The Effect of Pomegranate Peel Extract on the Oxidative and Inflammatory Status in the Spleens of Rats with Metabolic Syndrome
Abstract
1. Introduction
2. Results
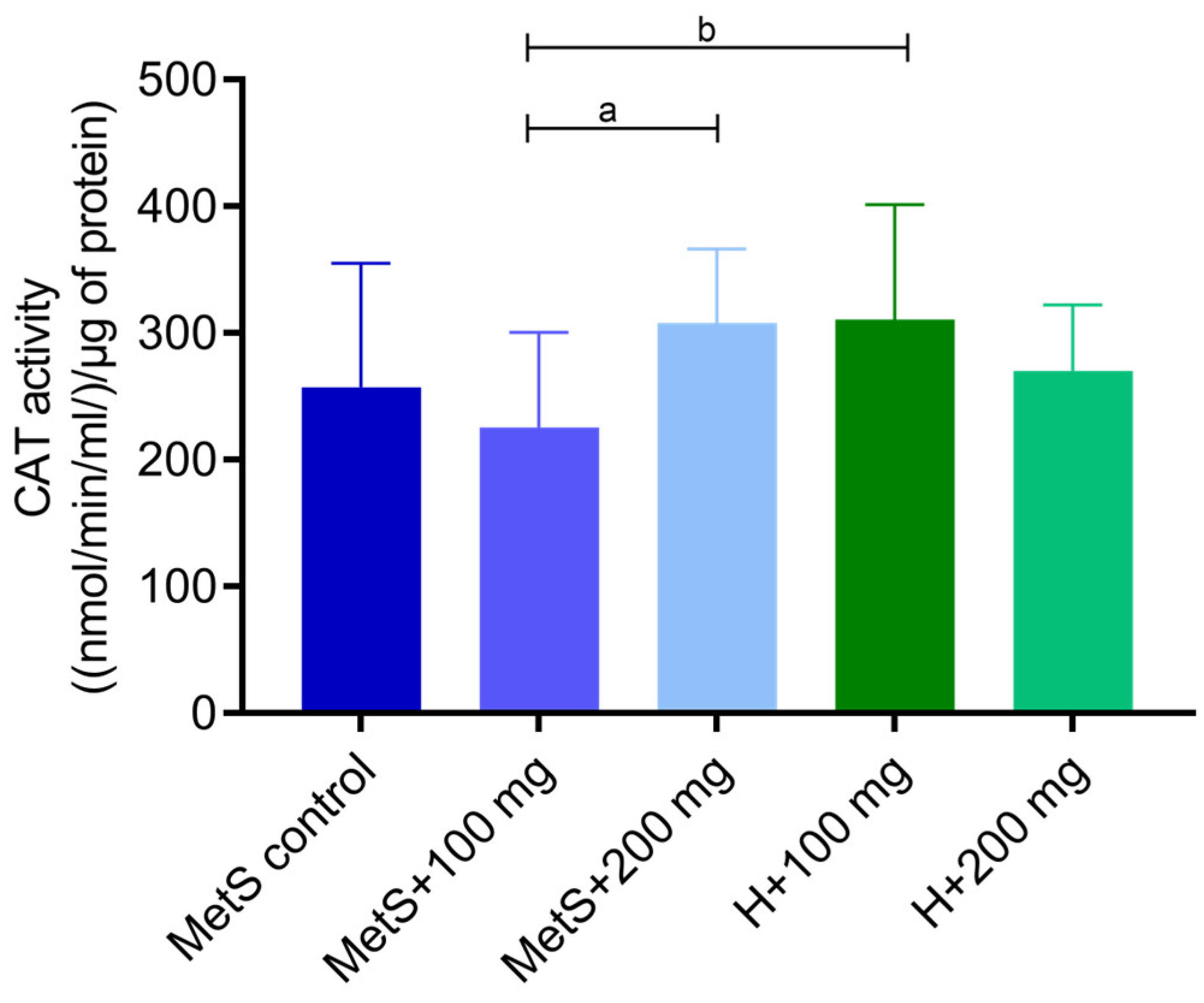
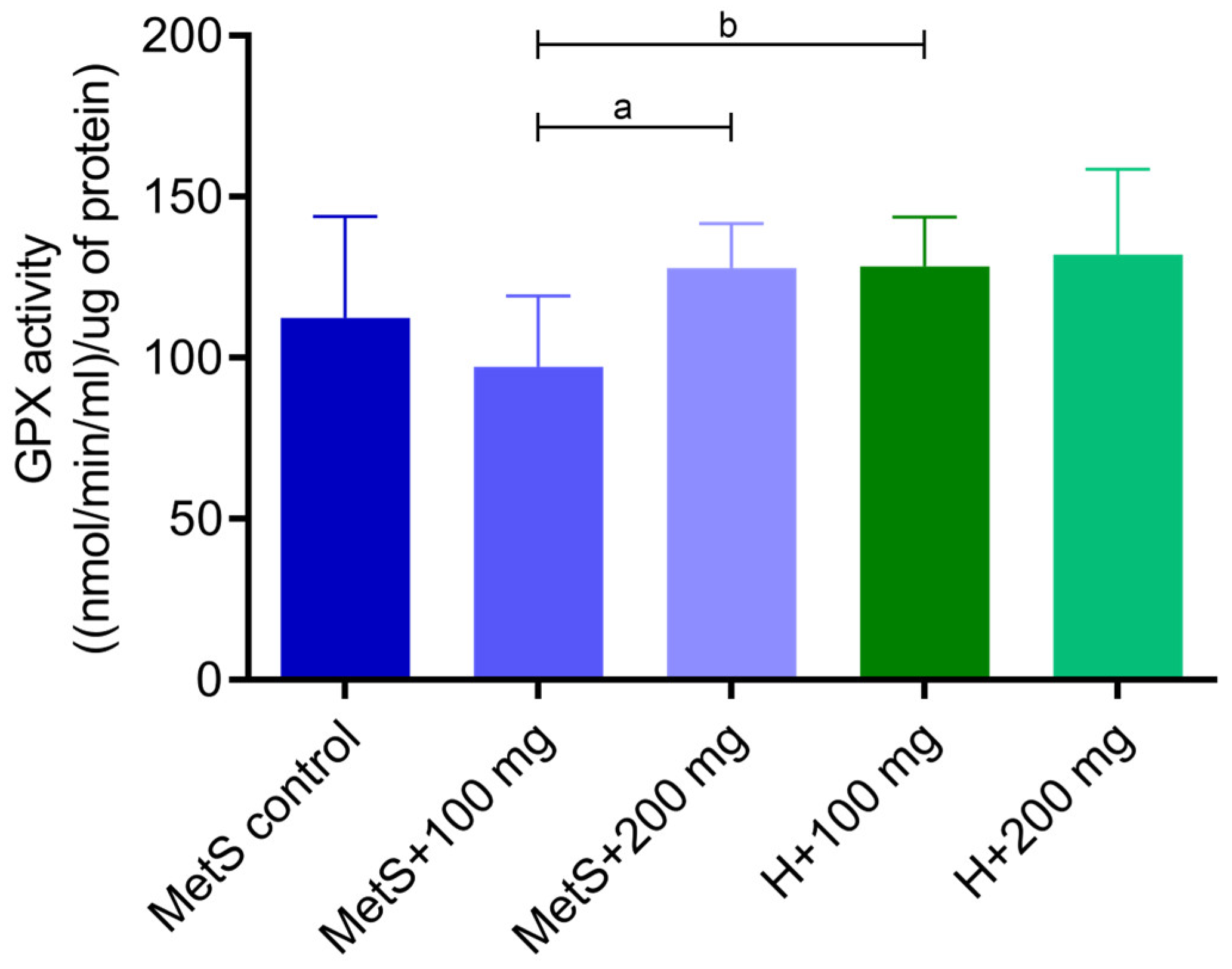
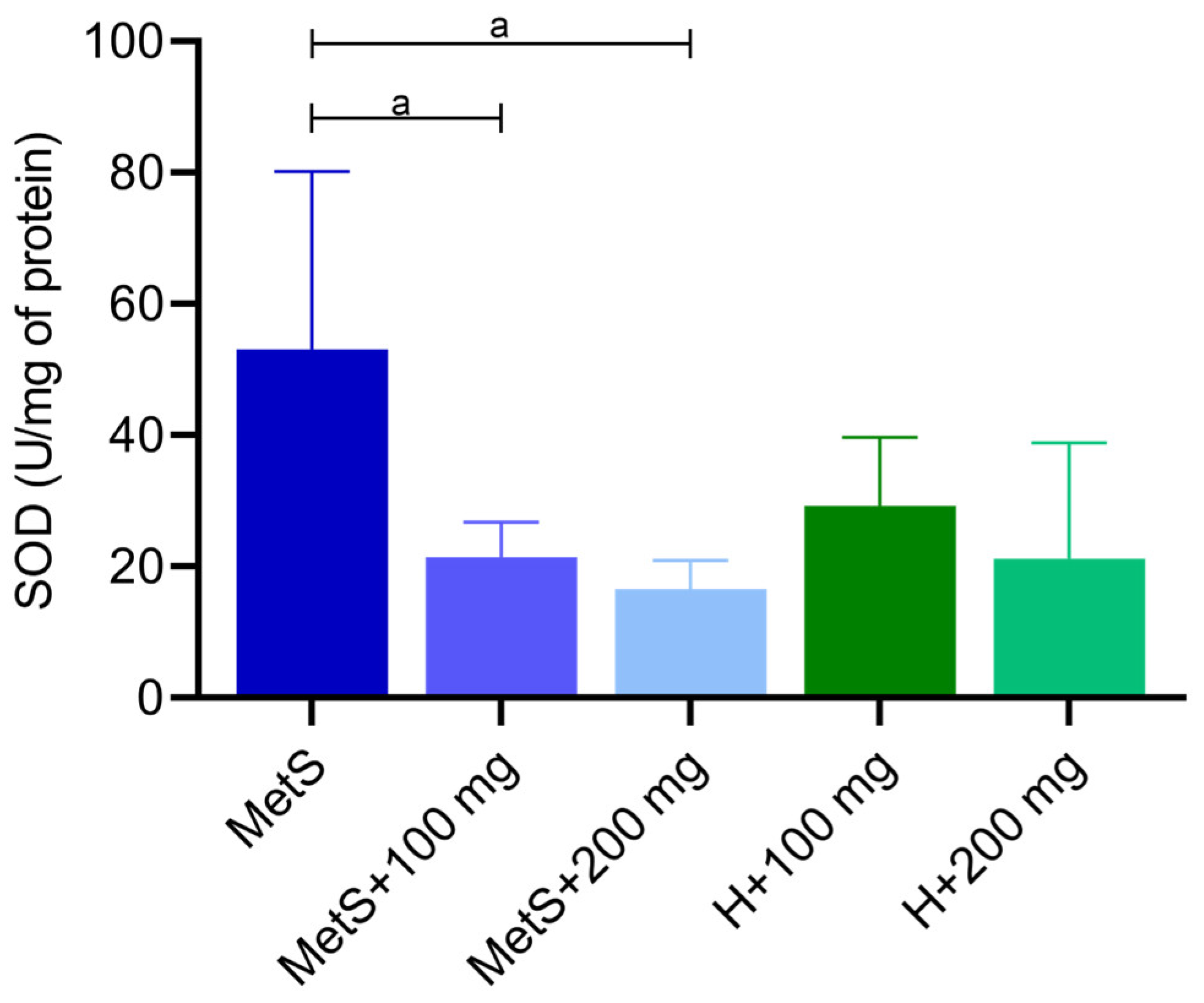
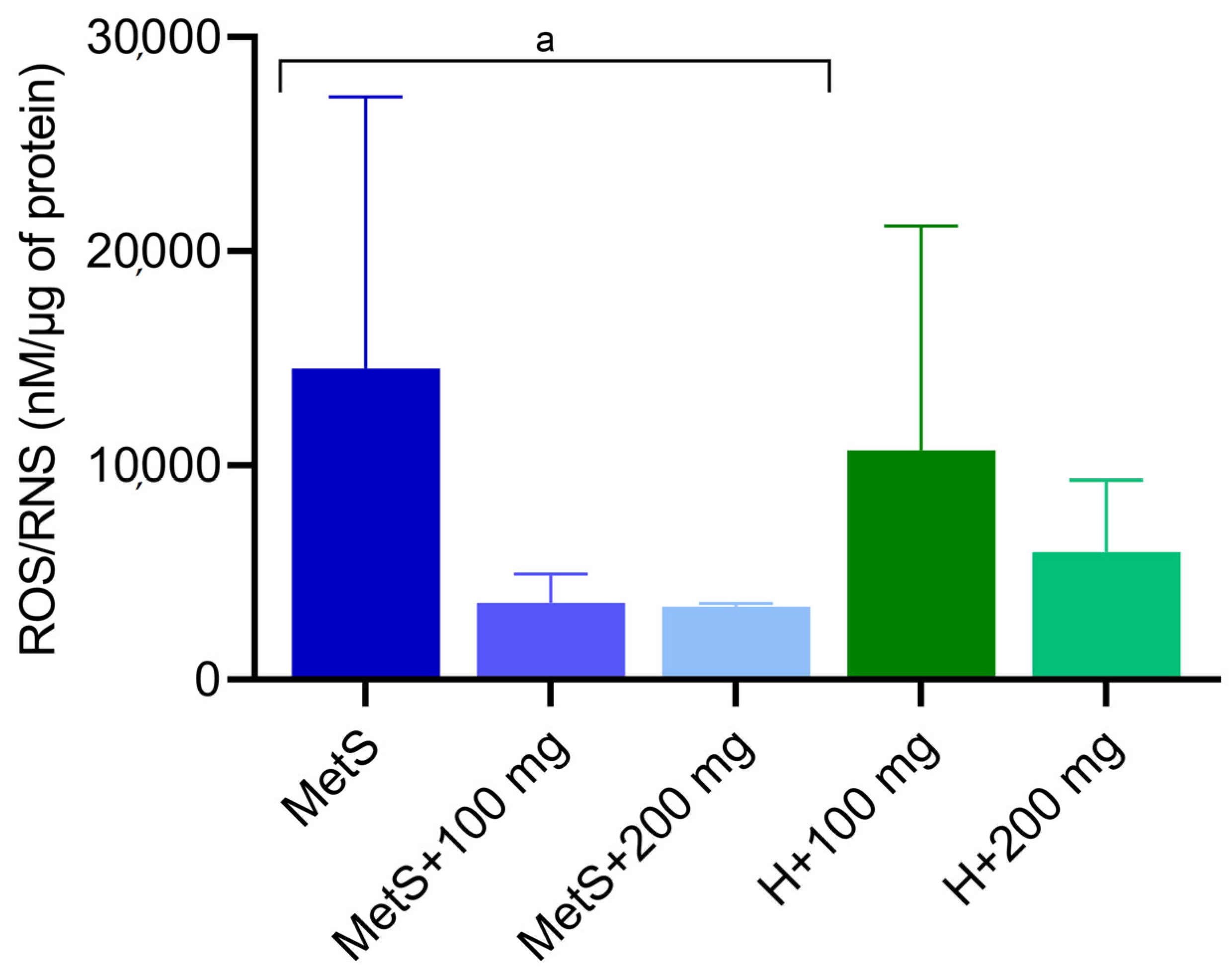
2.1. Oxidative Status
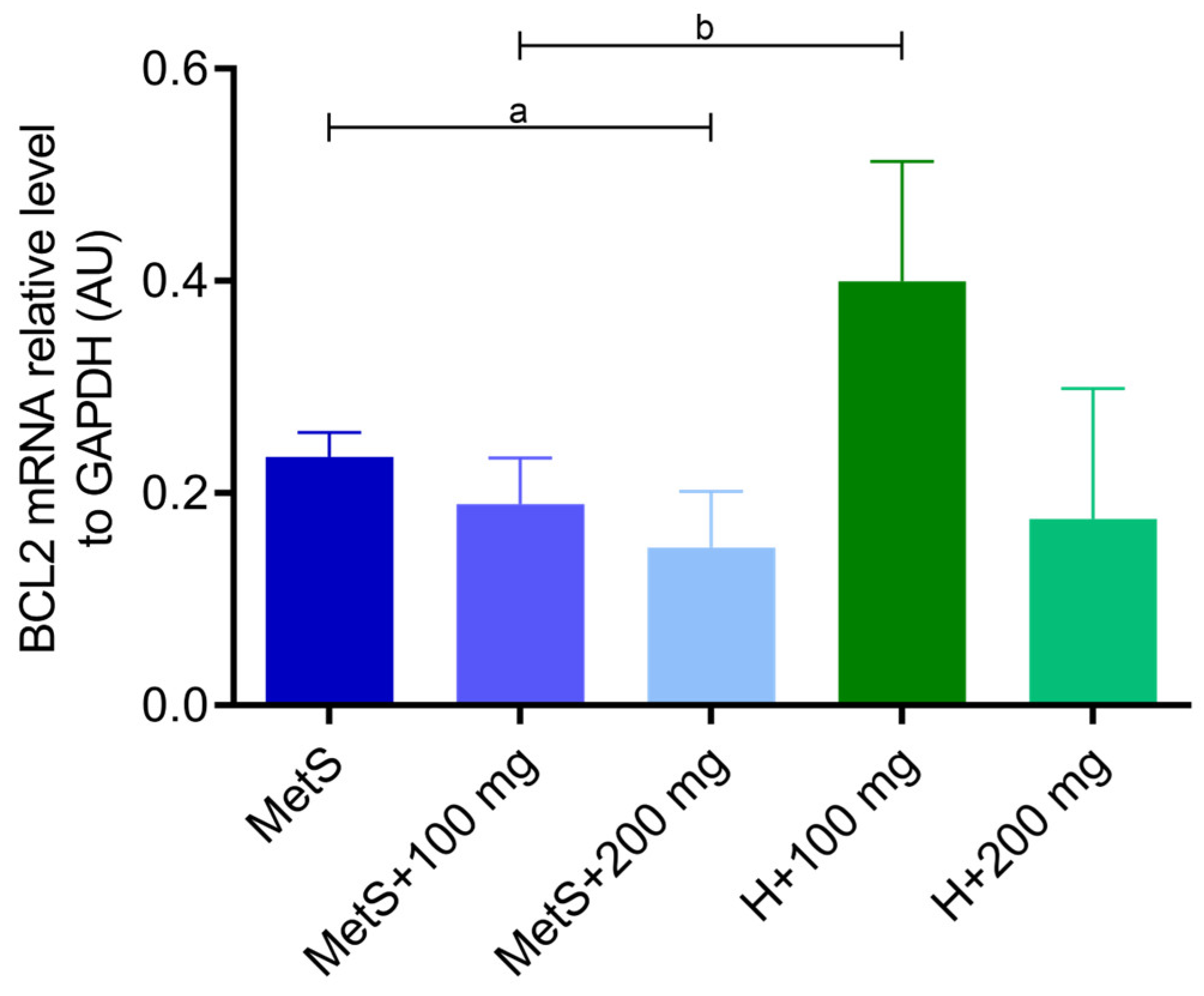
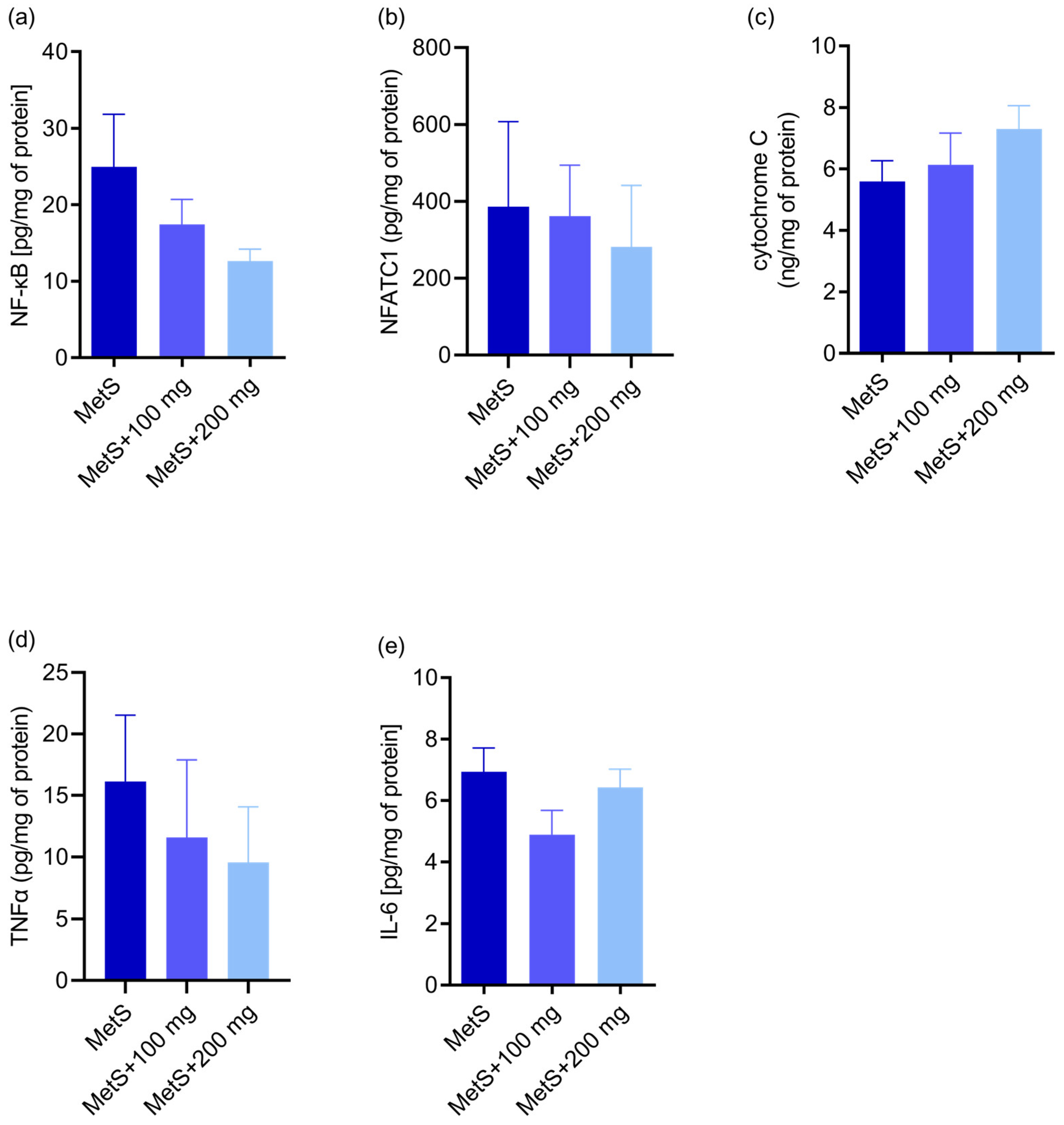
2.2. Apoptosis and Inflammation
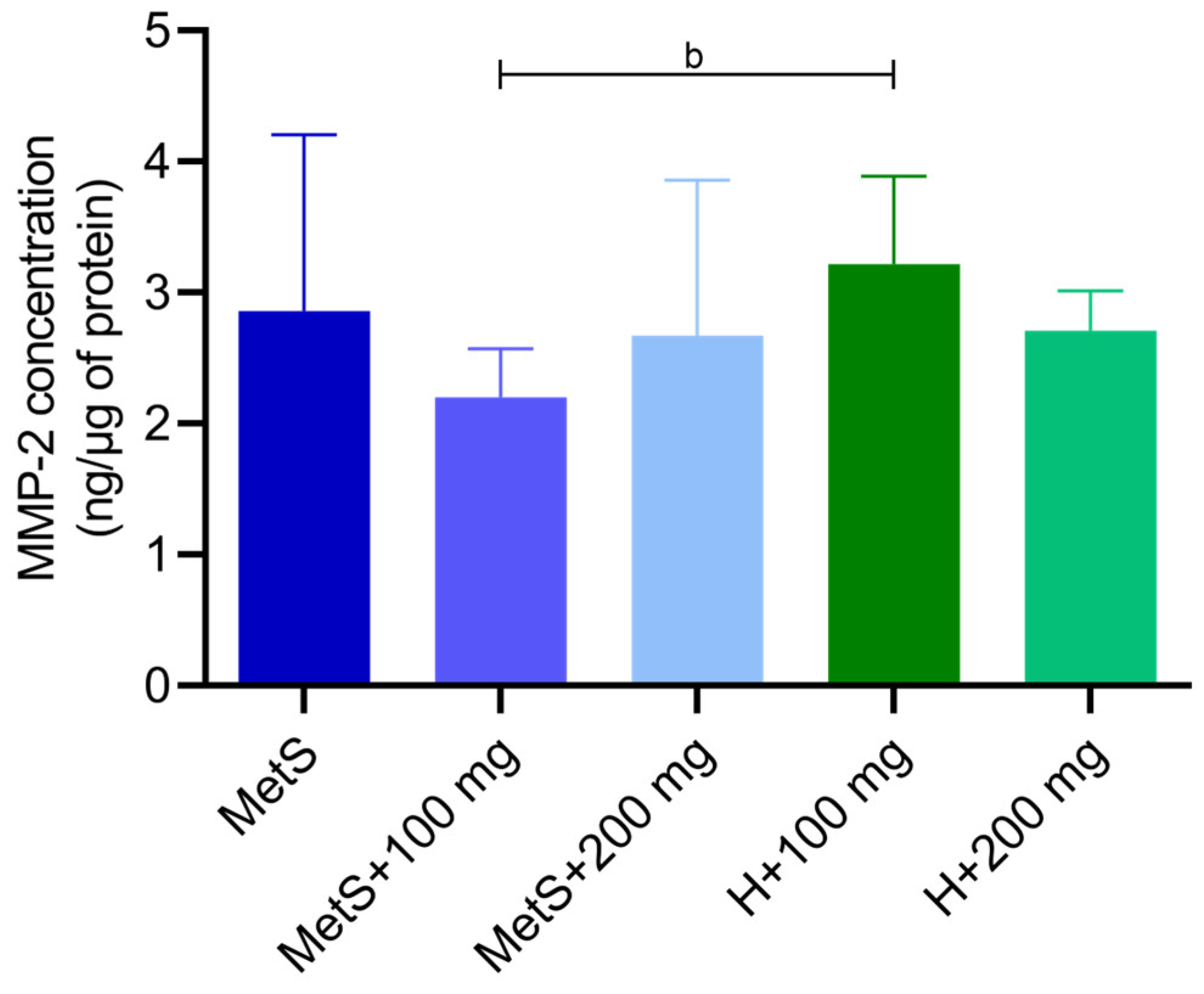
2.3. MMP-9 and MMP-2 Activity and MMP-2 Concentration
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Preparation of Polyphenol Extract
4.3. Spleen Isolation
4.4. Preparation of Tissue Homogenates
4.5. RNA Isolation and qRT-PCR
4.6. Enzyme-Linked Immunosorbent Assays
4.7. Glutathione Peroxidase Activity
4.8. Lipid Oxidation Level
4.9. Superoxide Dismutase Activity
4.10. ROS/RNS
4.11. Catalase Activity
4.12. Zymography
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishfaq, M.; Chen, C.; Bao, J.; Zhang, W.; Wu, Z.; Wang, J.; Liu, Y.; Tian, E.; Hamid, S.; Li, R.; et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019, 98, 6296–6310. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Fontana, S.; Laforgia, F.; Dragone, T.; Jirillo, E.; Passantino, L. Administration of a Polyphenol-Enriched Feed to Farmed Sea Bass (Dicentrarchus labrax L.) Modulates Intestinal and Spleen Immune Responses. Oxidative Med. Cell. Longev. 2015, 2016, 2827567. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free. Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef]
- Fathy, S.M.; Abdelkader, I.Y. Effect of resveratrol on the inflammatory status and oxidative stress in thymus gland and spleen of sulfoxaflor-treated rats. Environ. Toxicol. 2021, 36, 1326–1337. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokkas, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, J.; Zhang, Z.; Wang, H.; Guo, Y.; Liu, M.; Tian, C.; Zhang, P.; Yang, J.; et al. The protective effect of the flavonoid fraction of Abutilon theophrasti Medic. leaves on LPS-induced acute lung injury in mice via the NF-κB and MAPK signalling pathways. Biomed. Pharmacother. 2018, 109, 1024–1031. [Google Scholar] [CrossRef]
