State-of-the-Art Carbon-Nanotubes-Reinforced Copper-Based Composites: The Interface Design of CNTs and Cu Matrix
Abstract
:1. Introduction

| Method | Precursor | Product Description | Interface Features of Cu and CNTs | Ref. | |
|---|---|---|---|---|---|
| Brush coating | Cu foils and carboxyl-modified SWCNTs | Cu-CNT-Cu layer by layer | Physical binding | Cu diffusion in CNTs | [43] |
| Electrospinning-based polymer nanofiber templating and magnetron-sputtering | Cu tapes and carboxyl-modified SWCNTs | [66] | |||
| Ball milling, hot pressing, and high-pressure torsion | Electrolytic Cu powder and MWCNTs | CNT/Cu composite | [67] | ||
| Co-deposition and SPS | CNTs and CuSO4·5H2O | Clear and tightly bonded interface | [68] | ||
| In situ catalytic growth and SPS | Cu-Al2O3, C2H4, H2 | CNT/Cu-Al2O3 composite | Tightly bonded interface | [65] | |
| Template, cold pressing, and SPS | CNTs, RGO, CuSO4·5H2O, and Cu powder | CNTs-RGO/Cuf ®Cu | Physical binding between CNTs and Cu | [69] | |
| refined two-step organic–aqueous electrodeposition and hot compression | CNTs, Cu(CH3COO)2·H2O, CuSO4·5H2O | layered CNT/Cu composite | --- | [70] | |
| Electroless deposition | CuSO4·5H2O and MWCNTs | CNT/Cu thin film | Chemical bonding | Carboxyl or tiol | [14] |
| Wet-chemical, ball milling, and SPS | Cu(CH3COO)2·H2O and graphitized CNTs | CNT/Cu composite | Cu2O | [21] | |
| Wet mixing, thermal reduction, and SPS | Modified MWCNTs and Cu powders | [71] | |||
| Molecular-level mixing, ball milling, and thermal reduction | Cross-linked modified CNTs and Cu (CH3COO)2·H2O | [72] | |||
| Vacuum mixing and SPS | Modified MWCNTs, fine and coarse Cu powders | [73] | |||
| Electroless deposition, slurry dispersion, ball milling, cold isostatic pressing, vacuum sintering, and hot extrusion | Modified MWCNTs, Cu and TiB2 powders | Cu matrix composites reinforced with MWCNTs and TiB2 microparticles | [74,75] | ||
| Spraying pyrolysis, low-energy ball milling, and SPS | Modified and W-coated MWCNTs, Cu powders | CNT-W/Cu composite | [76] | ||
| Electroless deposition, ball milling, internal oxidation, and hot extrusion | CNTs, gas-atomized Cu-0.8Al powders, and Cu2O powders | Cu-Al2O3-CNT composite | CuxOy | [77] | |
| Electrodeposition, ball milling, and SPS | AgNO3, MWCNTs, and electrolytic Cu powder | Nanocomposites of Ag-nanoparticle-coated CNTs are uniformly distributed in Cu | Metallurgical bonding | Ag improved surface wettability | [78] |
| Ball milling and SPS | Cu powder, 4 at%Ni-1 at%Y catalyzed SWCNTs | --- | Ni-decorated SWCNTs | [79] | |
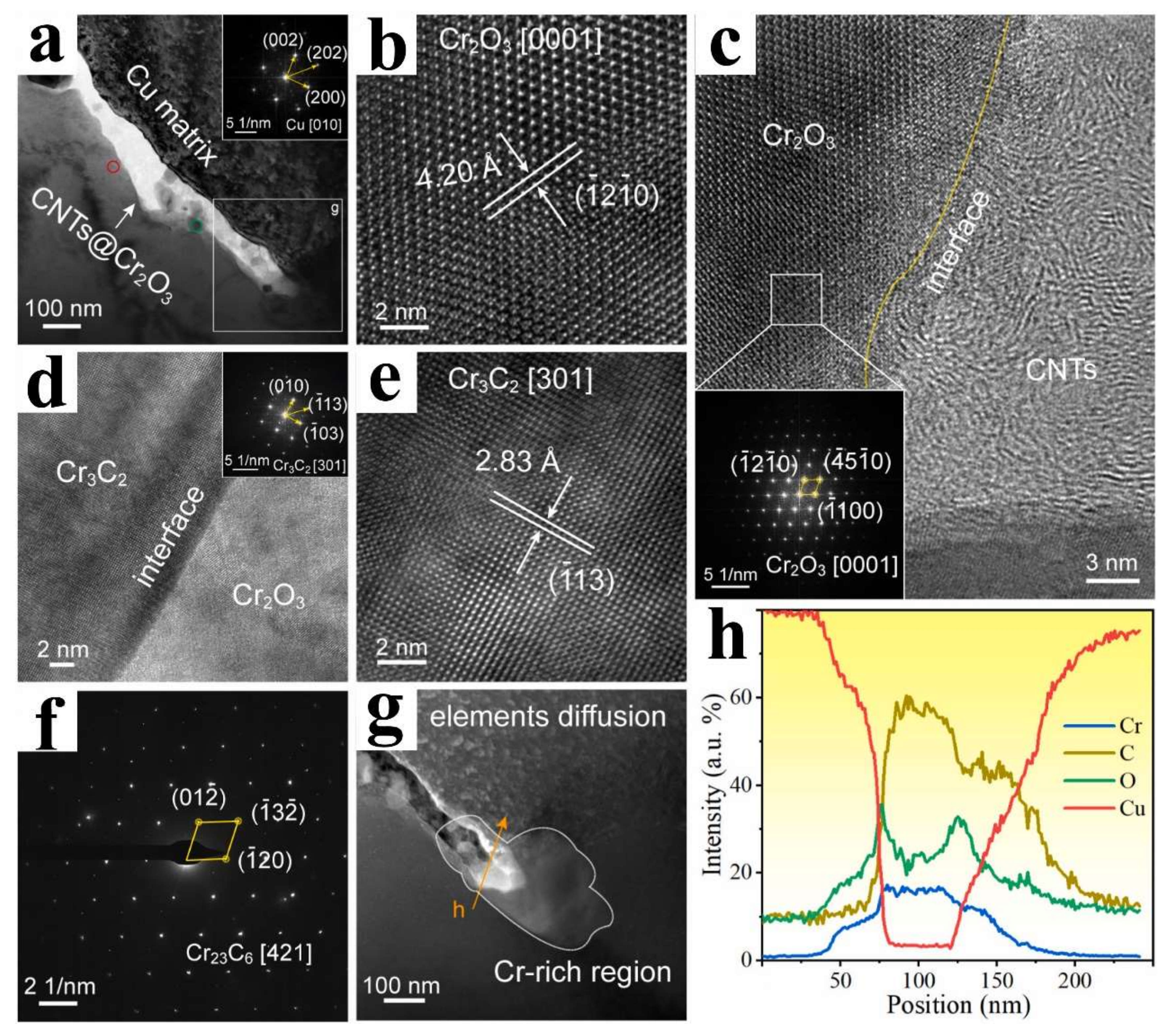
| Ball milling, vacuum hot-pressed sintering, and hot rolling | CNTs, Cr2(SO4)3 and Cu powder | CNT/Cu composite | Cr3C2 and Cr23C6 | [80] | |
| Catalyzed CVD and SPS | CuCr alloy, Cu powder, H2, and C2H4 | Chemical and metallurgical bonding | Cu2O and Cr3C2 | [64] | |
| Chemical unzipping, ball milling, and SPS | CuCr alloy powder, CNTs, H2SO4, and KMnO4 | CNT/CuCr composite | Cr7C3 and Cr23C6 | [81] | |
| Chemical modification and plating, ball milling, and fast hot-pressing sintering | CNTs, Cu powder | CNT/Cu composite | Cu2O and Sn and Ag diffused to Cu | [82] | |
| Interface Features | Strengthening Phase | Modified Group | Content | Electrical Conductivity (IACS%) | Ref. |
|---|---|---|---|---|---|
| Physical binding | MWCNTs | --- | 4 vol% | 82.5 ± 1.1 | [67] |
| CNTs | 1.5 vol% | 92.3 | [68] | ||
| --- | 81.4 | [70] | |||
| CNTs and Al2O3 | --- | 83.2 | [65] | ||
| CNTs and RGO | 0.064 vol% | 93.26 | [69] | ||
| Chemical bonding | modified SWCNTs | -COOH | ~0.45–0.5 vol% | --- | [43] |
| ~0.2 vol% | --- | [66] | |||
| 0.01 wt% | 96 | [83] | |||
| Modified MWCNTs | -OH | ~16 wt%, ~45 vol% | 0.345 | [14] | |
