Control of HSV-1 Infection: Directions for the Development of CRISPR/Cas-Based Therapeutics and Diagnostics
Abstract
1. Introduction
2. Molecular Mechanisms of HSV-1 Infection: The Basis for CRISPR/Cas9 Targets Selection
3. Directions for the Use of CRISPR/Cas Systems in the Control of HSV-1 Infections
3.1. The First Direction: The Search for Cellular and Viral Genes as Effective Targets for CRISPR/Cas Systems
3.2. The Second Direction: The Potential of the CRISPR/Cas9 System as an Anti-HSV-1 Therapy
3.2.1. Standard Chemotherapy Against HSV-1
3.2.2. HSV-1 Targeting In Vivo by CRISPR/Cas9 System
3.2.3. Improving the Delivery of CRISPR/Cas Systems In Vivo
3.2.4. Disadvantages of CRISPR/Cas Systems as Anti-HSV-1 Therapeutics
3.3. The Third Direction: CRISPR/Cas-Based HSV-1 Detection
- Serologic: anti-HSV-1 IgM and anti-HSV-1 IgG assays.
- Immunocyto- and histochemical techniques are employed for the detection of viral antigens (proteins).
- Culture: the detection of infectious active virus and the isolation of HSV-1 from clinical samples.
- Molecular–biological: the detection of viral DNA and individual nucleotide sequences.
3.3.1. The Serological Diagnosis of Herpesvirus Infections
3.3.2. HSV-1 Antigen Detection in Clinical Specimens
3.3.3. Detection of HSV-1 by Culture Methods
3.3.4. Molecular Biological Methods—Detection of Virus Nucleic Acids by PCR
3.3.5. Molecular Biological Methods—CRISPR/Cas-Based Diagnosis of Viral Infections
3.4. The Fourth Direction: CRISPR/Cas-Based Validation of Drug-Resistant HSV-1 Mutations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Virgin, H.W.; Wherry, E.J.; Ahmed, R. Redefining chronic viral infection. Cell 2009, 138, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Kushch, A.A.; Kisteneva, L.B.; Klimova, R.R.; Cheshik, S.G. The role of herpesviruses in development of diseases of the urogenital tract and infertility in women. Vopr. Virusol. 2021, 65, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Zuckerman, R.A.; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13526. [Google Scholar] [CrossRef]
- Mielcarska, M.B.; Skowronska, K.; Wyzewski, Z.; Toka, F.N. Disrupting neurons and glial cells oneness in the brain-the possible causal role of herpes simplex virus type 1 (HSV-1) in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 23, 242. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Alvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farias, M.A.; Henriquez, A.; Agurto-Munoz, C.; Gonzalez, P.A. Current antivirals and novel botanical molecules interfering with herpes simplex virus infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef]
- Gege, C.; Bravo, F.J.; Uhlig, N.; Hagmaier, T.; Schmachtenberg, R.; Elis, J.; Burger-Kentischer, A.; Finkelmeier, D.; Hamprecht, K.; Grunwald, T.; et al. A helicase-primase drug candidate with sufficient target tissue exposure affects latent neural herpes simplex virus infections. Sci. Transl. Med. 2021, 13, eabf8668. [Google Scholar] [CrossRef]
- Gege, C.; Kleymann, G. Helicase-primase inhibitors from Medshine Discovery Inc. (WO2018/127207 and WO2020/007355) for the treatment of herpes simplex virus infections—Structure proposal for Phaeno Therapeutics drug candidate HN0037. Expert Opin. Ther. Pat. 2022, 32, 933–937. [Google Scholar] [CrossRef]
- Shiraki, K.; Yasumoto, S.; Toyama, N.; Fukuda, H. Amenamevir, a Helicase-Primase Inhibitor, for the Optimal Treatment of Herpes Zoster. Viruses 2021, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.B.; Shabbir, M.Z.; Wu, Q.; Mahmood, S.; Sajid, A.; Maan, M.K.; Ahmed, S.; Naveed, U.; Hao, H.; Yuan, Z. CRISPR-cas system: Biological function in microbes and its use to treat antimicrobial resistant pathogens. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, E.; Papanikolaou, E.; Marinos, E.; Anagnou, N.P.; Pappa, K.I. Mimiviruses: Giant viruses with novel and intriguing features (Review). Mol. Med. Rep. 2022, 25, 207. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Cerci, B.; Uzay, I.A.; Kara, M.K.; Dincer, P. Clinical trials and promising preclinical applications of CRISPR/Cas gene editing. Life Sci. 2023, 312, 121204. [Google Scholar] [CrossRef]
- Xin, C.; Yin, J.; Yuan, S.; Ou, L.; Liu, M.; Zhang, W.; Hu, J. Comprehensive assessment of miniature CRISPR-Cas12f nucleases for gene disruption. Nat. Commun. 2022, 13, 5623. [Google Scholar] [CrossRef]
- Li, F.; Wing, K.; Wang, J.H.; Luu, C.D.; Bender, J.A.; Chen, J.; Wang, Q.; Lu, Q.; Nguyen Tran, M.T.; Young, K.M.; et al. Comparison of CRISPR/Cas endonucleases for in vivo retinal gene editing. Front. Cell Neurosci. 2020, 14, 570917. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- He, X.; Urip, B.A.; Zhang, Z.; Ngan, C.C.; Feng, B. Evolving AAV-delivered therapeutics towards ultimate cures. J. Mol. Med. 2021, 99, 593–617. [Google Scholar] [CrossRef]
- Gasiunas, G.; Young, J.K.; Karvelis, T.; Kazlauskas, D.; Urbaitis, T.; Jasnauskaite, M.; Grusyte, M.M.; Paulraj, S.; Wang, P.H.; Hou, Z.; et al. A catalogue of biochemically diverse CRISPR-Cas9 orthologs. Nat. Commun. 2020, 11, 5512. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Miranda-Saksena, M.; Denes, C.E.; Diefenbach, R.J.; Cunningham, A.L. Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton. Viruses 2018, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Pinninti, S.G.; Kimberlin, D.W. Neonatal herpes simplex virus infections. Semin. Perinatol. 2018, 42, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gnann, J.W., Jr.; Whitley, R.J. Herpes Simplex Encephalitis: An Update. Curr. Infect. Dis. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Fields, B.N.; Knipe, D.M.; Howley, P.M. Fields Virology, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Szpara, M.L.; Gatherer, D.; Ochoa, A.; Greenbaum, B.; Dolan, A.; Bowden, R.J.; Enquist, L.W.; Legendre, M.; Davison, A.J. Evolution and diversity in human herpes simplex virus genomes. J. Virol. 2014, 88, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Trus, B.L.; Booy, F.P.; Newcomb, W.W.; Brown, J.C.; Homa, F.L.; Thomsen, D.R.; Steven, A.C. The herpes simplex virus procapsid: Structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J. Mol. Biol. 1996, 263, 447–462. [Google Scholar] [CrossRef]
- Owen, D.J.; Crump, C.M.; Graham, S.C. Tegument Assembly and Secondary Envelopment of Alphaherpesviruses. Viruses 2015, 7, 5084–5114. [Google Scholar] [CrossRef]
- Satoh, T.; Arii, J.; Suenaga, T.; Wang, J.; Kogure, A.; Uehori, J.; Arase, N.; Shiratori, I.; Tanaka, S.; Kawaguchi, Y.; et al. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 2008, 132, 935–944. [Google Scholar] [CrossRef]
- Suenaga, T.; Satoh, T.; Somboonthum, P.; Kawaguchi, Y.; Mori, Y.; Arase, H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 866–871. [Google Scholar] [CrossRef]
- Arii, J.; Goto, H.; Suenaga, T.; Oyama, M.; Kozuka-Hata, H.; Imai, T.; Minowa, A.; Akashi, H.; Arase, H.; Kawaoka, Y.; et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 2010, 467, 859–862. [Google Scholar] [CrossRef]
- Gianni, T.; Salvioli, S.; Chesnokova, L.S.; Hutt-Fletcher, L.M.; Campadelli-Fiume, G. alphavbeta6- and alphavbeta8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013, 9, e1003806. [Google Scholar] [CrossRef]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell Infect. Microbiol. 2020, 10, 617578. [Google Scholar] [CrossRef] [PubMed]
- Karasneh, G.A.; Shukla, D. Herpes simplex virus infects most cell types in vitro: Clues to its success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef]
