Fast Colorimetric Detection of H2O2 and Glucose: A Way Based on Magnetic Nanoparticles Composed of Fe3(PO4)2·8H2O Isolated from Burkholderia cepacia CG-1
Abstract
1. Introduction
2. Results
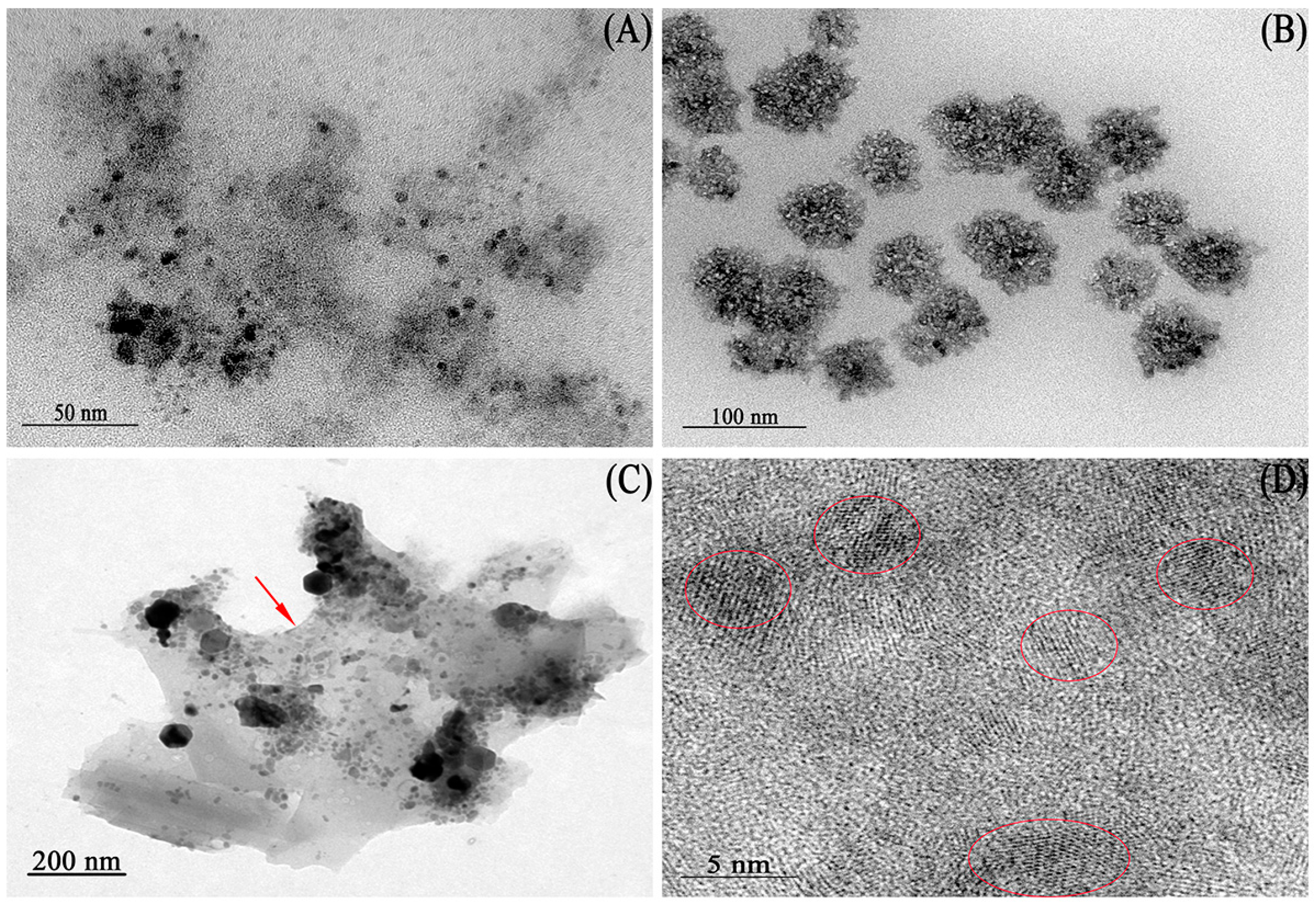
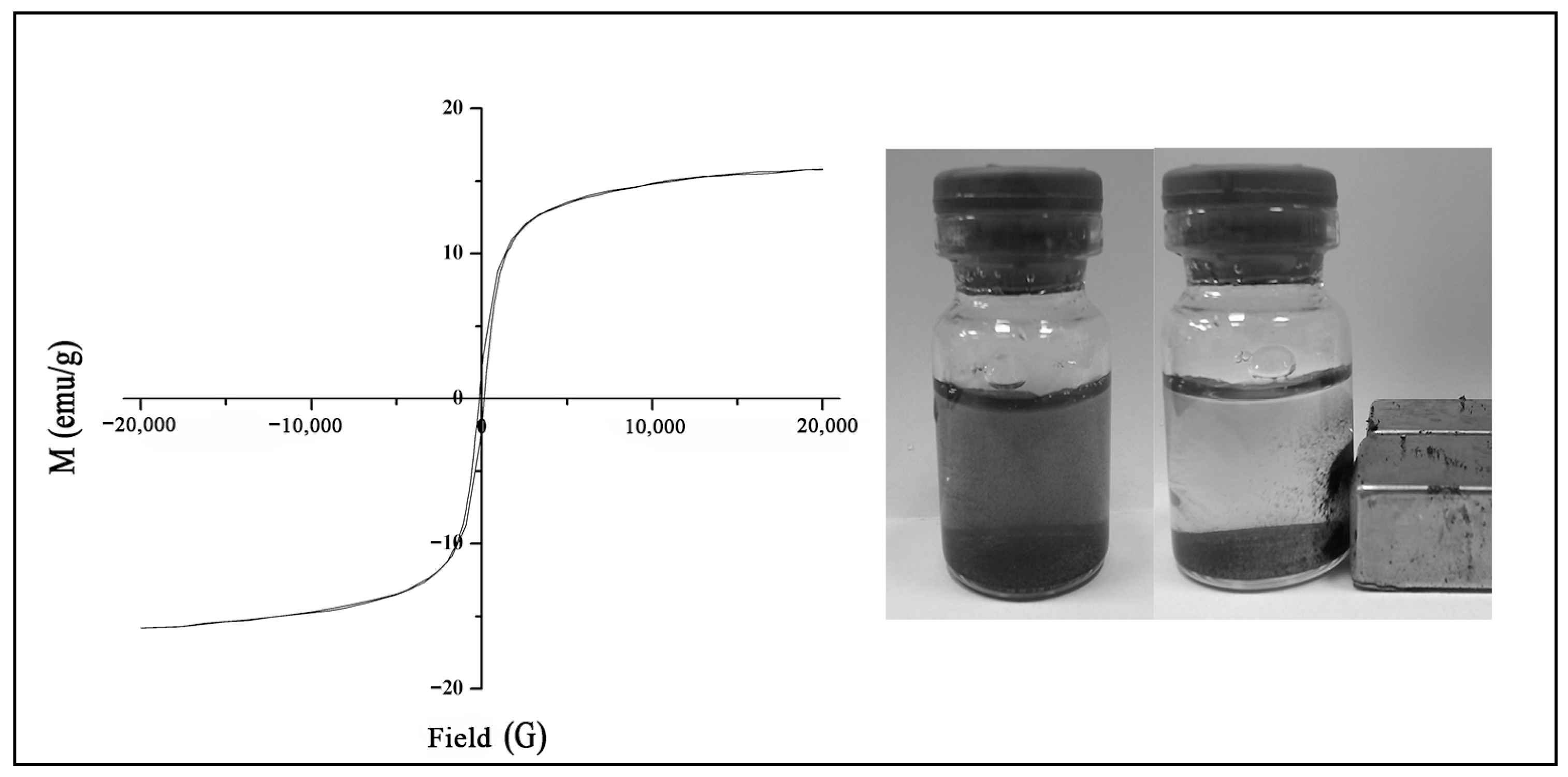
2.1. Characterization of Magnetic Nanoparticles (MNPs)
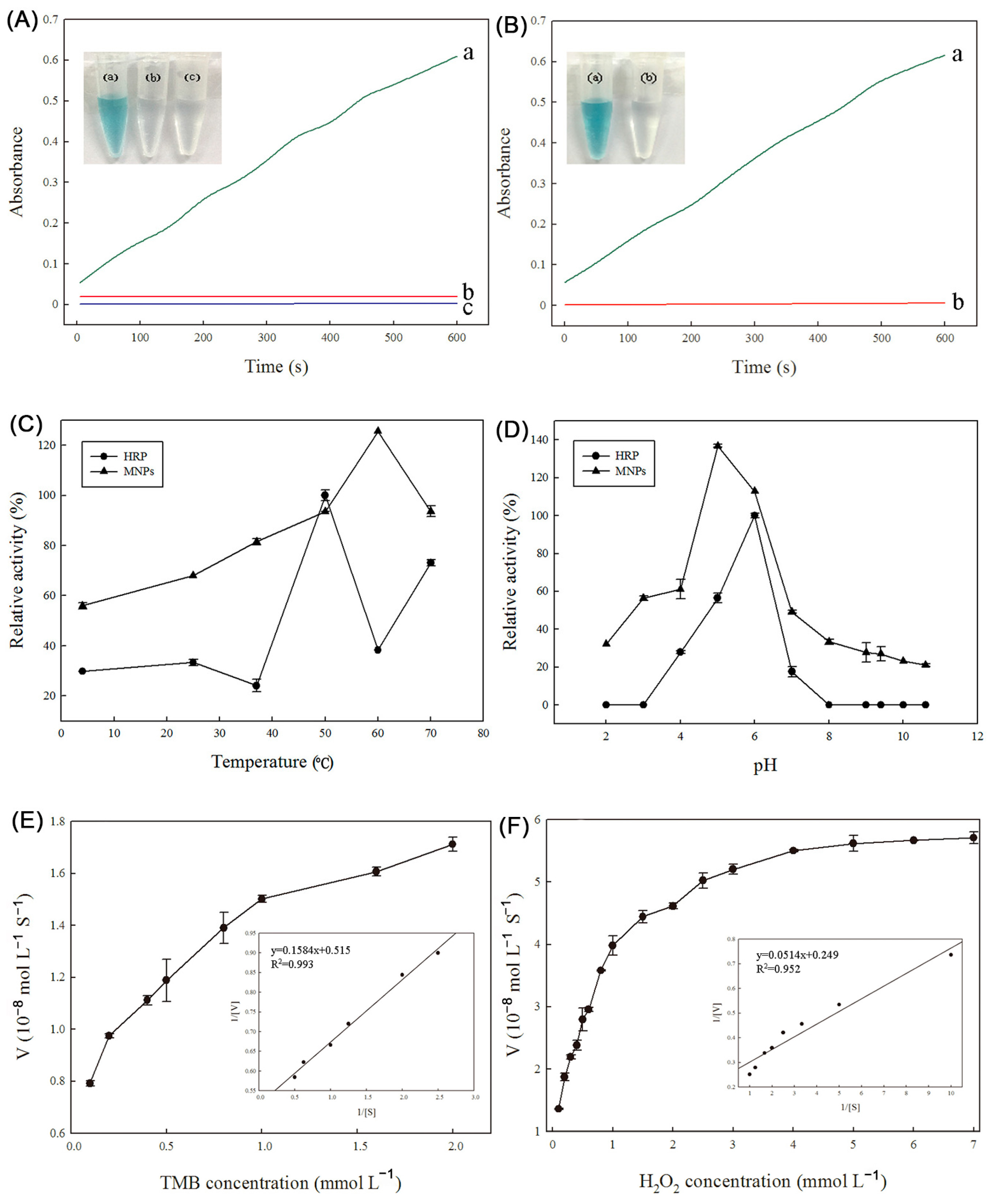
2.2. Peroxidase-Like Activity of the Fe3(PO4)2·8H2O Magnetic Nanoparticles (MNPs)
2.3. Stability of Peroxidase Activity of Fe3(PO4)2·8H2O MNPs and HRP
2.4. Kinetic Assay of Peroxidase-Like Activity of Fe3(PO4)2·8H2O MNPs
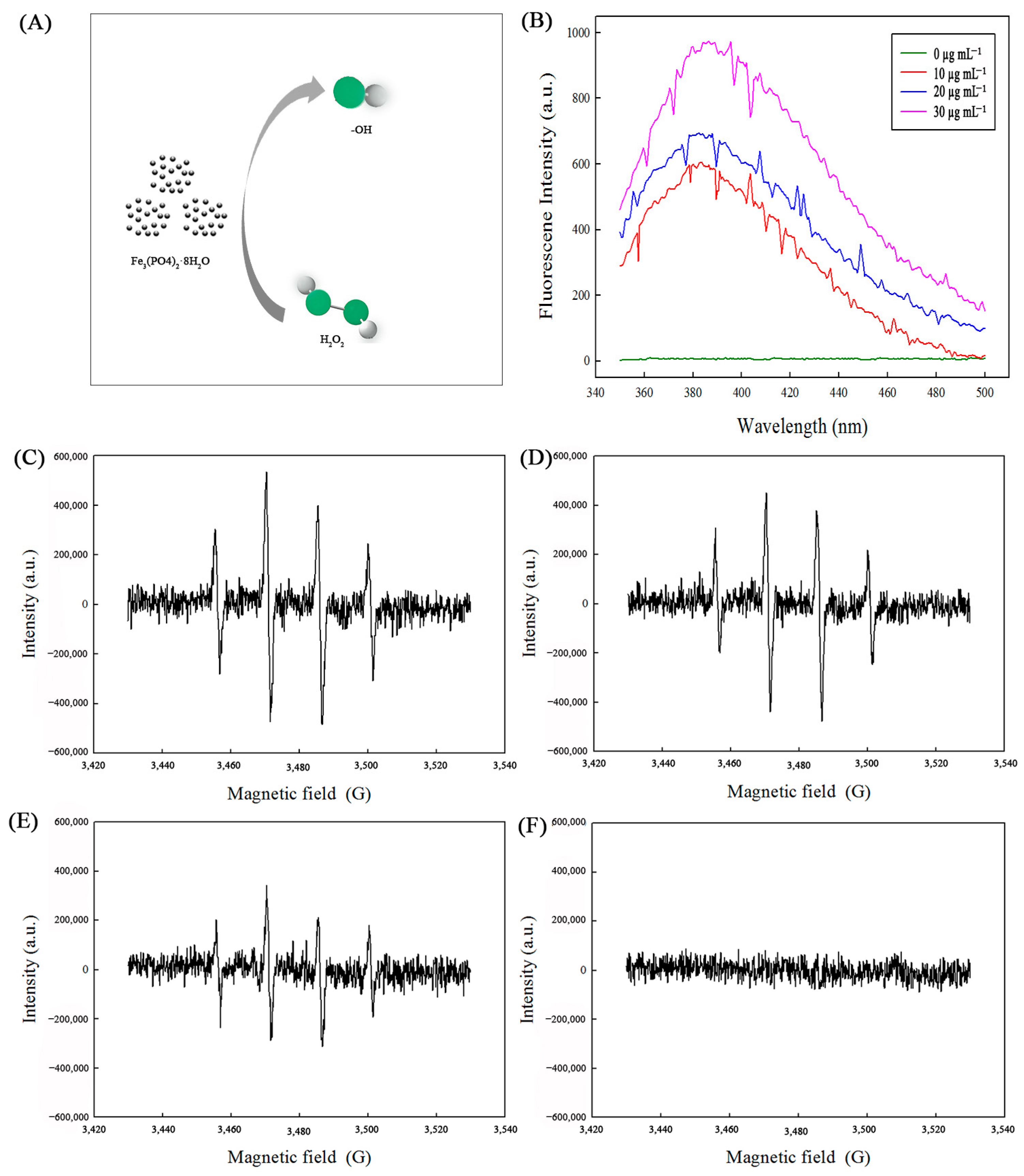
2.5. Reaction Mechanism for the Catalytic Activity of Fe3(PO4)2·8H2O MNPs as Peroxidase Mimetics
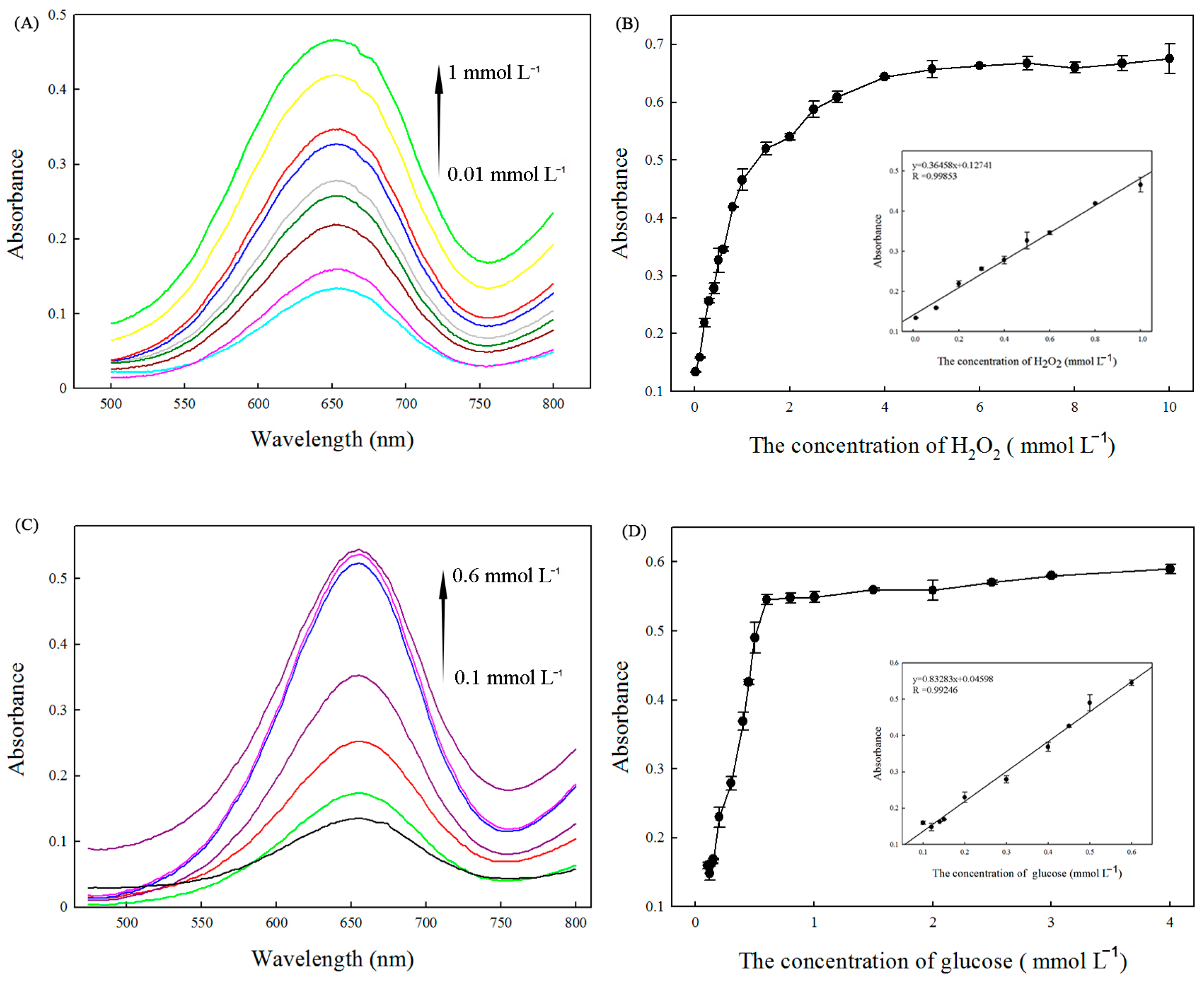
2.6. Detection of H2O2 and Glucose Using Fe3(PO4)2·8H2O MNPs as Peroxidase Mimetics
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strain and Culture
4.3. Production and Extraction of Magnetic Nanoparticles
4.4. Characterization of Magnetic Nanoparticles
4.5. Kinetic Analysis
4.6. Comparison of the Stability of Fe3(PO4)2·8H2O MNPs and HRP
4.7. Measurement of Hydroxyl Radical Formation
4.8. H2O2 Detection Using MNPs as Peroxidase Mimetics
4.9. Glucose Detection Using MNPs and Glucose Oxidase