| -SH | 1.41 | ||||
| -COOH | 0.4 wt.% | 93.6 | [21] | ||
| Oxygen-containing groups | 0.8 wt% | 92.3 | [76] | ||
| 1 wt% | 85.4 ± 0.6 | [73] | |||
| Diazotizing and oxygen-containing groups | 1 vol% | 92.2 ± 0.4 | [72] | ||
| CNTs and Al2O3 | --- | 0.6 vol% CNTs and 3.5 vol% Al2O3 | 72.1 ± 0.8 | [77] | |
| CNTs and TiB2 | Oxygen-containing groups | 5.8 vol% (xCNTs + yTiB2), x = 0, 1.4, 2.8, 4.2, 5.8, x + y = 5.8 | 58.9 (x = 0), 56.9 (x = 1.4), 56.6 (x = 2.8), 59.2 (x = 4.2), 66.8 (x = 5.8) | [75] | |
| Metallurgical bonding | Ni-decorated SWCNTs | --- | 0.05 wt% | 94.3 ± 0.9 | [79] |
| MWCNTs | Ag nanoparticles | 0.75 wt% (CNT-Ag composite powder) | 93.6 | [78] | |
| Multiple interface bonding | Cr3C2 and Cu2O | 0.5 vol% | 92.9 | [64] |
| Interface Features | Strengthening Phase | Content | Hardness (HV) | Tensile Strength (MPa) | Ductility (%) | Ref. |
|---|---|---|---|---|---|---|
| Physical binding | MWCNTs | 4 vol% | --- | 474.3 ± 12.7 | 11.0 ± 0.5 | [67] |
| CNTs | 1.5 vol% | --- | 290 | 15 | [68] | |
| --- | --- | 392 | --- | [70] | ||
| CNTs and Al2O3 | 123.3 | 280 | --- | [65] | ||
| CNTs and RGO | 0.064 vol% | 135.7~141.79 at the pure Cu region, 154.23~161.84 at the skeleton region | 382 ± 2 | 43 ± 0.7 | [69] | |
| Chemical bonding | -COOH-modified SWCNTs | ~0.45–0.5 vol% | --- | ~110 | --- | [43] |
| ~0.2 vol% | --- | ~118 | --- | [66] | ||
| 0.01 wt% | 61 | --- | --- | [83] | ||
| HNO3-oxidized MWCNTs | 15 vol% | 126 | --- | --- | [20] | |
| -COOH-modified MWCNTs | 0.4 wt.% | --- | 272 | 14.3 | [21] | |
| -OH- and -COOH-modified MWCNTs | 0.5 wt% | --- | 269.9 | 16.9 | [71] | |
| -OH- and/or -COOH-modified MWCNTs | 0.6 wt% | --- | 226 | 33 | [76] | |
| 1 wt% | 113 ± 4 | --- | --- | [73] | ||
| 3 vol% | --- | 865 | [46] | |||
| -OH- and/or -COOH-modified and diazotized MWCNTs | 1 vol% | --- | 299 ± 8 | 16 ± 3 | [72] | |
| H2SO4 (98%)- and HNO3 (68%)-oxidized MWCNTs and TiB2 | 1.2 vol% MWCNTs and 4.8 vol% TiB2 | --- | 375 ± 5 | 32.4 ± 2.3 | [74] | |
| CNTs and Al2O3 | 1.2 vol% CNTs and 3.5 vol% Al2O3 | --- | 510 ± 6 | 20.2 | [77] | |
| Metallurgical bonding | Ag-coated MWCNTs | 0.75 wt% | 85.25 | 314 | 24.8 | [78] |
| Ni-decorated SWCNTs | 0.05 wt% | 79.1 ± 2.6 | 223.5 ± 6.5 | 48 ± 2.5 | [79] | |
| Cr2O3-decorated CNTs | 0.75 vol% | 117.3 ± 5.2 | --- | --- | [80] | |
| Multiple interface bonding | CNTs prepared via CuCr-alloy-catalyzed CVD | 0.5 vol% | 102.5 | 275 | 24 | [64] |
| Partially unzipped CNTs | 2 vol% | --- | 382.9 | 37.02 | [81] | |
| -OH- and/or -COOH-modified CNTs | 0.75 vol% | --- | 340 | 18.4 | [82] |

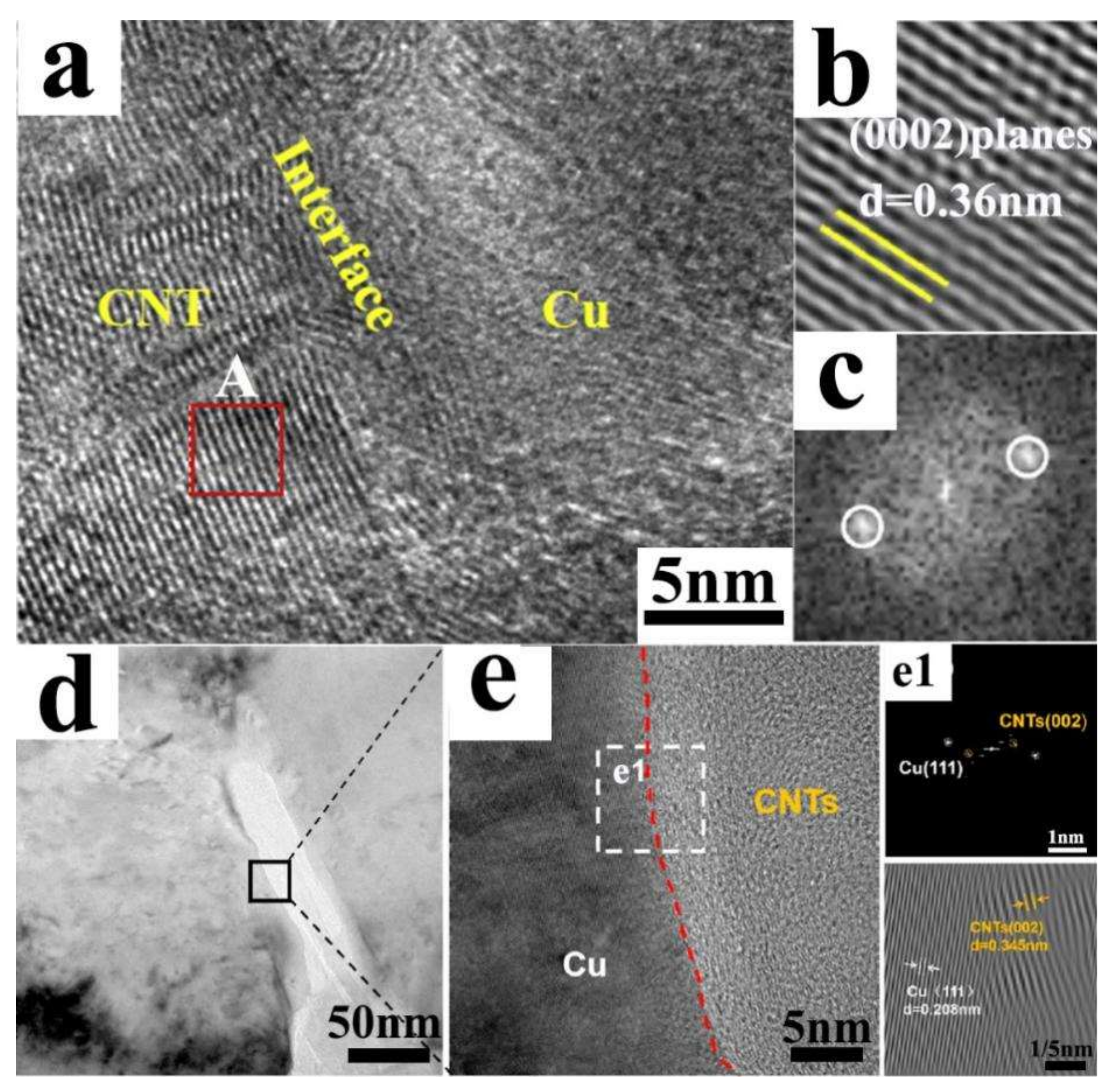
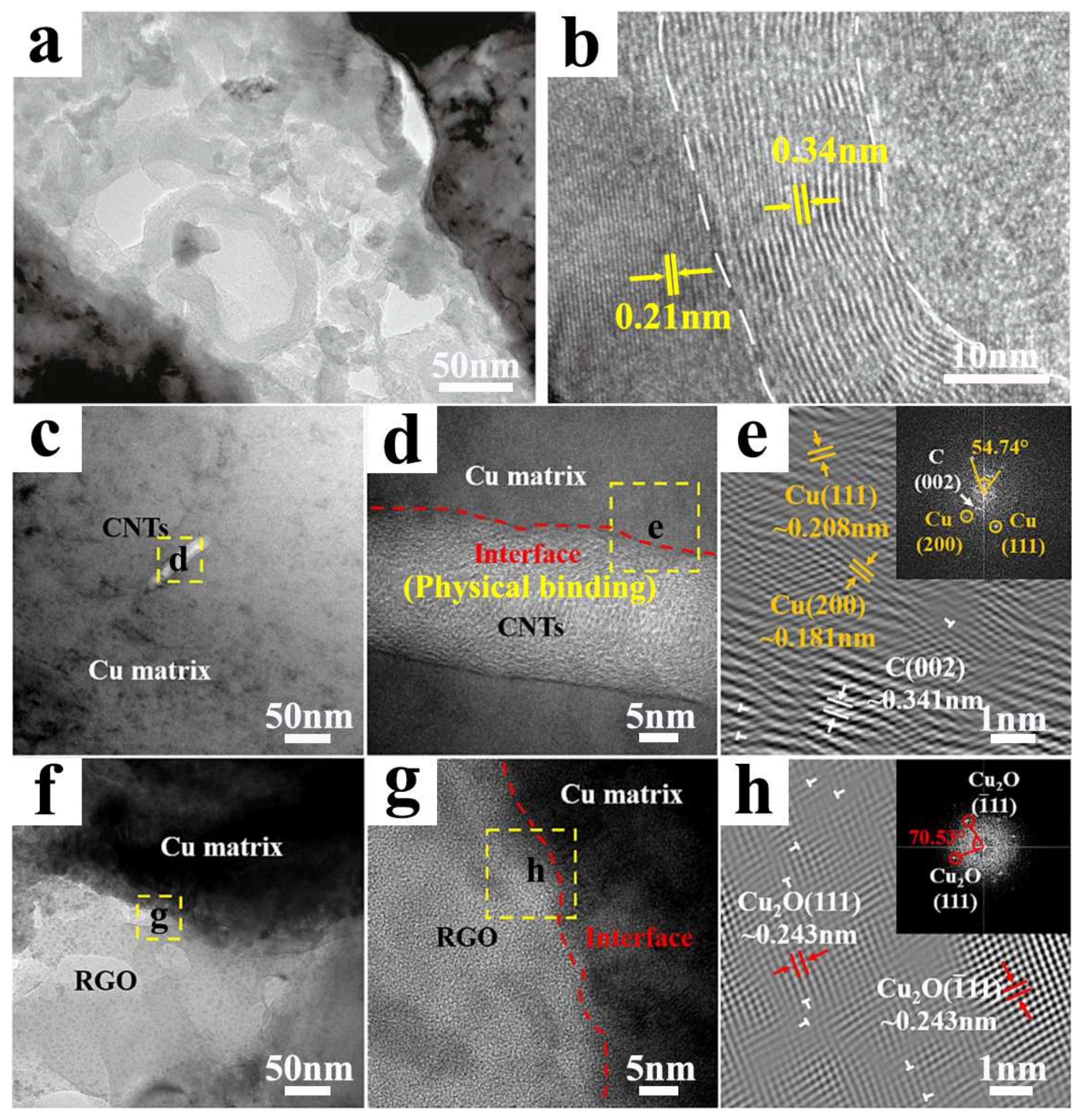
2. Physical Binding Interface
3. Chemical Bonding Interface
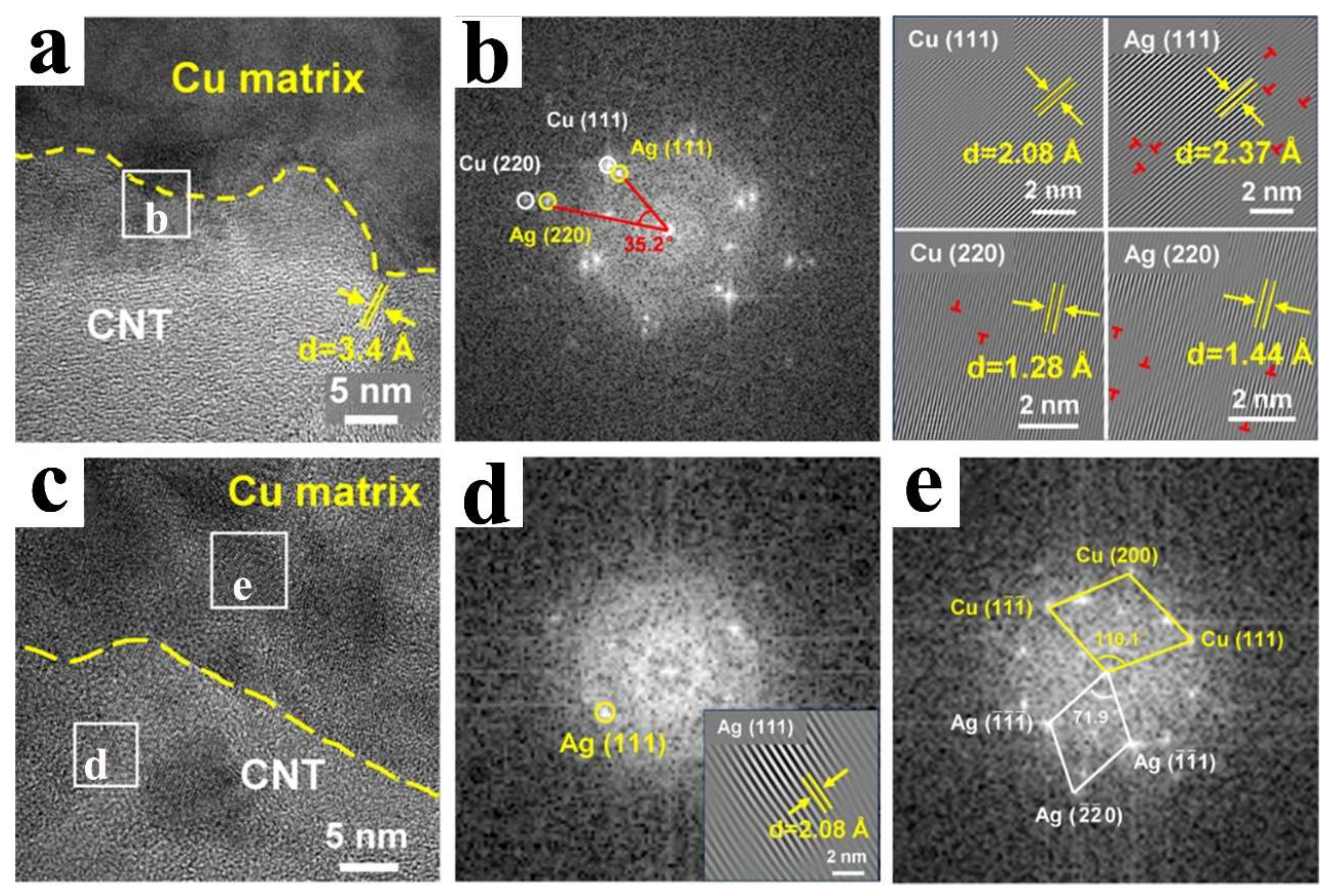
4. Metallurgical Bonding Interface
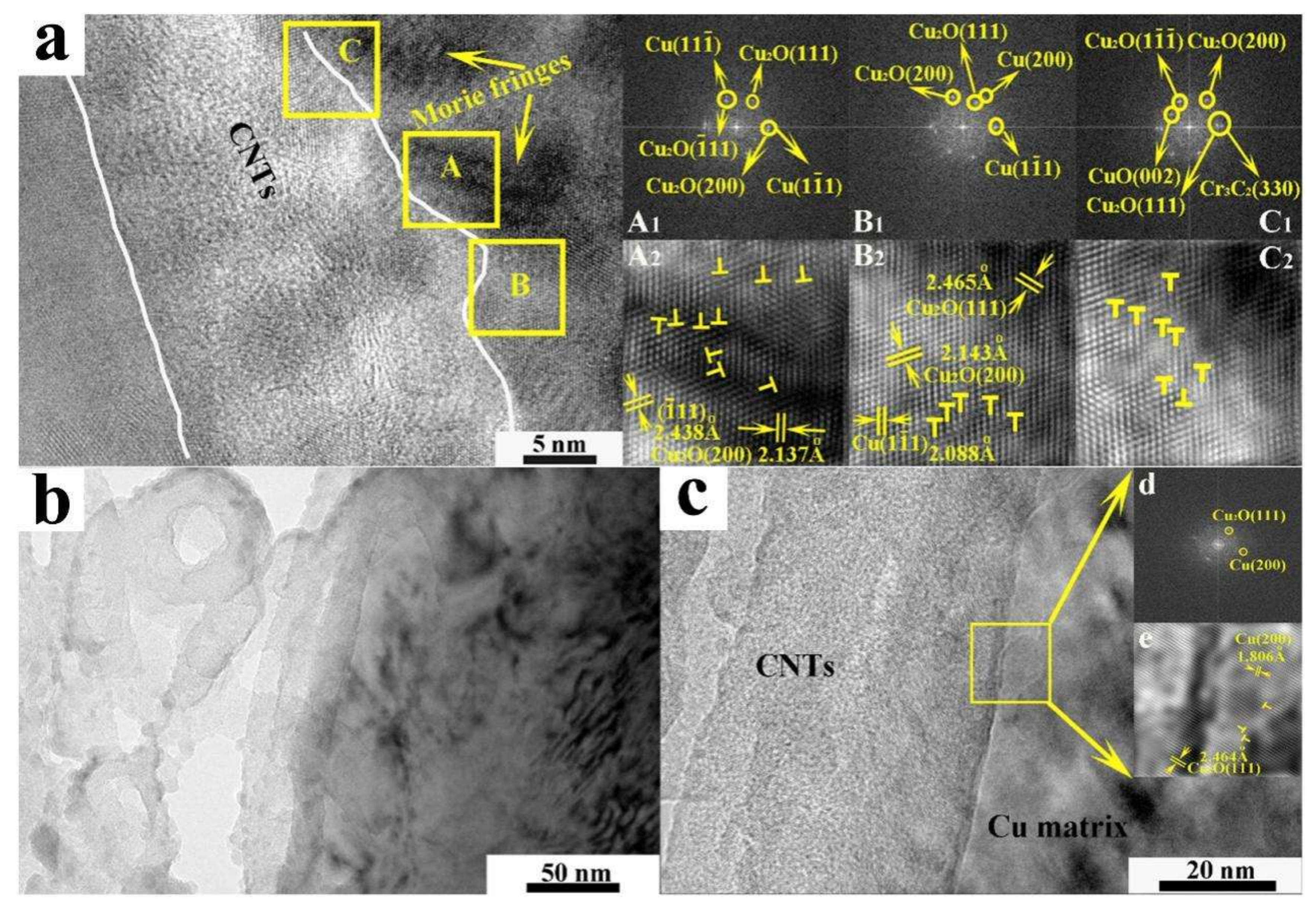
5. Multiple Interface Bonding Modes
6. Mutual Infiltration Interface
7. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Ma, Z.; Lei, C.; Meng, L.; Fang, Y.; Liu, J.; Wang, H. High Strength and High Conductivity Cu Alloys: A Review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Danoglidis, P.A.; Falara, M.G.; Nitodas, S.F. Fresh and Mechanical Properties, and Strain Sensing of Nanomodified Cement Mortars: The Effects of MWCNT Aspect Ratio, Density and Functionalization. Cem. Concr. Compos. 2017, 82, 137–151. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, S.; Kang, M.; Liu, Y.; Zhang, F.; Qu, W.; Guan, Y.; Li, S. The Thermal Behavior and Flame Retardant Performance of Phase Change Material Microcapsules with Modified Carbon Nanotubes. Energy 2022, 240, 122821. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Hu, X.; Li, H.; Shi, Z.; Yue, K.T.; Zi, J.; Gu, Z.; Wu, X.; Lian, Z.; Zhan, Y.; et al. Abnormal Anti-Stokes Raman Scattering of Carbon Nanotubes. Phys. Rev. B 2002, 66, 035413. [Google Scholar] [CrossRef]