- Villanueva-Valencia, J.R.; Tsimtsirakis, E.; Evilevitch, A. Role of HSV-1 Capsid Vertex-Specific Component (CVSC) and Viral Terminal DNA in Capsid Docking at the Nuclear Pore. Viruses 2021, 13, 2515. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Miranda-Saksena, M.; Boadle, R.A.; Kelly, B.J.; Diefenbach, R.J.; Alam, W.; Cunningham, A.L. Ultrastructural visualization of individual tegument protein dissociation during entry of herpes simplex virus 1 into human and rat dorsal root ganglion neurons. J. Virol. 2012, 86, 6123–6137. [Google Scholar] [CrossRef] [PubMed]
- Read, G.S. Virus-encoded endonucleases: Expected and novel functions. Wiley Interdiscip. Rev. RNA 2013, 4, 693–708. [Google Scholar] [CrossRef]
- Dauber, B.; Pelletier, J.; Smiley, J.R. The herpes simplex virus 1 vhs protein enhances translation of viral true late mRNAs and virus production in a cell type-dependent manner. J. Virol. 2011, 85, 5363–5373. [Google Scholar] [CrossRef]
- Muylaert, I.; Elias, P. Knockdown of DNA ligase IV/XRCC4 by RNA interference inhibits herpes simplex virus type I DNA replication. J. Biol. Chem. 2007, 282, 10865–10872. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Zhang-Ding, Z.; Xin, C.; Liu, M.; Wang, Y.; Ai, C.; Hu, J. In-depth assessment of the PAM compatibility and editing activities of Cas9 variants. Nucleic Acids Res. 2021, 49, 8785–8795. [Google Scholar] [CrossRef]
- Ibanez, F.J.; Farias, M.A.; Gonzalez-Troncoso, M.P.; Corrales, N.; Duarte, L.F.; Retamal-Diaz, A.; Gonzalez, P.A. Experimental dissection of the lytic replication cycles of herpes simplex viruses in vitro. Front. Microbiol. 2018, 9, 2406. [Google Scholar] [CrossRef]
- Dunn, L.E.M.; Birkenheuer, C.H.; Baines, J.D. A Revision of Herpes Simplex Virus Type 1 Transcription: First, Repress; Then, Express. Microorganisms 2024, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Kuddus, R.; DeLuca, N.A. Repression of activator-mediated transcription by herpes simplex virus ICP4 via a mechanism involving interactions with the basal transcription factors TATA-binding protein and TFIIB. Mol. Cell. Biol. 1995, 15, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Rivas, T.; Goodrich, J.A.; Kugel, J.F. The herpes simplex virus 1 protein ICP4 acts as both an activator and a repressor of host genome transcription during infection. Mol. Cell. Biol. 2021, 41, e0017121. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, M.; Cheng, A.; Jia, R.; Yang, Q.; Wu, Y.; Zhu, D.; Zhao, X.; Chen, S.; Liu, M.; et al. The role of VP16 in the life cycle of Alphaherpesviruses. Front. Microbiol. 2020, 11, 1910. [Google Scholar] [CrossRef]
- Wysocka, J.; Herr, W. The herpes simplex virus VP16-induced complex: The makings of a regulatory switch. Trends Biochem. Sci. 2003, 28, 294–304. [Google Scholar] [CrossRef]
- Dremel, S.E.; DeLuca, N.A. Herpes simplex viral nucleoprotein creates a competitive transcriptional environment facilitating robust viral transcription and host shut off. Elife 2019, 8, e51109. [Google Scholar] [CrossRef]
- Sandri-Goldin, R.M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998, 12, 868–879. [Google Scholar] [CrossRef]
- Tang, S.; Patel, A.; Krause, P.R. Herpes simplex virus ICP27 regulates alternative pre-mRNA polyadenylation and splicing in a sequence-dependent manner. Proc. Natl. Acad. Sci. USA 2016, 113, 12256–12261. [Google Scholar] [CrossRef]
- Fox, H.L.; Dembowski, J.A.; DeLuca, N.A. A herpesviral immediate early protein promotes transcription elongation of viral transcripts. mBio 2017, 8, e00745-17. [Google Scholar] [CrossRef]
- Packard, J.E.; Dembowski, J.A. HSV-1 DNA replication-coordinated regulation by viral and cellular factors. Viruses 2021, 13, 2015. [Google Scholar] [CrossRef]
- Lehman, I.R.; Boehmer, P.E. Replication of herpes simplex virus DNA. J. Biol. Chem. 1999, 274, 28059–28062. [Google Scholar] [CrossRef] [PubMed]
- Bermek, O.; Willcox, S.; Griffith, J.D. DNA replication catalyzed by herpes simplex virus type 1 proteins reveals trombone loops at the fork. J. Biol. Chem. 2015, 290, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Koff, A.; Schwedes, J.F.; Tegtmeyer, P. Herpes simplex virus origin-binding protein (UL9) loops and distorts the viral replication origin. J. Virol. 1991, 65, 3284–3292. [Google Scholar] [CrossRef]
- Aslani, A.; Olsson, M.; Elias, P. ATP-dependent unwinding of a minimal origin of DNA replication by the origin-binding protein and the single-strand DNA-binding protein ICP8 from herpes simplex virus type I. J. Biol. Chem. 2002, 277, 41204–41212. [Google Scholar] [CrossRef] [PubMed]
- Makhov, A.M.; Lee, S.S.; Lehman, I.R.; Griffith, J.D. Origin-specific unwinding of herpes simplex virus 1 DNA by the viral UL9 and ICP8 proteins: Visualization of a specific preunwinding complex. Proc. Natl. Acad. Sci. USA 2003, 100, 898–903. [Google Scholar] [CrossRef]
- Lee, C.K.; Knipe, D.M. An immunoassay for the study of DNA-binding activities of herpes simplex virus protein ICP8. J. Virol. 1985, 54, 731–738. [Google Scholar] [CrossRef]
- Darwish, A.S.; Grady, L.M.; Bai, P.; Weller, S.K. ICP8 filament formation is essential for replication compartment formation during herpes simplex virus infection. J. Virol. 2015, 90, 2561–2570. [Google Scholar] [CrossRef]
- Bermek, O.; Williams, R.S. The three-component helicase/primase complex of herpes simplex virus-1. Open Biol. 2021, 11, 210011. [Google Scholar] [CrossRef] [PubMed]
- Deiss, L.P.; Frenkel, N. Herpes simplex virus amplicon: Cleavage of concatemeric DNA is linked to packaging and involves amplification of the terminally reiterated a sequence. J. Virol. 1986, 57, 933–941. [Google Scholar] [CrossRef]
- Kosz-Vnenchak, M.; Coen, D.M.; Knipe, D.M. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J. Virol. 1990, 64, 5396–5402. [Google Scholar] [CrossRef]
- Jacobson, J.G.; Leib, D.A.; Goldstein, D.J.; Bogard, C.L.; Schaffer, P.A.; Weller, S.K.; Coen, D.M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 1989, 173, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.V.; Cox, B.; Ariza, M.E. Herpesviruses dUTPases: A New Family of Pathogen-Associated Molecular Pattern (PAMP) Proteins with Implications for Human Disease. Pathogens 2016, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Grady, L.M.; Szczepaniak, R.; Murelli, R.P.; Masaoka, T.; Le Grice, S.F.J.; Wright, D.L.; Weller, S.K. The exonuclease activity of herpes simplex virus 1 UL12 is required for production of viral DNA that can be packaged to produce infectious virus. J. Virol. 2017, 91, e01380-17. [Google Scholar] [CrossRef]
- Dogrammatzis, C.; Waisner, H.; Kalamvoki, M. “Non-essential” proteins of HSV-1 with essential roles in vivo: A comprehensive review. Viruses 2020, 13, 17. [Google Scholar] [CrossRef]
- Schumacher, A.J.; Mohni, K.N.; Kan, Y.; Hendrickson, E.A.; Stark, J.M.; Weller, S.K. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog. 2012, 8, e1002862. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.E.; Weller, S.K. The role of DNA recombination in herpes simplex virus DNA replication. IUBMB Life 2003, 55, 451–458. [Google Scholar] [CrossRef]
- Drake, J.W.; Hwang, C.B. On the mutation rate of herpes simplex virus type 1. Genetics 2005, 170, 969–970. [Google Scholar] [CrossRef]
- Reuven, N.B.; Antoku, S.; Weller, S.K. The UL12.5 gene product of herpes simplex virus type 1 exhibits nuclease and strand exchange activities but does not localize to the nucleus. J. Virol. 2004, 78, 4599–4608. [Google Scholar] [CrossRef]
