High Absorption and Elasticity of a Novel Transgenic Silk with Egg Case Silk Protein from Nephila clavata
Abstract
:1. Introduction
2. Results
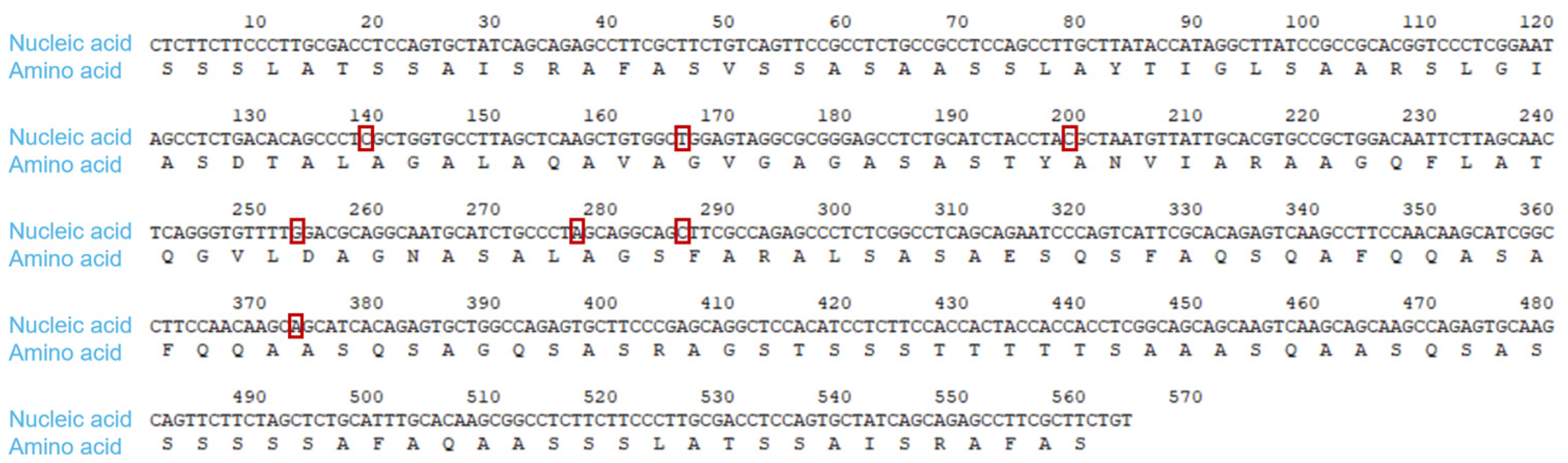
2.1. Optimize the Sequence of the Gene of Interest
2.2. piggyBac Vector Design and Construction
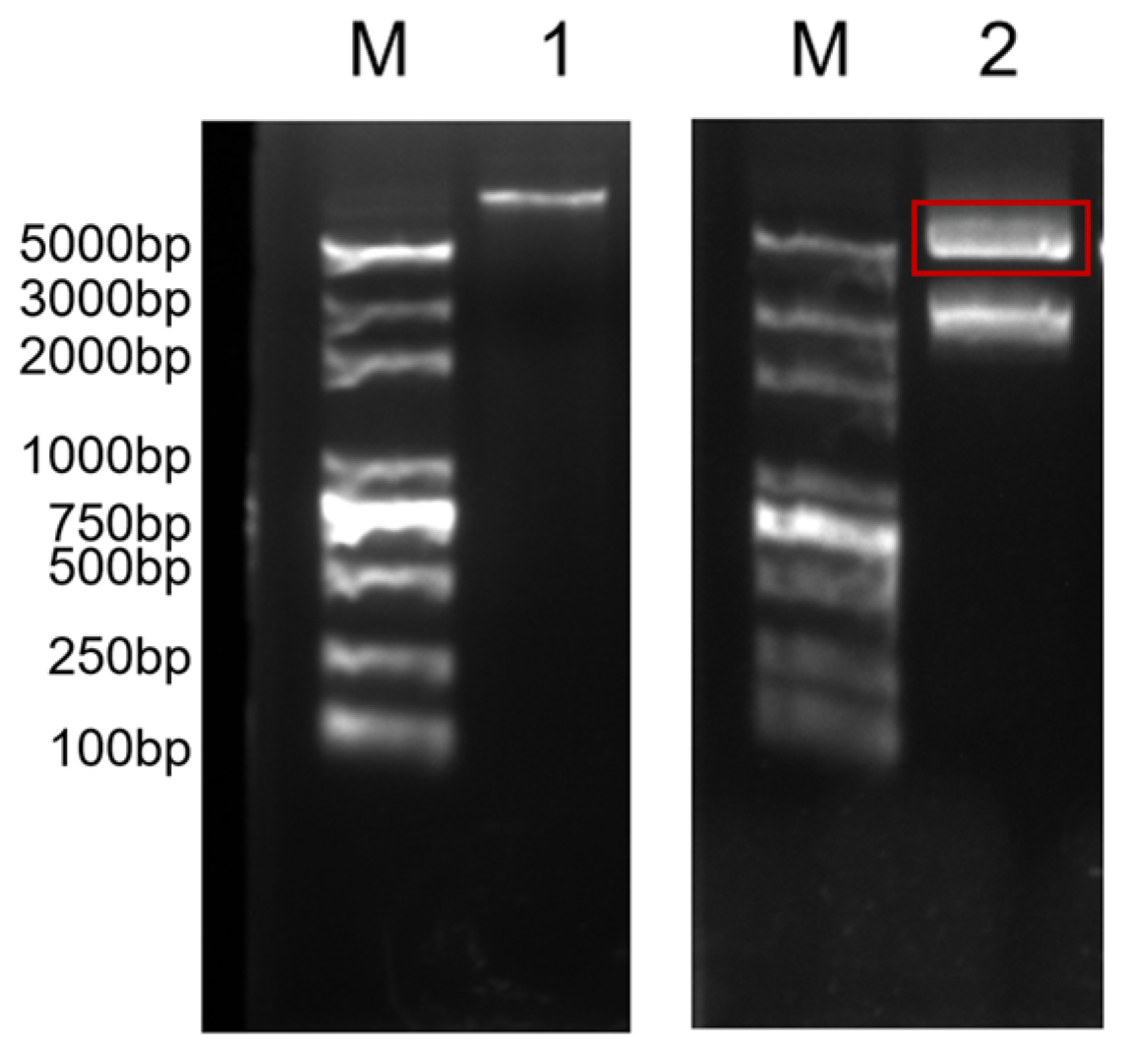
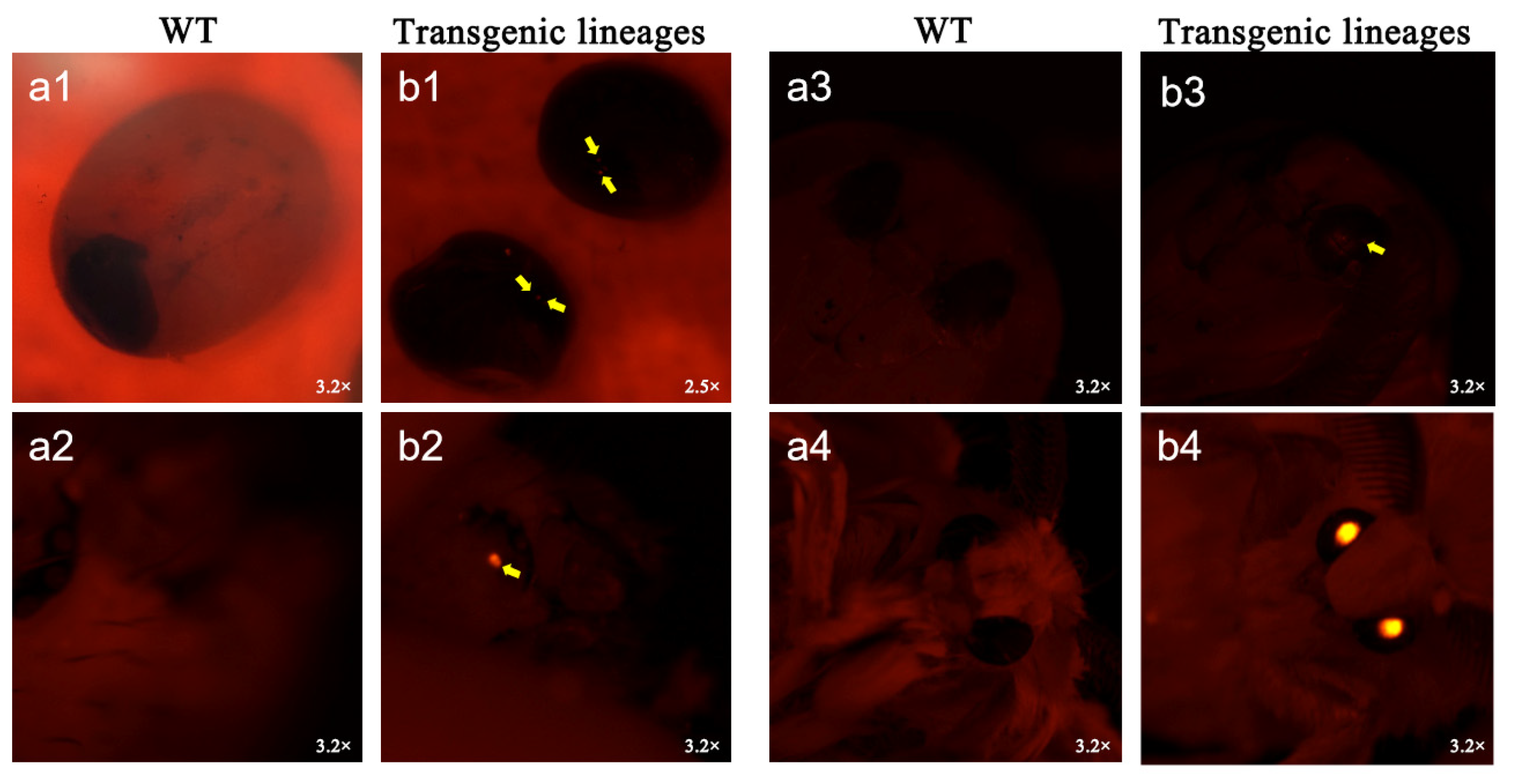
2.3. Silkworm Transformation
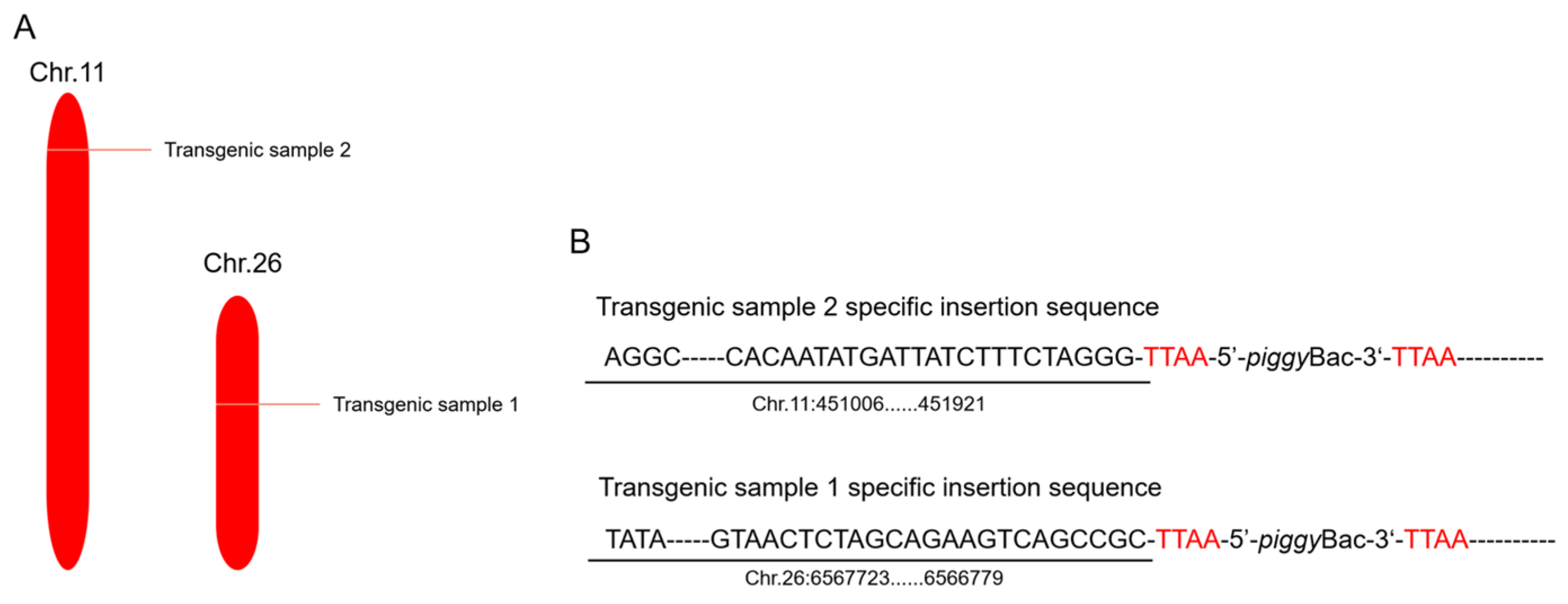
2.4. Inverse PCR Analysis
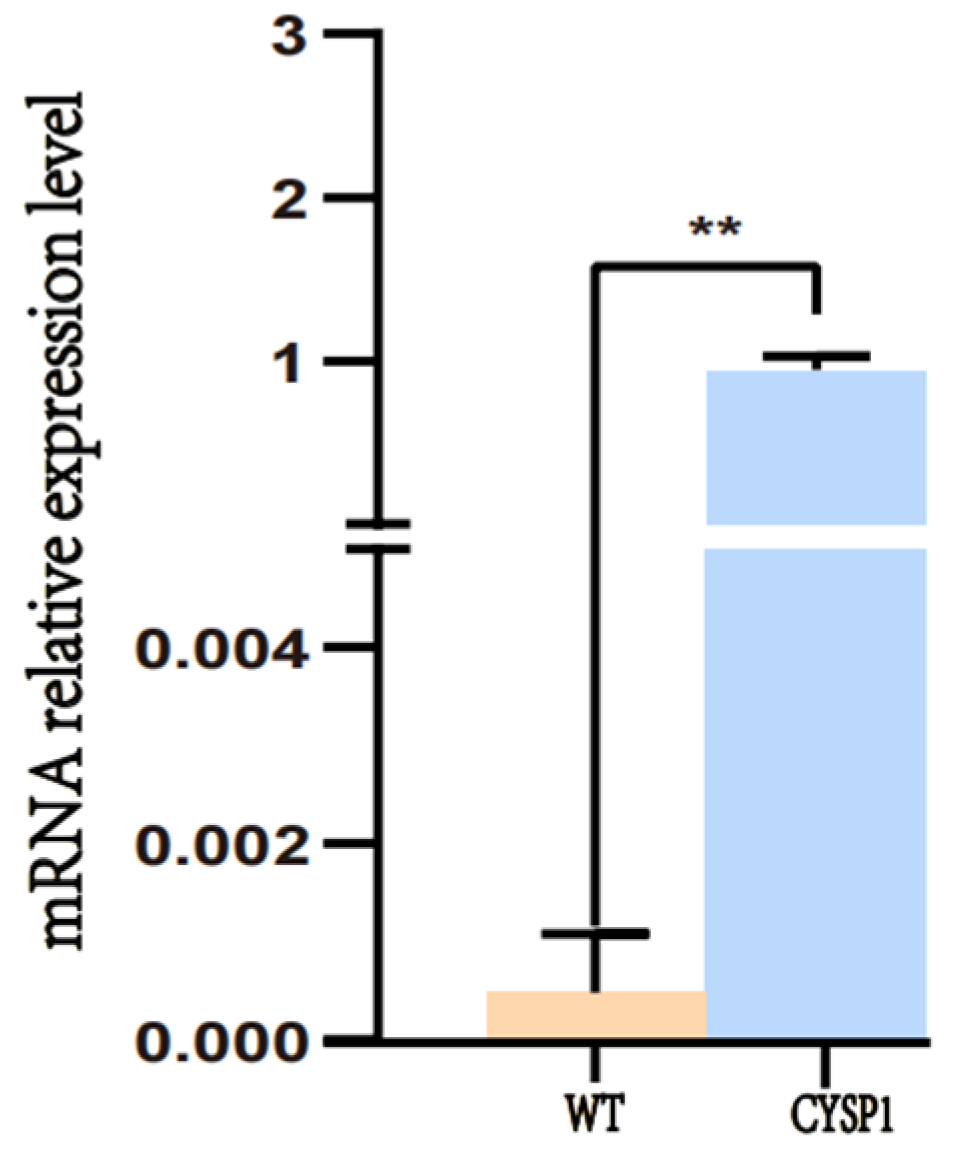
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
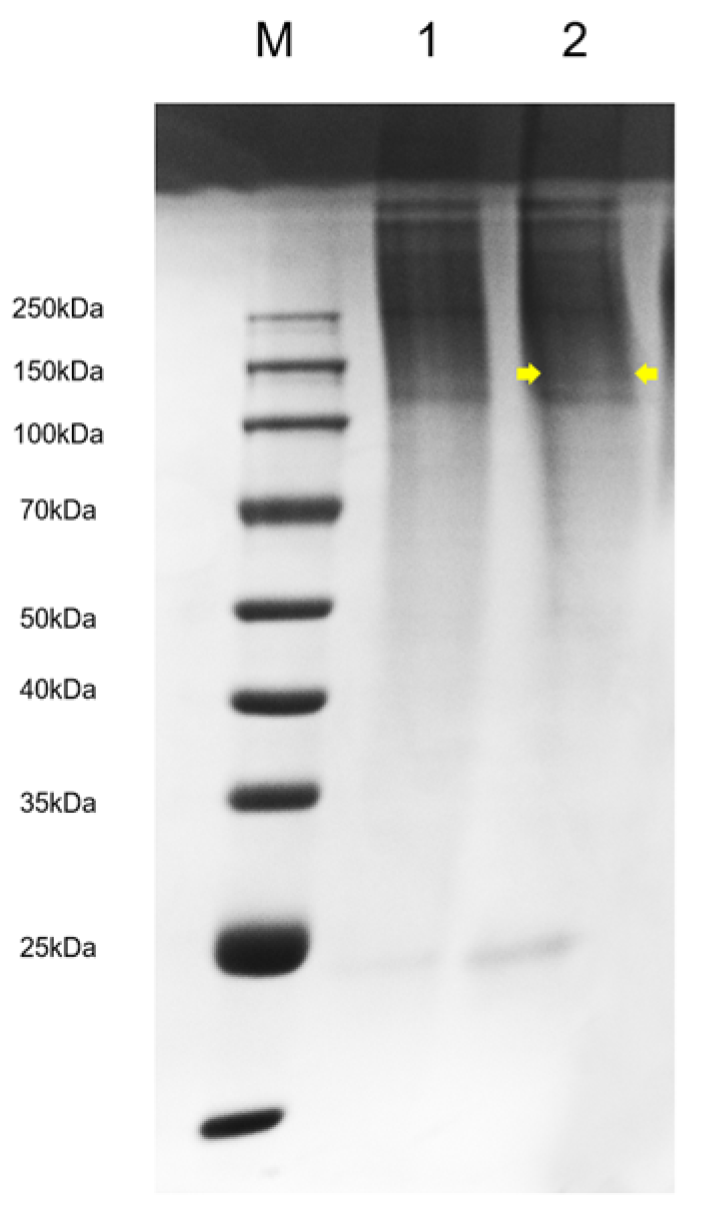
2.6. SDS-PAGE
2.7. Amino Acid Analysis
2.8. Scanning Electron Microscopy
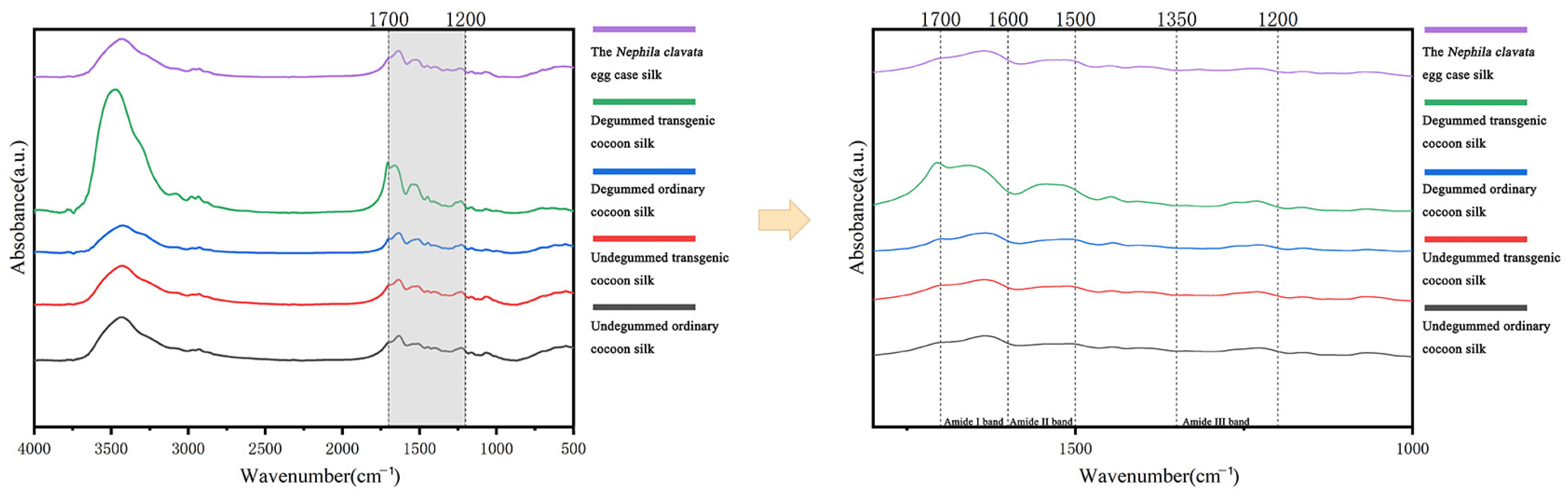
2.9. Fourier-Transform Infrared Spectroscopy
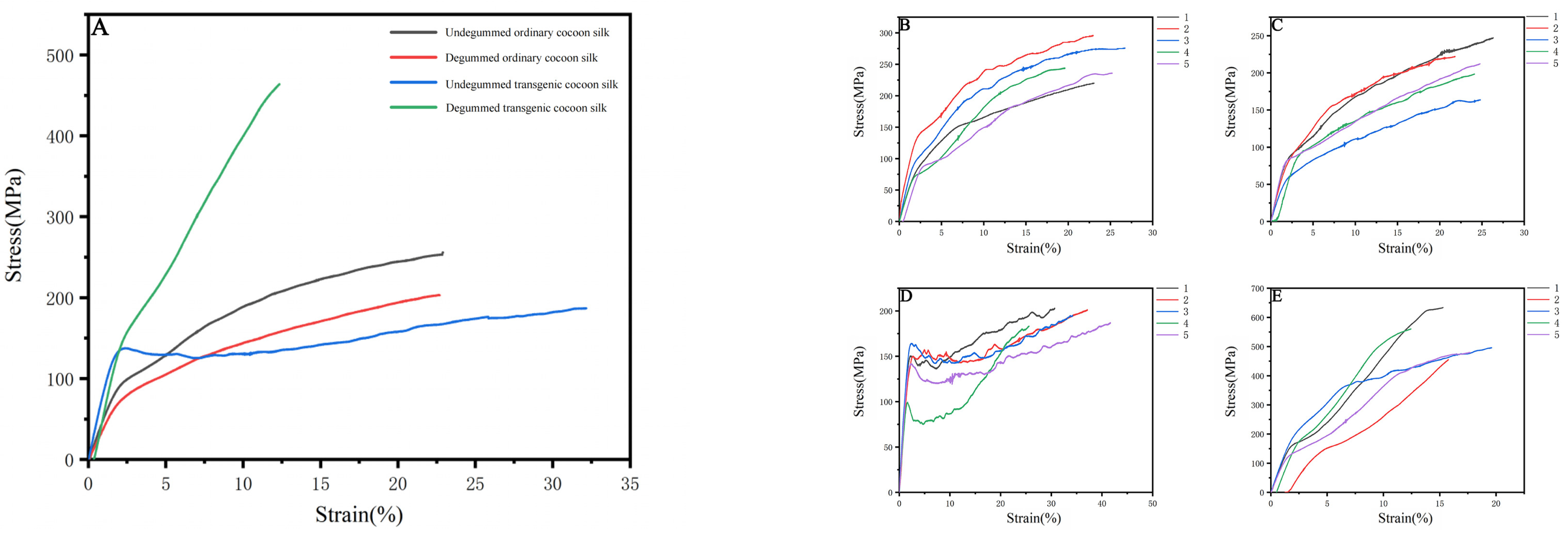
2.10. Mechanical Testing of Transgenic Silk Fibers
2.11. Water Absorption Performance Test
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. piggyBac Vector Construction
4.3. Silkworm Transformation
4.4. Inverse PCR Analysis
4.5. Quantitative Real-Time PCR Analysis
4.6. SDS-PAGE
4.7. Amino Acid Analysis
4.8. Scanning Electron Microscope
4.9. Fourier-Transform Infrared Spectroscopy
4.10. Mechanical Testing of Transgenic Silk Fibers
4.11. Water Absorption Performance Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J. Structure and Biological Spinning Process of Silk and Spider Silk. Adv. Text. Technol. 2004, 1, 40–42. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y.; Shi, Q. Research Status of Structure Biomimetic Materials. Mater. Rep. 2005, 6, 28–31. [Google Scholar]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, K.; Verdonk, P.; Forsyth, R.; Almqvist, K.F.; Van Nimmen, E.; Gheysens, T.; Mertens, J.; Van Langenhove, L.; Kiekens, P.; Verbruggen, G. Biocompatibility and biodegradability of spider egg sac silk. J. Mater. Sci. Mater. Med. 2008, 19, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.B. Biology of Spiders. Fla. Entomol. 2001, 84, 324. [Google Scholar] [CrossRef]
- Hu, X.; Lawrence, B.; Kohler, K.; Falick, A.M.; Moore, A.M.F.; McMullen, E.; Jones, P.R.; Vierra, C. Araneoid Egg Case Silk: A Fibroin with Novel Ensemble Repeat Units from the Black Widow Spider, Latrodectus hesperus. Biochemistry 2005, 44, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhao, T.; SiMa, Y.; Zhang, Y.; Nakagaki, K.; Miao, Y.; Shiomi, K.; Kajiura, Z.; Nagata, Y.; Nakagaki, M. Unique Molecular Architecture of Egg Case Silk Protein in a Spider, Nephila clavata. J. Biochem. 2005, 138, 593–604. [Google Scholar] [CrossRef]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent Advances in Development of Functional Spider Silk-Based Hybrid Materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.K.; Jin, C. Mechanical Properties of Spider Silk. Foreign Text. Technol. 2004, 2, 7–9. [Google Scholar]
- Chaoran, R.; Min, L. Research Progress on the Application of Spider Silk in the Medical Field. Chin. J. Med. Res. Clin. 2007, 5, 35–38. [Google Scholar]
