Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases
Abstract
1. Introduction
2. Hybridization Strategy
3. Marine Peptides—Characteristics and Biological Activity of Selected Compounds
3.1. Fish Peptides
| Peptide Source | Peptide Sequence (One-Letter Amino Acid Code) | Displayed Activity | Ref. |
|---|---|---|---|
| Tuna | AEPAPAPAPAPEPAPAPA, GEPGPAG, LPGGGPVL, AAAPAPAPAPAPA, AGLYPGA | antioxidative | [50,51] |
| LPHVLTPEAGAT and PTAEGGVYMVT | anticancer | [52] | |
| ICY, LSFR, IYSP | antihypertensive (ACE-inhibitory) and antioxidative | [55] | |
| GILTLK | antimicrobial | [74] | |
| WPEAAELMMEVDP | antioxidative | [75] | |
| GDLGKTTTVSNWSPPKYKDTP | antihypertensive | [54] | |
| Mackerel | LDIQKEV, TAAIVNTA | antioxidative | [76] |
| Shark | CF, EY, MF, FE | antihypertensive (ACE-inhibitory) | [56] |
| MLVGPIGAAKVVYEQ-XX X—unknown amino acid residues not defined by the authors | hepatoprotective, immunomodulatory, antidiabetic, antioxidative | [60] | |
| Sole | GFFALIPKIISSPLFKTLLSAVGSALSSSGGQE (called pardaxin) | antimicrobial, antitumor, increase in dopamine release | [61,77,78,79] |
| MIFPGAGGPEL | antihypertensive | [80] | |
| Hagfish | GWFKKAWRKVKNAGRRVLKGVGIHYGVGLI | antimicrobial (including antifungal activity) | [81,82] |
| Cod | TGGGNV, TCSP | antioxidative, ACE-inhibitory | [83] |
| Herring | PPVEEP, GPAGDPA, GADPEDVIVS | antidiabetic | [84] |
| Salmon | WA, WM, VW, MW, IW, LW, FL | ACE-inhibitory | [85] |
| GPAE | antidiabetic | [86] | |
| Sardine | LKVGGKGY, LY, YL, GRP, RFH, GWAP | ACE-inhibitory | [87] |
3.2. Marine Snail Peptides
3.3. Algae and Macroalgae Peptides
4. Marine Peptide-Based Hybrids—Are There Any?
Marine Peptide-Based Hybrids and Their Efficacy in Preclinical Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kleczkowska, P. Chimeric structures in mental illnesses—“Magic” Molecules Specified for Complex Disorders. Int. J. Mol. Sci. 2022, 23, 3739. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Ghasemi, J.B. Dual-acting of hybrid compounds—A New Dawn in the Discovery of Multi-target Drugs: Lead Generation Approaches. Curr. Top. Med. Chem. 2017, 17, 1096–1114. [Google Scholar] [CrossRef]
- Müller-Schiffmann, A.; Sticht, H.; Korth, C. Hybrid compounds: From simple combinations to nanomachines. BioDrugs 2012, 26, 21–31. [Google Scholar] [CrossRef]
- Korth, C.; Klingenstein, R.; Müller-Schiffmann, A. Hybrid molecules synergistically acting against protein aggregation diseases. Curr. Top. Med. Chem. 2013, 13, 2484–2490. [Google Scholar] [CrossRef]
- Muchowska, A.; Redkiewicz, P.; Różycki, K.; Matalińska, J.; Lipiński, P.F.J.; Czuwara, J.; Kosson, P. The analgesic hybrid of dermorphin/substance P and analog of enkephalin improve wound healing in streptozotocin-induced diabetic rats. Wound Repair Regen. 2020, 28, 177–184. [Google Scholar] [CrossRef]
- Foran, S.E.; Carr, D.B.; Lipkowski, A.W.; Maszczynska, I.; Marchand, J.E.; Misicka, A.; Beinborn, M.; Kopin, A.S.; Kream, R.M. Inhibition of morphine tolerance development by a substance P-opioid peptide chimera. J. Pharmacol. Exp. Ther. 2000, 295, 1142–1148. [Google Scholar]
- Mollica, A.; Costante, R.; Stefanucci, A.; Pinnen, F.; Luisi, G.; Pieretti, S.; Borsodi, A.; Bojnik, E.; Benyhe, S. Hybrid peptides endomorphin-2/DAMGO: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2013, 68, 167–177. [Google Scholar] [CrossRef]
- Wagner, H.; Efferth, T. Introduction: Novel hybrid combinations containing synthetic or antibiotic drugs with plant-derived phenolic or terpenoid compounds. Phytomedicine 2017, 37, 1–3. [Google Scholar] [CrossRef]
- Xu, W.; Popovich, D.G. Bioactive hybrid compounds from Myrtaceae: Chemical classification and biological activities. Stud. Natur. Prod. Chem. 2023, 77, 65–109. [Google Scholar]
- Pratt, J.H. A reappraisal of researches leading to the discovery of insulin. J. Hist. Med. 1954, 9, 281–289. [Google Scholar] [CrossRef]
