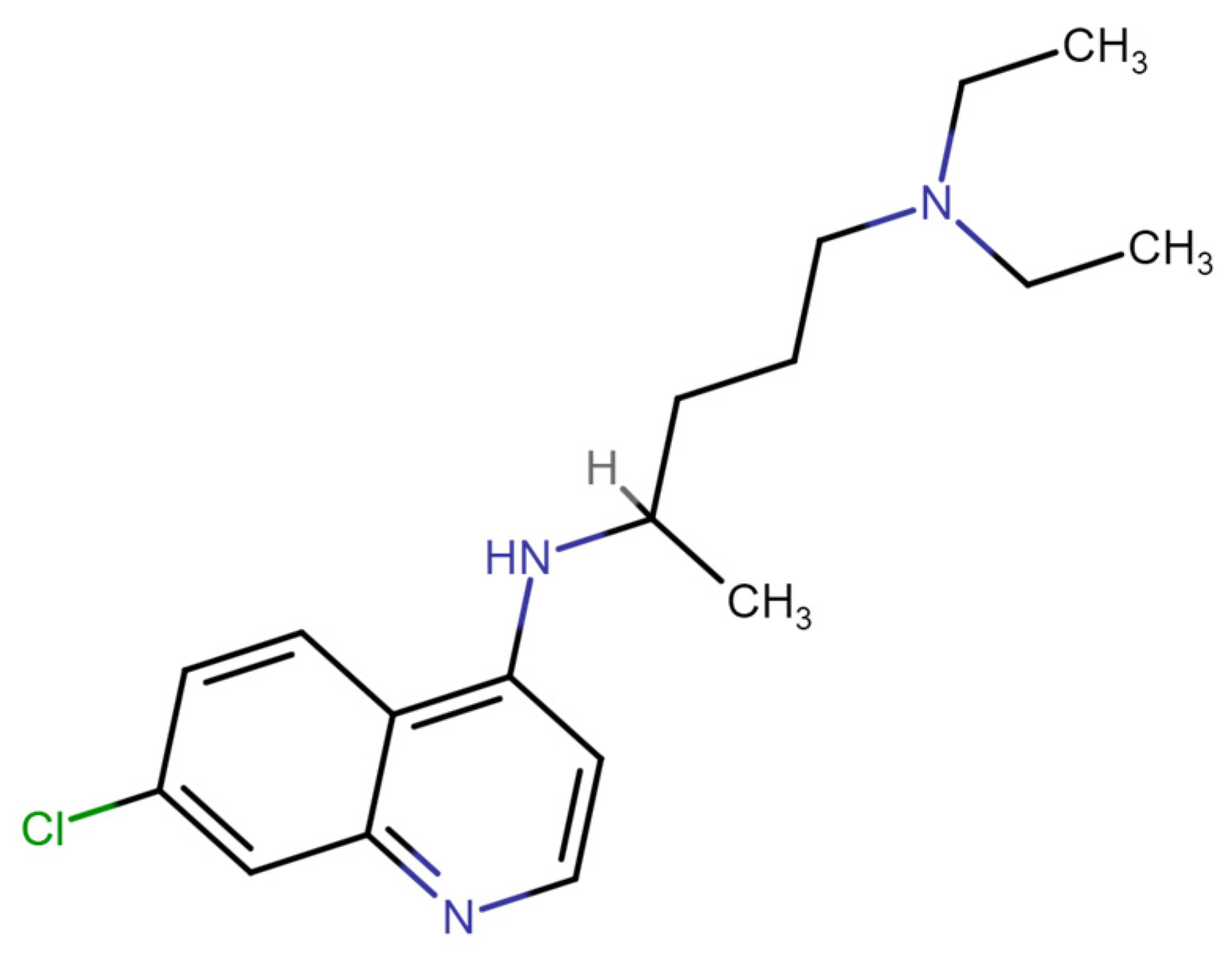
Mechanism of DNA Intercalation by Chloroquine Provides Insights into Toxicity
Abstract
1. Introduction
2. Results
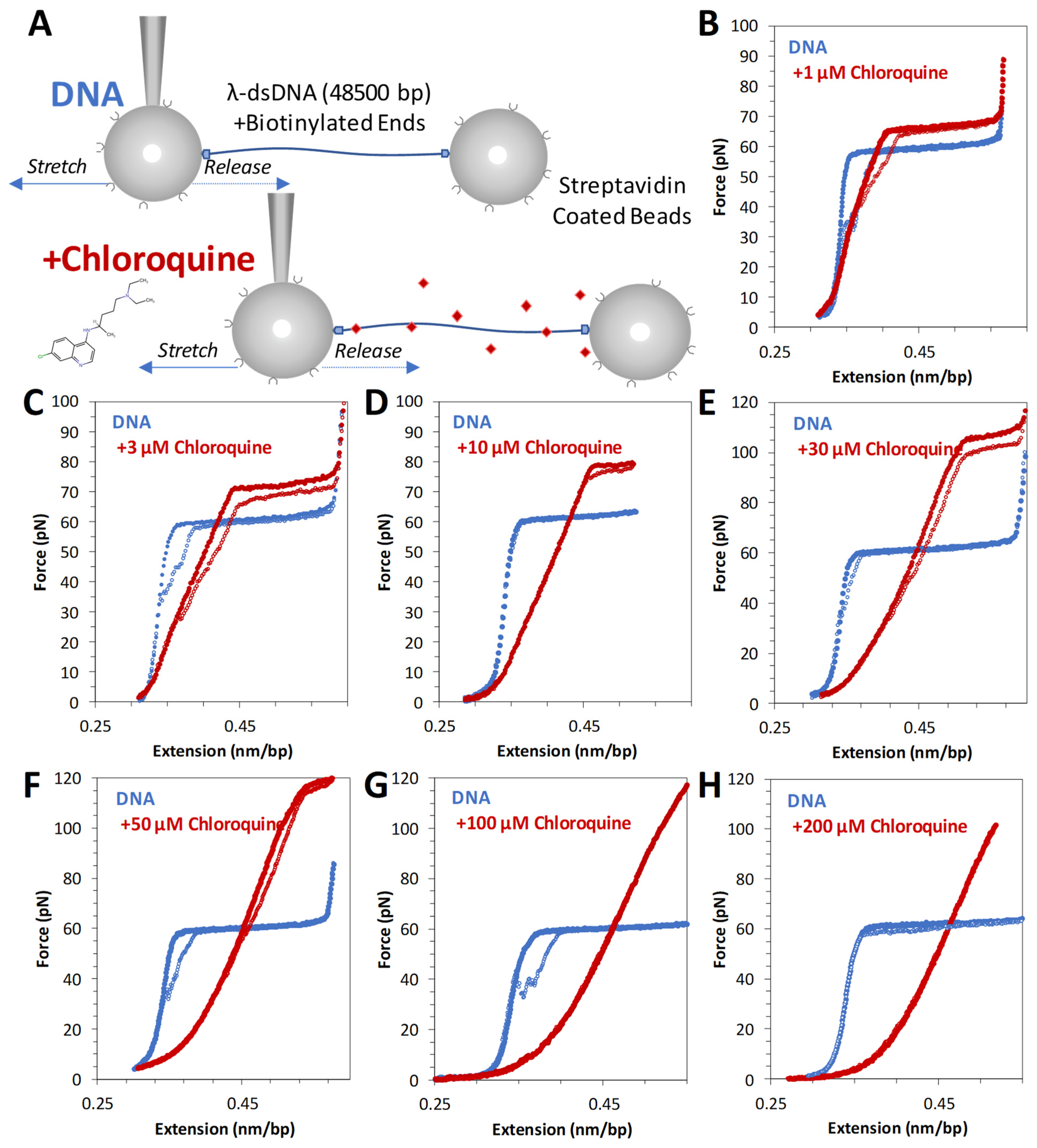
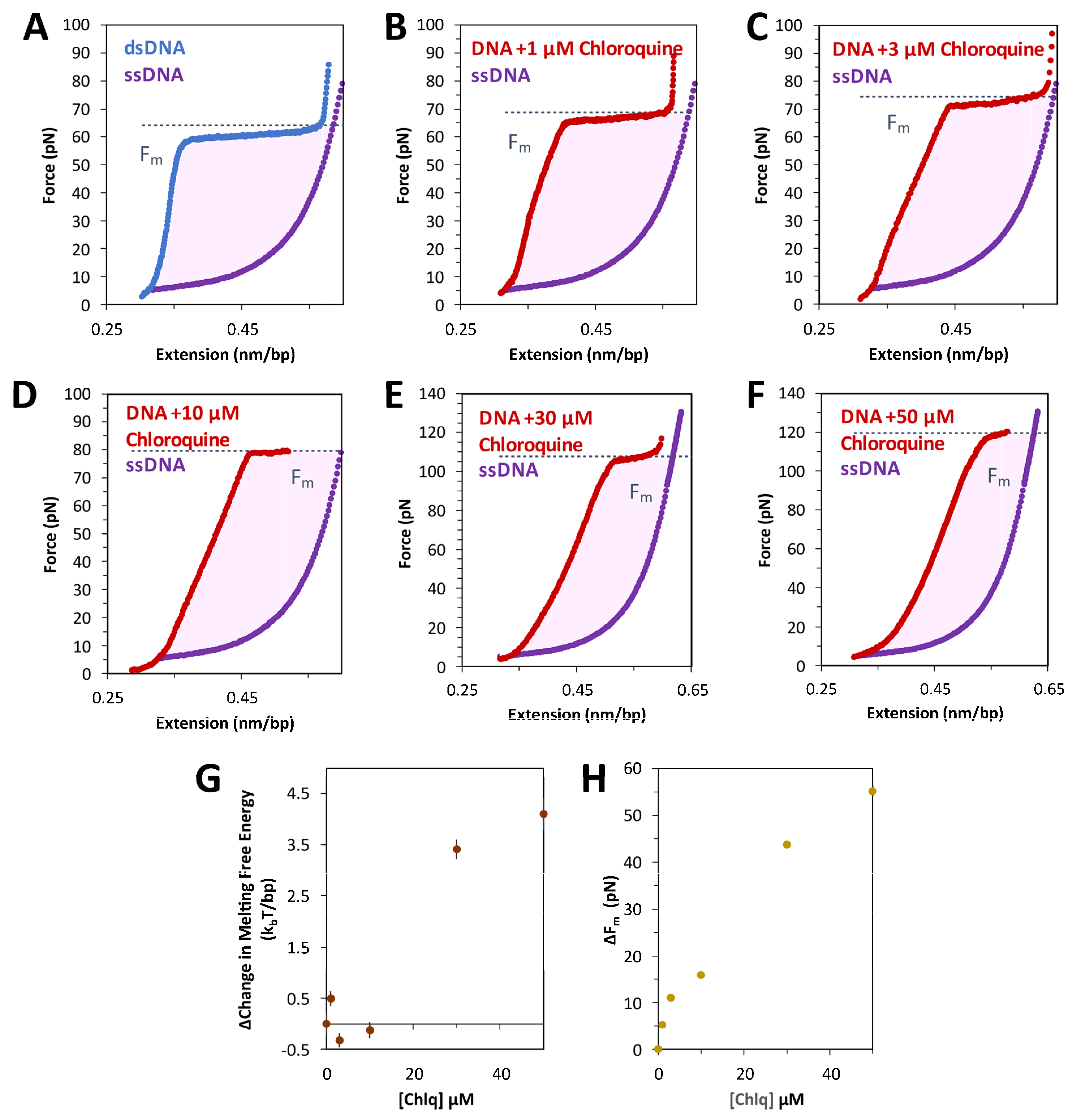
2.1. Force Extension of dsDNA + Chloroquine in Optical Tweezers