- Eberhard, W.G. How Micrathena duodecimspinosa (Araneae: Araneidae) uses the elasticity of her dragline to hide her egg sac. J. Arachnol. 2015, 43, 417–418. [Google Scholar] [CrossRef]
- Abraham, A.; Joseph, M.M.; Minu, M. Antimicrobial activities of natural and recombinant spider silk—A review. Uttar Pradesh J. Zool. 2021, 41, 106–112. [Google Scholar]
- Shao, J. Analyses on Property Speciality of Spider Silk. Cotton Text. Technol. 2005, 11, 17–21. [Google Scholar]
- Dong, J.; Zhao, K.; Chen, J.; Wang, L. Research Status and Prospect of Spider Silk Fiber. Adv. Text. Technol. 2019, 27, 15–19. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Y.; Xu, S. Application of Bombyx mori as Model Organism in Modern Biology. Lab. Anim. Comp. Med. 2009, 29, 61–65. [Google Scholar]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.-J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C. Study on New Approach of Silk Utilization. J. Anhui Agric. Sci. 2000, 4, 540–542. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, X.; Zha, X.; He, Y.; Gong, C. Research Progress on Transgenic Silkworm. Jiangsu Seric. 2001, 2, 6–10. [Google Scholar]
- Wu, J.; Luo, M.; Hao, Z.; Xie, Y.; Huang, X.; Ma, W.; Tian, N. Transgenic Technologies and Applications in Silkworm Bombyx mori. Guangdong Seric. 2019, 53, 1–5. [Google Scholar]
- Balu, B.; Shoue, D.A.; Fraser, M.J.; Adams, J.H. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc. Natl. Acad. Sci. USA 2005, 102, 16391–16396. [Google Scholar] [CrossRef]
