Metabonomics and Transcriptomics Analyses Reveal the Underlying HPA-Axis-Related Mechanisms of Lethality in Larimichthys polyactis Exposed to Underwater Noise Pollution
Abstract
:1. Introduction
2. Results
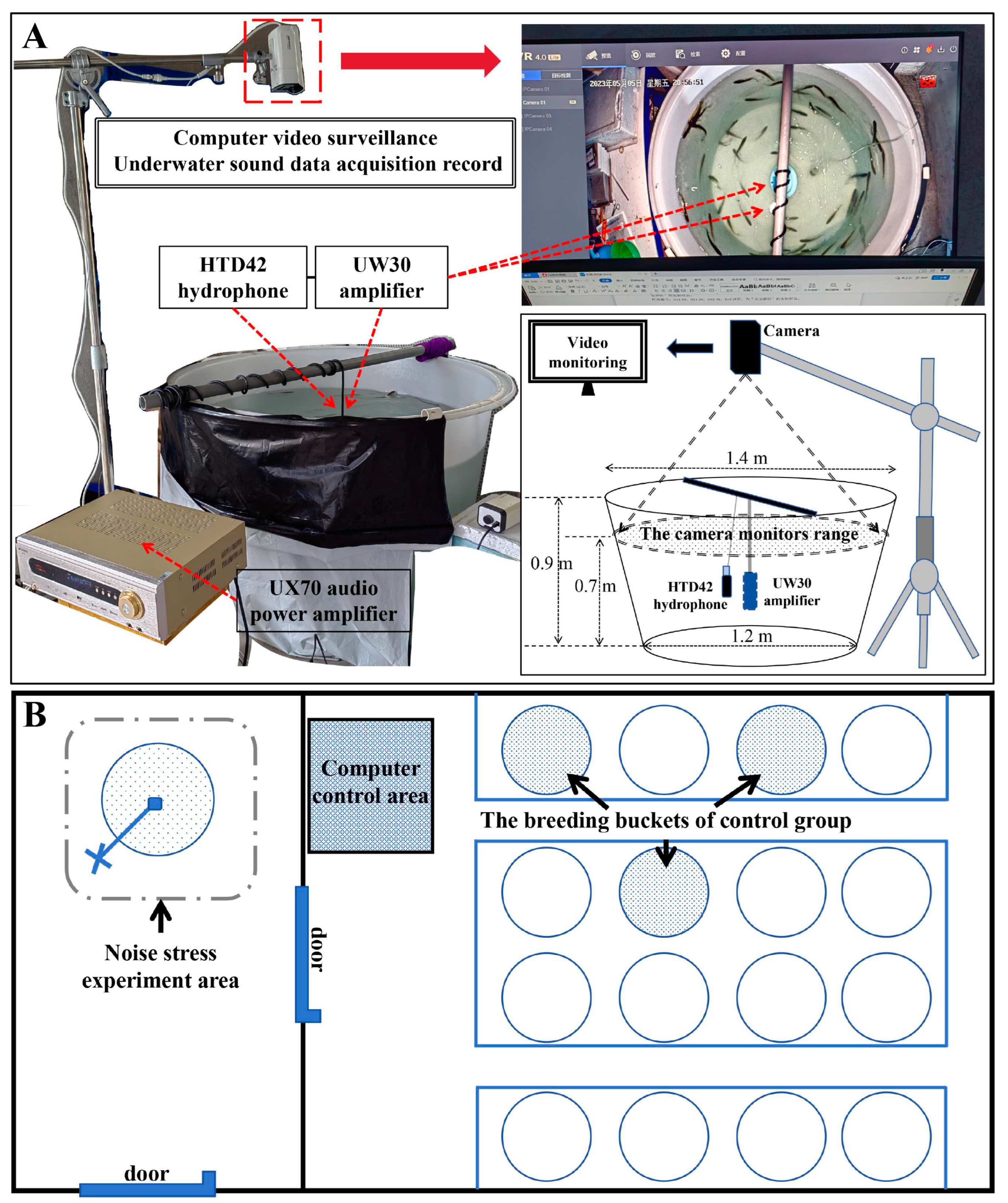
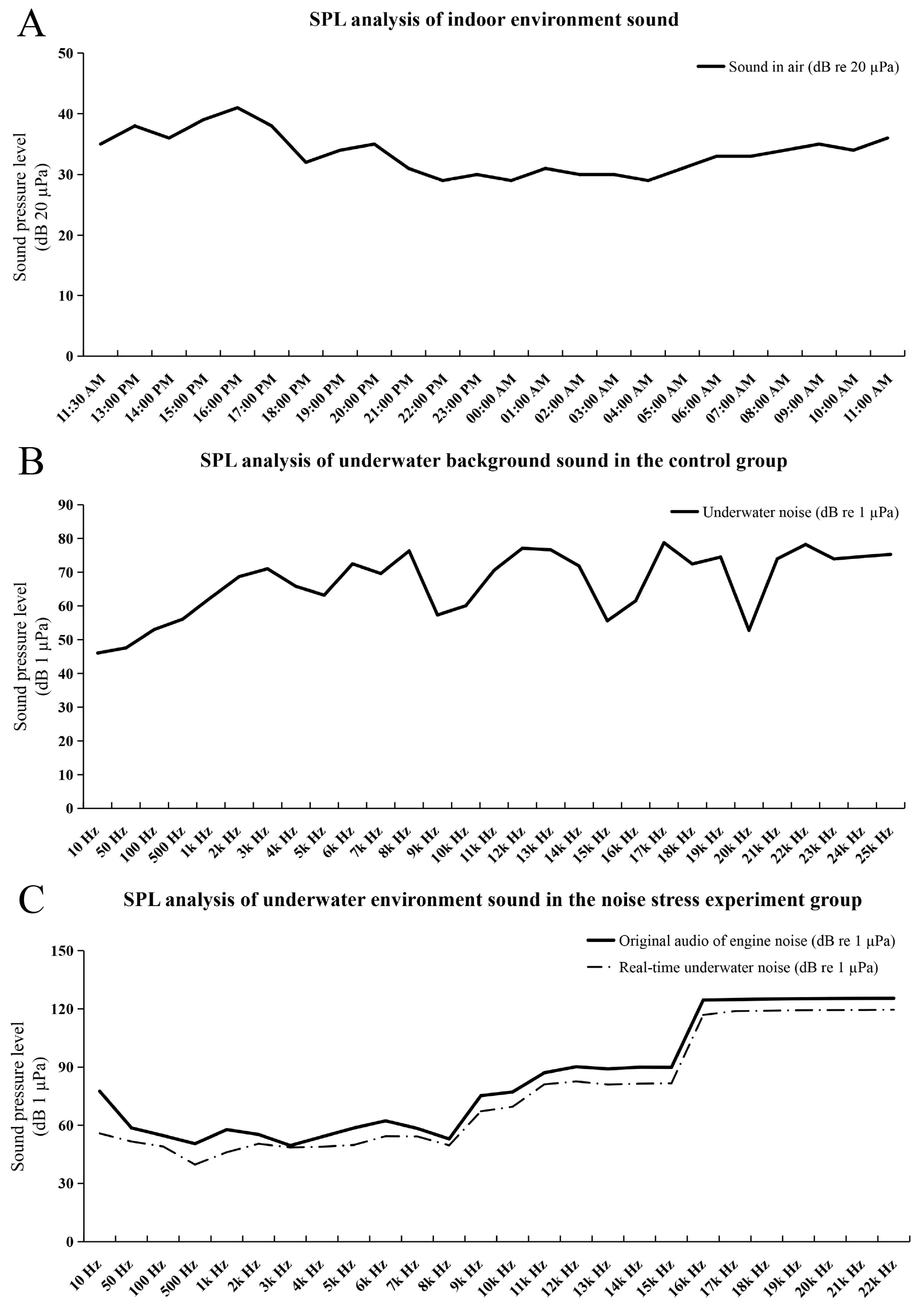
2.1. The Results of Noise Stress Experiments and Background Noise Detection
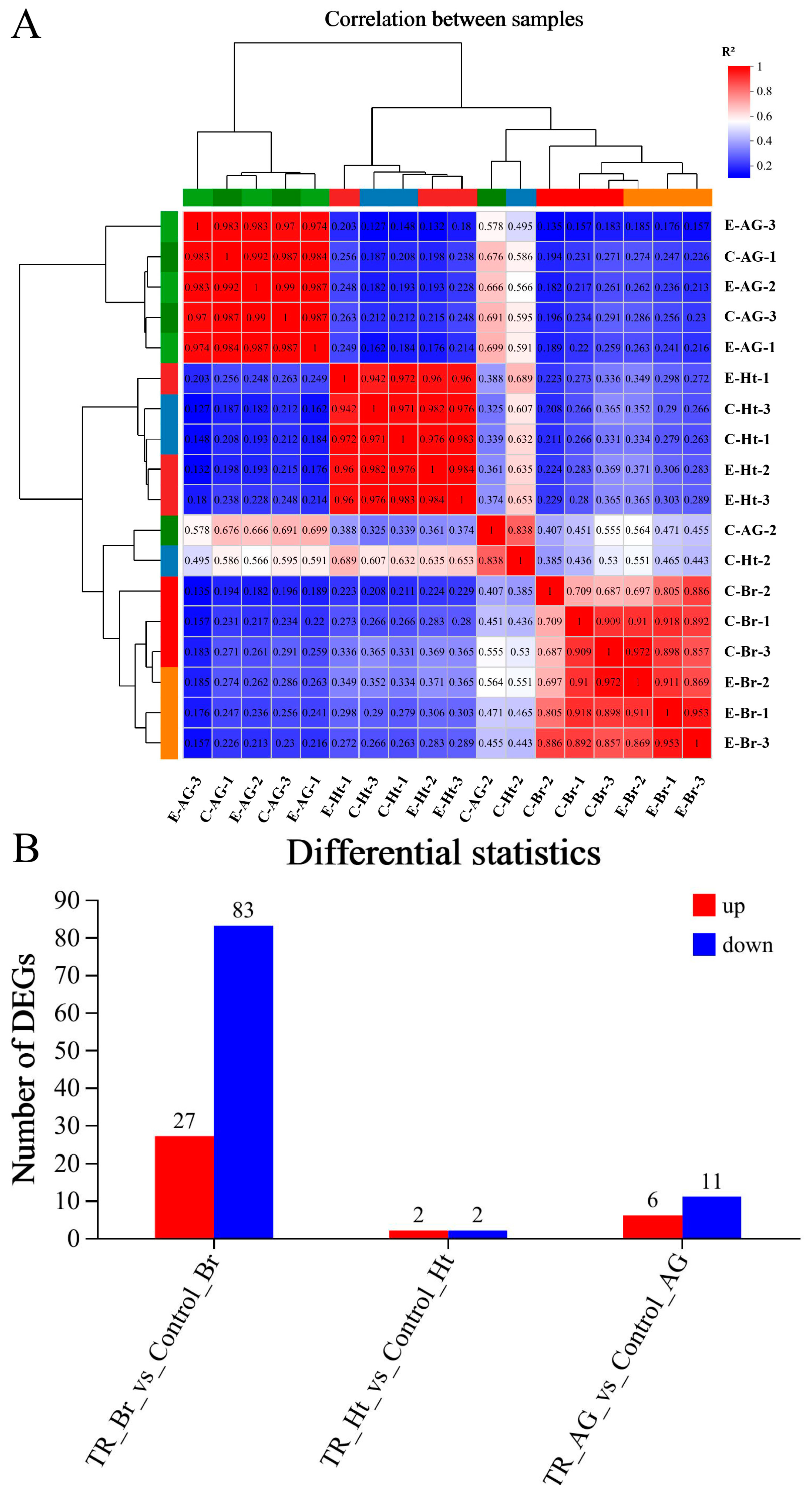
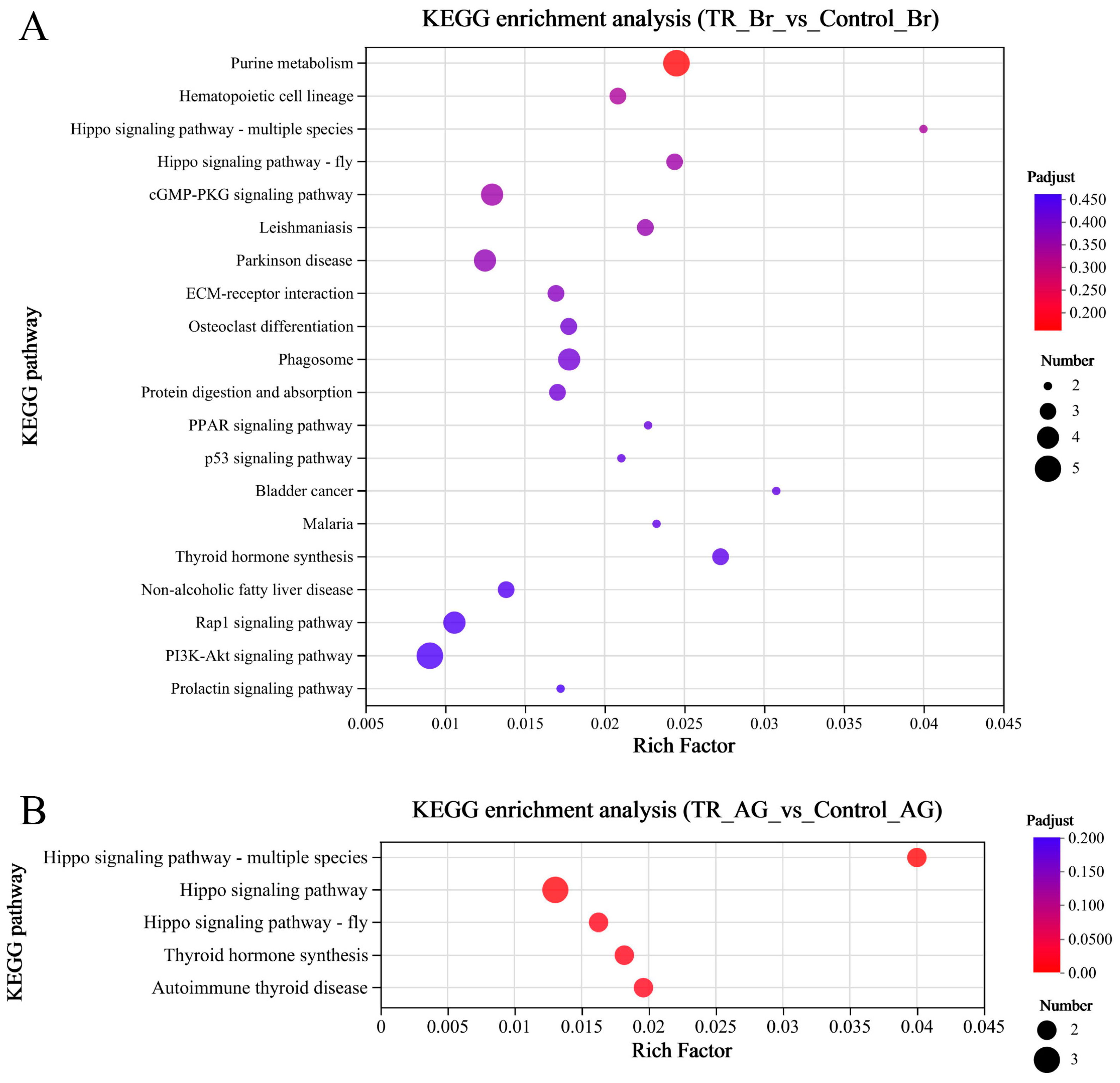
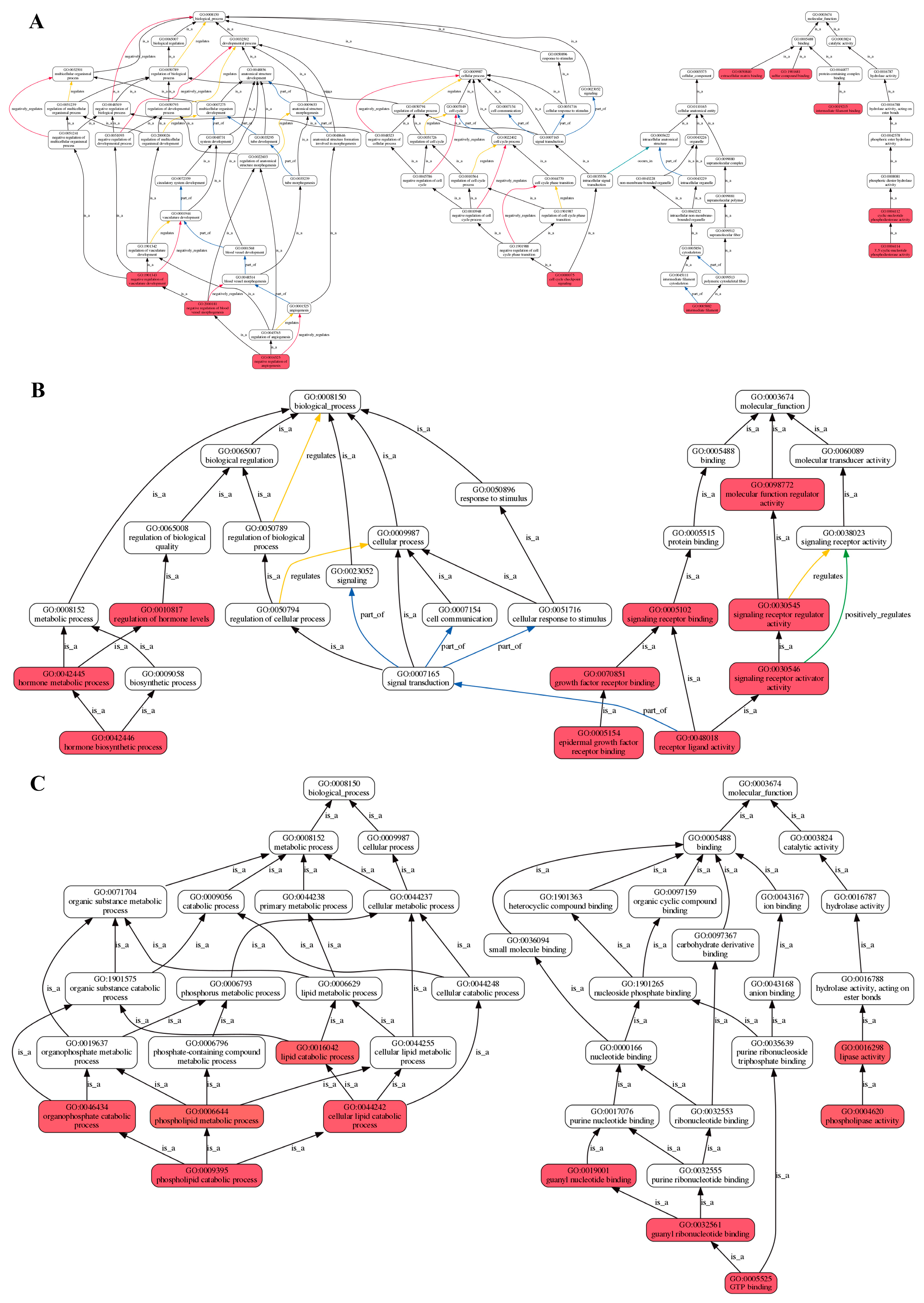
2.2. Analysis of Transcriptomic Data
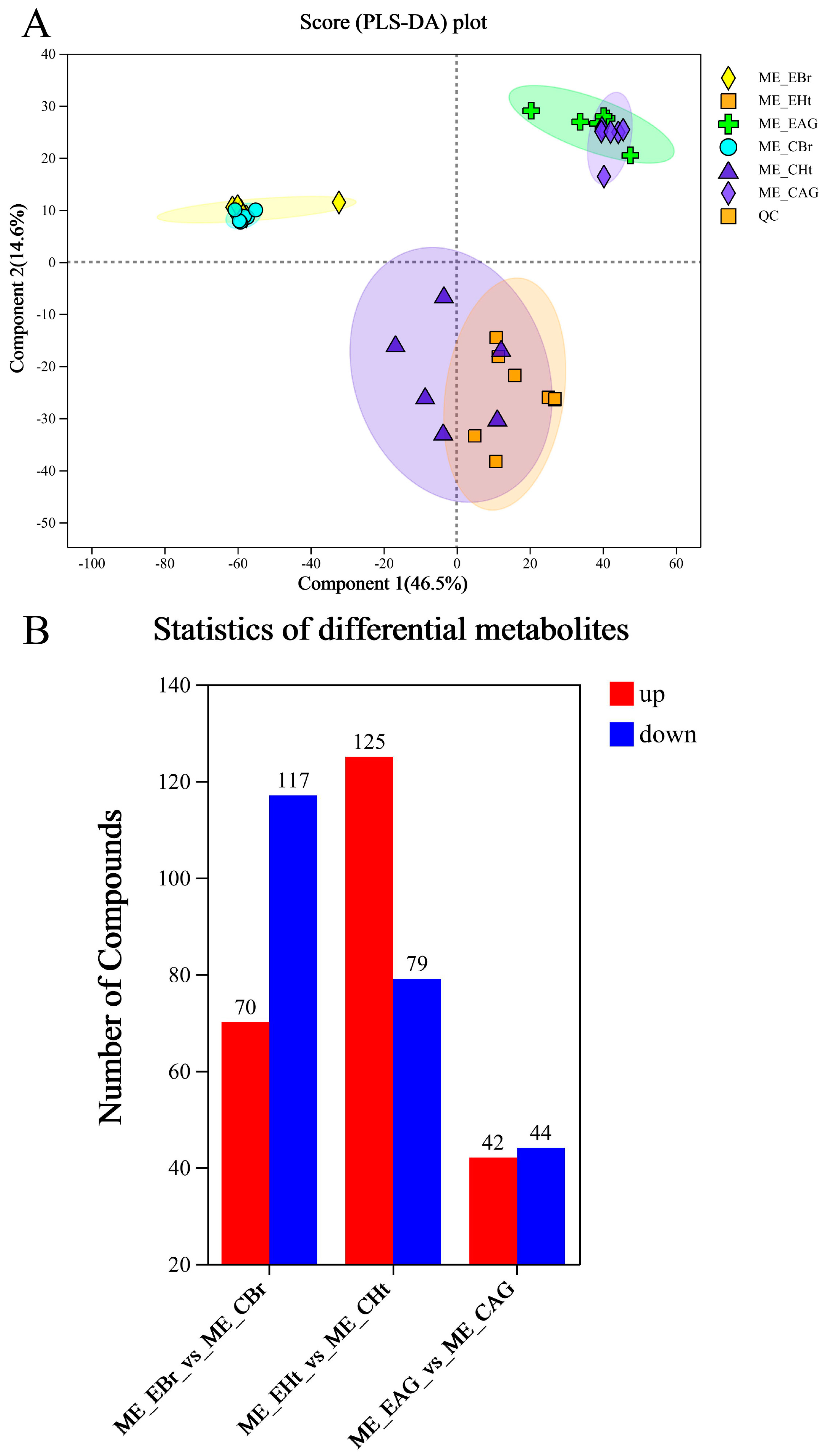
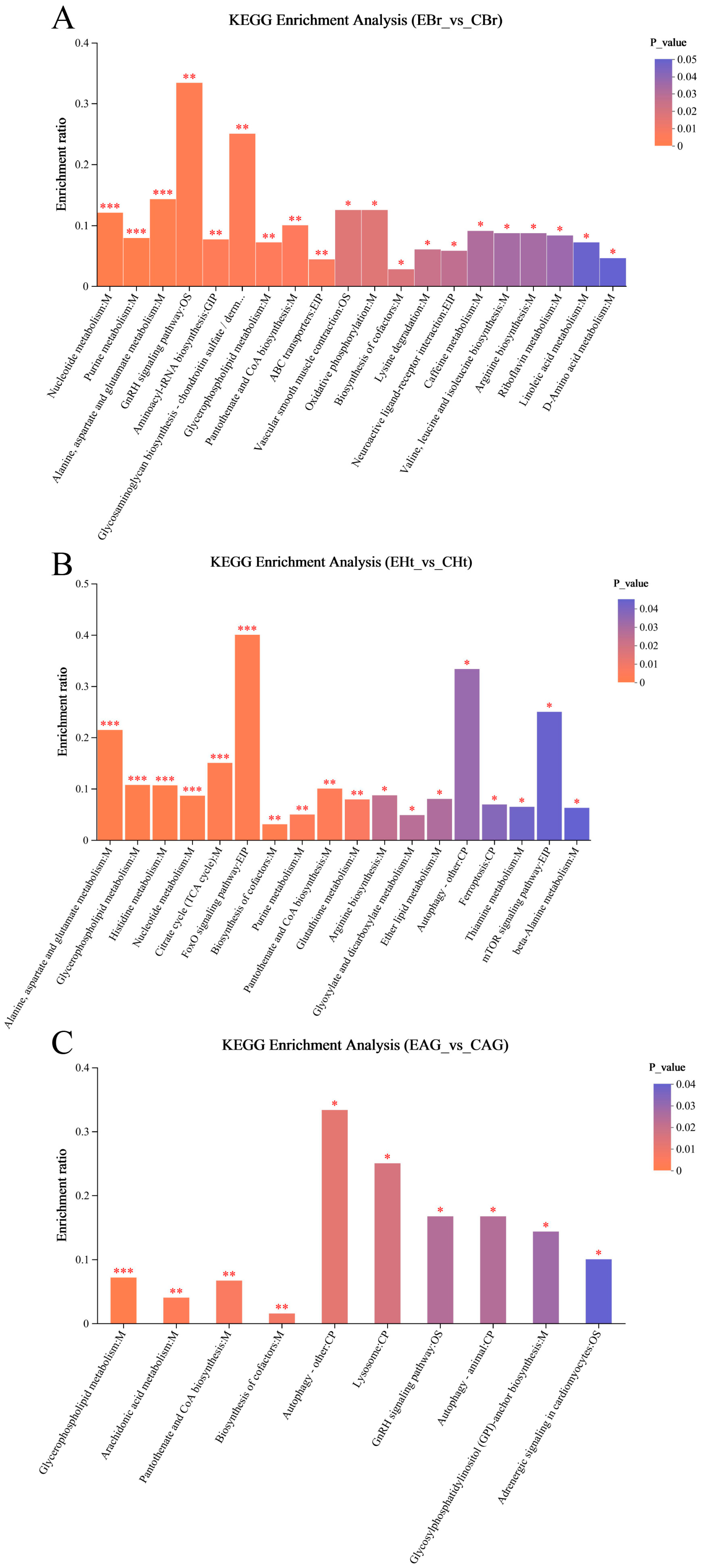
2.3. Analysis of Metabonomic Data
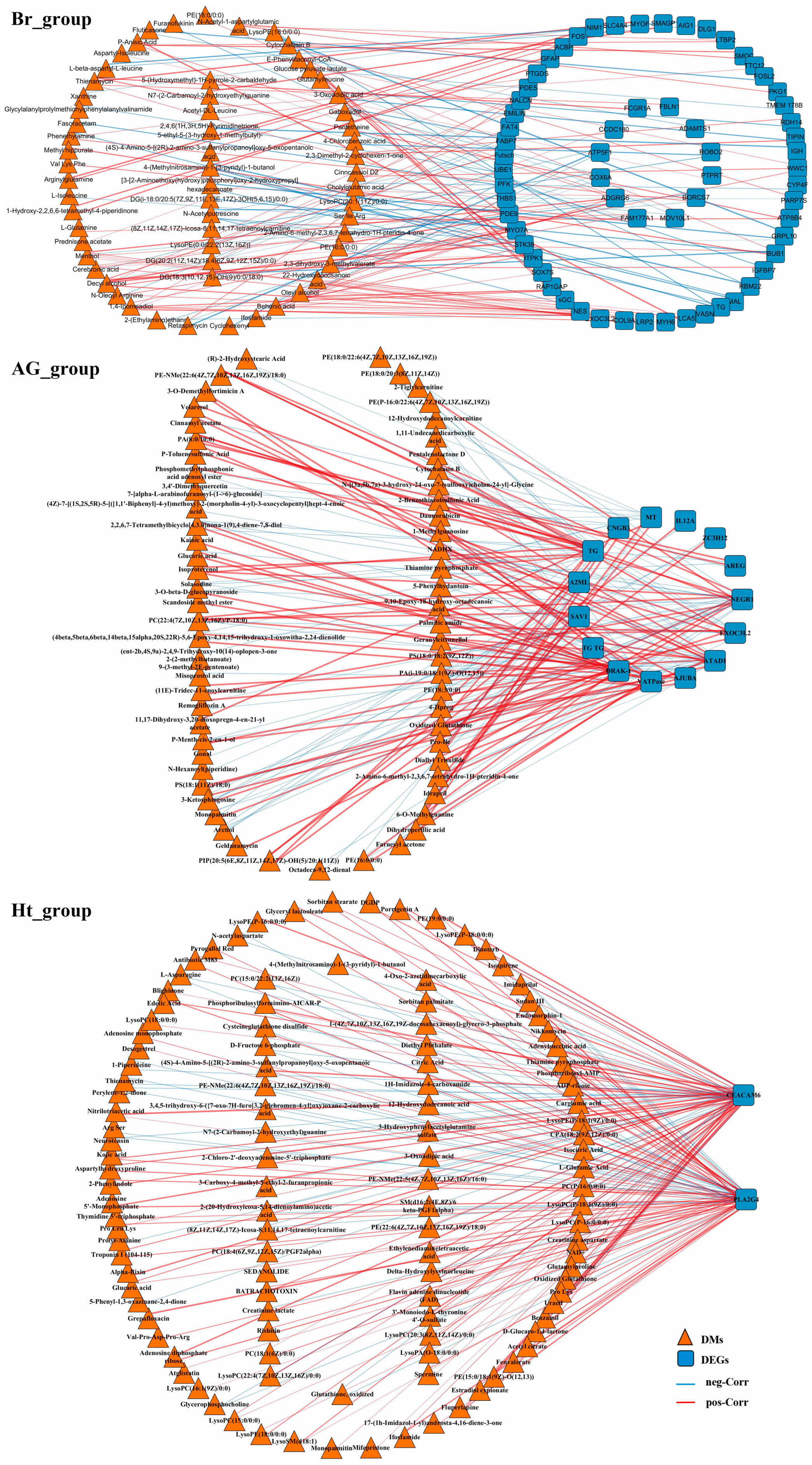
2.4. Correlation Analysis Between the Transcriptomic and Metabonomic Data
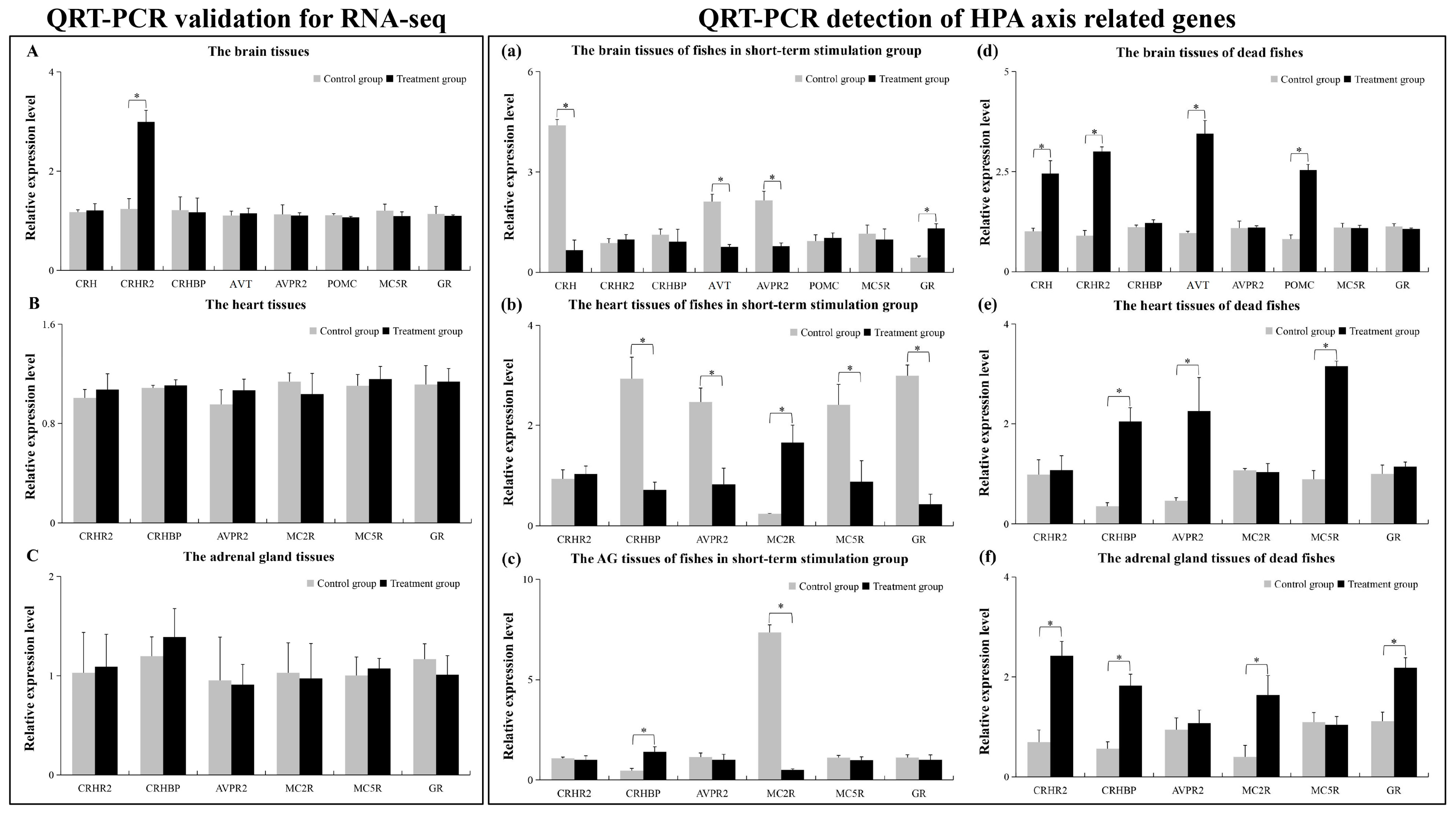
2.5. The qRT-PCR Results
3. Discussion
3.1. Strong Noise Severely Disturbed the Expression and Regulation of Important Genes
3.2. Strong-Noise-Induced Metabolic Disorders
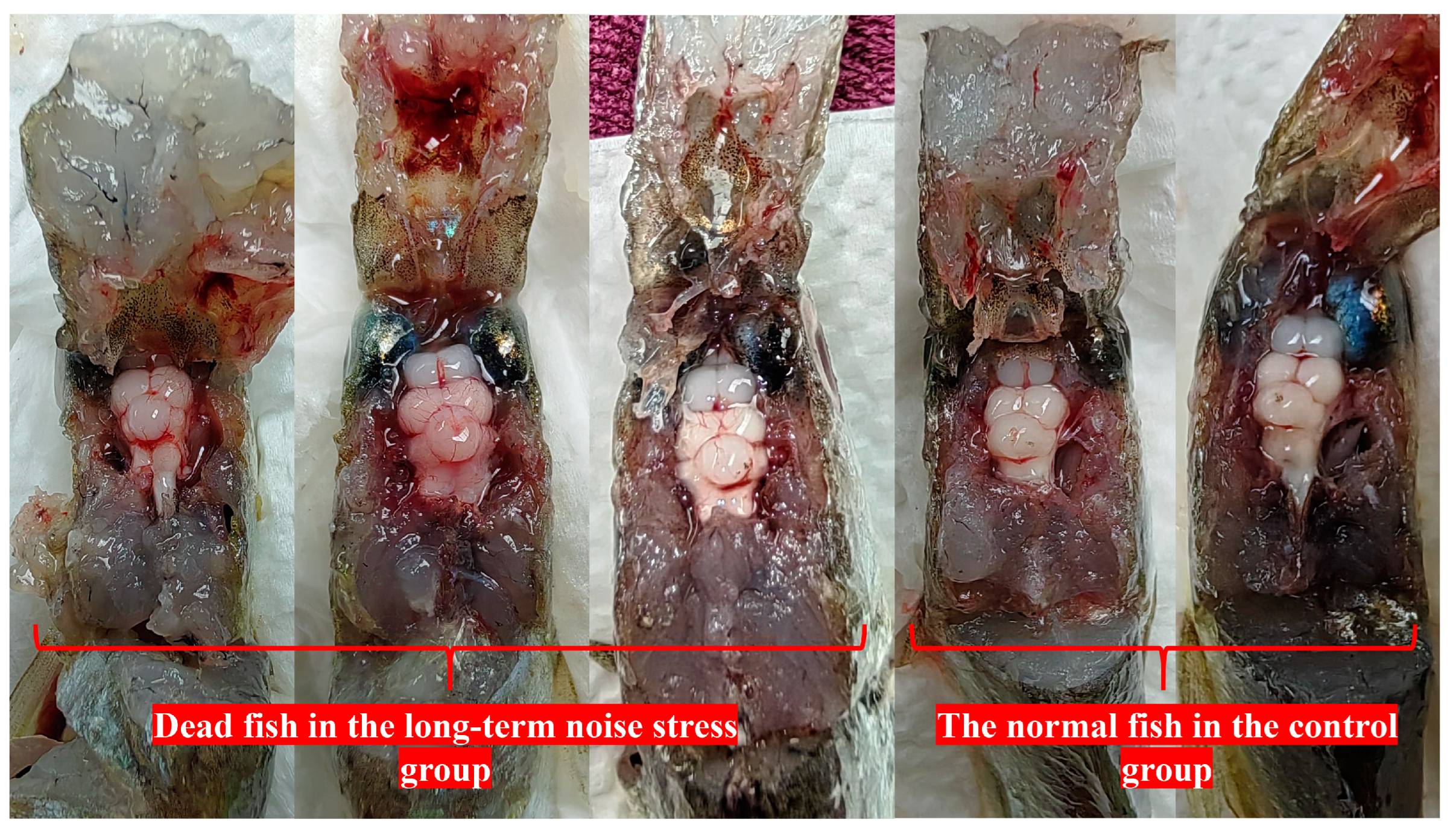
3.3. The Processes and Causes of Death Induced by Loud Noise
4. Materials and Methods
4.1. Animals
4.2. Noise Stress Experiment
4.3. Total RNA Isolation and Illumina Sequencing
4.4. Alignment of Transcriptomic Data
4.5. Functional Annotation and Pathway Enrichment Analysis
4.6. Metabolite Extraction and UPLC-MS/MS Analysis
4.7. Data Analysis and Statistics of Metabolites
4.8. Differential Identification and Pathway Enrichment Analysis of Metabolites
4.9. QRT-PCR Validation and Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chahouri, A.; Elouahmani, N.; Ouchene, H. Recent progress in marine noise pollution: A thorough review. Chemosphere 2021, 291, 132983. [Google Scholar] [CrossRef] [PubMed]
- Breeze, H.; Nolet, V.; Thomson, D.; Wright, A.J.; Marotte, E.; Sanders, M. Efforts to advance underwater noise management in Canada: Introduction to the Marine Pollution Bulletin Special Issue. Mar. Pollut. Bull. 2022, 178, 113596. [Google Scholar] [CrossRef] [PubMed]
- Erbe, C.; Marley, S.A.; Schoeman, R.P.; Smith, J.N.; Trigg, L.E.; Embling, C.B. The Effects of Ship Noise on Marine Mammals—A Review. Front. Mar. Sci. 2019, 6, 606. [Google Scholar] [CrossRef]
- Tervo, O.M.; Blackwell, S.B.; Ditlevsen, S.; Conrad, A.S.; Samson, A.L.; Garde, E.; Hansen, R.G.; Peter, H.-J.M. Narwhals react to ship noise and airgun pulses embedded in background noise. Biol. Lett. 2021, 17, 20210220. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.S.; Haxel, J.H.; Olsen, A.; Burnett, J.D.; Smith, A.; Chandler, T.E.; Nieukirk, S.L.; Larson, S.E.; Hunt, K.E.; Torres, L.G. Effects of vessel traffic and ocean noise on gray whale stress hormones. Sci. Rep. 2022, 12, 18580. [Google Scholar] [CrossRef]