- Wodlej, C.; Riedl, S.; Rinner, B.; Leber, R.; Drechsler, C.; Voelker, D.R.; Choi, J.-Y.; Lohner, K.; Zweytick, D. Interaction of two antitumor peptides with membrane lipids–Influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS ONE 2019, 14, e0211187. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Negi, B.; Kumar, D.; Rawat, D.S. Marine peptides as anticancer agents: A remedy to mankind by nature. Curr. Protein Pept. Sci. 2017, 18, 885–904. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Kosson, P.; Ballet, S.; Van den Eynde, I.; Tsuda, Y.; Tourwé, D.; Lipkowski, A.W. PK20, a new opioid-neurotensin hybrid peptide that exhibits central and peripheral antinociceptive effects. Mol. Pain. 2010, 6, 86. [Google Scholar] [CrossRef]
- Silbert, B.S.; Lipkowski, A.W.; Cepeda, M.S.; Szyfelbein, S.K.; Osgood, P.F.; Carr, D.B. Analgesic activity of novel bivalent opioid peptide compared to morphine via different routes administration. Agents Action 1991, 33, 382–387. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Hermans, E.; Kosson, P.; Kowalczyk, A.; Lesniak, A.; Pawlik, K.; Bojnik, E.; Benyhe, S.; Nowicka, B.; Bujalska-Zadrozny, M.; et al. Antinociceptive effect induced by a combination of opioid and neurotensin moieties vs. their hybrid peptide [Ile9]PK20 in an acute pain treatment in rodents. Brain Res. 2016, 1648, 172–180. [Google Scholar] [CrossRef]
- Klingenstein, R.; Lober, S.; Kujala, P.; Godsave, S.; Leliveld, S.R.; Gmeiner, P.; Peters, P.J.; Korth, C. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabilizing detergent-resistant membrane compartments. J. Neurochem. 2006, 98, 748–759. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Kawalec, M.; Bujalska-Zadrozny, M.; Filip, M.; Zablocka, B.; Lipkowski, A.W. Effects of the hybridization of opioid and neurotensin pharmacophores on cell survival in rat organotypic hippocampal slice cultures. Neurotox. Res. 2015, 28, 352–360. [Google Scholar] [CrossRef]
- Bądzyńska, B.; Lipkowski, A.W.; Sadowski, J. An antihypertensive opioid: Biphalin, a synthetic non-addictive enkephalin analog decreases blood pressure in spontaneously hypertensive rats. Pharmacol. Rep. 2016, 68, 51–55. [Google Scholar] [CrossRef]
- Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 668–680. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Wetzler, M.; Hamilton, P. Peptides as therapeutics. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing: Cambridge, UK, 2018; pp. 215–230. [Google Scholar]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Barman, P.; Joshi, S.; Sharma, S.; Preet, S.; Sharma, S.; Saini, A. Strategic approaches to improvise peptide drugs as next generation therapeutics. Int. J. Pept. Res. Ther. 2023, 29, 61. [Google Scholar] [CrossRef]
- Shinnar, A.E.; Butler, K.L.; Park, H.J. Cathelicidin family of antimicrobial peptides: Proteolytic processing and protease resistance. Bioorg. Chem. 2003, 31, 425–436. [Google Scholar] [CrossRef]
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Schultz, A.W.; Oh, D.C.; Carney, J.R.; Williamson, R.T.; Udwary, D.W.; Jensen, P.R.; Gould, S.J.; Fenical, W.; Moore, B.S. Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides of marine actinobacterial origin. J. Am. Chem. Soc. 2008, 130, 4507–4516. [Google Scholar] [CrossRef]
- Pettit, G.R.; Cichacz, Z.; Barkoczy, J.; Dorsaz, A.C.; Herald, D.L.; Williams, M.D.; Doubek, D.L.; Schmidt, J.M.; Tackett, L.P.; Brune, D.C.; et al. Isolation and structure of the marine sponge cell growth inhibitory cyclic peptide phakellistatin 1. J. Nat. Prod. 1993, 56, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux, D.; Munoz, M.; Bulet, P.; Bachere, E. Penaeidins, a family of antimicrobial peptides from penaeid shrimp. Cell. Mol. Life Sci. 2000, 57, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Patocka, J.; Nepovimova, E.; Oleksak, P.; Valis, M.; Wu, W.; Kuca, K. Marine invertebrate peptides: Antimicrobial peptides. Front. Microbiol. 2021, 12, 785085. [Google Scholar] [CrossRef]