- Handler, A.M. Use of the piggyBac transposon for germ-line transformation of insects. Insect Biochem. Mol. Biol. 2002, 32, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Hino, R.; Tomita, M.; Yoshizato, K. The generation of germline transgenic silkworms for the production of biologically active recombinant fusion proteins of fibroin and human basic fibroblast growth factor. Biomaterials 2006, 27, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.-L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.L.; Da Rocha, M.; Besse, A.; Mauchamp, B.; Chavancy, G. 3×P3-EGFP marker facilitates screening for transgenic silkworm Bombyx mori L. from the embryonic stage onwards. Insect Biochem. Mol. Biol. 2002, 32, 247–253. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, B.; Wang, F. Insect transgenic vector-piggyBac transposon. Acta Sericologica Sin. 2000, 1, 98–101. [Google Scholar] [CrossRef]
- Teule, F.; Miao, Y.G.; Sohn, B.H.; Kim, Y.S.; Hull, J.J.F., Jr.; Lewis, R.V.; Jarvis, D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. USA 2012, 109, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Hino, R.; Ogawa, S.; Iizuka, M.; Adachi, T.; Shimizu, K.; Sotoshiro, H.; Yoshizato, K. A germline transgenic silkworm that secretes recombinant proteins in the sericin layer of the cocoon. Transgenic Res. 2007, 16, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dong, Q.; Yu, Y.; Bao, L.; Li, D.; Huang, M.; Chen, Y.; XinTan, A. Mass spider silk production through targeted gene replacement in Bombyx mori. Proc. Natl. Acad. Sci. USA 2018, 115, 8757–8762. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Zhou, Y.; Ma, S.; Zhou, X.; Xu, S.; Yang, Y.; Sun, Y.; Xia, Q.; Zhu, H.; Wang, S.; et al. High-strength and ultra-tough whole spider silk fibers spun from transgenic silkworms. Matter 2023, 6, 3661–3683. [Google Scholar] [CrossRef]
- Rauscher, S.; Baud, S.; Miao, M.; Keeley, F.W.; Pomès, R. Proline and Glycine Control Protein Self-Organization into Elastomeric or Amyloid Fibrils. Structure 2006, 14, 1667–1676. [Google Scholar] [CrossRef]
- Lee, M.; Kwon, J.; Na, S. Mechanical behavior comparison of spider and silkworm silks using molecular dynamics at atomic scale. Phys. Chem. Chem. Phys. 2016, 18, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.P.; Kiselev, G.O.; Nikolaeva, V.O.; Seisenbaeva, G.; Kessler, V.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Hybrid Spider Silk with Inorganic Nanomaterials. Nanomaterials 2020, 10, 1853. [Google Scholar] [CrossRef] [PubMed]
- Van Nimmen, E.; Gellynck, K.; Gheysens, T.; Van Langenhove, L.; Mertens, J. Modeling of the stress-strain behavior of egg sac silk of the spider Araneus diadematus. J. Arachnol. 2005, 33, 629–639. [Google Scholar] [CrossRef]
- GB/T 8939-2008; Sanitary Absorbent Pads (Panty Liner). China Pulp and Paper Research Institute: Beijing, China, 2008.




