Molecular Subgroups of HRD Positive Ovarian Cancer and Their Prognostic Significance
Abstract
1. Introduction
2. Results
2.1. HRD and BRCA Mutational Status
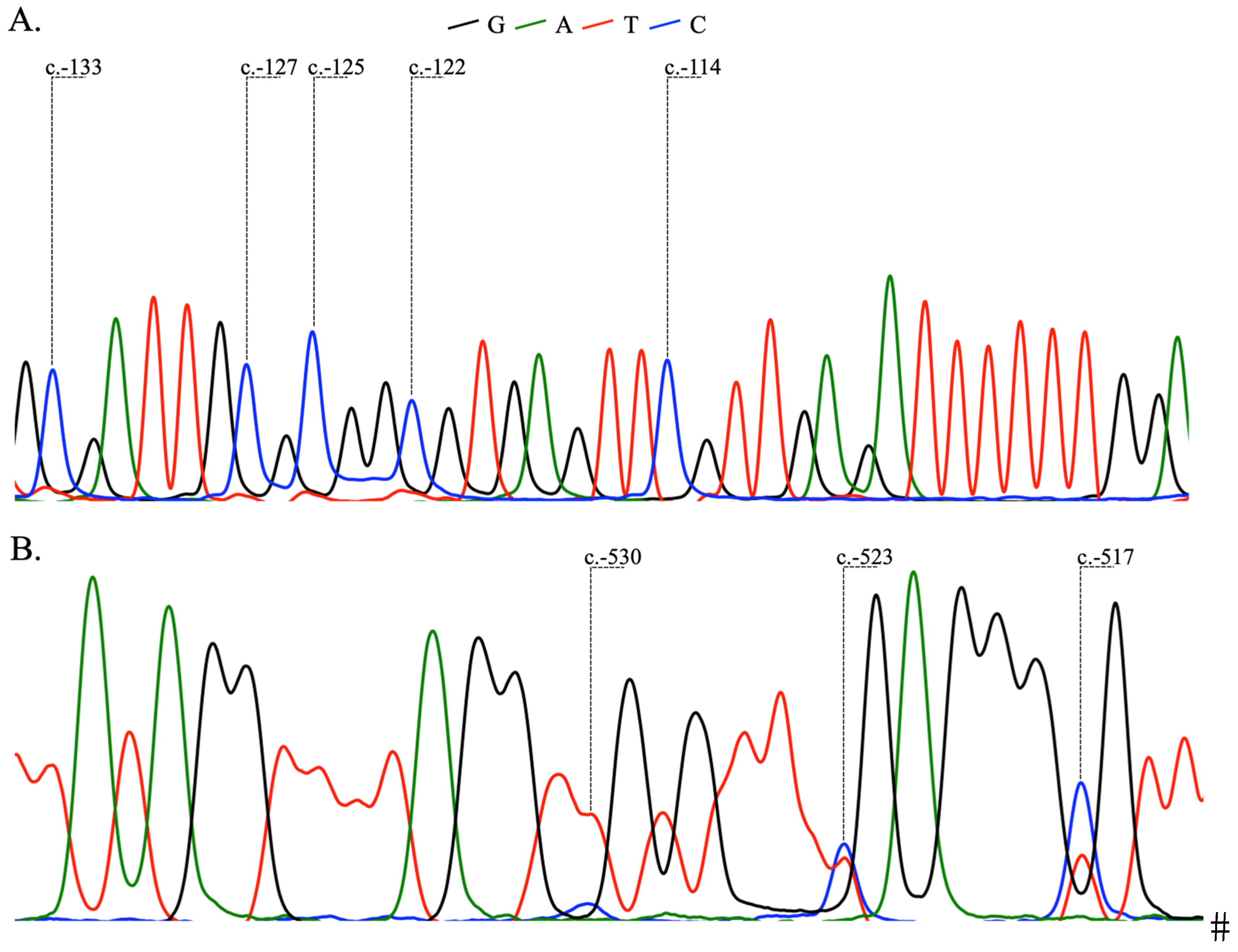
2.2. Analysis of BRCA1 and BRCA2 Methylation
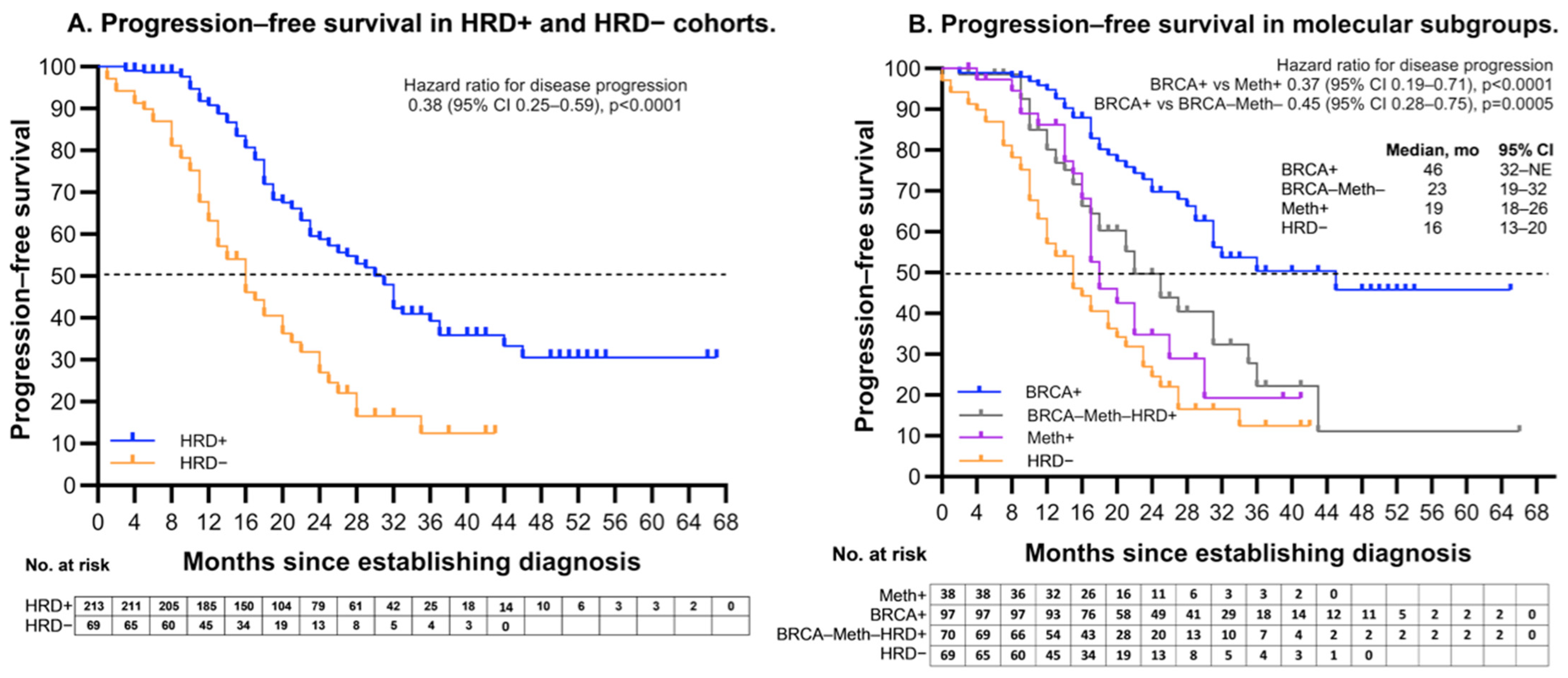
2.3. Molecular Subgroups
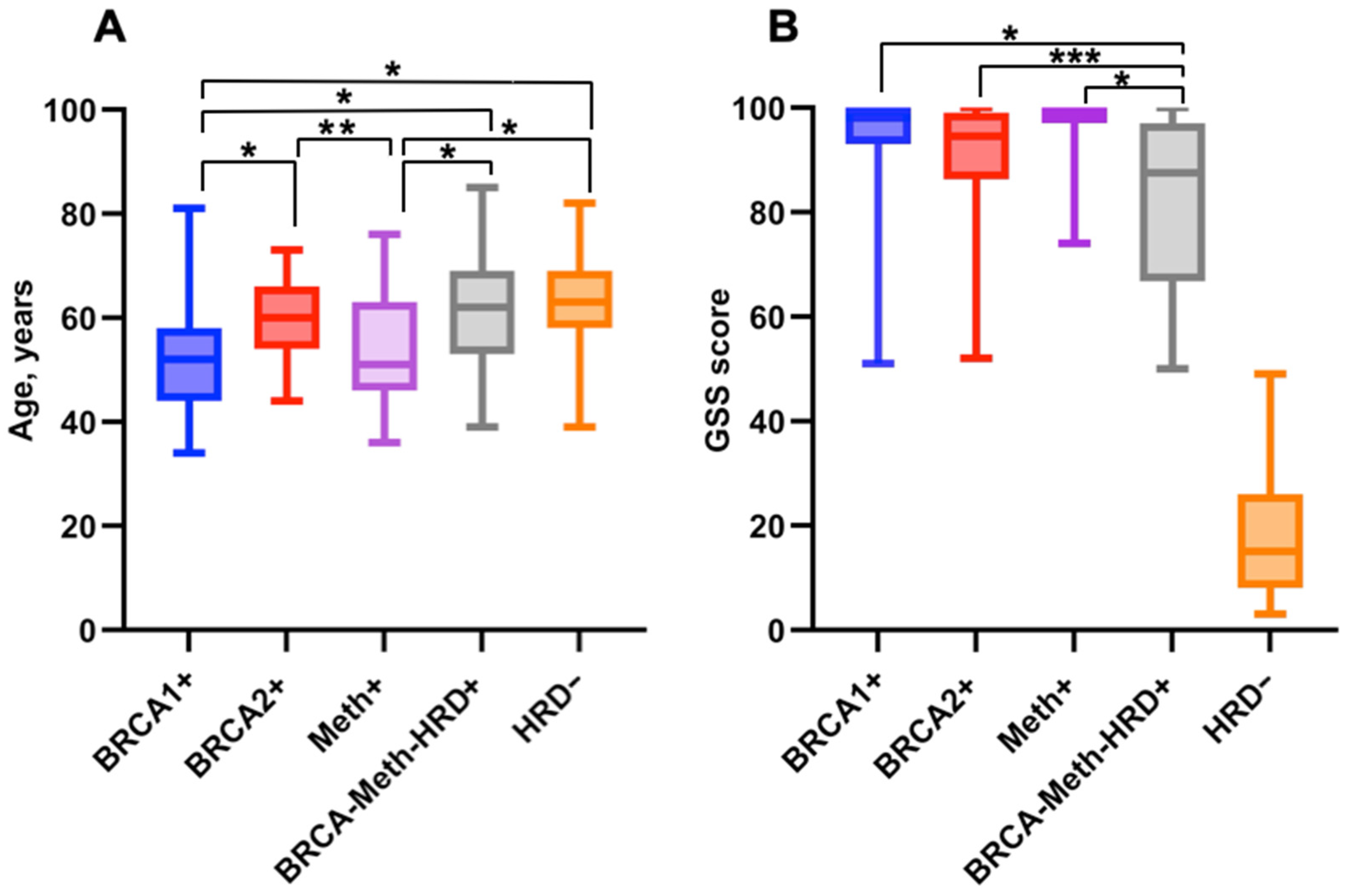
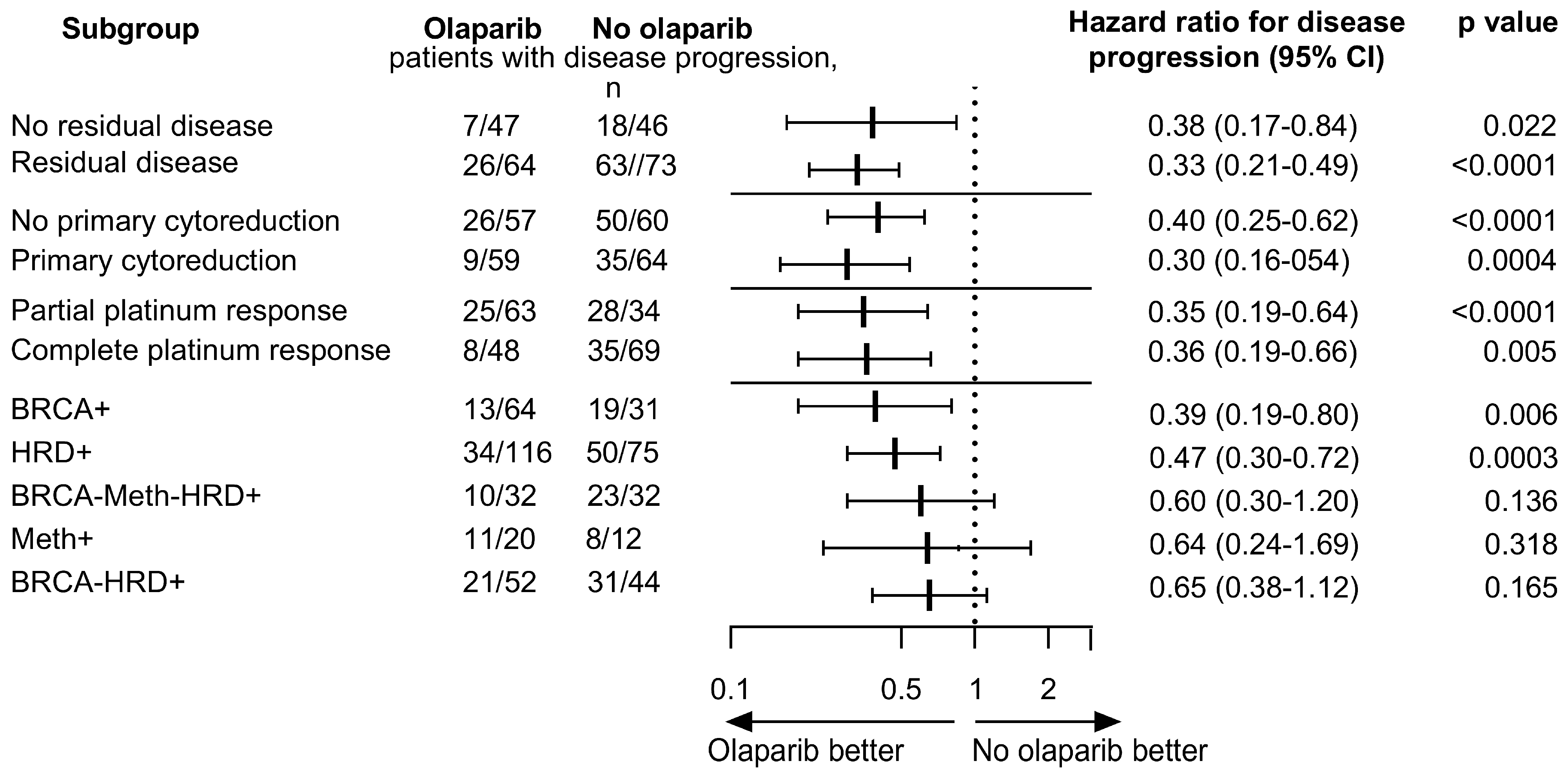
2.4. Association Between Molecular Subgroups and Clinical Characteristics
3. Discussion
4. Material and Methods
4.1. Study Cohort
4.2. Histopathological Specimens
4.3. Outcomes
4.4. Sample Preparation
4.5. Analysis of CpG Island Methylation
4.6. NGS Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The Histologic Type and Stage Distribution of Ovarian Carcinomas of Surface Epithelial Origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Mouret-Reynier, M.A.; Harter, P.; Cropet, C.; Caballero, C.; Wolfrum-Ristau, P.; Satoh, T.; Vergote, I.; Parma, G.; Nøttrup, T.J.; et al. Updated progression-free survival and final overall survival with maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Int. J. Gynecol. Cancer 2023, 34, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Rempel, E.; Kluck, K.; Beck, S.; Ourailidis, I.; Kazdal, D.; Neumann, O.; Volckmar, A.L.; Kirchner, M.; Goldschmid, H.; Pfarr, N.; et al. Pan-cancer analysis of genomic scar patterns caused by homologous repair deficiency (HRD). NPJ Precis. Oncol. 2022, 6, 36. [Google Scholar] [CrossRef]