- Kyhn, L.A.; Sveegaard, S.; Tougaard, J. Underwater noise emissions from a drillship in the Arctic. Mar. Pollut. Bull. 2014, 86, 424–433. [Google Scholar] [CrossRef]
- Tougaard, J.; Hermannsen, L.; Madsen, P.T. How loud is the underwater noise from operating offshore wind turbines? J. Acoust. Soc. Am. 2020, 148, 2885–2893. [Google Scholar] [CrossRef]
- Scholik, A.R.; Yan, H.Y. Effects of Boat Engine Noise on the Auditory Sensitivity of the Fathead Minnow, Pimephales promelas. Environ. Biol. Fishes 2002, 63, 203–209. [Google Scholar] [CrossRef]
- Putland, R.L.; Merchant, N.D.; Farcas, A.; Radford, C.A. Vessel noise cuts down communication space for vocalizing fish and marine mammals. Glob. Chang. Biol. 2017, 24, 1708–1721. [Google Scholar] [CrossRef]
- Di Franco, E.; Pierson, P.; Di Iorio, L.; Calò, A.; Cottalorda, J.; Derijard, B.; Di Franco, A.; Galvé, A.; Guibbolini, M.; Lebrun, J.; et al. Effects of marine noise pollution on Mediterranean fishes and invertebrates: A review. Mar. Pollut. Bull. 2020, 159, 111450. [Google Scholar] [CrossRef]
- Gendron, G.; Tremblay, R.; Jolivet, A.; Olivier, F.; Chauvaud, L.; Winkler, G.; Audet, C. Anthropogenic boat noise reduces feeding success in winter flounder larvae (Pseudopleuronectes americanus). Environ. Biol. Fishes 2020, 103, 1079–1090. [Google Scholar] [CrossRef]
- Van der Knaap, I.; Ashe, E.; Hannay, D.; Bergman, A.G.; Nielsen, K.A.; Lo, C.F.; Williams, R. Behavioural responses of wild Pacific salmon and herring to boat noise. Mar. Pollut. Bull. 2022, 174, 113257. [Google Scholar] [CrossRef] [PubMed]
- Weilgart, L. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Popper, A.N.; Hastings, M. The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 2009, 75, 455–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-T.; Akamatsu, T.; Nowacek, D.P.; Yuan, J.; Zhou, L.; Lei, P.-Y.; Li, J.; Duan, P.-X.; Wang, K.-X.; Wang, D. Soundscape of an Indo-Pacific humpback dolphin (Sousa chinensis) hotspot before windfarm construction in the Pearl River Estuary, China: Do dolphin engage in noise avoidance and passive eavesdropping behavior? Mar. Pollut. Bull. 2019, 140, 509–522. [Google Scholar] [CrossRef]
- Kusku, H.; Yigit, U.; Yilmaz, S.; Yigit, M.; Ergün, S. Acoustic effects of underwater drilling and piling noise on growth and physiological response of Nile tilapia (Oreochromis niloticus). Aquac. Res. 2020, 51, 3166–3174. [Google Scholar] [CrossRef]
- Mills, S.C.; Beldade, R.; Henry, L.; Laverty, D.; Nedelec, S.L.; Simpson, S.D.; Radford, A.N. Hormonal and behavioural effects of motorboat noise on wild coral reef fish. Environ. Pollut. 2020, 262, 114250. [Google Scholar] [CrossRef]
