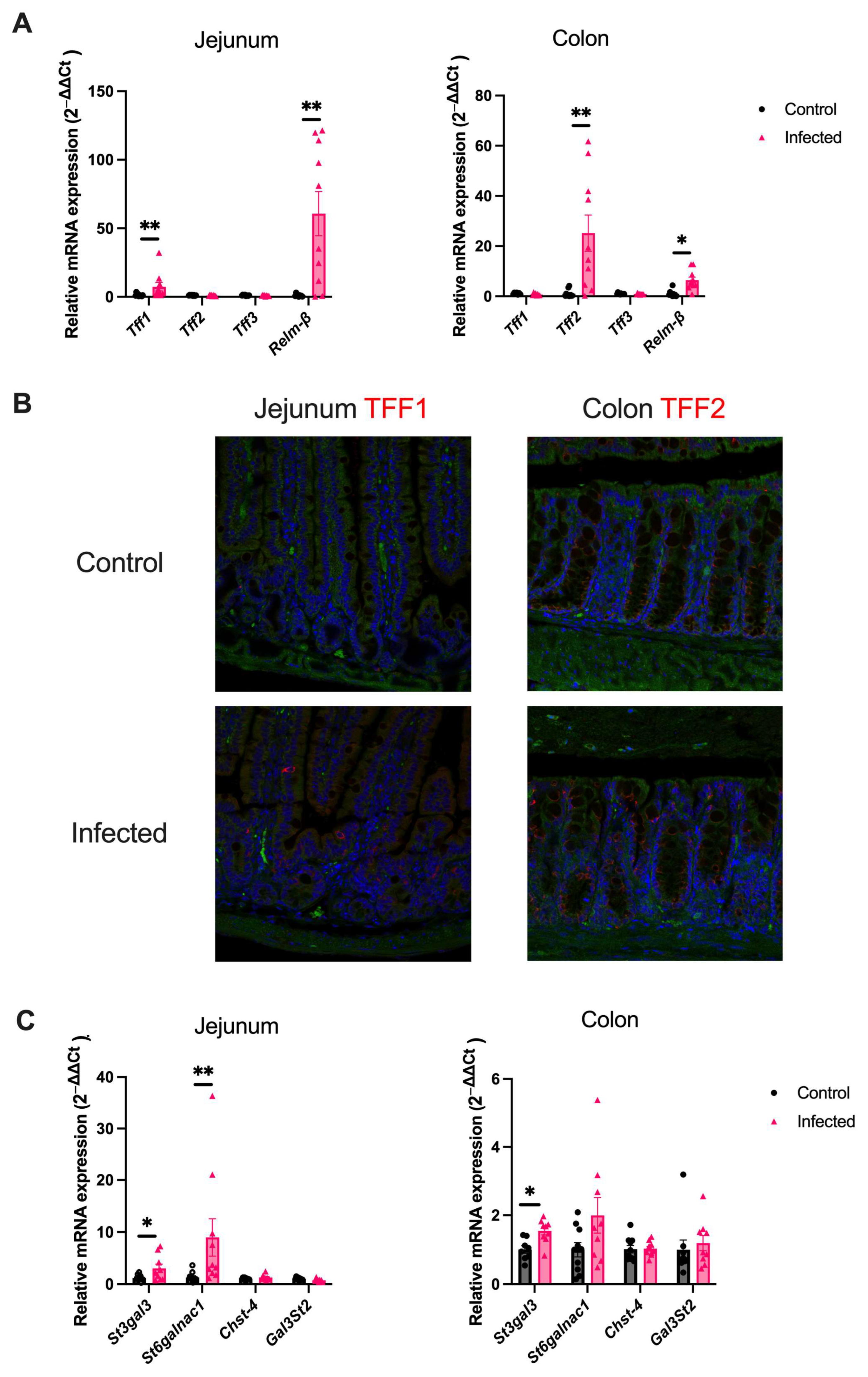
2.2. H. polygyrus Upregulates Goblet-Cell Derived Proteins and Mucin Sialylation Throughout the Intestine
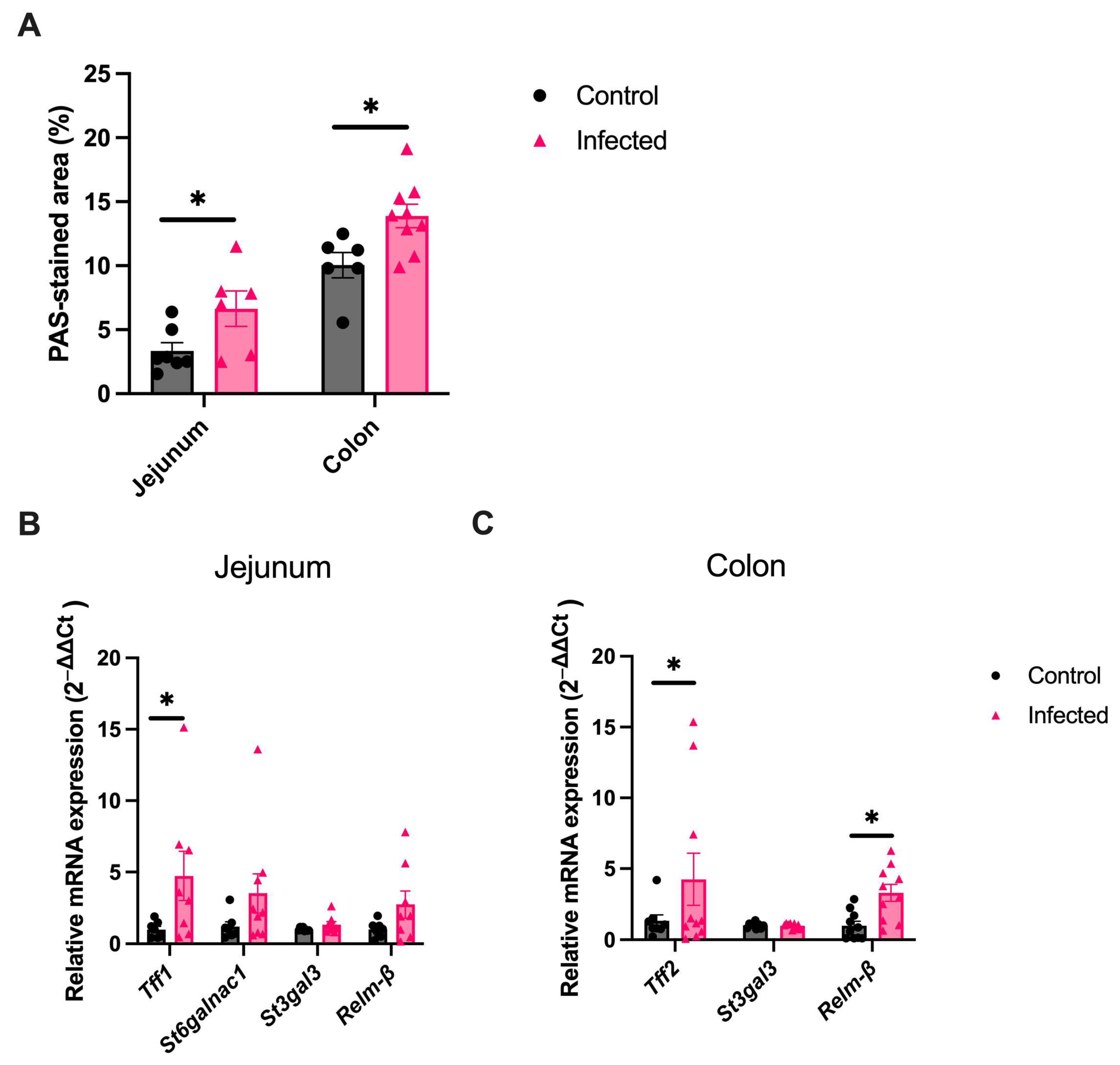
2.3. Goblet Cell Changes Are Sustained into the Chronic Infection Phase
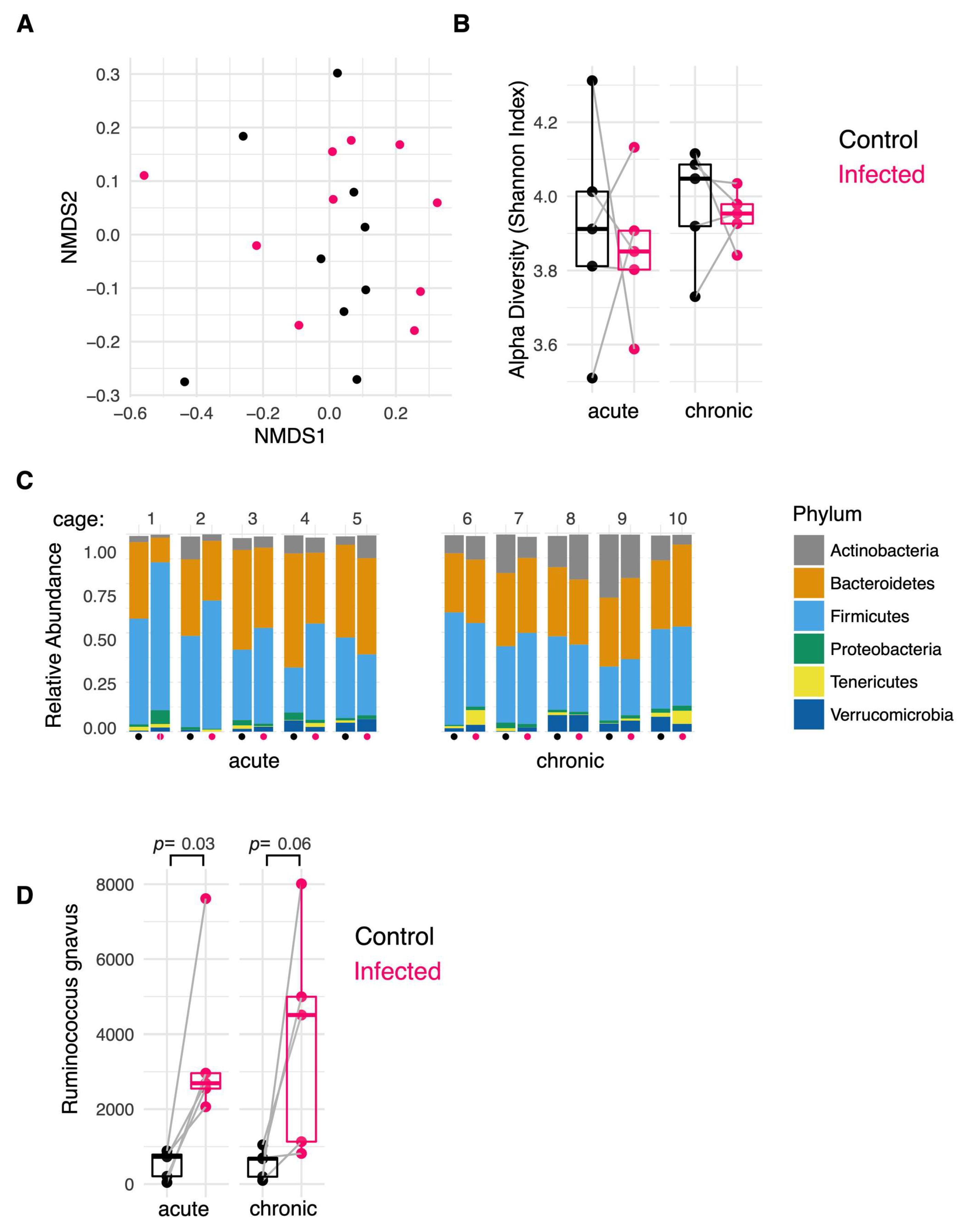
2.4. H. polygyrus Modifies the Colonic Mucosa-Associated Microbiome
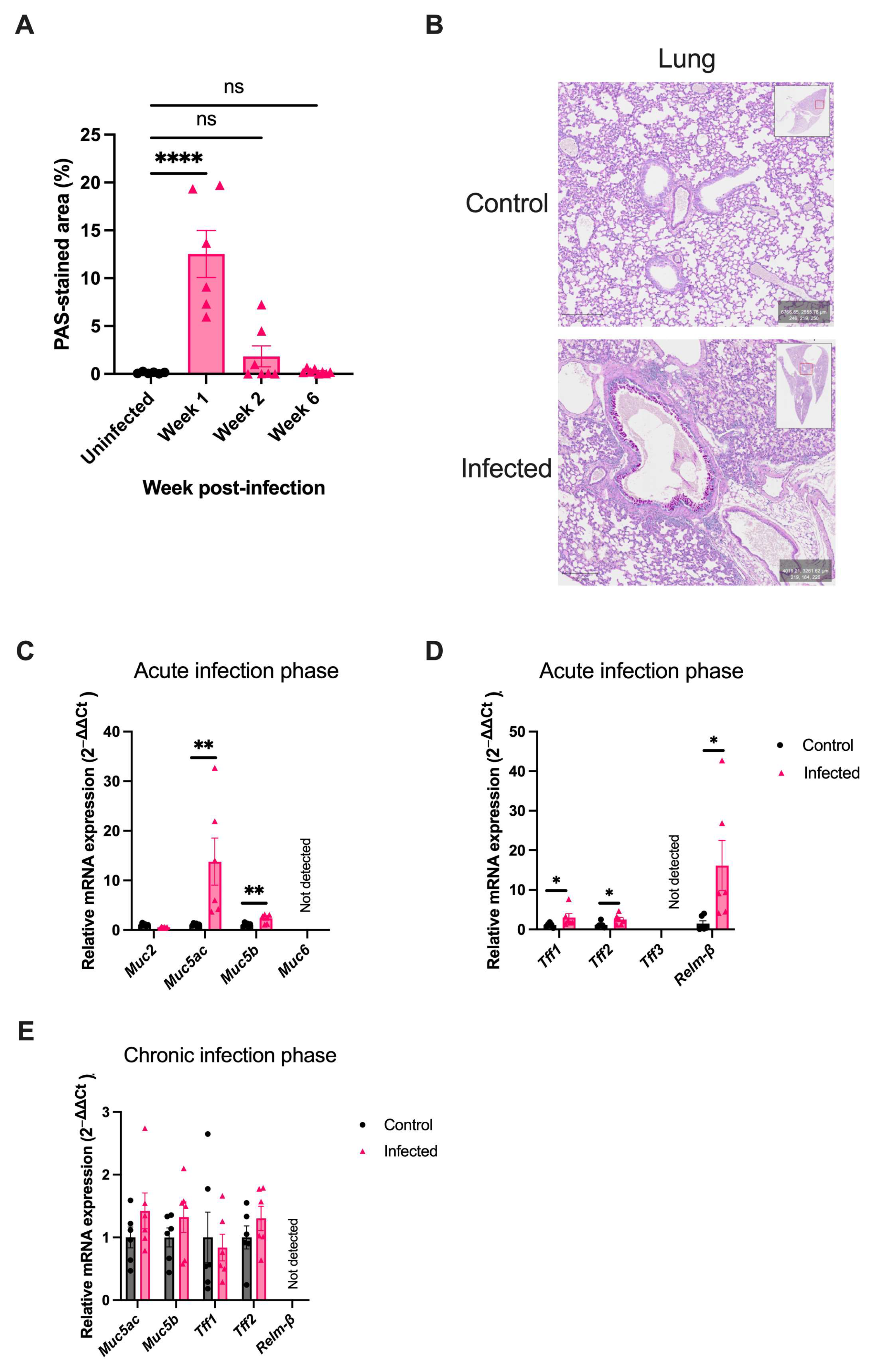
2.5. H. polygyrus Transiently Alters Mucus Composition in the Lung
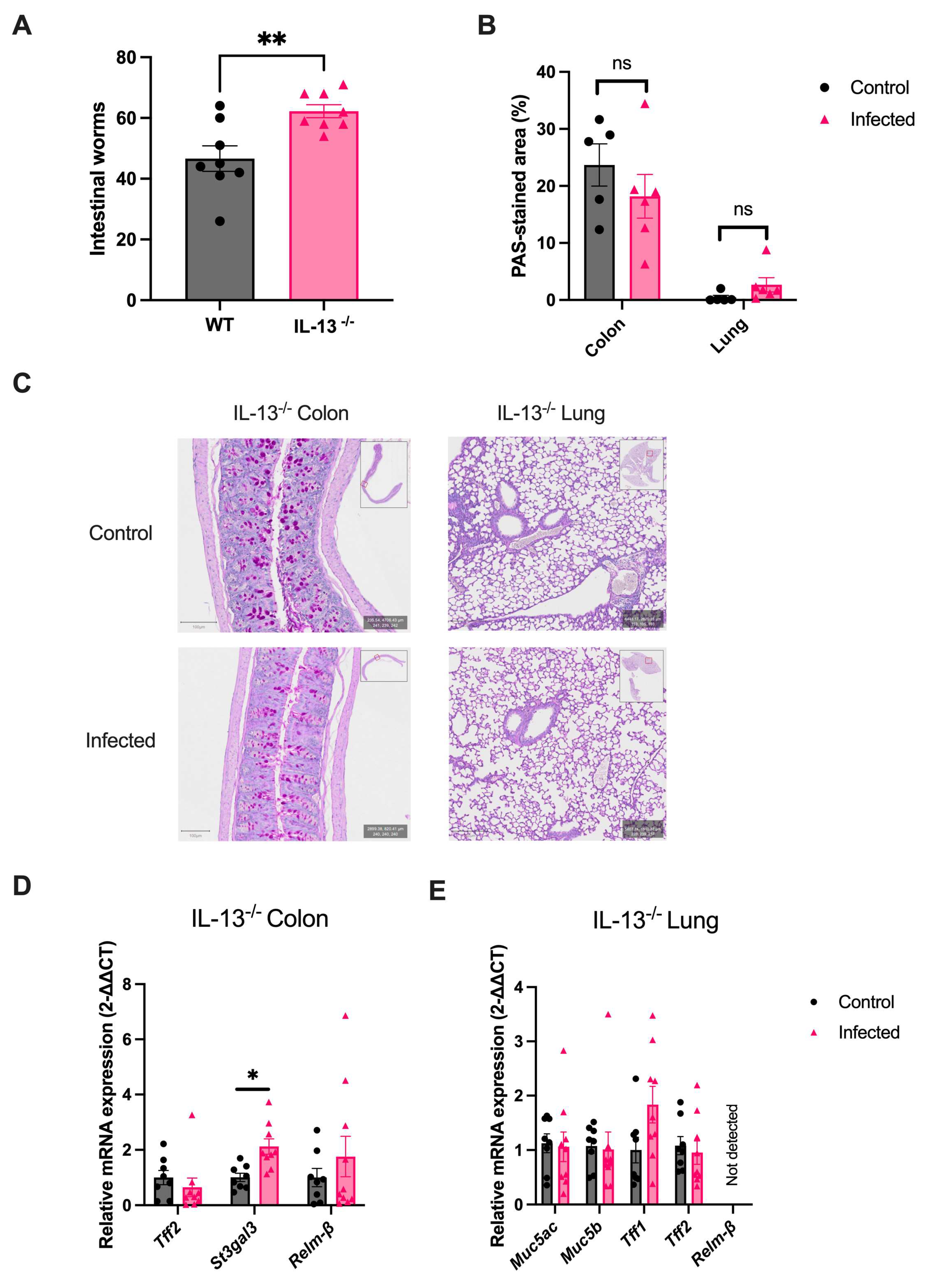
2.6. IL-13 Orchestrates the Helminth-Mediated Systemic Mucus Response
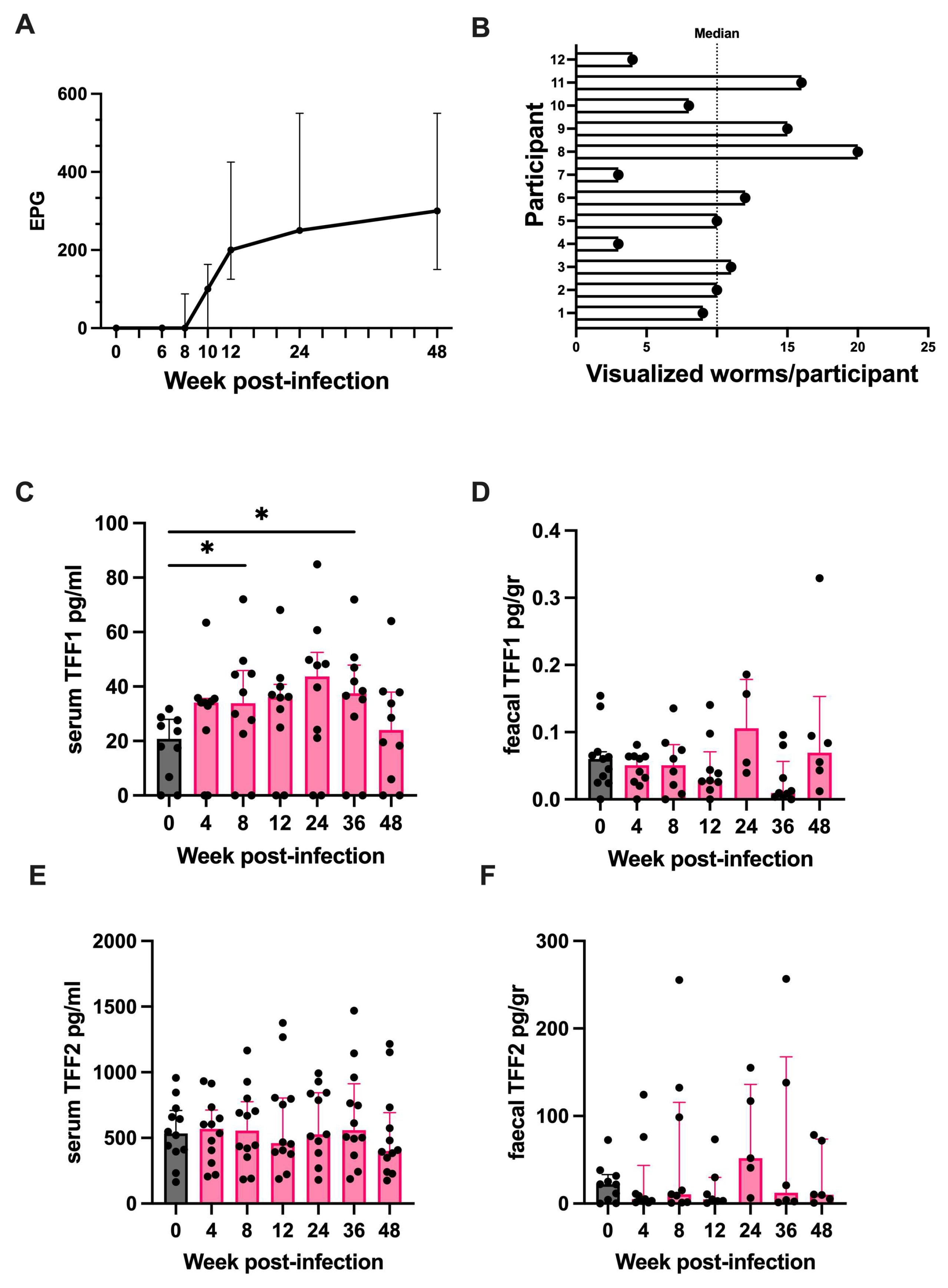
2.7. Trefoil Factor 1 Is Upregulated in Humans Experimentally Challenged with Human Hookworm
3. Discussion
4. Materials and Methods
4.1. Mice and H. polygyrus Infection
4.2. Human Experimental N. americanus Infection
4.3. Histology and Immunofluorescence Microscopy
4.4. Quantification of Histological Staining
4.5. RNA Isolation and Real-Time qPCR
4.6. Microbiota Analysis
4.7. Quantification of Human Serum and Faecal Proteins
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil-Transmitted Helminth Infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Mules, T.C.; Inns, S.; Le Gros, G. Helminths’ Therapeutic Potential to Treat Intestinal Barrier Dysfunction. Allergy 2023, 78, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Palma, M.; Bleich, D.; Loke, P.; Gause, W.C. Systemic Impact of Intestinal Helminth Infections. Mucosal Immunol. 2014, 7, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xu, N.; Wang, X.; Vallée, I.; Liu, M.; Liu, X. Helminth Therapy for Immune-Mediated Inflammatory Diseases: Current and Future Perspectives. J. Inflamm. Res. 2022, 15, 475–491. [Google Scholar] [CrossRef]
- Mules, T.C.; Lavender, B.; Maclean, K.; Vacca, F.; Noble, S.-L.; Yumnam, B.; Te Kawa, T.; Cait, A.; Tang, J.; O’Sullivan, D.; et al. Controlled Hookworm Infection for Medication-Free Maintenance in Patients with Ulcerative Colitis: A Pilot, Double-Blind, Randomized Control Trial. Inflamm. Bowel Dis. 2023, 30, 735–745. [Google Scholar] [CrossRef]