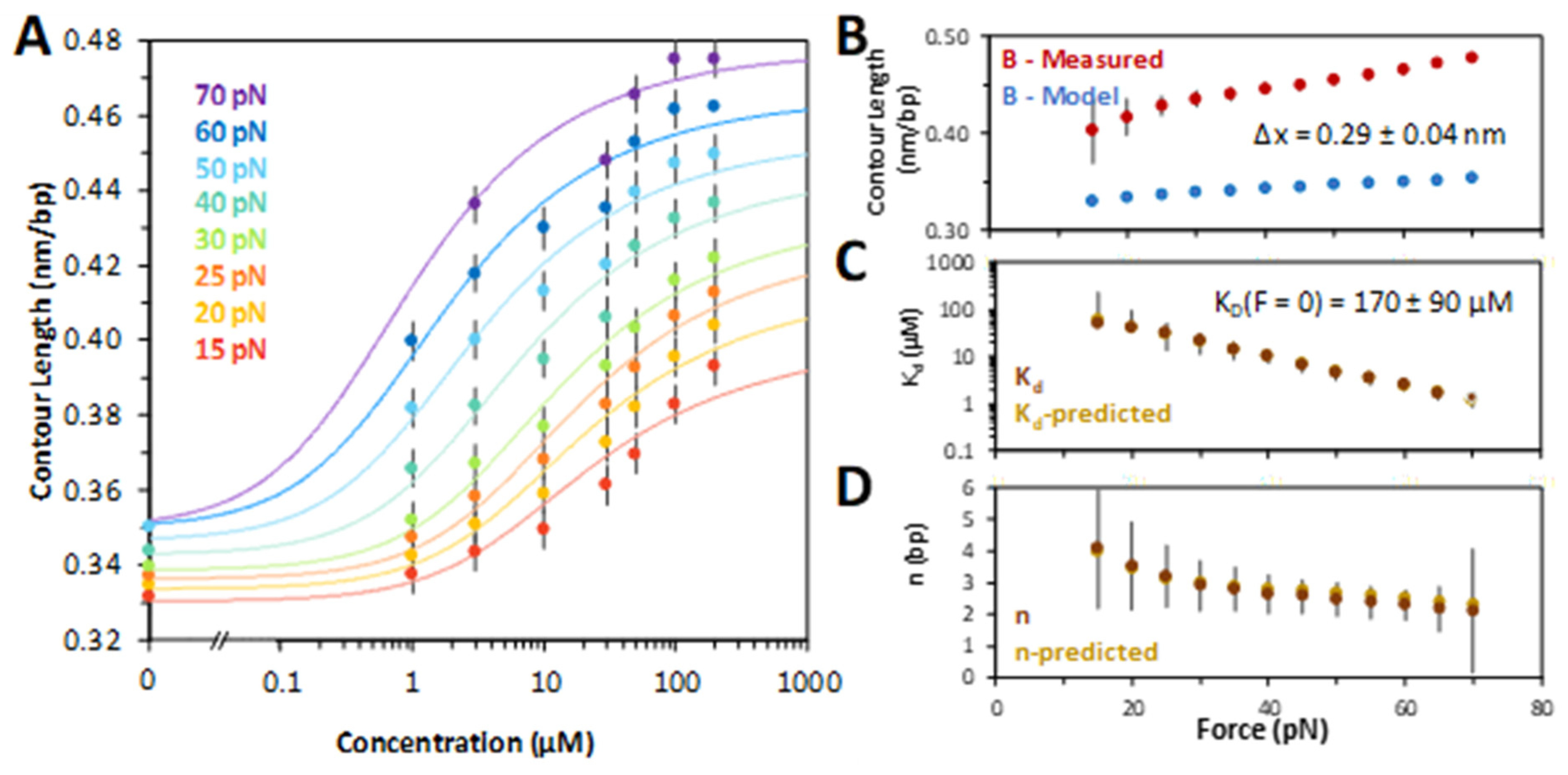
2.2. Characterizing Chloroquine Binding to dsDNA
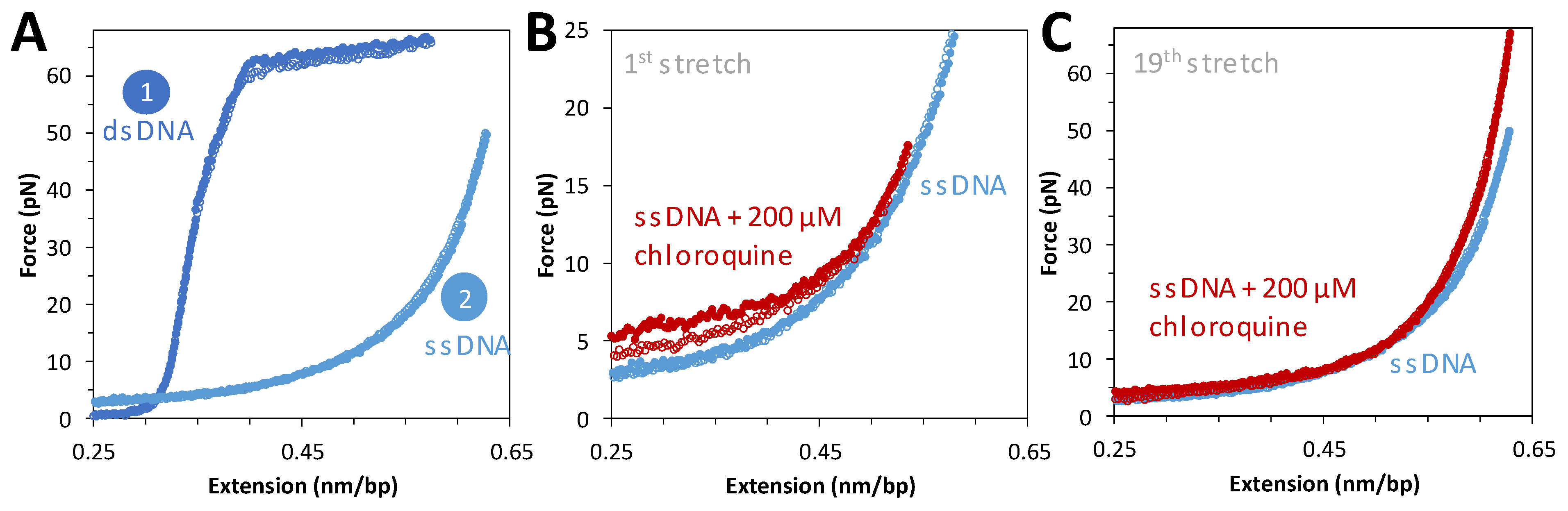
2.3. Chloroquine Binding to ssDNA Is Weaker Than to B-DNA
2.4. Chloroquine Intercalation Stabilizies B-DNA
