Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions
Abstract
1. Introduction
2. Results
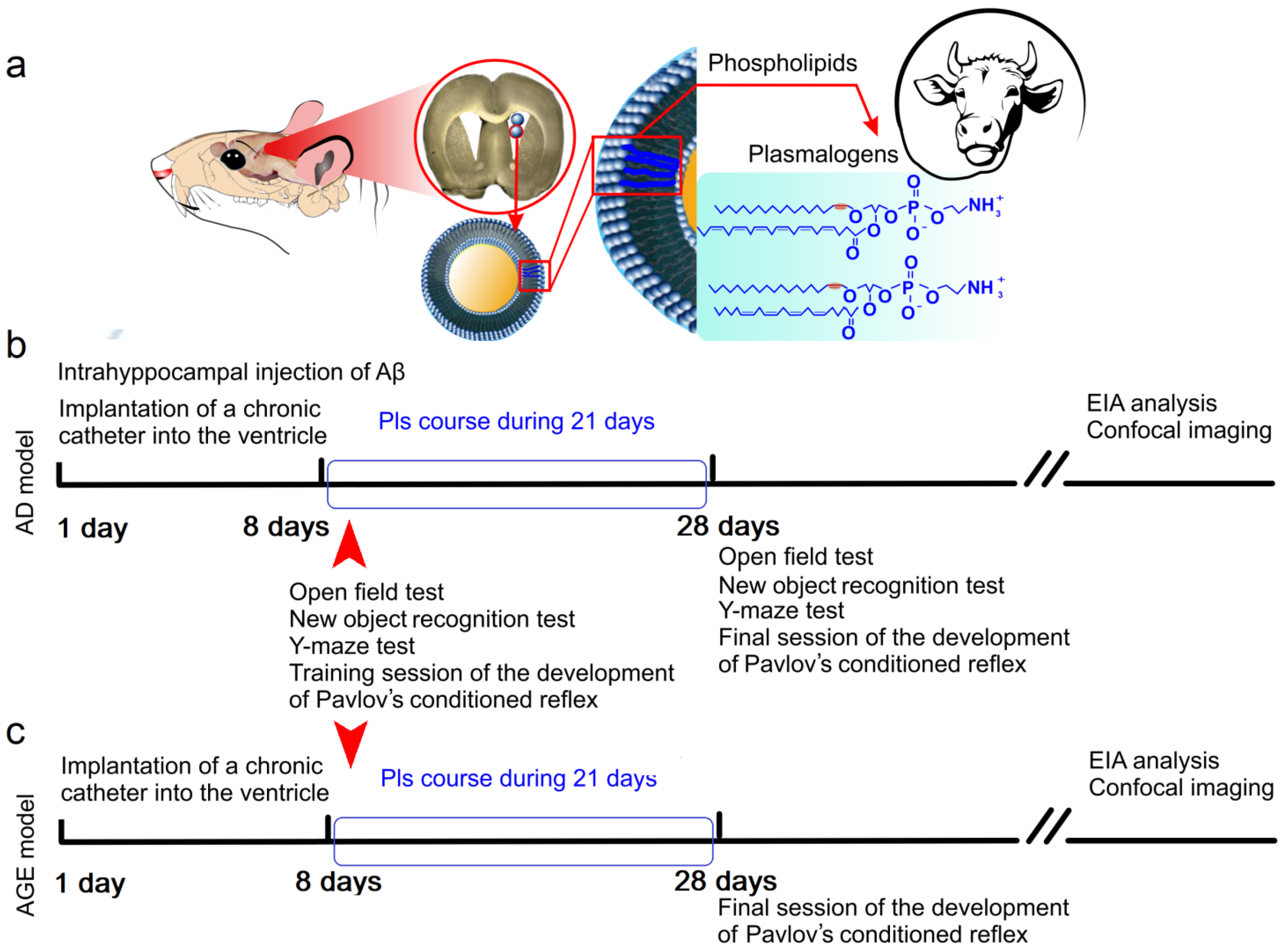
2.1. Effects of Pls on Clearance of Aβ from the Brain and Cognitive Functions: A Model of the Early Stages of AD
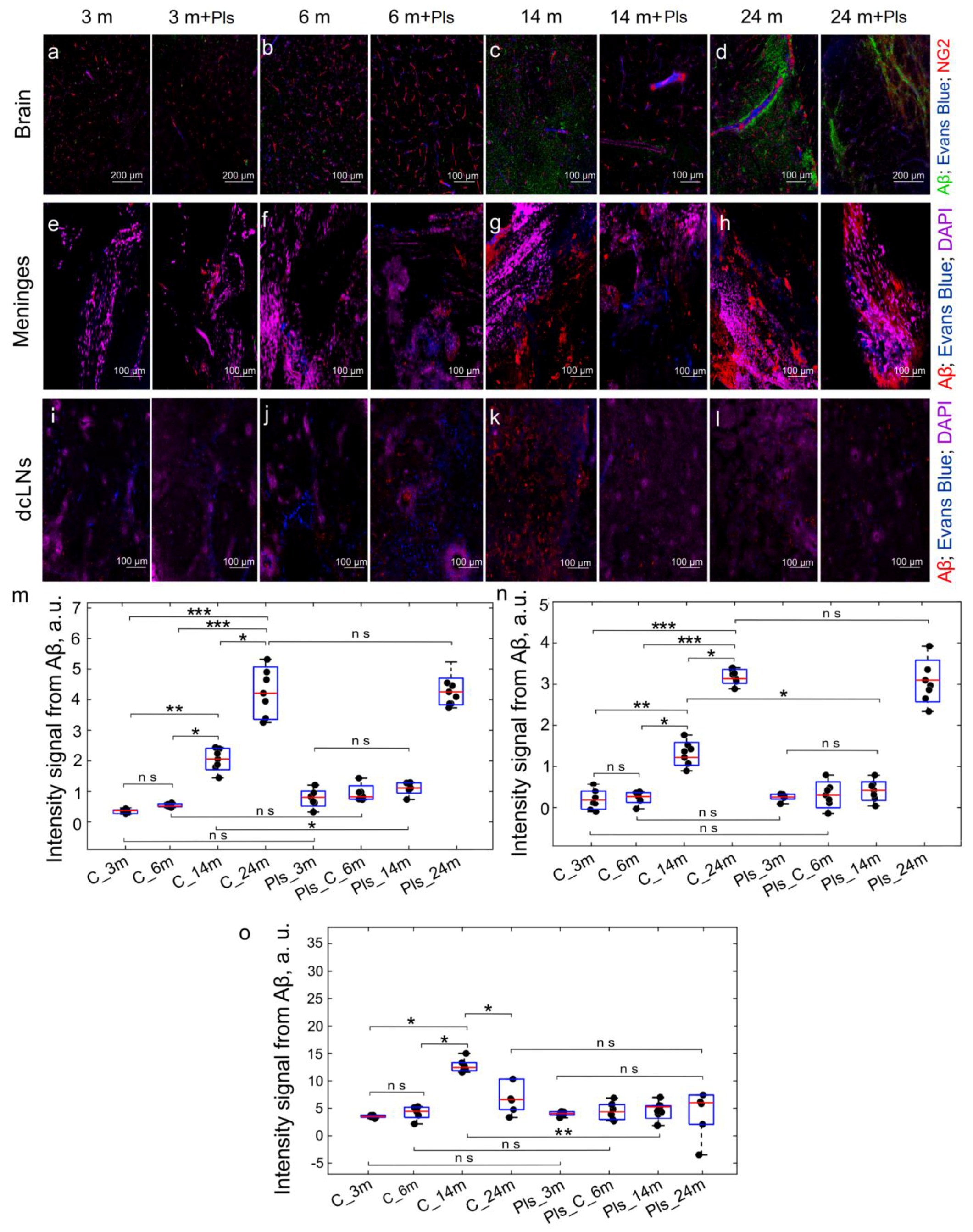
2.2. Effects of Pls on Clearance of Aβ from the Brain and Cognitive Functions: An Age Model of Aβ Plague Formation
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Preparation of Liposomes with Pls and Mode of Administration to Mice
4.3. An Injected Model of Early Stages of AD
4.4. Behavioral Testing
4.5. Immunohistochemical Assay and Confocal Imaging
4.6. ELISA Analysis of Brain Tissues
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Sig. Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kim, K.; Lim, H.J.; Kim, H.Y.; Shin, J.; Park, I.; Cho, I.; Kim, H.Y.; Kim, S.; McLean, C.; et al. Early onset diagnosis in Alzheimer’s disease patients via amyloid-β oligomers-sensing probe in cerebrospinal fluid. Nat. Commun. 2024, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gouras, G.K.; Almeida, C.G.; Takahashi, R.H. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol. Aging 2005, 26, 1235–1244. [Google Scholar] [CrossRef]
- Naslund, J.; Haroutunian, V.; Mohs, R.; Davis, K.L.; Davies, P.; Greengard, P.; Buxbaum, J.D. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 2000, 283, 1571–1577. [Google Scholar] [CrossRef]
- Wertkin, A.M.; Turner, R.S.; Pleasure, S.J.; Golde, T.E.; Younkin, S.G.; Trojanowski, J.Q.; Lee, V.M. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular beta-amyloid or A4 peptides. Proc. Natl. Acad. Sci. USA 1993, 90, 9513–9517. [Google Scholar] [CrossRef]
- Haass, C.; Schlossmacher, M.G.; Hung, A.Y.; Vigo-Pelfrey, C.; Mellon, A.; Ostaszewski, B.L.; Lieberburg, I.; Koo, E.H.; Schenk, D.; Teplow, D.B.; et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 1992, 359, 322–325. [Google Scholar] [CrossRef]
- Masi, M.; Biundo, F.; Fiou, A.; Racchi, M.; Pascale, A.; Buoso, E. The Labyrinthine Landscape of APP Processing: State of the Art and Possible Novel Soluble APP-Related Molecular Players in Traumatic Brain Injury and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 6639. [Google Scholar] [CrossRef]
- Almeida, Z.L.; Vaz, D.C.; Brito, R.M.M. Morphological and Molecular Profiling of Amyloid-β Species in Alzheimer’s Pathogenesis. Mol. Neurobiol. 2024, 1–29. [Google Scholar] [CrossRef]
- Müller, U.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Grimm, M.O.; Mett, J.; Stahlmann, C.P.; Grösgen, S.; Haupenthal, V.J.; Blümel, T.; Hundsdörfer, B.; Zimmer, V.C.; Mylonas, N.T.; Tanila, H.; et al. APP intracellular domain derived from amyloidogenic β- and γ-secretase cleavage regulates neprilysin expression. Front. Aging Neurosci. 2015, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Lanni, C.; Schettini, G.; Govoni, S.; Racchi, M. Beta-Amyloid precursor protein metabolism: Focus on the functions and degradation of its intracellular domain. Pharmacol. Res. 2010, 62, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, L.; Sang, S.; Pan, X.; Zhong, C. Physiological Roles of β-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology. Neurosci. Bull. 2023, 39, 1289–1308. [Google Scholar] [CrossRef]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fa, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef]
