The Placenta as the Main Source of Serotonin in Ontogenetic Dynamics: Inflammation-Induced Modulation of Placental Serotonin Can Be Prevented by Immunoglobulin Administration
Abstract
:1. Introduction
2. Results
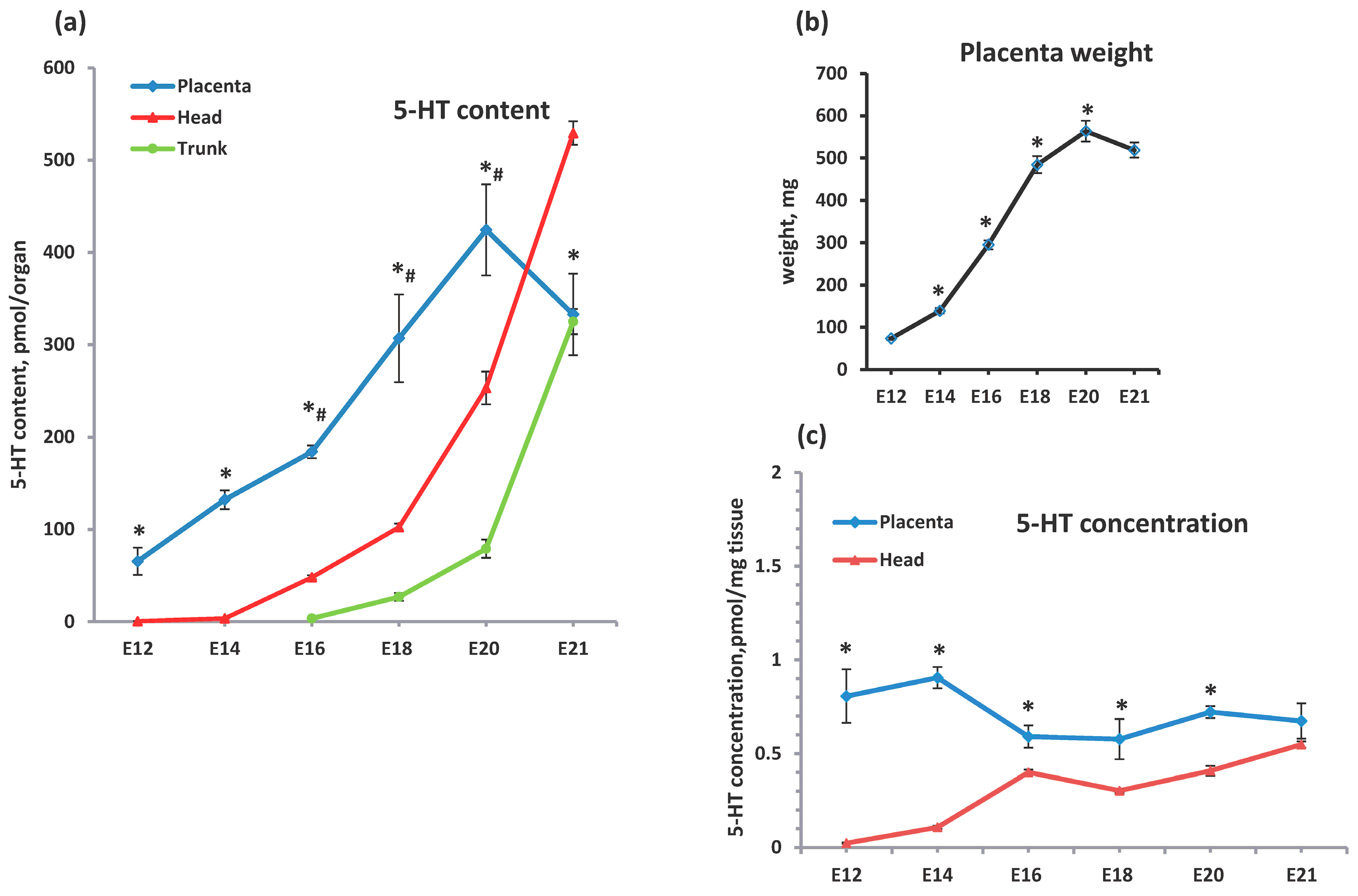
2.1. Comparative Analysis of the Serotonin Sources in the Feto-Placental Unit During Ontogenetic Dynamics
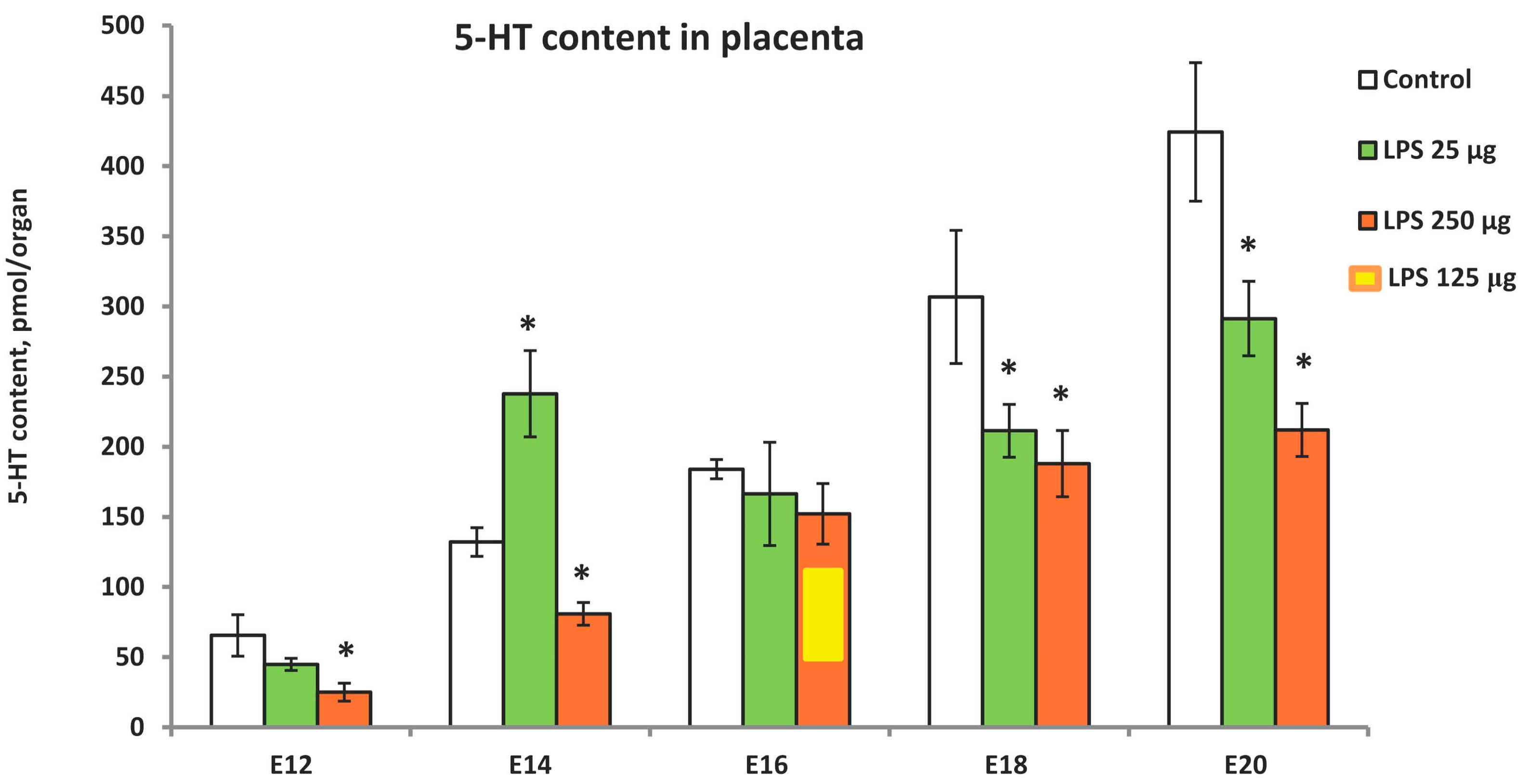
2.2. LPS-Induced Modulation of Placental Serotonin Levels in Ontogenetic Dynamics
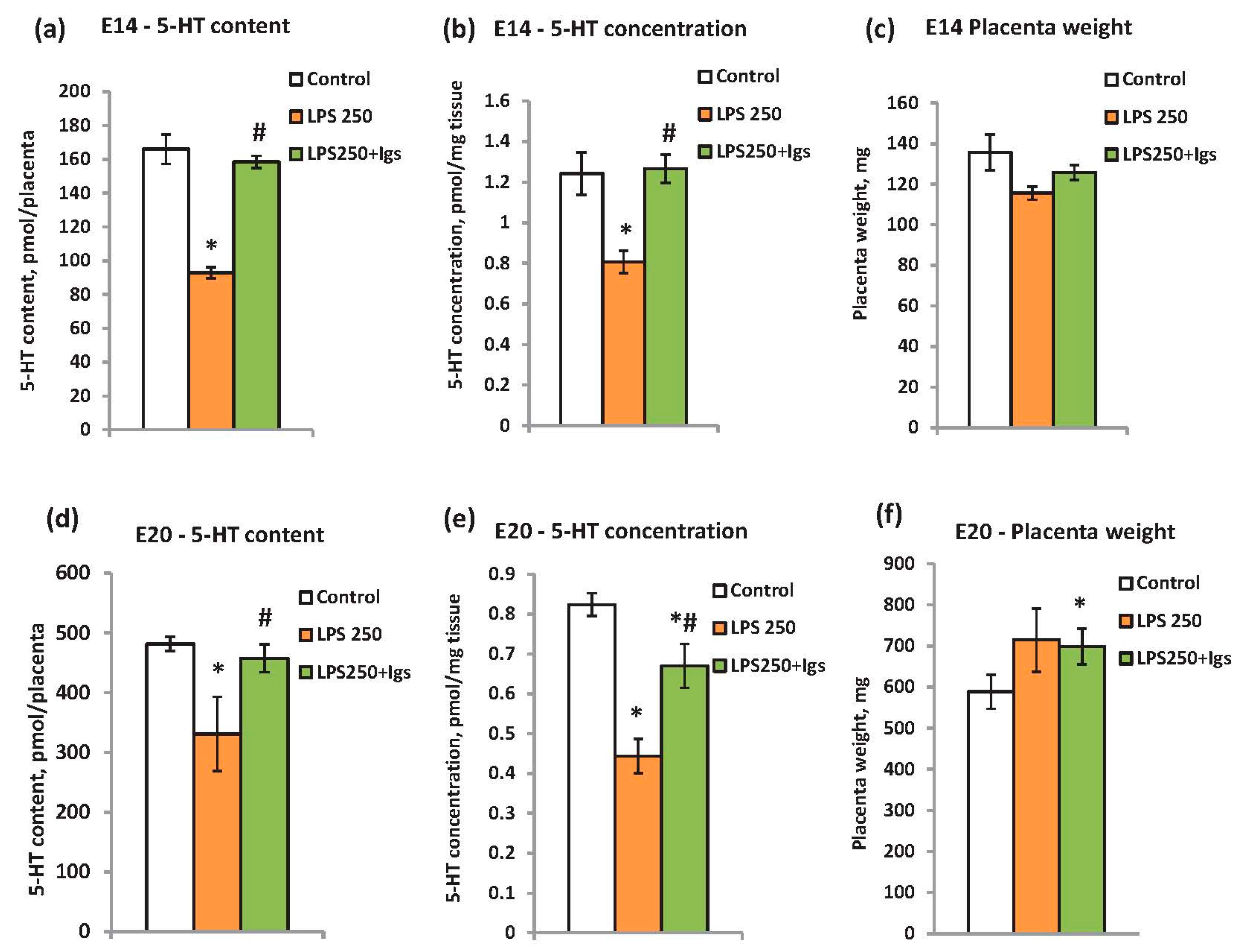
2.3. Immunoglobulin Administration Is Able to Prevent LPS-Induced Modulation of Placental Serotonin Levels
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Immunohistochemistry
4.3. High-Performance Liquid Chromatography with Fluorescent Detection (HPLC-FLD)
4.4. Statistical Analysis
4.5. Chemicals and Reagents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rossant, J.; Cross, J.C. Placental Development: Lessons from Mouse Mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental Adaptations to the Maternal–Fetal Environment: Implications for Fetal Growth and Developmental Programming. Reprod. BioMed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Goeden, N.; Chen, K.; Wilson, M.L.; King, J.; Shih, J.C.; Blakely, R.D.; Deneris, E.S.; Levitt, P. A Transient Placental Source of Serotonin for the Fetal Forebrain. Nature 2011, 472, 347–350. [Google Scholar] [CrossRef]
- Perić, M.; Bečeheli, I.; Čičin-Šain, L.; Desoye, G.; Štefulj, J. Serotonin System in the Human Placenta—The Knowns and Unknowns. Front. Endocrinol. 2022, 13, 1061317. [Google Scholar] [CrossRef]
