Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review
Abstract
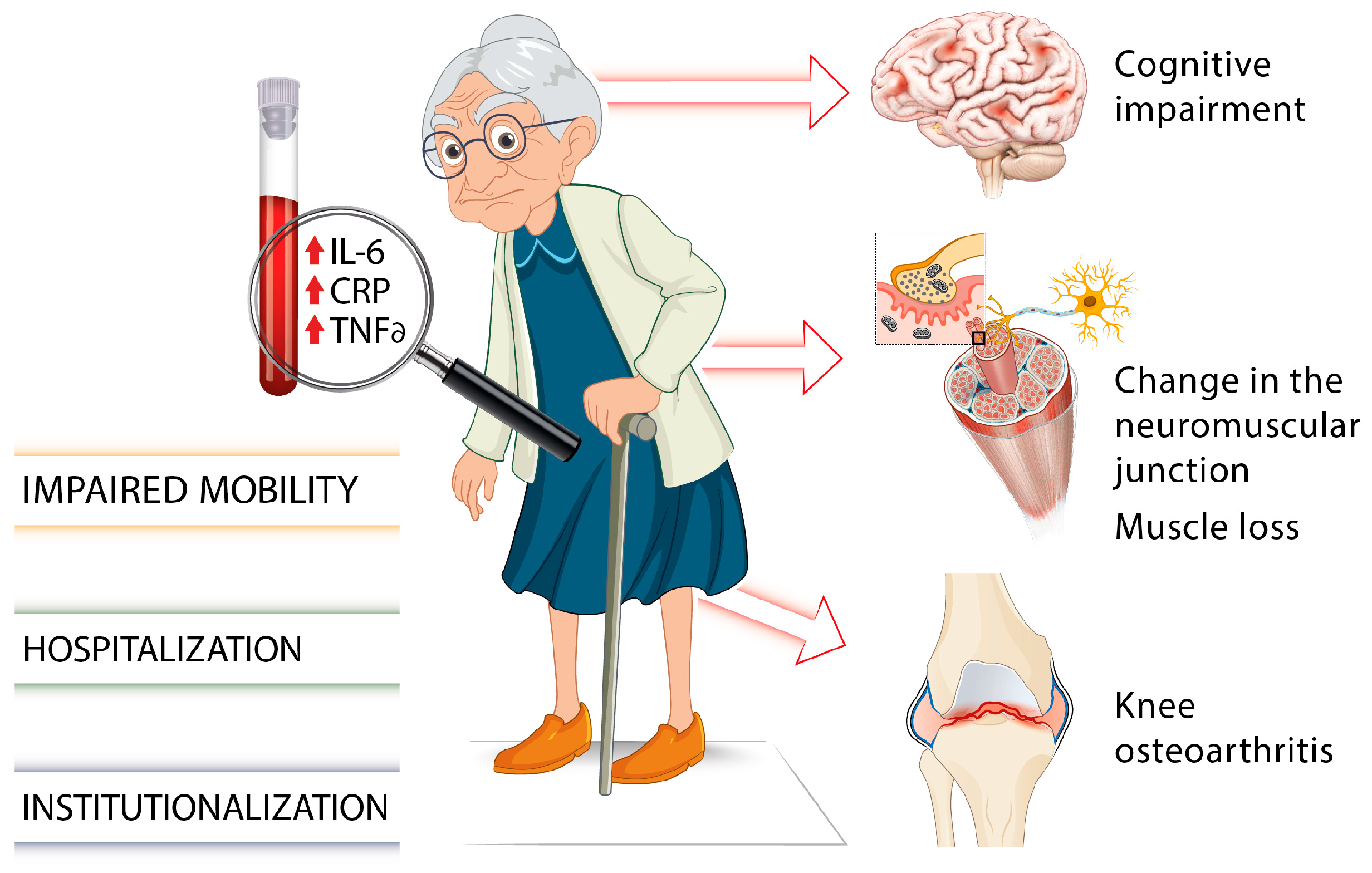
:1. Introduction
2. Methods
2.1. Identifying the Research Question
- Are proinflammatory markers a positive predictor and risk factor for gait impairment?
- Which proinflammatory markers represent a risk factor for slow gait speed and its complications?
