Manganese(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Complexes
2.2. Structure of the Complexes
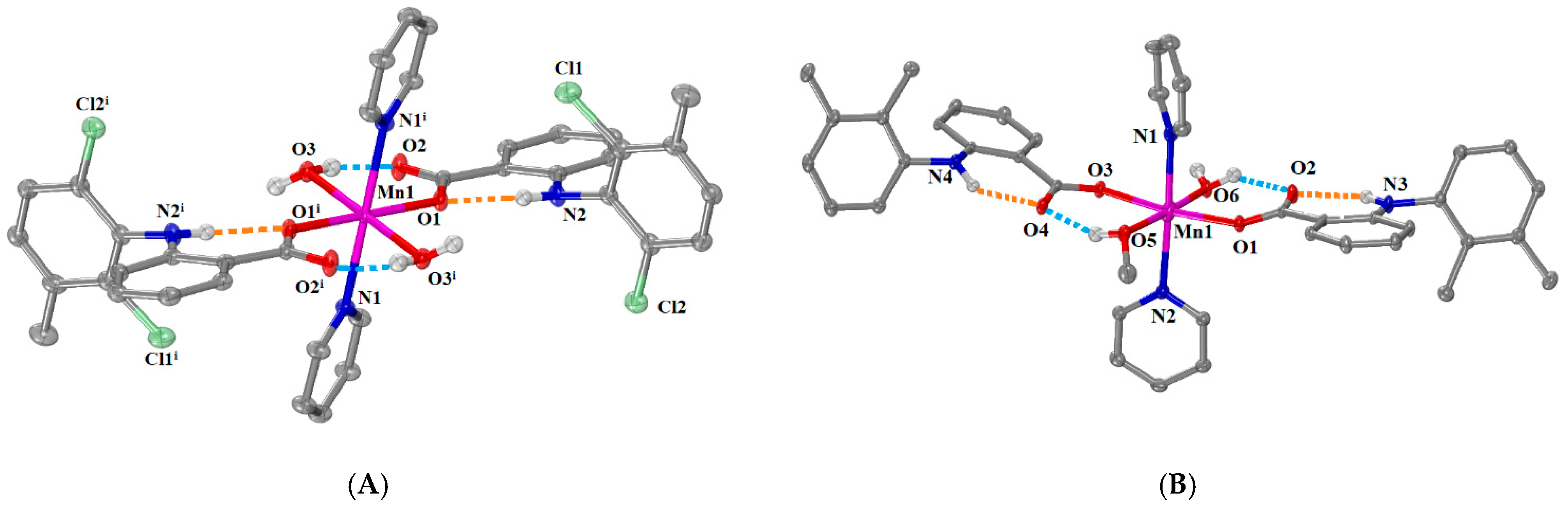
2.2.1. Structure of Complexes 1 and 2
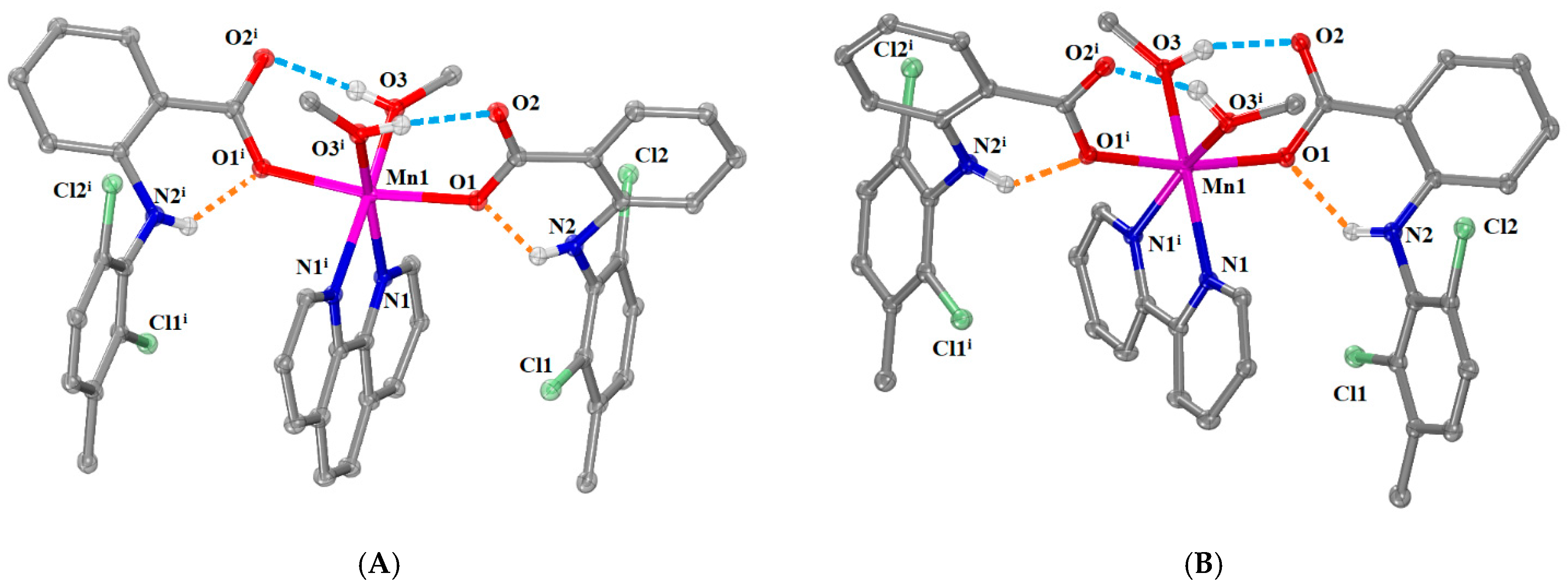
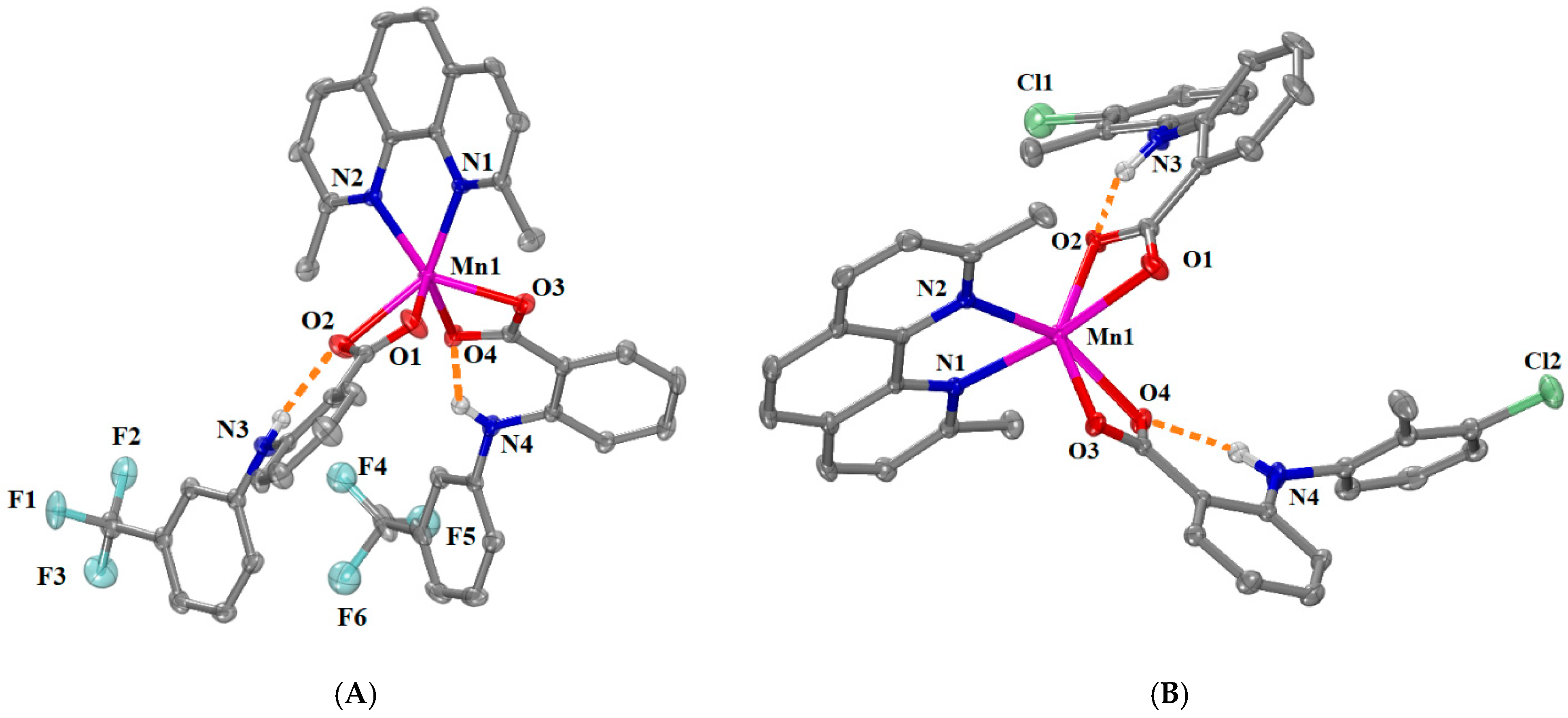
2.2.2. Structure of Complexes 3 and 4
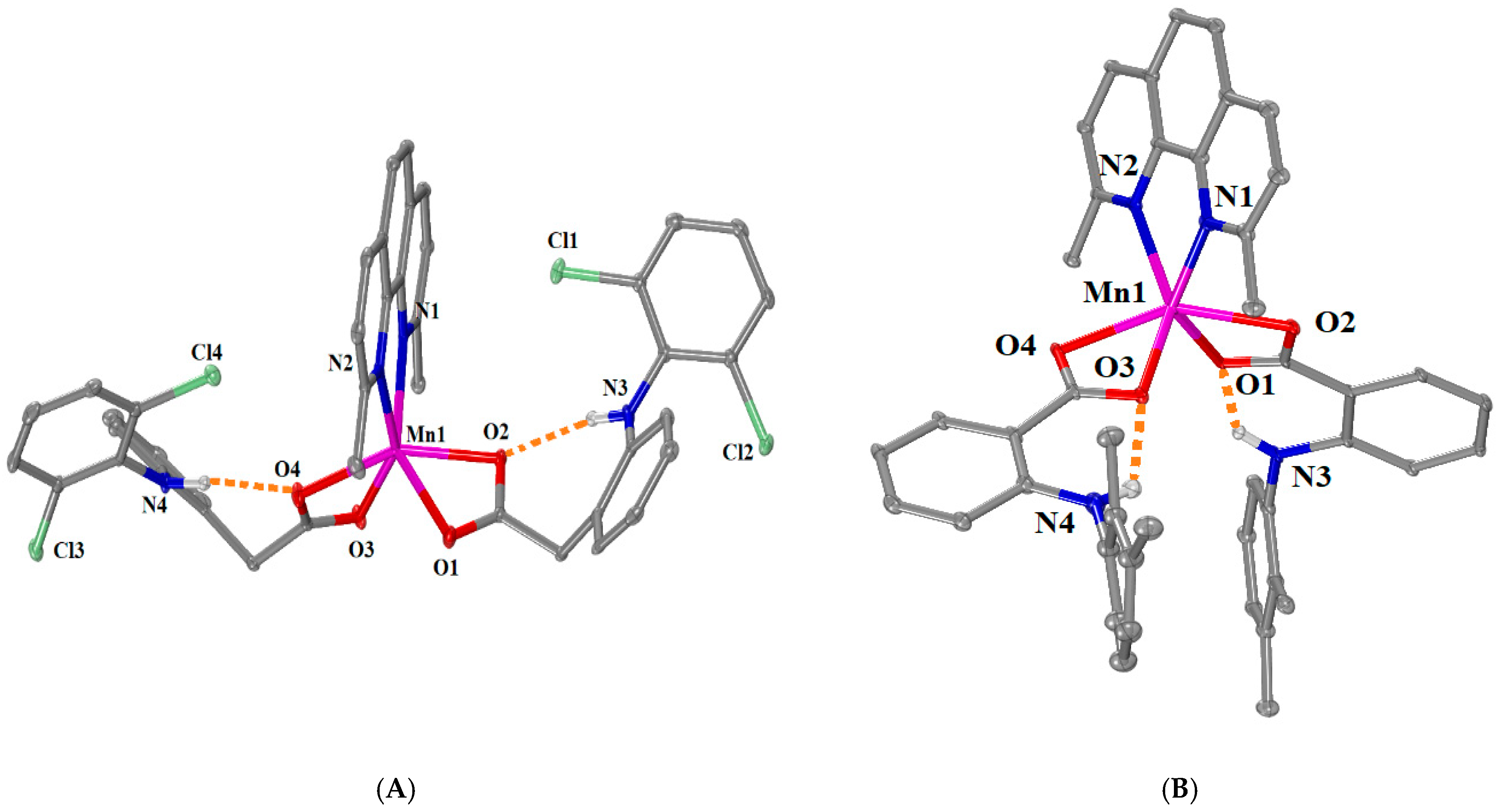
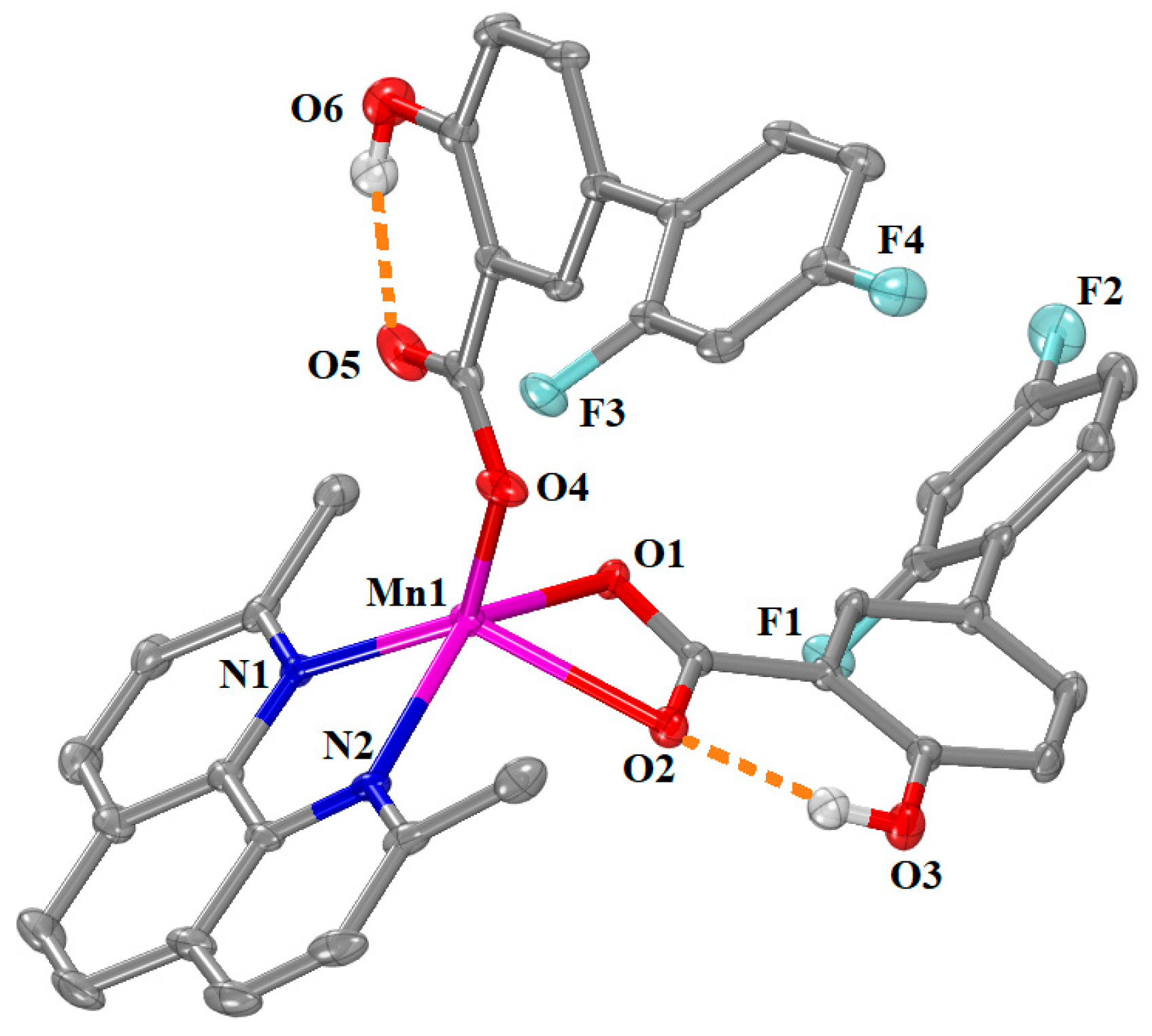
2.2.3. Structure of Complexes 5–9
2.3. Antioxidant Activity of the Complexes
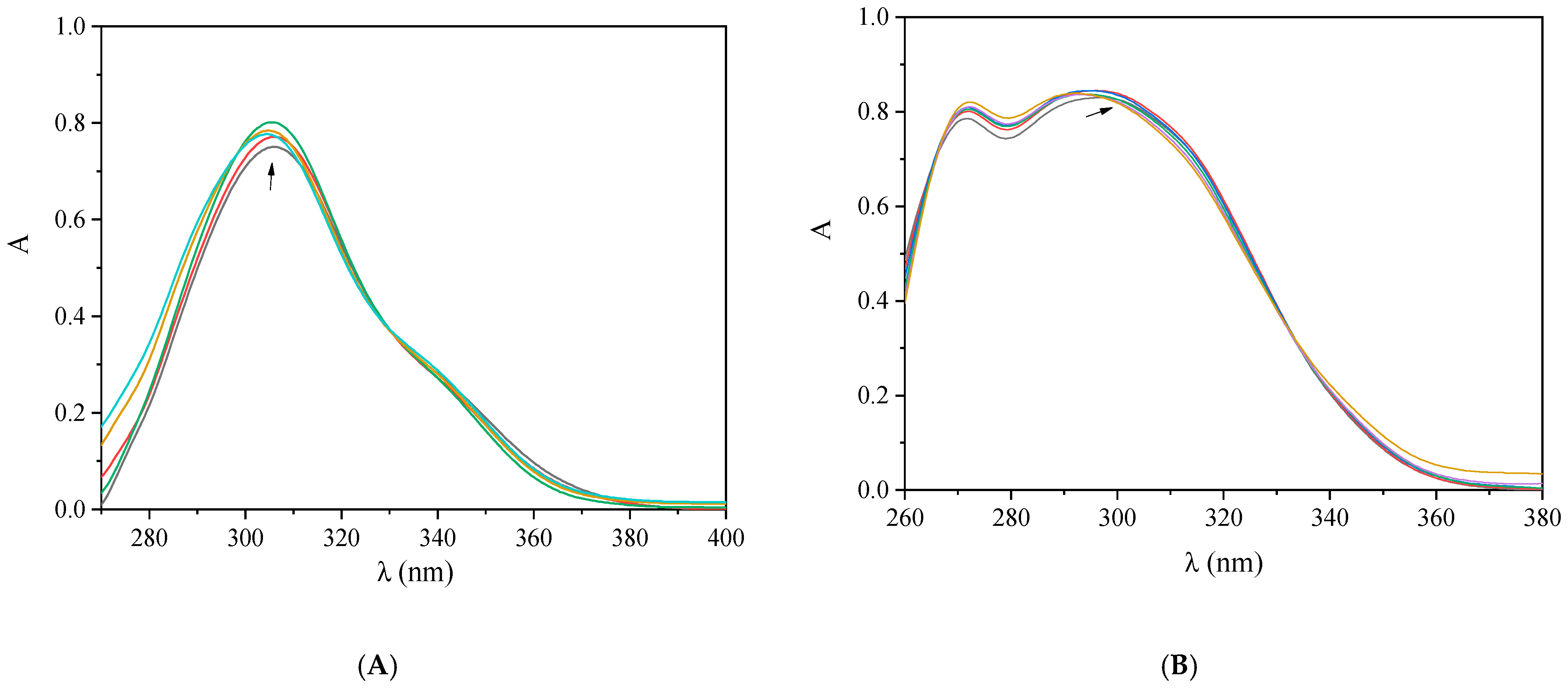
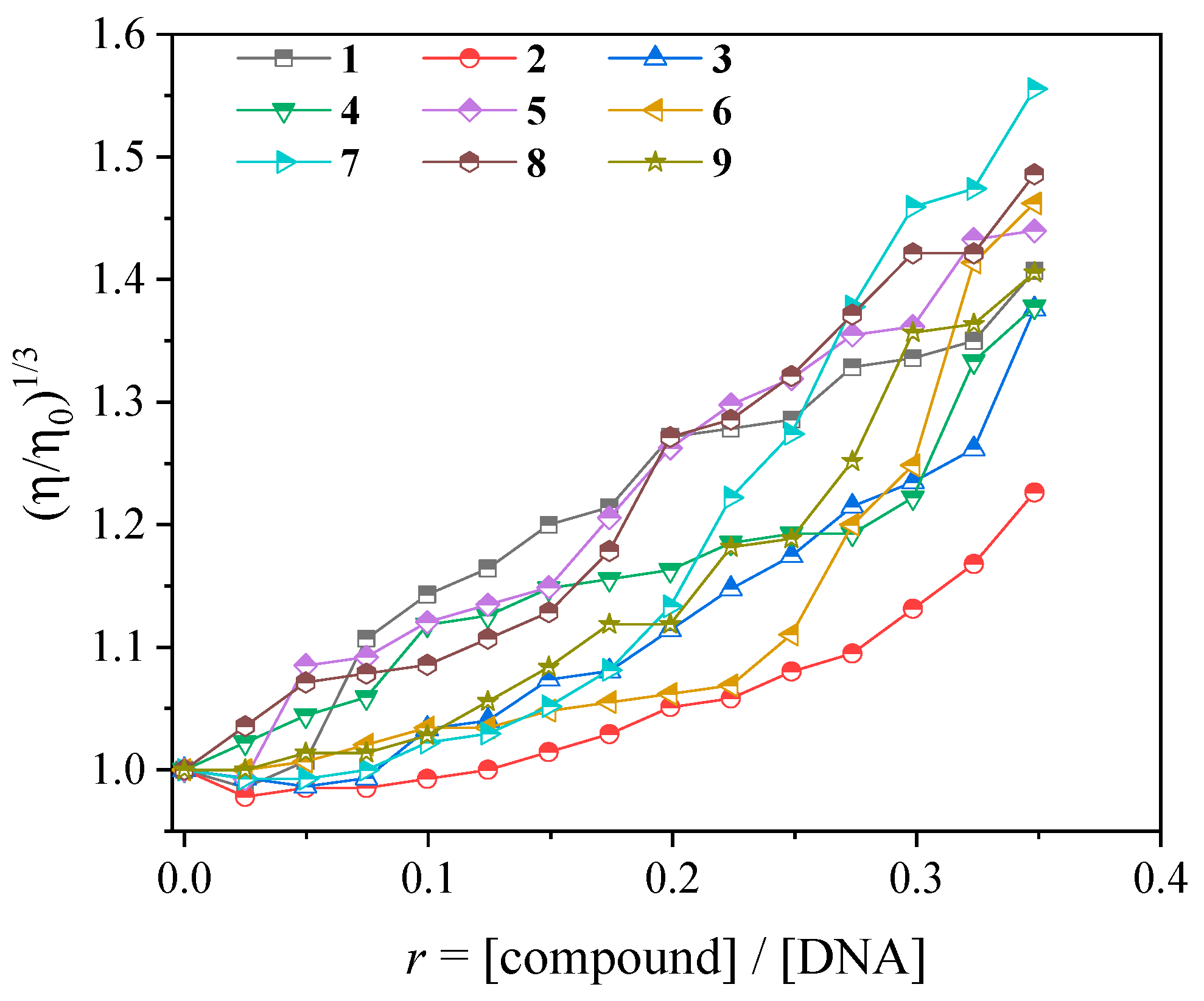
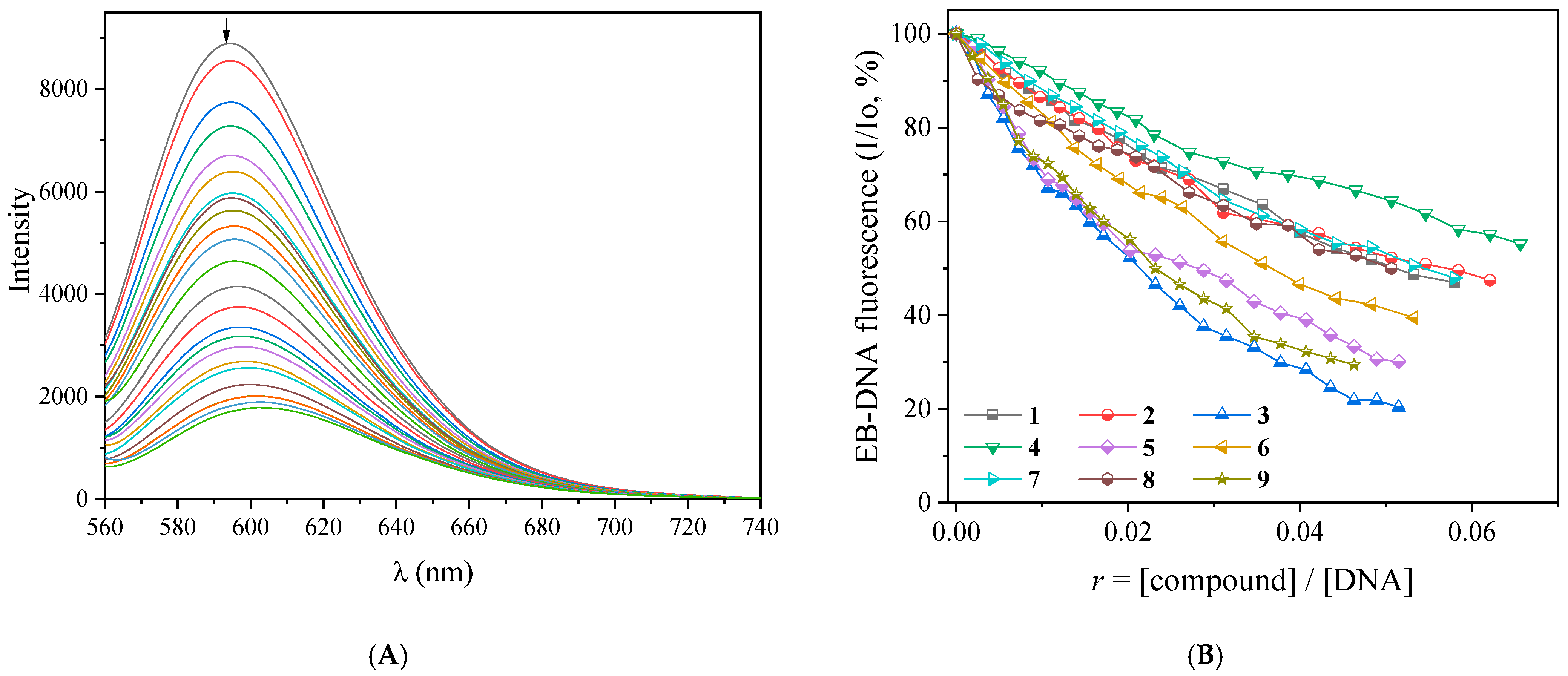
2.4. Interaction of the Complexes with CT DNA
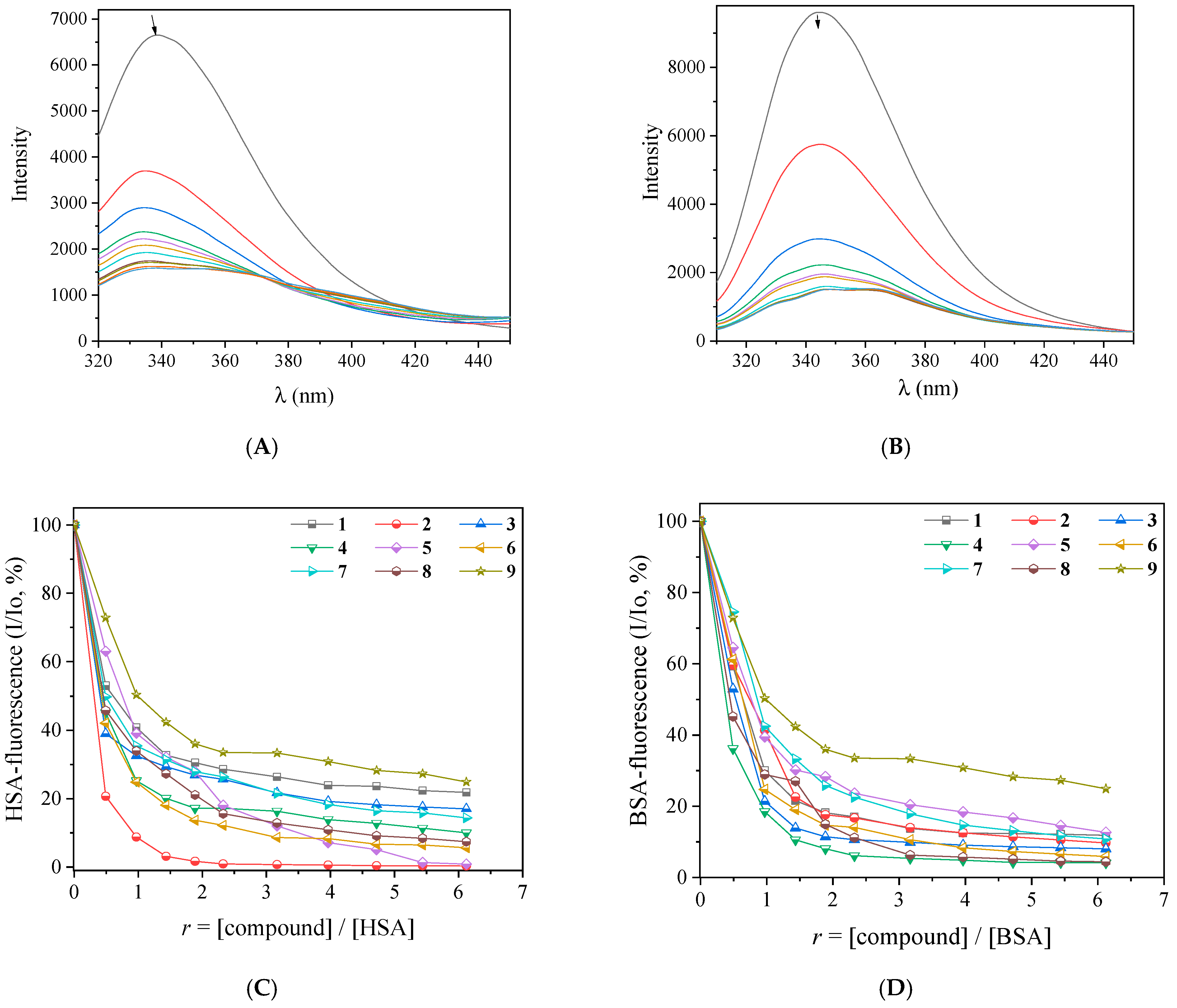
2.5. Interaction of the Complexes with Albumins
3. Materials and Methods
3.1. Materials—Instrumentation—Physical Measurements
3.2. Synthesis of the Complexes
3.2.1. Synthesis of the Complexes Bearing N Donors (Complexes 1 and 2)
3.2.2. Synthesis of the Complexes Bearing N,N’–Donors (Complexes 3–9)
3.3. Single-Crystal X-Ray Crystallography
3.4. In Vitro Biological Activity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
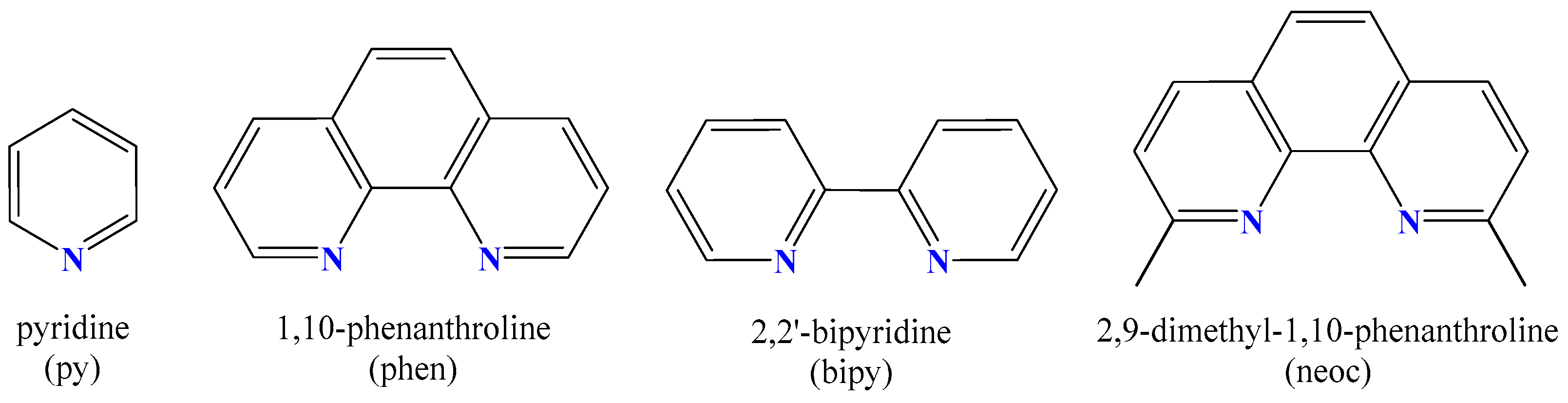
Abbreviations
| ABTS | 2,2′–azinobis(3–ethylbenzothiazoline–6–sulfonic acid) radical cation |
| ABTS% | ABTS scavenging activity |
| bipy | 2,2′–bipyridine |
| BSA | bovine serum albumin |
| CT | calf–thymus |
| dicl– | anion of diclofenac |
| difl–2 | dianion of diflunisal |
| EB | ethidium bromide, 3,8–diamino–5–ethyl–6–phenyl–phenanthridinium bromide |
| fluf– | flufenamato anion |
| Hdicl | diclofenac |
| Hdifl− | anion of diflunisal |
| Hfluf | flufenamic acid |
| Hmeclf | meclofenamic acid |
| Hmef | mefenamic acid |
| HSA | human serum albumin |
| Htolf | tolfenamic acid |
| H2difl | diflunisal |
| K | SA binding constant |
| Kb | DNA binding constant |
| kq | quenching constant |
| KSV | Stern–Volmer constant |
| meclf− | meclofenamato anion |
| mef− | mefenamato anion |
| neoc | neocuproine, 2,9–dimethyl–1,10–phenanthroline |
| NSAID | non-steroidal anti-inflammatory drug |
| phen | 1,10–phenqnthroline |
| py | pyridine |
| r | [compound]/[DNA] or [SA] ratio |
| SA | serum albumin |
| trolox | 6–hydroxy–2,5,7,8–tetramethylchromane–2–carboxylic acid |
| tolf− | tolfenamato anion |
| Δν(COO) | νasym(COO) − νsym(COO) |
References
- Mullins, C.S.; Pecoraro, V.L. Reflections on Small Molecule Manganese Models That Seek to Mimic Photosynthetic Water Oxidation Chemistry. Coord. Chem. Rev. 2008, 252, 416–443. [Google Scholar] [CrossRef]
- Wu, A.J.; Penner-Hahn, J.E.; Pecoraro, V.L. Structural, Spectroscopic, and Reactivity Models for the Manganese Catalases. Chem. Rev. 2004, 104, 903–938. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Krstić, N.S.; Nikolić, R.S.; Stanković, M.N.; Nikolić, N.G.; Đorđević, D.M. Coordination Compounds of M(II) Biometal Ions with Acid-Type Anti-Inflammatory Drugs as Ligands—A Review. Trop. J. Pharm. Res. 2015, 14, 337–349. [Google Scholar] [CrossRef]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-Induced Neurotoxicity: A Review of Its Behavioral Consequences and Neuroprotective Strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sadler, P.J. Metals in Medicine. Angew. Chem. Int. Ed. 1999, 38, 1512–1531. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn(II), Co(II) and Zn(II) Complexes with Heterocyclic Substituted Thiosemicarbazones: Synthesis, Characterization, X-Ray Crystal Structures and Antitumor Comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.F.; Chen, Q.Y.; Qi, Y.; Fu, H.J.; Li, Z.; Zhao, K.-D.; Gao, J. Anticancer Activity, Attenuation on the Absorption of Calcium in Mitochondria, and Catalase Activity for Manganese Complexes of N-Substituted Di(Picolyl)Amine. Inorg. Chem. 2011, 50, 6929–6937. [Google Scholar] [CrossRef]
- Zampakou, M.; Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Papadopoulos, A.N.; Psomas, G. Structurally Diverse Manganese(II)-Diclofenac Complexes Showing Enhanced Antioxidant Activity and Affinity to Serum Albumins in Comparison to Sodium Diclofenac. Eur. J. Inorg. Chem. 2015, 2015, 2285–2294. [Google Scholar] [CrossRef]
- Zampakou, M.; Balala, S.; Perdih, F.; Kalogiannis, S.; Turel, I.; Psomas, G. Structure, Antimicrobial Activity, Albumin- and DNA-Binding of Manganese(II)-Sparfloxacinato Complexes. RSC Adv. 2015, 5, 11861–11872. [Google Scholar] [CrossRef]
- Dimiza, F.; Raptopoulou, C.P.; Psycharis, V.; Papadopoulos, A.N.; Psomas, G. Manganese(II) Complexes with the Non-Steroidal Anti-Inflammatory Drugs Naproxen and Mefenamic Acid: Synthesis, Structure, Antioxidant Capacity, and Interaction with Albumins and DNA. New J. Chem. 2018, 42, 16666–16681. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, Structure and Antimicrobial Activity of Manganese(II) and Cobalt(II) Complexes of the Polyether Ionophore Antibiotic Sodium Monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Barmpa, A.; Frousiou, O.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Manganese(II) Complexes of the Quinolone Family Member Flumequine: Structure, Antimicrobial Activity and Affinity for Albumins and Calf-Thymus DNA. Polyhedron 2018, 145, 166–175. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, K.; Sharma, C. New 14-Membered Octaazamacrocyclic Complexes: Synthesis, Spectral, Antibacterial and Antifungal Studies. Eur. J. Med. Chem. 2010, 45, 1230–1236. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Metal Complexes and Their Effect at the Cellular Level. Eur. J. Inorg. Chem. 2016, 2016, 3048–3071. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and Zinc(II) Coordination Compounds of Non-Steroidal Anti-Inflammatory Drugs: Structural Features and Antioxidant Activity. Coord. Chem. Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Kim, K.S.; Yoon, J.H.; Kim, J.K.; Baek, S.J.; Eling, T.E.; Lee, W.J.; Ryu, J.H.; Lee, J.G.; Lee, J.H.; Yoo, J.B. Cyclooxygenase Inhibitors Induce Apoptosis in Oral Cavity Cancer Cells by Increased Expression of Nonsteroidal Anti-Inflammatory Drug-Activated Gene. Biochem. Biophys. Res. Commun. 2004, 325, 1298–1303. [Google Scholar] [CrossRef]
- Ho Woo, D.; Han, I.S.; Jung, G. Mefenamic Acid-Induced Apoptosis in Human Liver Cancer Cell-Lines through Caspase-3 Pathway. Life Sci. 2004, 75, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Muranaka, S.; Fujita, H.; Kanno, T.; Tamai, H.; Utsumi, K. Molecular Mechanism of Diclofenac-Induced Apoptosis of Promyelocytic Leukemia: Dependency on Reactive Oxygen Species, Akt, Bid, Cytochrome c, and Caspase Pathway. Free Radic. Biol. Med. 2004, 37, 1290–1299. [Google Scholar] [CrossRef]