- Pan, W.; Liu, X.; Ge, F.; Han, J.; Zheng, T. Perinerin, a novel antimicrobial peptide purified from the clamworm Perinereis aibuhitensis grube and its partial characterization. J. Biochem. 2004, 135, 297–304. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial peptides of fish: Innocuous alternatives to antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Kapil, S.; Sharma, V. D-Amino acids in antimicrobial peptides: A potential approach to treat and combat antimicrobial resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine natural peptides: Determination of absolute configuration using liquid chromatography methods and evaluation of bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Aillaud, I.; Kaniyappan, S.; Chandupatla, R.R.; Ramirez, L.M.; Alkhashrom, S.; Eichler, J.; Horn, A.H.C.; Zweckstetter, M.; Mandelkow, E.; Sticht, H.; et al. A novel D-amino acid peptide with therapeutic potential (ISAD1) inhibits aggregation of neurotoxic disease-relevant mutant Tau and prevents Tau toxicity in vitro. Alzheimers Res. Ther. 2022, 14, 15. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine bioactive peptides-an overview of generation, structure and application with a focus on food sources. Mar. Drugs 2020, 18, 424. [Google Scholar] [CrossRef]
- Giordano, D. Bioactive molecules from extreme environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, D.A.; Schmeing, T.M. Biosynthesis of depsipeptides, or Depsi: The peptides with varied generations. Protein Sci. 2020, 29, 2316–2347. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Tao, J.; Xu, S.; Bai, X.; Zhang, H. Marine organisms as a prolific source of bioactive depsipeptides. Mar. Drugs 2023, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Nič, M.; Jirát, J.; Košata, B.; Jenkins, A.; McNaught, A. IUPAC Compendium of Chemical Terminology; IUPAC: Research Triagle Park, NC, USA, 2009. [Google Scholar]
- Ariyoshi, Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993, 4, 139–144. [Google Scholar] [CrossRef]
- Ukeda, H.; Matsuda, H.; Kuroda, H.; Osajima, K.; Matsufuji, H.; Osajima, Y. Preparation and separation of angiotensin I converting enzyme inhibitory peptides. Nippon Nogeikagaku Kaishi 1991, 65, 1223–1228. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Kohama, Y.; Matsumoto, S.; Oka, H.; Teramoto, T.; Okabe, M.; Mimura, T. Isolation of angiotensin-converting enzyme-inhibitor from tuna muscle. Biochem. Biophys. Res. Commun. 1988, 155, 332–337. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Wang, C.; Wen, Z.; Zheng, B. The antioxidant mechanism of peptides extracted from tuna protein revealed using a molecular docking simulation. Antioxidants 2024, 13, 166. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Li-Chan, E.C.; Jao, C.-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, B.; Chen, H.; Wan, P.; Chen, D.; Ye, Z.; Duan, A.; Chen, X.; Sun, H.; Pan, J. Tuna trimmings (Thunnas albacares) hydrolysate alleviates immune stress and intestinal mucosal injury during chemotherapy on mice and identification of potentially active peptides. Curr. Res. Food Sci. 2023, 7, 100547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Suo, S.-K.; Zheng, S.-L.; Chi, C.-F.; Luo, H.-Y.; Wang, B. Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells. Front. Nutr. 2022, 9, 957778. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, H.L.; Chen, X.L.; Sun, C.Y.; Zhang, Y.Z.; Zhou, B.C. Purification and identification of novel angiotensin-I-converting enzyme inhibitory peptides from shark meat hydrolysate. Process Biochem. 2008, 43, 457–461. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y. Sharks: A potential source of antiangiogenic factors and tumor treatments. Mar. Biotechnol. 2002, 4, 521–525. [Google Scholar] [CrossRef]
- Kern, B.E.; Balcom, J.H.; Antoniu, B.A.; Warshaw, A.L.; Fernández-del Castillo, C. Troponin I peptide (Glu94-Leu123), a cartilage-derived angiogenesis inhibitor: In vitro and in vivo effects on human endothelial cells and on pancreatic cancer. J. Gastrointest. Surg. 2003, 7, 961–968, discussion 969. [Google Scholar] [CrossRef]
