Remodeling of the Intracardiac Ganglia During the Development of Cardiovascular Autonomic Dysfunction in Type 2 Diabetes: Molecular Mechanisms and Therapeutics
Abstract
1. Introduction
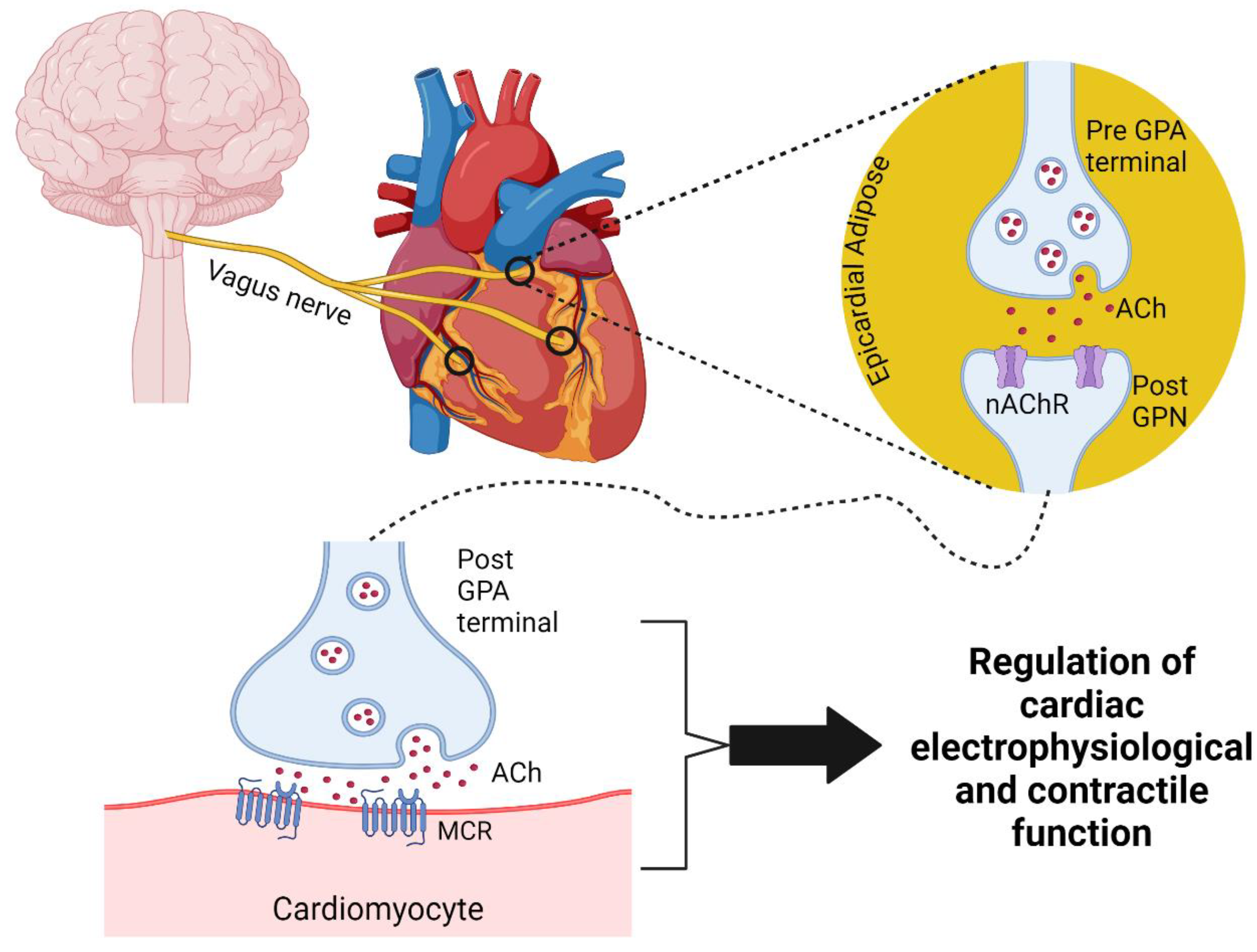
2. The Intrinsic Cardiac Nervous System
2.1. Anatomy of the Cardiac Parasympathetic Nervous System and Intrinsic Cardiac Nervous System
2.2. Neurophysiology of the Cardiac Parasympathetic Nervous System and Intracardiac Ganglia
3. Remodeling of CVP Parasympathetic Neurons and Its Role in Cardiac Vagal Withdrawal, Malignant Ventricular Arrhythmia, and Sudden Cardiac Death in T2DM
3.1. Cardiac Parasympathetic Withdrawal in T2DM
3.2. Structural Remodeling of Intracardiac Ganglia in T2DM
3.3. Functional Remodeling of Intracardiac Ganglia Neurons in T2DM
3.4. Changes in M2 Muscarinic Receptors in T2DM Rat Hearts
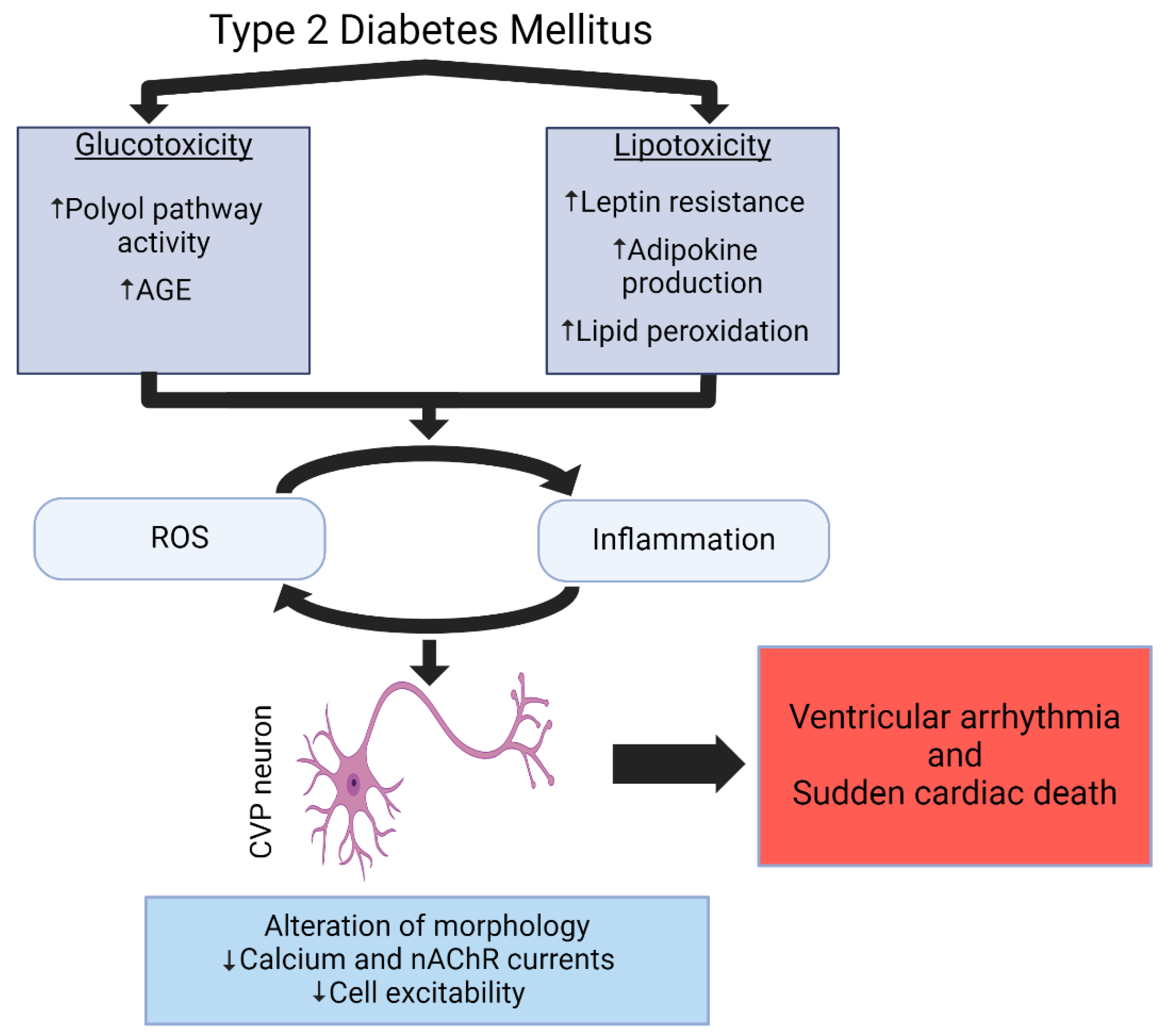
4. Cellular and Molecular Mechanisms Contributing to T2DM-Induced Cardiovascular Parasympathetic Withdrawal and Ventricular Arrhythmogenesis
4.1. Insulin Resistance, Glucose Toxicity, and Oxidative Stress
4.2. Lipotoxicity and Leptin Resistance
4.3. Inflammation
4.4. Vitamin Deficiencies
5. Potential Therapeutic Modalities to Treat Parasympathetic Withdrawal and Ventricular Arrhythmogenesis in T2DM Patients
5.1. Glycemic Control
5.2. Pharmacological Intervention
| Pharmaceutical Class | Citation | Treatment | Summary of Findings |
|---|---|---|---|
| Metformin | [247,248,249] | Metformin | Decreased incidence of ventricular arrhythmia and SCD |
| DPP-4 inhibitors | [253] | Teneligliptin | Improved heart rate in response to standing and heart rate in response to valsalva maneuver |
| [254,255] | Meta-analyses including sitagliptin, alogliptin, saxaglipitin, vidaglipitin, and linaglipitin | Induced sympathetic activation and heart failure | |
| GLP-1 receptor agonists | [259] | Liraglutide | Increased heart rate and reduced HRV |
| [260] | Liraglutide and glimepiride | No change in sympathovagal tone | |
| [261] | Meta-analysis including exenatide and liraglutide | No change in sympathovagal tone | |
| [262] | Meta-analysis including lixisenatide, liraglutide, semaglutide, exenatide, albiglutide, dulaglutide, and efpeglenatide | No impact on arrhythmia development | |
| SGLT2 inhibitors | [264] | Dapagliflozin | Improved HRV and reduced frequency of premature ventricular contractions |
| [265] | Empagliflozin | Reduced occurrence of ventricular arrhythmia | |
| [266] | Meta-analysis including dapagliflozin and empagliflozin | No changes in cardiovascular autonomic dysfunction | |
| ACE inhibitors | [267] | Rampiril | Improved E/I ratio during deep breathing test |
| [268] | Quinapril | Improved E/I ratio and mean circular resultant during deep breathing test | |
| [269] | Trandoapril | No changes in cardiovascular autonomic dysfunction | |
| Antioxidant therapy | [270] | Vitamin C | Improved HRV and decreased heart rate |
| [271] | Vitamin E | Increased high power components of HRV | |
| [272,273] | α-Lipoic acid | No change in HRV |
5.3. Antioxidant Therapy
5.4. Vagal Nerve Stimulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.; Handelsman, Y.; Heile, M.; Shannon, M. Burden of illness in type 2 diabetes mellitus. J. Manag. Care Spec. Pharm. 2018, 24, S5–S13. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, H.; Li, Z. The pathogenesis of diabetes. Int. J. Mol. Sci. 2023, 24, 6978. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Y.N.; Su, Y.T.; Shan, S.G.; Qian, W.B.; Zhang, F.X.; Li, M.X.; Zhang, Z.W. Molecular mechanisms of diabetic nephropathy: A narrative review. Cell Biol. Int. 2024, 48, 1240–1253. [Google Scholar] [CrossRef]
- Tang, Q.; Buonfiglio, F.; Böhm, E.W.; Zhang, L.; Pfeiffer, N.; Korb, C.A.; Gericke, A. Diabetic retinopathy: New treatment approaches targeting redox and immune mechanisms. Antioxidants 2024, 13, 594. [Google Scholar] [CrossRef]
- Strand, N.; Anderson, M.A.; Attanti, S.; Gill, B.; Wie, C.; Dawodu, A.; Pagan-Rosado, R.; Harbell, M.W.; Maloney, J.A. Diabetic neuropathy: Pathophysiology review. Curr. Pain. Headache Rep. 2024, 28, 481–487. [Google Scholar] [CrossRef]
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Jouven, X.; Lemaitre, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.P.; Siscovick, D.S. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, S.; Zhang, Y.; Zhang, X.; Xu, Q.; Xia, X.; Wang, P.; Luo, Y.; Wu, S.; Wang, A. Association of cumulative blood glucose load with cardiovascular risk and all-cause mortality. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102900. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.M.; Cubbon, R.M. Sudden cardiac death in patients with diabetes mellitus and chronic heart failure. Diabetes Vasc. Dis. Res. 2015, 12, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mhaimeed, O.; Pillai, K.; Dargham, S.; Al Suwaidi, J.; Jneid, H.; Abi Khalil, C. Type 2 diabetes and in-hospital sudden cardiac arrest in ST-elevation myocardial infarction in the US. Front. Cardiovasc. Med. 2023, 10, 1175731. [Google Scholar] [CrossRef]
- Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Abe, R.A.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Mohammed, L. Type 2 diabetes and causes of sudden cardiac death: A systematic review. Cureus 2021, 13, e18145. [Google Scholar] [CrossRef]
- Vahatalo, J.H.; Huikuri, H.V.; Holmstrom, L.T.A.; Kentta, T.V.; Haukilahti, M.A.E.; Pakanen, L.; Kaikkonen, K.S.; Tikkanen, J.; Perkiomaki, J.S.; Myerburg, R.J.; et al. Association of silent myocardial infarction and sudden cardiac death. JAMA Cardiol. 2019, 4, 796–802. [Google Scholar] [CrossRef]
- Watanabe, E.; Tanabe, T.; Osaka, M.; Chishaki, A.; Takase, B.; Niwano, S.; Watanabe, I.; Sugi, K.; Katoh, T.; Takayanagi, K.; et al. Sudden cardiac arrest recorded during Holter monitoring: Prevalence, antecedent electrical events, and outcomes. Heart Rhythm. 2014, 11, 1418–1425. [Google Scholar] [CrossRef]
- Deo, R.; Albert, C.M. Epidemiology and genetics of sudden cardiac death. Circulation 2012, 125, 620–637. [Google Scholar] [CrossRef]
- Chakraborty, P.; Nattel, S.; Nanthakumar, K.; Connelly, K.A.; Husain, M.; Po, S.S.; Ha, A.C.T. Sudden cardiac death due to ventricular arrhythmia in diabetes mellitus: A bench to bedside review. Heart Rhythm. 2024, 21, 1827–1837. [Google Scholar] [CrossRef]
- Rawshani, A.; McGuire, D.K.; Omerovic, E.; Sattar, N.; McMurray, J.J.V.; Smith, U.; Redfors, B.; Bergfeldt, L.; Eliasson, B.; Boren, J.; et al. Cardiac arrhythmias and conduction abnormalities in patients with type 2 diabetes. Sci. Rep. 2023, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Glucose-lowering agents and risk of ventricular arrhythmias and sudden cardiac death: A comprehensive review ranging from sulphonylureas to SGLT2 inhibitors. Diabetes Metab. 2022, 48, 101405. [Google Scholar] [CrossRef] [PubMed]
- Reaven, P.D.; Emanuele, N.V.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; McCarren, M.; Duckworth, W.C.; Hayward, R.A. Intensive glucose control in patients with type 2 diabetes—15-year follow-up. N. Engl. J. Med. 2019, 380, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [PubMed]