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotrophic and neurotoxic effects of amyloid protein: Reversal by tachykinin neuropeptides. Science 1990, 250, 279–282. [Google Scholar] [CrossRef]
- Plant, L.D.; Boyle, J.P.; Smith, I.F.; Peers, C.; Pearson, H.A. The production of amyloid peptide is a critical requirement for the viability of central neurons. J. Neurosci. 2003, 23, 5531–5535. [Google Scholar] [CrossRef]
- López-Toledano, M.A.; Shelanski, M.L. Neurogenic effect of amyloid peptide in the development of neural stem cells. J. Neurosci. 2004, 24, 5439–5444. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Luna, R.; Colín-Barenque, L. Amyloid beta: Multiple mechanisms of toxicity and only some protective effects? Oxidative Med. Cell. Longev. 2014, 2014, 795375. [Google Scholar] [CrossRef]
- Grimm, M.O.; Grimm, H.S.; Pätzold, A.J.; Zinser, E.G.; Halonen, R.; Duering, M.; Tschäpe, J.-A.; Strooper, B.D.; Müller, U.; Shen, J. Regulation of cholesterol and sphingomyelin metabolism by amyloid-and presenilin. Nat. Cell Biol. 2005, 7, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sheng, Z.H. Energy matters: Presynaptic metabolism and the maintenance of synaptic transmission. Nat. Rev. Neurosci. 2022, 23, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.S.; Gordon, B.A.; Couture, L.E.; Flores, S.; Xiong, C.; Morris, J.C.; Raichle, M.E.; Benzinger, T.L.-S.; Vlassenko, A.G. Spatiotemporal relationship between subthreshold amyloid accumulation and aerobic glycolysis in the human brain. Neurobiol. Aging 2020, 96, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Moghekar, A.; Rao, S.; Li, M.; Ruben, D.; Mammen, A.; Tang, X.; O’Brien, R.J. Large quantities of Abeta peptide are constitutively released during amyloid precursor protein metabolism in vivo and in vitro. J. Biol. Chem. 2011, 286, 15989–15997. [Google Scholar] [CrossRef]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef]
- Crouch, P.J.; Harding, S.M.; White, A.R.; Camakaris, J.; Bush, A.I.; Masters, C.L. Mechanisms of A beta-mediated neurodegeneration in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2008, 40, 181–198. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- O’brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Janelidze, S.; Ossenkoppele, R.; Kvartsberg, H.; Brinkmalm, A.; Mattsson-Carlgren, N.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; et al. Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 2021, 144, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Andersson, E.; Janelidze, S.; Ossenkoppele, R.; Insel, P.; Strandberg, O.; Zetterberg, H.; Rosen, H.J.; Rabinovici, G.; Chai, X.; et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci. Adv. 2020, 6, eaaz2387. [Google Scholar] [CrossRef]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.; Maloney, J.; et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef]