- Elliott, D.E.; Setiawan, T.; Metwali, A.; Blum, A.; Urban, J.F.; Weinstock, J.V. Heligmosomoides polygyrus Inhibits Established Colitis in IL-10-Deficient Mice. Eur. J. Immunol. 2004, 34, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Zhao, A.; Madden, K.B.; Elfrey, J.E.; Tuft, B.A.; Sullivan, C.A.; Urban, J.F.; Shea-Donohue, T. Anti-Inflammatory Mechanisms of Enteric Heligmosomoides polygyrus Infection against Trinitrobenzene Sulfonic Acid-Induced Colitis in a Murine Model. Infect Immun. 2008, 76, 4772–4782. [Google Scholar] [CrossRef]
- Kitagaki, K.; Businga, T.R.; Racila, D.; Elliott, D.E.; Weinstock, J.V.; Kline, J.N. Intestinal Helminths Protect in a Murine Model of Asthma. J. Immunol. 2006, 177, 1628–1635. [Google Scholar] [CrossRef]
- McFarlane, A.J.; McSorley, H.J.; Davidson, D.J.; Fitch, P.M.; Errington, C.; Mackenzie, K.J.; Gollwitzer, E.S.; Johnston, C.J.C.; MacDonald, A.S.; Edwards, M.R.; et al. Enteric Helminth-Induced Type I Interferon Signaling Protects against Pulmonary Virus Infection through Interaction with the Microbiota. J. Allergy Clin. Immunol. 2017, 140, 1068–1078.e6. [Google Scholar] [CrossRef]
- Varyani, F.; Fleming, J.O.; Maizels, R.M. Helminths in the Gastrointestinal Tract as Modulators of Immunity and Pathology. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G537–G549. [Google Scholar] [CrossRef]
- Wolff, M.J.; Broadhurst, M.J.; Loke, P. Helminthic Therapy: Improving Mucosal Barrier Function. Trends Parasitol. 2012, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural Weakening of the Colonic Mucus Barrier Is an Early Event in Ulcerative Colitis Pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Nyström, E.E.L.; Martinez-Abad, B.; Arike, L.; Birchenough, G.M.H.; Nonnecke, E.B.; Castillo, P.A.; Svensson, F.; Bevins, C.L.; Hansson, G.C.; Johansson, M.E.V. An Intercrypt Subpopulation of Goblet Cells Is Essential for Colonic Mucus Barrier Function. Science 2021, 372, eabb1590. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy Partners: The Mucus Barrier and Gut Microbiome in Ulcerative Colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Vacca, F.; Le Gros, G. Tissue-Specific Immunity in Helminth Infections. Mucosal Immunol. 2022, 15, 1212–1223. [Google Scholar] [CrossRef]