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Inter. 2017, 9, 10027–10033. [Google Scholar] [CrossRef]
- Dutta, A.K.; Das, S.; Samanta, S.; Samanta, P.K.; Adhikary, B.; Biswas, P. CuS nanoparticles as a mimic peroxidase for colorimetric estimation of human blood glucose level. Talanta 2013, 107, 361–367. [Google Scholar] [CrossRef]
- Dong, J.; Song, L.; Yin, J.J.; He, W.; Wu, Y.; Gu, N.; Zhang, Y. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl. Mater. Inter. 2014, 6, 1959–1970. [Google Scholar] [CrossRef]
- Yu, F.; Huang, Y.; Cole, A.J.; Yang, V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Feng, Y.B.; Hong, L.; Chen, Q.Y.; Wu, L.F.; Lin, X.-H.; Xia, X.-H. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef]
- Islam, T.; Peng, C.; Ali, I. Morphological and cellular diversity of magnetotactic bacteria: A review. J. Basic Microbiol. 2018, 58, 378–389. [Google Scholar] [CrossRef]
- Blakemore, R.P. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Li, K.; Chen, C.; Chen, C.; Wang, Y.; Wei, Z.; Pan, W.; Song, T. Magnetosomes extracted from Magnetospirillum magneticum strain AMB-1 showed enhanced peroxidase-like activity under visible-light irradiation. Enzyme Microb. Technol. 2015, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.P. Magnetotactic bacteria. Annu. Rev. Microbiol. 1982, 36, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Bazylinski, L.D. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Zhang, M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert. Opin. Drug Del. 2013, 10, 73–87. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes eliminate intra-cellular reactive oxygen species in Magnetospirillum gryphiswaldense MSR-1. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef]
- Pan, Y.; Li, N.; Mu, J.S.; Zhou, R.H.; Xu, Y.; Cui, D.Z.; Zhao, M. Biogenic magnetic nanoparticles from Burkholderia sp. YN01 exhibiting intrinsic peroxidase-like activity and their applications. Appl. Microbiol. Biot. 2015, 99, 703–715. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.; Zhang, S.; López, V.M.; Wang, W. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- Yu, P.; Yongming, W.; Xingyuan, F.; Webin, W.; Xiumin, W.; Cui, D.; Min, Z. Bacterial intracellular nanoparticles exhibiting antioxidant properties and the significance of their formation in ROS detoxification. Environ. Microbiol. Rep. 2019, 11, 140–146. [Google Scholar]
- Abagana, A.Y.; Zhao, M.; Alshahrani, M.Y.; Rehman, K.U.; Andleeb, S.; Wang, J.Y.; Bukhari, S.M. Hydrogen iron oxide from an Acinetobacter strain exhibiting intrinsic peroxidase-like activity and its catalytic mechanism and applications. Biomass Convers. Biorefin. 2022, 14, 3453–3462. [Google Scholar] [CrossRef]
