Cobalt(II) and Manganese(II) Complexes of Sodium Monensinate A Bearing Nitrate Co-Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Description of the Crystal Structures of Complexes 2 and 3
2.2. Spectral Characterization of Complexes 2 and 3

2.3. Antimicrobial Properties of 1–3
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of Complexes 2 and 3
3.3. X-Ray Crystallography
3.4. Physical Measurements
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agtarap, A.; Chamberlin, J.W. Monensin, a new biologically active compound. IV. Chemistry. Antimicrob. Agents Chemother. 1967, 7, 359–362. Available online: https://pubmed.ncbi.nlm.nih.gov/5596160 (accessed on 19 July 2024). [PubMed]
- Chappel, L.R. The site of action of the anticoccidial salinomycin (Coxistac). J. Parasitol. 1979, 65, 137–143. Available online: https://pubmed.ncbi.nlm.nih.gov/448588/ (accessed on 19 July 2024). [CrossRef] [PubMed]
- Long, P.L.; Jeffers, T.K. Studies on the stage of action of ionophorous antibiotics against Eimeria. J. Parasitol. 1982, 68, 363–371. Available online: https://pubmed.ncbi.nlm.nih.gov/7097439 (accessed on 19 July 2024). [CrossRef]
- Augustine, P.C.; Smith, C.K.; Danforth, H.D.; Ruff, M.D. Effect of ionophorous anticoccidials on invasion and development of Eimeria: Comparison of sensitive and resistant isolates and correlation with drug uptake. Poult. Sci. 1987, 66, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Koinarski, V.; Sherkov, S.N. Effect of anticoccidial preparations in the prevention of coccidiosis in turkeys caused by Eimeria adenoides. Vet. Med. Nauki 1987, 24, 81–85. Available online: https://pubmed.ncbi.nlm.nih.gov/3672911 (accessed on 19 July 2024).
- Folz, S.D.; Lee, B.L.; Nowakowski, L.H.; Conder, G.A. Anticoccidial evaluation of halofuginone, lasalocid, maduramicin, monensin and salinomycin. Vet. Parasitol. 1988, 28, 1–9. [Google Scholar] [CrossRef]
- Augustine, P.C.; Watkins, K.L.; Danforth, H.D. Effect of monensin on ultrastructure and cellular invasion by the turkey coccidia Eimeria adenoeides and Eimeria meleagrimitis. Poult. Sci. 1992, 71, 970–978. [Google Scholar] [CrossRef]
- Varga, I.; Sreter, T. Efficacy of a monensin-duokvin combination against Eimeria acervulina in chickens. Folia Parasitol. 1996, 43, 153–155. Available online: https://pubmed.ncbi.nlm.nih.gov/8768461 (accessed on 19 July 2024).