- Pringsheim, T.; Davenport, W.J.; Dodick, D. Acute Treatment and Prevention of Menstrually Related Migraine Headache. Neurology 2008, 70, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, R.S.; Hruby, V.J. Analgesics. In Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006; pp. 19–55. ISBN 978-0-444-52166-8. [Google Scholar]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Marnett, A.B.; Kozak, K.R.; Remmel, R.P.; Marnett, L.J. Biochemically Based Design of Cyclooxygenase-2 (COX-2) Inhibitors: Facile Conversion of Nonsteroidal Antiinflammatory Drugs to Potent and Highly Selective COX-2 Inhibitors. Proc. Natl. Acad. Sci. USA 2000, 97, 925–930. [Google Scholar] [CrossRef]
- Wechter, W.J.; Murray, E.D.; Kantoci, D.; Quiggle, D.D.; Leipold, D.D.; Gibson, K.M.; McCracken, J.D. Treatment and Survival Study in the C57BL/6J-APCMin/+ (Min) Mouse with R-Flurbiprofen. Life Sci. 2000, 66, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Lawton, G.M.; Chapman, P.J. Diflunisal—A Long-Acting Non-Steroidal Anti-Inflammatory Drug. Aust. Dent. J. 1993, 38, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, G.; Pipilas, A.; Mussinelli, R.; Gopal, D.M.; Berk, J.L.; Connors, L.H.; Vellanki, N.; Hellawell, J.; Siddiqi, O.K.; Fox, J.; et al. Stabilization of Cardiac Function with Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J. Card. Fail 2020, 26, 753–759. [Google Scholar] [CrossRef]
- Moilanen, E.; Kankaanranta, H. Tolfenamic Acid and Leukotriene Synthesis Inhibition. Pharmacol. Toxicol. 1994, 75, 60–63. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Guzik, H.; Guzik, U. Non-Steroidal Anti-Inflammatory Drugs in the Era of the Covid-19 Pandemic in the Context of the Human and the Environment. Sci. Total Environ. 2022, 834, 155317. [Google Scholar] [CrossRef] [PubMed]
- Kelleni, M.T. Early Use of Non-Steroidal Anti-Inflammatory Drugs in COVID-19 Might Reverse Pathogenesis, Prevent Complications and Improve Clinical Outcomes. Biomed. Pharmacother. 2021, 133, 110982. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. The Elements of Life and Medicines. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 2014018. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.F.; Monteiro, L.P.G.; Gomes, A.C.C.; Martel, F.; Santos, T.M.; Ferreira, B.J.M.L. NSAID-Based Coordination Compounds for Biomedical Applications: Recent Advances and Developments. Int. J. Mol. Sci. 2022, 23, 2855. [Google Scholar] [CrossRef] [PubMed]
- Geromichalos, G.D.; Tarushi, A.; Lafazanis, K.; Pantazaki, A.A.; Kessissoglou, D.P.; Psomas, G. In Vitro and in Silico Study of the Biological Activity of Manganese(III) Inverse-[9-MC-3]-Metallacrowns and Manganese(II) Complexes with the Anti-Inflammatory Drugs Diclofenac or Indomethacin. J. Inorg. Biochem. 2018, 187, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tarushi, A.; Geromichalos, G.D.; Lafazanis, K.; Raptopoulou, C.P.; Psycharis, V.; Lalioti, N.; Pantazaki, A.A.; Kessissoglou, D.P.; Tangoulis, V.; Psomas, G. A Step-Ladder Manganese(III) Metallacrown Hosting Mefenamic Acid and a Manganese(II)-Mefanamato Complex: Synthesis, Characterization and Cytotoxic Activity. New J. Chem. 2018, 42, 6955–6967. [Google Scholar] [CrossRef]