- Ni, J.; Guo, W.; Zhao, Q.; Cheng, X.; Xu, X.; Zhou, R.; Gu, H.; Chen, C.; Chen, X. Homologous Recombination Deficiency Associated With Response to Poly (ADP-ribose) Polymerase Inhibitors in Ovarian Cancer Patients: The First Real-World Evidence From China. Front. Oncol. 2022, 11, 746571. [Google Scholar] [CrossRef]
- Kekeeva, T.; Andreeva, Y.; Tanas, A.; Kalinkin, A.; Khokhlova, S.; Tikhomirova, T.; Tyulyandina, A.; Popov, A.; Kuzmenko, M.; Volkonsky, M.; et al. HRD Testing of Ovarian Cancer in Routine Practice: What Are We Dealing With? Int. J. Mol. Sci. 2023, 24, 10497. [Google Scholar] [CrossRef]
- Swisher, E.M.; Kwan, T.T.; Oza, A.M.; Tinker, A.V.; Ray-Coquard, I.; Oaknin, A.; Coleman, R.L.; Aghajanian, C.; Konecny, G.E.; O’malley, D.M.; et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat. Commun. 2021, 12, 2487. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef]
- Kalachand, R.D.; Stordal, B.; Madden, S.; Chandler, B.; Cunningham, J.; Goode, E.L.; Ruscito, I.; Braicu, E.I.; Sehouli, J.; Ignatov, A.; et al. BRCA1Promoter Methylation and Clinical Outcomes in Ovarian Cancer: An Individual Patient Data Meta-Analysis. JNCI J. Natl. Cancer Inst. 2020, 112, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, O.; Nguyen, M.; Shield-Artin, K.; Tinker, A.V.; Teng, N.N.H.; Harrell, M.I.; Kuiper, M.J.; Ho, G.Y.; Barker, H.; Jasin, M.; et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2017, 7, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Sopik, V.; Rosen, B.; Fan, I.; McLaughlin, J.R.; Risch, H.; Sun, P.; Narod, S.A.; Akbari, M.R. Frequency of germline PALB2 mutations among women with epithelial ovarian cancer. Fam. Cancer 2016, 16, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, Y.; Zhang, M.; Shi, X.; Li, Z.; Xu, X.; Sun, T.; Dong, Y.; Xue, C.; Zhu, X.; et al. HRD effects on first-line adjuvant chemotherapy and PARPi maintenance therapy in Chinese ovarian cancer patients. NPJ Precis. Oncol. 2023, 7, 51. [Google Scholar] [CrossRef]
- Feng, Z.; Shao, D.; Cai, Y.; Bi, R.; Ju, X.; Chen, D.; Song, C.; Chen, X.; Li, J.; An, N.; et al. Homologous recombination deficiency status predicts response to plati-num-based chemotherapy in Chinese patients with high-grade serous ovarian carcinoma. J. Ovarian Res. 2023, 16, 53. [Google Scholar] [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. A randomised phase III trial of carboplatin compared with docetaxel in BRCA1/2 mutated and pre-specified triple negative breast cancer “BRCAness” subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- Stefansson, O.A.; Villanueva, A.; Vidal, A.; Martí, L.; Esteller, M. BRCA1 epigenetic inactivation predicts sensitivity to plati-num-based chemotherapy in breast and ovarian cancer. Epigenetics 2012, 7, 1225–1229. [Google Scholar] [CrossRef]
- Birgisdottir, V.; Stefansson, O.A.; Bodvarsdottir, S.K.; Hilmarsdottir, H.; Jonasson, J.G.; Eyfjord, J.E. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006, 8, R38. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Patch, A.-M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, O.; Topp, M.; Nesic, K.; Lieschke, E.; Ho, G.Y.; Harrell, M.I.; Zapparoli, G.V.; Hadley, A.; Holian, R.; Boehm, E.; et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat. Commun. 2018, 9, 3970. [Google Scholar] [CrossRef]