- Choi, D.-S.; Ward, S.J.; Messaddeq, N.; Launay, J.-M.; Maroteaux, L. 5-HT2B Receptor-Mediated Serotonin Morphogenetic Functions in Mouse Cranial Neural Crest and Myocardiac Cells. Development 1997, 124, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M.; Wilkie, M.B.; Wu, C.; Singh, S. Expression of 5-HT2A, 5-HT2B and 5-HT2C Receptors in the Mouse Embryo. Int. J. Dev. Neurosci. 2000, 18, 653–662. [Google Scholar] [CrossRef]
- Azmitia, E.C. Modern Views on an Ancient Chemical: Serotonin Effects on Cell Proliferation, Maturation, and Apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.A.; Lambert, W.H.; Lauder, J.M. Serotonin and Serotonin-like Substances as Regulators of Early Embryogenesis and Morphogenesis. Cell Tissue Res. 2001, 305, 177–186. [Google Scholar] [CrossRef]
- Kindt, K.S.; Tam, T.; Whiteman, S.; Schafer, W.R. Serotonin Promotes Go-Dependent Neuronal Migration in Caenorhabditis Elegans. Curr. Biol. 2002, 12, 1738–1747. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-Induced Increases in Adult Cell Proliferation and Neurogenesis Are Mediated Through Different and Common 5-HT Receptor Subtypes in the Dentate Gyrus and the Subventricular Zone. Neuropsychopharmacology 2004, 29, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Torii, M.; Wang, L.; Rakic, P.; Levitt, P. Serotonin Modulates the Response of Embryonic Thalamocortical Axons to Netrin-1. Nat. Neurosci. 2007, 10, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Côté, F.; Fligny, C.; Bayard, E.; Launay, J.-M.; Gershon, M.D.; Mallet, J.; Vodjdani, G. Maternal Serotonin Is Crucial for Murine Embryonic Development. Proc. Natl. Acad. Sci. USA 2007, 104, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Levitt, P. Placental Source for 5-HT That Tunes Fetal Brain Development. Neuropsychopharmacology 2012, 37, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Voronezhskaya, E.E.; Melnikova, V.I.; Ivashkin, E.G. Monoamines as Adaptive Developmental Regulators: Phenomenon and Mechanisms of Action. Neurosci. Behav. Physiol. 2021, 71, 295–305. [Google Scholar] [CrossRef]