- de Moura-Tonello, S.C.; Porta, A.; Marchi, A.; de Almeida, F.A.; Francisco, C.O.; Rehder-Santos, P.; Milan-Mattos, J.C.; Simoes, R.P.; Gois, M.O.; Catai, A.M. Cardiovascular variability analysis and baroreflex estimation in patients with type 2 diabetes in absence of any manifest neuropathy. PLoS ONE 2016, 11, e0148903. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tanaka, A.; Sata, M.; Dai, K.; Shibata, Y.; Inoue, Y.; Ikenaga, H.; Kishimoto, S.; Ogasawara, K.; Takashima, A.; et al. alpha-Glucosidase inhibitor miglitol attenuates glucose fluctuation, heart rate variability and sympathetic activity in patients with type 2 diabetes and acute coronary syndrome: A multicenter randomized controlled (MACS) study. Cardiovasc. Diabetol. 2017, 16, 86–0571. [Google Scholar] [CrossRef]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjornsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef]
- Thaung, H.P.; Baldi, J.C.; Wang, H.Y.; Hughes, G.; Cook, R.F.; Bussey, C.T.; Sheard, P.W.; Bahn, A.; Jones, P.P.; Schwenke, D.O.; et al. Increased efferent cardiac sympathetic nerve activity and defective intrinsic heart rate regulation in type 2 diabetes. Diabetes 2015, 64, 2944–2956. [Google Scholar] [CrossRef]
- Ruiz, J.; Monbaron, D.; Parati, G.; Perret, S.; Haesler, E.; Danzeisen, C.; Hayoz, D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 2005, 46, 162–167. [Google Scholar] [CrossRef]
- Sorski, L.; Gidron, Y. The vagal nerve, inflammation, and diabetes-A holy triangle. Cells 2023, 12, 1632. [Google Scholar] [CrossRef]
- Ziegler, D.; Strom, A.; Bonhof, G.; Puttgen, S.; Bodis, K.; Burkart, V.; Mussig, K.; Szendroedi, J.; Markgraf, D.F.; Roden, M.; et al. Differential associations of lower cardiac vagal tone with insulin resistance and insulin secretion in recently diagnosed type 1 and type 2 diabetes. Metabolism 2018, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Cai, J.; Brancati, F.L.; Folsom, A.; Barnes, R.W.; Tyroler, H.A.; Heiss, G. Association of vagal tone with serum insulin, glucose, and diabetes mellitus; The ARIC Study. Diabetes Res. Clin. Pract. 1995, 30, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, J.; Dajani, A.H.; Cha, S.; Hanna, P.; Challita, R.; Hoover, D.B.; Ajijola, O.A.; Shivkumar, K.; Ardell, J.L. Vagal nerve stimulation reduces ventricular arrhythmias and mitigates adverse neural cardiac remodeling post-myocardial infarction. JACC Basic. Transl. Sci. 2023, 8, 1100–1118. [Google Scholar] [CrossRef] [PubMed]
- Kalla, M.; Herring, N.; Paterson, D.J. Cardiac sympatho-vagal balance and ventricular arrhythmia. Auton. Neurosci. 2016, 199, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.A. Vagal modulation of cardiac ventricular arrhythmia. Exp. Physiol. 2014, 99, 295–299. [Google Scholar] [CrossRef]
- Vaseghi, M.; Shivkumar, K. The role of the autonomic nervous system in sudden cardiac death. Prog. Cardiovasc. Dis. 2008, 50, 404–419. [Google Scholar] [CrossRef]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef]
- Verrier, R.L.; Antzelevitch, C. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr. Opin. Cardiol. 2004, 19, 2–11. [Google Scholar] [CrossRef]
- Duraes Campos, I.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell Cardiol. 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Dyavanapalli, J. Novel approaches to restore parasympathetic activity to the heart in cardiorespiratory diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1153–H1161. [Google Scholar] [CrossRef]
- Fedele, L.; Brand, T. The intrinsic cardiac nervous system and its role in cardiac pacemaking and conduction. J. Cardiovasc. Dev. Dis. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, V.; Schwertfeger, E.; Rutz, T.; Beyersdorf, F.; Rump, L.C. Acetylcholine release in human heart atrium. Circulation 2001, 103, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, H.; Zheng, H.; Zhang, L.; Tran, T.P.; Muelleman, R.L.; Li, Y.L. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. Am. J. Physiol. Cell Physiol. 2012, 302, C1119–C1127. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, D.; Tu, H.; Li, Y.L. Reduced cell excitability of cardiac postganglionic parasympathetic neurons correlates with myocardial infarction-induced fatal ventricular arrhythmias in type 2 diabetes mellitus. Front. Neurosci. 2021, 15, 721364. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Tu, H.; Muelleman, R.L.; Wang, W.Z.; Li, Y.L. Nicotinic acetylcholine receptors and cardiac vagal activity in rats with type 2 diabetes. Diabetes Metab. 2015, S13, 012. [Google Scholar]
- Machhada, A.; Ang, R.; Ackland, G.L.; Ninkina, N.; Buchman, V.L.; Lythgoe, M.F.; Trapp, S.; Tinker, A.; Marina, N.; Gourine, A.V. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm. 2015, 12, 2285–2293. [Google Scholar] [CrossRef]
- Batulevicius, D.; Frese, T.; Peschke, E.; Pauza, D.H.; Batuleviciene, V. Remodeling of the intracardiac ganglia in diabetic Goto-Kakizaki rats: An anatomical study. Cardiovasc. Diabetol. 2013, 12, 85. [Google Scholar]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Robinson, B.F.; Epstein, S.E.; Beiser, G.D.; Braunwald, E. Control of heart rate by the autonomic nervous system. Circ. Res. 1966, 19, 400–411. [Google Scholar] [CrossRef]
- Khan, A.A.; Lip, G.Y.H.; Shantsila, A. Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. Eur. J. Clin. Investig. 2019, 49, e13174. [Google Scholar] [CrossRef]
- Gorky, J.; Schwaber, J. Conceptualization of a parasympathetic endocrine system. Front. Neurosci. 2019, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Zera, T.; Paton, J.F.R. Chapter 10—Respiratory–cardiovascular interactions. In Handbook of Clinical Neurology; Chen, R., Guyenet, P.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 279–308. [Google Scholar]
- Giannino, G.; Braia, V.; Griffith Brookles, C.; Giacobbe, F.; D’Ascenzo, F.; Angelini, F.; Saglietto, A.; De Ferrari, G.M.; Dusi, V. The intrinsic cardiac nervous system: From pathophysiology to therapeutic implications. Biology 2024, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 2005, 209, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Murphy, D.A.; Yuan, B.-X.; MacDonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Aksu, T.; Gupta, D.; Pauza, D.H. Anatomy and physiology of intrinsic cardiac autonomic nervous system: Da vinci anatomy card #2. JACC Case Rep. 2021, 3, 625–629. [Google Scholar]
- Armour, J.A. Intrinsic cardiac neurons. J. Cardiovasc. Electrophysiol. 1991, 2, 331–341. [Google Scholar] [CrossRef]
- Tompkins, J.D.; Hoover, D.B.; Havton, L.A.; Patel, J.C.; Cho, Y.; Smith, E.H.; Biscola, N.P.; Ajijola, O.A.; Shivkumar, K.; Ardell, J.L. Comparative specialization of intrinsic cardiac neurons in humans, mice, and pigs. bioRxiv 2024. [Google Scholar] [CrossRef]
- Singh, S.; Johnson, P.I.; Lee, R.E.; Orfei, E.; Lonchyna, V.A.; Sullivan, H.J.; Montoya, A.; Tran, H.; Wehrmacher, W.H.; Wurster, R.D. Topography of cardiac ganglia in the adult human heart. J. Thorac. Cardiovasc. Surg. 1996, 112, 943–953. [Google Scholar]
- Richardson, R.J.; Grkovic, I.; Anderson, C.R. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 2003, 314, 337–350. [Google Scholar] [CrossRef]
- Lizot, G.; Pasqualin, C.; Tissot, A.; Pagès, S.; Faivre, J.-F.; Chatelier, A. Molecular and functional characterization of the mouse intrinsic cardiac nervous system. Heart Rhythm. 2022, 19, 1352–1362. [Google Scholar] [CrossRef]
- Sampaio, K.N.; Mauad, H.; Spyer, K.M.; Ford, T.W. Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp. Physiol. 2003, 88, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Achanta, S.; Gorky, J.; Leung, C.; Moss, A.; Robbins, S.; Eisenman, L.; Chen, J.; Tappan, S.; Heal, M.; Farahani, N.; et al. A comprehensive integrated anatomical and molecular atlas of rat intrinsic cardiac nervous system. iScience 2020, 23, 101140. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevicius, R.; Saburkina, I.; Rysevaite, K.; Vaitkeviciene, I.; Pauziene, N.; Zaliunas, R.; Schauerte, P.; Jalife, J.; Pauza, D.H. Nerve supply of the human pulmonary veins: An anatomical study. Heart Rhythm. 2009, 6, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Pauza, D.H.; Skripka, V.; Pauziene, N.; Stropus, R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000, 259, 353–382. [Google Scholar] [CrossRef]