| Genetically Modified Silkworm Strains | Transgenic Plasmid Concentration (ng/μL) | Coenzyme Plasmid hsp Concentration (ng/μL) | Number of Eggs Injected (Grains) | Number of Eggs Hatched (Grains) | Hatchability (%) | Number of Moth Areas in the G1 Generation | Number of Positive Moth Areas in the G1 Generation | Positivity Rate (%) |
|---|---|---|---|---|---|---|---|---|
| Nc-4xCYSP1 | 716.5 | 719.5 | 640 | 26 | 4% | 20 | 1 | 5% |
| Relative Amino Acid Contents (%) | Ordinary Silkworm Cocoon | Genetically Modified Silkworm Cocoon | Egg Case Silk of Nephila clavata | ||
|---|---|---|---|---|---|
| Degummed Silk | Undegummed Silk | Degummed Silk | Undegummed Silk | Egg Case Silk | |
| Aspartic acid | 2.59 | 7.46 | 2.73 | 7.92 | 4.79 |
| Threonine | 1.38 | 3.47 | 1.41 | 3.54 | 2.78 |
| Serine | 13.13 | 16.34 | 13.41 | 16.49 | 9.87 |
| Glutamic acid | 2.36 | 3.41 | 2.28 | 3.47 | 15.74 |
| Glycine | 26.36 | 21.35 | 25.94 | 21.22 | 13.51 |
| Alanine | 28.07 | 21.33 | 27.91 | 19.88 | 16.67 |
| Cystine | 3.57 | 3.37 | 3.49 | 3.62 | 3.65 |
| Valine | 3.53 | 3.50 | 3.60 | 3.47 | 3.48 |
| Methionine | 0.62 | 0.51 | 0.57 | 0.48 | 0.85 |
| Isoleucine | 0.70 | 0.77 | 0.78 | 0.76 | 1.65 |
| Leucine | 0.76 | 1.00 | 0.82 | 1.06 | 5.93 |
| Tyrosine | 12.70 | 10.28 | 12.59 | 9.83 | 6.22 |
| Phenylalanine | 1.53 | 1.50 | 1.62 | 1.78 | 2.69 |
| Histidine | 0.55 | 1.06 | 0.54 | 1.28 | 1.09 |
| Lysine | 0.56 | 1.67 | 0.58 | 1.76 | 2.68 |
| Arginine | 0.81 | 2.15 | 0.89 | 2.28 | 4.41 |
| Proline | 0.78 | 0.83 | 0.84 | 1.24 | 4.05 |
| Acidic amino acids | 4.95 | 10.87 | 5.01 | 11.39 | 20.53 |