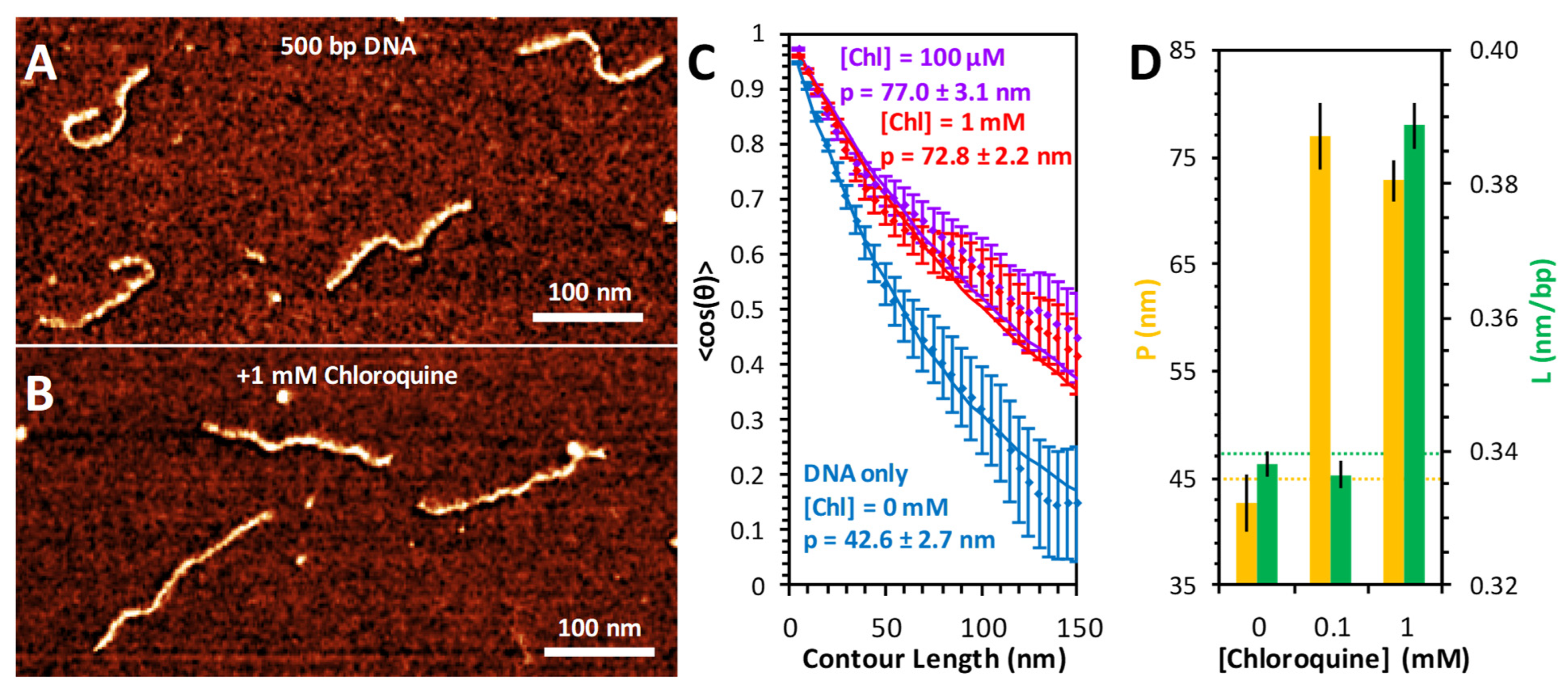
2.5. AFM Measures Distinct Binding Modes
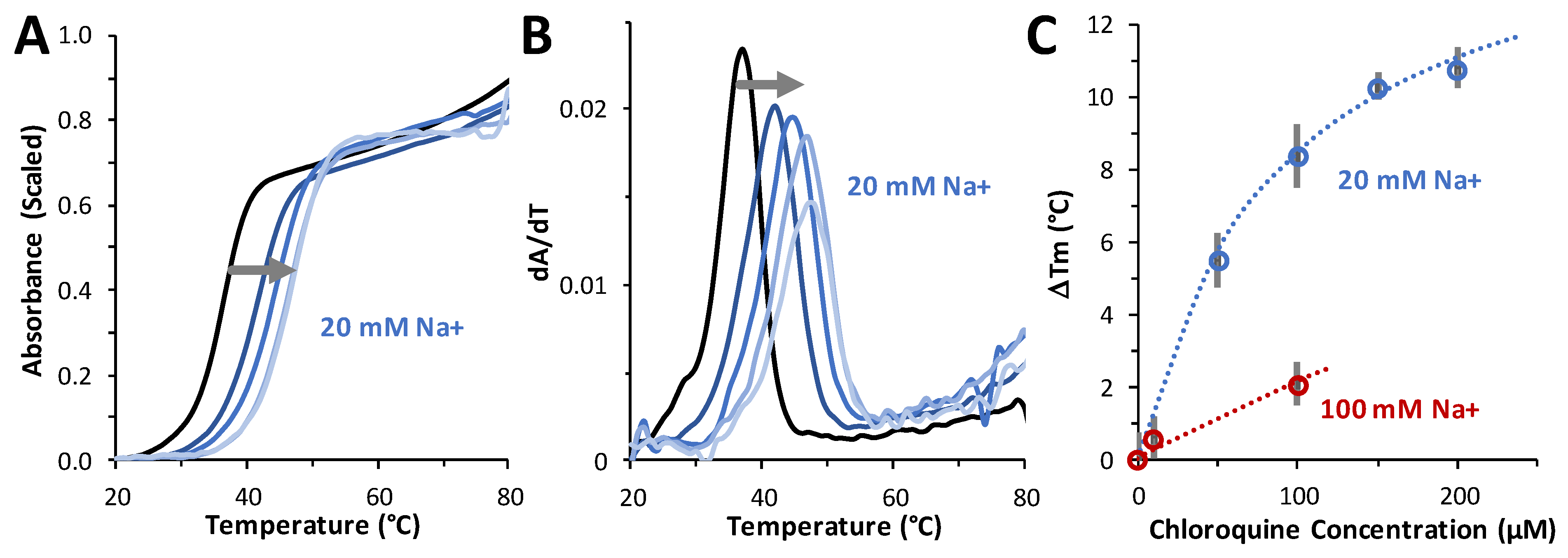
2.6. Chloroquine Increases dsDNA Melting Temperature
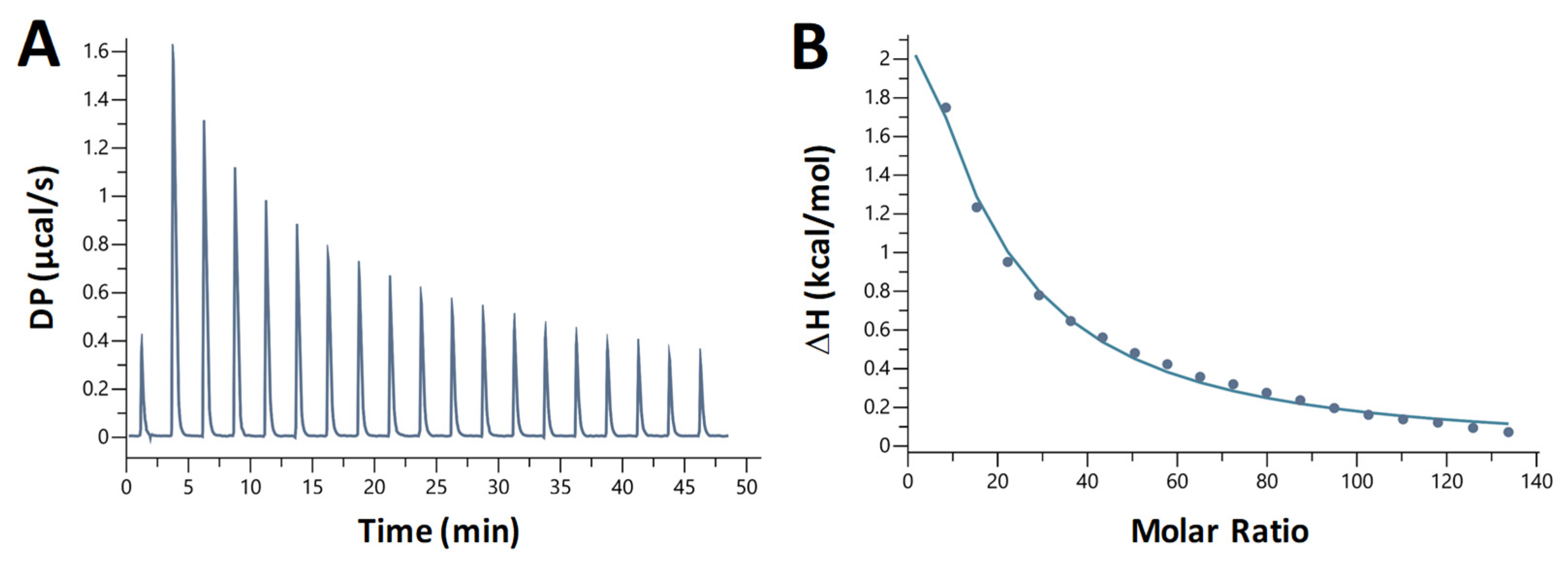
2.7. Chloroquine-B DNA Binding Is Entropically Driven and Endothermic
3. Discussion
4. Materials and Methods
4.1. Samples and Solutions
4.2. Optical Tweezers
4.3. Models of Polymer Elasticity
4.4. Deducing Binding Affinity
4.5. Calculating the Energy of Overstretching
4.6. Atomic Force Microscopy
4.7. Thermal Melting Studies
4.8. Isothermal Titration Calorimetry
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoekenga, M.T. The treatment of acute malaria with single oral doses of amodiaquin, chloroquine, hydroxychloroquine and pyrimethamine. Am. J. Trop. Med. Hyg. 1954, 3, 833–838. [Google Scholar] [CrossRef]
- Fong, W.; To, K.K.W. Repurposing Chloroquine Analogs as an Adjuvant Cancer Therapy. Recent Patents Anti-Cancer Drug Discov. 2021, 16, 204–221. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.S.; Zou, C.; Zhang, J. Chloroquine against malaria, cancers and viral diseases. Drug Discov. Today 2020, 25, 2012–2022. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine [Press Release]. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and#:~:text=Today%2C%20the%20U.S.%20Food%20and,clinical%20trial%20was%20unavailable%2C%20or (accessed on 28 December 2023).