- Bąska, P.; Norbury, L.J. The Role of the Intestinal Epithelium in the “Weep and Sweep” Response during Gastro—Intestinal Helminth Infections. Animals 2022, 12, 175. [Google Scholar] [CrossRef]
- Glover, M.; Colombo, S.A.P.; Thornton, D.J.; Grencis, R.K. Trickle Infection and Immunity to Trichuris muris. PLoS Pathog. 2019, 15, e1007926. [Google Scholar] [CrossRef]
- Marillier, R.G.; Michels, C.; Smith, E.M.; Fick, L.C.; Leeto, M.; Dewals, B.; Horsnell, W.G.; Brombacher, F. IL-4/IL-13 Independent Goblet Cell Hyperplasia in Experimental Helminth Infections. BMC Immunol. 2008, 9, 11. [Google Scholar] [CrossRef]
- Shekels, L.L.; Anway, R.E.; Lin, J.; Kennedy, M.W.; Garside, P.; Lawrence, C.E.; Ho, S.B. Coordinated Muc2 and Muc3 Mucin Gene Expression in Trichinella spiralis Infection in Wild-Type and Cytokine-Deficient Mice. Dig. Dis. Sci. 2001, 46, 1757–1764. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Wang, H.; Ghia, J.-E.; Haq, N.; Deng, Y.; Velcich, A.; Grencis, R.K.; Thornton, D.J.; Khan, W.I. Mucin Gene Deficiency in Mice Impairs Host Resistance to an Enteric Parasitic Infection. Gastroenterology 2010, 138, 1763–1771. [Google Scholar] [CrossRef]
- Tsubokawa, D.; Goso, Y.; Nakamura, T.; Maruyama, H.; Yatabe, F.; Kurihara, M.; Ichikawa, T.; Ishihara, K. Rapid and Specific Alterations of Goblet Cell Mucin in Rat Airway and Small Intestine Associated with Resistance against Nippostrongylus brasiliensis Reinfection. Exp. Parasitol. 2012, 130, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.R.; Yang, J.-Q.; Hogan, S.P.; Groschwitz, K.; Khodoun, M.; Munitz, A.; Orekov, T.; Perkins, C.; Wang, Q.; Brombacher, F.; et al. Intestinal Epithelial Cell Secretion of RELM-Beta Protects against Gastrointestinal Worm Infection. J. Exp. Med. 2009, 206, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Rani, R.; Dienger, K.; Lewkowich, I.; Fox, J.G.; Perkins, C.; Lewis, L.; Finkelman, F.D.; Smith, D.E.; Bryce, P.J.; et al. Trefoil Factor 2 Rapidly Induces Interleukin 33 to Promote Type 2 Immunity during Allergic Asthma and Hookworm Infection. J. Exp. Med. 2012, 209, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Belle, N.M.; Ji, Y.; Herbine, K.; Wei, Y.; Park, J.; Zullo, K.; Hung, L.