- Hanna, P.; Dacey, M.J.; Brennan, J.; Moss, A.; Robbins, S.; Achanta, S.; Biscola, N.P.; Swid, M.A.; Rajendran, P.S.; Mori, S.; et al. Innervation and neuronal control of the mammalian sinoatrial node a comprehensive atlas. Circ. Res. 2021, 128, 1279–1296. [Google Scholar] [CrossRef]
- Bell, C. Chapter 46—Regulation of metabolism by the autonomic nervous system. In Primer on the Autonomic Nervous System, 4th ed.; Biaggioni, I., Browning, K., Fink, G., Jordan, J., Low, P.A., Paton, J.F.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 263–266. [Google Scholar]
- Apelt-Glitz, K.; Alken, F.-A.; Jungen, C.; Scherschel, K.; Klöcker, N.; Meyer, C. Respiratory and heart rate dynamics during peripheral chemoreceptor deactivation compared to targeted sympathetic and sympathetic/parasympathetic (co-)activation. Auton. Neurosci. 2022, 241, 103009. [Google Scholar] [CrossRef]
- Elia, A.; Fossati, S. Autonomic nervous system and cardiac neuro-signaling pathway modulation in cardiovascular disorders and Alzheimer’s disease. Front. Physiol. 2023, 14, 1060666. [Google Scholar] [CrossRef]
- Tanaka, K.; Chiba, T. The vagal origin of preganglionic fibers containing nitric oxide synthase in the guinea-pig heart. Neurosci. Lett. 1998, 252, 135–138. [Google Scholar] [CrossRef]
- Sato, A.; Kojima, F.; Hayashi, T.; Arichi, S.; Maruo, Y.; Ishibashi, H.; Eto, K. The KCNQ channel inhibitor XE991 suppresses nicotinic acetylcholine receptor-mediated responses in rat intracardiac ganglion neurons. Pharmacol. Rep. 2022, 74, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Tu, H.; Hu, W.; Duan, B.; Zimmerman, M.C.; Li, Y.L. Hydrogen peroxide scavenging restores N-type calcium channels in cardiac vagal postganglionic neurons and mitigates myocardial infarction-evoked ventricular arrhythmias in type 2 diabetes mellitus. Front. Cardiovasc. Med. 2022, 9, 871852. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Remme, C.A.; Schumacher, C.A.; Scicluna, B.P.; Wolswinkel, R.; de Jonge, B.; Bezzina, C.R.; Veldkamp, M.W. Functional NaV1.8 channels in intracardiac neurons. Circ. Res. 2012, 111, 333–343. [Google Scholar] [CrossRef]
- Xu, Z.J.; Adams, D.J. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J. Physiol. 1992, 456, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A. The little brain on the heart. Cleve Clin. J. Med. 2007, 74, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Hoard, J.L.; Hoover, D.B.; Mabe, A.M.; Blakely, R.D.; Feng, N.; Paolocci, N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 2008, 156, 129–142. [Google Scholar] [CrossRef]
- Tomas, R.; Kristina, R.-K.; Neringa, P.; Hermanas, I.; Dainius, P.H. Intrinsic cardiac neurons of the adult pigs: Chemical types, abundance, parameters and distribution within ganglionated plexus. Ann. Anat. Anat. Anz. 2022, 243, 151935. [Google Scholar] [CrossRef]
- Horackova, M.; Croll, R.P.; Hopkins, D.A.; Losier, A.M.; Armour, J.A. Morphological and immunohistochemical properties of primary long-term cultures of adult guinea-pig ventricular cardiomyocytes with peripheral cardiac neurons. Tissue Cell 1996, 28, 411–425. [Google Scholar] [CrossRef]
- Brown, D.A. Acetylcholine and cholinergic receptors. Brain Neurosci. Adv. 2019, 3, 2398212818820506. [Google Scholar] [CrossRef]
- Peralta, E.G.; Ashkenazi, A.; Winslow, J.W.; Smith, D.H.; Ramachandran, J.; Capon, D.J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987, 6, 3923–3929. [Google Scholar] [CrossRef]
- Brann, M.R.; Ellis, J.; Jørgensen, H.; Hill-Eubanks, D.; Jones, S.V.P. Chapter 12: Muscarinic acetylcholine receptor subtypes: Localization and structure/function. In Progress in Brain Research; Cuello, A.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 121–127. [Google Scholar]
- Wang, H.; Han, H.; Zhang, L.; Shi, H.; Schram, G.; Nattel, S.; Wang, Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol. Pharmacol. 2001, 59, 1029. [Google Scholar] [CrossRef]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.D.; Belevych, A.E. Muscarinic regulation of cardiac ion channels. Br. J. Pharmacol. 2003, 139, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Sachse, F.B.; Moreno-Galindo, E.G.; Navarro-Polanco, R.A.; Tristani-Firouzi, M.; Seemann, G. Modeling effects of voltage dependent properties of the cardiac muscarinic receptor on human sinus node function. PLoS Comput. Biol. 2018, 14, e1006438. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, I.; Chong, A.C.Y.; Carcouet, A.; Waard, S.D.; Charpentier, F.; Ronjat, M.; Waard, M.D.; Isbrandt, D.; Wickman, K.; Vincent, A.; et al. Inhibition of G protein-gated K+ channels by tertiapin-Q rescues sinus node dysfunction and atrioventricular conduction in mouse models of primary bradycardia. Sci. Rep. 2020, 10, 9835. [Google Scholar] [CrossRef] [PubMed]
- Borst, M.M.; Szalai, P.; Herzog, N.; Kübler, W.; Strasser, R.H. Transregulation of adenylyl-cyclase-coupled inhibitory receptors in heart failure enhances anti-adrenergic effects on adult rat cardiomyocytes. Cardiovasc. Res. 1999, 44, 113–120. [Google Scholar] [CrossRef]
- Wei, J.W.; Wang, M.C. Muscarinic M2 receptors coupled to inhibition of adenylate cyclase in rat heart. Chin. J. Physiol. 1990, 33, 315–327. [Google Scholar]
- Ashton, J.L.; Prince, B.; Sands, G.; Argent, L.; Anderson, M.; Smith, J.E.G.; Tedoldi, A.; Ahmad, A.; Baddeley, D.; Pereira, A.G.; et al. Electrophysiology and 3D-imaging reveal properties of human intracardiac neurons and increased excitability with atrial fibrillation. J. Physiol. 2024. [Google Scholar] [CrossRef]
- van Weperen, V.Y.H.; Vos, M.A.; Ajijola, O.A. Autonomic modulation of ventricular electrical activity: Recent developments and clinical implications. Clin. Auton. Res. 2021, 31, 659–676. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Nakamura, K.; Ajijola, O.A.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Myocardial infarction induces structural and functional remodeling of the intrinsic cardiac nervous system. J. Physiol. 2016, 594, 321–341. [Google Scholar] [CrossRef]
- Zoppini, G.; Cacciatori, V.; Raimondo, D.; Gemma, M.; Trombetta, M.; Dauriz, M.; Brangani, C.; Pichiri, I.; Negri, C.; Stoico, V.; et al. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: The verona newly diagnosed type 2 diabetes study (VNDS). Diabetes Care 2015, 38, 1487–1493. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Dubb, K.; Raymond, N.T.; Begum, S.; Altaf, Q.A.; Sadiqi, H.; Piya, M.K.; Stevens, M.J. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: A cohort study. Diabetologia 2014, 57, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hanna, P.; Rajendran, P.S.; Shivkumar, K. Neuromodulation for ventricular tachycardia and atrial fibrillation: A clinical scenario-based review. JACC Clin. Electrophysiol. 2019, 5, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Jungen, C.; Scherschel, K.; Flenner, F.; Jee, H.; Rajendran, P.; De Jong, K.A.; Nikolaev, V.; Meyer, C.; Ardell, J.L.; Tompkins, J.D. Increased arrhythmia susceptibility in type 2 diabetic mice related to dysregulation of ventricular sympathetic innervation. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1328–H1341. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, L.A. Diabetes and arrhythmias: Pathophysiology, mechanisms and therapeutic outcomes. Front. Physiol. 2018, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.P.; Neeraja, M.; Kumar, S.C.; Farha, N.; Vijitha, P. Cardiovascular parasympathetic changes in type-II diabetic mellitus. Int. J. Integr. Med. Sci. 2016, 3, 374–383. [Google Scholar] [CrossRef]