- Martin, M.; Gridley, T.; Elwen, S.H.; Charrier, I. Assessment of the impact of anthropogenic airborne noise on the behaviour of Cape fur seals during the breeding season in Namibia. J. Exp. Mar. Biol. Ecol. 2022, 550. [Google Scholar] [CrossRef]
- Tu, Z.; Tang, L.; Khan, F.U.; Hu, M.; Shen, H.; Wang, Y. Low-frequency noise impairs righting reflex behavior by disrupting central nervous system in the sea slug Onchidium reevesii. Sci. Total Environ. 2024, 918, 170552. [Google Scholar] [CrossRef]
- Cox, T.M.; Ragen, T.J.; Read, A.J.; Vos, E.; Baird, R.W.; Balcomb, K.; Barlow, J.; Caldwell, J.; Cranford, T.; Crum, L.; et al. Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manag. 2005, 7, 177–187. [Google Scholar] [CrossRef]
- Cox, K.; Brennan, L.P.; Gerwing, T.G.; Dudas, S.E.; Juanes, F. Sound the alarm: A meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob. Chang. Biol. 2018, 24, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Chen, C.-F.; Chuah, Y.-C.; Zhuang, C.-R.; Chen, I.-H.; Mooney, T.A.; Stott, J.; Blanchard, M.; Jen, I.-F.; Chou, L.-S. Anthropogenic Sound Exposure-Induced Stress in Captive Dolphins and Implications for Cetacean Health. Front. Mar. Sci. 2021, 8, 606736. [Google Scholar] [CrossRef]
- Ortiz, R.M.; Worthy, G.A. Effects of capture on adrenal steroid and vasopressin concentrations in free-ranging bottlenose dolphins (Tursiops truncatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 125, 317–324. [Google Scholar] [CrossRef] [PubMed]
- A Romano, T.; Keogh, M.J.; Kelly, C.; Feng, P.; Berk, L.; E Schlundt, C.; A Carder, D.; Finneran, J.J. Anthropogenic sound and marine mammal health: Measures of the nervous and immune systems before and after intense sound exposure. Can. J. Fish. Aquat. Sci. 2004, 61, 1124–1134. [Google Scholar] [CrossRef]
- Rolland, R.M.; McLellan, W.A.; Moore, M.J.; Harms, C.A.; Burgess, E.A.; Hunt, K.E. Fecal glucocorticoids and anthropogenic injury and mortality in North Atlantic right whales Eubalaena glacialis. Endanger. Species Res. 2017, 34, 417–429. [Google Scholar] [CrossRef]
- Smith, G.W.; Aubry, J.-M.; Dellu, F.; Contarino, A.; Bilezikjian, L.M.; Gold, L.H.; Chen, R.; Marchuk, Y.; Hauser, C.; A Bentley, C.; et al. Corticotropin Releasing Factor Receptor 1–Deficient Mice Display Decreased Anxiety, Impaired Stress Response, and Aberrant Neuroendocrine Development. Neuron 1998, 20, 1093–1102. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metabolism 2002, 51, 5–10. [Google Scholar] [CrossRef]