- Reuven, N.B.; Willcox, S.; Griffith, J.D.; Weller, S.K. Catalysis of strand exchange by the HSV-1 UL12 and ICP8 proteins: Potent ICP8 recombinase activity is revealed upon resection of dsDNA substrate by nuclease. J. Mol. Biol. 2004, 342, 57–71. [Google Scholar] [CrossRef]
- Pheasant, K.; Moller-Levet, C.S.; Jones, J.; Depledge, D.; Breuer, J.; Elliott, G. Nuclear-cytoplasmic compartmentalization of the herpes simplex virus 1 infected cell transcriptome is co-ordinated by the viral endoribonuclease vhs and cofactors to facilitate the translation of late proteins. PLoS Pathog. 2018, 14, e1007331. [Google Scholar] [CrossRef]
- Elliott, G.; Pheasant, K.; Ebert-Keel, K.; Stylianou, J.; Franklyn, A.; Jones, J. Multiple Posttranscriptional Strategies To Regulate the Herpes Simplex Virus 1 vhs Endoribonuclease. J. Virol. 2018, 92, e00818-18. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.R.; Dembowski, J.A. Fashionably late: Temporal regulation of HSV-1 late gene transcription. PLoS Pathog. 2022, 18, e1010536. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, M.; Johns, H.L.; Sayers, C.L.; Gonzalez-Lopez, C.; Smith, G.L.; Elliott, G. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. 2012, 31, 4204–4220. [Google Scholar] [CrossRef]
- Gao, M.; Matusick-Kumar, L.; Hurlburt, W.; DiTusa, S.F.; Newcomb, W.W.; Brown, J.C.; McCann, P.J., 3rd; Deckman, I.; Colonno, R.J. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 1994, 68, 3702–3712. [Google Scholar] [CrossRef]
- Dohner, K.; Ramos-Nascimento, A.; Bialy, D.; Anderson, F.; Hickford-Martinez, A.; Rother, F.; Koithan, T.; Rudolph, K.; Buch, A.; Prank, U.; et al. Importin alpha1 is required for nuclear import of herpes simplex virus proteins and capsid assembly in fibroblasts and neurons. PLoS Pathog. 2018, 14, e1006823. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, S.; Zhang, L.; Baines, J.; Roller, R.; Ames, J.; Yang, M.; Wang, J.; Chen, D.; Liu, Y.; et al. Herpes simplex virus 1 induces phosphorylation and reorganization of lamin A/C through the gamma134.5 protein that facilitates nuclear egress. J. Virol. 2016, 90, 10414–10422. [Google Scholar] [CrossRef]
- Bjerke, S.L.; Roller, R.J. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology 2006, 347, 261–276. [Google Scholar] [CrossRef]
- Ahmad, I.; Wilson, D.W. HSV-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef]
- Buch, A.; Muller, O.; Ivanova, L.; Dohner, K.; Bialy, D.; Bosse, J.B.; Pohlmann, A.; Binz, A.; Hegemann, M.; Nagel, C.H.; et al. Inner tegument proteins of Herpes Simplex Virus are sufficient for intracellular capsid motility in neurons but not for axonal targeting. PLoS Pathog. 2017, 13, e1006813. [Google Scholar] [CrossRef]
- Orzalli, M.H.; Conwell, S.E.; Berrios, C.; DeCaprio, J.A.; Knipe, D.M. Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA. Proc. Natl. Acad. Sci. USA 2013, 110, E4492–E4501. [Google Scholar] [CrossRef]
- Cohen, C.; Corpet, A.; Roubille, S.; Maroui, M.A.; Poccardi, N.; Rousseau, A.; Kleijwegt, C.; Binda, O.; Texier, P.; Sawtell, N.; et al. Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 Chaperone Axis. PLoS Pathog. 2018, 14, e1007313. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.C.; Giordani, N.V.; Kwiatkowski, D.L. Epigenetic regulation of latent HSV-1 gene expression. Biochim. Biophys. Acta 2010, 1799, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Garza, H.H., Jr.; Su, Y.H.; Meegalla, R.; Hanna, L.A.; Loutsch, J.M.; Thompson, H.W.; Varnell, E.D.; Bloom, D.C.; Block, T.M. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J. Virol. 1997, 71, 6555–6559. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.A.H.; Foley, J.W.; Davison, A.J.; Sommer, M.; Liu, D.; Sung, P.; Moffat, J.; Zerboni, L.; Arvin, A.M. The latency-associated transcript locus of herpes simplex virus 1 is a virulence determinant in human skin. PLoS Pathog. 2020, 16, e1009166. [Google Scholar] [CrossRef]
- Wechsler, S.L.; Nesburn, A.B.; Watson, R.; Slanina, S.M.; Ghiasi, H. Fine mapping of the latency-related gene of herpes simplex virus type 1: Alternative splicing produces distinct latency-related RNAs containing open reading frames. J. Virol. 1988, 62, 4051–4058. [Google Scholar] [CrossRef]
- Block, T.M.; Hill, J.M. The latency associated transcripts (LAT) of herpes simplex virus: Still no end in sight. J. Neurovirol 1997, 3, 313–321. [Google Scholar] [CrossRef]
- Grams, T.R.; Edwards, T.G.; Bloom, D.C. HSV-1 LAT promoter deletion viruses exhibit strain-specific and LAT-dependent epigenetic regulation of latent viral genomes in human neurons. J. Virol. 2023, 97, e0193522. [Google Scholar] [CrossRef]
- Umbach, J.L.; Kramer, M.F.; Jurak, I.; Karnowski, H.W.; Coen, D.M.; Cullen, B.R. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 2008, 454, 780–783. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, L.S.; Wang, J.; Cai, W.Q.; Cui, W.; Song, T.J.; Peng, X.C.; Ma, Z.; Xiang, Y.; Cui, S.Z.; et al. Multifunctional Non-Coding RNAs Mediate Latent Infection and Recurrence of Herpes Simplex Viruses. Infect. Drug Resist. 2021, 14, 5335–5349. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; MacGibeny, M.A.; Enquist, L.W. Latent versus productive infection: The alpha herpesvirus switch. Future Virol. 2018, 13, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Suzich, J.B.; Cliffe, A.R. Strength in diversity: Understanding the pathways to herpes simplex virus reactivation. Virology 2018, 522, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, A.R.; Arbuckle, J.H.; Vogel, J.L.; Geden, M.J.; Rothbart, S.B.; Cusack, C.L.; Strahl, B.D.; Kristie, T.M.; Deshmukh, M. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 2015, 18, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Linderman, J.A.; Kobayashi, M.; Rayannavar, V.; Fak, J.J.; Darnell, R.B.; Chao, M.V.; Wilson, A.C.; Mohr, I. Immune Escape via a Transient Gene Expression Program Enables Productive Replication of a Latent Pathogen. Cell Rep. 2017, 18, 1312–1323. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mandarino, A.; Chao, M.V.; Mohr, I.; Wilson, A.C. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog. 2012, 8, e1002540. [Google Scholar] [CrossRef]
- Canova, P.N.; Charron, A.J.; Leib, D.A. Models of Herpes Simplex Virus Latency. Viruses 2024, 16, 747. [Google Scholar] [CrossRef]
- Puschnik, A.S.; Majzoub, K.; Ooi, Y.S.; Carette, J.E. A CRISPR toolbox to study virus-host interactions. Nat. Rev. Microbiol. 2017, 15, 351–364. [Google Scholar] [CrossRef]
- Suzuki, T.; Sato, Y.; Okuno, Y.; Goshima, F.; Mikami, T.; Umeda, M.; Murata, T.; Watanabe, T.; Watashi, K.; Wakita, T.; et al. Genome-wide CRISPR screen for HSV-1 host factors reveals PAPSS1 contributes to heparan sulfate synthesis. Commun. Biol. 2022, 5, 694. [Google Scholar] [CrossRef]
- Johnson, K.E.; Bottero, V.; Flaherty, S.; Dutta, S.; Singh, V.V.; Chandran, B. IFI16 restricts HSV-1 replication by accumulating on the hsv-1 genome, repressing HSV-1 gene expression, and directly or indirectly modulating histone modifications. PLoS Pathog. 2014, 10, e1004503. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, F.; Zhang, Y.; Wang, T.; Gao, W.; Yu, S.; Sun, H.; Pu, J.; Sun, Y.; Wang, M.; et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat. Microbiol. 2021, 6, 932–945. [Google Scholar] [CrossRef]