- Ahmed, A.; Abagana, A.; Cui, D.; Zhao, M. De Novo Iron Oxide Hydroxide, Ferrihydrite Produced by Comamonas testosteroni Exhibiting Intrinsic Peroxidase-Like Activity and Their Analytical Applications. BioMed Res. Int. 2019, 14, 7127869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hai, X.; Xia, C.; Chen, X.W.; Wang, J.H. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 248, 374–384. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of transition metal ions on the TiO2-assisted photodegradation of dyes under visible irradiation: A probe for the interfacial electron transfer process and reaction mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Ana Peigneux, A.; Tercedor, C.V.; Moreno, R.L.; González, T.P.; Vivas, M.A.F.; López, C.J. Learning from magnetotactic bacteria: A review on the synthesis of biomimetic nanoparticles mediated by magnetosome-associated proteins. J. Struct. Biol. 2016, 196, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Azam, H.M.; Finneran, K.T. Ferric iron amendment increases Fe(III)-reducing microbial diversity and carbon oxidation in on-site wastewater systems. Chemosphere 2013, 90, 1431443. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kevin, T.F. Fe(III) reduction-mediated phosphate removal as vivianite (Fe3(PO4)2▪8H2O) in septic system wastewater. Chemosphere 2014, 97, 1–9. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Y.; Zhao, Z.; Song, W.; Cheng, Y. The photo-catalytic activities of MP (M=Ba, Ca, Cu, Sr, Ag; P=PO43−, HPO42−) microparticles. Appl. Surf. Sci. 2014, 292, 570–575. [Google Scholar] [CrossRef]
- Chen, C.; Liu, G.B.; Wang, Y.; Li, J.L.; Liu, H. Preparation and electrochemical properties of LiFePO4/C nanocomposite using FePO4·2H2O nanoparticles by introduction of Fe3(PO4)2·8H2O at low cost. Electrochim. Acta 2013, 113, 464–469. [Google Scholar] [CrossRef]
- Shi, W.B.; Wang, Q.L.; Long, Y.J.; Cheng, Z.L.; Chen, S.H.; Zheng, H.Z.; Huang, Y.M. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 695–6697. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, W.; Zheng, S.; Zhang, C.; Xian, Y. SDS-MoS2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta 2015, 141, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, D.; Wang, X.; Qi, B.; Zhao, M. Polyoxometalates as peroxidase mimetics and their applications in H2O2 and glucose detection. Biosens. Bioelectron. 2012, 36, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sens. Actuators B Chem. 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Li, H.; Zhu, R.; Shao, Q.; Yang, S.; Xu, J. NiO nanoparticles modified with 5,10,15,20-tetrakis (4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens. Bioelectron. 