- Navolokin, N.; Adushkina, V.; Zlatogorskaya, D.; Telnova, V.; Evsiukova, A.; Vodovozova, E.; Eroshova, A.; Dosadina, E.; Diduk, S.; Semyachkina-Glushkovskaya, O. Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer’s Disease. Pharmaceuticals 2024, 17, 788. [Google Scholar] [CrossRef]
- Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 20, 1598–1695.
- Milton, N. SARS-CoV-2 amyloid, is COVID-19-exacerbated dementia an amyloid disorder in the making? Front. Dement. 2023, 2, 1233340. [Google Scholar] [CrossRef]
- Rudnicka-Drożak, E.; Drożak, P.; Mizerski, G.; Zaborowski, T.; Ślusarska, B.; Nowicki, G.; Drożak, M. Links Between COVID-19 and Alzheimer’s Disease—What Do We Already Know? Int. J. Environ. Res. Public Health 2023, 20, 2146. [Google Scholar] [CrossRef]
- Rahman, M.R.; Akter, R.; Neelotpol, S.; Mayesha, I.I.; Afrose, A. The Neuropathological Impacts of COVID-19: Challenges and Alternative Treatment Options for Alzheimer’s Like Brain Changes on Severely SARS-CoV-2 Infected Patients. Am. J. Alzheimers Dis. Other Dement. 2023, 38, 15333175231214974. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401—A clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers Res. Ther. 2016, 8, 14. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Howard, R.; Liu, K.Y. Questions EMERGE as Biogen claims aducanumab turnaround. Nat. Rev. Neurol 2020, 16, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.; Kepp, K.; Herrup, K. Lecanemab and Donanemab as Therapies for Alzheimer’s Disease: An Illustrated Perspective on the Data. eNeuro 2024, 11, ENEURO.0319-23.2024. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef]
- Gu, J.; Chen, L.; Sun, R.; Wang, J.-L.; Wang, J.; Lin, Y.; Lei, S.; Zhang, Y.; Lv, D.; Jiang, F.; et al. Plasmalogens Eliminate Aging-Associated Synaptic Defects and Microglia-Mediated Neuroinflammation in Mice. Front. Mol. Biosci. 2022, 9, 815320. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 100. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. eBioMedicine 2017, 17, 199–205. [Google Scholar] [CrossRef]
- Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.V.; Gvozdenko, T.A. Plasmalogens in the Pathophysiology and Therapy of Age-Specific Diseases. Adv. Gerontol. 2020, 10, 272–281. [Google Scholar] [CrossRef]
- Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. [Google Scholar] [CrossRef] [PubMed]