- Ren, X.-N.; Xia, M.; Yan, Q.-Z.; Ge, C.-C. Controllable Preparation of Tungsten/Tungsten Carbide Nanowires or Nanodots in Nanostructured Carbon with Hollow Macroporous Core/Mesoporous Shell. Chin. Phys. B 2017, 26, 038103. [Google Scholar] [CrossRef]
- Wang, C.; Dong, B.; Gao, G.-Y.; Xu, M.-W.; Li, H.-L. A Study on Microhardness and Tribological Behavior of Carbon Nanotubes Reinforced AMMA-CNTs Copolymer Nanocomposites. Mater. Sci. Eng. A 2008, 478, 314–318. [Google Scholar] [CrossRef]
- Zhan, P.; Jia, Y.; Zhai, W.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. A Fibrous Flexible Strain Sensor with Ag Nanoparticles and Carbon Nanotubes for Synergetic High Sensitivity and Large Response Range. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107431. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K. Melt-Mixed Carbon Nanotubes/Polymer Nanocomposites. Polym. Compos. 2019, 40, 4473–4488. [Google Scholar] [CrossRef]
- Zhao, S.; Song, Z.; Cui, J.; Li, C.; Yan, Y. Improving Dispersion and Integration of Single-Walled Carbon Nanotubes in Epoxy Composites by Using a Reactive Noncovalent Dispersant. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4548–4556. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Garg, A.; Chalak, H.D.; Belarbi, M.-O.; Zenkour, A.M.; Sahoo, R. Estimation of Carbon Nanotubes and Their Applications as Reinforcing Composite Materials–An Engineering Review. Compos. Struct. 2021, 272, 114234. [Google Scholar] [CrossRef]
- Ren, X.; Hussain, M.I.; Chang, Y.; Ge, C. State-of-the-Art Review on Amorphous Carbon Nanotubes: Synthesis, Structure, and Application. Int. J. Mol. Sci. 2023, 24, 17239. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Kunjiappan, S.; Palanisamy, P. A Brief Review of Carbon Nanotube Reinforced Metal Matrix Composites for Aerospace and Defense Applications. Int. Nano Lett. 2021, 11, 321–345. [Google Scholar] [CrossRef]
- Daneshvar, F.; Zhang, T.; Aziz, A.; Sue, H.-J.; Welland, M.E. Tuning the Composition and Morphology of Carbon Nanotube-Copper Interface. Carbon 2020, 157, 583–593. [Google Scholar] [CrossRef]
- Hjortstam, O.; Isberg, P.; Söderholm, S.; Dai, H. Can We Achieve Ultra-Low Resistivity in Carbon Nanotube-Based Metal Composites? Appl. Phys. A 2004, 78, 1175–1179. [Google Scholar] [CrossRef]
- Uddin, S.M.; Mahmud, T.; Wolf, C.; Glanz, C.; Kolaric, I.; Volkmer, C.; Höller, H.; Wienecke, U.; Roth, S.; Fecht, H.-J. Effect of Size and Shape of Metal Particles to Improve Hardness and Electrical Properties of Carbon Nanotube Reinforced Copper and Copper Alloy Composites. Compos. Sci. Technol. 2010, 70, 2253–2257. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Joo, S.-J.; Kim, H.-S. Copper Nanoparticle/Multiwalled Carbon Nanotube Composite Films with High Electrical Conductivity and Fatigue Resistance Fabricated via Flash Light Sintering. ACS Appl. Mater. Interfaces 2015, 7, 25413–25423. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Masquelier, N.; Lassila, S.; Aromaa, J.; Janas, D.; Forsén, O.; Lundström, M. Corrosion Behaviour of Cast and Deformed Copper-Carbon Nanotube Composite Wires in Chloride Media. J. Alloys Compd. 2018, 746, 218–226. [Google Scholar] [CrossRef]
- Arnaud, C.; Lecouturier, F.; Mesguich, D.; Ferreira, N.; Chevallier, G.; Estournès, C.; Weibel, A.; Laurent, C. High Strength–High Conductivity Double-Walled Carbon Nanotube–Copper Composite Wires. Carbon 2016, 96, 212–215. [Google Scholar] [CrossRef]