- Wang, Z.; Suo, X.; Xia, X.; Chen, J. Influence of monensin on cation influx and Na+-K+-ATPase activity of Eimeria tenella sporozoites in vitro. J. Parasitol. 2006, 92, 1092–1096. Available online: http://www.jstor.org/stable/40058627 (accessed on 19 July 2024). [CrossRef]
- Chapman, H.D.; Jeffers, T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult. Sci. 2015, 94, 943–946. [Google Scholar] [CrossRef]
- Vereecken, M.; Dehaeck, B.; Berge, A.C.; Marien, M.; Geerinckx, M.; De Gussem, K. Synergistic effect of a combination of nicarbazin and monensin against coccidiosis in the chicken caused by Eimeria spp. Avian Pathol. 2020, 49, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Si, H.; Liu, X.; Suo, X.; Hu, D. Early transcriptional response to monensin in sensitive and resistant strains of Eimeria tenella. Front. Microbiol. 2022, 13, 934153. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Jeffers, T.K.; Williams, R.B. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 2010, 89, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. 2009. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores—Promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef]
- Schaffhausen, J. Remaining hurdles to effective cancer therapy. Trends Pharmacol. Sci. 2015, 36, v. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer mechanisms of salinomycin in breast cancer and its clinical applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef] [PubMed]
- Harrowfield, J.M. Biological coordination chemistry, a confluence of chemistry and biochemistry. Compt. Rend. Chim. 2005, 8, 199–210. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Ferreira, L.M.; Branco, P.S. Molecules of natural origin, semi-synthesis and synthesis with anti-inflammatory and anticancer utilities. Curr. Pharm. Des. 2012, 18, 3979–4046. [Google Scholar] [CrossRef]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef]
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive compound collections: From design to target identification. Chem. Eng. Progr. 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Antoszczak, M.; Huczyński, A. Salinomycin and its derivatives—A new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 2019, 176, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, V.K.; Mishra, A.; Singh, A.A.; Prasad, G.; Singh, A.K. Recent advancements in coordination compounds and their potential clinical application in the management of diseases: An up-to-date review. Polyhedron 2023, 241, 116485. [Google Scholar] [CrossRef]
