Inert Gas Mild Pressure Action on Healthy Humans: The “IPA” Study
Abstract
:1. Introduction
2. Results
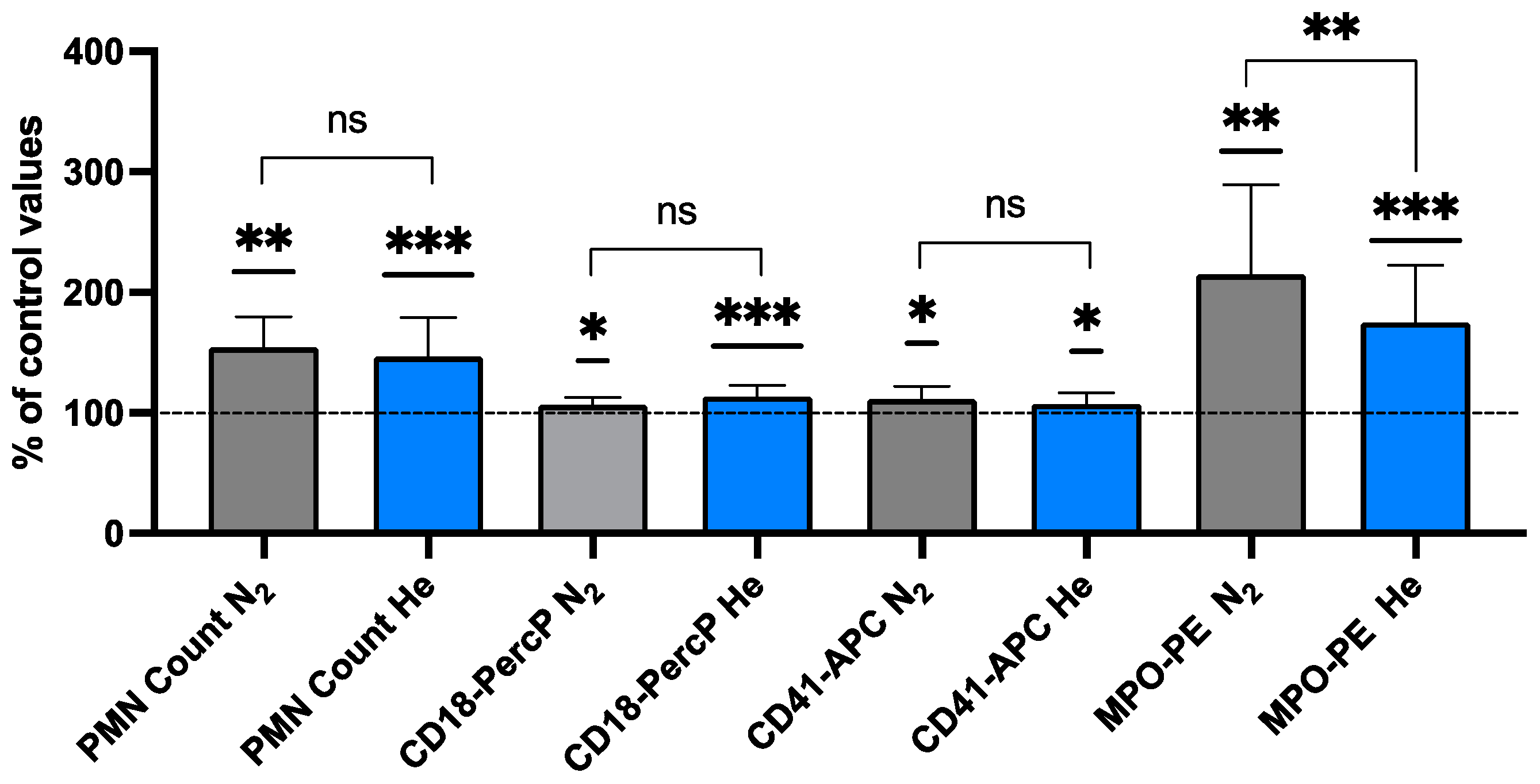
2.1. Neutrophil Activation Elicited by Inert Gas
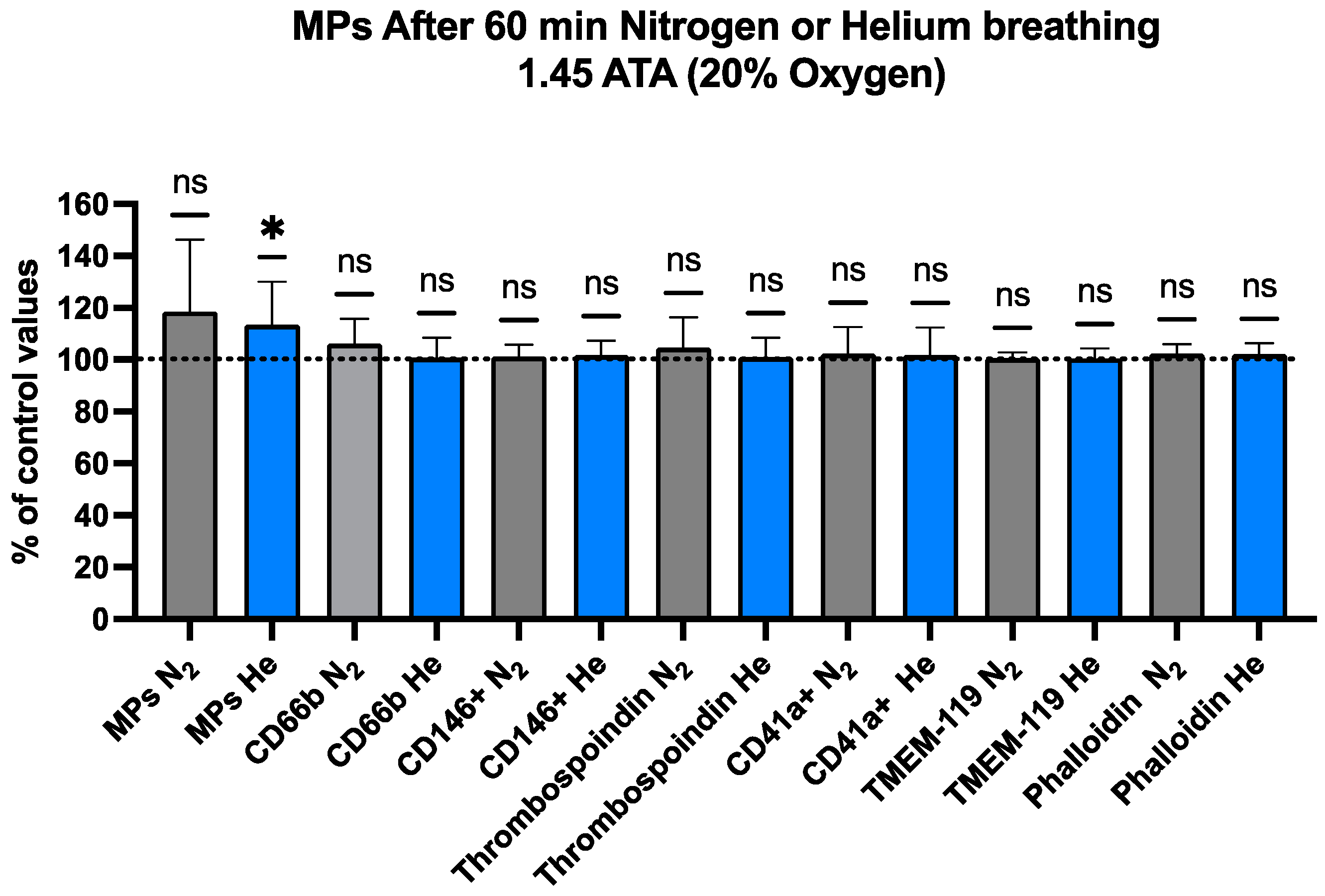
2.2. Blood-Borne EVs Elicited by Pressurized Inert Gas Exposure
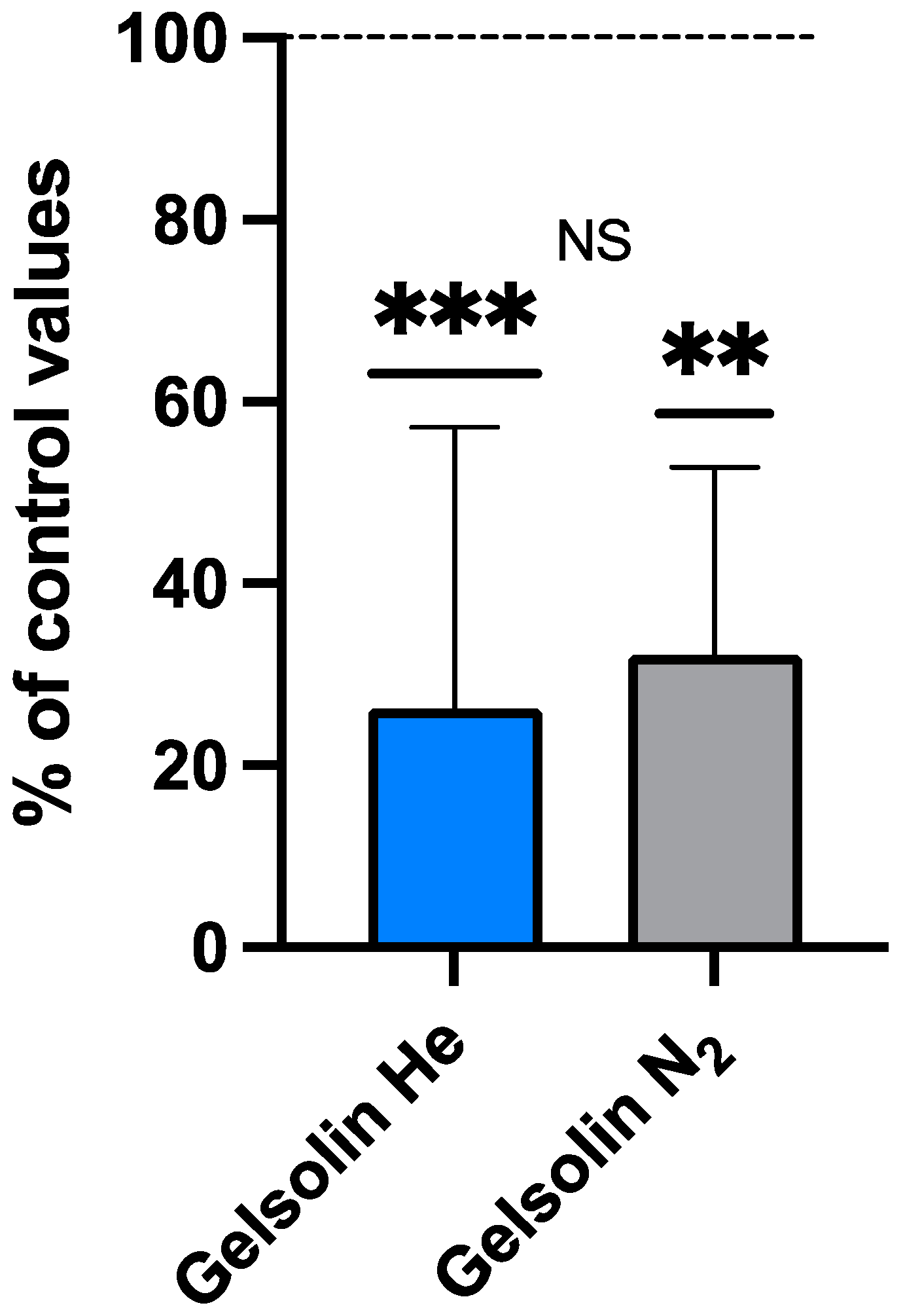
2.3. IL-1β, iNOS, and Gelsolin Changes
Plasma Gelsolin (pGSN) After Inert Gas Exposure
2.4. Oxyinflammation After Inert Gas Exposure
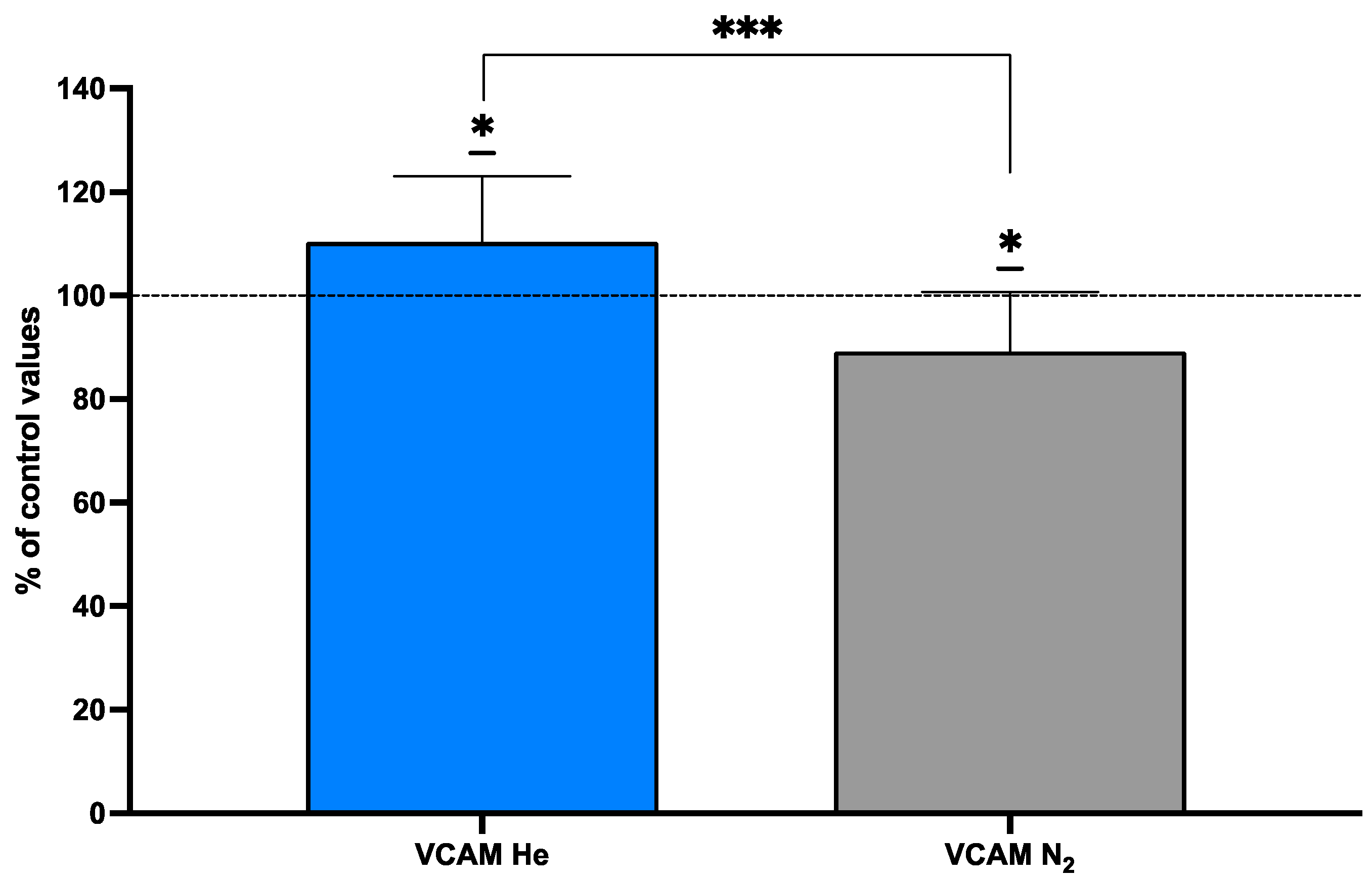
2.5. Endothelial Markers After Inert Gas Exposure
3. Discussion
4. Materials and Methods
4.1. Experimental Protocol
4.2. Experimental Protocol for Microparticle Analysis
4.3. Reagents (Microparticle Analysis)
4.4. Neutrophil Activation Analysis
4.5. IL-1β and NOS2 Measurements
4.6. Gelsolin Assay
4.7. Exosome/Lipophilic Particles Assay
4.8. Blood Collection for Other Biomarkers
Vascular Cell Adhesion Protein (VCAM-1)
4.9. Saliva Collection and Biomarker Assessment
4.9.1. Reactive Oxygen Species (ROS)
4.9.2. Total Antioxidant Capacity (TAC)
4.10. Urine Collection
4.10.1. Lipid Peroxidation (8-iso-PGF2α)
4.10.2. Interleukin-6 (IL-6)
4.10.3. NO Metabolites (NOx)
4.10.4. Neopterin
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCS | Decompression sickness |
| EVs | Extracellular vesicles |
| IL-1β | Interleukin-1β |
| Il-6 | Interleukin-6 |
| MPO | Myeloperoxidase |
| MPs | Blood-borne microparticles |
| NOx | Nitric oxide metabolites |
| pGSN | Plasma gelsolin |
| PO2 | Oxygen partial pressure |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| TAC | Total antioxidant capacity |
| TMEM-119 | Transmembrane protein 119 |
| TLR4 | Toll-like receptor 4 |
| TSP | Thrombospondin 1 |
| VCAM-1 | Vascular cell adhesion protein 1 |
| 8-iso-PGF2α | 8-isoprostane |
References
- Tuominen, L.J.; Tuohinen, S.; Lundell, R.V.; Raisanen-Sokolowski, A.K.; Wuorimaa, T. The effect of a single closed-circuit rebreather decompression dive in extremely cold water to cardiac function. Eur. J. Appl. Physiol. 2024, 124, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Imbert, J.P.; Matity, L.; Massimelli, J.Y.; Bryson, P. Review of saturation decompression procedures used in commercial diving. Diving Hyperb. Med. 2024, 54, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J. Decompression illness: A comprehensive overview. Diving Hyperb. Med. 2024, 54, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Yoder, T.L.; Taber, K.A.; Howle, L.E.; Moon, R.E. Pushing Scuba to New Heights: Approach, Decompression, and Logistical Considerations for High-Altitude Diving. High. Alt. Med. Biol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Breen, I.D.; Stepanek, J.; Marks, L.; Yale, K.; Mesinkovska, N.; Swanson, D. Clinical Significance of Mottling Rashes in Diving Decompression Sickness. Aerosp. Med. Hum. Perform. 2024, 95, 695–702. [Google Scholar] [CrossRef]
- Germonpré, P.; Lafère, P.; Portier, W.; Germonpré, F.L.; Marroni, A.; Balestra, C. Increased Risk of Decompression Sickness When Diving With a Right-to-Left Shunt: Results of a Prospective Single-Blinded Observational Study (The “Carotid Doppler” Study). Front. Physiol. 2021, 12, 763408. [Google Scholar] [CrossRef]
- Arieli, R. Distal arterial bubble: An alternative mechanism underlying vestibular decompression illness. J. Appl. Physiol. 2023, 134, 593–594. [Google Scholar] [CrossRef]
- Arieli, R. Do skin rash and cutis marmorata stem from lamellar bodies within the skin? Diving Hyperb. Med. 2018, 48, 114. [Google Scholar] [CrossRef]