- Rajkumar, K.; Aravindan, S. Tribological Studies on Microwave Sintered Copper–Carbon Nanotube Composites. Wear 2011, 270, 613–621. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Hu, Y.; Liu, Y.; Bao, R.; Li, F.; Fang, D.; Li, C.; Yi, J. Enhancement of Mechanical Properties and Conductivity in Carbon Nanotubes (CNTs)/Cu Matrix Composite by Surface and Intratube Decoration of CNTs. Mater. Sci. Eng. A 2021, 816, 141248. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A Sensitive Nonenzymatic Glucose Sensor in Alkaline Media with a Copper Nanocluster/Multiwall Carbon Nanotube-Modified Glassy Carbon Electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, W.-D.; Gunasekaran, S. An Amperometric Non-Enzymatic Glucose Sensor by Electrodepositing Copper Nanocubes onto Vertically Well-Aligned Multi-Walled Carbon Nanotube Arrays. Biosens. Bioelectron. 2010, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Wang, M.; Chatrathi, M.P. Carbon-Nanotube/Copper Composite Electrodes for Capillary Electrophoresis Microchip Detection of Carbohydrates. Analyst 2004, 129, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Bajwa, S.Z.; Mansoor, S.; Briddon, R.W.; Khan, W.S.; Scheffler, B.E.; Amin, I. Evaluation of Carbon Nanotube Based Copper Nanoparticle Composite for the Efficient Detection of Agroviruses. J. Hazard. Mater. 2018, 346, 27–35. [Google Scholar] [CrossRef]
- Yang, C.; Chan, P.C.H.; Fu, Y.; Chuang, Y.C.; Liu, C.Y. Copper/Carbon Nanotube Composite Interconnect for Enhanced Electromigration Resistance. In Proceedings of the 2008 58th Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 27–30 May 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 412–420. [Google Scholar]
- Feng, Y.; Burkett, S.L. Fabrication and Electrical Performance of through Silicon via Interconnects Filled with a Copper/Carbon Nanotube Composite. J. Vac. Sci. Technol. B 2015, 33, 022004. [Google Scholar] [CrossRef]
- Han, B.; Guo, E.; Xue, X.; Zhao, Z.; Li, T.; Xu, Y.; Luo, L.; Hou, H. Fabricating and Strengthening the Carbon Nanotube/Copper Composite Fibers with High Strength and High Electrical Conductivity. Appl. Surf. Sci. 2018, 441, 984–992. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Peltonen, A.; Aromaa, J.; Janas, D.; Lundström, M.; Wilson, B.P.; Koziol, K.; Forsén, O. Carbon Nanotube-Copper Composites by Electrodeposition on Carbon Nanotube Fibers. Carbon 2016, 107, 281–287. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, L.; Xue, W.; Li, W. Fabrication of Superaligned Carbon Nanotubes Reinforced Copper Matrix Laminar Composite by Electrodeposition. Trans. Nonferrous Met. Soc. China 2015, 25, 2994–3001. [Google Scholar] [CrossRef]
- Chai, G.; Sun, Y.; Sun, J.J.; Chen, Q. Mechanical Properties of Carbon Nanotube–Copper Nanocomposites. J. Micromech. Microeng. 2008, 18, 035013. [Google Scholar] [CrossRef]
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and Mechanical Properties of Carbon Nanotube Reinforced Copper Nanocomposites Fabricated by Electroless Deposition Process. Mater. Sci. Eng. A 2009, 513–514, 247–253. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.-H.; Hu, Z.-Y.; Song, Q.; Yin, S.-P.; Kang, Z.; Li, S.-L. Improvement of Interfacial Interaction and Mechanical Properties in Copper Matrix Composites Reinforced with Copper Coated Carbon Nanotubes. Mater. Sci. Eng. A 2018, 715, 163–173. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, D.-B.; Tan, Z.; Fan, G.; Guo, Q.; Su, Y.; Li, Z.; Zhang, D. Enhanced Mechanical Properties and High Electrical Conductivity in Multiwalled Carbon Nanotubes Reinforced Copper Matrix Nanolaminated Composites. Mater. Sci. Eng. A 2018, 729, 452–457. [Google Scholar] [CrossRef]
- Kim, K.T.; Cha, S.I.; Hong, S.H.; Hong, S.H. Microstructures and Tensile Behavior of Carbon Nanotube Reinforced Cu Matrix Nanocomposites. Mater. Sci. Eng. A 2006, 430, 27–33. [Google Scholar] [CrossRef]
