A New Probiotic Formulation Promotes Resolution of Inflammation in a Crohn’s Disease Mouse Model by Inducing Apoptosis in Mucosal Innate Immune Cells
Abstract
:1. Introduction
2. Results
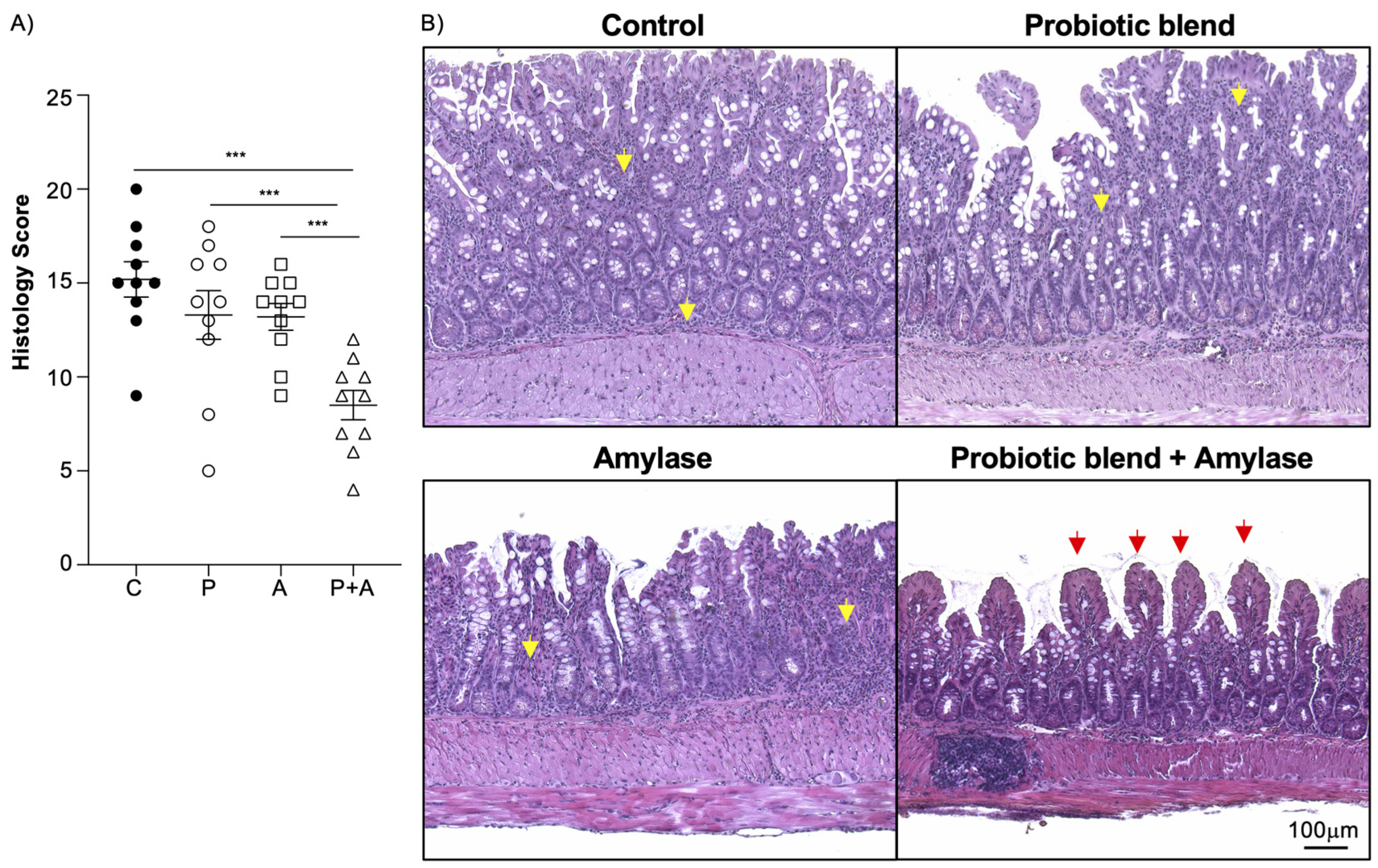
2.1. Amylase and Probiotics Are Both Necessary to Ameliorate Inflammation in SAMP Mice
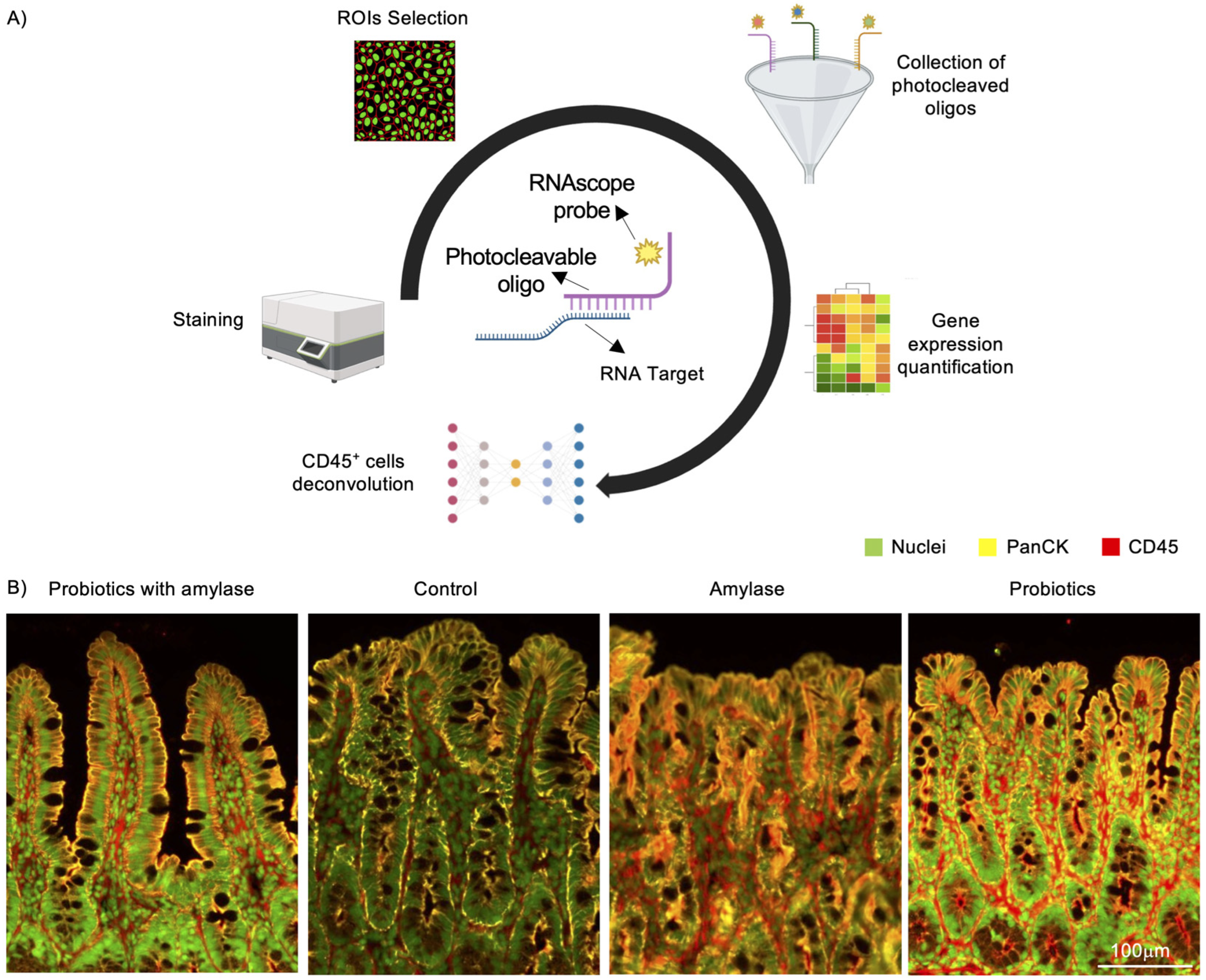
2.2. Digital Spatial Profiling of Genetic Expression Performed on Mucosal Ileal Tissue in SAMP Mice
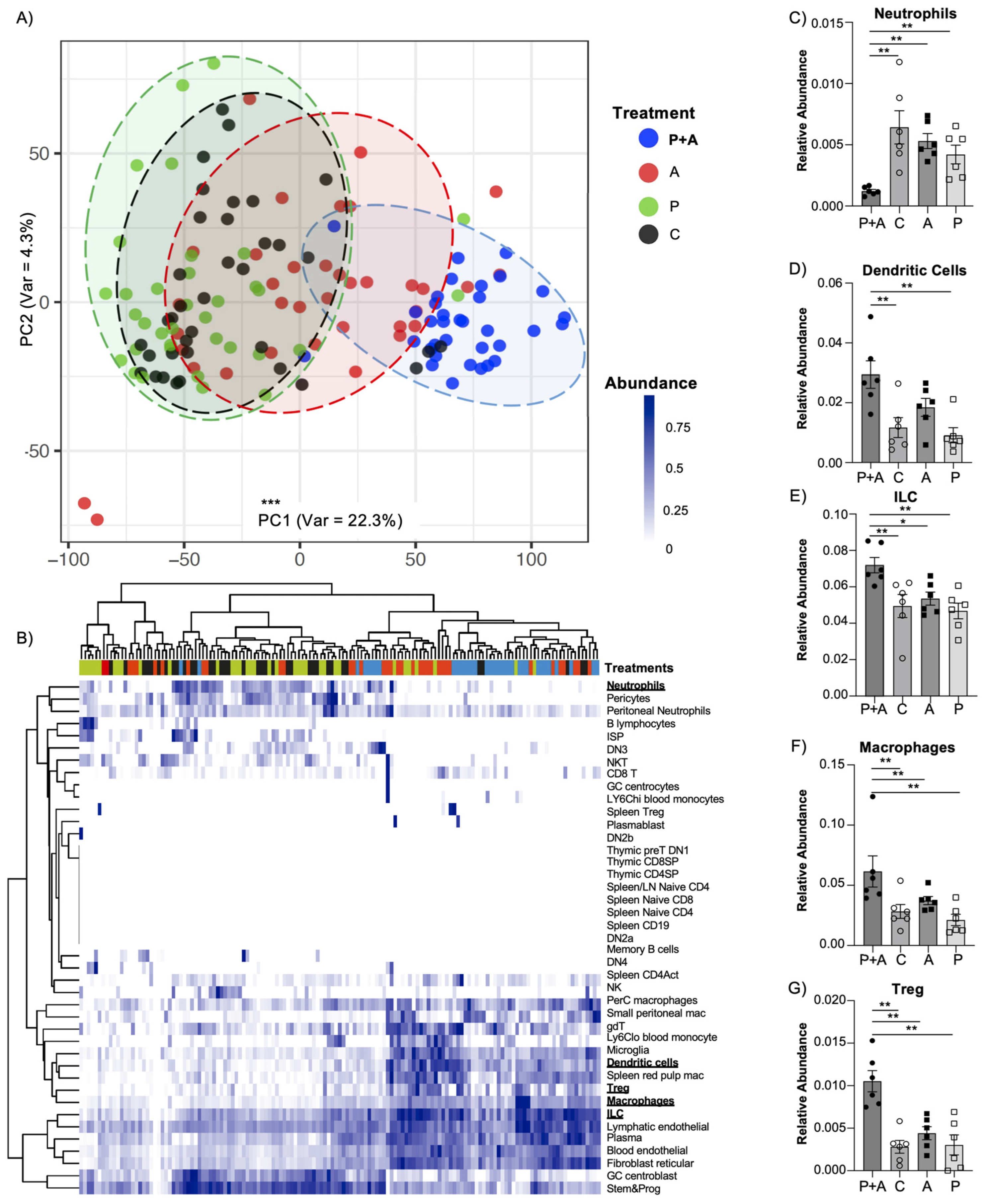
2.3. Probiotics Mix Coupled with Amylase Exerts an Enhanced Immunomodulatory Effect in the Mucosal Layer
2.4. Probiotic Plus Amylase Blend Stimulates Apoptosis in Immune Cell Subsets
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Test Materials and Administration Protocol
4.3. Histology
4.4. GeoMx NanoString Data Processing and Analysis
4.5. TUNEL Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef]
- Aleksandrowicz, A.; Carolak, E.; Dutkiewicz, A.; Blachut, A.; Waszczuk, W.; Grzymajlo, K. Better together-Salmonella biofilm-associated antibiotic resistance. Gut Microbes 2023, 15, 2229937. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Sartor, R.B. Intestinal bacterial biofilms modulate mucosal immune responses. J. Immunol. Sci. 2018, 2, 13–18. [Google Scholar]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tsolis, R.M.; Baumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Aas, J.; Gessert, C.E.; Bakken, J.S. Recurrent Clostridium difficile colitis: Case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin. Infect. Dis. 2003, 36, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Smug, L.N.; Salminen, S.; Sanders, M.E.; Ebner, S. Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benef. Microbes 2014, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clement, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- So, D.; Quigley, E.M.M.; Whelan, K. Probiotics in irritable bowel syndrome and inflammatory bowel disease: Review of mechanisms and effectiveness. Curr. Opin. Gastroenterol. 2023, 39, 103–109. [Google Scholar] [CrossRef]
- Hager, C.L.; Isham, N.; Schrom, K.P.; Chandra, J.; McCormick, T.; Miyagi, M.; Ghannoum, M.A. Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. mBio 2019, 10, 2. [Google Scholar] [CrossRef]
- Pizarro, T.T.; Pastorelli, L.; Bamias, G.; Garg, R.R.; Reuter, B.K.; Mercado, J.R.; Chieppa, M.; Arseneau, K.O.; Ley, K.; Cominelli, F. SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 2011, 17, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; Osme, A.; Ghannoum, M.; Cominelli, F. A Novel Probiotic Combination Ameliorates Crohn’s Disease-Like Ileitis by Increasing Short-Chain Fatty Acid Production and Modulating Essential Adaptive Immune Pathways. Inflamm. Bowel Dis. 2023, 29, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Danaher, P.; Kim, Y.; Nelson, B.; Griswold, M.; Yang, Z.; Piazza, E.; Beechem, J.M. Advances in mixed cell deconvolution enable quantification of cell types in spatial transcriptomic data. Nat. Commun. 2022, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil apoptosis: Relevance to the innate immune response and inflammatory disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Steukers, L.; Glorieux, S.; Vandekerckhove, A.P.; Favoreel, H.W.; Nauwynck, H.J. Diverse microbial interactions with the basement membrane barrier. Trends Microbiol. 2012, 20, 147–155. [Google Scholar] [CrossRef]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, Development, and Cross-Species Interactions in Biofilms. Front. Microbiol. 2021, 12, 757327. [Google Scholar] [CrossRef]