- Huang, F.J.; Lv, Z.B.; Li, Q.; Wei, L.J.; Zhang, L.; Wu, W.T. Study on hepatoprotective effect of peptide S-8300 from shark liver. World J. Gastroenterol. 2005, 11, 1809–1812. [Google Scholar] [CrossRef]
- Huang, F.; Wu, W. Antidiabetic effect of a new peptide from Squalus mitsukurii liver (S-8300) in alloxan-diabetes. Clin. Exp. Pharmacol. Physiol. 2005, 32, 521–525. [Google Scholar] [CrossRef]
- Huang, T.C.; Lee, J.F.; Chen, J.Y. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar. Drugs 2011, 9, 1995–2009. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.C.; Chen, J.Y. Pardaxin-induced apoptosis enhances antitumor activity in HeLa cells. Peptides 2011, 32, 1110–1116. [Google Scholar] [CrossRef]
- Mulero, I.; Noga, E.J.; Meseguer, J.; Garcia-Ayala, A.; Mulero, V. The antimicrobial peptides piscidins are stored in the granules of professional phagocytic granulocytes of fish and are delivered to the bacteria-containing phagosome upon phagocytosis. Dev. Comp. Immunol. 2008, 32, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Calavia, P.; González-Acosta, S.; Otazo-Pérez, A.; López, M.R.; Morales-delaNuez, A.; Pérez de la Lastra, J.M. Teleost piscidins-in silico perspective of natural peptide antibiotics from marine sources. Antibiotics 2023, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Kung, C.W.; Chen, J.Y. Antiviral activity by fish antimicrobial peptides of epinecidin-1 and hepcidin 1–5 against nervous necrosis virus in medaka. Peptides 2010, 31, 1026–1033. [Google Scholar] [CrossRef]
- Pan, C.Y.; Chen, J.Y.; Lin, T.L.; Lin, C.H. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides 2009, 30, 1058–1068. [Google Scholar] [CrossRef]
- Niu, S.F.; Jin, Y.; Xu, X.; Qiao, Y.; Wu, Y.; Mao, Y.; Su, Y.Q.; Wang, J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish Immunol. 2013, 35, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Figueras, A.; Novoa, B. A novel hepcidin-like in turbot (Scophthalmus maximus L.) highly expressed after pathogen challenge but not after iron overload. Fish Shellfish Immunol. 2012, 32, 879–889. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lee, S.C.; Rajanbabu, V.; Lin, C.H.; Chen, J.Y. Insights into the antibacterial and immunomodulatory functions of tilapia hepcidin (TH)2–3 against Vibrio vulnificus infection in mice. Dev. Comp. Immunol. 2012, 36, 166–173. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, W.J.; Lin, T.L. A fish antimicrobial peptide, tilapia hepcidin TH2–3, shows potent antitumor activity against human fibrosarcoma cells. Peptides 2009, 30, 1636–1642. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.; Chen, J.Y. Characteristics of the antitumor activities in tumor cells and modulation of the inflammatory response in RAW264.7 cells of a novel antimicrobial peptide, chrysophsin-1, from the red sea bream (Chrysophrys major). Peptides 2011, 32, 900–910. [Google Scholar] [CrossRef]
- Lin, W.J.; Chien, Y.L.; Pan, C.Y.; Lin, T.L.; Chen, J.Y.; Chiu, S.J.; Hui, C.F. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides 2009, 30, 283–290. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Doucette, C.D.; Pinto, D.M.; Patrzykat, A.; Douglas, S.; Hoskin, D.W. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011, 13, R102. [Google Scholar] [CrossRef] [PubMed]
- Rigano, F.; Arena, P.; Mangraviti, D.; Donnarumma, D.; Dugo, P.; Donato, P.; Mondello, L.; Micalizzi, G. Identification of high-value generating molecules from the wastes of tuna fishery industry by liquid chromatography and gas chromatography hyphenated techniques with automated sample preparation. J. Sep. Sci. 2021, 44, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Je, J.-Y.; Kim, S.-K. Antihypertensive effect of angiotensin I converting enzyme inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Chen, X.; Li, R.; Li, K.; Liu, S.; Li, P. Purification and identification of antioxidative peptides from mackerel (pneumatophorus japonicus) protein. RSC Adv. 2018, 8, 20488. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Lin, C.-N.; Chiou, M.-T.; Yu, C.Y.; Chen, J.-Y.; Chien, C.-H. The antimicrobial peptide pardaxin exerts potent anti-tumor activity against canine perianal gland adenoma. Oncotarget 2015, 6, 2290–2301. [Google Scholar] [CrossRef]