- Feng, J.; Du, X.; Liu, H.; Sui, X.; Zhang, C.; Tang, Y.; Zhang, J. Manganese-Mefenamic Acid Complexes Exhibit High Lipoxygenase Inhibitory Activity. Dalton Trans. 2014, 43, 10930–10939. [Google Scholar] [CrossRef]
- Klepcová, Z.; Špaková, I.; Madreiter-Sokolowski, C.T.; Graier, W.; Kalinová, K.; Samoľová, E.; Smolková, R.; Smolko, L.; Rabajdová, M. Investigation of Novel Mn(II) Fenamato Complexes with Neocuproine and Their Effects on Endometrial Cell Lines. New J. Chem. 2023, 47, 13088–13097. [Google Scholar] [CrossRef]
- Borkar, A.; Nnabuike, G.G.; Obaleye, J.A.; Harihar, S.; Patil, A.S.; Butcher, R.J.; Salunke-Gawali, S. Manganese (II)-Imidazole Complexes of the Non-Steroidal Anti-Inflammatory Drug Mefenamic Acid: Synthesis, and Structural Studies. Inorganica Chim. Acta 2020, 512, 119878. [Google Scholar] [CrossRef]
- Dimiza, F.; Hatzidimitriou, A.G.; Sanakis, Y.; Papadopoulos, A.N.; Psomas, G. Trinuclear and Tetranuclear Iron(III) Complexes with Fenamates: Structure and Biological Profile. J. Inorg. Biochem. 2021, 218, 111410. [Google Scholar] [CrossRef] [PubMed]
- Dimiza, F.; Barmpa, A.; Chronakis, A.; Hatzidimitriou, A.G.; Sanakis, Y.; Papadopoulos, A.N.; Psomas, G. Iron(III) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure, Antioxidant and Anticholinergic Activity, and Interaction with Biomolecules. Int. J. Mol. Sci. 2023, 24, 6391. [Google Scholar] [CrossRef]
- Perontsis, S.; Hatzidimitriou, A.G.; Psomas, G. Coordination Compounds of Cobalt(II) with Carboxylate Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Profile. Dalton Trans. 2024, 53, 15215–15235. [Google Scholar] [CrossRef]
- Nnabuike, G.G.; Salunke-Gawali, S.; Patil, A.S.; Butcher, R.J.; Obaleye, J.A.; Ashtekar, H.; Prakash, B. Cobalt(II) Complexes Containing Mefenamic Acid with Imidazole and Pyridine Based Auxiliary Ligands: Synthesis, Structural Investigation and Cytotoxic Evaluation. J. Mol. Struct. 2023, 1285, 135519. [Google Scholar] [CrossRef]
- Smolko, L.; Smolková, R.; Samoľová, E.; Morgan, I.; Saoud, M.; Kaluđerović, G.N. Two Isostructural Co(II) Flufenamato and Niflumato Complexes with Bathocuproine: Analogues with a Different Cytotoxic Activity. J. Inorg. Biochem. 2020, 210, 111160. [Google Scholar] [CrossRef] [PubMed]
- Smolková, R.; Smolko, L.; Samoľová, E.; Dušek, M. Co(II) Fenamato, Tolfenamato and Niflumato Complexes with Neocuproine: Synthesis, Crystal Structure, Spectral Characterization and Biological Activity. J. Mol. Struct. 2023, 1272, 134772. [Google Scholar] [CrossRef]
- Barmpa, A.; Geromichalos, G.D.; Hatzidimitriou, A.G.; Psomas, G. Nickel(II)–Meclofenamate Complexes: Structure, in Vitro and in Silico DNA– and Albumin–Binding Studies, Antioxidant and Anticholinergic Activity. J. Inorg. Biochem. 2021, 222, 111507. [Google Scholar] [CrossRef]
- Totta, X.; Papadopoulou, A.A.; Hatzidimitriou, A.G.; Papadopoulos, A.; Psomas, G. Synthesis, Structure and Biological Activity of Nickel(II) Complexes with Mefenamato and Nitrogen-Donor Ligands. J. Inorg. Biochem. 2015, 145, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Malis, G.; Bakali, A.S.; Hatzidimitriou, A.G.; Psomas, G. Copper(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs and Neocuproine: Structure and Biological Evaluation. J. Mol. Struct. 2024, 1303, 137590. [Google Scholar] [CrossRef]