- Lautridou, J.; Buzzacott, P.; Belhomme, M.; Dugrenot, E.; Lafère, P.; Balestra, C.; Guerrero, F. Evidence of Heritable Determinants of Decompression Sickness in Rats. Med. Sci. Sports Exerc. 2017, 49, 2433–2438. [Google Scholar] [CrossRef]
- Buzzacott, P.; Lambrechts, K.; Mazur, A.; Wang, Q.; Papadopoulou, V.; Theron, M.; Balestra, C.; Guerrero, F. A ternary model of decompression sickness in rats. Comput. Biol. Med. 2014, 55, 74–78. [Google Scholar] [CrossRef]
- Druelle, A.; Castagna, O.; Roffi, R.; Louge, P.; Faivre, A.; Blatteau, J.E. Taravana syndrome and posterior reversible encephalopathy syndrome: A microbubble hypothesis for neurological accidents in breath-hold divers. Front. Physiol. 2024, 15, 1478650. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, K.; Germonpre, P.; Vandenheede, J.; Delorme, M.; Lafere, P.; Balestra, C. Mini Trampoline, a New and Promising Way of SCUBA Diving Preconditioning to Reduce Vascular Gas Emboli? Int. J. Environ. Res. Public. Health 2022, 19, 5410. [Google Scholar] [CrossRef] [PubMed]
- Germonpre, P.; Balestra, C. Preconditioning to Reduce Decompression Stress in Scuba Divers. Aerosp. Med. Hum. Perform. 2017, 88, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Arieli, R. Gas micronuclei underlying decompression bubbles may explain the influence of oxygen enriched gases during decompression on bubble formation and endothelial function in self-contained underwater breathing apparatus diving. Croat. Med. J. 2019, 60, 388. [Google Scholar] [CrossRef]
- Blatteau, J.E.; Souraud, J.B.; Gempp, E.; Boussuges, A. Gas nuclei, their origin, and their role in bubble formation. Aviat. Space Environ. Med. 2006, 77, 1068–1076. [Google Scholar]
- Vezzoli, A.; Mrakic-Sposta, S.; Brizzolari, A.; Balestra, C.; Camporesi, E.M.; Bosco, G. Oxy-Inflammation in Humans during Underwater Activities. Int. J. Mol. Sci. 2024, 25, 3060. [Google Scholar] [CrossRef]
- Balestra, C.; Leveque, C.; Mrakic-Sposta, S.; Vezzoli, A.; Wauthy, P.; Germonpre, P.; Tillmans, F.; Guerrero, F.; Lafere, P. Physiology of deep closed circuit rebreather mixed gas diving: Vascular gas emboli and biological changes during a week-long liveaboard safari. Front. Physiol. 2024, 15, 1395846. [Google Scholar] [CrossRef]
- Arieli, R. Taravana, vestibular decompression illness, and autochthonous distal arterial bubbles. Respir. Physiol. Neurobiol. 2019, 259, 119–121. [Google Scholar] [CrossRef]
- Barak, O.F.; Janjic, N.; Drvis, I.; Mijacika, T.; Mudnic, I.; Coombs, G.B.; Thom, S.R.; Madic, D.; Dujic, Z. Vascular dysfunction following breath-hold diving. Can. J. Physiol. Pharmacol. 2020, 98, 124–130. [Google Scholar] [CrossRef]
- Vince, R.V.; McNaughton, L.R.; Taylor, L.; Midgley, A.W.; Laden, G.; Madden, L.A. Release of VCAM-1 associated endothelial microparticles following simulated SCUBA dives. Eur. J. Appl. Physiol. 2009, 105, 507–513. [Google Scholar] [CrossRef]
- Madden, L.A.; Chrismas, B.C.; Mellor, D.; Vince, R.V.; Midgley, A.W.; McNaughton, L.R.; Atkins, S.L.; Laden, G. Endothelial function and stress response after simulated dives to 18 msw breathing air or oxygen. Aviat. Space Environ. Med. 2010, 81, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Bhopale, V.M.; Yang, M.; Bushmann, K.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Dujic, Z. Microparticle production, neutrophil activation and intravascular bubbles following open-water SCUBA diving. J. Appl. Physiol. 2012, 112, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Yang, M.; Bhopale, V.M.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Madden, D.; Lozo, M.; et al. Bubbles, microparticles and neutrophil activation: Changes with exercise level and breathing gas during open-water SCUBA diving. J. Appl. Physiol. 2013, 114, 1396–1405. [Google Scholar] [CrossRef]