- Akbel, E.; Arslan-Acaroz, D.; Demirel, H.H.; Kucukkurt, I.; Ince, S. The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats: The protective role of resveratrol. Toxicol. Res. 2018, 7, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.R.; Karam, H.M. Resveratrol attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR signaling pathway. Environ. Toxicol. 2019, 35, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Afshari-Kaveh, M.; Abbasalipourkabir, R.; Nourian, A.; Ziamajidi, N. The Protective Effects of Vitamins A and E on Titanium Dioxide Nanoparticles (nTiO2)-Induced Oxidative Stress in the Spleen Tissues of Male Wistar Rats. Biol. Trace Element Res. 2020, 199, 3677–3687. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin Alleviates Lipopolysaccharide-Induced Liver Inflammation in Chicken by Suppressing TLR4-Mediated NF-κB Pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Fahmy, H.A.; Farag, M.A. Ongoing and potential novel trends of pomegranate fruit peel; a comprehensive review of its health benefits and future perspectives as nutraceutical. J. Food Biochem. 2021, 46, e14024. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Wang, L.; Irfan, S.; Safdar, M.N.; Murtaza, M.A.; Nadeem, M.; Mahmood, S.; Mueen-Ud-Din, G.; Nadeem, H.R. A comprehensive review on phytochemistry, bioactivity and medicinal value of bioactive compounds of pomegranate (Punica granatum). Orient. Pharm. Exp. Med. 2021, 23, 37–57. [Google Scholar] [CrossRef]
- Niewiadomska, J.; Kasztura, M.; Janus, I.; Chełmecka, E.; Stygar, D.M.; Frydrychowski, P.; Wojdyło, A.; Noszczyk-Nowak, A. Punica granatum L. Extract Shows Cardioprotective Effects Measured by Oxidative Stress Markers and Biomarkers of Heart Failure in an Animal Model of Metabolic Syndrome. Antioxidants 2023, 12, 1152. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Fan, R.-F.; Liu, J.-X.; Yan, Y.-X.; Wang, L.; Wang, Z.-Y. Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult. Sci. 2020, 99, 5430–5439. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G. Spleen: A new role for an old player? World J. Gastroenterol. 2011, 17, 3776–3784. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, R.A.; Naguib, M.; Rashed, L.A. Spleen size in patients with metabolic syndrome and its relation to metabolic and inflammatory parameters. Egypt. J. Intern. Med. 2018, 30, 78–82. [Google Scholar] [CrossRef]