2.2. Literature Search Methodology
3. Results
3.1. CRP
3.2. Relationship of CRP with Other Biomarkers
3.3. IL-6 and TNF-Alpha
3.4. Relationship between IL-6 and TNF-Alpha and Other Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montero-Odasso, M.; Schapira, M.; Soriano, E.R.; Varela, M.; Kaplan, R.; Camera, L.A.; Mayorga, L.M. Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 896–901. [Google Scholar] [CrossRef]
- Verghese, J.; LeValley, A.; Hall, C.B.; Katz, M.J.; Ambrose, A.F.; Lipton, R.B. Epidemiology of Gait Disorders in Community-Residing Older Adults. J. Am. Geriatr. Soc. 2006, 54, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918. [Google Scholar] [CrossRef]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50. [Google Scholar] [CrossRef]
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory Markers and Gait Speed Decline in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 1083–1089. [Google Scholar] [CrossRef]
- Hardy, S.E.; Perera, S.; Roumani, Y.F.; Chandler, J.M.; Studenski, S.A. Improvement in Usual Gait Speed Predicts Better Survival in Older Adults. J. Am. Geriatr. Soc. 2007, 55, 1727–1734. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Lévy, V.; Sebbane, G.; Boubaya, M.; Landre, T.; Bloch-Queyrat, C.; Paillaud, E.; Zelek, L. Slow gait speed is an independent predictor of early death in older cancer outpatients: Results from a prospective cohort study. J. Nutr. Health Aging 2017, 21, 202–206. [Google Scholar] [CrossRef]
- Zhu, S.; Patel, K.V.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M. Predictors of Interleukin-6 Elevation in Older Adults. J. Am. Geriatr. Soc. 2009, 57, 1672–1677. [Google Scholar] [CrossRef]
- Hacıdursunoğlu Erbaş, D.; Çınar, F.; Eti Aslan, F. Elderly patients and falls: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 2953–2966. [Google Scholar] [CrossRef]
- World Health Organization. Falls. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 20 July 2023).
- Almada, M.; Brochado, P.; Portela, D.; Midão, L.; Costa, E. Prevalence of falls and associated factors among community-dwelling older adults: A cross-sectional study. J. Frailty Aging 2021, 10, 10–16. [Google Scholar] [CrossRef]
- Gambaro, E.; Gramaglia, C.; Azzolina, D.; Campani, D.; Molin, A.D.; Zeppegno, P. The complex associations between late life depression, fear of falling and risk of falls. A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101532. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Henein, M.Y. Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2670. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M.; Teague, A.M.; Casanegra, A.I. Monitored Daily Ambulatory Activity, Inflammation, and Oxidative Stress in Patients with Claudication. Angiology 2014, 65, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.P.A.; Zunzunegui, M.-V.; Li, A.; Phillips, S.P.; Guralnik, J.M.; Guerra, R.O. Association between C-reactive protein and physical performance in older populations: Results from the International Mobility in Aging Study (IMIAS). Age Ageing 2016, 45, 274–280. [Google Scholar] [CrossRef]
- Edd, S.N.; Favre, J.; Blazek, K.; Omoumi, P.; Asay, J.L.; Andriacchi, T.P. Altered gait mechanics and elevated serum pro-inflammatory cytokines in asymptomatic patients with MRI evidence of knee cartilage loss. Osteoarthr. Cartil. 2017, 25, 899–906. [Google Scholar] [CrossRef]
- Mabey, T. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95. [Google Scholar] [CrossRef]
- Shultz, S.P.; Buck, A.N.; Fink, P.W.; Kung, S.M.; Ward, M.J.; Antal, Z.; Backus, S.I.; Kraszewski, A.P.; Hillstrom, H.J. Body mass affects kinetic symmetry and inflammatory markers in adolescent knees during gait. Clin. Biomech. 2023, 102, 105887. [Google Scholar] [CrossRef]
- Addison, O.; Drummond, M.J.; Lastayo, P.C.; Dibble, L.E.; Wende, A.R.; McClain, D.A.; Marcus, R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging 2014, 18, 532–538. [Google Scholar] [CrossRef]