- Hadad, R.; Akobe, S.F.; Weber, P.; Madsen, C.V.; Larsen, B.S.; Madsbad, S.; Nielsen, O.W.; Dominguez, M.H.; Haugaard, S.B.; Sajadieh, A. Parasympathetic tonus in type 2 diabetes and pre-diabetes and its clinical implications. Sci. Rep. 2022, 12, 18020. [Google Scholar] [CrossRef]
- Rasmussen, T.; Finnerup, N.B.; Singer, W.; Jensen, T.S.; Hansen, J.; Terkelsen, A.J. Preferential impairment of parasymathetic autonomic function in type 2 diabetes. Auton. Neurosci. 2022, 243, 103026. [Google Scholar] [CrossRef]
- Spallone, V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: What Iis defined, what is new, and what is unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef]
- Serhiyenko, V.A.; Serhiyenko, A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Sudo, S.Z.; Montagnoli, T.L.; Rocha, B.S.; Santos, A.D.; de Sa, M.P.L.; Zapata-Sudo, G. Diabetes-induced cardiac autonomic neuropathy: Impact on heart function and prognosis. Biomedicines 2022, 10, 3258. [Google Scholar] [CrossRef]
- Julu, P. Vagolytic effect of diabetes mellitus. Brain 1993, 116, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, A.; Harsoda, J.M. Sympathetic overactivity and parasympathetic impairment in type 2 diabetes: An analysis of cardiovascular autonomic functions. Cureus 2024, 16, e59561. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Strom, A.; Kupriyanova, Y.; Bierwagen, A.; Bonhof, G.J.; Bodis, K.; Mussig, K.; Szendroedi, J.; Bobrov, P.; Markgraf, D.F.; et al. Association of lower cardiovagal tone and baroreflex sensitivity with higher liver fat content early in type 2 diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 1130–1138. [Google Scholar] [CrossRef]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef]
- John, A.P.P.; Udupa, K.; Avangapur, S.; Sujan, M.U.; Inbaraj, G.; Vasuki, P.P.; Mahadevan, A.; Anilkumar, R.; Shekar, M.A.; Sathyaprabha, T.N. Cardiac autonomic dysfunctions in type 2 diabetes mellitus: An investigative study with heart rate variability measures. Am. J. Cardiovasc. Dis. 2022, 12, 224–232. [Google Scholar]
- Tu, H.; Zhang, D.; Li, Y.L. Cellular and molecular mechanisms underlying arterial baroreceptor remodeling in cardiovascular diseases and diabetes. Neurosci. Bull. 2019, 35, 98–112. [Google Scholar] [CrossRef]
- Ai, J.; Epstein, P.N.; Gozal, D.; Yang, B.; Wurster, R.; Cheng, Z.J. Morphology and topography of nucleus ambiguus projections to cardiac ganglia in rats and mice. Neuroscience 2007, 149, 845–860. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, H.; Yu, J.; Wurster, R.D.; Gozal, D. Attenuation of baroreflex sensitivity after domoic acid lesion of the nucleus ambiguus of rats. J. Appl. Physiol. 2003, 96, 1137–1145. [Google Scholar] [CrossRef]
- Bakkar, N.Z.; Mougharbil, N.; Mroueh, A.; Kaplan, A.; Eid, A.H.; Fares, S.; Zouein, F.A.; El-Yazbi, A.F. Worsening baroreflex sensitivity on progression to type 2 diabetes: Localized vs. systemic inflammation and role of antidiabetic therapy. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E835–E851. [Google Scholar] [CrossRef]
- Sailaja, A.N.; Nanda, N.; Suryanarayana, B.S.; Pal, G.K. Hypertension attenuates the link of osteoprotegerin to reduced baroreflex sensitivity in type 2 diabetes mellitus patients on oral antidiabetic and antihypertensive therapy—A cross sectional study. BMC Endocr. Disord. 2022, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Sanya, E.O.; Brown, C.M.; Dutsch, M.; Zikeli, U.; Neundorfer, B.; Hilz, M.J. Impaired cardiovagal and vasomotor responses to baroreceptor stimulation in type II diabetes mellitus. Eur. J. Clin. Investig. 2003, 33, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Raczak, G.; Szwoch, M.; Wabich, E.; Świątczak, M.; Daniłowicz-Szymanowicz, L. Baroreflex sensitivity but not microvolt T-wave alternans can predict major adverse cardiac events in ischemic heart failure. Cardiol. J. 2022, 29, 1004–1012. [Google Scholar] [CrossRef]
- Garcia, R.; Degand, B.; Fraty, M.; Le Marcis, V.; Bidegain, N.; Laude, D.; Tavernier, M.; Le Gal, F.; Hadjadj, S.; Saulnier, P.-J.; et al. Baroreflex sensitivity assessed with the sequence method is associated with ventricular arrhythmias in patients implanted with a defibrillator for the primary prevention of sudden cardiac death. Arch. Cardiovasc. Dis. 2019, 112, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.; Talanki Manjunatha, R.; Bollu, B.; Jhaveri, S.; Avanthika, C.; Reddy, N.; Saha, T.; Gandhi, F. A comprehensive review of neuronal changes in diabetics. Cureus 2021, 13, e19142. [Google Scholar] [CrossRef]
- Lund, D.D.; Subieta, A.R.; Pardini, B.J.; Chang, K.S. Alterations in cardiac parasympathetic indices in STZ-induced diabetic rats. Diabetes 1992, 41, 160–166. [Google Scholar] [CrossRef]
- Kamal, A.A.J.; Tay, S.S.W.; Wong, W.C. The cardiac ganglia in streptozotocin-induced diabetic rats. Arch. Histol. Cytol. 1991, 54, 41–49. [Google Scholar] [CrossRef][Green Version]
- Tsujimura, T.; Nunotani, H.; Fushimi, H.; Inoue, T. Morphological changes in autonomic ganglionic cells of the heart in diabetic patients. Diabetes Res. Clin. Pract. 1986, 2, 133–137. [Google Scholar] [CrossRef]
- Jungen, C.; Scherschel, K.; Eickholt, C.; Kuklik, P.; Klatt, N.; Bork, N.; Salzbrunn, T.; Alken, F.; Angendohr, S.; Klene, C.; et al. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat. Commun. 2017, 8, 14155. [Google Scholar] [CrossRef]
- Xu, Z.J.; Adams, D.J. Voltage-dependent sodium and calcium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J. Physiol. 1992, 456, 425–441. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; LaCroix, C.; Freeling, J. Specific subtypes of nicotinic cholinergic receptors involved in sympathetic and parasympathetic cardiovascular responses. Neurosci. Lett. 2009, 462, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Orr-Urtreger, A.; Korczyn, A.D. The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: Lessons from knockout mice. Progress. Neurobiol. 2002, 68, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jiang, H. Role of nicotinic acetylcholine receptors in cardiovascular physiology and pathophysiology: Current trends and perspectives. Curr. Vasc. Pharmacol. 2021, 19, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Koster, J.C.; Permutt, M.A.; Nichols, C.G. Diabetes and insulin secretion: The ATP-sensitive K+ channel (KATP) connection. Diabetes 2005, 54, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Stakenborg, N.; Gomez-Pinilla, P.J.; Verlinden, T.J.M.; Wolthuis, A.M.; D’Hoore, A.; Farré, R.; Herijgers, P.; Matteoli, G.; Boeckxstaens, G.E. Comparison between the cervical and abdominal vagus nerves in mice, pigs, and humans. Neurogastroenterol. Motil. 2020, 32, e13889. [Google Scholar] [CrossRef]
- Tomankova, H.; Valuskova, P.; Varejkova, E.; Rotkova, J.; Benes, J.; Myslivecek, J. The M2 muscarinic receptors are essential for signaling in the heart left ventricle during restraint stress in mice. Stress 2015, 18, 208–220. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, C.; Freeling, J.; Giles, A.; Wess, J.; Li, Y.F. Deficiency of M2 muscarinic acetylcholine receptors increases susceptibility of ventricular function to chronic adrenergic stress. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H810–H820. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Tu, H.; Whitney, L.; Carpenter, K.; Li, Y.L. Correlation of leptin resistance with attenuation of cardiac vagal activation in type 2 diabetes. Circulation 2024, 135, AMo135. [Google Scholar] [CrossRef]