- Shukla, A.K.; Nayan, N.; Murty, S.V.S.N.; Sharma, S.C.; Chandran, P.; Bakshi, S.R.; George, K.M. Processing of Copper–Carbon Nanotube Composites by Vacuum Hot Pressing Technique. Mater. Sci. Eng. A 2013, 560, 365–371. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Arshad, S.N.; Mo, C.B.; Hong, S.H. Extraordinary Strengthening Effect of Carbon Nanotubes in Metal-Matrix Nanocomposites Processed by Molecular-Level Mixing. Adv. Mater. 2005, 17, 1377–1381. [Google Scholar] [CrossRef]
- Kim, K.T.; Eckert, J.; Menzel, S.B.; Gemming, T.; Hong, S.H. Grain Refinement Assisted Strengthening of Carbon Nanotube Reinforced Copper Matrix Nanocomposites. Appl. Phys. Lett. 2008, 92, 121901. [Google Scholar] [CrossRef]
- Kim, K.T.; Cha, S.I.; Hong, S.H. Hardness and Wear Resistance of Carbon Nanotube Reinforced Cu Matrix Nanocomposites. Mater. Sci. Eng. A 2007, 449–451, 46–50. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, K.; Shuai, J.; Hou, Z.; Zhu, L.; Li, W. Toward High Strength and High Electrical Conductivity in Super-Aligned Carbon Nanotubes Reinforced Copper. Adv. Eng. Mater. 2018, 20, 1700805. [Google Scholar] [CrossRef]
- Chen, W.X.; Tu, J.P.; Wang, L.Y.; Gan, H.Y.; Xu, Z.D.; Zhang, X.B. Tribological Application of Carbon Nanotubes in a Metal-Based Composite Coating and Composites. Carbon 2003, 8, 215–222. [Google Scholar] [CrossRef]
- Tu, J.P.; Yang, Y.Z.; Wang, L.Y.; Ma, X.C.; Zhang, X.B. Tribological Properties of Carbon-Nanotube-Reinforced Copper Composites. Tribol. Lett. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Jiang, H.; Cooke, L.; Srivilliputhur, K.; McGuire, M.A.; Meyer, H.M.I.; Yoon, M.; Haynes, J.; Nawaz, K.; Lupini, A.R.; Li, K.; et al. Copper–Carbon Nanotube Composites Enabled by Brush Coating for Advanced Conductors. ACS Appl. Nano Mater. 2024, 7, 11176–11183. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One Hundred Fold Increase in Current Carrying Capacity in a Carbon Nanotube–Copper Composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef]
- Sundaram, R.; Sekiguchi, A.; Chen, G.; Futaba, D.; Yamada, T.; Kokubo, K.; Hata, K. Influence of Carbon Nanotube Attributes on Carbon Nanotube/Cu Composite Electrical Performances. C 2021, 7, 78. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.-H.; Hu, Z.-Y.; Wang, F.-C.; Li, S.-L.; Korznikov, E.; Zhao, X.-C.; Liu, Y.; Liu, Z.-F.; Kang, Z. Synergistic Strengthening Effect of Nanocrystalline Copper Reinforced with Carbon Nanotubes. Sci. Rep. 2016, 6, 26258. [Google Scholar] [CrossRef]
- Xiong, L.; Shuai, J.; Liu, K.; Hou, Z.; Zhu, L.; Li, W. Enhanced Mechanical and Electrical Properties of Super-Aligned Carbon Nanotubes Reinforced Copper by Severe Plastic Deformation. Compos. Part B Eng. 2019, 160, 315–320. [Google Scholar] [CrossRef]
- Tian, D.; Liu, Y.; Yu, J.; Zhao, Q.; Tao, J.; Wu, Z.; Zhang, J.; Fan, Y.; Liu, Y.; Li, C.; et al. A Study of Silver Decoration on Carbon Nanotubes via Ultrasonic Chemical Synthesis and Their Reinforced Copper Matrix Composites. Nanomaterials 2023, 13, 887. [Google Scholar] [CrossRef]
- Lahiri, D.; Agarwal, A. Carbon Nanotube Reinforced Metal Matrix Composites—A Review AU—Bakshi, S R. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Yang, P.; You, X.; Yi, J.; Fang, D.; Bao, R.; Shen, T.; Liu, Y.; Tao, J.; Li, C. Influence of Dispersion State of Carbon Nanotubes on Electrical Conductivity of Copper Matrix Composites. J. Alloys Compd. 2018, 752, 376–380. [Google Scholar] [CrossRef]
- Chen, L.; Hou, Z.; Liu, Y.; Luan, C.; Zhu, L.; Li, W. High Strength and High Ductility Copper Matrix Composite Reinforced by Graded Distribution of Carbon Nanotubes. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106063. [Google Scholar] [CrossRef]