- Bautmans, I.; Njemini, R.; Predom, H.; Lemper, J.-C.; Mets, T. Muscle Endurance in Elderly Nursing Home Residents Is Related to Fatigue Perception, Mobility, and Circulating Tumor Necrosis Factor-Alpha, Interleukin-6, and Heat Shock Protein 70. J. Am. Geriatr. Soc. 2008, 56, 389–396. [Google Scholar] [CrossRef]
- Broström, E.; Esbjörnsson, A.-C.; von Heideken, J.; Larsson, P.; Wretenberg, P.; Iversen, M. Change in Gait Deviation Index after anti-tumour necrosis factor-α treatment in individuals with rheumatoid arthritis: A pilot study. Scand. J. Rheumatol. 2013, 42, 356–361. [Google Scholar] [CrossRef]
- Oda, R.; Fujiwara, H.; Tokunaga, D.; Nakamura, S.; Taniguchi, D.; Kawahito, Y.; Seno, T.; Matsui, T.; Kubo, T. How do anti-TNF therapies affect gait function in patients with rheumatoid arthritis? Int. J. Rheum. Dis. 2014, 17, 57–62. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Green, D.; Tan, J.; Liao, Y.; Pearce, W.H.; Schneider, J.R.; et al. Circulating Blood Markers and Functional Impairment in Peripheral Arterial Disease. J. Am. Geriatr. Soc. 2008, 56, 1504–1510. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Leng, X.; Miller, M.E.; Kitzman, D.W.; Pahor, M.; Berry, M.J.; Marsh, A.P.; Kritchevsky, S.B.; Nicklas, B.J. Chronic Inflammation Is Associated with Low Physical Function in Older Adults Across Multiple Comorbidities. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 455–461. [Google Scholar] [CrossRef]
- Hsu, F.-C.; Kritchevsky, S.B.; Liu, Y.; Kanaya, A.; Newman, A.B.; Perry, S.E.; Visser, M.; Pahor, M.; Harris, T.B.; Nicklas, B.J.; et al. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory Markers and Incident Mobility Limitation in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.-C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 Level and the Development of Disability in Older Persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Brunetti, N.; Martelli, M.; Talerico, T.; Bastagli, L.; Muscari, A.; Mariani, E. Peripheral blood markers of inflammation and functional impairment in elderly community-dwellers. Exp. Gerontol. 2004, 39, 1415–1422. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Beavers, D.P.; Kritchevsky, S.B.; Gill, T.M.; Ambrosius, W.T.; Anton, S.D.; Fielding, R.A.; King, A.C.; Rejeski, W.J.; Lovato, L.; McDermott, M.M.; et al. Elevated IL-6 and CRP Levels Are Associated with Incident Self-Reported Major Mobility Disability: A Pooled Analysis of Older Adults with Slow Gait Speed. J. Gerontol. Ser. A 2021, 76, 2293–2299. [Google Scholar] [CrossRef]
- Custodero, C.; Pahor, M.; Mazzoccoli, C.; Manini, T.M.; Anton, S.D.; Mazzocca, A.; Lozupone, M.; Panza, F.; Sabbà, C.; Solfrizzi, V. Effect of change of interleukin-6 over time on gait speed response: Results from the lifestyle interventions and independence for elders study. Mech. Ageing Dev. 2023, 210, 111763. [Google Scholar] [CrossRef]
- Nadkarni, N.K.; Boudreau, R.M.; Studenski, S.A.; Lopez, O.L.; Liu, G.; Kritchevsky, S.; Yaffe, K.; Newman, A.B.; Rosano, C. Slow gait, white matter characteristics, and prior 10-year interleukin-6 levels in older adults. Neurology 2016, 87, 1993–1999. [Google Scholar] [CrossRef]
- Felicio, D.C.; Pereira, D.S.; Assumpção, A.M.; de Jesus-Moraleida, F.R.; de Queiroz, B.Z.; da Silva, J.P.; Rosa, N.M.d.B.; Dias, J.M.D.; Pereira, L.S.M. Inflammatory mediators, muscle and functional performance of community-dwelling elderly women. Arch. Gerontol. Geriatr. 2014, 59, 549–553. [Google Scholar] [CrossRef]