- Lee, L.M.; Chang, C.K.; Cheng, K.C.; Kou, D.H.; Liu, I.M.; Cheng, J.T. Increase of cardiac M2-muscarinic receptor gene expression in type-1 but not in type-2 diabetic rats. Neurosci. Lett. 2008, 441, 201–204. [Google Scholar] [CrossRef]
- Gallego, M.; Zayas-Arrabal, J.; Alquiza, A.; Apellaniz, B.; Casis, O. Electrical features of the diabetic myocardium. Arrhythmic and cardiovascular safety considerations in diabetes. Front. Pharmacol. 2021, 12, 687256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, Y.; Jin, J.; Chen, Y.; Chen, C.; Chen, Z.; Huang, H.; Xu, L. Insulin resistance is independently associated with cardiovascular autonomic neuropathy in type 2 diabetes. J. Diabetes Investig. 2021, 12, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Lin, Y.S.; Dagher, Z.; Saha, A.; Luo, Z.; Ido, Y.; Ruderman, N.B. Hyperglycemia and insulin resistance: Possible mechanisms. Ann. N. Y Acad. Sci. 2002, 967, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Stringer, D.M.; Zahradka, P.; Taylor, C.G. Glucose transporters: Cellular links to hyperglycemia in insulin resistance and diabetes. Nutr. Rev. 2015, 73, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Tomlinson, D.R.; Gardiner, N.J. Glucose neurotoxicity. Nat. Rev. Neurosci. 2008, 9, 36–45. [Google Scholar] [CrossRef]
- Giaccari, A.; Sorice, G.; Muscogiuri, G. Glucose toxicity: The leading actor in the pathogenesis and clinical history of type 2 diabetes—Mechanisms and potentials for treatment. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 365–377. [Google Scholar] [CrossRef]
- Oates, P.J. Polyol pathway and diabetic peripheral neuropathy. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2002; pp. 325–392. [Google Scholar]
- Whyte, K.A.; Hogg, R.C.; Dyavanapalli, J.; Harper, A.A.; Adams, D.J. Reactive oxygen species modulate neuronal excitability in rat intrinsic cardiac ganglia. Auton. Neurosci. 2009, 150, 45–52. [Google Scholar] [CrossRef]
- Uehara, K.; Yamagishi, S.I.; Otsuki, S.; Chin, S.; Yagihashi, S. Effects of polyol pathway hyperactivity on protein kinase C activity, nociceptive peptide expression, and neuronal structure in dorsal root ganglia in diabetic mice. Diabetes 2004, 53, 3239–3247. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef]
- Chang, G.J.; Yeh, Y.H.; Chen, W.J.; Ko, Y.S.; Pang, J.S.; Lee, H.Y. Inhibition of advanced glycation end products formation attenuates cardiac electrical and mechanical remodeling and vulnerability to tachyarrhythmias in diabetic rats. J. Pharmacol. Exp. Ther. 2019, 368, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, G.; Sekeroglu, M.R.; Erdogan, E.; Ozturk, M. The effect of non-enzymatic glycation of extracellular matrix proteins on axonal regeneration in vitro. Acta Neuropathol. 2006, 112, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Erbas, T.; Casellini, C.M. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J. Diabetes Investig. 2013, 4, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. 2009, 33, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Cellek, S.; Qu, W.; Schmidt, A.M.; Moncada, S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia 2004, 47, 331–339. [Google Scholar] [CrossRef][Green Version]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J. Polyol pathway and redox balance in diabetes. Pharmacol. Res. 2022, 182, 106326. [Google Scholar] [CrossRef]
- Kaur, N. Diabetic autonomic neuropathy: Pathogenesis to pharmacological management. J. Diabetes Metab. 2014, 5, 7. [Google Scholar] [CrossRef]
- Gabbay, K.H. The sorbitol pathway and the complications of diabetes. N. Engl. J. Med. 1973, 288, 831–836. [Google Scholar]
- Gugliucci, A. Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Maekawa, K.; Tanimoto, T.; Okada, S.; Suzuki, T.; Suzuki, T.; Yabe-Nishimura, C. Expression of aldose reductase and sorbitol dehydrogenase genes in Schwann cells isolated from rat: Effects of high glucose and osmotic stress. Mol. Brain Res. 2001, 87, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Natesan, V.; Kim, S.J. Diabetic nephropathy—A review of risk factors, progression, mechanism, and dietary management. Biomol. Ther. 2021, 29, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.W.; Pop-Busui, R. Diabetic neuropathy: Mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Van Huysen, C.; Fathallah, L.; Cao, X.C.; Greene, D.A.; Stevens, M.J. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002, 16, 123–125. [Google Scholar] [CrossRef]
- Li, Q.R.; Wang, Z.; Zhou, W.; Fan, S.R.; Ma, R.; Xue, L.; Yang, L.; Li, Y.S.; Tan, H.L.; Shao, Q.H.; et al. Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen. Res. 2016, 11, 345–351. [Google Scholar]
- Cameron, N.E.; Cotter, M.A.; Maxfield, E.K. Anti-oxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia 1993, 36, 299–304. [Google Scholar] [CrossRef]
- Campanucci, V.; Krishnaswamy, A.; Cooper, E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 2010, 66, 827–834. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, W.; Tu, H.; Wadman, M.; Li, Y.L. H2O2-REST signaling pathway is involved in cardiac vagal dysfunction and myocardial infarction-evoked ventricular arrhythmia in type 2 diabetes. Physiology 2023, 38, 5727640. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, S.; Basta, G. An update on advanced glycation endproducts and atherosclerosis. Biofactors 2012, 38, 266–274. [Google Scholar] [CrossRef]
- Popescu, S.; Timar, B.; Baderca, F.; Simu, M.; Diaconu, L.; Velea, I.; Timar, R. Age as an independent factor for the development of neuropathy in diabetic patients. Clin. Interv. Aging 2016, 11, 313–318. [Google Scholar] [PubMed]
- Papachristou, S.; Pafili, K.; Trypsianis, G.; Papazoglou, D.; Vadikolias, K.; Papanas, N. Skin advanced glycation end products as a screening tool of neuropathy in type 2 diabetes mellitus. J. Diabetes Complicat. 2022, 36, 108356. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.P.; Graaff, R.; Hoogenberg, K.; Lefrandt, J.D.; Baynes, J.W.; Gans, R.O.; Smit, A.J. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia 2005, 48, 1637–1644. [Google Scholar] [CrossRef]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef]
- Hirose, A.; Tanikawa, T.; Mori, H.; Okada, Y.; Tanaka, Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010, 584, 61–66. [Google Scholar] [CrossRef]
- Sango, K.; Horie, H.; Okamura, A.; Inoue, S.; Takenaka, T. Diabetes impairs DRG neuronal attachment to extracellular matrix proteins in vitro. Brain Res. Bull. 1995, 37, 533–537. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Uemura, T.; Etcheverry, S.B.; Cortizo, A.M. Advanced glycation endproducts interfere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int. J. Biochem. Cell Biol. 2004, 36, 840–848. [Google Scholar] [CrossRef]
- Dhar, S.; Sun, Z.; Meininger, G.A.; Hill, M.A. Nonenzymatic glycation interferes with fibronectin-integrin interactions in vascular smooth muscle cells. Microcirculation 2017, 24, e12347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.J.; King, R.H.M.; Lewin, J.; Thomas, P.K. Effects of nonenzymatic glycosylation of extracellular matrix components on cell survival and sensory neurite extension in cell culture. J. Neurol. 2002, 249, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, N.P.; Vaegter, C.B.; Andersen, H.; Ostergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bao, W.; Men, X.; Liu, Y.; Sun, J.; Li, J.; Liu, H.; Cai, H.; Zhang, W.; Lou, J.; et al. Interleukin-10 protects Schwann cells against advanced glycation end products-induced apoptosis via NF-kappaB suppression. Exp. Clin. Endocrinol. Diabetes 2020, 128, 89–96. [Google Scholar] [PubMed]