- Oliveira, M.; Cunha, E.; Tavares, L.; Serrano, I.P. aeruginosa interactions with other microbes in biofilms during co-infection. AIMS Microbiol. 2023, 9, 612–646. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Nacato-Toapanta, A.L.; Enriquez-Martinez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle royale: Immune response on biofilms—Host-pathogen interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef]
- Ikeda, Y.; Saigo, N.; Nagasaki, Y. Direct evidence for the involvement of intestinal reactive oxygen species in the progress of depression via the gut-brain axis. Biomaterials 2023, 295, 122053. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, B.; Veres, G.; Dezsofi, A.; Rusai, K.; Vannay, A.; Mraz, M.; Majorova, E.; Arato, A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin. Exp. Immunol. 2008, 151, 34–41. [Google Scholar] [CrossRef]
- Sanchez-Munoz, F.; Fonseca-Camarillo, G.C.; Villeda-Ramirez, M.A.; Barreto-Zuniga, R.; Bojalil, R.; Dominguez-Lopez, A.; Uribe, M.; Yamamoto-Furusho, J.K. TLR9 mRNA expression is upregulated in patients with active ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1267–1268. [Google Scholar] [CrossRef]
- van Maren, W.W.; Jacobs, J.F.; de Vries, I.J.; Nierkens, S.; Adema, G.J. Toll-like receptor signalling on Tregs: To suppress or not to suppress? Immunology 2008, 124, 445–452. [Google Scholar] [CrossRef]