- Kameneva, P.; Melnikova, V.I.; Kastriti, M.E.; Kurtova, A.; Kryukov, E.; Murtazina, A.; Faure, L.; Poverennaya, I.; Artemov, A.V.; Kalinina, T.S.; et al. Serotonin Limits Generation of Chromaffin Cells during Adrenal Organ Development. Nat. Commun. 2022, 13, 2901. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, N.S.; Voronova, S.N.; Voronezhskaya, E.E.; Melnikova, V.I. Prenatal Stress and Adaptive Behavior of Offspring: The Role of Placental Serotonin. Dokl. Biochem. Biophys. 2022, 503, 104–107. [Google Scholar] [CrossRef]
- Goeden, N.; Velasquez, J.; Arnold, K.A.; Chan, Y.; Lund, B.T.; Anderson, G.M.; Bonnin, A. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. 2016, 36, 6041–6049. [Google Scholar] [CrossRef]
- Karahoda, R.; Robles, M.; Marushka, J.; Stranik, J.; Abad, C.; Horackova, H.; Tebbens, J.D.; Vaillancourt, C.; Kacerovsky, M.; Staud, F. Prenatal Inflammation as a Link between Placental Expression Signature of Tryptophan Metabolism and Preterm Birth. Human Mol. Genet. 2021, 30, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.H. Maternal Infection: Window on Neuroimmune Interactions in Fetal Brain Development and Mental Illness. Curr. Opin. Neurobiol. 2002, 12, 115–118. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune Involvement in Schizophrenia and Autism: Etiology, Pathology and Animal Models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Levitt, P. Fetal, Maternal, and Placental Sources of Serotonin and New Implications for Developmental Programming of the Brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of Autoimmune and Inflammatory Diseases with Intravenous Immune Globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef]