- Nesic, K.; Kondrashova, O.; Hurley, R.M.; McGehee, C.D.; Vandenberg, C.J.; Ho, G.-Y.; Lieschke, E.; Dall, G.; Bound, N.; Shield-Artin, K.; et al. Acquired RAD51C Promoter Methylation Loss Causes PARP Inhibitor Resistance in High-Grade Serous Ovarian Carcinoma. Cancer Res. 2021, 81, 4709–4722. [Google Scholar] [CrossRef]
- Velazquez, C.; Orhan, E.; Tabet, I.; Fenou, L.; Orsetti, B.; Adélaïde, J.; Guille, A.; Thézénas, S.; Crapez, E.; Colombo, P.-E.; et al. BRCA1-methylated triple negative breast cancers previously exposed to neoadjuvant chemotherapy form RAD51 foci and respond poorly to olaparib. Front. Oncol. 2023, 13, 1125021. [Google Scholar] [CrossRef]
- Menghi, F.; Banda, K.; Kumar, P.; Straub, R.; Dobrolecki, L.; Rodriguez, I.V.; Yost, S.E.; Chandok, H.; Radke, M.R.; Somlo, G.; et al. Genomic and epigenomic BRCA alterations predict adaptive resistance and response to platinum-based therapy in patients with triple-negative breast and ovarian carcinomas. Sci. Transl. Med. 2022, 14, eabn1926. [Google Scholar] [CrossRef]
- Lincoln, S.E.; Hambuch, T.; Zook, J.M.; Bristow, S.L.; Hatchell, K.; Truty, R.; Kennemer, M.; Shirts, B.H.; Fellowes, A.; Chowdhury, S.; et al. One in seven pathogenic variants can be challenging to detect by NGS: An analysis of 450,000 patients with implications for clinical sensitivity and genetic test implementation. Anesthesia Analg. 2021, 23, 1673–1680. [Google Scholar] [CrossRef]