- Abu-Raya, S.; Bloch-Shilderman, E.; Lelkes, P.I.; Trembovler, V.; Shohami, E.; Gutman, Y.; Lazarovici, P. Characterization of pardaxin-induced dopamine release from pheochromocytoma cells: Role of calcium and eicosanoids. J. Pharmacol. Exp. Ther. 1999, 288, 399–406. [Google Scholar]
- Lazarovici, P. The structure and function of pardaxin. J. Toxicol. Toxin Rev. 2002, 21, 391–421. [Google Scholar] [CrossRef]
- Jung, W.K.; Mendis, E.; Je, J.Y.; Park, P.J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2005, 94, 26–32. [Google Scholar] [CrossRef]
- Bhusal, A.; Nam, Y.; Seo, D.; Rahman, M.H.; Hwang, E.M.; Kim, S.C.; Lee, W.H.; Suk, K. Cathelicidin-related antimicrobial peptide promotes neuroinflammation through astrocyte-microglia communication in experimental autoimmune encephalomyelitis. Glia 2022, 70, 1902–1926. [Google Scholar] [CrossRef]
- Basanez, G.; Shinnar, A.E.; Zimmerberg, J. Interaction of hagfish cathelicidin antimicrobial peptides with model lipid membranes. FEBS Lett. 2002, 532, 115–120. [Google Scholar] [CrossRef]
- Ngo, D.H.; Ryu, B.; Vo, T.S.; Himaya, S.W.A.; Wijesekara, I.; Kim, S.K. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Marnila, P.; Hiidenhovi, J.; Valimaa, A.L.; Granato, D.; Makinen, S. Baltic herring hydrolysates: Identification of peptides, in silico DPP-4 prediction, and their effects on an in vivo mice model of obesity. Food Res. Int. 2004, 191, 114696. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Isolation of peptides with angiotensin I-converting enzyme inhibitory effect derived from hydrolysate of upstream chum salmon muscle. J. Food Sci. 2003, 68, 1611–1614. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Hunag, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Matsufuji, H.; Matsui, T.; Seki, E.; Osajima, K.; Nakashima, H.; Osajima, K. Angiotensin Iconverting enzyme inhibitory peptides in an alkaline protease hydrolyzate derived from sardine muscle. Biosci. Biotechnol. Biochem. 1994, 58, 2244–2245. [Google Scholar] [CrossRef]
- Cruz, L.J.; de Santoz, V.; Zafaralla, G.C.; Ramilo, C.A.; Zeikus, R.; Gray, W.R.; Olivera, B.M. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms. J. Biol. Chem. 1987, 262, 15821–15824. [Google Scholar] [CrossRef]
- Cottrell, G.A.; Twarog, B.M. Proceedings: Active factors in the venom duct of Conus californicus. Br. J. Pharmacol. 1972, 44, 365P–366P. [Google Scholar]
- Green, B.R.; Olivera, B.M. Venom peptides from cone snails: Pharmacological Probes for Voltage-Gated Sodium Channels. Curr. Top Membr. 2016, 78, 65–86. [Google Scholar]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteom. 2019, 190, 12–20. [Google Scholar] [CrossRef]
- Rauck, R.L.; Wallac, M.S.; Leong, M.S.; Minehart, M.; Webster, L.R.; Charapata, S.G.; Abraham, J.E.; Buffington, D.E.; Ellis, D.; Kartzinel, R.; et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J. Pain Symptom Manag. 2006, 31, 393–406. [Google Scholar] [CrossRef]
- Frank, M. Natural Peptide Toxins. In Comprehensive Natural Products, 2nd ed.; Hiu, L.W., Begley, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 511–538. [Google Scholar]
- Ohizumi, Y.; Nakamura, H.; Kobayashi, J. Presynaptic inhibitory effect of geographutoxin II, a new peptide toxin from Conus geographus venom, in the guinea-pig vas deferens. Eur. J. Pharmacol. 1986, 120, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Ohizumi, Y.; Minoshima, S.; Takahashi, M.; Kajiwara, A.; Nakamura, H.; Kobayashi, J. Geographutoxin II, a novel peptide inhibitor of Na channels of skeletal muscles and autonomic nerves. J. Pharmacol. Exp. Ther. 1986, 239, 243–248. [Google Scholar] [PubMed]