- Durandy, A.; Kaveri, S.V.; Kuijpers, T.W.; Basta, M.; Miescher, S.; Ravetch, J.V.; Rieben, R. Intravenous Immunoglobulins—Understanding Properties and Mechanisms. Clin. Exp. Immunol. 2009, 158, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Ballow, M. The IgG Molecule as a Biological Immune Response Modifier: Mechanisms of Action of Intravenous Immune Serum Globulin in Autoimmune and Inflammatory Disorders. J. Allergy Clin. Immunol. 2011, 127, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Soto, Á.; Simón-Fuentes, M.; De Las Casas-Engel, M.; Cuevas, V.D.; López-Bravo, M.; Domínguez-Andrés, J.; Saz-Leal, P.; Sancho, D.; Ardavín, C.; Ochoa-Grullón, J.; et al. IVIg Promote Cross-Tolerance against Inflammatory Stimuli In Vitro and In Vivo. J. Immunol. 2018, 201, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kyvelidou, C.; Sotiriou, D.; Zerva, I.; Athanassakis, I. Protection Against Lipopolysaccharide-Induced Immunosuppression by IgG and IgM. Shock 2018, 49, 474–482. [Google Scholar] [CrossRef]
- Abad, C.; Karahoda, R.; Kastner, P.; Portillo, R.; Horackova, H.; Kucera, R.; Nachtigal, P.; Staud, F. Profiling of Tryptophan Metabolic Pathways in the Rat Fetoplacental Unit during Gestation. Int. J. Mol. Sci. 2020, 21, 7578. [Google Scholar] [CrossRef]
- Branchek, T.A.; Gershon, M.D. Time Course of Expression of Neuropeptide Y, Calcitonin Gene-related Peptide, and NADPH Diaphorase Activity in Neurons of the Developing Murine Bowel and the Appearance of 5-hydroxytryptamine in Mucosal Enterochromaffin Cells. J. Comp. Neurol. 1989, 285, 262–273. [Google Scholar] [CrossRef]
- Goeden, N.; Bonnin, A. Ex Vivo Perfusion of Mid-to-Late-Gestation Mouse Placenta for Maternal-Fetal Interaction Studies during Pregnancy. Nat. Protoc. 2013, 8, 66–74. [Google Scholar] [CrossRef]