- Barmpa, A.; Hatzidimitriou, A.G.; Psomas, G. Copper(II) Complexes with Meclofenamate Ligands: Structure, Interaction with DNA and Albumins, Antioxidant and Anticholinergic Activity. J. Inorg. Biochem. 2021, 217, 111357. [Google Scholar] [CrossRef] [PubMed]
- Tarushi, A.; Kakoulidou, C.; Raptopoulou, C.P.; Psycharis, V.; Kessissoglou, D.P.; Zoi, I.; Papadopoulos, A.N.; Psomas, G. Zinc Complexes of Diflunisal: Synthesis, Characterization, Structure, Antioxidant Activity, and in Vitro and in Silico Study of the Interaction with DNA and Albumins. J. Inorg. Biochem. 2017, 170, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kakoulidou, C.; Gritzapis, P.S.; Hatzidimitriou, A.G.; Fylaktakidou, K.C.; Psomas, G. Zn(II) Complexes of (E)-4-(2-(Pyridin-2-Ylmethylene)Hydrazinyl)Quinazoline in Combination with Non-Steroidal Anti-Inflammatory Drug Sodium Diclofenac: Structure, DNA Binding and Photo-Cleavage Studies, Antioxidant Activity and Interaction with Albumin. J. Inorg. Biochem. 2020, 211, 111194. [Google Scholar] [CrossRef]
- Altay, A.; Caglar, S.; Caglar, B.; Sahin, O. Synthesis, Structural, Thermal Elucidation and in Vitro Anticancer Activity of Novel Silver(I) Complexes with Non-Steroidal Anti-Inflammatory Drugs Diclofenac and Mefenamic Acid Including Picoline Derivatives. Polyhedron 2018, 151, 160–170. [Google Scholar] [CrossRef]
- Zhang, L.L.; Huang, X.; Azam, M.; Yuan, H.X.; Ma, F.J.; Cheng, Y.Z.; Zhang, L.P.; Sun, D. Silver(I) Complexes with Mefenamic Acid and Nitrogen Heterocyclic Ligands: Synthesis, Characterization, and Biological Evaluation. Inorg. Chem. 2024, 63, 12624–12634. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Papatriantafyllopoulou, C.; Papachristodoulou, C.; Hatzidimitriou, A.G.; Hadjikakou, S.K. New Apoptosis Inducers Containing Anti-Inflammatory Drugs and Pnictogen Derivatives: A New Strategy in the Development of Mitochondrial Targeting Chemotherapeutics. J. Med. Chem. 2023, 66, 4131–4149. [Google Scholar] [CrossRef] [PubMed]
- Banti, C.N.; Gkaniatsou, E.I.; Kourkoumelis, N.; Manos, M.J.; Tasiopoulos, A.J.; Bakas, T.; Hadjikakou, S.K. Assessment of Organotins against the Linoleic Acid, Glutathione and CT-DNA. Inorganica Chim. Acta 2014, 423, 98–106. [Google Scholar] [CrossRef]
- Johnson, A.; Olelewe, C.; Kim, J.H.; Northcote-Smith, J.; Mertens, R.T.; Passeri, G.; Singh, K.; Awuah, S.G.; Suntharalingam, K. The Anti-Breast Cancer Stem Cell Properties of Gold(I)-Non-Steroidal Anti-Inflammatory Drug Complexes. Chem. Sci. 2022, 14, 557–565. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Q.Y.; Liu, C.M.; Wang, Y.L.; Chen, L. Dinuclear Lanthanide–Carboxylate Compounds: Field-Induced Slow Relaxation of Magnetization for Dysprosium(III) Analogue. Aust. J. Chem. 2014, 68, 488–492. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; Wiley: Hoboken, NJ, USA, 2008; pp. 1–408. [Google Scholar] [CrossRef]