- Rivas, M.N.; Koh, Y.T.; Chen, A.; Nguyen, A.; Lee, Y.H.; Lawson, G.; Chatila, T.A. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J. Clin. Invest. 2012, 122, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Laukova, M.; Glatman Zaretsky, A. Regulatory T cells as a therapeutic approach for inflammatory bowel disease. Eur. J. Immunol. 2023, 53, e2250007. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Dong, C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Savio, L.E.B.; Coutinho-Silva, R.; Ojcius, D.M. The role of NOD-like receptors in innate immunity. Front. Immunol. 2023, 14, 1122586. [Google Scholar] [CrossRef]
- Kijima, M.; Yamagishi, H.; Hara, Y.; Kasai, M.; Takami, Y.; Takemura, H.; Miyanari, Y.; Shinkai, Y.; Mizuta, R. Histone H1 quantity determines the efficiency of chromatin condensation in both apoptotic and live cells. Biochem. Biophys. Res. Commun. 2019, 512, 202–207. [Google Scholar] [CrossRef]
- Kratzmeier, M.; Albig, W.; Meergans, T.; Doenecke, D. Changes in the protein pattern of H1 histones associated with apoptotic DNA fragmentation. Biochem. J. 1999, 337, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sustrova, T.; Ondrackova, P.; Leva, L.; Kolarova, M.; Kulich, P.; Sladek, Z. Effect of probiotics on the viability of porcine and human neutrophils in vitro. Veterinární Medicína 2017, 62, 637–646. [Google Scholar] [CrossRef]
- Kanzato, H.; Fujiwara, S.; Ise, W.; Kaminogawa, S.; Sato, R.; Hachimura, S. Lactobacillus acidophilus strain L-92 induces apoptosis of antigen-stimulated T cells by modulating dendritic cell function. Immunobiology 2008, 213, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Pishkhan Dibazar, S.; Allahyari, S.; Javadi, M.; Farasat, A.; Darzi, S. Probiotic Saccharomyces cerevisiae var. boulardii supernatant inhibits survivin gene expression and induces apoptosis in human gastric cancer cells. Food Sci. Nutr. 2021, 9, 692–700. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Wang, Y.; Xu, L.; Guo, Y.; Wang, Y.; Wang, L.; Guo, C. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology 2021, 10, 1868122. [Google Scholar] [CrossRef]