-Y.; Srivatsa, S.; Young, T.; Oniskey, T.; et al. TFF3 Interacts with LINGO2 to Regulate EGFR Activation for Protection against Colitis and Gastrointestinal Helminths. Nat. Commun. 2019, 10, 4408. [Google Scholar] [CrossRef]
- Soga, K.; Yamauchi, J.; Kawai, Y.; Yamada, M.; Uchikawa, R.; Tegoshi, T.; Mitsufuji, S.; Yoshikawa, T.; Arizono, N. Alteration of the Expression Profiles of Acidic Mucin, Sialytransferase, and Sulfotransferases in the Intestinal Epithelium of Rats Infected with the Nematode Nippostrongylus brasiliensis. Parasitol. Res. 2008, 103, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Kawai, Y.; Yamada, M.; Uchikawa, R.; Tegoshi, T.; Arizono, N. Altered Expression of Goblet Cell- and Mucin Glycosylation-Related Genes in the Intestinal Epithelium during Infection with the Nematode Nippostrongylus brasiliensis in Rat. APMIS 2006, 114, 270–278. [Google Scholar] [CrossRef]
- Tsubokawa, D.; Ishiwata, K.; Goso, Y.; Yokoyama, T.; Kanuka, H.; Ishihara, K.; Nakamura, T.; Tsuji, N. Induction of Sda-Sialomucin and Sulfated H-Sulfomucin in Mouse Small Intestinal Mucosa by Infection with Parasitic Helminth. Exp. Parasitol. 2015, 153, 165–173. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Dawson, P.A.; Lourie, R.; Hutson, P.; Tong, H.; Grencis, R.K.; McGuckin, M.A.; Thornton, D.J. Immune-Driven Alterations in Mucin Sulphation Is an Important Mediator of Trichuris muris Helminth Expulsion. PLoS Pathog. 2017, 13, e1006218. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal Mucus Components and Secretion Mechanisms: What We Do and Do Not Know. Exp. Mol. Med. 2023, 55, 651–691. [Google Scholar] [CrossRef]