- Wong, R.J.; Ahmed, A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J. Hepatol. 2014, 6, 263–273. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Pizza, G.; De Rosa, A.; Nedi, V.; Rossi, A.; Orio, F.; Lombardi, G.; Colao, A.; Tarantino, G. Liver-spleen axis, insulin-like growth factor-(IGF)-I axis and fat mass in overweight/obese females. J. Transl. Med. 2011, 9, 136. [Google Scholar] [CrossRef]
- Tsushima, Y.; Endo, K. Spleen Enlargement in Patients with Nonalcoholic Fatty Liver. Dig. Dis. Sci. 2000, 45, 196–200. [Google Scholar] [CrossRef]
- Tarantino, G.; Conca, P.; Pasanisi, F.; Ariello, M.; Mastrolia, M.; Arena, A.; Vecchione, R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur. J. Gastroenterol. Hepatol. 2009, 21, 504–511. [Google Scholar] [CrossRef]
- Rousset, S.; Emre, Y.; Joinlambert, O.; Hurtaud, C.; Ricquier, D.; Cassarddoulcier, A. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine 2006, 35, 135–142. [Google Scholar] [CrossRef]
- Latini, G.; De Mitri, B.; Del Vecchio, A.; Chitano, G.; De Felice, C.; Zetterström, R. Foetal growth of kidneys, liver and spleen in intrauterine growth restriction: “programming” causing “metabolic syndrome” in adult age. Acta Paediatr. 2004, 93, 1635–1639. [Google Scholar] [CrossRef]
- Tarantino, G.; Colicchio, P.; Conca, P.; Finelli, C.; Di Minno, M.N.D.; Tarantino, M.; Capone, D.; Pasanisi, F. Young adult obese subjects with and without insulin resistance: What is the role of chronic inflammation and how to weigh it non-invasively? J. Inflamm. 2009, 6, 1–6. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pr. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mahesar, S.A.; Kori, A.H.; Sherazi, S.T.H.; Kandhro, A.A.; Laghari, Z.H. Pomegranate (Punica Granatum) Seed Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Zug, Switzerland, 2019; pp. 691–709. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2012, 4, 19–39. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free. Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Radajewska, A.; Szyller, J.; Niewiadomska, J.; Noszczyk-Nowak, A.; Bil-Lula, I. Punica granatum L. Polyphenolic Extract as an Antioxidant to Prevent Kidney Injury in Metabolic Syndrome Rats. Oxidative Med. Cell. Longev. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Gheorghe, A.; de Heredia, F.P.; Hunsche, C.; Redondo, N.; Díaz, L.E.; Hernández, O.; Marcos, A.; De la Fuente, M. Oxidative stress and immunosenescence in spleen of obese mice can be reversed by 2-hydroxyoleic acid. Exp. Physiol. 2017, 102, 533–544. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Introduction to Free Radicals and the Body’s Antioxidant Defense. In Antioxidants in Food, Vitamins and Supplements; Dasgupta, A., Klein, K., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 1–18. [Google Scholar] [CrossRef]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. Int. J. Mol. Sci. 2020, 21, 2035. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Aradhya, S.M.; Divakar, S. Isolation and identification of a radical scavenging antioxidant—Punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chem. 2004, 87, 551–557. [Google Scholar] [CrossRef]
- Liu, H.; Ren, J.; Chen, H.; Huang, Y.; Li, H.; Zhang, Z.; Wang, J. Resveratrol Protects against Cigarette Smoke–Induced Oxidative Damage and Pulmonary Inflammation. J. Biochem. Mol. Toxicol. 2014, 28, 465–471. [Google Scholar] [CrossRef]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef]
- Chang, C.-C. Effect of Resveratrol on Oxidative and Inflammatory Stress in Liver and Spleen of Streptozotocin-Induced Type 1 Diabetic Rats. Chin. J. Physiol. 2012, 55, 192–201. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Lv, L.; Li, P.; Ma, C.; He, S.; Zeng, J.; Ping, F.; Zhang, H.; Li, W.; et al. Relationship between Decreased Serum Superoxide Dismutase Activity and Metabolic Syndrome: Synergistic Mediating Role of Insulin Resistance and β-Cell Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Denisenko, Y.K.; Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.V.; Gvozdenko, T.A.; Kantur, T.A. Lipid-Induced Mechanisms of Metabolic Syndrome. J. Obes. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- De Leon, J.A.D.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, e61122. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Aviram, M. Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages. J. Nutr. Biochem. 2005, 16, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Song, E.-K.; Park, H.; Kim, H.-S. Additive effect of walnut and chokeberry on regulation of antioxidant enzyme gene expression and attenuation of lipid peroxidation in d-galactose-induced aging-mouse model. Nutr. Res. 2019, 70, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Amri, Z.; Ghorbel, A.; Turki, M.; Akrout, F.M.; Ayadi, F.; Elfeki, A.; Hammami, M. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Khorramabadi, R.M.; Assadollahi, V.; Khosravi, P.; Venol, A.C.; Veiskerami, S.; Ahmadvand, H. The effects of pomegranate peel extract on the gene expressions of antioxidant enzymes in a rat model of alloxan-induced diabetes. Arch. Physiol. Biochem. 2021, 129, 870–878. [Google Scholar] [CrossRef]
- Parsaeyan, N.; Mozaffari–Khosravi, H.; Mozayan, M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012, 11, 11. [Google Scholar] [CrossRef]
- Sohrab, G.; Angoorani, P.; Tohidi, M.; Tabibi, H.; Kimiagar, M.; Nasrollahzadeh, J. Pomegranate (Punicagranatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: A randomized double-blind clinical trial. Food Nutr. Res. 2015, 59, 28551. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Grujić, V.R.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57. [Google Scholar] [CrossRef]
- Oliva, M.E.; Cian, R.E.; Ferreira, M.d.R.; Garzón, A.G.; Drago, S.R.; D’Alessandro, M.E. In vivo and in silico study of antioxidant and anti-inflammatory effects on the liver-spleen axis of microencapsulated brewers’ spent grain peptides. Food Funct. 2023, 14, 5290–5300. [Google Scholar] [CrossRef]
- Oveland, E.; Karlsen, T.V.; Haslene-Hox, H.; Semaeva, E.; Janaczyk, B.; Tenstad, O.; Wiig, H. Proteomic Evaluation of Inflammatory Proteins in Rat Spleen Interstitial Fluid and Lymph during LPS-Induced Systemic Inflammation Reveals Increased Levels of ADAMST1. J. Proteome Res. 2012, 11, 5338–5349. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; He, F.; Huang, M.; Peng, M.; Zhou, Z.; Liu, F.; Dai, Y.-S. NFATc3 deficiency reduces the classical activation of adipose tissue macrophages. J. Mol. Endocrinol. 2018, 61, 79–89. [Google Scholar] [CrossRef]