- Montalban, G.; Bonache, S.; Moles-Fernández, A.; Gisbert-Beamud, A.; Tenés, A.; Bach, V.; Carrasco, E.; López-Fernández, A.; Stjepanovic, N.; Balmaña, J.; et al. Screening of BRCA1/2 deep intronic regions by targeted gene sequencing identifies the first germline BRCA1 variant causing pseudoexon activation in a patient with breast/ovarian cancer. J. Med. Genet. 2019, 56, 63–74. [Google Scholar] [CrossRef]
- Szafron, L.A.; Sobiczewski, P.; Dansonka-Mieszkowska, A.; Kupryjanczyk, J.; Szafron, L.M. An Analysis of Genetic Polymor-phisms in 76 Genes Related to the Development of Ovarian Tumors of Different Aggressiveness. Int. J. Mol. Sci. 2024, 25, 10876. [Google Scholar] [CrossRef]
- Su, R.; Liu, Y.; Wu, X.; Xiang, J.; Xi, X. Dynamically Accumulating Homologous Recombination Deficiency Score Served as an Important Prognosis Factor in High-Grade Serous Ovarian Cancer. Front. Mol. Biosci. 2021, 8, 762741. [Google Scholar] [CrossRef]
- Yuan, W.; Ni, J.; Wen, H.; Shi, W.; Chen, X.; Huang, H.; Zhang, X.; Lu, X.; Zhu, C.; Dong, H.; et al. Genomic Scar Score: A robust model predicting homologous recom-bination deficiency based on genomic instability. BJOG 2022, 129 (Suppl. S2), 14–22. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients (%) | |||
|---|---|---|---|
| HGSOC Samples | BRCA1 Exon1 Methylation | BRCA2 Exon1 Methylation | BRCA2 Promoter Methylation |
| All | 52/352 (15%) | 0/352 | 28/352 (8%) |
| BRCA+ | 0/102 | 0/102 | 10/102 (10%) |
| BRCA-/HRD+ | 52/126 (42%) | 0/126 | 10/126 (8%) |
| HRD- | 0/124 | 0/124 | 7/124 (6%) |
| Molecular Subgroups | ||||
|---|---|---|---|---|
| HRD+ | HRD− | |||