- West, A.; Zoni, V.; Teague, W.E., Jr.; Leonard, A.N.; Vanni, S.; Gawrisch, K.; Tristram-Nagle, S.; Sachs, J.N.; Klauda, J.B. How Do Ethanolamine Plasmalogens Contribute to Order and Structure of Neurological Membranes? J. Phys. Chem. B 2020, 124, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sheikh, A.M.; Haque, A.; Osago, H.; Sakai, H.; Shibly, A.Z.; Yano, S.; Michikawa, M.; Hossain, S.; Tabassum, S.; et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Macala, L.J.; Yu, R.K.; Ando, S. Analysis of Brain Lipids by High Performance Thin-Layer Chromatography and Densitometry. J. Lipid Res. 1983, 24, 1243–1250. [Google Scholar] [CrossRef]
- Udagawa, J.; Hino, K. Plasmalogen in the brain: Effects on cognitive functions and behaviors attributable to its properties. Brain Res. Bull. 2022, 188, 197–202. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ifuku, M.; Take, S.; Kawamura, J.; Miake, K.; Katafuchi, T. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS ONE 2013, 8, 83508. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular Anatomy of a Trafficking Organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From Peroxisomal Disorders to Common Neurodegenerative Diseases—The Role of Ether Phospholipids in the Nervous System. FEBS Lett. 2017, 591, 2761–2788. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens inhibit neuroinflammation and promote cognitive function. Brain Res. Bull. 2023, 192, 56–61. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Bizien, T.; Gorshkova, Y.E.; Deng, Y. Plasmalogen-Based Liquid Crystalline Multiphase Structures Involving Docosapentaenoyl Derivatives Inspired by Biological Cubic Membranes. Front. Cell Dev. Biol. 2021, 9, 617984. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; König, T.; Scholze, P.; Berger, M.L.; Zeitler, G.; Wiesinger, C.; Gundacker, A.; Pollak, D.D.; Huck, S.; Just, W.W.; et al. Disturbed Neurotransmitter Homeostasis in Ether Lipid Deficiency. Hum. Mol. Genet. 2019, 28, 2046–2061. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rojo, N.; Riezman, H. On the Road to Unraveling the Molecular Functions of Ether Lipids. FEBS Lett. 2019, 593, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Koivuniemi, A. The Biophysical Properties of Plasmalogens Originating from Their Unique Molecular Architecture. FEBS Lett. 2017, 591, 2700–2713. [Google Scholar] [CrossRef]
- Mandel, H.; Sharf, R.; Berant, M.; Wanders, R.J.; Vreken, P.; Aviram, M. Plasmalogen phospholipids are involved in HDL-mediated cholesterol efflux: Insights from investigations with plasmalogen-deficient cells. Biochem. Biophys. Res. Commun. 1998, 250, 369–373. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Ellison, D.W.; Beal, M.F.; Martin, J.B. Phosphoethanolamine and ethanolamine are decreased in Alzheimer’s disease and Huntington’s disease. Brain Res. 1987, 417, 389–392. [Google Scholar] [CrossRef]
- Rothhaar, T.L.; Grösgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdörfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O. Plasmalogens inhibit APP processing by directly affecting γ-secretase activity in Alzheimer’s disease. Sci. World J. 2012, 2012, 141240. [Google Scholar] [CrossRef]