- Vlassara, H.; Brownlee, M.; Cerami, A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1981, 78, 5190–5192. [Google Scholar] [CrossRef]
- Ooi, H.; Furukawa, A.; Takeuchi, M.; Koriyama, Y. Toxic advanced glycation end-products inhibit axonal elongation mediated by β-tubulin aggregation in mice optic nerves. Int. J. Mol. Sci. 2024, 25, 7409. [Google Scholar] [CrossRef]
- Grimm, S.; Ott, C.; Hörlacher, M.; Weber, D.; Höhn, A.; Grune, T. Advanced-glycation-end-product-induced formation of immunoproteasomes: Involvement of RAGE and Jak2/STAT1. Biochem. J. 2012, 448, 127–139. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, W.; Huang, X.R.; Oldfield, M.; Schmidt, A.M.; Cooper, M.E.; Lan, H.Y. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am. J. Pathol. 2004, 164, 1389–1397. [Google Scholar] [CrossRef]
- Guimarães, E.L.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Deng, J.; Zhang, T.; Li, H.-M.; Liang, Q.-Z.; Zhang, K.-L. Enhanced expression of RAGE/NADPH oxidase signaling pathway and increased level of oxidative stress in brains of rats with chronic fluorosis and the protective effects of blockers. J. Trace Elem. Med. Biol. 2023, 80, 127288. [Google Scholar] [CrossRef]
- Verma, S.; Alam, R.; Ahmad, I.; Singla, D.; Ali, K.; Hussain, M.E. Effect of glycemic control and disease duration on cardiac autonomic function and oxidative stress in type 2 diabetes mellitus. J. Diabetes Metab. Disord. 2018, 17, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Adithan, C.; Ananthanarayanan, P.H.; Pal, P.; Nanda, N.; Durgadevi, T.; Lalitha, V.; Syamsunder, A.N.; Dutta, T.K. Sympathovagal imbalance contributes to prehypertension status and cardiovascular risks attributed by insulin resistance, inflammation, dyslipidemia and oxidative stress in first degree relatives of type 2 diabetics. PLoS ONE 2013, 8, e78072. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Cakici, M.; Polat, M.; Suner, A.; Zencir, C.; Ardic, I. Relation of epicardial fat thickness with carotid intima-media thickness in patients with type 2 diabetes mellitus. Int. J. Endocrinol. 2013, 2013, 769175. [Google Scholar] [CrossRef][Green Version]
- Groves, E.M.; Erande, A.S.; Le, C.; Salcedo, J.; Hoang, K.C.; Kumar, S.; Mohar, D.S.; Saremi, F.; Im, J.; Agrawal, Y.; et al. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am. J. Cardiol. 2014, 114, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Hong, Y.S.; Lee, H.; Oh, J.Y.; Sung, Y.A.; Kim, Y.; Song, D.K.; Hong, Y.S.; Lee, H.; Oh, J.Y.; et al. Increased epicardial adipose tissue thickness in type 2 diabetes mellitus and obesity. Diabetes Metab. J. 2015, 39, 405–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, W.; Xie, S.; Deng, W. Epicardial adipose tissue: A potential therapeutic target for cardiovascular diseases. J. Cardiovasc. Transl. Res. 2024, 17, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Chen, J.; Guo, H.; Ren, L.; Chen, X.; Chen, Y.; Sun, Y. Independent association between epicardial adipose tissue volume and recurrence of idiopathic ventricular tachycardia after ablation. Rev. Cardiovasc. Med. 2023, 24, 189. [Google Scholar] [CrossRef]
- Sepehri Shamloo, A.; Schoene, K.; Stauber, A.; Darma, A.; Dagres, N.; Dinov, B.; Bertagnolli, L.; Hilbert, S.; Mussigbrodt, A.; Husser, D.; et al. Epicardial adipose tissue thickness as an independent predictor of ventricular tachycardia recurrence following ablation. Heart Rhythm. 2019, 16, 1492–1498. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Gonzalez, N.; Moreno-Villegas, Z.; Gonzalez-Bris, A.; Egido, J.; Lorenzo, O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Jayaraj, R.; Dey, A.; Vellingiri, B.; Ganesan, R. Epicardial adipose tissue and cardiac lipotoxicity: A review. Life Sci. 2023, 328, 121913. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Strom, A.; Strassburger, K.; Knebel, B.; Bonhof, G.J.; Kotzka, J.; Szendroedi, J.; Roden, M. Association of cardiac autonomic dysfunction with higher levels of plasma lipid metabolites in recent-onset type 2 diabetes. Diabetologia 2021, 64, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Romer, A.; Linn, T.; Petry, S.F. Lipotoxic impairment of mitochondrial function in beta-cells: A review. Antioxidants 2021, 10, 293. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Li, R.L.; Wang, L.Y.; Duan, H.X.; Zhang, Q.; Guo, X.; Wu, C.; Peng, W. Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Front. Pharmacol. 2022, 13, 937289. [Google Scholar] [CrossRef]
- Rumora, A.E.; Guo, K.; Hinder, L.M.; O’Brien, P.D.; Hayes, J.M.; Hur, J.; Feldman, E.L. A high-fat diet disrupts nerve lipids and mitochondrial function in murine models of neuropathy. Front. Physiol. 2022, 13, 921942. [Google Scholar] [CrossRef] [PubMed]
- Oltman, C.L.; Coppey, L.J.; Gellett, J.S.; Davidson, E.P.; Lund, D.D.; Yorek, M.A. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E113–E122. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Hasanova, N.; Julius, U.; Obrosova, I.G. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: Role for oxidative-nitrosative stress. Free Radic. Biol. Med. 2012, 52, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Toczylowski, K.; Hirnle, T.; Harasiuk, D.; Zabielski, P.; Lewczuk, A.; Dmitruk, I.; Ksiazek, M.; Sulik, A.; Gorski, J.; Chabowski, A.; et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J. Transl. Med. 2019, 17, 310. [Google Scholar] [CrossRef]
- Rodriguez, A.; Becerril, S.; Hernandez-Pardos, A.W.; Fruhbeck, G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef]
- Jung, C.H.; Jung, S.H.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Association of serum omentin levels with cardiac autonomic neuropathy in patients with type 2 diabetes mellitus: A hospital-based study. Cardiovasc. Diabetol. 2015, 14, 140. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Jung, S.H.; Mok, J.O. Association of serum adipocytokine levels with cardiac autonomic neuropathy in type 2 diabetic patients. Cardiovasc. Diabetol. 2012, 11, 24. [Google Scholar] [CrossRef]
- Lai, Y.-R.; Chiu, W.-C.; Cheng, B.-C.; Chen, J.-F.; Tsai, N.-W.; Huang, C.-C.; Lu, C.-H. Associations between adipocytokines and severity of cardiovascular autonomic neuropathy in well-controlled type 2 diabetes and prediabetes. Preprint 2020. [CrossRef]
- Patraca, I.; Martinez, N.; Busquets, O.; Marti, A.; Pedros, I.; Beas-Zarate, C.; Marin, M.; Ettcheto, M.; Sureda, F.; Auladell, C.; et al. Anti-inflammatory role of leptin in glial cells through p38 MAPK pathway inhibition. Pharmacol. Rep. 2017, 69, 409–418. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Vilarino-Garcia, T.; Sanchez-Margalet, V. Role of leptin in inflammation and vice versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Leptin stimulates the release of pro-inflammatory cytokines in hypothalamic astrocyte cultures from adult and aged rats. Metab. Brain Dis. 2018, 33, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Both, E.; Hutanu, A.; Sular, F.L.; Roiban, A.L. Correlations of serum leptin and leptin resistance with depression and anxiety in patients with type 2 diabetes. Psychiatry Clin. Neurosci. 2019, 73, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Tank, J.; Diedrich, A.; Hilzendeger, A.; Plehm, R.; Bader, M.; Luft, F.C.; Jordan, J.; Gross, V. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 2009, 53, 387–392. [Google Scholar] [CrossRef]