- Ackerman, Z.; Grozovski, M.; Oron-Herman, M.; Rosenthal, T.; Sela, B.; Amir, G. Effects of Antihypertensive and Triglyceride Lowering Agents on Splenocyte Apoptosis in Rats with Fatty Liver. Basic Clin. Pharmacol. Toxicol. 2013, 113, 37–42. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Briscini, L.; Tonello, C.; Dioni, L.; Carruba, M.O.; Nisoli, E. Bcl-2 and Bax are involved in the sympathetic protection of brown adipocytes from obesity-linked apoptosis. FEBS Lett. 1998, 431, 80–84. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.-Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020, 131, 110769. [Google Scholar] [CrossRef]
- Zhao, F.; Pang, W.; Zhang, Z.; Zhao, J.; Wang, X.; Liu, Y.; Wang, X.; Feng, Z.; Zhang, Y.; Sun, W.; et al. Pomegranate extract and exercise provide additive benefits on improvement of immune function by inhibiting inflammation and oxidative stress in high-fat-diet-induced obesity in rats. J. Nutr. Biochem. 2016, 32, 20–28. [Google Scholar] [CrossRef]
- Nasr, M.; Naeem, S.A.; El-Shenbaby, I.; Mohamed, F.M.A.; Mahmoud, S.M.; Abuamara, T.M.M.; Abd-Elhay, W.M.; Elbayoumy, F.M.A.E.; Elkot, A.; Shikhon, T.; et al. Pomegranate Seeds and Peel Ethanolic Extracts Anticancer Potentials and Related Genetic, Histological, Immunohistochemical, Apoptotic and Oxidative Stress Profiles: In vitro Study. J. Exp. Pharmacol. 2023, 15, 191–205. [Google Scholar] [CrossRef]
- Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Afaq, F.; Mukhtar, H. Photochemopreventive Effect of Pomegranate Fruit Extract on UVA-mediated Activation of Cellular Pathways in Normal Human Epidermal Keratinocytes. Photochem. Photobiol. 2006, 82, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Rusolo, F.; De Vito, V.; Moccia, S.; Picariello, G.; Capone, F.; Guerriero, E.; Castello, G.; Volpe, M.G. Potential Anti-Inflammatory Effects of the Hydrophilic Fraction of Pomegranate (Punica granatum L.) Seed Oil on Breast Cancer Cell Lines. Molecules 2014, 19, 8644–8660. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138. [Google Scholar] [CrossRef]