- Khaleghi, E.; Torikachvili, M.; Meyers, M.A.; Olevsky, E.A. Magnetic Enhancement of Thermal Conductivity in Copper–Carbon Nanotube Composites Produced by Electroless Plating, Freeze Drying, and Spark Plasma Sintering. Mater. Lett. 2012, 79, 256–258. [Google Scholar] [CrossRef]
- Zhao, S.; Zheng, Z.; Huang, Z.; Dong, S.; Luo, P.; Zhang, Z.; Wang, Y. Cu Matrix Composites Reinforced with Aligned Carbon Nanotubes: Mechanical, Electrical and Thermal Properties. Mater. Sci. Eng. A 2016, 675, 82–91. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. Load Transfer Strengthening in Carbon Nanotubes Reinforced Metal Matrix Composites via In-Situ Tensile Tests. Compos. Sci. Technol. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline Materials. In Advanced Structural and Functional Materials; Bunk, W.G.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–37. ISBN 978-3-642-49263-1. [Google Scholar]
- Cho, S.; Kikuchi, K.; Kawasaki, A. On the Role of Amorphous Intergranular and Interfacial Layers in the Thermal Conductivity of a Multi-Walled Carbon Nanotube–Copper Matrix Composite. Acta Mater. 2012, 60, 726–736. [Google Scholar] [CrossRef]
- Neubauer, E.; Kitzmantel, M.; Hulman, M.; Angerer, P. Potential and Challenges of Metal-Matrix-Composites Reinforced with Carbon Nanofibers and Carbon Nanotubes. Compos. Sci. Technol. 2010, 70, 2228–2236. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, K.; Sun, W.; Ding, M.; Wang, Y.; Kong, X.; Jia, D.; Wu, M.; Fu, Y. Enhancement Mechanisms of Mechanical, Electrical and Thermal Properties of Carbon Nanotube-Copper Composites: A Review. J. Mater. Res. Technol. 2024, 32, 1395–1415. [Google Scholar] [CrossRef]
- Yermembetova, A.; Rahimi, R.M.; Kim, C.-E.; Skinner, J.L.; Andriolo, J.M.; Murphy, J.P.; Bahr, D.F. Nanomechanics and Testing of Core-Shell Composite Ligaments for High Strength, Light Weight Foams. MRS Adv. 2017, 2, 3577–3583. [Google Scholar] [CrossRef]
- Jenei, P.; Gubicza, J.; Yoon, E.Y.; Kim, H.S.; Lábár, J.L. High Temperature Thermal Stability of Pure Copper and Copper–Carbon Nanotube Composites Consolidated by High Pressure Torsion. Compos. Part A Appl. Sci. Manuf. 2013, 51, 71–79. [Google Scholar] [CrossRef]
- Jenei, P.; Yoon, E.Y.; Gubicza, J.; Kim, H.S.; Lábár, J.L.; Ungár, T. Microstructure and Hardness of Copper–Carbon Nanotube Composites Consolidated by High Pressure Torsion. Mater. Sci. Eng. A 2011, 528, 4690–4695. [Google Scholar] [CrossRef]
- Yang, P.; You, X.; Yi, J.; Fang, D.; Bao, R.; Shen, T.; Liu, Y.; Tao, J.; Li, C.; Tan, S.; et al. Simultaneous Achievement of High Strength, Excellent Ductility, and Good Electrical Conductivity in Carbon Nanotube/Copper Composites. J. Alloys Compd. 2018, 752, 431–439. [Google Scholar] [CrossRef]
- Vignesh Babu, R.; Kanagaraj, S. Thermal, Electrical and Mechanical Characterization of Microwave Sintered Copper/Carbon Nanotubes (CNT) Composites against Sintering Duration, CNT Diameter and Its Concentration. J. Mater. Process. Technol. 2018, 258, 296–309. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Liu, P. Preparation of CNTs/Cu Composites with Good Electrical Conductivity and Excellent Mechanical Properties. Mater. Sci. Eng. A 2020, 771, 138656. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Liu, P.; Zhou, H.; Fu, S.; Li, W.; Liu, X.; Ma, F.; Wu, Z. Preparation and Mechanical Properties of Copper Matrix Composites Reinforced by Carbon Nanotubes and Al2O3. Adv. Eng. Mater. 2021, 23, 2001490. [Google Scholar] [CrossRef]