- Martin, C.J.; Peters, K.N.; Behar, S.M. Macrophages clean up: Efferocytosis and microbial control. Curr. Opin. Microbiol. 2014, 17, 17–23. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigon, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Angulo, S.; Morales, A.; Danese, S.; Llacuna, L.; Masamunt, M.C.; Pultz, N.; Cifone, M.G.; De Simone, C.; Delgado, S.; Vila, J.; et al. Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS ONE 2011, 6, e16953. [Google Scholar] [CrossRef]
- Luo, D.; Schowengerdt, K.O., Jr.; Stegner, J.J.; May, W.S., Jr.; Koenig, J.M. Decreased functional caspase-3 expression in umbilical cord blood neutrophils is linked to delayed apoptosis. Pediatr. Res. 2003, 53, 859–864. [Google Scholar] [CrossRef]
- Akhter, A.; Gavrilin, M.A.; Frantz, L.; Washington, S.; Ditty, C.; Limoli, D.; Day, C.; Sarkar, A.; Newland, C.; Butchar, J.; et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009, 5, e1000361. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Ju, E.; Park, K.A.; Shen, H.M.; Hur, G.M. The resurrection of RIP kinase 1 as an early cell death checkpoint regulator-a potential target for therapy in the necroptosis era. Exp. Mol. Med. 2022, 54, 1401–1411. [Google Scholar] [CrossRef]
- Yarur, A.J.; Strobel, S.G.; Deshpande, A.R.; Abreu, M.T. Predictors of aggressive inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 652–659. [Google Scholar]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Corridoni, D.; Kodani, T.; Rodriguez-Palacios, A.; Pizarro, T.T.; Xin, W.; Nickerson, K.P.; McDonald, C.; Ley, K.F.; Abbott, D.W.; Cominelli, F. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc. Natl. Acad. Sci. USA 2013, 110, 16999–17004. [Google Scholar] [CrossRef]