- Maciel-Flores, C.E.; Lozano-Alvarez, J.A.; Bivián-Castro, E.Y. Recently reported biological activities and action targets of Pt(II)- and Cu(II)-based complexes. Molecules 2024, 29, 1066. [Google Scholar] [CrossRef] [PubMed]
- Pantcheva, I.N.; Mitewa, M.I.; Sheldrick, W.S.; Oppel, I.M.; Zhorova, R.; Dorkov, P. First divalent metal complexes of the polyether ionophore monensin A: X-ray structures of [Co(Mon)2(H2O)2] and [Mn(Mon)2(H2O)2] and their properties. Curr. Drug Discov. Technol. 2008, 5, 154–161. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. First solid state alkaline-earth complexes of monensic A acid: X-ray crystal Structure of [M(Mon)2(H2O)2] (M = Mg, Ca), spectral properties and cytotoxicity against Gram-positive bacteria. BioMetals 2010, 23, 59–70. [Google Scholar] [CrossRef]
- Pantcheva, I.N.; Ivanova, J.; Zhorova, R.; Mitewa, M.; Simova, S.; Mayer-Figge, H.; Sheldrick, W.S. Nickel(II) and zinc(II) dimonensinates: Crystal structure, spectral properties and bactericidal activity. Inorganica Chim. Acta 2010, 363, 1879–1886. [Google Scholar] [CrossRef]
- Ivanova, J.; Pantcheva, I.N.; Zhorova, R.; Momekov, G.; Simova, S.; Stoyanova, R.; Zhecheva, E.; Ivanova, S.; Mitewa, M. Synthesis, Spectral properties, antibacterial and antitumor activity of salinomycin complexes with the transition metal ions Co(II), Ni(II), Cu(II) and Zn(II). J. Chem. Chem. Eng. David Publ. 2012, 6, 551–562. [Google Scholar]
- Pantcheva, I.; Dimitrova, R.; Ivanova, V.; Nedzhib, A.; Dorkov, P.; Dinev, D.; Spasov, R.; Alexandrova, R. Spectral properties and biological activity of La(III) and Nd(III) monensinates. Open Chem. 2019, 17, 1423–1434. [Google Scholar] [CrossRef]
- Pashkunova-Martic, I.; Kukeva, R.; Stoyanova, R.; Pantcheva, I.; Dorkov, P.; Friske, J.; Hejl, M.; Jakupec, M.; Hohagen, M.; Legin, A.; et al. Novel salinomycin-based paramagnetic complexes—First evaluation of their potential theranostic properties. Pharmaceutics 2022, 14, 2319. [Google Scholar] [CrossRef]
- Petkov, N.; Tadjer, A.; Encheva, E.; Cherkezova-Zheleva, Z.; Paneva, D.; Stoyanova, R.; Kukeva, R.; Dorkov, P.; Pantcheva, I. Experimental and DFT study of monensinate and salinomycinate complexes containing {Fe3(µ3–O)}7+ core. Molecules 2024, 26, 364. [Google Scholar] [CrossRef] [PubMed]