- Kositsawat, J.; Kuo, C.-L.; Barry, L.C.; Melzer, D.; Bandinelli, S.; Ferrucci, L.; Wu, R.; Kuchel, G.A. Interaction Between Vitamin D and Interleukin 6 on Slow Gait Speed: 6-Year Follow-up Data of Older Adults from InCHIANTI. J. Gerontol. Ser. A 2020, 75, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Steptoe, A.; Cadar, D.; Akbaraly, T.N.; Kivimäki, M.; Zaninotto, P. Association of 10-Year C-Reactive Protein Trajectories with Markers of Healthy Aging: Findings from the English Longitudinal Study of Aging. J. Gerontol. Ser. A 2019, 74, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kositsawat, J.; Barry, L.C.; Kuchel, G.A. C-Reactive Protein, Vitamin D Deficiency, and Slow Gait Speed. J. Am. Geriatr. Soc. 2013, 61, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 2012, 41, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-K.; Bean, J.F.; Yen, C.-J.; Leveille, S.G. Linking C-Reactive Protein to Late-Life Disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, W.; Zhou, C.; Zhu, Y.; Gu, M.; Liu, B.; Ren, H.; Yang, X. Association between levels of high-sensitivity C-reactive protein in plasma and freezing of gait in Parkinson’s disease. Aging Clin. Exp. Res. 2022, 34, 1865–1872. [Google Scholar] [CrossRef]
- Bai, A.; Shi, H.; Huang, X.; Xu, W.; Deng, Y. Association of C-Reactive Protein and Motoric Cognitive Risk Syndrome in Community-Dwelling Older Adults: The China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2021, 25, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Roose, S.P.; Zhang, J.; Wall, M.; Rutherford, B.R.; Ayonayon, H.N.; Butters, M.A.; Harris, T.; Newman, A.B.; Satterfield, S.; et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Antonio, L.; Dedeyne, L.; O’Neill, T.W.; Vanderschueren, D.; Rastrelli, G.; Maggi, M.; Bártfai, G.; Casanueva, F.F.; Giwercman, A.; et al. Inflammatory markers are associated with quality of life, physical activity, and gait speed but not sarcopenia in aged men (40–79 years). J. Cachexia Sarcopenia Muscle 2021, 12, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Heumann, Z.; Youssim, I.; Kizony, R.; Friedlander, Y.; Shochat, T.; Weiss, R.; Hochner, H.; Agmon, M. The Relationships of Fibrinogen and C-Reactive Protein with Gait Performance: A 20-Year Longitudinal Study. Front. Aging Neurosci. 2022, 14, 761948. [Google Scholar] [CrossRef]
- Langmann, G.A.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Inflammatory Markers and Frailty in Long-Term Care Residents. J. Am. Geriatr. Soc. 2017, 65, 1777–1783. [Google Scholar] [CrossRef]
- Dupont, J.; Vercauteren, L.; Amini, N.; Lapauw, L.; De Schaepdryver, M.; Poesen, K.; Dedeyne, L.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are inflammatory markers associated with sarcopenia-related traits in older adults with sarcopenia?—A cross-sectional analysis of the ENHANce study. Exp. Gerontol. 2023, 178, 112196. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Barrett, B.B.; Englund, D.A.; Liu, C.; Travison, T.G.; Cederholm, T.; Koochek, A.; von Berens, Å.; Gustafsson, T.; Benard, T.; et al. Circulating interleukin-6 is associated with skeletal muscle strength, quality, and functional adaptation with exercise training in mobility-limited older adults. J. Frailty Aging 2020, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Lohman, M.; Dumenci, L.; Lapane, K.L. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am. J. Geriatr. Psychiatry 2013, 21, 560–569. [Google Scholar] [CrossRef]
- Sipilä, S.; Tirkkonen, A.; Savikangas, T.; Hänninen, T.; Laukkanen, P.; Alen, M.; Fielding, R.A.; Kivipelto, M.; Kulmala, J.; Rantanen, T.; et al. Effects of physical and cognitive training on gait speed and cognition in older adults: A randomized controlled trial. Scand. J. Med. Sci. Sports 2021, 31, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Broadbent, J.M.; Cohen, H.J.; d’Arbeloff, T.; Elliott, M.; Hancox, R.J.; Harrington, H.; Hogan, S.; et al. Association of Neurocognitive and Physical Function with Gait Speed in Midlife. JAMA Netw. Open 2019, 2, e1913123. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef]