| Clinical characteristic | BRCA+ (n = 97) | Meth+ (n = 38) | BRCA–Meth– (n = 70) | GSS– (n = 69) |
| Age | ||||
| Mean | 56 | 52 | 62 | 65 |
| Range | 32–82 | 36–76 | 39–85 | 39–82 |
| Stage | ||||
| III | 65/97 (67%) | 28/38 (74%) | 47/70 (67%) | 43/69 (62%) |
| IV | 32/97 (33%) | 10/38 (26%) | 23/70 (33%) | 26/69 (38%) |
| Timing of cytoreductive surgery | ||||
| Primary debulking surgery | 48/95 (51%) | 17/31 (55%) | 35/57 (61%) | 23/57 (40%) |
| Interval cytoreduction | 37/95 (39%) | 10/31 (32%) | 12/57 (21%) | 21/57 (37%) |
| Not perfomed | 10/95 (10%) | 4/31 (13%) | 10/57 (18%) | 13/57 (23%) |
| Unknown | 2 | 7 | 13 | 12 |
| Cytoreductive surgery outcome | ||||
| Cytoreductive surgery without residual macroscopic disease | 38/92 (41%) | 14/31 (45%) | 20/48 (42%) | 21/59 (36%) |
| Cytoreductive surgery with residual macroscopic disease | 44/92 (48%) | 13/31 (42%) | 18/48 (37%) | 25/59 (42%) |
| No cytoreductive surgery | 10/92 (11%) | 4/31 (13%) | 10/48 (21%) | 13/59 (22%) |
| Unknown | 5 | 7 | 22 | 10 |
| Numbers of cycles of first-line platinum-based chemotherapy | ||||
| 1–5 | 5/88 (6%) | 2/24 (8%) | 8/61 (13%) | 15/67 (22%) |
| 6 | 79/88 (90%) | 21/24 (88%) | 50/61 (78%) | 47/67 (70%) |
| 7–8 | 4/88 (4%) | 1/24 (4%) | 3/61 (5%) | 5/67 (8%) |
| Unknown | 9 | 14 | 9 | 2 |
| Disease response to first-line chemotherapy | ||||
| Complete response/NED | 43/94 (46%) | 17/32 (53%) | 33/58 (57%) | 24/59 (40%) |
| Partial response | 47/94 (50%) | 10/32 (31%) | 19/58 (33%) | 21/59 (36%) |
| Stable disease | 4/94 (4%) | 2/32 (6%) | 6/58 (10%) | 7/59 (12%) |
| Progressive disease | 0 | 3/32 (10%) | 0 | 7/59 (12%) |