- Petkov, N.; Tadjer, A.; Simova, S.; Cherkezova-Zheleva, Z.; Paneva, D.; Stoyanova, R.; Kukeva, R.; Dorkov, P.; Pantcheva, I. Synthesis, spectral characterization and structural modelling of di- and trinuclear iron(III) monensinates with different bridging patterns. Inorganics 2024, 12, 114. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.A.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, structure and antimicrobial activity of manganese(II) and cobalt(II) complexes of the polyether ionophore antibiotic sodium monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Pantcheva, I.N.; Dorkov, P.; Atanasov, V.N.; Mitewa, M.; Shivachev, B.L.; Nikolova, R.P.; Mayer-Figge, H.; Sheldrick, W.S. Crystal structure and properties of the copper(II) complex of sodium monensin A. J. Inorg. Biochem. 2009, 103, 1419–1424. [Google Scholar] [CrossRef]
- Pantcheva, I.; Petkov, N.; Encheva, E.; Kolev, S.; Simova, S.; Tsanev, A.; Dorkov, P.; Ugrinov, A. Heteronuclear complexes of Hg(II) and Zn(II) with sodium monensinate as a ligand. Molecules 2024, 29, 3106. [Google Scholar] [CrossRef]
- Huczynski, A.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. Molecular structure of the 1:1 inclusion complex of monensin A sodium salt with acetonitrile. J. Mol. Struct. 2007, 832, 84–89. [Google Scholar] [CrossRef]
- Warzeska, S.T.; Miccichè, F.; Mimmi, M.C.; Bouwman, E.; Kooijman, H.; Spek, A.L.; Reedijk, J. Tuning the coordination mode in mononuclear manganese complexes by changing the steric bulk of the carboxylates. J. Chem. Soc. Dalton Trans. 2001, 3507–3512. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Lin, J.-L.; Kong, Z.-P. Syntheses, crystal structures, and thermal behavior of Co(phen)(HL)2 and [Co2(phen)2(H2O)4L2]·H2O with H2L = pimelic acid. Z. Anorg. Allg. Chem. 2003, 629, 357–361. [Google Scholar] [CrossRef]
- Park, G.S.; Kim, H.U.; Kim, K.M.; Lee, G.H.; Park, S.K. Solvothermal synthesis, crystal structure, and magnetic properties of [Co3(SDA)3(DMF)2]: 2-D layered metal-organic framework derived from 4,4′-stilbenedicarboxylic acid (H2SDA). Bull. Kor. Chem. Soc. 2006, 27, 443–446. [Google Scholar]
- Ribar, B.; Milinski, N.; Herak, R.; Krstanovic, I.; Djuric, S. The crystal structure of cobalt nitrate dihydrate, Co(NO3)2·2H2O. Z. Kristallogr. 1976, 144, 133–138. [Google Scholar] [CrossRef]
- Blake, A.J.; Gould, R.O.; Grant, C.M.; Milne, P.E.Y.; Winpenny, R.E.P. Use of a hexanuclear copper complex as a ligand transfer agent: Crystal structures of hexakis(6-methyl-2-pyridone)iron(III) nitrate and tetrakis(6-methyl-2-pyridone)bis(nitrato) cobalt(II). Polyhedron 1994, 13, 187–191. [Google Scholar] [CrossRef]
- Abboud, K.A.; Palenik, R.C.; Palenik, G.J. Aqua [2,6-diacetylpyridinedi(benzoic acid hydrazone)] nitratocobalt(II) nitrate at 173 K. Acta Cryst. C Crystal Str. Comm. 1996, 52, 2994–2996. [Google Scholar] [CrossRef]