- Pontier, J.M.; Gempp, E.; Ignatescu, M. Blood platelet-derived microparticles release and bubble formation after an open-sea dive. Appl. Physiol. Nutr. Metab. 2012, 37, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.; Thom, S.R.; Yang, M.; Bhopale, V.M.; Milovanova, T.N.; Ljubkovic, M.; Dujic, Z. High intensity cycling before SCUBA diving reduces post-decompression microparticle production and neutrophil activation. Eur. J. Appl. Physiol. 2014, 114, 1955–1961. [Google Scholar] [CrossRef]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Huang, S.; Milovanova, T.N. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J. Appl. Physiol. 2011, 110, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bennett, M.; Banham, N.D.; Chin, W.; Blake, D.F.; Rosen, A.; Pollock, N.W.; Madden, D.; Barak, O.; Marroni, A.; et al. Association of microparticles and neutrophil activation with decompression sickness. J. Appl. Physiol. 2015, 119, 427–434. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Yang, M. Provocative decompression causes diffuse vascular injury in mice mediated by microparticles containing interleukin-1beta. J. Appl. Physiol. 2018, 125, 1339–1348. [Google Scholar] [CrossRef]
- Thom, S.R.; Yang, M.; Bhopale, V.M.; Milovanova, T.N.; Bogush, M.; Buerk, D.G. Intra-microparticle nitrogen dioxide is a bubble nucleation site leading to decompression-induced neutrophil activation and vascular injury. J. Appl. Physiol. 2013, 114, 550–558. [Google Scholar] [CrossRef]
- Yang, M.; Milovanova, T.N.; Bogush, M.; Uzan, G.; Bhopale, V.M.; Thom, S.R. Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries. J. Appl. Physiol. 2012, 112, 204–211. [Google Scholar] [CrossRef]
- Yang, M.; Kosterin, P.; Salzberg, B.M.; Milovanova, T.N.; Bhopale, V.M.; Thom, S.R. Microparticles generated by decompression stress cause central nervous system injury manifested as neurohypophiseal terminal action potential broadening. J. Appl. Physiol. 2013, 115, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Van Hoy, S.E. Integrating Alternative Ultrasound Technology into Saturation Facility Operations. Int. J. Appl. Technol. Leadersh. 2024, 3, 1–10. [Google Scholar]
- OJ, O.N.; Haftel, A.; Murphy-Lavoie, H.M. Hyperbaric Treatment of Compressed Air Workers, Caissons, Tunneling, Bounce Diving, and Saturation Diving; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kageyama, N.; Sawamura, T. Effect of hyperbaric exposure on cognitive performance: An investigation conducting numerical Stroop tasks during a simulated 440 m sea water saturation diving. J. Physiol. Anthropol. 2024, 43, 24. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Yang, M. Neutrophils generate microparticles during exposure to inert gases due to cytoskeletal oxidative stress. J. Biol. Chem. 2014, 289, 18831–18845. [Google Scholar] [CrossRef]
- Bhopale, V.M.; Ruhela, D.; Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Levinson, S.L.; DiNubile, M.J.; Thom, S.R. Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. 2021, 130, 1604–1613. [Google Scholar] [CrossRef]
- Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Bhopale, V.M.; Yang, M.; Thom, S.R. Microparticle and interleukin-1β production with human simulated compressed air diving. Sci. Rep. 2019, 9, 13320. [Google Scholar] [CrossRef]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Räisänen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévénez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. [Google Scholar] [CrossRef]