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Leach, S.; Pasco, J.A. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci. Rep. 2020, 10, 10367. [Google Scholar] [CrossRef]
- Fornage, M.; Chiang, Y.A.; O’Meara, E.S.; Psaty, B.M.; Reiner, A.P.; Siscovick, D.S.; Tracy, R.P.; Longstreth, W.T., Jr. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain. Stroke 2008, 39, 1952–1959. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef]
- Wersching, H.; Duning, T.; Lohmann, H.; Mohammadi, S.; Stehling, C.; Fobker, M.; Conty, M.; Minnerup, J.; Ringelstein, E.; Berger, K.; et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010, 74, 1022–1029. [Google Scholar] [CrossRef]
- Benson, R.R.; Guttmann, C.R.G.; Wei, X.; Warfield, S.K.; Hall, C.; Schmidt, J.A.; Kikinis, R.; Wolfson, L.I. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002, 58, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Kuller, L.H.; Chung, H.; Arnold, A.M.; Longstreth, W.T.; Newman, A.B. Subclinical Brain Magnetic Resonance Imaging Abnormalities Predict Physical Functional Decline in High-Functioning Older Adults. J. Am. Geriatr. Soc. 2005, 53, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.F.; Chan, Y.H.; Seetharaman, S.; Denishkrshna, A.; Au, L.; Kwek, S.C.; Chen, M.Z.; Ng, S.E.; Hui, R.J.Y.; Merchant, R.A. Impact of Exercise and Cognitive Stimulation Therapy on Physical Function, Cognition and Muscle Mass in Pre-Frail Older Adults in the Primary Care Setting: A Cluster Randomized Controlled Trial. J. Nutr. Health Aging 2023, 27, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.W.; Qiao, Y.; Gmelin, T.; Santanasto, A.J.; Boudreau, R.M.; Walston, J.D.; Perls, T.T.; Christensen, K.; Newman, A.B.; Glynn, N.W.; et al. Association of fatigue, inflammation, and physical activity on gait speed: The Long Life Family Study. Aging Clin. Exp. Res. 2022, 34, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; Kümmer, A.; Cardoso, F.; Teixeira, A.L. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci. Lett. 2010, 468, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Kadomura, T.; Ohta, K.; Koyama, K.; Okuda, H.; Kobayashi, M.; Ishii, C.; Fujiwara, Y.; Nishioka, T.; Ohmae, Y.; et al. Analyses of laboratory data and establishment of reference values and intervals for healthy elderly people. J. Nutr. Health Aging 2012, 16, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Thyagarajan, B.; Sun, F.; Honig, L.S.; Schupf, N.; Cosentino, S.; Feitosa, M.F.; Wojczynski, M.; Newman, A.B.; Montano, M.; et al. Age and Sex Distributions of Age-Related Biomarker Values in Healthy Older Adults from the Long Life Family Study. J. Am. Geriatr. Soc. 2016, 64, e189–e194. [Google Scholar] [CrossRef]
- Arbeev, K.G.; Ukraintseva, S.V.; Akushevich, I.; Kulminski, A.M.; Arbeeva, L.S.; Akushevich, L.; Culminskaya, I.V.; Yashin, A.I. Age trajectories of physiological indices in relation to healthy life course. Mech. Ageing Dev. 2011, 132, 93–102. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the Reliability and Validity of a Shorter Walk Test Compared with the 10-Meter Walk Test for Measurements of Gait Speed in Healthy, Older Adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Giannouli, V.; Markopoulou, A.; Kiosseoglou, G.; Kosmidis, M.H. Neuropsychological functioning in patients with interstitial lung disease. Appl. Neuropsychol. Adult 2022, 29, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.S.; Pin, T.W. Reliability, validity and minimal detectable change of 2-minute walk test, 6-minute walk test and 10-meter walk test in frail older adults with dementia. Exp. Gerontol. 2019, 115, 9–18. [Google Scholar] [CrossRef] [PubMed]

| Reference | Sample Characteristics | Inflammatory Biomarker | Gait Assessment | Design of the Study | Main Findings |
|---|---|---|---|---|---|