- Kwaskowska-Chȩć, E.; Kubiak, M.; Glowiak, T.; Ziółkowski, J.J. Synthesis, crystal and molecular structure of the novel complex [dinitrato{N,N-bis (1-methylbenzimidazol-2-ylmethyl) methyl-amine}] cobalt(II). Trans. Met. Chem. 1998, 23, 641–643. [Google Scholar] [CrossRef]
- Tiliakos, M.; Cordopatis, P.; Terzis, A.; Raptopoulou, C.P.; Perlepes, S.P.; Manessi-Zoupa, E. Reactions of 3d-metal nitrates with N,N′-bis (2-pyridyl)urea (LH2): Preparation, X-ray crystal structures and spectroscopic studies of the products trans- [M(II)(ONO2)2(LH2)2] (M=Mn, Fe, Co, Ni, Cu, Zn) and mer- [Co(III)(LH)2](NO3)·MeOH. Polyhedron 2001, 20, 2203–2214. [Google Scholar] [CrossRef]
- Yi, T.; Ho-Chol, C.; Gao, S.; Kitagawa, S. Tuning of the spin states in trinuclear cobalt compounds of pyridazine by the second simple bridging ligand. Eur. J. Inorg. Chem. 2006, 2006, 1381–1387. [Google Scholar] [CrossRef]
- Alsalme, A.; Al-Farhan, K.; Ghazzali, M.; Khair, M.; Khan, R.A.; Reedijk, J. Structure of bis(nitrato)tetrakis(pyrazole)cobalt(II): Fine tuning in the stabilization of coordination entities by using intramolecular hydrogen bonding. Inorg. Chim. Acta 2013, 407, 7–10. [Google Scholar] [CrossRef]
- Hadadzadeh, H.; Salimi, M.; Weil, M.; Behnamfar, M.T.; Darabi, F. Complex conversion of the redox pair CoIII-NO2 to CoII-NO3: Synthesis, crystal structure and DNA-binding of trans, trans, trans-[Co(py)2(H2O)2(NO3)2]. Polyhedron 2013, 53, 179–186. [Google Scholar] [CrossRef]
- Marandi, F.; Moeini, K.; Krautscheid, H. A pyridil-triazine complex of Co(II): Spectroscopic, structural, and docking studies. J. Struct. Chem. 2021, 62, 590–597. [Google Scholar] [CrossRef]
- Kleywegt, G.Y.; Wiesmeijer, W.G.R.; Van Driel, G.J.; Driessen, W.L.; Reedijk, J.; Noordik, J.H. Unidentate versus symmetrically and unsymmetrically bidentate nitrate co-ordination in pyrazole-containing chelates. The crystal and molecular structures of (nitrato-O) [tris(3,5-dimethylpyrazol-l-ylmethyl)amine] copper(II) nitrate, (nitrato-O,O’) [tris(3,5-dimethyl pyrazol-1-ylmethyl)amine] nickel(II) nitrate, and (nitrato-O)(nitrato-O,O’) [tris(3,5-dimethylpyrazol-l-ylmethyl)-amine] cadmium(II). J. Chem. Soc. Dalton Trans. 1985, 2177–2184. [Google Scholar] [CrossRef]
- Milinski, N.; Ribár, B.; Culum, Z.; Djuric, S. The crystal structure of manganese nitrate monohydrate. Acta Cryst. 1977, B33, 1678–1682. [Google Scholar] [CrossRef]