- Hernandez, S.; Lazcano, R.; Serrano, A.; Powell, S.; Kostousov, L.; Mehta, J.; Khan, K.; Lu, W.; Solis, L.M. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx((R)) Digital Spatial Profiler. Front. Oncol. 2022, 12, 890410. [Google Scholar] [CrossRef]
- Dessau, R.B.; Pipper, C.B. ‘‘R”—Project for statistical computing. Ugeskr. Laeger 2008, 170, 328–330. [Google Scholar]
- Han, X.; Wang, R.; Zhou, Y.; Fei, L.; Sun, H.; Lai, S.; Saadatpour, A.; Zhou, Z.; Chen, H.; Ye, F.; et al. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 2018, 172, 1091–1107.e1017. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef]
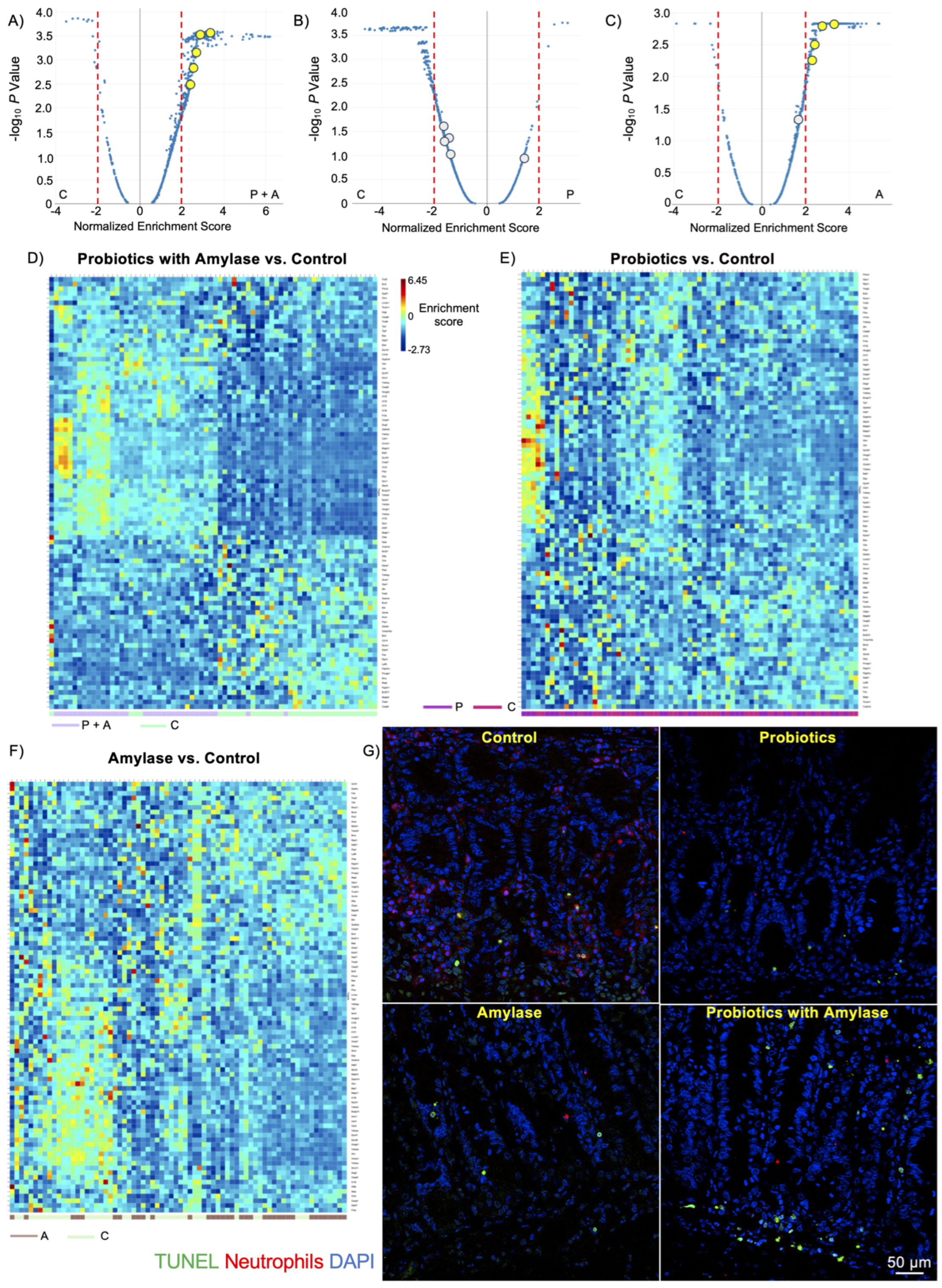
| Normalized Enrichment Score | P + A vs. C | P vs. C | A vs. C | P + A vs. P | P vs. A | P + A vs. A |
|---|---|---|---|---|---|---|
| Apoptosis | 3.08 | 1.87 | 3.51 | 2.93 | 3.61 | 2.69 |
| Apoptotic Cleavage of Cellular Proteins | 2.61 | 1.45 | 2.28 | 2.66 | 2.65 | 2.56 |
| Apoptotic Execution Phase | 3.20 | 1.46 | 3.38 | 3.16 | 3.49 | 2.96 |
| Apoptotic Factor-Mediated Response | 2.22 | −0.71 | 1.69 | 2.15 | 1.67 | 2.22 |
| Intrinsic Pathway of Apoptosis | 2.56 | 1.17 | 2.57 | 2.46 | 2.71 | 2.18 |
| Normalized Enrichment Score | P + A vs. C | P vs. C | A vs. C | P + A vs. P | P vs. A | P + A vs. A |
|---|---|---|---|---|---|---|
| TLR1:TLR2 Cascade | 2.08 | −1.86 | 2.25 | 2.04 | −2.45 | 2.14 |
| TLR2 Cascade | 2.08 | −1.86 | 2.25 | 2.04 | −2.45 | 2.14 |
| TLR3 Cascade | 2.29 | −1.86 | 2.33 | 2.22 | −2.49 | 2.41 |