- Chen, W.; Zhen, J.T.; Tan, T. Review of the regulatory mechanisms of stress responses in animals. Swine Ind. Sci. 2021, 38, 35–38. [Google Scholar] [CrossRef]
- Solomon-Lane, T.K.; Hofmann, H.A. Early-life social environment alters juvenile behavior and neuroendocrine function in a highly social cichlid fish. Horm. Behav. 2019, 115, 104552. [Google Scholar] [CrossRef]
- Davidson, J.; Bebak, J.; Mazik, P. The effects of aquaculture production noise on the growth, condition factor, feed conversion, and survival of rainbow trout, Oncorhynchus mykiss. Aquaculture 2009, 288, 337–343. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Simpson, S.D.; Morley, E.L.; Nedelec, B.; Radford, A.N. Impacts of regular and random noise on the behaviour, growth and development of larval Atlantic cod (Gadus morhua). Proc. R. Soc. B Biol. Sci. 2015, 282, 20151943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liang, X.; Ye, T.; Zhang, Y.; Lou, B. Metabonomics and Transcriptomics Analyses Reveal the Development Process of the Auditory System in the Embryonic Development Period of the Small Yellow Croaker Under Background Noise. Int. J. Mol. Sci. 2024, 25, 1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, G.; Zhao, L.; Yang, Q.; Gao, T. Assessment of fishery resources using environmental DNA: Small yellow croaker (Larimichthys polyactis) in East China Sea. PLoS ONE 2020, 15, e0244495. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Xu, W.; Zhan, W.; Zou, H.; Lin, J. Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure. Animals 2022, 12, 2061. [Google Scholar] [CrossRef]
- Xie, Q.; Zhan, W.; Shi, J.; Liu, F.; Niu, B.; He, X.; Liu, M.; Wang, J.; Liang, Q.; Xie, Y.; et al. Whole-genome assembly and annotation for the little yellow croaker (Larimichthys polyactis) provide insights into the evolution of hermaphroditism and gonochorism. Mol. Ecol. Resour. 2023, 23, 632–658. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A.; Olsen, M.L. AP-1 and the injury response of the GFAP gene. J. Neurosci. Res. 2018, 97, 149–161. [Google Scholar] [CrossRef]
- Demircan, K.; Yonezawa, T.; Takigawa, T.; Topcu, V.; Erdogan, S.; Ucar, F.; Armutcu, F.; Yigitoglu, M.R.; Ninomiya, Y.; Hirohata, S. ADAMTS1, ADAMTS5, ADAMTS9 and aggrecanase-generated proteoglycan fragments are induced following spinal cord injury in mouse. Neurosci. Lett. 2013, 544, 25–30. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Li, L.; Yao, L.; Ma, Z.; Li, J. ADAM10- and γ-secretase-dependent cleavage of the transmembrane protein PTPRT attenuates neurodegeneration in the mouse model of Alzheimer’s disease. FASEB J. 2022, 37, e22734. [Google Scholar] [CrossRef]