- Fruhbeck, G.; Catalan, V.; Rodriguez, A.; Ramirez, B.; Becerril, S.; Portincasa, P.; Gomez-Ambrosi, J. Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Sci. Rep. 2017, 7, 2752. [Google Scholar] [CrossRef]
- Yagishita, Y.; Uruno, A.; Fukutomi, T.; Saito, R.; Saigusa, D.; Pi, J.; Fukamizu, A.; Sugiyama, F.; Takahashi, S.; Yamamoto, M. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 2017, 18, 2030–2044. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.; Abdul-Hadi, M.; Naji, M.; Shams, H.; Sami, O.; Al-Harchan, N.-A.; Al-Gareeb, A. Oxidative stress injury and glucolipotoxicity in type 2 diabetes mellitus: The potential role of metformin and sitagliptin. Biomed. Biotechnol. Res. J. (BBRJ) 2020, 4, 166–172. [Google Scholar] [CrossRef]
- Bakkar, N.Z.; Dwaib, H.S.; Fares, S.; Eid, A.H.; Al-Dhaheri, Y.; El-Yazbi, A.F. Cardiac autonomic neuropathy: A progressive consequence of chronic low-grade inflammation in type 2 diabetes and related metabolic disorders. Int. J. Mol. Sci. 2020, 21, 9005. [Google Scholar] [CrossRef]
- Cerf, M.E. Cardiac glucolipotoxicity and cardiovascular outcomes. Medicina 2018, 54, 70. [Google Scholar] [CrossRef]
- Herder, C.; Schamarek, I.; Nowotny, B.; Carstensen-Kirberg, M.; Strassburger, K.; Nowotny, P.; Kannenberg, J.M.; Strom, A.; Puttgen, S.; Mussig, K.; et al. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart 2017, 103, 63–70. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Radeke, H.H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989, 263, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J. On the immunometabolic role of NF-kappaB in adipocytes. Immunometabolism 2022, 4, e220003. [Google Scholar] [CrossRef]
- Sen, A.; Mohanraj, P.S.; Ranjan, A.; Rajendran, V.; ArulVijayaVani, S.; Balan, Y.; Bansal, A. Unraveling the role of tumor necrosis factor-alpha in diabetic peripheral neuropathy: A systematic review and Meta-analysis. Cureus 2023, 15, e49926. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Munshi, A. Role of miRNAs in diabetic neuropathy: Mechanisms and possible interventions. Mol. Neurobiol. 2022, 59, 1836–1849. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, C.; Xu, H. Downregulation of miR-145-5p elevates retinal ganglion cell survival to delay diabetic retinopathy progress by targeting FGF5. Biosci. Biotechnol. Biochem. 2019, 83, 1655–1662. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef]
- Maser, R.E.; Lenhard, M.J.; Pohlig, R.T. Vitamin D insufficiency is associated with reduced parasympathetic nerve fiber function in type 2 diabetes. Endocr. Pract. 2015, 21, 174–181. [Google Scholar] [CrossRef]
- Jung, C.H.; Jung, S.H.; Kim, K.J.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. The relationship between vitamin D status and cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2015, 12, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiong, J.; Cai, H.; Zou, R.; Li, F.; Wang, Y.; Wang, C. Effects of vitamin D deficiency on the function of the cardiac autonomic nervous system in rats. Cardiovasc. Ther. 2022, 2022, 4366948. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Jensen, J.S.; Ridderstrale, M.; Vistisen, D.; Jorgensen, M.E.; Fleischer, J. Vitamin B12 deficiency is associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Meher, M.; Panda, J.K. Impact of glycemic control over cardiac autonomic neuropathy. J. Diabetes Metab. Disord. 2020, 19, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.B.; Gentil, P.; Seguro, C.S.; de Oliveira, G.T.; Silva, M.S.; Zamuner, A.R.; Beltrame, T.; Rebelo, A.C.S. High fasting glycemia predicts impairment of cardiac autonomic control in adults with type 2 diabetes: A case-control study. Front. Endocrinol. 2021, 12, 760292. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Laitinen, T.P.; Lipponen, J.A.; Cornforth, D.J.; Jelinek, H.F. Cardiac autonomic dysfunction in type 2 diabetes—Effect of hyperglycemia and disease duration. Front. Endocrinol. 2014, 5, 130. [Google Scholar] [CrossRef]
- Jacobson, A.M. Impact of improved glycemic control on quality of life in patients with diabetes. Endocr. Pract. 2004, 10, 502–508. [Google Scholar] [CrossRef]
- Cassidy, S.; Vaidya, V.; Houghton, D.; Zalewski, P.; Seferovic, J.P.; Hallsworth, K.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. Unsupervised high-intensity interval training improves glycaemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomised controlled trial. Diabetes Vasc. Dis. Res. 2019, 16, 69–76. [Google Scholar] [CrossRef]
- Stahn, A.; Pistrosch, F.; Ganz, X.; Teige, M.; Koehler, C.; Bornstein, S.; Hanefeld, M. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: Silent hypoglycemias and silent arrhythmias. Diabetes Care 2014, 37, 516–520. [Google Scholar] [CrossRef]
- Li, G.; Zhong, S.; Wang, X.; Zhuge, F. Association of hypoglycaemia with the risks of arrhythmia and mortality in individuals with diabetes—A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1222409. [Google Scholar] [CrossRef]
- Achmad, C.; Lim, N.S.; Pramudyo, M.; Iqbal, M.; Karwiky, G.; Febrianora, M.; Natalia, N. Relation between glycemic control and cardiac autonomic neuropathy in patients with diabetes mellitus type 2. Curr. Probl. Cardiol. 2023, 48, 101135. [Google Scholar] [CrossRef] [PubMed]
- Ostropolets, A.; Elias, P.A.; Reyes, M.V.; Wan, E.Y.; Pajvani, U.B.; Hripcsak, G.; Morrow, J.P. Metformin is associated with a lower risk of atrial fibrillation and ventricular arrhythmias compared with sulfonylureas: An observational study. Circ. Arrhythm. Electrophysiol. 2021, 14, e009115. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.L.; Hui, J.M.H.; Lee, Y.H.A.; Satti, D.I.; Shum, Y.K.L.; Kiu, P.T.H.; Wai, A.K.C.; Liu, T.; Wong, W.T.; Chan, J.S.K.; et al. Sulfonylurea is associated with higher risks of ventricular arrhythmia or sudden cardiac death compared with metformin: A population-based cohort study. J. Am. Heart Assoc. 2022, 11, e026289. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Reynier, P.; Douros, A.; Yu, O.H.Y.; Filion, K.B. Sulphonylureas versus metformin and the risk of ventricular arrhythmias among people with type 2 diabetes: A population-based cohort study. Diabetes Obes. Metab. 2023, 25, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Manica, D.; Sandri, G.; da Silva, G.B.; Manica, A.; da Silva Rosa Bonadiman, B.; Dos Santos, D.; Flores, E.M.M.; Bolzan, R.C.; Barcelos, R.C.S.; Tomazoni, F.; et al. Evaluation of the effects of metformin on antioxidant biomarkers and mineral levels in patients with type II diabetes mellitus: A cross-sectional study. J. Diabetes Complicat. 2023, 37, 108497. [Google Scholar] [CrossRef]
- Kristofi, R.; Eriksson, J.W. Metformin as an anti-inflammatory agent: A short review. J. Endocrinol. 2021, 251, R11–R22. [Google Scholar] [CrossRef]
- Sakata, N. The anti-inflammatory effect of metformin: The molecular targets. Genes. Cells 2024, 29, 183–191. [Google Scholar] [CrossRef]
- Syngle, A.; Chahal, S.; Vohra, K. Efficacy and tolerability of DPP4 inhibitor, teneligliptin, on autonomic and peripheral neuropathy in type 2 diabetes: An open label, pilot study. Neurol. Sci. 2021, 42, 1429–1436. [Google Scholar] [CrossRef]
- Sano, M. Mechanism by which dipeptidyl peptidase-4 inhibitors increase the risk of heart failure and possible differences in heart failure risk. J. Cardiol. 2019, 73, 28–32. [Google Scholar] [CrossRef]
- Packer, M. Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Clues from laboratory models and clinical trials. Circ. Res. 2018, 122, 928–932. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Ussher, J.R.; McLean, B.A.; Cao, X.; Kabir, M.G.; Mulvihill, E.E.; Mighiu, A.S.; Zhang, H.; Ludwig, A.; Seeley, R.J.; et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 2017, 6, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of cardiac ventricular excitability by GLP-1 (glucagon-like peptide-1). Circ. Arrhythm. Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef] [PubMed]