| Verghese et al., 2012 [7] | 333 participants. Age: 80.27 (5.48) | IL-6 and TNFα | GAITRite®. Participants walked for two trials (2–3 h apart) on a walkway 15 feet long | Cross-sectional and longitudinal analyses | IL-6 at baseline predicted future risk of gait speed decline; each one-unit increase in log IL-6 (which corresponds to an increase of 1.7 times in untransformed IL-6 levels) |
| Penninx et al., 2004 [30] | 2979 participants. Age: 73.5 ± 2.9 | IL-6, TNFα, CRP, IL-6R, IL-2R, TNFsR1, and TNFsR2 | Difficulty or inability to walk a quarter of a mile or climb 10 steps in two consecutive six-month follow-up assessments. | Prospective cohort study | Persons who developed incident mobility limitation had significantly higher serum levels of IL-6 (2.18 pg/mL), TNFα (3.36 pg/mL), and CRP (2.31 mg/L) at baseline. |
| Ferrucci et al., 1999 [31] | 350 participants. Age: 79.4 ± 0.3 | IL-6 | Difficulty in mobility or ADL (walking half a mile or climbing a flight of stairs, walking across a small room, bathing, transferring from bed to chair, and using the toilet). | Longitudinal case-based design. | Older persons who are completely independent in ADL and mobility and have circulating levels of IL-6 greater than 2.5 pg/mL are at higher risk of functional decline over the subsequent 4 years |
| Beavers et al., 2021 [36] | 1732 participants. Age: 77.5 ± 5.1 | IL-6 and CRP | Walking 400 m in less than 15 min. | Observational pooled analysis | High baseline IL-6 and CRP were associated with an increased risk of major mobility disability among older adults with slow gait speed (<1.0 m/s) |
| Custodero et al., 2023 [37] | 1300 participants. Age: 79.14 ± 5.27 | IL-6 | 400 m gait speed | Multicenter single-blind randomized clinical trial | No effects were observed on 400 m gait speed for wider range of variation of plasma IL-6 levels |
| Nadkarni et al., 2016 [38] | 179 participants. Age: 83.1 (2.7) | IL-6 | Gait speed was measured on a 4-m-long GaitMatII (EQ Inc., Chalfont, PA, USA). | Prospective cohort study | Sustained exposure to high IL-6 over 10 years rather than the rate of change in IL-6 or an isolated high IL-6 level may adversely affect gait speed |
| Carvalho Felicio et al., 2014 [39] | 221 participants. Age: 71.07 ± 4.93 | IL-6 and sTNFR1 | Q&Q stopwatch (CBM Corp., Japan, Tokyo) was used to measure habitual and fast gait velocity over 10 m. | Cross-sectional study | There was no negative correlation between inflammatory mediators and muscle or physical performance in elderly women. |
| Kositsawat et al., 2020 [40] | Participants: 713 at 3-year visit and 600 at 6-year visit. Age: 74.6 (7.1) | IL-6, CRP and IGF-1 | Gait speed was assessed over a 4-m walk at every visit. | Prospective observational cohort study | Participants with slow gait speed (<0.8 m/s) had high CRP (≥3 mg/L) and high IL-6 (≥2.87 pg/mL) |
| Lassale et al., 2019 [41] | 2437 participants aged 47–87 years | hs-CRP | Gait speed was measured directly, with respondents aged 60 years and older walking a distance of 8 feet (2.4 m) twice: the mean speed (m/s) of the two trials was used. | Prospective cohort study | Stable-high trajectory (5.7 mg/L) was associated with low grip strength, walking speed, and balance impairment, but the precision of the estimates was low and they did not reach statistical significance at the conventional level |
| Kositsawat et al., 2013 [42] | 1826 participants aged 50–85 years | CRP and Vitamin D | Participants were asked to walk 20 feet (6.1 m) unassisted or with or without a cane or a walker at usual speed. Slow gait speed was defined as <0.8 m/s. | Cross-sectional study | Participants with high CRP without severe vitamin D deficiency were more likely to have slow gait speed. |
| Verghese et al., 2012 [43] | 624 participants. Age: 80.34 (5.37) | hs-CRP | Subjects walked on an instrumented walkway (GAITRite®, CIR systems, Havertown, PA, USA). | Prospective cohort | Elevated hs-CRP levels (≥3 mg/L) at baseline were associated with a faster annual decline in gait velocity of 0.91 cm/s |