| Basic amino acids | 1.92 | 4.88 | 2.01 | 5.32 | 8.18 |
| Polar amino acids | 37.65 | 49.21 | 37.92 | 50.19 | 51.23 |
| Non-polar amino acids | 62.35 | 50.79 | 62.08 | 49.89 | 48.83 |
| Sample | β-Sheets (1600–1640 cm−1) | Random Curls/α-Helices (1640–1660 cm−1) | β-Turns (1660–1700 cm−1) |
|---|---|---|---|
| Undegummed ordinary cocoon silk | 44.25% | 41.57% | 14.18% |
| Undegummed transgenic cocoon silk | 43.93% | 41.73% | 14.33% |
| Degummed ordinary cocoon silk | 40.33% | 38.87% | 20.80% |
| Degummed transgenic cocoon silk | 30.87% | 44.91% | 24.21% |
| Nephila clavata egg case silk | 40.63% | 42.12% | 17.26% |
| Sample | Strength (MPa) | Elongation (%) | Young’s Modulus (GPa) | Toughness (MJ/m3) |
|---|---|---|---|---|
| Undegummed ordinary cocoon silk | 255.664 ± 40.336 | 22.908 ± 3.812 | 4.650 ± 0.1455 | 41.673 ± 14.697 |
| Degummed ordinary cocoon silk | 203.112 ± 44.074 | 22.691 ± 3.633 | 3.833 ± 0.0924 | 32.176 ± 13.514 |
| Undegummed transgenic cocoon silk | 186.936 ± 16.178 | 32.169 ± 9.500 | 7.998 ± 0.3392 | 47.411 ± 18.561 |
| Degummed transgenic cocoon silk | 463.912 ± 8.515 | 12.340 ± 7.271 | 8.211 ± 0.2878 | 32.673 ± 38.707 |
| Bombyx mori silk (mulberry silk) [33] | 500–600 | 18 | 9.600 ± 0.6000 | 70 |
| Egg case silk (Araneomorpha) [34] | 400 ± 50 | 5–20 | 8.700 ± 0.9000 | - |
| Sample | Mass Before Water Absorption (g) | Mass After Water Absorption (g) | Water Absorption Rate (%) | |
|---|---|---|---|---|
| Ordinary D9L silk | 0.010 | Sample | Mass (g) | 8.46 |