- Vacca, F.; Mules, T.C.; Camberis, M.; Lavender, B.; Noble, S.L.; Cait, A.; Maclean, K.; Mamum, J.; Yumnam, B.; Te Kawa, T.; et al. Controlled infection with cryopreserved human hookworm induces CTLA-4 expression on Tregs and upregulates tryptophan metabolism. Gut Microbes 2024, 16, 2416517. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Filbey, K.J.; Maizels, R.M. Immunity to the Model Intestinal Helminth Parasite Heligmosomoides polygyrus. Semin. Immunopathol. 2012, 34, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Juge, N. Relationship between Mucosa-Associated Gut Microbiota and Human Diseases. Biochem. Soc. Trans. 2022, 50, 1225–1236. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.; Ma, C.; Matthaei, K.I.; Collins, S.M. Stat6 Dependent Goblet Cell Hyperplasia during Intestinal Nematode Infection. Parasite Immunol. 2001, 23, 39–42. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, G.J.; Bancroft, A.; Grencis, R.K.; McKenzie, A.N. A Distinct Role for Interleukin-13 in Th2-Cell-Mediated Immune Responses. Curr. Biol. 1998, 8, 339–342. [Google Scholar] [CrossRef]
- McDermott, J.R.; Humphreys, N.E.; Forman, S.P.; Donaldson, D.D.; Grencis, R.K. Intraepithelial NK Cell-Derived IL-13 Induces Intestinal Pathology Associated with Nematode Infection. J. Immunol. 2005, 175, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Adewale, B.; Heintz, J.R.; Pastore, C.F.; Rossi, H.L.; Hung, L.-Y.; Rahman, N.; Bethony, J.; Diemert, D.; Babatunde, J.A.; Herbert, D.R. Parasitic Helminth Infections in Humans Modulate Trefoil Factor Levels in a Manner Dependent on the Species of Parasite and Age of the Host. PLoS Negl. Trop. Dis. 2021, 15, e0009550. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Cain, K.C.; Shulman, R.J.; Burr, R.L.; Ko, C.; Hollister, E.B.; Callen, N.; Zia, J.; Han, C.J.; Jarrett, M.E. Stool and Urine Trefoil Factor 3 Levels: Associations with Symptoms, Intestinal Permeability, and Microbial Diversity in Irritable Bowel Syndrome. Benef. Microbes 2018, 9, 345–355. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M.E.V. The Role of Goblet Cells and Mucus in Intestinal Homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Yao, Y.; Kim, G.; Shafer, S.; Chen, Z.; Kubo, S.; Ji, Y.; Luo, J.; Yang, W.; Perner, S.P.; Kanellopoulou, C.; et al. Mucus Sialylation Determines Intestinal Host-Commensal Homeostasis. Cell 2022, 185, 1172–1188.e28. [Google Scholar] [CrossRef]