- Ohizumi, Y.; Nakamura, H.; Kobayashi, J.; Catterall, W.A. Specific inhibition of [3H]saxitoxin binding to skeletal muscle sodium channels by geographutoxin II, a polypeptide channel blocker. J. Biol. Chem. 1986, 261, 6149. [Google Scholar] [CrossRef]
- Moczydlowski, E.; Olivera, B.M.; Gray, W.R.; Strichartz, G.A. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and p-conotoxins. Proc. Natl. Acad. Sci. USA 1986, 83, 5321. [Google Scholar] [CrossRef]
- Layer, R.T.; McIntosh, J.M. Conotoxins: Therapeutic Potential and Application. Mar. Drugs 2006, 4, 119–142. [Google Scholar] [CrossRef]
- Malmberg, A.B.; Gilbert, H.; McCabe, R.T.; Basbaum, A.I. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain 2003, 101, 109–116. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Mao, X.; Zhang, X. Novel peptides with anti-proliferation activity from the Porphyra haitanesis hydrolysate. Process Biochem. 2017, 60, 98–107. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.-R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Chen, M.-F.; Zhang, Y.Y.; Di He, M.; Li, C.Y.; Zhou, C.X.; Hong, P.Z.; Qian, Z.-J. Antioxidant peptide purified from enzymatic hydrolysates of isochrysis zhanjiangensis and its protective effect against ethanol induced oxidative stress of HepG2 Cells. Biotechnol. Bioprocess Eng. 2019, 24, 308–317. [Google Scholar] [CrossRef]
- Ramachandran, J. Structure, function and therapeutic potential of omega conopeptides: Novel blockers of neuronal calcium channels. Proc. Indian Acad. Sci (Chem. Sci.) 1994, 106, 1383–1387. [Google Scholar] [CrossRef]
- Nadasdi, L.; Yamashiro, D.; Chung, D.; Tarczy-Hornoch, K.; Adriaenssens, P.; Ramachandran, J. Structure-activity analysis of a conus peptide blocker of n-type neuronal calcium channels. Biochemistry 1995, 34, 8076–8081. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.; Craik, D.J.; Adams, D.J. Conotoxin modulation of voltage-gated sodium channels. Int. J. Biochem. Cell Biol. 2008, 40, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Uta, D.; Ohashi, M.; Hoshino, R.; Baba, H. Omega-conotoxin MVIIA reduces neuropathic pain after spinal cord injury by inhibiting N-type voltage-dependent calcium channels on spinal dorsal horn. Front. Neurosci. 2024, 18, 1366829. [Google Scholar] [CrossRef]
- Tombaccini, D.; Adeyemo, O.M.; Pollard, H.B.; Feuerstein, G. Monoclonal antibodies against the presynaptic calcium channel antagonist ω-conotoxin GVI A from cone snail poison. FEBS Lett. 1990, 261, 71–75. [Google Scholar] [CrossRef]
- Teta, R.; Irollo, E.; Della Sala, G.; Pirozzi, G.; Mangoni, A.; Costantino, V. Smenamides A and B, chlorinated peptide/polyketide hybrids containing a dolapyrrolidinone unit from the Caribbean sponge Smenospongia aurea. Evaluation of their role as leads in antitumor drug research. Mar. Drugs 2013, 11, 4451–4463. [Google Scholar] [CrossRef]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the Jamaicamides, New Mixed Polyketide-Peptide Neurotoxins from the Marine Cyanobacterium Lyngbya majuscule. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef]
- Caso, A.; Laurenzana, I.; Lamorte, D.; Trino, S.; Esposito, G.; Piccialli, V.; Costantino, V. Smenamide A analogues. Synthesis and biological activity on multiple myeloma cells. Mar. Drugs 2018, 16, 206. [Google Scholar] [CrossRef]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Kanamori, Y.; Ohno, O.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Ōmura, S.; et al. Janadolide, a cyclic polyketide-peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016, 79, 1862–1866. [Google Scholar] [CrossRef]
- Chung, J.H.; Tang, A.H.; Geraghty, K.; Corcillus, L.; Kaiser, M.; Payne, R.J. Total synthesis and antitripanosomal activity of janadolide and simplified analogues. Org. Lett. 2020, 22, 3089–3093. [Google Scholar] [CrossRef]
- Available online: https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/232326/2/dykkk00092.pdf (accessed on 27 September 2024).