| Unknown | 3 | 6 | 12 | 10 |
| Response chemotherapy rates | ||||
| Disease control rate | 94/94 (100%) | 29/32 (90%) | 58/58 (100%) | 52/59 (88%) |
| Objective response rate | 90/94 (96%) | 27/32 (84%) | 52/58 (90%) | 45/59 (76%) |
| PARP-inhibitors maintenance therapy | ||||
| Yes | 64/95 (67%) | 20/32 (63%) | 32/64 (50%) | 3/63 (5%) |
| No | 31/95 (33%) | 12/32 (37%) | 32/64 (50%) | 60/63 (95%) |
| Unknown | 2 | 6 | 6 | 6 |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| p value | HR (95% CI) | p value | HR (95% CI) | |
| Age (≥60 vs. <60 years) | 0.008 | 1.70 (1.15–2.52) | 0.69 | 1.10 (0.69–1.74) |
| FIGO stage (IV vs. III) | 0.007 | 1.70 (1.15–2.49) | 0.87 | 1.04 (0.67–1.60) |
| BRCA1 methylation (Meth+ vs. Meth-) | 0.15 | 1.45 (0.87–2.42) | 0.48 | 1.24 (0.68–2.25) |
| BRCA mutation (BRCA+ vs. BRCA-) | <0.0001 | 0.34 (0.00–0.53) | 0.002 | 0.45 (0.00–0.75) |
| Olaparib maintence (olaparib vs. no olaparib) | <0.0001 | 0.39 (0.00–0.58) | 0.003 | 0.50 (0.00–0.79) |
| Timing of cytoreductive surgery (primary vs. non-primary) | <0.0001 | 0.40 (0.00–0.61) | 0.004 | 0.52 (0.00–0.82) |
| Residual disease (no residual vs. residual) | <0.0001 | 0.36 (0.00–0.56) | 0.001 | 0.43 (0.00–0.69) |
| Chemotherapy response (CR/PR vs. SD/PD) | <0.0001 | 0.33 (0.00–0.53) | 0.01 | 0.52 (0.00–0.86) |
| Gene | Primer (5′ to 3′) | Annealing, °C |
|---|---|---|
| BRCA1 exon1 F BRCA1 exon1 R | GTATTTTGAGAGGTTGTTGTTTAG TACCTTTACCCAAAACAAAAAATAAA | 62 |
| BRCA2 exon1 F BRCA2 exon1 R | GGTTTATTTAGGTTTGATTTT ATCACAAATCTATCCCCTCAC | 60 |