- Li, K.; McGuire, M.; Lupini, A.; Skolrood, L.; List, F.; Ozpineci, B.; Ozcan, S.; Aytug, T. Copper–Carbon Nanotube Composites Enabled by Electrospinning for Advanced Conductors. ACS Appl. Nano Mater. 2020, 3, 6863–6875. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Mousa Mirabad, H.; Alipour, S.; Kim, H.S. Enhanced Tensile Properties and Electrical Conductivity of Cu-CNT Nanocomposites Processed via the Combination of Flake Powder Metallurgy and High Pressure Torsion Methods. Mater. Sci. Eng. A 2020, 773, 138888. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Tao, J.; Liu, Y.; Bao, R.; Li, C.; Li, F.; Chen, X.; Ye, D.; Yi, J. Synergistic Optimization of Properties in Carbon Nanotubes Reinforced Cu Matrix Composites Prepared by Co-Deposition. Ceram. Int. 2024, 50, 18337–18346. [Google Scholar] [CrossRef]
- You, X.; Yan, A.; Liu, Y.; Zhao, Q.; Fan, Y.; Liu, Y.; Li, C.; Yi, J. A Comparison Study of the Strengthening Effect of Carbon Nanomaterial Reinforcements in the 3D Skeleton-Reinforced Copper Matrix Composites. Diam. Relat. Mater. 2024, 141, 110580. [Google Scholar] [CrossRef]
- Liu, D.; Wang, P.; Zhang, X.; Chen, C.; Zou, J.; Hou, L.; Zhao, J.; Xue, J.; Ding, F.; Gao, Z.; et al. Synergistically Improved Mechanical, Thermal, and Ampacity Performances of Carbon Nanotube/Copper Composite Conductors Based on Network Confinement Effects. Carbon 2023, 201, 837–846. [Google Scholar] [CrossRef]
- Zhang, W.; You, X.; Fang, D.; Yang, P.; Yi, J.; Yu, X.; Bao, R.; Li, C.; Liu, Y.; Tao, J.; et al. Influence of Acid-Treated Time of Carbon Nanotubes on Mechanical Property in Carbon Nanotubes Reinforced Copper Matrix Composites. Diam. Relat. Mater. 2020, 109, 108069. [Google Scholar] [CrossRef]
- Mu, S.; Tao, J.; Chen, X.; Liu, Y.; Bao, R.; Li, C.; Li, F.; Yi, J. The Configuration Design of Cross-Linked CNT Networks to Realize Heterostructure in Cu Matrix Composite towards Prominent Mechanical-Electrical Property Synergy. J. Mater. Res. Technol. 2024, 30, 7447–7461. [Google Scholar] [CrossRef]
- Mishra, S.; Mohapatra, S.; Mudliyar, B.S.; Das, S.; Das, K. Synergistic Effect of Bimodal Structure and Cu-Coated CNTs on the Properties of Cu/CNTs Composites. J. Alloys Compd. 2024, 971, 172681. [Google Scholar] [CrossRef]
- Long, F.; Guo, X.; Song, K.; Jia, S.; Yakubov, V.; Li, S.; Yang, Y.; Liang, S. Synergistic Strengthening Effect of Carbon Nanotubes (CNTs) and Titanium Diboride (TiB2) Microparticles on Mechanical Properties of Copper Matrix Composites. J. Mater. Res. Technol. 2020, 9, 7989–8000. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Song, K.; Shaolin, L.; Jiang, F.; Wang, X. Arc Erosion Resistance of Hybrid Copper Matrix Composites Reinforced with CNTs and Micro-TiB2 Particles. J. Mater. Res. Technol. 2021, 11, 1469–1479. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, R.; Yi, J.; Guo, S.; Tao, J.; Li, C.; Fang, D.; Liu, Y.; Li, F. Improving Comprehensive Performance of Copper Matrix Composite by Spray Pyrolysis Fabricated CNT/W Reinforcement. J. Alloys Compd. 2020, 833, 154940. [Google Scholar] [CrossRef]
- Long, F.; Guo, X.; Song, K.; Liu, J.; Wang, X.; Yang, Y.; Li, S. An Internal-Oxidation-Based Strategy Induced High-Density Alumina in-Situ Nanoprecipitation and Carbon Nanotube Interface Optimization for Co-Reinforcing Copper Matrix Composites. Compos. Part B Eng. 2022, 229, 109455. [Google Scholar] [CrossRef]
- Yang, G.; Wang, R.; Fang, D.; Hu, T.; Bao, C.; Yi, J. Nano-Silver Modified Carbon Nanotubes to Reinforce the Copper Matrix Composites and Their Mechanical Properties. Adv. Powder Technol. 2022, 33, 103672. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, T.; Deng, Z.-Y.; Zhao, X. Enhancement of Ultimate Tensile Strength and Ductility of Copper Matrix Composites Using a Low Content of Single-Wall Carbon Nanotubes. J. Alloys Compd. 2023, 968, 172225. [Google Scholar] [CrossRef]