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Okeanina sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Kaneda, M.; Kawaguchi, S.; Fujii, N.; Ohno, H.; Oishi, S. Structure-activity relationship study on odoamide: Insights into the bioactivities of aurilide-family hybrid peptide-polyketides. ACS Med. Chem. Lett. 2018, 9, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Murakami, M. Kasumigamide, an antialgal peptide from the cyanobacterium Microcystis aeruginosa. J. Org. Chem. 2000, 65, 5898–5900. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Egami, Y.; Kimura, M.; Wakimoto, T.; Abe, I. Metagenomic analysis of the sponge Discodermia reveals the production of the cyanobacterial natural product kasumigamide by ‘Entotheonella’. PLoS ONE 2016, 11, e0164468. [Google Scholar] [CrossRef]
- Seo, C.; Yim, J.H.; Lee, H.K.; Park, S.M.; Sohn, J.H.; Oh, H. Stereocalpin A, a bioactive cyclic depsipeptide from the Antarctic lichen Stereocaulon alpinum. Tetrahedron Lett. 2008, 49, 29–31. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Aknin, M.; Neumann, D.; Ben-Califa, N.; Kashman, Y. Taumycin A and B, two bioactive lipodepsipeptides from the Madagascar sponge Fascaplysinopsis sp. Org. Lett. 2008, 10, 4307–4309. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Teng, D.; Li, Z.; Wang, X.; Mao, R.; Wang, X.; Hao, Y.; Wang, J. Antibacterial and detoxifying activity of NZ17074 analogues with multi-layers of selective antimicrobial actions against Escherichia coli and Salmonella enteritidis. Sci. Rep. 2017, 7, 3392. [Google Scholar] [CrossRef]
- Li, T.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. C-terminal mini-PEGylation of a marine peptide N6 had potent antibacterial and anti-inflammatory properties against Escherichia coli and Salmonella strains in vitro and in vivo. BMC Microbiol. 2022, 22, 128. [Google Scholar] [CrossRef]
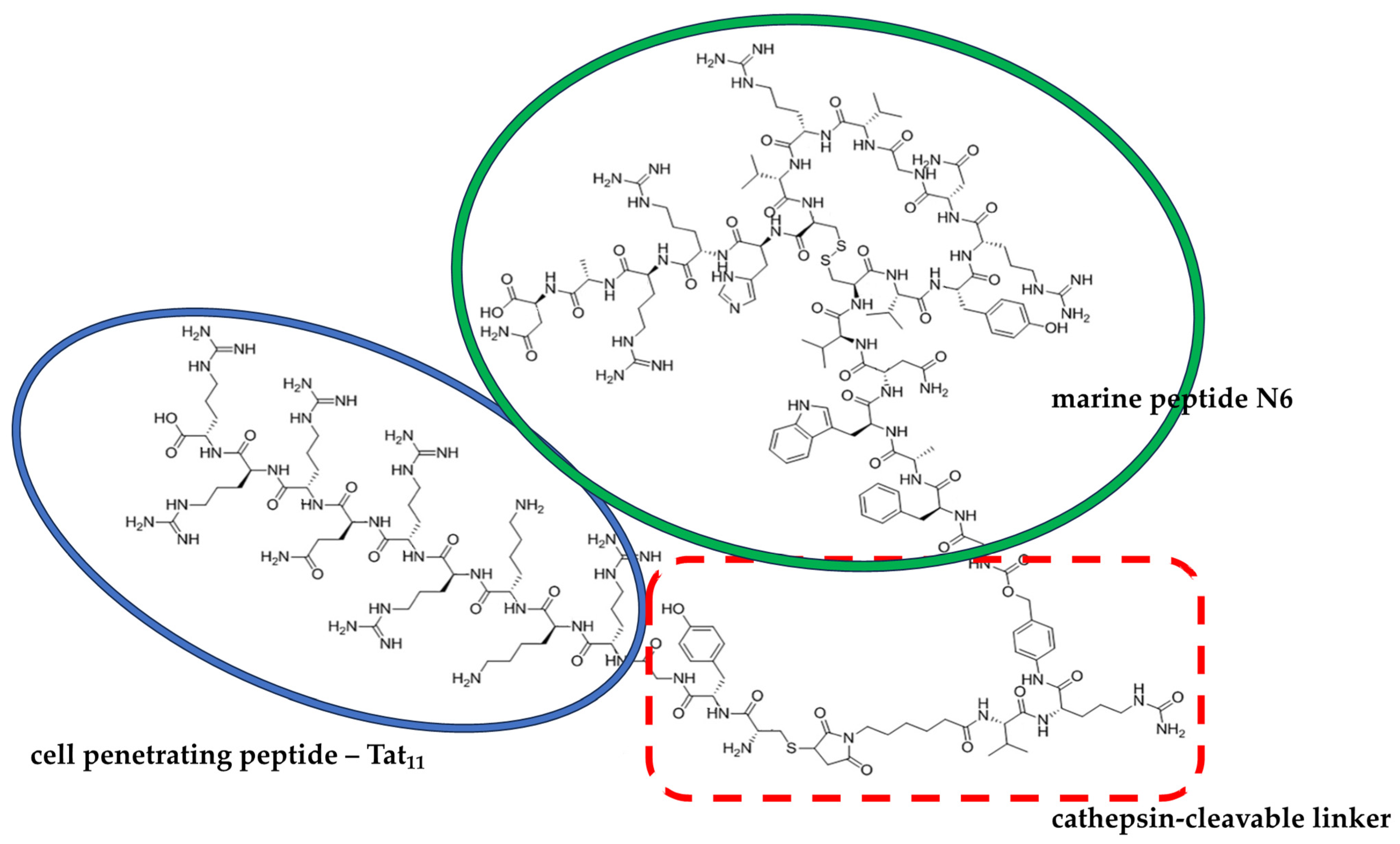