| Kuo et al., 2006 [44] | 1680 participants. Age >60 years old | hs-CRP | 20-foot timed walk test. | Cross-sectional study | CRP had an inverse relationship to leg power and walking speed |
| Liu et al., 2022 [45] | 217 participants. Aged: 68.83 ± 9.50 | CRP | Freezing of gait was diagnosed in Parkinson’s disease patients who scored at least 1 on the third Freezing of Gait score. | Prospective study | A cut-off level of 0.935 mg/L distinguished patients with or without Freezing of gait (FOG). hs-CRP levels in plasma can be used as a sensitive biomarker of FOG and of Parkinson’s disease progression |
| Bai et al., 2021 [46] | 5642 participants. Age: 68.13 ± 6.55 | CRP | The average time respondents took to walk along a straight path twice was used to compute gait speed. | Longitudinal Study | Participants with higher CRP levels had an increased likelihood of motoric cognitive risk syndrome |
| Brown et al., 2016 [47] | 3075 participants. Age: 73.6 (2.87) | IL-6 | Participants’ usual walking speed was assessed over 6, 10, and 20 m. | Longitudinal Study | Slow gait was associated with inflammation and depression |
| Ravaglia et al., 2004 [34] | 739 participants. Age: 74.0 ± 6.6 | hs-CRP, fibrinogen, leucocyte count, cholesterol, and albumin | Participants underwent the Tinetti test for gait and balance. | Cross-sectional study | CRP (>0.4 mg/dL) was consistently associated with a Tinetti test score below 19 |
| Dupont et al., 2021 [48] | 2577 participants. Aged: 59.66 ± 11.00 | hs-CRP, WBC, and Albumin | Physical performance was assessed by gait speed. | Cross-sectional study | An increase in hs-CRP levels was negatively associated with gait speed; only significant in older adults, but not in middle-aged men. |
| Heumann et al., 2022 [49] | 373 participants. Aged: 64 ± 13 | CRP, Fibrinogen | Participants were asked to walk for 1 min or ten meters as a Single Task (ST) and a Dual Task (DT). | Population-based longitudinal study | Gait speed under ST was negatively associated with baseline levels of CRP |
| Langmann et al., 2017 [50] | 178 participants | IL-6, TNFα, hs-CRP | Gait speed was assessed with the Fried frailty index on a 6-m walk | Prospective study | Higher baseline hs-CRP and IL-6 levels were associated with worse ADL performance, physical performance, and gait speed at 12 months |
| Dupont et al., 2023 [51] | 40 participants. Aged: 77.1 (6.8) | IL-6, TNFα, CRP, IL-1β, IL-8 | The subject was instructed to walk six meters at a usual pace. | Cross-sectional study | CRP, IL-6, and TNFα have an inverse correlation with the SF-36 physical component score. This study highlights an important role of gender |
| Grosicki et al., 2020 [52] | 95 participants. Aged: 78 (5) | IL-6 | Walking speed over 400 m (m) | Randomized controlled trial | Reductions in IL-6 were associated with gait speed improvements in “higher” IL-6 individuals (>1.36 pg/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brognara, L.; Luna, O.C.; Traina, F.; Cauli, O. Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1368. https://doi.org/10.3390/ijms25031368
Brognara L, Luna OC, Traina F, Cauli O. Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(3):1368. https://doi.org/10.3390/ijms25031368
Chicago/Turabian StyleBrognara, Lorenzo, Oscar Caballero Luna, Francesco Traina, and Omar Cauli. 2024. "Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review" International Journal of Molecular Sciences 25, no. 3: 1368. https://doi.org/10.3390/ijms25031368
APA StyleBrognara, L., Luna, O. C., Traina, F., & Cauli, O. (2024). Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. International Journal of Molecular Sciences, 25(3), 1368. https://doi.org/10.3390/ijms25031368