- Luo, S.; Yu, W.; Song, M.; Yi, J.; Guo, B.; Yu, Z.; Li, W. Tailoring the Interface with the In-Situ Formed Chromium Oxide and Carbide for Higher Mechanical Properties of Copper Matrix Composites. Ceram. Int. 2023, 49, 28107–28117. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Lei, Q.; Li, W.; Gan, X.; Zhou, K. Enhanced Mechanical Properties of Alloyed Copper Matrix Composites Reinforced with Partially-Unzipped Carbon Nanotubes. Mater. Sci. Eng. A 2020, 792, 139552. [Google Scholar] [CrossRef]
- Luo, S.; Chen, B.; Song, M.; Zhang, Z.; Yi, J.; Zhou, S.; Guo, B.; Yu, Z.; Li, W. Improving the Strength-Ductility Synergy of Carbon Nanotubes Reinforced Cu Matrix Composites through Interfacial Regulation. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107787. [Google Scholar] [CrossRef]
- Castellanos-Leal, E.; Martínez-Guerra, E.; Chavez-Valdez, A.; Arizmendi-Morquecho, A. Effect of the Reinforcement Phase on the Electrical and Mechanical Properties of Cu–SWCNTs Nanocomposites. Diam. Relat. Mater. 2024, 142, 110765. [Google Scholar] [CrossRef]
- Ren, X.; Xia, M.; Yan, Q.; Ge, C. Controllable Modification of Nanostructured Carbon with Hollow Macroporous Core/Mesoporous Shell and Its Application as Templates in Aqueous Solution. Chem. Phys. Lett. 2016, 662, 286–290. [Google Scholar] [CrossRef]
- Yan, K.Y.; Xue, Q.Z.; Zheng, Q.B.; Hao, L.Z. The Interface Effect of the Effective Electrical Conductivity of Carbon Nanotube Composites. Nanotechnology 2007, 18, 255705. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Sekiguchi, A.; Yamada, T.; Kokubo, K.; Hata, K. Improving Carbon Nanotube/Copper Film Composite Electrical Performances by Tailoring Oxygen Interface through Gaseous Ozone Treatment of Carbon Nanotube Films. Synth. Met. 2022, 288, 117103. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhao, Q.; Jiang, H.; Yu, X.; Fan, Y.; Liu, Y.; Li, C.; Yi, J. Molecular Dynamics Study of the Interface Fine Structure and Mechanical Properties of Ni@SWCNT/Cu Nanocrystalline Composite Materials. Mater. Sci. Eng. A 2024, 901, 146523. [Google Scholar] [CrossRef]
- Long, R.; Liu, Y.; Tao, J.; Zhang, H.; Liu, Y.; Bao, R.; Li, F.; Li, C.; Yi, J. Synergistic Influence of Carbon Nanotube-Graphene Oxide Hybrid and Nanosized Interfacial TiC on the Mechanical Performance of Cu Matrix Composites. J. Mater. Res. Technol. 2023, 25, 2866–2879. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Q.; Zhou, L.; Chen, H.; Zhan, K.; Liu, J.; Ding, R.; You, S.; Zhao, B.; Ji, V. Microstructure, Properties and Synergetic Effect of Graphene Oxide-Functionalized Carbon Nanotubes Hybrid Reinforced Copper Matrix Composites Prepared by DC Electrodeposition. Carbon 2023, 212, 118157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Chang, Y.; Ge, C. State-of-the-Art Carbon-Nanotubes-Reinforced Copper-Based Composites: The Interface Design of CNTs and Cu Matrix. Int. J. Mol. Sci. 2024, 25, 12957. https://doi.org/10.3390/ijms252312957
Ren X, Chang Y, Ge C. State-of-the-Art Carbon-Nanotubes-Reinforced Copper-Based Composites: The Interface Design of CNTs and Cu Matrix. International Journal of Molecular Sciences. 2024; 25(23):12957. https://doi.org/10.3390/ijms252312957
Chicago/Turabian StyleRen, Xiaona, Yue Chang, and Changchun Ge. 2024. "State-of-the-Art Carbon-Nanotubes-Reinforced Copper-Based Composites: The Interface Design of CNTs and Cu Matrix" International Journal of Molecular Sciences 25, no. 23: 12957. https://doi.org/10.3390/ijms252312957
APA StyleRen, X., Chang, Y., & Ge, C. (2024). State-of-the-Art Carbon-Nanotubes-Reinforced Copper-Based Composites: The Interface Design of CNTs and Cu Matrix. International Journal of Molecular Sciences, 25(23), 12957. https://doi.org/10.3390/ijms252312957