- Hino, K.; Kaneko, S.; Harasawa, T.; Kimura, T.; Takei, S.; Shinohara, M.; Yamazaki, F.; Morita, S.Y.; Sato, S.; Kubo, Y.; et al. Change in Brain Plasmalogen Composition by Exposure to Prenatal Undernutrition Leads to Behavioral Impairment of Rats. J. Neurosci. 2019, 39, 7689–7702. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens, the vinyl ether-linked glycerophospholipids, enhance learning and memory by regulating brain-derived neurotrophic factor. Front. Cell Dev. Biol. 2022, 10, 828282. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Zhang, L.; Ding, L.; Xie, W.; Jiang, X.; Xue, C.; Zhang, T.; Wang, Y. EPAenriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine enhance BDNF/TrkB/CREB signaling and inhibit neuronal apoptosis in vitro and in vivo. Food Funct. 2020, 11, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Hossain, M.S.; Mawatari, S. Therapeutic efficacy of plasmalogens for Alzheimer’s disease, mild cognitive impairment, and Parkinson’s disease in conjunction with a new hypothesis for the etiology of Alzheimer’s disease. Adv. Exp. Med. Biol. 2020, 1299, 195–212. [Google Scholar] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Biological functions of plasmalogens. Adv. Exp. Med. Biol. 2020, 1299, 171–193. [Google Scholar]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of Blood Plasmalogens and Clinical Symptoms in Parkinson’s Disease by Oral Administration of Ether Phospholipids: A Preliminary Report. Park. Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Halupczok, S.; Rodenberg-Frank, E.; Valerius, K.P.; Baumgart-Vogt, E. Endogenous Murine Amyloid-β Peptide Assembles into Aggregates in the Aged C57BL/6J Mouse Suggesting These Animals as a Model to Study Pathogenesis of Amyloid-β Plaque Formation. J. Alzheimers Dis. 2018, 61, 1425–1450. [Google Scholar] [CrossRef]
- Facchinetti, R.; Bronzuoli, M.R.; Scuderi, C. An Animal Model of Alzheimer Disease Based on the Intrahippocampal Injection of Amyloid β-Peptide. Methods Mol. Biol. 2018, 1727, 343–352. [Google Scholar]
- Park, J.; Lee, S.Y.; Shon, J.; Kim, K.; Lee, H.J.; Kim, K.A.; Lee, B.Y.; Oh, S.H.; Kim, N.K.; Kim, O.J. Adalimumab improves cognitive impairment, exerts neuroprotective effects and attenuates neuroinflammation in an Aβ1-40-injected mouse model of Alzheimer’s disease. Cytotherapy 2019, 21, 671–682. [Google Scholar] [CrossRef]
- Aquino, R.; de Concini, V.; Dhenain, M.; Lam, S.; Gosset, D.; Baquedano, L.; Forero, M.G.; Menuet, A.; Baril, P.; Pichon, C. Intrahippocampal Inoculation of Aβ1–42 Peptide in Rat as a Model of Alzheimer’s Disease Identified MicroRNA-146a-5p as Blood Marker with Anti-Inflammatory Function in Astrocyte Cells. Cells 2023, 12, 694. [Google Scholar] [CrossRef]
- Kobro-Flatmoen, A.; Hormann, T.M.; Gouras, G. Intracellular Amyloid-β in the Normal Rat Brain and Human Subjects and Its relevance for Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.L.; Miller, T.M. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 2013, 75, e50326. [Google Scholar] [CrossRef]