- Yadav, S.S.; Mandal, R.K.; Singh, M.K.; Dwivedi, P.; Sethi, R.; Usman, K.; Khattri, S. Cumulative Risk of Metabolic Syndrome Correlated with the Coexistence of (-1306C/T) and Altered Circulating MMP2 level. Exp. Clin. Endocrinol. Diabetes 2016, 124, 380–384. [Google Scholar] [CrossRef]
- Yadav, S.S.; Mandal, R.K.; Singh, M.K.; Usman, K.; Khattri, S. Genetic Variants of Matrix Metalloproteinase (MMP2) Gene Influence Metabolic Syndrome Susceptibility. Genet. Test. Mol. Biomarkers 2014, 18, 88–92. [Google Scholar] [CrossRef]
- Berg, G.; Miksztowicz, V.; Schreier, L. Metalloproteinases in metabolic syndrome. Clin. Chim. Acta 2011, 412, 1731–1739. [Google Scholar] [CrossRef]
- Khattri, S.; Mandal, R.; Pant, K.; Singh, M.; Usman, K.; Yadav, S.; Dwivedi, P. Significance of impaired serum gelatinases activities in metabolic syndrome. Toxicol. Int. 2014, 21, 108–111. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, F.M.; Jacob-Ferreira, A.L.; Gomes, V.A.; Casella-Filho, A.; Chagas, A.C.; Marcaccini, A.M.; Gerlach, R.F.; Tanus-Santos, J.E. Increased circulating levels of matrix metalloproteinase (MMP)-8, MMP-9, and pro-inflammatory markers in patients with metabolic syndrome. Clin. Chim. Acta 2009, 403, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Plaksej, R.; Przewlocka-Kosmala, M.; Kuliczkowska-Plaksej, J.; Bednarek-Tupikowska, G.; Mazurek, W. Matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in premenopausal obese women: Relationship to cardiac function. Int. J. Obes. 2008, 32, 763–771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miksztowicz, V.; Morales, C.; Zago, V.; Friedman, S.; Schreier, L.; Berg, G. Effect of insulin-resistance on circulating and adipose tissue MMP-2 and MMP-9 activity in rats fed a sucrose-rich diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Act on 15 January 2015 on Protection of Animals Used in Scientific or Educational Procedures; Act on the protection of animals used for scientific or educational purposes; Ministry of Sciences and Higher Education: Jabłonna, Poland, 2015.