- Velasquez, J.C.; Goeden, N.; Bonnin, A. Placental Serotonin: Implications for the Developmental Effects of SSRIs and Maternal Depression. Front. Cell. Neurosci. 2013, 7, 47. [Google Scholar] [CrossRef]
- Chen, H.J.; Antonson, A.M.; Rajasekera, T.A.; Patterson, J.M.; Bailey, M.T.; Gur, T.L. Prenatal Stress Causes Intrauterine Inflammation and Serotonergic Dysfunction, and Long-Term Behavioral Deficits through Microbe- and CCL2-Dependent Mechanisms. Transl. Psychiatry 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Lee, S.K. Immune Modulation of i.v. Immunoglobulin in Women with Reproductive Failure. Reprod. Med. Biol. 2018, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hanswijk, S.I.; Spoelder, M.; Shan, L.; Verheij, M.M.M.; Muilwijk, O.G.; Li, W.; Liu, C.; Kolk, S.M.; Homberg, J.R. Gestational Factors throughout Fetal Neurodevelopment: The Serotonin Link. Int. J. Mol. Sci. 2020, 21, 5850. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Hallenbeck, J.M. Effects of Intrauterine Inflammation on Developing Rat Brain. J. Neurosci. Res. 2002, 70, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Dammann, O.; O’Shea, T.M. Cytokines and Perinatal Brain Damage. Clin. Perinatol. 2008, 35, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.; Tremblay, L.; Lepage, M.; Sébire, G. IL-1 Receptor Antagonist Protects against Placental and Neurodevelopmental Defects Induced by Maternal Inflammation. J. Immunol. 2010, 184, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Kinoshita, M.; Inoue, K.; Ohara, J.; Moriyama, K. Immunoglobulin for Treating Bacterial Infections: One More Mechanism of Action. Antibodies 2019, 8, 52. [Google Scholar] [CrossRef]
- Gelfand, E.W. Intravenous Immune Globulin in Autoimmune and Inflammatory Diseases. N. Engl. J. Med. 2012, 367, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Brown, A.G.; Breen, K.; Anton, L.; Maubert, M.; Burd, I. Intrauterine Inflammation, Insufficient to Induce Parturition, Still Evokes Fetal and Neonatal Brain Injury. Int. J. Dev. Neurosci. 2011, 29, 663–671. [Google Scholar] [CrossRef]