- Cao, W.; Ji, Z.; Zhu, S.; Wang, M.; Sun, R. Bioinformatic identification and experiment validation reveal 6 hub genes, promising diagnostic and therapeutic targets for Alzheimer’s disease. BMC Med. Genom. 2024, 17, 6. [Google Scholar] [CrossRef]
- Iacomino, M.; Doliana, R.; Marchese, M.; Capuano, A.; Striano, P.; Spessotto, P.; Bosisio, G.; Iodice, R.; Manganelli, F.; Lanteri, P.; et al. Distal motor neuropathy associated with novel EMILIN1 mutation. Neurobiol. Dis. 2020, 137, 104757. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Kong, Y.; Zou, Z.; Chen, L.; Liang, G. Long non-coding RNA MIR17HG impedes FOSL2-mediated transcription activation of HIC1 to maintain a pro-inflammatory phenotype of microglia during intracerebral haemorrhage. Eur. J. Neurosci. 2023, 58, 4107–4122. [Google Scholar] [CrossRef] [PubMed]
- Coscia, F.; Taler-Verčič, A.; Chang, V.T.; Sinn, L.; O’reilly, F.J.; Izoré, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D.; et al. The structure of human thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Pabbidi, M.R.; Ji, X.; Maxwell, J.T.; Mignery, G.A.; Samarel, A.M.; Lipsius, S.L. Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP3-Dependent Ca2+ Signaling in Atrial Myocytes. PLoS ONE 2016, 11, e0168505. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-P.; Cheng, M.-L.; Wang, C.-H.; Huang, S.-S.; Hsieh, P.-S.; Chang, C.-C.; Kuo, C.-Y.; Chen, K.-H.; Hung, L.-M. Therapeutic potential of cPLA2 inhibitor to counteract dilated-cardiomyopathy in cholesterol-treated H9C2 cardiomyocyte and MUNO rat. Pharmacol. Res. 2020, 160, 105201. [Google Scholar] [CrossRef]
- Bonetti, A.; Allegri, L.; Baldan, F.; Contin, M.; Battistella, C.; Damante, G.; Marchini, M.; Ortolani, F. Critical Involvement of Calcium-Dependent Cytosolic Phospholipase A2α in Aortic Valve Interstitial Cell Calcification. Int. J. Mol. Sci. 2020, 21, 6398. [Google Scholar] [CrossRef]
- Parra, L.G.; Erjavec, L.C.; Casali, C.I.; Velazquez, A.Z.; Weber, K.; Setton-Avruj, C.P.; Tome, M.d.C.F. Cytosolic phospholipase A2 regulates lipid homeostasis under osmotic stress through PPARγ. FEBS J. 2023, 291, 722–743. [Google Scholar] [CrossRef]
- Song, C.Y.; Khan, N.S.; Liao, F.-F.; Wang, B.; Shin, J.S.; Bonventre, J.V.; Malik, K.U. Brain Cytosolic Phospholipase A2α Mediates Angiotensin II-Induced Hypertension and Reactive Oxygen Species Production in Male Mice. Am. J. Hypertens. 2018, 31, 622–629. [Google Scholar] [CrossRef]
- Diaconu, A.; Coculescu, B.-I.; Manole, G.; Vultur, H.; Coculescu, E.C.; Stocheci, C.M.; Tudorache, I.-S.; Dincă, A.-L.; Dincă, V.G. Lipoprotein-associated phospholipase A2 (Lp-PLA2)—Possible diagnostic and risk biomarker in chronic ischaemic heart disease. J. Enzym. Inhib. Med. Chem. 2020, 36, 68–73. [Google Scholar] [CrossRef]