- Kumarathurai, P.; Anholm, C.; Larsen, B.S.; Olsen, R.H.; Madsbad, S.; Kristiansen, O.; Nielsen, O.W.; Haugaard, S.B.; Sajadieh, A. Effects of liraglutide on heart rate and heart rate variability: A randomized, double-blind, placebo-controlled crossover study. Diabetes Care 2017, 40, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, T.; Santos-Pardo, I.; Fang, X.; Cao, Y.; Hedberg, F.; Jendle, J. Heart rate variability in type 2 diabetic subjects randomized to liraglutide or glimepiride treatment, both in combination with metformin: A randomized, open, parallel-group study. Endocrinol. Diabetes Metab. 2019, 2, e00058. [Google Scholar] [CrossRef]
- Greco, C.; Santi, D.; Brigante, G.; Pacchioni, C.; Simoni, M. Effect of the glucagon-like peptide-1 receptor agonists on autonomic function in subjects with diabetes: A systematic review and Meta-analysis. Diabetes Metab. J. 2022, 46, 901–911. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Ye, H.; Wang, Y.; Wang, L.; Zhang, X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 2022, 13, 910256. [Google Scholar] [CrossRef]
- Chao, E.C. SGLT-2 inhibitors: A new mechanism for glycemic control. Clin. Diabetes 2014, 32, 4–11. [Google Scholar] [CrossRef]
- Balcioglu, A.S.; Celik, E.; Sahin, M.; Gocer, K.; Aksu, E.; Aykan, A.C. Dapagliflozin improves cardiac autonomic function measures in type 2 diabetic patients with cardiac autonomic neuropathy. Anatol. J. Cardiol. 2022, 26, 832–840. [Google Scholar] [CrossRef]
- Fujiki, S.; Iijima, K.; Nakagawa, Y.; Takahashi, K.; Okabe, M.; Kusano, K.; Owada, S.; Kondo, Y.; Tsujita, K.; Shimizu, W.; et al. Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: The EMPA-ICD trial. Cardiovasc. Diabetol. 2024, 23, 224. [Google Scholar] [CrossRef]
- Patoulias, D.; Katsimardou, A.; Fragakis, N.; Papadopoulos, C.; Doumas, M. Effect of SGLT-2 inhibitors on cardiac autonomic function in type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Acta Diabetol. 2023, 60, 1–8. [Google Scholar] [CrossRef]
- Chindhalore, C.A.; Dakhale, G.N.; Kamble, P.H.; Rathod, B.D.; Kumbhalkar, S.; Phatak, M.S. Effect of ramipril on cardiac autonomic neuropathy in patients with type II diabetes mellitus. Cureus 2023, 15, e36209. [Google Scholar] [CrossRef] [PubMed]
- Didangelos, T.; Tziomalos, K.; Margaritidis, C.; Kontoninas, Z.; Stergiou, I.; Tsotoulidis, S.; Karlafti, E.; Mourouglakis, A.; Hatzitolios, A.I. Efficacy of administration of an angiotensin converting enzyme inhibitor for two years on autonomic and peripheral neuropathy in patients with diabetes mellitus. J. Diabetes Res. 2017, 2017, 6719239. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.A.; Williamson, S.; Abbott, C.; Carrington, A.L.; Iqbal, J.; Schady, W.; Boulton, A.J. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: Randomised double-blind controlled trial. Lancet 1998, 352, 1978–1981. [Google Scholar] [CrossRef] [PubMed]
- Fabiyi-Edebor, T.D. Vitamin C ameliorated cardiac autonomic neuropathy in type 2 diabetic rats. World J. Diabetes 2020, 11, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Manzella, D.; Barbieri, M.; Ragno, E.; Paolisso, G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am. J. Clin. Nutr. 2001, 73, 1052–1057. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, S.J.; Lee, Y.C.; Lee, Y.H.; Lee, J.E.; Kim, C.H.; Min, K.W.; Cha, B.Y. Effects of high-dose alpha-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea. Diabetes Metab. J. 2017, 41, 275–283. [Google Scholar] [CrossRef]
- Ziegler, D.; Schatz, H.; Conrad, F.; Gries, F.A.; Ulrich, H.; Reichel, G. Effects of treatment with the antioxidant α-lipoic acid on cardiac autonomic neuropathy in NIDDM patients: A 4-month randomized controlled multicenter trial (DEKAN Study). Diabetes Care 1997, 20, 369–373. [Google Scholar] [CrossRef]
- Mendoza-Nunez, V.M.; Garcia-Martinez, B.I.; Rosado-Perez, J.; Santiago-Osorio, E.; Pedraza-Chaverri, J.; Hernandez-Abad, V.J. The effect of 600 mg alpha-lipoic acid supplementation on oxidative stress, inflammation, and RAGE in older adults with type 2 diabetes mellitus. Oxid. Med. Cell Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef]
- Hoang, J.D.; Yamakawa, K.; Rajendran, P.S.; Chan, C.A.; Yagishita, D.; Nakamura, K.; Lux, R.L.; Vaseghi, M. Proarrhythmic effects of sympathetic activation are mitigated by vagal nerve stimulation in infarcted hearts. JACC Clin. Electrophysiol. 2022, 8, 513–525. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, Y.; Ning, X.; Tang, M.; Zhao, Y.; Li, Z.; Zhang, S. Vagus nerve stimulation in early stage of acute myocardial infarction prevent ventricular arrhythmias and cardiac remodeling. Front. Cardiovasc. Med. 2021, 8, 648910. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Tipton, M.J.; Shattock, M.J. Autonomic conflict exacerbates long QT associated ventricular arrhythmias. J. Mol. Cell. Cardiol. 2018, 116, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, Y.; Xue, F.-S.; Yuan, Y.-J.; Xiong, J.; Li, R.-P.; Liao, X.; Liu, J.-H. Postconditioning with vagal stimulation attenuates local and systemic inflammatory responses to myocardial ischemia reperfusion injury in rats. Inflamm. Res. 2012, 61, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Imai, M.; Jiang, A.; Wang, M.; Sabbah, H. Chronic therapy with selective electric vagus nerve stimulation normalizes plasma concentration of tissue necrosis factor-alpha, interleukin-6 and B-Type natriuretic peptide in dogs with heart failure. Am. Coll. Cardiol. 2006, 47, 77A–78A. [Google Scholar]
- Samniang, B.; Shinlapawittayatorn, K.; Chunchai, T.; Pongkan, W.; Kumfu, S.; Chattipakorn, S.C.; KenKnight, B.H.; Chattipakorn, N. Vagus nerve stimulation improves cardiac function by preventing mitochondrial dysfunction in obese-insulin resistant rats. Sci. Rep. 2016, 6, 19749. [Google Scholar] [CrossRef]
- Payne, S.C.; Ward, G.; Fallon, J.B.; Hyakumura, T.; Prins, J.B.; Andrikopoulos, S.; MacIsaac, R.J.; Villalobos, J. Blood glucose modulation and safety of efferent vagus nerve stimulation in a type 2 diabetic rat model. Physiol. Rep. 2022, 10, e15257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, A.J.; Li, Y.-L. Remodeling of the Intracardiac Ganglia During the Development of Cardiovascular Autonomic Dysfunction in Type 2 Diabetes: Molecular Mechanisms and Therapeutics. Int. J. Mol. Sci. 2024, 25, 12464. https://doi.org/10.3390/ijms252212464
Evans AJ, Li Y-L. Remodeling of the Intracardiac Ganglia During the Development of Cardiovascular Autonomic Dysfunction in Type 2 Diabetes: Molecular Mechanisms and Therapeutics. International Journal of Molecular Sciences. 2024; 25(22):12464. https://doi.org/10.3390/ijms252212464
Chicago/Turabian StyleEvans, Anthony J., and Yu-Long Li. 2024. "Remodeling of the Intracardiac Ganglia During the Development of Cardiovascular Autonomic Dysfunction in Type 2 Diabetes: Molecular Mechanisms and Therapeutics" International Journal of Molecular Sciences 25, no. 22: 12464. https://doi.org/10.3390/ijms252212464
APA StyleEvans, A. J., & Li, Y.-L. (2024). Remodeling of the Intracardiac Ganglia During the Development of Cardiovascular Autonomic Dysfunction in Type 2 Diabetes: Molecular Mechanisms and Therapeutics. International Journal of Molecular Sciences, 25(22), 12464. https://doi.org/10.3390/ijms252212464