- Keuleers, R.; Papaefstathiou, G.S.; Raptopoulou, C.P.; Tangoulis, V.; Desseyn, H.O.; Perlepes, S.P. Tris (N,N-dimethylurea) bis (nitrato-O,O’) manganese(II), the first example of a seven-coordinate manganese(II) complex with a monodentate organic ligand. Inorg. Chem. Comm. 1999, 2, 472–475. [Google Scholar] [CrossRef]
- Yang, E.-C.; Harden, N.; Wernsdorfer, W.; Zakharov, L.; Brechin, E.K.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Mn4 single-molecule magnets with a planar diamond core and S = 9. Polyhedron 2003, 22, 1857–1863. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 64–67. [Google Scholar]
- Svorec, J.; Polakovičová, P.; Moncol, J.; Kuchtanin, V.; Breza, M.; Šoralová, S.; Padělková, Z.; Mrozinski, J.; Lis, T.; Segľa, P. Structural, magnetic and quantum-chemical study of dinuclear copper(II) thiophenecarboxylate and furancarboxylate complexes. Polyhedron 2014, 81, 216–226. [Google Scholar] [CrossRef]
- Scatena, R.; Massignani, S.; Lanza, A.E.; Zorzi, F.; Monari, M.; Nestola, F.; Pettinari, C.; Pandolfo, L. Synthesis of coordination polymers and discrete complexes from the reaction of copper(II) carboxylates with pyrazole: Role of carboxylates basicity. Cryst. Growth Des. 2022, 22, 1032–1044. [Google Scholar] [CrossRef]
- SADABS, v 2016/2 Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2016.
- SAINTV8.38A Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2018.
- SHELXTL 2018, Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. B 2013, 69, 249–259. [Google Scholar] [CrossRef]

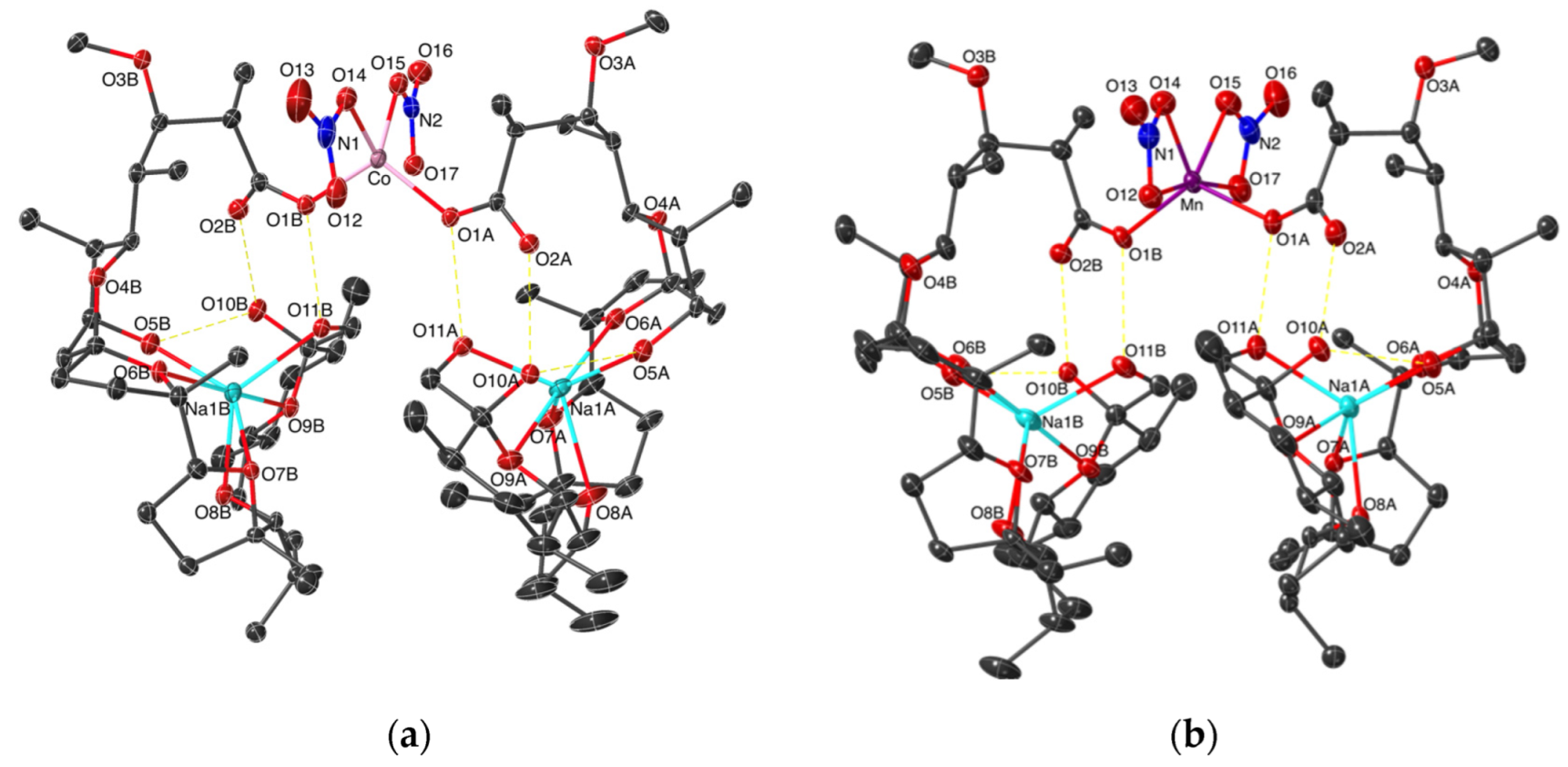
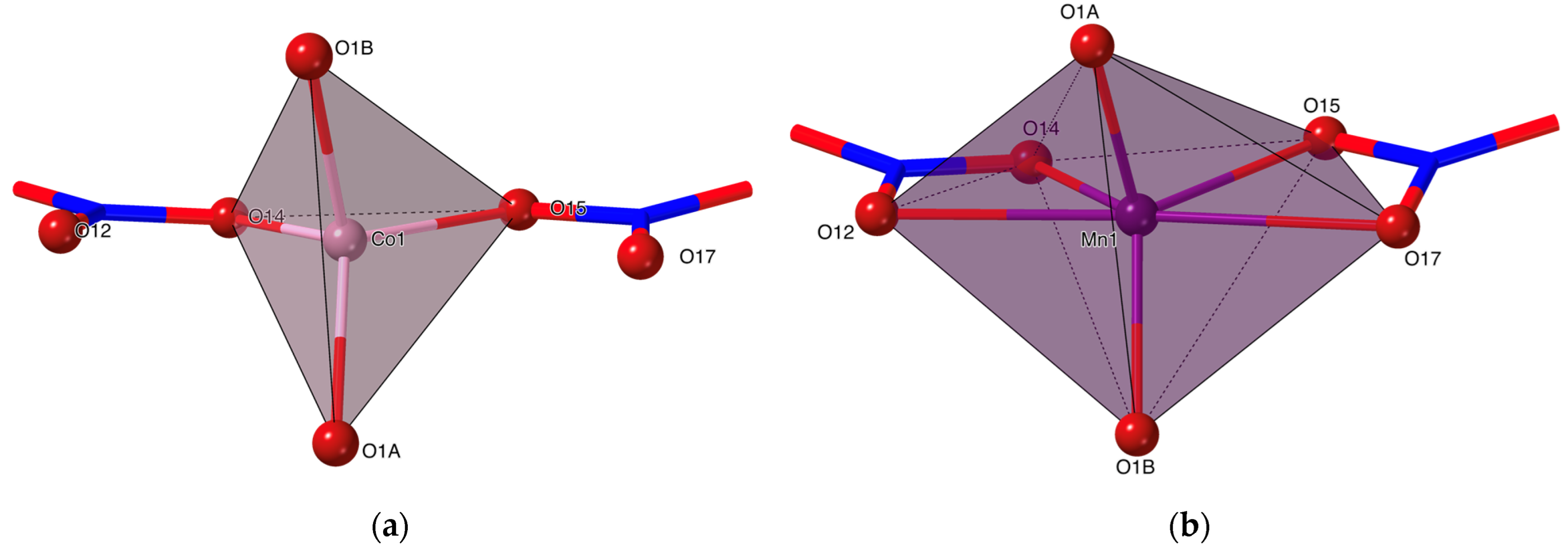
| [Co(MonNa)2(NO3)2], 2 | [Mn(MonNa)2(NO3)2], 3 | |
|---|---|---|
| M-O1A | 1.985(5) | 2.057(4) |
| M-O1B | 1.978(5) | 2.077(4) |
| M-O12 | 2.682(7) | 2.377(5) |
| M-O13 | 3.903(6) | 3.902 (6) |
| M-O14 | 1.997(5) | 2.202(5) |
| M-O15 | 2.012(6) | 2.149(5) |
| M-O16 | 3.876(6) | 3.928(5) |
| M-O17 | 2.564(6) | 2.489(5) |
| M-N1 | 2.736(7) | 2.680(7) |
| M-N2 | 2.671(7) | 2.725(6) |
| O1A-M-O1B | 112.8(2) | 121.5(2) |
| O1A-M-O14 | 114.9(2) | 107.8(2) |
| O1A-M-O15 | 117.9(2) | 104.0(2) |
| O1B-M-O14 | 109.7(2) | 117.8(2) |
| O1B-M-O15 | 108.8(2) | 117.6(2) |
| M-N1-O13 | 160.1(8) | 172.6(6) |
| M-N2-O16 | 164.5(6) | 169.7(6) |
| M-O12-N1 | 79.4(5) | 89.0(4) |
| M-O14-N1 | 110.9(5) | 97.5(4) |
| M-O15-N2 | 105.9(5) | 102.2(4) |
| M-O17-N2 | 81.0(4) | 86.8(4) |
| Bond | MonNa [38], 295 K | Complex 2, 296 K | MonNa 1, 107 K | Complex 3, 107 K |
|---|---|---|---|---|