- Lifantseva, N.V.; Koneeva, T.O.; Voronezhskaya, E.E.; Melnikova, V.I. Expression of Components of the Serotonergic System in the Developing Rat Thymus. Dokl. Biochem. Biophys. 2017, 477, 401–404. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarenko, N.; Lifantseva, N.; Voronova, S.; Melnikova, V. The Placenta as the Main Source of Serotonin in Ontogenetic Dynamics: Inflammation-Induced Modulation of Placental Serotonin Can Be Prevented by Immunoglobulin Administration. Int. J. Mol. Sci. 2024, 25, 13532. https://doi.org/10.3390/ijms252413532
Bondarenko N, Lifantseva N, Voronova S, Melnikova V. The Placenta as the Main Source of Serotonin in Ontogenetic Dynamics: Inflammation-Induced Modulation of Placental Serotonin Can Be Prevented by Immunoglobulin Administration. International Journal of Molecular Sciences. 2024; 25(24):13532. https://doi.org/10.3390/ijms252413532
Chicago/Turabian StyleBondarenko, Nadezhda, Nadezhda Lifantseva, Svetlana Voronova, and Victoria Melnikova. 2024. "The Placenta as the Main Source of Serotonin in Ontogenetic Dynamics: Inflammation-Induced Modulation of Placental Serotonin Can Be Prevented by Immunoglobulin Administration" International Journal of Molecular Sciences 25, no. 24: 13532. https://doi.org/10.3390/ijms252413532
APA StyleBondarenko, N., Lifantseva, N., Voronova, S., & Melnikova, V. (2024). The Placenta as the Main Source of Serotonin in Ontogenetic Dynamics: Inflammation-Induced Modulation of Placental Serotonin Can Be Prevented by Immunoglobulin Administration. International Journal of Molecular Sciences, 25(24), 13532. https://doi.org/10.3390/ijms252413532