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of Trefoil Factors on the Viscoelastic Properties of Mucus Gels. Eur. J. Clin. Investig. 2002, 32, 519–527. [Google Scholar] [CrossRef]
- Propheter, D.C.; Chara, A.L.; Harris, T.A.; Ruhn, K.A.; Hooper, L.V. Resistin-like Molecule β Is a Bactericidal Protein That Promotes Spatial Segregation of the Microbiota and the Colonic Epithelium. Proc. Natl. Acad. Sci. USA 2017, 114, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Okumura, R.; Matsuzaki, T.; Nakatani, A.; Sakaki, K.; Okamoto, S.; Ishibashi, A.; Tani, H.; Horikiri, M.; Kobayashi, N.; et al. Sialylation Shapes Mucus Architecture Inhibiting Bacterial Invasion in the Colon. Mucosal. Immunol. 2023, 16, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Mules, T.C.; Tang, J.S.; Vacca, F.; Yumnam, B.; Schmidt, A.; Lavender, B.; Maclean, K.; Noble, S.; Waugh, C.; Van Ginkel, R.; et al. Modulation of Intestinal Epithelial Permeability by Chronic Small Intestinal Helminth Infections. Immunol. Cell Biol. 2024, 102, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Braga Emidio, N.; Brierley, S.M.; Schroeder, C.I.; Muttenthaler, M. Structure, Function, and Therapeutic Potential of the Trefoil Factor Family in the Gastrointestinal Tract. ACS Pharmacol. Transl. Sci. 2020, 3, 583–597. [Google Scholar] [CrossRef]
- Rio, M.-C.; Chenard, M.-P.; Wolf, C.; Marcellin, L.; Tomasetto, C.; Lathe, R.; Bellocq, J.-P.; Chambon, P. Induction of pS2 and hSP Genes as Markers of Mucosal Ulceration of the Digestive Tract. Gastroenterology 1991, 100, 375–379. [Google Scholar] [CrossRef]
- Wright, N.A.; Poulsom, R.; Stamp, G.; Van Noorden, S.; Sarraf, C.; Elia, G.; Ahnen, D.; Jeffery, R.; Longcroft, J.; Pike, C.; et al. Trefoil Peptide Gene Expression in Gastrointestinal Epithelial Cells in Inflammatory Bowel Disease. Gastroenterology 1993, 104, 12–20. [Google Scholar] [CrossRef]
- Longman, R.J.; Poulsom, R.; Corfield, A.P.; Warren, B.F.; Wright, N.A.; Thomas, M.G. Alterations in the Composition of the Supramucosal Defense Barrier in Relation to Disease Severity of Ulcerative Colitis. J. Histochem. Cytochem. 2006, 54, 1335–1348. [Google Scholar] [CrossRef]