- Li, Z.; Teng, D.; Mao, R.; Wang, X.; Hao, Y.; Wang, X.; Wang, J. Improved antibacterial activity of the marine peptide N6 against intracellular salmonella typhimurium by conjugating with the cell-penetrating peptide Tat11via a cleavable linker. J. Med. Chem. 2018, 61, 7991–8000. [Google Scholar] [CrossRef]
- Hamann, M.T.; Scheuer, P.J.; Kahalalide, F. A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J. Am. Chem. Soc. 1993, 115, 5825–5826. [Google Scholar] [CrossRef]
- Kita, M.; Hirayama, Y.; Sugiyama, M.; Kigoshi, H. Development of highly cytotoxic and actin-depolymerizing biotin derivatives of Aplyronine A. Angew. Chemie 2011, 50, 9871–9874. [Google Scholar] [CrossRef]
- Piggott, A.M.; Karuso, P. Rapid identification of a protein binding partner for the marine natural product kahalalide F by using reverse chemical proteomics. ChemBioChem 2008, 9, 524–530. [Google Scholar] [CrossRef]


| Compound | Amio Acid Sequence |
|---|---|
| SNX-111 | CKGKGAKCSRLMYDCCTGSCRSGKC |
| SNX-183 | CKLKGQSCRKTSYDCCSGSCGRRRGKC |
| SNX-202 * | CKLKGQSCSRLMYDCCSGSCGRRRG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, M.; Glowacka, M.; Kamysz, W.; Kleczkowska, P. Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases. Int. J. Mol. Sci. 2024, 25, 12601. https://doi.org/10.3390/ijms252312601
Bauer M, Glowacka M, Kamysz W, Kleczkowska P. Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases. International Journal of Molecular Sciences. 2024; 25(23):12601. https://doi.org/10.3390/ijms252312601
Chicago/Turabian StyleBauer, Marta, Magdalena Glowacka, Wojciech Kamysz, and Patrycja Kleczkowska. 2024. "Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases" International Journal of Molecular Sciences 25, no. 23: 12601. https://doi.org/10.3390/ijms252312601
APA StyleBauer, M., Glowacka, M., Kamysz, W., & Kleczkowska, P. (2024). Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases. International Journal of Molecular Sciences, 25(23), 12601. https://doi.org/10.3390/ijms252312601