- Balestra, C.; Mrakic-Sposta, S.; Virgili, F. Oxygen Variations-Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue? Int. J. Mol. Sci. 2023, 24, 13472. [Google Scholar] [CrossRef]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpre, P.; Lambrechts, K.; Lafere, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins-Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Doubt, T.J. Cardiovascular and thermal responses to SCUBA diving. Med. Sci. Sports Exerc. 1996, 28, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Sureda, A.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic. Res. 2007, 41, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.L.; Sarandy, M.M.; Novaes, R.D.; Morais-Santos, M.; Gonçalves, R.V. OxInflammation Affects Transdifferentiation to Myofibroblasts, Prolonging Wound Healing in Diabetes: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8992. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadottir, T. Nrf2 mediates redox adaptations to exercise. Redox Biology 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mrakic-Sposta, S.; Moretti, S.; Balestra, C.; Rubini, A. Spirometry and oxidative stress after rebreather diving in warm water. Undersea Hyperb. Med. 2018, 45, 191–198. [Google Scholar] [CrossRef]
- Morrow, J.D.; Chen, Y.; Brame, C.J.; Yang, J.; Sanchez, S.C.; Xu, J.; Zackert, W.E.; Awad, J.A.; Roberts, L.J. The isoprostanes: Unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab. Rev. 1999, 31, 117–139. [Google Scholar] [CrossRef]
- Liu, T.; Stern, A.; Roberts, L.J.; Morrow, J.D. The isoprostanes: Novel prostaglandin-like products of the free radical-catalyzed peroxidation of arachidonic acid. J. Biomed. Sci. 1999, 6, 226–235. [Google Scholar] [CrossRef]
- Tranfo, G.; Marchetti, E.; Pigini, D.; Miccheli, A.; Spagnoli, M.; Sciubba, F.; Conta, G.; Tomassini, A.; Fattorini, L. Targeted and untargeted metabolomics applied to occupational exposure to hyperbaric atmosphere. Toxicol. Lett. 2020, 328, 28–34. [Google Scholar] [CrossRef]
- Theunissen, S.; Guerrero, F.; Sponsiello, N.; Cialoni, D.; Pieri, M.; Germonpre, P.; Obeid, G.; Tillmans, F.; Papadopoulou, V.; Hemelryck, W.; et al. Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 2013, 40, 135–144. [Google Scholar] [PubMed]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Pieri, M.; Marroni, A. Altered Venous Blood Nitric Oxide Levels at Depth and Related Bubble Formation During Scuba Diving. Front. Physiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares NO without oxidative damage. Med. Sci. Sports Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Mestre-Alfaro, A.; Tur, J.A.; Pons, A. Scuba diving activates vascular antioxidant system. Int. J. Sports Med. 2012, 33, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Bigley, N.J.; Perymon, H.; Bowman, G.C.; Hull, B.E.; Stills, H.F.; Henderson, R.A. Inflammatory cytokines and cell adhesion molecules in a rat model of decompression sickness. J. Interf. Cytokine Res. 2008, 28, 55–63. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafere, P.; Germonpre, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- Wang, H.T.; Fang, Y.Q.; Bao, X.C.; Yuan, H.R.; Ma, J.; Wang, F.F.; Zhang, S.; Li, K.C. Expression changes of TNF-alpha, IL-1beta and IL-6 in the rat lung of decompression sickness induced by fast buoyancy ascent escape. Undersea Hyperb. Med. 2015, 42, 23–31. [Google Scholar]
- Brizzolari, A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Barassi, A.; Paganini, M.; Cialoni, D.; Mrakic-Sposta, S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. Int. J. Environ. Res. Public. Health 2023, 20, 3157. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic-Sposta, S.; Lafere, P.; Vezzoli, A.; Germonpre, P.; Beer, A.; Mievis, S.; Virgili, F.; Lambrechts, K.; Theunissen, S.; et al. Oxidative Stress Response’s Kinetics after 60 Minutes at Different (30% or 100%) Normobaric Hyperoxia Exposures. Int. J. Mol. Sci. 2022, 24, 664. [Google Scholar] [CrossRef]