- Szorcsik, A.; Nagy, L.; Sletten, J.; Szalontai, G.; Kamu, E.; Fiore, T.; Pellerito, L.; Kálmán, E. Preparation and Structural Studies on Dibutyltin(IV) Complexes with Pyridine Mono- and Dicarboxylic Acids. J. Organomet. Chem. 2004, 689, 1145–1154. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Cini, R.; Giorgi, G.; Cinquantini, A.; Rossi, C.; Sabat, M. Metal Complexes of the Antiinflammatory Drug Piroxicam. Inorg. Chem. 1990, 29, 5197–5200. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Ntella, M.; Mpompou, L.; Karallaki, F.; Athanasios, P.; Hadjipavlou-Litina, D.; Lazari, D. Study of the Antioxidant Activity of Thymus sibthorpii Bentham (Lamiaceae). J. Enzyme Inhib. Med. Chem. 2016, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, S.; Chen, Y.; Feng, F.; Qu, W.; Sun, H. Donepezil-Based Multi-Functional Cholinesterase Inhibitors for Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2018, 158, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.B.; Everette, J.D. Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K.; Pierre, V.C. Metallo-Intercalators and Metallo-Insertors. Chem. Commun. 2007, 44, 4565–4579. [Google Scholar] [CrossRef]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal Complex Interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M.; Rehmann, J.P.; Meshoyrer, R.; Kumar, C.V.; Turro, N.J.; Barton, J.K. Mixed-Ligand Complexes of Ruthenium(II): Factors Governing Binding to DNA. J. Am. Chem. Soc. 2002, 111, 3051–3058. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G.H.; Meehan, T. Polycyclic Aromatic Hydrocarbons Physically Intercalate into Duplex Regions of Denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Dimitrakopoulou, A.; Dendrinou-Samara, C.; Pantazaki, A.A.; Alexiou, M.; Nordlander, E.; Kessissoglou, D.P. Synthesis, Structure and Interactions with DNA of Novel Tetranuclear, [Mn4(II/II/II/IV)] Mixed Valence Complexes. J. Inorg. Biochem. 2008, 102, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Luis García-Giménez, J.; González-Álvarez, M.; Liu-González, M.; Macías, B.; Borrás, J.; Alzuet, G. Toward the Development of Metal-Based Synthetic Nucleases: DNA Binding and Oxidative DNA Cleavage of a Mixed Copper(II) Complex with N-(9H-Purin-6-Yl)Benzenesulfonamide and 1,10-Phenantroline. Antitumor Activity in Human Caco-2 Cells and Jurkat T Lymphocytes. Evaluation of P53 and Bcl-2 Proteins in the Apoptotic Mechanism. J. Inorg. Biochem. 2009, 103, 923–934. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Zhao, G.; Lin, H.; Zhu, S.; Sun, H.; Chen, Y. Dinuclear Palladium(II) Complexes Containing Two Monofunctional [Pd(En)(Pyridine)Cl]+ Units Bridged by Se or S. Synthesis, Characterization, Cytotoxicity and Kinetic Studies of DNA-Binding. J. Inorg. Biochem. 1998, 70, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.P.; Greenstock, C.L. Fluorescence Lifetime Analysis of DNA Intercalated Ethidium Bromide and Quenching by Free Dye. Biophys. Chem. 1994, 50, 305–312. [Google Scholar] [CrossRef]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal Structure of Human Serum Albumin Complexed with Fatty Acid Reveals an Asymmetric Distribution of Binding Sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.E.; Christ, D.D. Chapter 33. Plasma Protein Binding of Drugs. Annu. Rep. Med. Chem. 1996, 31, 327–336. [Google Scholar] [CrossRef]
- Shamsi, A.; Mohammad, T.; Anwar, S.; Alajmi, M.F.; Hussain, A.; Hassan, M.I.; Ahmad, F.; Islam, A. Probing the Interaction of Rivastigmine Tartrate, an Important Alzheimer’s Drug, with Serum Albumin: Attempting Treatment of Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 148, 533–542. [Google Scholar] [CrossRef]