- Ansari, M.A.; Dutta, S.; Veettil, M.V.; Dutta, D.; Iqbal, J.; Kumar, B.; Roy, A.; Chikoti, L.; Singh, V.V.; Chandran, B. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-beta responses. PLoS Pathog. 2015, 11, e1005019. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.R.; Lum, K.K.; Kennedy, M.A.; Cristea, I.M. The nuclear DNA sensor IFI16 indiscriminately binds to and diminishes accessibility of the HSV-1 genome to suppress infection. mSystems 2022, 7, e0019822. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhou, Y.; Wang, S.; Gao, M.; Xia, Y.; Li, Y.; Zhong, Y.; Xu, W.; Bai, L.; Fu, B.; et al. Genome-Wide CRISPR-Cas9 Screen Identifies SMCHD1 as a Restriction Factor for Herpesviruses. mBio 2023, 14, e0054923. [Google Scholar] [CrossRef]
- Turner, E.M.; Brown, R.S.; Laudermilch, E.; Tsai, P.L.; Schlieker, C. The Torsin activator LULL1 is required for efficient growth of herpes simplex virus 1. J. Virol. 2015, 89, 8444–8452. [Google Scholar] [CrossRef] [PubMed]
- Rampello, A.J.; Prophet, S.M.; Schlieker, C. The role of Torsin AAA+ proteins in preserving nuclear envelope integrity and safeguarding against disease. Biomolecules 2020, 10, 468. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Li, G.; Huang, S.; Xu, J.; Ding, Q.; Hong, J. Targeting NECTIN-1 based on CRISPR/Cas9 system attenuated the herpes simplex virus infection in human corneal epithelial cells in vitro. Transl. Vis. Sci. Technol. 2022, 11, 8. [Google Scholar] [CrossRef]
- Shukla, N.D.; Tiwari, V.; Valyi-Nagy, T. Nectin-1-specific entry of herpes simplex virus 1 is sufficient for infection of the cornea and viral spread to the trigeminal ganglia. Mol. Vis. 2012, 18, 2711–2716. [Google Scholar]
- Neuhausser, W.M.; Oh, H.S.; Eggan, P.; Angelova, M.; Kirchner, R.; Eggan, K.C.; Knipe, D.M. Screening Method for CRISPR/Cas9 Inhibition of a Human DNA Virus: Herpes Simplex Virus. Bio Protoc. 2020, 10, e3748. [Google Scholar] [CrossRef] [PubMed]
- Roehm, P.C.; Shekarabi, M.; Wollebo, H.S.; Bellizzi, A.; He, L.; Salkind, J.; Khalili, K. Inhibition of HSV-1 replication by gene editing strategy. Sci. Rep. 2016, 6, 23146. [Google Scholar] [CrossRef]
- Ying, M.; Wang, H.; Liu, T.; Han, Z.; Lin, K.; Shi, Q.; Zheng, N.; Ye, T.; Gong, H.; Xu, F. CLEAR Strategy Inhibited HSV Proliferation Using Viral Vectors Delivered CRISPR-Cas9. Pathogens 2023, 12, 814. [Google Scholar] [CrossRef]
- van Diemen, F.R.; Kruse, E.M.; Hooykaas, M.J.; Bruggeling, C.E.; Schurch, A.C.; van Ham, P.M.; Imhof, S.M.; Nijhuis, M.; Wiertz, E.J.; Lebbink, R.J. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 2016, 12, e1005701. [Google Scholar] [CrossRef] [PubMed]
- Karpov, D.S.; Demidova, N.A.; Kulagin, K.A.; Shuvalova, A.I.; Kovalev, M.A.; Simonov, R.A.; Karpov, V.L.; Snezhkina, A.V.; Kudryavtseva, A.V.; Klimova, R.R.; et al. Complete and Prolonged Inhibition of Herpes Simplex Virus Type 1 Infection In Vitro by CRISPR/Cas9 and CRISPR/CasX Systems. Int. J. Mol. Sci. 2022, 23, 14847. [Google Scholar] [CrossRef] [PubMed]
- Karpov, D.S.; Karpov, V.L.; Klimova, R.R.; Demidova, N.A.; Kushch, A.A. A plasmid-expressed CRISPR/Cas9 system suppresses replication of HSV type I in a Vero cell culture. Mol. Biol. 2019, 53, 70–78. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Makvandi, M.; Abbasi, S.; Azadmanesh, K.; Teimoori, A. Developing oncolytic Herpes simplex virus type 1 through UL39 knockout by CRISPR-Cas9. Iran. J. Basic. Med. Sci. 2020, 23, 937–944. [Google Scholar] [CrossRef]
- Xu, X.; Fan, S.; Zhou, J.; Zhang, Y.; Che, Y.; Cai, H.; Wang, L.; Guo, L.; Liu, L.; Li, Q. The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of alpha-4 gene transcription. Virol. J. 2016, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, N.; Fani, M.; Jamehdor, S.; Nahidsamiei, R.; Makvandi, M.; Kaboli, S.; Teimoori, A.; Thekkiniath, J. A knockdown of the herpes simplex virus type-1 gene in all-in-one CRISPR vectors. Folia Histochem. Cytobiol. 2020, 58, 174–181. [Google Scholar] [CrossRef]
- Gao, J.; Yan, X.; Banfield, B.W. Comparative analysis of UL16 mutants derived from multiple strains of herpes simplex virus 2 (HSV-2) and HSV-1 reveals species-specific requirements for the UL16 protein. J. Virol. 2018, 92, e00629-18. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, S.; Liang, P.; Zheng, Q.; Liu, M.; Zhao, Q.; Ren, J.; Cui, J.; Huang, J.; Liu, Y.; et al. Single AAV-mediated CRISPR-SaCas9 inhibits HSV-1 replication by editing ICP4 in trigeminal ganglion neurons. Mol. Ther. Methods Clin. Dev. 2020, 18, 33–43. [Google Scholar] [CrossRef]
- Oh, H.S.; Neuhausser, W.M.; Eggan, P.; Angelova, M.; Kirchner, R.; Eggan, K.C.; Knipe, D.M. Herpesviral lytic gene functions render the viral genome susceptible to novel editing by CRISPR/Cas9. Elife 2019, 8, e51662. [Google Scholar] [CrossRef]
- Amrani, N.; Luk, K.; Singh, P.; Shipley, M.; Isik, M.; Donadoni, M.; Bellizzi, A.; Khalili, K.; Sariyer, I.K.; Neumann, D.; et al. CRISPR-Cas9-mediated genome editing delivered by a single AAV9 vector inhibits HSV-1 reactivation in a latent rabbit keratitis model. Mol. Ther. Methods Clin. Dev. 2024, 32, 101303. [Google Scholar] [CrossRef]
- Yin, D.; Ling, S.; Wang, D.; Dai, Y.; Jiang, H.; Zhou, X.; Paludan, S.R.; Hong, J.; Cai, Y. Targeting herpes simplex virus with CRISPR-Cas9 cures herpetic stromal keratitis in mice. Nat. Biotechnol. 2021, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, A.; Cakir, S.; Donadoni, M.; Sariyer, R.; Liao, S.; Liu, H.; Ruan, G.X.; Gordon, J.; Khalili, K.; Sariyer, I.K. Suppression of HSV-1 infection and viral reactivation by CRISPR-Cas9 gene editing in 2D and 3D culture models. Mol. Ther. Nucleic Acids 2024, 35, 102282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Yu, J.; Wan, Y.; Zhang, C.; Zhang, H.; Cao, Y. Construction of an IL12 and CXCL11 armed oncolytic herpes simplex virus using the CRISPR/Cas9 system for colon cancer treatment. Virus Res. 2022, 323, 198979. [Google Scholar] [CrossRef]
- Bi, Y.; Sun, L.; Gao, D.; Ding, C.; Li, Z.; Li, Y.; Cun, W.; Li, Q. High-efficiency targeted editing of large viral genomes by RNA-guided nucleases. PLoS Pathog. 2014, 10, e1004090. [Google Scholar] [CrossRef]
- Vasques Raposo, J.; Rodrigues Carvalho Barros, L.; Ribas Torres, L.; Barbosa da Silva Pinto, R.; De Oliveira Lopes, A.; Mello de Souza, E.; Hernan Bonamino, M.; Salete de Paula, V. CRISPR/Cas-9 vector system: Targets UL-39 and inhibits Simplexvirus humanalpha1 (HSV-1) replication in vitro. Cell. Mol. Biol 2023, 69, 19–23. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Fan, S.; Cui, P.; Feng, M.; Wang, L.; Zhang, Y.; Liao, Y.; Zhang, X.; Li, Q. Attenuated phenotypes and analysis of a herpes simplex virus 1 strain with partial deletion of the UL7, UL41 and LAT genes. Virol. Sin. 2017, 32, 404–414. [Google Scholar] [CrossRef]
- Finnen, R.L.; Banfield, B.W. CRISPR/Cas9 mutagenesis of UL21 in multiple strains of herpes simplex virus reveals differential requirements for pUL21 in viral replication. Viruses 2018, 10, 258. [Google Scholar] [CrossRef]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Practical updates in clinical antiviral resistance testing. J. Clin. Microbiol. 2024, 62, e0072823. [Google Scholar] [CrossRef]
- Wei, A.; Yin, D.; Zhai, Z.; Ling, S.; Le, H.; Tian, L.; Xu, J.; Paludan, S.R.; Cai, Y.; Hong, J. In vivo CRISPR gene editing in patients with herpetic stromal keratitis. Mol. Ther. 2023, 31, 3163–3175. [Google Scholar] [CrossRef]
- Farooq, A.V.; Shukla, D. Herpes simplex epithelial and stromal keratitis: An epidemiologic update. Surv. Ophthalmol. 2012, 57, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Alio, J.L. Ocular surgery after herpes simplex and herpes zoster keratitis. Int. Ophthalmol. 2020, 40, 3599–3612. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef]