- Deng, S.; Guo, A.; Huang, Z.; Guan, K.; Zhu, Y.; Chan, C.; Gui, J.; Song, C.; Li, X. The exploration of neuroinflammatory mechanism by which CRHR2 deficiency induced anxiety disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 128, 110844. [Google Scholar] [CrossRef]
- Coste, S.C.; Kesterson, R.A.; Heldwein, K.A.; Stevens, S.L.; Heard, A.D.; Hollis, J.H.; Murray, S.E.; Hill, J.K.; Pantely, G.A.; Hohimer, A.R.; et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000, 24, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.R.; Kulkarni, P.; Cai, X.; Iriah, S.; Aggarwal, D.; Lu, S.-F.; Simon, N.G.; Madularu, D.; Ferris, C.F. Treating head injury using a novel vasopressin 1a receptor antagonist. Neurosci. Lett. 2020, 714, 134565. [Google Scholar] [CrossRef] [PubMed]
- Pittman, Q.J. Vasopressin and central control of the cardiovascular system: A 40-year retrospective. J. Neuroendocr. 2021, 33, e13011. [Google Scholar] [CrossRef] [PubMed]
- Harno, E.; Ramamoorthy, T.G.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef] [PubMed]
- Stojiljković, A.S.; Čupić, Ž.; Maćešić, S.; Ivanović-Šašić, A.; Kolar-Anić, L. Influence of arginine vasopressin on the ultradian dynamics of Hypothalamic-Pituitary-Adrenal axis. Front. Endocrinol. 2022, 13, 976323. [Google Scholar] [CrossRef]
- Fam, B.S.d.O.; Vargas-Pinilla, P.; Paré, P.; Landau, L.; Viscardi, L.H.; Pissinatti, A.; Falótico, T.; Maestri, R.; Bortolini, M.C. Exploring the diversity of AVPR2 in Primates and its evolutionary implications. Genet. Mol. Biol. 2023, 46, e20230045. [Google Scholar] [CrossRef]
- Fridmanis, D.; Roga, A.; Klovins, J. ACTH Receptor (MC2R) Specificity: What Do We Know About Underlying Molecular Mechanisms? Front. Endocrinol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, X.; Zhou, R.; Gong, R. Melanocortin 5 receptor signaling pathway in health and disease. Cell. Mol. Life Sci. 2020, 77, 3831–3840. [Google Scholar] [CrossRef]
- Xia, H.; Wang, H.; Fu, L.; Wang, S.; Li, L.; Ru, G.; He, X.; Tong, X.; Mou, X.; Huang, D. Decreased CRHBP expression is predictive of poor prognosis in patients with hepatocellular carcinoma. Oncol. Lett. 2018, 16, 3681–3689. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, T.-N.; Knight, J.K.; Goodwin, J.E. The Glucocorticoid Receptor in Cardiovascular Health and Disease. Cells 2019, 8, 1227. [Google Scholar] [CrossRef]