| TLR4 Cascade | 1.96 | −2.05 | 2.33 | 1.90 | −2.68 | 2.07 |
| TLR5 Cascade | 2.13 | −1.92 | 2.20 | 2.08 | −2.41 | 2.21 |
| TLR6:TLR2 Cascade | 2.08 | −1.86 | 2.25 | 2.04 | −2.46 | 2.14 |
| TLR7/8 Cascade | 2.02 | −1.98 | 1.96 | 2.01 | −2.25 | 2.10 |
| TLR9 Cascade | 1.90 | −1.91 | 1.85 | 1.89 | −2.19 | 1.99 |
| TLR10 Cascade | 2.13 | −1.92 | 2.20 | 2.08 | −2.41 | 2.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Salvo, C.; Osme, A.; Ghannoum, M.; Cominelli, F.; Di Martino, L. A New Probiotic Formulation Promotes Resolution of Inflammation in a Crohn’s Disease Mouse Model by Inducing Apoptosis in Mucosal Innate Immune Cells. Int. J. Mol. Sci. 2024, 25, 12066. https://doi.org/10.3390/ijms252212066
De Salvo C, Osme A, Ghannoum M, Cominelli F, Di Martino L. A New Probiotic Formulation Promotes Resolution of Inflammation in a Crohn’s Disease Mouse Model by Inducing Apoptosis in Mucosal Innate Immune Cells. International Journal of Molecular Sciences. 2024; 25(22):12066. https://doi.org/10.3390/ijms252212066
Chicago/Turabian StyleDe Salvo, Carlo, Abdullah Osme, Mahmoud Ghannoum, Fabio Cominelli, and Luca Di Martino. 2024. "A New Probiotic Formulation Promotes Resolution of Inflammation in a Crohn’s Disease Mouse Model by Inducing Apoptosis in Mucosal Innate Immune Cells" International Journal of Molecular Sciences 25, no. 22: 12066. https://doi.org/10.3390/ijms252212066
APA StyleDe Salvo, C., Osme, A., Ghannoum, M., Cominelli, F., & Di Martino, L. (2024). A New Probiotic Formulation Promotes Resolution of Inflammation in a Crohn’s Disease Mouse Model by Inducing Apoptosis in Mucosal Innate Immune Cells. International Journal of Molecular Sciences, 25(22), 12066. https://doi.org/10.3390/ijms252212066