| 1 | 0.116 | |||
| 2 | 0.057 | |||
| 3 | 0.069 | |||
| 4 | 0.089 | |||
| 5 | 0.092 | |||
| Average | 0.0846 | |||
| Genetically recombinant Nephila clavata egg case silk | 0.010 | Sample | Mass (g) | 9.2 |
| 1 | 0.116 | |||
| 2 | 0.057 | |||
| 3 | 0.069 | |||
| 4 | 0.089 | |||
| 5 | 0.092 | |||
| Average | 0.0920 | |||
| Nephila clavata egg case silk | 0.010 | 0.0950 | 9.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, Y.; Luo, Y.; Zeng, D.; He, H.; Zhao, T. High Absorption and Elasticity of a Novel Transgenic Silk with Egg Case Silk Protein from Nephila clavata. Int. J. Mol. Sci. 2024, 25, 12793. https://doi.org/10.3390/ijms252312793
Wang Y, Lin Y, Luo Y, Zeng D, He H, Zhao T. High Absorption and Elasticity of a Novel Transgenic Silk with Egg Case Silk Protein from Nephila clavata. International Journal of Molecular Sciences. 2024; 25(23):12793. https://doi.org/10.3390/ijms252312793
Chicago/Turabian StyleWang, Yichen, Yuhang Lin, Yongkang Luo, Di Zeng, Haibo He, and Tianfu Zhao. 2024. "High Absorption and Elasticity of a Novel Transgenic Silk with Egg Case Silk Protein from Nephila clavata" International Journal of Molecular Sciences 25, no. 23: 12793. https://doi.org/10.3390/ijms252312793
APA StyleWang, Y., Lin, Y., Luo, Y., Zeng, D., He, H., & Zhao, T. (2024). High Absorption and Elasticity of a Novel Transgenic Silk with Egg Case Silk Protein from Nephila clavata. International Journal of Molecular Sciences, 25(23), 12793. https://doi.org/10.3390/ijms252312793