- Sarkar, C.; Lipinski, M.M. Glycerophospholipid dysregulation after traumatic brain injury. Neurochem. Int. 2024, 175, 105701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Zan, J.; Wu, C.; Tan, W. Untargeted lipidomics reveals progression of early Alzheimer’s disease in APP/PS1 transgenic mice. Sci. Rep. 2020, 10, 14509. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Hai, W.; Chen, S.; Zhang, M.; Jiang, X.; Tang, H. Multi-omics data reveals aberrant gut microbiota-host glycerophospholipid metabolism in association with neuroinflammation in APP/PS1 mice. Gut Microbes 2023, 15, 2282790. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Mu, J. Alteration of Glycerophospholipid Metabolism in Hippocampus of Post-stroke Depression Rats. Neurochem. Res. 2022, 47, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Wang, T.; Cai, W.; Hua, T.; Duan, J.; Zhang, X.; Zhu, Y.; Yao, L. Metabolic changes of glycerophospholipids during the reparative phase after myocardial infarction injury. Front. Cardiovasc. Med. 2023, 10, 1122571. [Google Scholar] [CrossRef]
- Qi, T.; Wu, L.; Yu, J.; Song, Z.; Liu, F.; Li, J.; Song, X.; Li, X. Acute low-dose phosphate disrupts glycerophospholipid metabolism and induces stress in juvenile turbot (Scophthalmus maximus). Sci. Total. Environ. 2022, 861, 160430. [Google Scholar] [CrossRef]
- A Miranda, R.; Torrezan, R.; de Oliveira, J.C.; Barella, L.F.; Franco, C.C.d.S.; Lisboa, P.C.; Moura, E.G.; Mathias, P.C.F. HPA axis and vagus nervous function are involved in impaired insulin secretion of MSG-obese rats. J. Endocrinol. 2016, 230, 27–38. [Google Scholar] [CrossRef]
- Cai, H.L.; Jiang, P.; Tan, Q.Y.; Dang, R.L.; Tang, M.M.; Xue, Y.; Deng, Y.; Zhang, B.K.; Fang, P.F.; Xu, P.; et al. Therapeutic efficacy of atypical antipsychotic drugs by targeting multiple stress-related metabolic pathways. Transl. Psychiatry 2017, 7, e1130. [Google Scholar] [CrossRef]
- Głombik, K.; Detka, J.; Budziszewska, B. Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease. Cells 2021, 10, 2937. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-Demiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Ertuglu, L.A.; Elijovich, F.; Laffer, C.L.; Kirabo, A. Salt-Sensitivity of Blood Pressure and Insulin Resistance. Front. Physiol. 2021, 12, 793924. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Klopfenstein, D.; Tang, H.; Ramirez, F.; Botvinnik, O.; Pedersen, B.; Flick, P.; Sato, K.; Mungall, C.; Schmitt, U.; Stupp, G.; et al. GOATOOLS: Tools for Gene Ontology. Zenodo. 2015. Available online: https://zenodo.org/records/819475 (accessed on 12 July 2023).
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Experiment Type | Stimulation Time | Dead Fish/Total | Occurrence Time |
|---|---|---|---|