- Broadhurst, P.L. Determinants of emotionality in rat: I. Situational factors. Br. J. Psychol. 1957, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, B.; Coper, H. Tests in rodents for assessing sensorimotor performance during aging. Adv. Psychol. 1996, 114, 201–233. [Google Scholar]
- Baerends, E.; Soud, K.; Folke, J.; Pedersen, A.K.; Henmar, S.; Konrad, L.; Lycas, M.D.; Mori, Y.; Pakkenberg, B.; Woldbye, D.; et al. Modeling the early stages of Alzheimer’s disease by administering intracerebroventricular injections of human native Aβ oligomers to rats. Acta Neuropathol. Commun. 2022, 10, 113. [Google Scholar] [CrossRef]
- Hascup, K.N.; Hascup, E.R. Altered neurotransmission prior to cognitive decline in AbetaPP/PS1 mice, a model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 44, 771–776. [Google Scholar] [CrossRef]
- Ma, S.; Zuo, Y. Synaptic modifications in learning and memory—A dendritic spine story. Semin. Cell Dev. Biol. 2022, 125, 84–90. [Google Scholar] [CrossRef]
- Li, R.; Xiong, W.; Li, B.; Li, Y.; Fang, B.; Wang, X.; Ren, F. Plasmalogen Improves Memory Function by Regulating Neurogenesis in a Mouse Model of Alzheimer’s Diseases. Int. J. Mol. Sci. 2023, 24, 12234. [Google Scholar] [CrossRef]
- Gage, F.H. Adult Neurogenesis in Mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Bielefeld, P.; Durá, I.; Danielewicz, J.; Lucassen, P.J.; Baekelandt, V.; Abrous, D.N.; Encinas, J.M.; Fitzsimons, C.P. Insult-Induced Aberrant Hippocampal Neurogenesis: Functional Consequences and Possible Therapeutic Strategies. Behav. Brain Res. 2019, 372, 112032. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar]
- Braverman, N.E.; Moser, A.B. Functions of Plasmalogen Lipids in Health and Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Blokina, I.; Iluykov, E.; Myagkov, D.; Tuktarov, D.; Popov, S.; Inozemzev, T.; Fedosov, I.; Shirokov, A.; Terskov, A.; Dmitrenko, A.; et al. Photobiomodulation Under Electroencephalographic Controls of Sleep for Stimulation of Lymphatic Removal of Toxins from Mouse Brain. J. Vis. Exp. 2024, 28, 208. [Google Scholar] [CrossRef]
- Cole, G.; Ma, Q.; Frautschy, S. Dietary fatty acids and the aging brain. Nutr. Rev. 2010, 68 (Suppl. S2), S102–S111. [Google Scholar] [CrossRef]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Henkel, N.; Eladawi, M.A.; Villarreal, E.C.; Kandimalla, P.; Lundh, A.; McCullumsmith, R.E.; Cuevas, E. Sex and Gender Differences in Alzheimer’s Disease: Genetic, Hormonal, and Inflammation Impacts. Int. J. Mol. Sci. 2024, 25, 8485. [Google Scholar] [CrossRef]
- Yamashita, S.; Honjo, A.; Aruga, M.; Nakagawa, K.; Miyazawa, T. Preparation of marine plasmalogen and selective identification of molecular species by LC-MS/MS. J. Oleo Sci. 2014, 63, 423–430. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar]