- Stella, L.; Capodilupo, A.L.; Bietti, M. A Reassessment of the Association between Azulene and (60)Fullerene. Possible Pitfalls in the Determination of Binding Constants through Fluorescence Spectroscopy. Chem. Commun. 2008, 39, 4744–4746. [Google Scholar] [CrossRef]
- Laitinen, O.H.; Hytönen, V.P.; Nordlund, H.R.; Kulomaa, M.S. Genetically Engineered Avidins and Streptavidins. Cell. Mol. Life Sci. 2006, 63, 2992–3017. [Google Scholar] [CrossRef]
- Marmur, J. A Procedure for the Isolation of Deoxyribonucleic Acid from Micro-Organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Bruker Analytical X-Ray Systems Inc. Apex2 Version 2 User Manual M86-E01078; Bruker Analytical X-Ray Systems: Madison, WI, USA, 2006. [Google Scholar]
- Siemens Industrial Automation Inc. SADABS Area–Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS Version 12: Software for Guided Crystal Structure Analysis. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological Evaluation of Several Coumarin Derivatives Designed as Possible Anti-Inflammatory/Antioxidant Agents. J. Enzyme Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Zhang, H.-M.; Zhang, G.-C.; Tao, W.-H.; Tang, S.-H. Interaction of the Flavonoid Hesperidin with Bovine Serum Albumin: A Fluorescence Quenching Study. J. Lumin. 2007, 126, 211–218. [Google Scholar] [CrossRef]

| Compound | ABTS% |
|---|---|
| Complex 1 | 39.18 ± 3.27 |
| Complex 2 | 92.56 ± 0.74 |
| Complex 3 | 18.66 ± 0.71 |
| Complex 4 | 98.69 ± 0.06 |
| Complex 5 | 99.20 ± 0.06 |
| Complex 6 | 98.69 ± 0.07 |
| Complex 7 | 29.70 ± 2.86 |
| Complex 8 | 99.63 ± 0.07 |
| Complex 9 | 25.80 ± 1.43 |
| Na meclf [39] | 59.48 ± 0.06 |
| Hmef [39] | 66.32 ± 0.38 |
| Na dicl [40] | 76.35 ± 0.75 |
| Hfluf [39] | 64.57 ± 0.43 |
| Htolf [39] | 59.43 ± 0.33 |
| H2difl [40] | 76.58 ± 0.74 |
| Trolox | 91.8 ± 0.17 |
| Compound | λmax (nm) (ΔA/A0 (%) a, Δλ (nm) b) | Kb (M–1) |
|---|---|---|
| Complex 1 | 306 (+10, −2) | 5.16 (±0.09) × 105 |
| Complex 2 | 306 (+7, 0); 345(<−50 c, elim d) | 3.71 (±0.15) × 105 |
| Complex 3 | 307(−6, +2) | 1.62 (±0.04) × 104 |
| Complex 4 | 287 (+15, −7); 319(+9, −2) | 6.25 (±0.35) × 104 |
| Complex 5 | 272 (+8, +2); 285(sh) (+7, −4) | 6.58 (±0.20) × 105 |
| Complex 6 | 271 (+3, 0); 289 (+2, −2) | 2.48 (±0.33) × 104 |
| Complex 7 | 275 (−6, 0) | 1.10 (±0.02) × 106 |
| Complex 8 | 271 (+5, +1); 296 (+2 +2) | 4.15 (±0.23) × 104 |
| Complex 9 | 269 (+6, 0) | 2.24 (±0.71) × 105 |
| Na meclf [48] | 302(−12, −1); 315(sh) (+5, −2) | 1.51 (±0.12) × 105 |
| Hmef [39] | 324(+10, 0) | 1.05 (±0.02) × 105 |
| Na dicl [40] | 295(−7.5, −5) | 3.16 (±0.14) × 104 |
| Hfluf [39] | 292(+40, +10); 344(<−50, 0) | 2.70 (±0.11) × 105 |
| Htolf [39] | 304 (+40, +5); 348 (<−50, −2) | 5.00 (±0.10) × 104 |
| H2difl [40] | 295 (+15, +2) | 3.08 (±0.15) × 103 |
| Compound | ΔI/Io (%) | KSV (M–1) | kq (M–1s–1) |
|---|---|---|---|
| Complex 1 | 53.0 | 1.15 (±0.17) × 105 | 4.99 (±0.15) × 1012 |
| Complex 2 | 52.7 | 2.30 (±0.53) × 105 | 1.00 (±0.155) × 1013 |
| Complex 3 | 79.6 | 1.18 (±0.32) × 106 | 5.15 (±0.14) × 1013 |
| Complex 4 | 44.8 | 1.54 (±0.31) × 105 | 6.69 (±0.13) × 1012 |
| Complex 5 | 69.9 | 7.37 (±0.05) × 105 | 3.20 (±0.21) × 1013 |