- Schneider-Crease, I.A.; Feder, J.A.; Baniel, A.; McCann, C.; Haile, A.A.; Abebe, B.; Fitzgerald, L.; Gomery, M.A.; Simberloff, R.A.; Petrie, Z.L.; et al. Urinary neopterin reflects immunological variation associated with age, helminth parasitism, and the microbiome in a wild primate. Sci. Rep. 2022, 12, 21307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Belhomme, M.; Guerrero, F.; Mazur, A.; Lambrechts, K.; Theron, M. Diving under a microscope--a new simple and versatile in vitro diving device for fluorescence and confocal microscopy allowing the controls of hydrostatic pressure, gas pressures, and kinetics of gas saturation. Microsc. Microanal. 2013, 19, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Bhat, A.R.; Arya, A.K.; Ruhela, D.; Qiao, G.; Li, X.; Tang, S.; Xu, S. Neuroinflammation with increased glymphatic flow in a murine model of decompression sickness. J. Neurophysiol. 2023, 129, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Jy, W.; Mao, W.W.; Horstman, L.; Tao, J.; Ahn, Y.S. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol. Dis. 1995, 21, 217–231. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Maitz, K.; Kindler, O.; Raftopoulou, S.; Kienzl, M.; Santiso, A.; Mihalic, Z.N.; Brcic, L.; Lindenmann, J.; Fediuk, M.; et al. Identification of Novel Low-Density Neutrophil Markers Through Unbiased High-Dimensional Flow Cytometry Screening in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 703846. [Google Scholar] [CrossRef]
- Cypryk, W.; Nyman, T.A.; Matikainen, S. From inflammasome to exosome-does extracellular vesicle secretion constitute an inflammasome-dependent immune response? Front. Immunol. 2018, 9, 2188. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Yang, M.; Barak, O.F.; Dujic, Z.; Madden, D.; Bhopale, V.M.; Bhullar, J.; Thom, S.R. Ascorbic acid supplementation diminishes microparticle elevations and neutrophil activation following SCUBA diving. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R338–R344. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Yang, M. Microparticle-induced vascular injury in mice following decompression is inhibited by hyperbaric oxygen: Effects on microparticles and interleukin-1beta. J. Appl. Physiol. 2019, 126, 1006–1014. [Google Scholar] [CrossRef]
- Ostrowski, K.; Hermann, C.; Bangash, A.; Schjerling, P.; Nielsen, J.N.; Pedersen, B.K. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J. Physiol. 1998, 513 Pt 3, 889–894. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar] [PubMed]
- Schubert, C.; Seizer, L.; Chamson, E.; König, P.; Sepp, N.; Ocaña-Peinado, F.M.; Schnapka-Köpf, M.; Fuchs, D. Real-Life Cause-Effect Relations Between Urinary IL-6 Levels and Specific and Nonspecific Symptoms in a Patient With Mild SLE Disease Activity. Front. Immunol. 2021, 12, 718838. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Sagar, A.; Peddada, N.; Choudhary, V.; Singh Chopra, B.; Garg, V.; Ashish. Plasma gelsolin levels decrease in diabetic state and increase upon treatment with F-actin depolymerizing versions of gelsolin. J. Diab Res. 2014, 2014, 152075. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lin, S.-T.; Chou, H.-C.; Lee, Y.-R.; Chan, H.-L. Proteomic analysis of retinopathy-related plasma biomarkers in diabetic patients. Arch. Biochem. Biophys. 2013, 529, 146–156. [Google Scholar] [CrossRef]