| Subunits A/B | Subunits A/B | |||
| Na-O5H | 2.341(2) | 2.330(6)/2.349(6) | 2.332(2) | 2.349(5)/2.327(6) |
| Na-O6 | 2.328(1) | 2.309(6)/2.312(5) | 2.313(2) | 2.319(4)/2.307(6) |
| Na-O7 | 2.603(1) | 2.453(6)/2.482(5) | 2.582(2) | 2.489(5)/2.470(1) |
| Na-O8 | 2.450(1) | 2.394(7)/2.383(6) | 2.436(2) | 2.376(5)/2.402(6) |
| Na-O9 | 2.409(1) | 2.439(6)/2.459(6) | 2.389(2) | 2.457(5)/2.423(6) |
| Na-O11H | 2.383(1) | 2.329(6)/2.340(6) | 2.370(2) | 2.329(5)/2.313(5) |
| O1-C1 | 1.245(2) | 1.281(9)/1.297(9) | 1.255(3) | 1.286(8)/1.284(7) |
| O2-C1 | 1.261(2) | 1.246(9)/1.255(9) | 1.266(3) | 1.228(9)/1.231(8) |
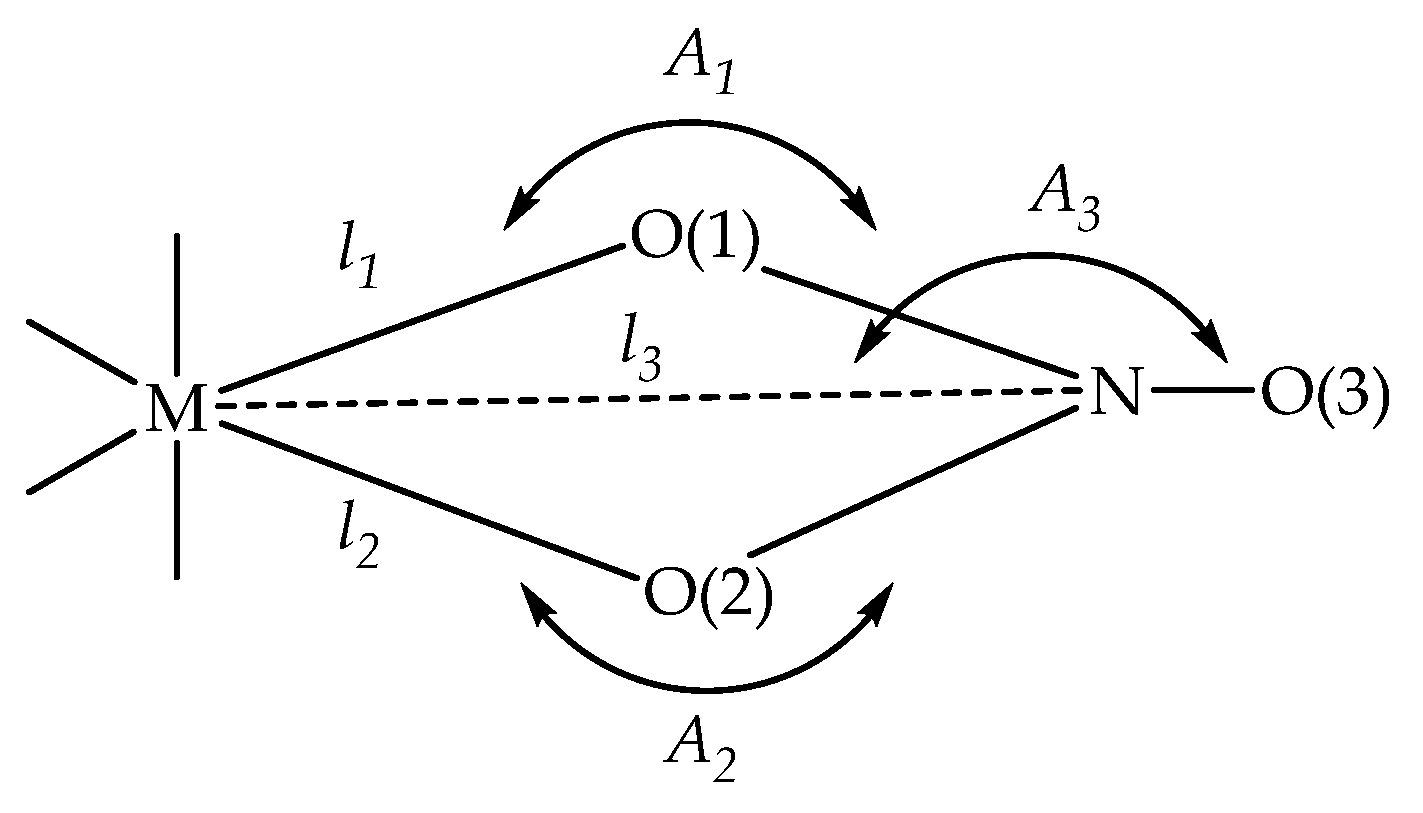
| Criteria | Unidentate | Bidentate |
|---|---|---|
| l2–l1, Å | >0.6 | <0.3 |
| A1–A2, ° | >28 | <14 |
| l3–l2, Å | <0.1 | >0.2 |
| A3, ° | <162 | >168 |
| Co(II) Complex 2 | Mn(II) Complex 3 | ||||||
|---|---|---|---|---|---|---|---|
| Nitrate 1 | Nitrate 2 | Nitrate 1 | Nitrate 2 | ||||
| l1: Co-O14 | 1.977 | l1: Co-O15 | 2.012 | l1: Mn-O14 | 2.202 | l1: Mn-O15 | 2.149 |
| l2: Co-O12 | 2.682 | l2: Co-O17 | 2.564 | l2: Mn-O12 | 2.377 | l2: Mn-O17 | 2.489 |
| l3: Co-N1 | 2.736 | l3: Co-N2 | 2.671 | l3: Mn-N1 | 2.680 | l3: Mn-N2 | 2.725 |
| A1 | 110.9 | 105.9 | 97.5 | 102.2 | |||
| A2 | 79.4 | 81.0 | 89.0 | 86.8 | |||
| l2–l1, Å | 0.705 | 0.552 | 0.175 | 0.340 | |||
| A1–A2, ° | 31.5 | 24.9 | 8.5 | 15.4 | |||
| l3–l2, Å | 0.054 | 0.107 | 0.303 | 0.236 | |||
| A3, ° | 160.1 | 164.5 | 172.6 | 169.7 | |||
| Bond | MonNa [38], 295 K | Complex 2, 296 K | MonNa 1, 107 K | Complex 3, 107 K |
|---|---|---|---|---|
| Subunits A/B | Subunits A/B | |||
| O1···HO11 | 2.554 | 2.760/2.695 | 2.539 | 2.673/2.729 |
| O2···HO10 | 2.684 | 2.642/2.624 | 2.677 | 2.627/2.624 |
| HO10···HO5 | 2.880 | 2.891/2.841 | 2.903 | 2.811/2.844 |
| N1S···HO5 | 2.980 | − | 2.925 | − |
| Bacterial Strain | Minimum Inhibitory Concentration, µM | ||||
|---|---|---|---|---|---|
| BS | BC | KR | SA | SS | |
| MonNa, 1 | 90.0 | 2.8 | 180 | 45.0 | 45.0 |
| [Co(MonNa)2(NO3)2], 2 | 19.7 | 2.5 | 39.3 | 19.7 | 9.8 |
| [Mn(MonNa)2(NO3)2], 3 | 10.0 | 5.0 | 40.0 | 20.0 | 20.0 |
| [Co(MonNa)2Cl2] | 20.6 | 5.2 | 41.2 | 20.6 | 20.6 |
| [Mn(MonNa)2Cl2] | 10.3 | 5.2 | 41.3 | 20.7 | 20.7 |
| Crystal Data | MonNa × MeCN, 1 | Complex 2 | Complex 3 |
|---|---|---|---|
| Chemical formula | C38H64NNaO11 | C73H123.5CoN2.5Na2O28 | C72H122MnN2Na2O28 |
| Mr | 733.89 | 1589.15 | 1564.63 |
| Crystal system, space group | Orthorhombic, P 21 21 21 | Monoclinic, P21 | Monoclinic, P21 |