- Asmamaw, M.; Zawdie, B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Sinclair, F.; Begum, A.A.; Dai, C.C.; Toth, I.; Moyle, P.M. Recent advances in the delivery and applications of nonviral CRISPR/Cas9 gene editing. Drug Deliv. Transl. Res. 2023, 13, 1500–1519. [Google Scholar] [CrossRef]
- Wilbie, D.; Walther, J.; Mastrobattista, E. Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc. Chem. Res. 2019, 52, 1555–1564. [Google Scholar] [CrossRef]
- Taha, E.A.; Lee, J.; Hotta, A. Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges. J. Control. Release 2022, 342, 345–361. [Google Scholar] [CrossRef]
- Asmamaw Mengstie, M. Viral vectors for the in vivo delivery of CRISPR components: Advances and challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Colon-Thillet, R.; Jerome, K.R.; Stone, D. Optimization of AAV vectors to target persistent viral reservoirs. Virol. J. 2021, 18, 85. [Google Scholar] [CrossRef]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV vector immunogenicity in humans: A long journey to successful gene transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Bijlani, S.; Pang, K.M.; Sivanandam, V.; Singh, A.; Chatterjee, S. The role of recombinant AAV in precise genome editing. Front. Genome Ed. 2021, 3, 799722. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Gilbert, L.A. The promise and challenge of in vivo delivery for genome therapeutics. ACS Chem. Biol. 2018, 13, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Aubert, M.; Strongin, D.E.; Roychoudhury, P.; Loprieno, M.A.; Haick, A.K.; Klouser, L.M.; Stensland, L.; Huang, M.L.; Makhsous, N.; Tait, A.; et al. Gene editing and elimination of latent herpes simplex virus in vivo. Nat. Commun. 2020, 11, 4148. [Google Scholar] [CrossRef]
- De Silva Feelixge, H.S.; Stone, D.; Roychoudhury, P.; Aubert, M.; Jerome, K.R. CRISPR/Cas9 and genome editing for viral disease-is resistance futile? ACS Infect. Dis. 2018, 4, 871–880. [Google Scholar] [CrossRef]
- Dash, P.K.; Kaminski, R.; Bella, R.; Su, H.; Mathews, S.; Ahooyi, T.M.; Chen, C.; Mancuso, P.; Sariyer, R.; Ferrante, P.; et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat. Commun. 2019, 10, 2753. [Google Scholar] [CrossRef]
- Yip, B.H. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules 2020, 10, 839. [Google Scholar] [CrossRef]
- Chen, Z.R.; Guo, J.Y.; He, L.; Liu, S.; Xu, J.Y.; Yang, Z.J.; Su, W.; Liu, K.; Gong, S.S.; Wang, G.P. Co-transduction of dual-adeno-associated virus vectors in the neonatal and adult mouse utricles. Front. Mol. Neurosci. 2022, 15, 1020803. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kubo, Y.; Izumida, M.; Matsuyama, T. Efficient viral delivery of Cas9 into human safe harbor. Sci. Rep. 2020, 10, 21474. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR gene therapy: Applications, limitations, and implications for the future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Liu, J.J.; Orlova, N.; Oakes, B.L.; Ma, E.; Spinner, H.B.; Baney, K.L.M.; Chuck, J.; Tan, D.; Knott, G.J.; Harrington, L.B.; et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature 2019, 566, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rube, H.T.; Vakulskas, C.A.; Behlke, M.A.; Bussemaker, H.J.; Pufall, M.A. Systematic in vitro profiling of off-target affinity, cleavage and efficiency for CRISPR enzymes. Nucleic Acids Res. 2020, 48, 5037–5053. [Google Scholar] [CrossRef] [PubMed]
- Ibraheim, R.; Tai, P.W.L.; Mir, A.; Javeed, N.; Wang, J.; Rodriguez, T.C.; Namkung, S.; Nelson, S.; Khokhar, E.S.; Mintzer, E.; et al. Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo. Nat. Commun. 2021, 12, 6267. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Merkel, O.M. Immunogenicity of Cas9 protein. J. Pharm. Sci. 2020, 109, 62–67. [Google Scholar] [CrossRef]
- Hakim, C.H.; Kumar, S.R.P.; Perez-Lopez, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; et al. Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat. Commun. 2021, 12, 6769. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Smirnov, V.; Volchkova, E.; Lukashev, A.; Chulanov, V. Gene editing by extracellular vesicles. Int. J. Mol. Sci. 2020, 21, 7362. [Google Scholar] [CrossRef]
- Li, A.; Tanner, M.R.; Lee, C.M.; Hurley, A.E.; De Giorgi, M.; Jarrett, K.E.; Davis, T.H.; Doerfler, A.M.; Bao, G.; Beeton, C.; et al. AAV-CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol. Ther. 2020, 28, 1432–1441. [Google Scholar] [CrossRef]
- Ronzitti, G.; Gross, D.A.; Mingozzi, F. Human immune responses to adeno-associated virus (AAV) vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: Immunology and formulation for clinical translation. Expert. Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.W.; Tay, T.; Sasaki, K.; Rotello, V.M. Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, M.; Yang, Y.; Li, W.; Bao, J.; Li, Y. The CRISPR/Cas9 system delivered by extracellular vesicles. Pharmaceutics 2023, 15, 984. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Soleymani, M.; Shahriyary, F.; Amirzargar, M.R.; Ofoghi, M.; Fattahi, M.D.; Safa, M. Viral vectors and extracellular vesicles: Innate delivery systems utilized in CRISPR/Cas-mediated cancer therapy. Cancer Gene Ther. 2023, 30, 936–954. [Google Scholar] [CrossRef]
- Karpov, D.S. CRISPR-Cas Systems and Genome Editing: Beginning the Era of CRISPR/Cas Therapies for Humans. Int. J. Mol. Sci. 2024, 25, 5292. [Google Scholar] [CrossRef]
- Adashi, E.Y.; Gruppuso, P.A.; Cohen, I.G. CRISPR Therapy of Sickle Cell Disease: The Dawning of the Gene Editing Era. Am. J. Med. 2024, 137, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Tycko, J.; Wainberg, M.; Marinov, G.K.; Ursu, O.; Hess, G.T.; Ego, B.K.; Aradhana; Li, A.; Truong, A.; Trevino, A.E.; et al. Mitigation of off-target toxicity in CRISPR-Cas9 screens for essential non-coding elements. Nat. Commun. 2019, 10, 4063. [Google Scholar] [CrossRef]
- Kovalev, M.A.; Davletshin, A.I.; Karpov, D.S. Engineering Cas9: Next generation of genomic editors. Appl. Microbiol. Biotechnol. 2024, 108, 209. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Lobato, G.; Zohren, J.; McCarthy, A.; Fogarty, N.M.E.; Kubikova, N.; Hardman, E.; Greco, M.; Wells, D.; Turner, J.M.A.; Niakan, K.K. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc. Natl. Acad. Sci. USA 2021, 118, e2004832117. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Mafessoni, F.; Gross, O.; Melamed-Bessudo, C.; Filler-Hayut, S.; Dahan-Meir, T.; Amsellem, Z.; Pawlowski, W.P.; Levy, A.A. CRISPR/Cas9-induced DNA breaks trigger crossover, chromosomal loss, and chromothripsis-like rearrangements. Plant Cell 2023, 35, 3957–3972. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, R.; Chaubey, B. CRISPR/Cas9: A tool to eradicate HIV-1. AIDS Res. Ther. 2022, 19, 58. [Google Scholar] [CrossRef]
- Lebbink, R.J.; de Jong, D.C.; Wolters, F.; Kruse, E.M.; van Ham, P.M.; Wiertz, E.J.; Nijhuis, M. A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Sci. Rep. 2017, 7, 41968. [Google Scholar] [CrossRef]
- Lin, C.; Li, H.; Hao, M.; Xiong, D.; Luo, Y.; Huang, C.; Yuan, Q.; Zhang, J.; Xia, N. Increasing the Efficiency of CRISPR/Cas9-mediated Precise Genome Editing of HSV-1 Virus in Human Cells. Sci. Rep. 2016, 6, 34531. [Google Scholar] [CrossRef]
- Ewaisha, R.; Anderson, K.S. Immunogenicity of CRISPR therapeutics-Critical considerations for clinical translation. Front. Bioeng. Biotechnol. 2023, 11, 1138596. [Google Scholar] [CrossRef]