| BRCA2 promoter F BRCA2 promoter R | TTGGGGAATAGGTTTTGAGAGAATATTT AATCCCAAACCACCCTACTTAAAAAAAC | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kekeeva, T.; Dudina, I.; Andreeva, Y.; Tanas, A.; Kalinkin, A.; Musatova, V.; Chernorubashkina, N.; Khokhlova, S.; Tikhomirova, T.; Volkonsky, M.; et al. Molecular Subgroups of HRD Positive Ovarian Cancer and Their Prognostic Significance. Int. J. Mol. Sci. 2024, 25, 13549. https://doi.org/10.3390/ijms252413549
Kekeeva T, Dudina I, Andreeva Y, Tanas A, Kalinkin A, Musatova V, Chernorubashkina N, Khokhlova S, Tikhomirova T, Volkonsky M, et al. Molecular Subgroups of HRD Positive Ovarian Cancer and Their Prognostic Significance. International Journal of Molecular Sciences. 2024; 25(24):13549. https://doi.org/10.3390/ijms252413549
Chicago/Turabian StyleKekeeva, Tatiana, Irina Dudina, Yulia Andreeva, Alexander Tanas, Alexey Kalinkin, Victoria Musatova, Natalia Chernorubashkina, Svetlana Khokhlova, Tatiana Tikhomirova, Mikhail Volkonsky, and et al. 2024. "Molecular Subgroups of HRD Positive Ovarian Cancer and Their Prognostic Significance" International Journal of Molecular Sciences 25, no. 24: 13549. https://doi.org/10.3390/ijms252413549
APA StyleKekeeva, T., Dudina, I., Andreeva, Y., Tanas, A., Kalinkin, A., Musatova, V., Chernorubashkina, N., Khokhlova, S., Tikhomirova, T., Volkonsky, M., Kutsev, S., Zaletaev, D., & Strelnikov, V. (2024). Molecular Subgroups of HRD Positive Ovarian Cancer and Their Prognostic Significance. International Journal of Molecular Sciences, 25(24), 13549. https://doi.org/10.3390/ijms252413549