- Ebert, M.P.A.; Hoffmann, J.; Haeckel, C.; Rutkowski, K.; Schmid, R.M.; Wagner, M.; Adler, G.; Schulz, H.U.; Roessner, A.; Hoffmann, W.; et al. Induction of TFF1 Gene Expression in Pancreas Overexpressing Transforming Growth Factor Alpha. Gut 1999, 45, 105–111. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-Evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef]
- Owen, C.D.; Tailford, L.E.; Monaco, S.; Šuligoj, T.; Vaux, L.; Lallement, R.; Khedri, Z.; Yu, H.; Lecointe, K.; Walshaw, J.; et al. Unravelling the Specificity and Mechanism of Sialic Acid Recognition by the Gut Symbiont Ruminococcus gnavus. Nat. Commun. 2017, 8, 2196. [Google Scholar] [CrossRef]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the Mucin-Degrading Capacity of the Human Gut Microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A Novel Ruminococcus Gnavus Clade Enriched in Inflammatory Bowel Disease Patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs. Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef] [PubMed]
- Grahnemo, L.; Nethander, M.; Coward, E.; Gabrielsen, M.E.; Sree, S.; Billod, J.-M.; Engstrand, L.; Abrahamsson, S.; Langhammer, A.; Hveem, K.; et al. Cross-Sectional Associations between the Gut Microbe Ruminococcus gnavus and Features of the Metabolic Syndrome: The HUNT Study. Lancet Diabetes Endocrinol. 2022, 10, 481–483. [Google Scholar] [CrossRef]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus Gnavus: Friend or Foe for Human Health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, S.; Kim, B.; Nam, M.H.; Ahn, Y.K.; Park, Y.M.; Jeong, S.; Park, M.J.; Song, K.B.; Lee, S.; et al. Ruminococcus Gnavus Ameliorates Atopic Dermatitis by Enhancing Treg Cell and Metabolites in BALB/c Mice. Pediatr. Allergy Immunol. 2022, 33, e13678. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Camberis, M.; Le Gros, G.; Urban, J. Animal Model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. In Current Protocols in Immunology; Coligan, J.E., Bierer, B.E., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; p. im1912s55. ISBN 978-0-471-14273-7. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Elderman, M.; Sovran, B.; Hugenholtz, F.; Graversen, K.; Huijskes, M.; Houtsma, E.; Belzer, C.; Boekschoten, M.; de Vos, P.; Dekker, J.; et al. The Effect of Age on the Intestinal Mucus Thickness, Microbiota Composition and Immunity in Relation to Sex in Mice. PLoS ONE 2017, 12, e0184274. [Google Scholar] [CrossRef]
- Schloss, P.D. Amplicon Sequence Variants Artificially Split Bacterial Genomes into Separate Clusters. mSphere 2021, 6, e00191-21. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Britton, G.J.; Chen-Liaw, A.; Cossarini, F.; Livanos, A.E.; Spindler, M.P.; Plitt, T.; Eggers, J.; Mogno, I.; Gonzalez-Reiche, A.S.; Siu, S.; et al. Limited Intestinal Inflammation despite Diarrhea, Fecal Viral RNA and SARS-CoV-2-Specific IgA in Patients with Acute COVID-19. Sci. Rep. 2021, 11, 13308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mules, T.C.; Vacca, F.; Cait, A.; Yumnam, B.; Schmidt, A.; Lavender, B.; Maclean, K.; Noble, S.-L.; Gasser, O.; Camberis, M.; et al. A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome. Int. J. Mol. Sci. 2024, 25, 12015. https://doi.org/10.3390/ijms252212015
Mules TC, Vacca F, Cait A, Yumnam B, Schmidt A, Lavender B, Maclean K, Noble S-L, Gasser O, Camberis M, et al. A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome. International Journal of Molecular Sciences. 2024; 25(22):12015. https://doi.org/10.3390/ijms252212015
Chicago/Turabian StyleMules, Thomas C., Francesco Vacca, Alissa Cait, Bibek Yumnam, Alfonso Schmidt, Brittany Lavender, Kate Maclean, Sophia-Louise Noble, Olivier Gasser, Mali Camberis, and et al. 2024. "A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome" International Journal of Molecular Sciences 25, no. 22: 12015. https://doi.org/10.3390/ijms252212015
APA StyleMules, T. C., Vacca, F., Cait, A., Yumnam, B., Schmidt, A., Lavender, B., Maclean, K., Noble, S.-L., Gasser, O., Camberis, M., Le Gros, G., & Inns, S. (2024). A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome. International Journal of Molecular Sciences, 25(22), 12015. https://doi.org/10.3390/ijms252212015