- Lee, P.S.; Patel, S.R.; Christiani, D.C.; Bajwa, E.; Stossel, T.P.; Waxman, A.B. Plasma gelsolin depletion and circulating actin in sepsis: A pilot study. PLoS ONE 2008, 3, e3712. [Google Scholar] [CrossRef]
- Lee, P.S.; Sampath, K.; Karumanchi, S.A.; Tamez, H.; Bhan, I.; Isakova, T.; Gutierrez, O.M.; Wolf, M.; Chang, Y.; Stossel, T.P.; et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. Am. Soc. Nephrol. 2009, 20, 1140–1148. [Google Scholar] [CrossRef]
- Osborn, T.M.; Verdrengh, M.; Stossel, T.P.; Tarkowski, A.; Bokarewa, M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R117. [Google Scholar] [CrossRef] [PubMed]
- Peddada, N.; Sagar, A.; Ashish; Garg, R. Plasma gelsolin: A general prognostic marker of health. Med. Hypotheses 2012, 778, 203–210. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Shishkova, E.; Miller, I.J.; Balnis, J.; Bernstein, M.N.; Peters-Clarke, T.M.; Meyer, J.G.; Quan, Q.; Muehlbauer, L.K.; Trujillo, E.A.; et al. Large-scale multi-omic analysis of COVID-19 severity. medRxiv 2020. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Arya, A.K.; Ruhela, D.; Bhat, A.R.; Mitra, N.; Hoffstad, O.; Malay, D.S.; Mirza, Z.K.; Lantis, J.C.; et al. Blood-Borne Microparticles Are an Inflammatory Stimulus in Type 2 Diabetes Mellitus. Immunohorizons 2023, 7, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Levental, I.; Durnás, B.; Janmey, P.; Bucki, R. Plasma gelsolin: Indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef] [PubMed]
- Van Damme-Ostapowicz, K.; Cybulski, M.; Kozakiewicz, M.; Krajewska-Kułak, E.; Siermontowski, P.; Sobolewski, M.; Kaczerska, D. Analysis of the Increase of Vascular Cell Adhesion Molecule-1 (VCAM-1) Expression and the Effect of Exposure in a Hyperbaric Chamber on VCAM-1 in Human Blood Serum: A Cross-Sectional Study. Medicina 2022, 58, 95. [Google Scholar] [CrossRef]
- Bao, X.C.; Shen, Q.; Fang, Y.Q.; Wu, J.G. Human Physiological Responses to a Single Deep Helium-Oxygen Diving. Front. Physiol. 2021, 12, 735986. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Li, Z.; Xu, G.; Bao, X. Hyperbaric oxygen-induced acute lung injury: A mouse model study on pathogenic characteristics and recovery dynamics. Front. Physiol. 2024, 15, 1474933. [Google Scholar] [CrossRef]
- MacLaughlin, K.J.; Barton, G.P.; Braun, R.K.; MacLaughlin, J.E.; Lamers, J.J.; Marcou, M.D.; Eldridge, M.W. Hyperbaric air mobilizes stem cells in humans; a new perspective on the hormetic dose curve. Front. Neurol. 2023, 14, 1192793. [Google Scholar] [CrossRef]
- MacLaughlin, K.J.; Barton, G.P.; Braun, R.K.; Eldridge, M.W. Effect of intermittent hyperoxia on stem cell mobilization and cytokine expression. Med. Gas. Res. 2019, 9, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guerrero, F.; Lambrechts, K.; Mazur, A.; Buzzacott, P.; Belhomme, M.; Theron, M. Simulated air dives induce superoxide, nitric oxide, peroxynitrite, and Ca(2+) alterations in endothelial cells. J. Physiol. Biochem. 2020, 76, 61–72. [Google Scholar] [CrossRef]
- Wang, Q.; Guerrero, F.; Mazur, A.; Lambrechts, K.; Buzzacott, P.; Belhomme, M.; Theron, M. Reactive Oxygen Species, Mitochondria, and Endothelial Cell Death during In Vitro Simulated Dives. Med. Sci. Sports Exerc. 2015, 47, 1362–1371. [Google Scholar] [CrossRef]
- Oei, G.T.M.L.; Smit, K.F.; vd Vondervoort, D.; Brevoord, D.; Hoogendijk, A.; Wieland, C.W.; Hollmann, M.W.; Preckel, B.; Weber, N.C. Effects of helium and air inhalation on the innate and early adaptive immune system in healthy volunteers ex vivo. J. Transl. Med. 2012, 10, 201. [Google Scholar] [CrossRef]