2015, 64, 147–153. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of methane by bacterial extracts. J. Biol. Chem. 1963, 238, 2882–2886. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D.D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–663. [Google Scholar] [CrossRef]


| Sample | Provided by Hospital (mmol L−1) | Experimental Result (mmol L−1 ± SD, n = 3) | RSD (%) |
|---|---|---|---|
| 1 | 5.6 | 5.45 ± 0.04 | 0.69 |
| 2 | 6.1 | 6.10 ± 0.08 | 1.27 |
| 3 | 5.6 | 5.73 ± 0.25 | 4.34 |
| 4 | 5.8 | 5.72 ± 0.04 | 0.61 |
| 5 | 5.5 | 5.35 ± 0.11 | 2.07 |
| Nanomaterial | Linear Range (μmol L−1) | Detection Limit (μmol L−1) | Reference |
|---|---|---|---|
| SDS-MoS2 | 2–100 * | 0.32 * | [31] |
| 5–500 ** | 0.57 ** | ||
| Fe3O4 | 5–100 * | 3 * | [32] |
| 50–1000 ** | 30 ** | ||
| H3PW12O40 | 0.134–67 * | 0.134 * | [33] |
| 0.1–100 ** | 0.1 ** | ||
| Por-Ceria | 10–100 * | 1.8 * | [34] |
| 40–150 ** | 19 ** | ||
| Nitrogen-doped graphene | 20–1170 * | 5.3 * | [35] |
| 25–375 ** | 16 ** | ||
| Graphene oxide | 0.05–1 * | 0.05 * | [36] |
| 1–20 ** | 1 ** | ||
| H2TCPP-NiO | 20–100 * | 8 * | [37] |
| 50–500 ** | 20 ** | ||
| Cu | 10–1000 * | 10 * | [38] |
| 100–2000 ** | 100 ** | ||
| Fe3(PO4)2·8H2O | 10–1000 * | 1 * | This work |