| Complex 6 | 60.6 | 4.29 (±0.32) × 105 | 1.86 (±0.18) × 1013 |
| Complex 7 | 52.1 | 5.55(±0.12) × 105 | 2.41 (±0.05) × 1013 |
| Complex 8 | 50.0 | 2.35 (±0.48) × 105 | 1.02 (±0.03) × 1013 |
| Complex 9 | 31.6 | 1.01 (±0.42) × 106 | 4.39 (±0.10) × 1013 |
| Na meclf [48] | 80.1 | 8.20 (±0.26) × 104 | 3.57 (±0.11) × 1012 |
| Hmef [39] | 80.0 | 1.58 (±0.06) × 105 | 6.87 (±0.26) × 1012 |
| Na dicl [40] | 65.0 | 2.47 (±0.06) × 105 | 1.07 (±0.03) × 1013 |
| Hfluf [39] | 67.0 | 6.34 (±0.30) × 105 | 2.76 (±0.13) × 1013 |
| Htolf [39] | 74.0 | 1.15 (±0.04) × 106 | 5.00 (±0.17) × 1013 |
| H2difl [40] | 65.0 | 8.59 (±0.35) × 105 | 3.73 (±0.15) × 1013 |
| Compound | kq(BSA) (M–1s–1) | Κ(BSA) (M–1) | kq(HSA) (M–1s–1) | Κ(HSA) (M–1) |
|---|---|---|---|---|
| Complex 1 | 6.88 (±0.05) × 1013 | 9.58 (±0.07) × 105 | 4.05 (±0.42) × 1013 | 8.83 (±0.24) × 105 |
| Complex 2 | 5.30 (±0.29) × 1013 | 1.03 (±0.16) × 106 | 1.87 (±0.01) × 1015 | 1.92 (±0.10) × 106 |
| Complex 3 | 1.34 (±0.16) × 1014 | 2.71 (±0.16) × 106 | 2.30 (±0.91) × 1013 | 8.73 (±0.06) × 105 |
| Complex 4 | 1.76 (±0.11) × 1014 | 9.61 (±0.06) × 105 | 4.69 (±0.15) × 1013 | 9.38 (±0.29) × 105 |
| Complex 5 | 3.46 (±0.16) × 1013 | 6.32 (±0.32) × 105 | 7.67 (±0.10) × 1013 | 3.09 (±0.21) × 105 |
| Complex 6 | 1.87 (±0.04) × 1014 | 1.08 (±0.38) × 106 | 9.06 (±0.35) × 1013 | 9.48 (±0.35) × 105 |
| Complex 7 | 4.62 (±0.11) × 1013 | 4.69 (±0.37) × 105 | 3.02 (±0.15) × 1013 | 8.33 (±0.04) × 105 |
| Complex 8 | 1.36 (±0.53) × 1014 | 7.23 (±0.23) × 105 | 6.79 (±0.14) × 1013 | 6.35 (±0.29) × 105 |
| Complex 9 | 2.99 (±0.16) × 1013 | 5.67 (±0.27) × 105 | 2.97 (±0.13) × 1012 | 5.44 (±0.24) × 105 |
| Na meclf [48] | 4.84 (±0.32) × 1013 | 1.78 (±0.11) × 106 | 2.98 (±0.31) × 1013 | 1.05 (±0.03) × 106 |
| Hmef [39] | 2.78 (±0.20) × 1013 | 1.35 (±0.22) × 105 | 7.13 (±0.34) × 1012 | 1.32 (±0.15) × 105 |
| Na dicl [40] | 8.11 (±0.34) × 1012 | 3.55 (±0.22) × 105 | 1.81 (±0.17) × 1012 | 1.63 (±0.15) × 105 |
| Hfluf [39] | 1.83 (±0.20) × 1013 | 1.06 (±0.04) × 106 | 1.86 (±0.21) × 1012 | 1.79 (±0.17) × 105 |
| Htolf [39] | 2.18 (±0.12) × 1013 | 1.60 (±0.14) × 105 | 6.10 (±0.38) × 1012 | 3.12 (±0.25) × 105 |
| H2difl [40] | 1.53 (±0.08) × 1013 | 1.93 (±0.15) × 105 | 2.67 (±0.16) × 1012 | 1.22 (±0.07) × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimiza, F.; Hatzidimitriou, A.G.; Psomas, G. Manganese(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Activity. Int. J. Mol. Sci. 2024, 25, 13457. https://doi.org/10.3390/ijms252413457
Dimiza F, Hatzidimitriou AG, Psomas G. Manganese(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Activity. International Journal of Molecular Sciences. 2024; 25(24):13457. https://doi.org/10.3390/ijms252413457
Chicago/Turabian StyleDimiza, Filitsa, Antonios G. Hatzidimitriou, and George Psomas. 2024. "Manganese(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Activity" International Journal of Molecular Sciences 25, no. 24: 13457. https://doi.org/10.3390/ijms252413457
APA StyleDimiza, F., Hatzidimitriou, A. G., & Psomas, G. (2024). Manganese(II) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure and Biological Activity. International Journal of Molecular Sciences, 25(24), 13457. https://doi.org/10.3390/ijms252413457