- Hild, M.; Schmidt, R. The mechanism of the collision-induced enhancement of the radiative transitions of oxygen. J. Phys. Chem. 1999, 103, 6091–6096. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Hampton, M.; Troiano, M.A.; Mirza, Z.; Malay, D.S.; Shannon, S.; Jennato, N.B.; Donohue, C.M.; Hoffstad, O.; Woltereck, D.; et al. Measurements of CD34+/CD45-dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes 2016, 65, 486–497. [Google Scholar] [CrossRef]
- Chen, J.K.; Zhan, Y.J.; Yang, C.S.; Tzeng, S.F. Oxidative stress-induced attenuation of thrombospondin-1 expression in primary rat astrocytes. J. Cell Biochem. 2011, 112, 59–70. [Google Scholar] [CrossRef]
- Tung, J.W.; Parks, D.R.; Moore, W.A.; Herzenberg, L.A.; Herzenberg, L.A. New approaches to fluorescence compensation and visualization of FACS data. Clin. Immunol. 2004, 110, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxid. Med. Cell Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Biagini, D.; Bondi, D.; Pietrangelo, T.; Vezzoli, A.; Lomonaco, T.; Di Francesco, F.; Verratti, V. OxInflammation at High Altitudes: A Proof of Concept from the Himalayas. Antioxidants 2022, 11, 368. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Green, H.J.; Fraser, I.G. Differential effects of exercise intensity on serum uric acid concentration. Med. Sci. Sports Exerc. 1988, 20, 55–59. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]



| Exposition | Baseline | After 120 min | p Value | Size Effect | n |
|---|---|---|---|---|---|
| He 1.45 ATA (20 kPa oxygen) | MPs/μL | MPs/μL | Paired t-test | Cohen’s d | |
| % CD18 | 1.31 ± 0.433 | 3.66± 1.579 | <0.001 *** | −1.581 | 14 |
| Median CD18 | 18.44 ± 5.826 | 20.72 ± 6.117 | <0.001 *** | −1.613 | 14 |
| % CD41 | 3.91 ± 1.933 | 6.11 ± 1.860 | 0.002 ** | −1.020 | 14 |
| Median CD41 | 57.52 ± 8.568 | 61.09 ± 1.869 | 0.012 * | −0.779 | 14 |
| % Gelsolin | 5.57 ± 1.223 | 3.38 ± 1.321 | <0.001 *** | 1.460 | 14 |
| Median—gelsolin | 25.86 ± 2.012 | 23.91 ± 1.652 | <0.001 *** | 1.508 | 14 |
| % MPO | 1.40 ± 0.547 | 3.49 ± 1.149 | <0.001 *** | −1.790 | 14 |
| Median MPO | 5.13 ± 1.178 | 5.13 ± 1.178 | <0.001 *** | −1.623 | 14 |
| N2 1.45 ATA (20 kPa oxygen) | |||||
| % CD18- | 1.43 ± 0.669 | 3.80 ± 1.446 | 0.003 ** | −1.768 | 7 |
| Median | 17.83 ± 4.247 | 18.92 ± 4.309 | 0.020 * | −1.186 | 7 |
| % CD41 | 4.13 ± 1.150 | 7.69 ± 3.037 | 0.009 ** | −1.453 | 7 |
| Median | 57.87 ± 6.199 | 64.08 ± 7.968 | 0.043 * | −0.964 | 7 |
| % Gelsolin | 4.81 ± 1.321 | 2.22 ± 1.064 | 0.001 ** | 2.232 | 7 |
| Median | 22.20 ± 4.128 | 17.13 ± 3.748 | 0.012 * | 1.331 | 7 |
| % MPO | 1.18 ± 0.536 | 3.41± 1.206 | <0.001 *** | −2.281 | 7 |
| Median | 2.32 ± 0.752 | 4.82 ± 1.659 | 0.002 ** | −1.970 | 7 |
| Exposure (60 min) | Baseline | 120 min Post | p Value | Size Effect | n |
|---|---|---|---|---|---|
| He 1.45 ATA (20 kPa oxygen) | MPs/μL | MPs/μL | Paired t-test | Cohen’s d | |
| Total MPs | 791.91 ± 120.230 | 889.24 ± 148.106 | 0.026 * | −0.6707 | 14 |
| CD66b+ | 9.43 ± 1.050 | 9.48 ± 1.158 | 0.775 | −0.0779 | 14 |
| CD146+ | 20.02 ± 0.689 | 20.35 ± 1.219 | 0.290 | −0.2950 | 14 |
| Thrombospoindin1 | 9.59 ± 0.832 | 9.68 ± 1.137 | 0.642 | −0.1272 | 14 |
| CD41a+ | 7.08 ± 1.552 | 7.16 ± 1.599 | 0.703 | −0.1042 | 14 |
| TMEM-119 | 29.67 ± 0.712 | 29.78 ± 1.032 | 0.705 | −0.1035 | 14 |
| Phalloidin | 17.51 ± 0.567 | 17.85 ± 0.815 | 0.104 | −0.4673 | 14 |
| N2 1.45 ATA (20 kPa oxygen) | |||||
| Total MPs | 742.13 ± 188.954 | 844.04± 141.385 | 0.115 | −0.589 | 9 |
| CD66b+ | 9.63± 0.736 | 10.15 ± 0.431 | 0.111 | −0.597 | 9 |
| CD146+ | 21.40 ± 1.533 | 21.57 ± 0.948 | 0.611 | −0.176 | 9 |
| Thrombospoindin1 | 8.81 ± 0.602 | 9.16 ± 0.530 | 0.295 | −0.374 | 9 |