- Niewiadomska, J.; Kumiega, E.; Płóciennik, M.; Gajek, J.; Noszczyk-Nowak, A. Effects of Punica granatum L. peel extract supplementation on body weight, cardiac function, and haematological and biochemical parameters in an animal model of metabolic syndrome. J. Veter. Res. 2023, 67, 219–232. [Google Scholar] [CrossRef] [PubMed]




| Rt | MS [M-H]− (m/z) | MS/MS [M-H]− (m/z) | Name of Compounds | Polyphenols Content |
|---|---|---|---|---|
| 1.67 | 331 | 271/169 | Galloyl-glucose | 2.00 ± 0.03 |
| 1.73 | 781 | 721/601 | Punicalin α/A | 3.11 ± 0.06 |
| 2.02 | 1083 | 611/331/146 | HHDP-galloyl-hexoside (punicalagin) | 4.20 ± 0.09 |
| 2.12 | 1083 | 781/622/301 | Punicalagin isomer | 14.82 ± 1.04 |
| 2.33 | 933 | 631/450/301 | Ellagitannin | 4.71 ± 0.40 |
| 2.87 | 1083 | 781/301 | HHDP-gallagyl-hexoside (punicalagin) | 93.91 ± 2.05 |
| 3.12 | 1085 | 907/783/301 | Ellagic acid derivative | 2.49 ± 0.53 |
| 3.69 | 1083 | 781/301 | HHDP-gallagyl-hexoside (punicalagin) | 157.0 ± 2.65 |
| 3.89 | 799 | 301 | Granatin A | 4.74 ± 0.32 |
| 5.08 | 783 | 481/301 | Ellagitannin | 25.86 ± 1.53 |
| 6.20 | 1085 | 933/301 | Digalloyl-gallagyl-hexoside | 10.37 ± 0.65 |
| 6.25 | 783 | 481/301 | Ellagitannin | 13.51 ± 0.99 |
| 6.38 | 463 | 301 | Ellagic acid-hexoside | 33.63 ± 1.23 |
| 6.89 | 951 | 907/635/301 | Galloyl-HHDP-DHHDP-hex (granatin B) | 2.68 ± 0.11 |
| Total (mg/g dw) | 373.05 |
| Gene (NM Code) | Forward Prime | Reverse Primer |
|---|---|---|
| BAX (NM_017059.2) | CACGTCTGCGGGGAGTCA | TAGGAAAGGAGGCCATCCCA |
| BCL2 (NM_016993.2) | GGTGAACTGGGGGAGGATTG | AGAGCGATGTTGTCCACCAG |
| GAPDH (NM_017008.4) | AGTGCCAGCCTCGTCTCATA | GATGGTGATGGGTTTCCCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak-Pasikowska, A.; Hałucha, K.; Kamińska, M.; Niewiadomska, J.; Noszczyk-Nowak, A.; Bil-Lula, I. The Effect of Pomegranate Peel Extract on the Oxidative and Inflammatory Status in the Spleens of Rats with Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 12253. https://doi.org/10.3390/ijms252212253
Rak-Pasikowska A, Hałucha K, Kamińska M, Niewiadomska J, Noszczyk-Nowak A, Bil-Lula I. The Effect of Pomegranate Peel Extract on the Oxidative and Inflammatory Status in the Spleens of Rats with Metabolic Syndrome. International Journal of Molecular Sciences. 2024; 25(22):12253. https://doi.org/10.3390/ijms252212253
Chicago/Turabian StyleRak-Pasikowska, Alina, Kornela Hałucha, Marta Kamińska, Joanna Niewiadomska, Agnieszka Noszczyk-Nowak, and Iwona Bil-Lula. 2024. "The Effect of Pomegranate Peel Extract on the Oxidative and Inflammatory Status in the Spleens of Rats with Metabolic Syndrome" International Journal of Molecular Sciences 25, no. 22: 12253. https://doi.org/10.3390/ijms252212253
APA StyleRak-Pasikowska, A., Hałucha, K., Kamińska, M., Niewiadomska, J., Noszczyk-Nowak, A., & Bil-Lula, I. (2024). The Effect of Pomegranate Peel Extract on the Oxidative and Inflammatory Status in the Spleens of Rats with Metabolic Syndrome. International Journal of Molecular Sciences, 25(22), 12253. https://doi.org/10.3390/ijms252212253



.jpg)