- Zhen, W.; Sheikh, F.; Breining, D.A.; Berry, G.J. Rapid diagnosis of herpes simplex virus 1 and 2 bloodstream infections utilizing a sample-to-answer platform. J. Clin. Microbiol. 2024, 62, e0013124. [Google Scholar] [CrossRef]
- Liermann, K.; Schafler, A.; Henke, A.; Sauerbrei, A. Evaluation of commercial herpes simplex virus IgG and IgM enzyme immunoassays. J. Virol. Methods 2014, 199, 29–34. [Google Scholar] [CrossRef]
- Aldisi, R.S.; Elsidiq, M.S.; Dargham, S.R.; Sahara, A.S.; Al-Absi, E.S.; Nofal, M.Y.; Mohammed, L.I.; Abu-Raddad, L.J.; Nasrallah, G.K. Performance evaluation of four type-specific commercial assays for detection of herpes simplex virus type 1 antibodies in a Middle East and North Africa population. J. Clin. Virol. 2018, 103, 1–7. [Google Scholar] [CrossRef]
- Crawford, K.H.D.; Selke, S.; Pepper, G.; Goecker, E.; Sobel, A.; Wald, A.; Johnston, C.; Greninger, A.L. Performance characteristics of highly automated HSV-1 and HSV-2 IgG testing. J. Clin. Microbiol. 2024, 62, e0026324. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.R.; Ashley-Morrow, R.; Swenson, P.; Hogrefe, W.R.; Handsfield, H.H.; Wald, A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex. Transm. Dis. 2005, 32, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.L.; Brandt, K.; Horsman, G.B. Comparison of Chemicon SimulFluor direct fluorescent antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and with cytospin direct immunofluorescence staining for detection of varicella-zoster virus. Clin. Diagn. Lab. Immunol. 2001, 8, 909–912. [Google Scholar] [CrossRef]
- LeGoff, J.; Pere, H.; Belec, L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol. J. 2014, 11, 83. [Google Scholar] [CrossRef]
- Satpathy, G.; Behera, H.S.; Sharma, A.; Mishra, A.K.; Mishra, D.; Sharma, N.; Tandon, R.; Agarwal, T.; Titiyal, J.S. A 20-year experience of ocular herpes virus detection using immunofluorescence and polymerase chain reaction. Clin. Exp. Optom. 2018, 101, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Gitman, M.R.; Ferguson, D.; Landry, M.L. Comparison of Simplexa HSV 1 & 2 PCR with culture, immunofluorescence, and laboratory-developed TaqMan PCR for detection of herpes simplex virus in swab specimens. J. Clin. Microbiol. 2013, 51, 3765–3769. [Google Scholar] [CrossRef]
- Nath, P.; Kabir, M.A.; Doust, S.K.; Ray, A. Diagnosis of Herpes Simplex Virus: Laboratory and Point-of-Care Techniques. Infect. Dis. Rep. 2021, 13, 518–539. [Google Scholar] [CrossRef]
- Muller, W.J.; Zheng, X. Laboratory Diagnosis of Neonatal Herpes Simplex Virus Infections. J. Clin. Microbiol. 2019, 57, e01460-18. [Google Scholar] [CrossRef]
- Dominguez, S.R.; Pretty, K.; Hengartner, R.; Robinson, C.C. Comparison of Herpes Simplex Virus PCR with Culture for Virus Detection in Multisource Surface Swab Specimens from Neonates. J. Clin. Microbiol. 2018, 56, e01460-18. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, Y.; Gao, H.; Zhang, S.; Zheng, J.; Lu, X.; Liu, S.; Zhou, H.; Hun, X. CRISPR/Cas12a-based MUSCA-PEC strategy for HSV-1 assay. Anal. Chim. Acta 2023, 1250, 340955. [Google Scholar] [CrossRef]
- Huang, M.; Chen, Y.; Zheng, L.; Yao, Y.F. Highly sensitive and naked-eye detection of herpes simplex virus type 1 using LAMP- CRISPR/Cas12 diagnostic technology and gold nanoparticles. Heliyon 2023, 9, e22146. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chan, C.; Springs, S.L.; Lee, Y.H.; Lu, T.K.; Yu, H. A warm-start digital CRISPR/Cas-based method for the quantitative detection of nucleic acids. Anal. Chim. Acta 2022, 1196, 339494. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Luo, H.; Zhou, H.; Zhang, Z.; Lan, Y.; Feng, Z.; Chen, W.; Zheng, H. A rapid isothermal CRISPR-Cas13a diagnostic test for genital herpes simplex virus infection. iScience 2024, 27, 108581. [Google Scholar] [CrossRef]
- Arshad, Z.; Alturkistani, A.; Brindley, D.; Lam, C.; Foley, K.; Meinert, E. Tools for the Diagnosis of Herpes Simplex Virus 1/2: Systematic Review of Studies Published Between 2012 and 2018. JMIR Public Health Surveill 2019, 5, e14216. [Google Scholar] [CrossRef]
- Scoular, A. Using the evidence base on genital herpes: Optimising the use of diagnostic tests and information provision. Sex. Transm. Infect. 2002, 78, 160–165. [Google Scholar] [CrossRef]
- Devireddy, B.; Kalin, W.; Laningham, F.; Naeem, F. An Enigmatic Case of a Febrile Infant With Seizures. Cureus 2022, 14, e25663. [Google Scholar] [CrossRef]
- Akkaya, O. Prevalence of Herpes Simplex Virus Infections in the Central Nervous System. Clin. Lab. 2021, 67, 1615. [Google Scholar] [CrossRef]
- Politza, A.J.; Nouri, R.; Guan, W. Digital CRISPR systems for the next generation of nucleic acid quantification. Trends Anal. Chem. 2023, 159, 116917. [Google Scholar] [CrossRef] [PubMed]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas systems for diagnosing infectious diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Kirby, E.N.; Shue, B.; Thomas, P.Q.; Beard, M.R. CRISPR Tackles Emerging Viral Pathogens. Viruses 2021, 13, 2157. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Mol. Cell 2019, 76, 826–837.e811. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Bohn-Wippert, K.; Schlattmann, P.; Zell, R.; Sauerbrei, A. Sequence Analysis of Herpes Simplex Virus 1 Thymidine Kinase and DNA Polymerase Genes from over 300 Clinical Isolates from 1973 to 2014 Finds Novel Mutations That May Be Relevant for Development of Antiviral Resistance. Antimicrob. Agents Chemother. 2015, 59, 4938–4945. [Google Scholar] [CrossRef]
- Schalkwijk, H.H.; Georgala, A.; Gillemot, S.; Temblador, A.; Topalis, D.; Wittnebel, S.; Andrei, G.; Snoeck, R. A Herpes Simplex Virus 1 DNA Polymerase Multidrug Resistance Mutation Identified in a Patient With Immunodeficiency and Confirmed by Gene Editing. J. Infect. Dis. 2023, 228, 1505–1515. [Google Scholar] [CrossRef]
- Zheng, S.; Verjans, G.; Evers, A.; van den Wittenboer, E.; Tjhie, J.H.T.; Snoeck, R.; Wiertz, E.; Andrei, G.; van Kampen, J.J.A.; Lebbink, R.J. CRISPR/Cas9-mediated genome editing of the thymidine kinase gene in a clinical HSV-1 isolate identifies F289S as novel acyclovir-resistant mutation. Antivir. Res. 2024, 228, 105950. [Google Scholar] [CrossRef]
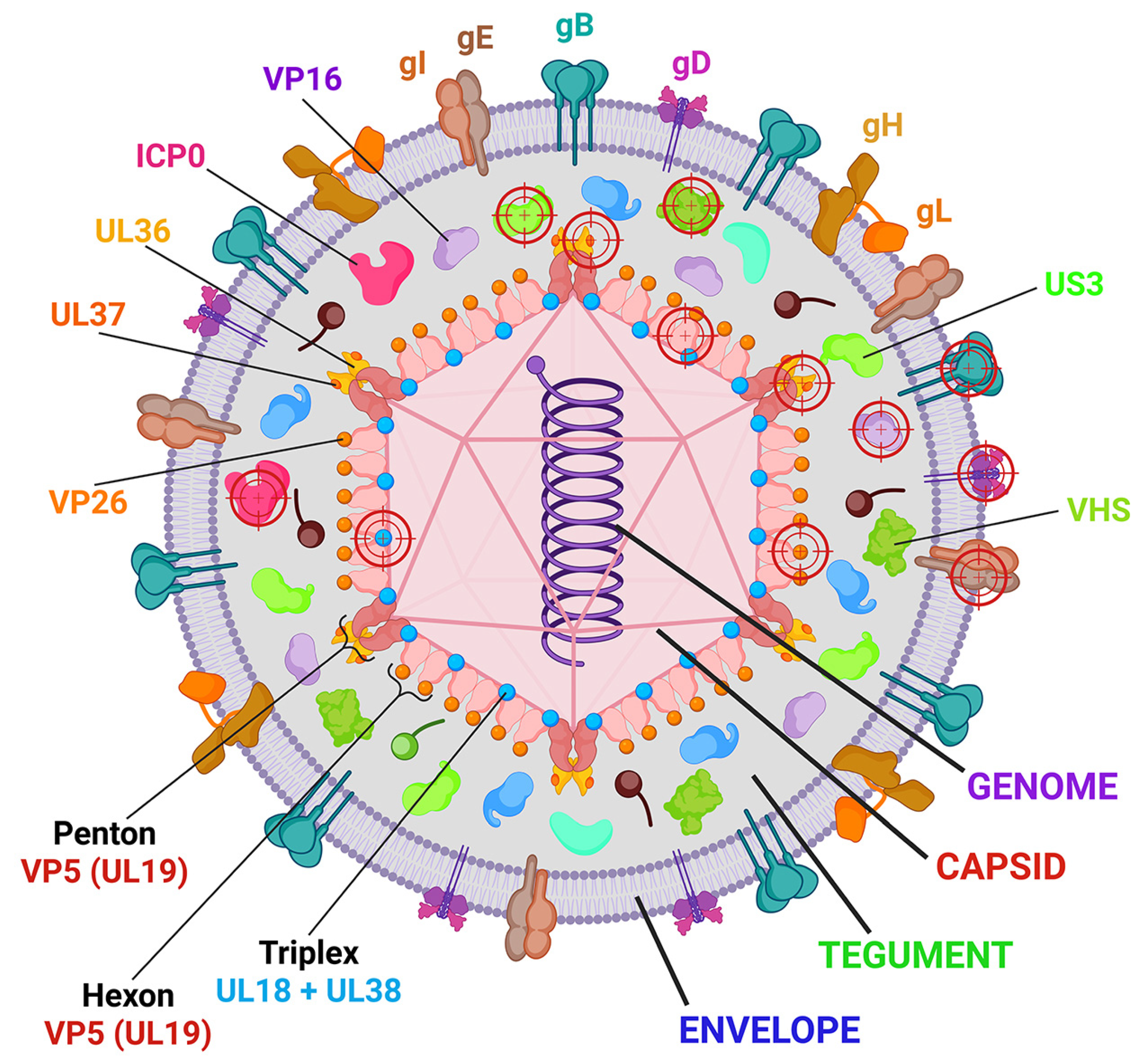
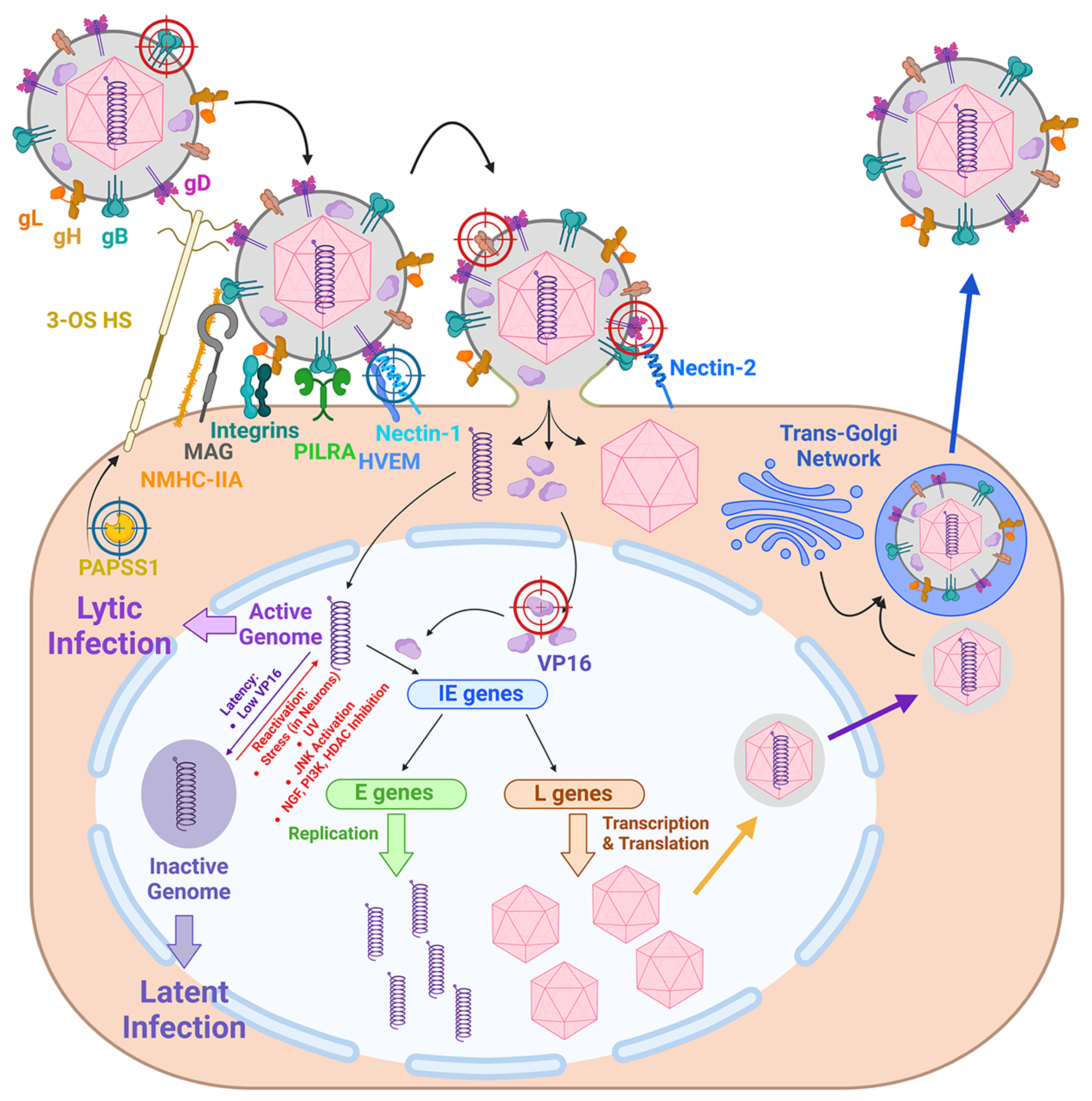
| Targeted Gene (Protein Name) | Function of Gene | Name of Cas Nuclease | Method or Vector Delivery | Anti-HSV-1 Activity | Reference |
|---|---|---|---|---|---|
| * Immediate early genes | |||||
| RL2 (ICP0) | RING-type E3 ubiquitin ligase acts in initial stages of infection or during HSV-1 reactivation, helps to evade cellular antiviral response | SpCas9 | Plasmid transfection | ICP0-mutants with indels demonstrated decreased reproduction | [110] |
| LVs with sgRNA and cells expressing Cas9 | Suppression of viral particle production | ||||
| LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication and increased protection of cells from infection | ||||
| SaCas9 | AAV2 with sgRNA and SaCas9 | Inhibition of viral replication in the Vero cell line and in cerebral organoids; suppression of the latent form of HSV-1 in cerebral organoids | [123] | ||
| SaCas9 | AAV9 with sgRNA and SaCas9 | Inhibition of HSV-1 infection and reactivation in a latent rabbit keratitis model | [121] | ||
| SaCas9 or SpCas9 | LVs with SaCas9 or SpCas9 and sgRNAs | Protection of cells from infection | [119] | ||
| RS1 (ICP4) | Major viral transcription factor | SaCas9 | LVs with SaCas9 and sgRNAs | Inhibition of viral particle production | [120] |