| CD41a+ | 5.70 ± 0.653 | 5.78 ± 0.411 | 0.685 | −0.140 | 9 |
| TMEM-119 | 30.07 ± 0.697 | 30.22 ± 0.317 | 0.535 | −0.216 | 9 |
| Phalloidin | 17.85 ± 0.932 | 18.21 ± 0.618 | 0.148 | −0.534 | 9 |
| Exposure (60 min) | Baseline | Post-120 min of Exposure | p Value | Size Effect | n |
|---|---|---|---|---|---|
| He 1.45 ATA (20 kPa oxygen) | Pre | Post | Wilcoxon | Cohen’s d | |
| IL-1β (pg/million MPs) | - | 33.7 ± 47.2 | - | - | 13 |
| iNOS (pg/million MPs) | 0.174 ± 0.0952 | 0.510 ± 0.258 | <0.001 *** | −1.00 | 14 |
| Gelsolin (μg/mL) | 190 ± 226.5 | 26.0 ± 22.3 | 0.931 | −0.0245 | 14 |
| N2 1.45 ATA (20 kPa oxygen) | |||||
| IL-1β (pg/million MPs) | 11.6 ± 6.14 | 38.1 ± 32.9 | 0.004 ** | −1.00 | 9 |
| iNOS (pg/million MPs) | 0.0503 ± 0.033 | 0.347 ± 0.126 | 0.001 ** | −1.00 | 9 |
| Gelsolin (μg/mL) | 147.7 ± 56.9 | 42.7 ± 26.9 | 0.004 ** | 1.36 | 9 |
| Exposure (60 min) | Baseline | Post-120 min of Exposure | p Value | Size Effect | n |
|---|---|---|---|---|---|
| He 1.45 ATA (20 kPa oxygen) | Value | Value | Paired-t test | Cohen’s d | |
| ROS—He (μmol⋅min−1) | 0.240 ± 0.011 | 0.308 ± 0.009 | <0.001 ** | −4.778 | 14 |
| TAC—He (mM) | 0.244 ± 0.061 | 0.238 ± 0.045 | 0.131 | 0.640 | 14 |
| 8-iso-PGF2 α -He (pg⋅mg−1 creatinine) | 224.3 ± 74.90 | 258.4 ± 61.63 | 0.190 | −0.552 | 14 |
| Neopterin—He (mM⋅mol−1 creatinine) | 7.07 ± 2.15 | 5.97 ± 1.90 | 0.046 * | −0.861 | 14 |
| IL-6—He (pg·mL−1) | 4.18 ± 1.50 | 3.43 ± 1.11 | 0.126 | −0.982 | 14 |
| NOx—He (µM) | 803 ± 428 | 449 ± 240 | 0.020 * | 1.022 | 14 |
| N2 1.45 ATA (20 kPa oxygen) | |||||
| ROS—N (μmol⋅min−1) | 0.238 ± 0.008 | 0.307 ± 0.018 | <0.001 *** | −6.085 | 9 |
| TAC—N (mM) | 0.255 ± 0.025 | 0.225 ± 0.060 | 0.189 | 0.121 | 9 |
| 8-iso-PGF2 ⍺ -N (pg·mg−1 creatinine) | 364.3 ± 80.56 | 413.1 ± 95.48 | 0.281 | −0.497 | 9 |
| Neopterin -N (mM⋅mol−1creatinine) | 7.87 ± 1.93 | 5.76 ± 1.68 | 0.071 | −0.857 | 9 |
| IL-6—N (pg·mL−1) | 3.82 ± 1.51 | 5.54 ± 1.98 | 0.012 * | 0.567 | 9 |
| NOx—N (µM) | 671 ± 293 | 493 ± 189 | 0.124 | 0.721 | 9 |
| Exposure (60 min) | Baseline | Post-120 min of Exposure | p Value | Size Effect | n |
|---|---|---|---|---|---|
| He 1.45 ATA (20 kPa oxygen) | Value | Value | Paired t-test | Cohen’s d | |
| VCAM-1—He (mg·L−1) | 580 ± 122 | 624 ± 126 | 0.402 | −0.349 | 14 |
| N2 1.45 ATA ((20 kPa oxygen) | |||||
| VCAM-1—N (mg·L−1) | 611 ± 116 | 561 ± 118 | 0.351 | 0.428 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, C.; Leveque, C.; Mrakic-Sposta, S.; Coulon, M.; Tumbarello, R.; Vezzoli, A.; Bosco, G.; Imtiyaz, Z.; Thom, S.R. Inert Gas Mild Pressure Action on Healthy Humans: The “IPA” Study. Int. J. Mol. Sci. 2024, 25, 12067. https://doi.org/10.3390/ijms252212067
Balestra C, Leveque C, Mrakic-Sposta S, Coulon M, Tumbarello R, Vezzoli A, Bosco G, Imtiyaz Z, Thom SR. Inert Gas Mild Pressure Action on Healthy Humans: The “IPA” Study. International Journal of Molecular Sciences. 2024; 25(22):12067. https://doi.org/10.3390/ijms252212067
Chicago/Turabian StyleBalestra, Costantino, Clément Leveque, Simona Mrakic-Sposta, Mathias Coulon, Romain Tumbarello, Alessandra Vezzoli, Gerardo Bosco, Zuha Imtiyaz, and Stephen R. Thom. 2024. "Inert Gas Mild Pressure Action on Healthy Humans: The “IPA” Study" International Journal of Molecular Sciences 25, no. 22: 12067. https://doi.org/10.3390/ijms252212067
APA StyleBalestra, C., Leveque, C., Mrakic-Sposta, S., Coulon, M., Tumbarello, R., Vezzoli, A., Bosco, G., Imtiyaz, Z., & Thom, S. R. (2024). Inert Gas Mild Pressure Action on Healthy Humans: The “IPA” Study. International Journal of Molecular Sciences, 25(22), 12067. https://doi.org/10.3390/ijms252212067