- Sarkar, D. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xue, G.; Wang, S.; Zhang, L.; Shi, C.; Xie, X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer’s disease mouse model. J. Alzheimers Dis. 2012, 31, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Sik, A.; Van Nieuwehuyzen, P.; Prickaerts, J.; Blokland, A. Performance of different mouse strains in an object recognition task. Behav. Brain Res. 2003, 147, 49–54. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Zaikin, A.; Ageev, V.; Ilyukov, E.; Myagkov, D.; Tuktarov, D.; Blokhina, I.; Shirokov, A.; Terskov, A.; et al. Technology of the photobiostimulation of the brain’s drainage system during sleep for improvement of learning and memory in male mice. Biomed. Opt. Express 2023, 15, 44–58. [Google Scholar] [CrossRef]
- Lederle, L.; Weber, S.; Wrigh, T.; Feyder, M.; Brigman, J.L.; Crombag, H.S.; Saksida, L.M.; Bussey, T.J.; Holmes, A. Reward-related behavioral paradigms for addiction research in the mouse: Performance of common inbred strains. PLoS ONE 2011, 6, e15536. [Google Scholar] [CrossRef]



| Tested Groups | no Pls | +Pls |
|---|---|---|
| AD mice | 23.43 ± 1.14 ### | 16.07 ± 2.14 † |
| 3-month-old mice | 10.18 ± 1.23 | 10.04 ± 1.32 |
| 6-month-old mice | 11.07 ± 1.01 | 11.12 ± 1.03 |
| 14-month-old mice | 14.29 ± 1.05 ** | 11.00 ± 1.01 † |
| 24-month-old mice | 18.83 ± 1.07 *** | 17.95 ± 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirokov, A.; Zlatogosrkaya, D.; Adushkina, V.; Vodovozova, E.; Kardashevskaya, K.; Sultanov, R.; Kasyanov, S.; Blokhina, I.; Terskov, A.; Tzoy, M.; et al. Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions. Int. J. Mol. Sci. 2024, 25, 12552. https://doi.org/10.3390/ijms252312552
Shirokov A, Zlatogosrkaya D, Adushkina V, Vodovozova E, Kardashevskaya K, Sultanov R, Kasyanov S, Blokhina I, Terskov A, Tzoy M, et al. Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions. International Journal of Molecular Sciences. 2024; 25(23):12552. https://doi.org/10.3390/ijms252312552
Chicago/Turabian StyleShirokov, Alexander, Daria Zlatogosrkaya, Viktoria Adushkina, Elena Vodovozova, Kristina Kardashevskaya, Ruslan Sultanov, Sergey Kasyanov, Inna Blokhina, Andrey Terskov, Maria Tzoy, and et al. 2024. "Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions" International Journal of Molecular Sciences 25, no. 23: 12552. https://doi.org/10.3390/ijms252312552
APA StyleShirokov, A., Zlatogosrkaya, D., Adushkina, V., Vodovozova, E., Kardashevskaya, K., Sultanov, R., Kasyanov, S., Blokhina, I., Terskov, A., Tzoy, M., Evsyukova, A., Dubrovsky, A., Tuzhilkin, M., Elezarova, I., Dmitrenko, A., Manzhaeva, M., Krupnova, V., Semiachkina-Glushkovskaia, A., Ilyukov, E., ... Semyachkina-Glushkovskaya, O. (2024). Plasmalogens Improve Lymphatic Clearance of Amyloid Beta from Mouse Brain and Cognitive Functions. International Journal of Molecular Sciences, 25(23), 12552. https://doi.org/10.3390/ijms252312552