| SaCas9 or SpCas9 | LVs with SaCas9 or SpCas9 and sgRNAs | Protection of cells from infection, reduction of viral infection | [119] | ||
| AAVs with SaCas9 and sgRNAs | Inhibition of viral replication and particle production | ||||
| SpCas9 | Plasmid transfection | Inhibition of viral replication and particle production | [111] | ||
| UL54 (ICP27) | Multifunctional regulator of viral and cellular gene expression, modulates splicing, 3′ processing, mRNA export, and inhibits host mRNA biogenesis | SpCas9 | Plasmid transfection | Inhibition of viral replication and particle production | [111] |
| SaCas9 | LVs with SaCas9 and sgRNAs | Inhibition of viral particle production | [120] | ||
| SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] | ||
| SaCas9 | AAV2 with sgRNA and SaCas9 | Inhibition of viral replication in the Vero cell line and in cerebral organoids. Suppression of the latent form of HSV-1 in cerebral organoids. | [123] | ||
| SaCas9 | AAV9 with sgRNA and SaCas9 | Inhibition of HSV-1 infection and reactivation in a latent rabbit keratitis model | [121] | ||
| US12 (ICP47) | Multifunctional protein, blocks mRNA splicing, inhibits peptide presentation on MHC-I, transports viral mRNA from nucleus to cytoplasm | SpCas9 | Plasmid transfection | The combination of ICP47 and ICP34.5 gene deletions suppresses viral replication and particle formation | [124] |
| Early genes | |||||
| UL5 | Helicase, essential for HSV-1 genome replication | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL8 | DNA helicase/primase complex-associated protein, essential for HSV-1 genome replication | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication and particle production | [112] |
| SpCas9 | mRNA (Cas9)-carrying LVs with sgRNA | Inhibition of viral particle production | [122] | ||
| SpCas9 | Plasmid transfection | Targeting together with UL29 reduces viral replication and particle production | [114] | ||
| UL9 | Origin binding protein | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL23 | TK | SpCas9 | Inhibition of viral replication | [125] | |
| UL29 (ICP8) | ssDNA-binding protein | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication and particle production | [112] |
| SpCas9 | mRNA (Cas9)-carrying LVs with sgRNA | Inhibition of viral particle production | [122] | ||
| SpCas9 | Plasmid transfection | Targeting together with UL8 reduces viral replication and reproduction | [114] | ||
| UL30 | DNA-polymerase | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| SpCas9 | Plasmid transfection | Reduction of viral replication and particle production | [113] | ||
| DpbCasX | Plasmid transfection | Reduction of viral replication and particle production | |||
| SaCas9 | LVs with SaCas9 and sgRNAs | Inhibition of viral particle production | [120] | ||
| UL39 (ICP6) | Large subunit of ribonucleotide reductase; autophosphorylates via unique N terminus but does not trans-phosphorylate | SpCas9 | Plasmid transfection | UL39 knockout decreases HSV-1 replication, reproduction, and spreading | [115] |
| SpCas9 | Plasmid transfection | Inhibition of viral replication | [126] | ||
| UL42 | Processivity factor, DNA-polymerase complex-associated protein, increasing the affinity of UL30 for viral DNA | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL48 (VP16) | Tegument protein, activates transcription of IE genes | SpCas9 | Plasmid transfection | Inhibition of viral replication and particle production | [111] |
| UL52 | DNA primase, essential for HSV-1 genome replication | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication and particle production | [112] |
| SpCas9 | Plasmid transfection | Targeting together with UL29 reduces viral replication and reproduction | [114] | ||
| US3 | Viral serine/threonine kinase | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| Late genes | |||||
| UL7 | Tegument protein involved in herpesvirus assembly | SpCas9 | Plasmid transfection | UL7 mutation (30 bp deletion) reduces replication and in vitro proliferation and decreases LAT mRNA levels in latent infection in mice | [116] |
| SpCas9 | Plasmid transfection | UL7 mutation (30 bp deletion) attenuates pathogenicity, decreases replication, causes non-lethal infections in mice, lowers viral loads in brain and trigeminal nerve | [127] | ||
| UL15 | Terminase complex protein | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL16 | Tegument protein is crucial for the egress of capsids from the nuclei and the acquisition of a viral envelope | SpCas9 | Plasmid transfection | UL16 knockout decreases HSV-1 replication, reproduction, and spreading | [118] |
| UL21 | Tegument protein, involved in cell-to-cell spread | SpCas9 | Plasmid transfection | UL21 knockout decreases HSV-1 replication, reproduction, spreading | [128] |
| UL19 (VP5) | Major capsid protein, forms an icosahedral capsid with a T = 16 symmetry consisting of 162 capsomers | SpCas9 | Plasmid transfection | Inhibition of viral replication and particle production | [113] |
| RL1 (ICP34.5) | Neurovirulence factor | SpCas9 | Plasmid transfection | ICP34.5 knockout (replacement of the ICP34.5 gene with a GFP expression cassette) inhibits viral replication and viral particle production | [124] |
| UL35 (VP26) | Small capsomere-interacting protein, participates in the assembly of the infectious particles, forms a layer between the capsid and the tegument | SpCas9 | Plasmid transfection | Effect is unknown due to high cellular toxicity of the CRISPR/Cas9 system targeted to the UL35 | [113] |
| UL36 | Inner tegument protein, deubiquitinase | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL37 | Inner tegument protein involved in capsid traffic and virion morphogenesis | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| UL41 (VHS) | Causes nonspecific degradation of mRNA after infection; shuts off host protein synthesis, enables sequential synthesis of viral proteins | SpCas9 | Plasmid transfection | Attenuated pathogenicity (30 bp deletion in UL7 gene and 59 bp deletion in the UL41 gene), inhibition of viral replication, non-lethal infections in mice, lower viral loads in nervous tissues | [127] |
| UL27 (gB) | Envelope glycoprotein that forms spikes at the surface of virion envelope, essential for the initial attachment to the host cell receptors, involved in fusion of viral and cellular membranes, together with gK, induces syncytia formation | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| US6 (gD) | Envelope glycoprotein that binds to several cell receptors, including HVEM, NECTIN1, and 3-O-sulfated heparan sulfate | SpCas9 | Plasmid transfection | Inhibition of viral replication and particle production | [111] |
| US8 (gE) | Envelope glycoprotein is important for viral egress and cell-to-cell spread | SpCas9 | LVs with sgRNA and LVs with Cas9 | Inhibition of viral replication | [112] |
| SpCas9 | Plasmid transfection | US6 knockout decreases HSV-1 replication, reproduction, and spreading | [117] | ||
| LAT | LAT transcript is responsible for HSV-1 latency establishment and maintenance | SpCas9 | Plasmid transfection | Attenuated pathogenicity (30 bp deletion in UL7 gene, 59 bp deletion in the UL41 gene, and 138 bp deletion in the LAT gene), reduced replication, non-lethal infections in mice, reduced viral load in neural tissues and decreased latency | [127] |
| Name | Mechanism of Action | Advantages | Disadvantages | FDA Approval or Clinical Trials |
|---|---|---|---|---|