| Temperature (K) | 107.00 | 296.15 | 106.99 |
| a (Å) | 12.7546(3) | 12.2955(3) | 12.2549(3) |
| b (Å) | 16.3176(4) | 23.9817(6) | 23.6776(6) |
| c (Å) | 18.4699(5) | 13.5662(4) | 13.6701(3) |
| V (Å3) | 3844.04(17) | 3997.63(18) | 3963.82(16) |
| Z | 4 | 2 | 2 |
| Radiation type, λ [Å] | Cu Kα, 1.54178 | ||
| µ (mm−1) | 0.844 | 2.465 | 2.134 |
| Crystal size (mm3) | 0.25 × 0.19 × 0.17 | 0.201 × 0.142 × 0.131 | 0.192 × 0.174 × 0.085 |
| Data collection | |||
| Diffractometer | Bruker APEX-II CCD | ||
| Absorption correction | Multi-scan | ||
| Tmin, Tmax | 0.610, 0.753 | 0.572, 0.753 | 0.559, 0.753 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 21,811, 6775, 6530 | 53,699, 13,879, 13,172 | 45,614, 14,062, 11,472 |
| Rint | 0.0475 | 0.0555 | 0.0720 |
| Refinement | |||
| R[F2 > 2σ(F2)], wR(F2), S | 0.0346, 0.0895, 1.028 | 0.0694, 0.1781, 1.088 | 0.0604, 0.1416, 1.022 |
| No. of reflections | 6775 | 13879 | 14062 |
| No. of parameters | 478 | 1003 | 1071 |
| No. of restraints | 0 | 7 | 40 |
| Δρmax, Δρmin (e Å−3) | 0.27/−0.34 | 0.45/−0.70 | 0.46/−0.47 |
| Flack x determined using quotients [(I+)-(I−)]/[(I+)+(I−)] [64] | |||
| Abs. str. param.: Flack Hooft | −0.02(9) −0.03(4) | 0.042(6) −0.0168(15) | 0.020(6) 0.019(8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkov, N.; Boyadzhiev, M.; Bozhilova, N.; Dorkov, P.; Encheva, E.; Ugrinov, A.; Pantcheva, I.N. Cobalt(II) and Manganese(II) Complexes of Sodium Monensinate A Bearing Nitrate Co-Ligands. Int. J. Mol. Sci. 2024, 25, 12129. https://doi.org/10.3390/ijms252212129
Petkov N, Boyadzhiev M, Bozhilova N, Dorkov P, Encheva E, Ugrinov A, Pantcheva IN. Cobalt(II) and Manganese(II) Complexes of Sodium Monensinate A Bearing Nitrate Co-Ligands. International Journal of Molecular Sciences. 2024; 25(22):12129. https://doi.org/10.3390/ijms252212129
Chicago/Turabian StylePetkov, Nikolay, Miroslav Boyadzhiev, Nikita Bozhilova, Petar Dorkov, Elzhana Encheva, Angel Ugrinov, and Ivayla N. Pantcheva. 2024. "Cobalt(II) and Manganese(II) Complexes of Sodium Monensinate A Bearing Nitrate Co-Ligands" International Journal of Molecular Sciences 25, no. 22: 12129. https://doi.org/10.3390/ijms252212129
APA StylePetkov, N., Boyadzhiev, M., Bozhilova, N., Dorkov, P., Encheva, E., Ugrinov, A., & Pantcheva, I. N. (2024). Cobalt(II) and Manganese(II) Complexes of Sodium Monensinate A Bearing Nitrate Co-Ligands. International Journal of Molecular Sciences, 25(22), 12129. https://doi.org/10.3390/ijms252212129