| Short-term stimulation group | 10 min (start at 16:02 p.m. on 4 May 2023) | No deaths | / |
| Control group (short-term experiment) | no treatment | No deaths | / |
| Long-term stimulation group | 6 days (start at 13:54 p.m. on 6 May 2023) | 8/30 | 7 May 2023, at 9:17 a.m., 2 dead fishes; 7 May 2023, at 14:31 p.m., 1 dead fish; 7 May 2023, at 19:12 p.m., 1 dead fish; 7 May 2023, at 20:25 p.m., 1 dead fish; 8 May 2023, at 12:14 p.m., 1 dead fish; 8 May 2023, at 17:40 p.m., 1 dead fish; 10 May 2023, at 9:30 a.m., 1 dead fish |
| Control group (long-term experiment) | no treatment | 1/30 | May 10, 2023, at 7:28 a.m., 1 dead fish |
| Gene | Br Group | AG Group | Ht Group | Sequence_ID |
|---|---|---|---|---|
| CRH | no | down | / | / | evm.TU.Scaffold11.445 |
| CRHR2 | yes | up | no | down | no | down | evm.TU.Scaffold165.70 |
| CRHBP | no | up | no | up | no | up | evm.TU.Scaffold2659.208 |
| AVT | no | up | / | / | evm.TU.Scaffold2659.150 |
| AVPR2 | no | up | no | up | no | up | evm.TU.Scaffold218.13 |
| POMC | no | up | no | up | no | up | evm.TU.Scaffold169.472 |
| no | up | no | down | no | down | evm.TU.scaffold20s2.1 | |
| MC2R | / | no | up | / | evm.TU.Scaffold95.252 |
| MC5R | no | up | no | up | no | up | evm.TU.Scaffold95.251 |
| GR | no | down | no | down | no | down | evm.TU.Scaffold306.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Zhang, Y.; Ye, T.; Liang, X.; Lou, B. Metabonomics and Transcriptomics Analyses Reveal the Underlying HPA-Axis-Related Mechanisms of Lethality in Larimichthys polyactis Exposed to Underwater Noise Pollution. Int. J. Mol. Sci. 2024, 25, 12610. https://doi.org/10.3390/ijms252312610
Jiang Q, Zhang Y, Ye T, Liang X, Lou B. Metabonomics and Transcriptomics Analyses Reveal the Underlying HPA-Axis-Related Mechanisms of Lethality in Larimichthys polyactis Exposed to Underwater Noise Pollution. International Journal of Molecular Sciences. 2024; 25(23):12610. https://doi.org/10.3390/ijms252312610
Chicago/Turabian StyleJiang, Qinghua, Yu Zhang, Ting Ye, Xiao Liang, and Bao Lou. 2024. "Metabonomics and Transcriptomics Analyses Reveal the Underlying HPA-Axis-Related Mechanisms of Lethality in Larimichthys polyactis Exposed to Underwater Noise Pollution" International Journal of Molecular Sciences 25, no. 23: 12610. https://doi.org/10.3390/ijms252312610
APA StyleJiang, Q., Zhang, Y., Ye, T., Liang, X., & Lou, B. (2024). Metabonomics and Transcriptomics Analyses Reveal the Underlying HPA-Axis-Related Mechanisms of Lethality in Larimichthys polyactis Exposed to Underwater Noise Pollution. International Journal of Molecular Sciences, 25(23), 12610. https://doi.org/10.3390/ijms252312610