| ACV | Inhibits DNA polymerase indirectly through viral TK | Standard therapy, proven effectiveness | Neurotoxicity, virus can develop resistance | FDA approved |
| Foscarnet | Directly inhibits DNA polymerase by blocking the pyrophosphate binding site and preventing the cleavage of pyrophosphate from deoxynucleotide triphosphates | Useful for treating infections caused by ACV-resistant herpesviruses | Nephrotoxicity, wide range of side effects, virus can develop resistance | FDA approved |
| Amenavir, Pritelivir | Inhibition of the HSV-1 helicase–primase complex | Useful for treating infections caused by ACV-resistant herpesviruses | Virus can develop resistance | Phase 3 clinical trials (NCT03073967, NCT01959295) |
| CRISPR/Cas systems | Damage to viral DNA by introduction of double-stranded breaks followed by action by error-prone cellular DNA repair pathways, leading to inactivation of important viral genes | A potentially effective and safe approach to treat both active and latent HSV-1 infection | Insufficient knowledge of side effects | Phase 1, 2 clinical trials (NCT04560790, NCT06474416, NCT06474442) |
| Methods | Principle | Sample | Sensitivity/ Specificity | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Serological methods Detection of antiviral antibodies | Anti-HSV-1 IgM ELISA | Serum, plasma, | 88–95.4%/ 99% | Detection of early, acute phase of infection | False negative and false positive results; low sensitivity | [180] |
| Anti-HSV-1 IgG ELISA | 96%/ 86% | Markers of current, chronic, or latent infection | High prevalence in human population | [181] | ||
| Modifications: HSV-1 IgG sandwich electrochemiluminescence immunoassays | 85.9%/ 98.7% | [182] | ||||
| HSV-1 IgG multiplexed microparticle immunoassay | 87.1%/ 98.2% | |||||
| HSV-1 IgG chemiluminescent immunoassays | 94.8%/ 90.4% | |||||
| Additional methods: Avidity of anti-HSV-1 detection | Serum, plasma | 100%/ 100% | Low avidity indicates an early stage of the disease; high avidity indicates an active infection | According to “HSV-1 IgG avidity-IMBIAN-ELISA” test | ||
| Anti-HSV-1/2 immunoblotting | Serum, plasma | 95%/ 100% | High sensitivity and specificity; confirms and clarifies ELISA results; detection of antibodies against certain clinically relevant viral antigens | Difficulty in interpreting results in some categories of patients; requires assessment by qualified specialists; expensive; labor-intensive and requires high qualifications of the researcher | [183] | |
| Detection of viral antigen | Immunofluorescence assays in situ (IFA) Enzyme immunoassay (immunoperoxidase) assays (EIA) in the infected cells | Swabs | 80–86%/ 98.3–100% | Rapid (<3 h) HSV-1 strains can be typed | Require fresh clinical samples; suboptimal sensitivity; requires high sample quality and high qualifications of the researcher | [184,185,186] |
| Smears from urogenital lesions | ||||||
| Smear or vesicular fluid of exudate from base of vesicle | ||||||
| Corneal scrapings/corneal grafts | ||||||
| Detection of virus by cell culture method | Clinical samples co-cultivation with the cultural cells | Swabs | 80–90%/ 100% | Detection of infectious virus in a sample; high specificity Allows virus isolation | Require rapid transport, cooled, protected from light in virus transport medium; labor-intensive; expensive; specialized laboratories; long time to get results (4–7 days) | [187,188] |
| Skin lesions | ||||||
| Vesicular fluid of exudate from base of vesicle | ||||||
| Mucosal sample without lesions | ||||||
| Conjunctival/corneal smears | ||||||
| Saliva | ||||||
| Urine | ||||||
| Blood | ||||||
| Biopsies | ||||||
| Modification: rapid culture method: co-cultivation of samples with sensitive cell culture for 24–48 h followed by IFA using monoclonal antibodies | The samples presented above | 95%/ 100% | Faster than the classical culture method (24–48 h); confirmation of the HSV-1 presence in the clinical samples; determination of the number of viral infected cells per 105 cells | Sample storage and transport conditions influence sensitivity; labor-intensive; expensive; specialized laboratories | [189] | |
| Molecular-biological methods | ||||||
| Detection of viral DNA and genes by PCR | HSV1 DNA detection and quantitation by classical or real-time PCR | Conjunctival/corneal smear | 98%/ 100% | High sensitivity | Can be performed only in specialized laboratories | [187,188,190] |
| Swabs | Currently “preferred” test (CDC 2010) | Not standardized | ||||
| Skin lesions | Allows virus detection and typing in the same test | Not validated for all types of samples | ||||
| Vesicular fluid or exudate from the base of the vesicle | Rapid (<3 h) | Risk of contamination (PCR) | ||||
| Mucosal samples | May be automated | May be relatively expensive (real-time PCR) | ||||
| Aqueous/vitreous humor | Labor efficient | Routine resistance genotyping not available | ||||
| Cortico-spinal fluid | ||||||
| Blood and blood cells | Method of choice for cerebrospinal fluid (CSF) | |||||
| Real-time PCR | ||||||
| Rapid amplification | ||||||
| Quantitative analysis | ||||||
| Method of choice for skin lesions | ||||||
| Reduced risk of contamination | ||||||
| CRISPR/Cas-based diagnostics | MUSCA-PEC assay coupled with CRISPR/Cas12a for HSV-1 detection | Serum | 96.2%/ 100% | High sensitivity and specificity | Not yet approved by the FDA | [191] |
| LAMP-Cas12 diagnostic technology combined with gold nanoparticles | Tear specimens | 93.9%/100% | High sensitivity and specificity; easy interpretation no equipment required (suitable for point-of-care detection of HSV-1) | Not yet approved by the FDA | [192] | |
| Warm-start rapid digital CRISPR approach (WS-RADICA) | Synthetic DNA fragments | 99.6%/100% | High sensitivity and specificity | Not yet approved by the FDA | [193] | |
| Rapid isothermal CRISPR-Cas13a diagnostic test (HSV-SHERLOCK assay) | Clinical samples, swabs | 96.2%/ 100% | High sensitivity and specificity; rapid | Not yet approved by the FDA; requires relatively expensive equipment (microplate reader) | [194] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnovtseva, A.O.; Demidova, N.A.; Klimova, R.R.; Kovalev, M.A.; Kushch, A.A.; Starodubova, E.S.; Latanova, A.A.; Karpov, D.S. Control of HSV-1 Infection: Directions for the Development of CRISPR/Cas-Based Therapeutics and Diagnostics. Int. J. Mol. Sci. 2024, 25, 12346. https://doi.org/10.3390/ijms252212346
Sosnovtseva AO, Demidova NA, Klimova RR, Kovalev MA, Kushch AA, Starodubova ES, Latanova AA, Karpov DS. Control of HSV-1 Infection: Directions for the Development of CRISPR/Cas-Based Therapeutics and Diagnostics. International Journal of Molecular Sciences. 2024; 25(22):12346. https://doi.org/10.3390/ijms252212346
Chicago/Turabian StyleSosnovtseva, Anastasiia O., Natalia A. Demidova, Regina R. Klimova, Maxim A. Kovalev, Alla A. Kushch, Elizaveta S. Starodubova, Anastasia A. Latanova, and Dmitry S. Karpov. 2024. "Control of HSV-1 Infection: Directions for the Development of CRISPR/Cas-Based Therapeutics and Diagnostics" International Journal of Molecular Sciences 25, no. 22: 12346. https://doi.org/10.3390/ijms252212346
APA StyleSosnovtseva, A. O., Demidova, N. A., Klimova, R. R., Kovalev, M. A., Kushch, A. A., Starodubova, E. S., Latanova, A. A., & Karpov, D. S. (2024). Control of HSV-1 Infection: Directions for the Development of CRISPR/Cas-Based Therapeutics and Diagnostics. International Journal of Molecular Sciences, 25(22), 12346. https://doi.org/10.3390/ijms252212346