| 100–600 ** | 5 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Wang, J.; Liu, Y.; Cui, D.; Zhao, M. Fast Colorimetric Detection of H2O2 and Glucose: A Way Based on Magnetic Nanoparticles Composed of Fe3(PO4)2·8H2O Isolated from Burkholderia cepacia CG-1. Int. J. Mol. Sci. 2024, 25, 12518. https://doi.org/10.3390/ijms252312518
Jia M, Wang J, Liu Y, Cui D, Zhao M. Fast Colorimetric Detection of H2O2 and Glucose: A Way Based on Magnetic Nanoparticles Composed of Fe3(PO4)2·8H2O Isolated from Burkholderia cepacia CG-1. International Journal of Molecular Sciences. 2024; 25(23):12518. https://doi.org/10.3390/ijms252312518
Chicago/Turabian StyleJia, Mingyu, Jueyu Wang, Yuxuan Liu, Daizong Cui, and Min Zhao. 2024. "Fast Colorimetric Detection of H2O2 and Glucose: A Way Based on Magnetic Nanoparticles Composed of Fe3(PO4)2·8H2O Isolated from Burkholderia cepacia CG-1" International Journal of Molecular Sciences 25, no. 23: 12518. https://doi.org/10.3390/ijms252312518
APA StyleJia, M., Wang, J., Liu, Y., Cui, D., & Zhao, M. (2024). Fast Colorimetric Detection of H2O2 and Glucose: A Way Based on Magnetic Nanoparticles Composed of Fe3(PO4)2·8H2O Isolated from Burkholderia cepacia CG-1. International Journal of Molecular Sciences, 25(23), 12518. https://doi.org/10.3390/ijms252312518