- Yang, J.; Guo, Z.; Liu, X.; Liu, Q.; Wu, M.; Yao, X.; Liu, Y.; Cui, C.; Li, H.; Song, C.; et al. Cytotoxicity Evaluation of Chloroquine and Hydroxychloroquine in Multiple Cell Lines and Tissues by Dynamic Imaging System and Physiologically Based Pharmacokinetic Model. Front. Pharmacol. 2020, 11, 574720. [Google Scholar] [CrossRef] [PubMed]
- Gies, V.; Bekaddour, N.; Dieudonné, Y.; Guffroy, A.; Frenger, Q.; Gros, F.; Rodero, M.P.; Herbeuval, J.P.; Korganow, A.S. Beyond Anti-viral Effects of Chloroquine/Hydroxychloroquine. Front. Immunol. 2020, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Dassarma, B.; Roy, S.; Chabalala, H.; Matsabisa, M.G. A review on possible modes of action of chloroquine/hydroxychloroquine: Repurposing against SAR-CoV-2 (COVID-19) pandemic. Int. J. Antimicrob. Agents 2020, 56, 106028. [Google Scholar] [CrossRef] [PubMed]
- Krogstad, D.J.; Schlesinger, P.H. The basis of antimalarial action: Non-weak base effects of chloroquine on acid vesicle pH. Am. J. Trop. Med. Hyg. 1987, 36, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fitch, C.D.; Dutta, P.; Kanjananggulpan, P.; Chevli, R. Ferriprotoporphyrin IX: A mediator of the antimalarial action of oxidants and 4-aminoquinoline drugs. Prog. Clin. Biol. Res. 1984, 155, 119–130. [Google Scholar] [PubMed]
- O’Brien, R.L.; Allison, J.L.; Hahn, F.E. Evidence for intercalation of chloroquine into DNA. Biochim. Biophys. Acta 1966, 129, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.A.; Goncalves, A.P.; Batista, J.A.D.; Bazoni, R.F.; Santos, A.A.; Rocha, M.S. New Insights into the Mechanism of Action of the Drug Chloroquine: Direct Interaction with DNA and Cytotoxicity. J. Phys. Chem. B 2022, 126, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Almaqwashi, A.A.; Paramanathan, T.; Rouzina, I.; Williams, M.C. Mechanisms of small molecule-DNA interactions probed by single-molecule force spectroscopy. Nucleic Acids Res. 2016, 44, 3971–3988. [Google Scholar] [CrossRef] [PubMed]
- Jabak, A.A.; Bryden, N.; Westerlund, F.; Lincoln, P.; McCauley, M.J.; Rouzina, I.; Williams, M.C.; Paramanathan, T. Left versus right: Exploring the effects of chiral threading intercalators using optical tweezers. Biophys. J. 2022, 121, 3745–3752. [Google Scholar] [CrossRef] [PubMed]
- Biebricher, A.S.; Heller, I.; Roijmans, R.F.H.; Hoekstra, T.P.; Peterman, E.J.G.; Wuite, G.J.L. The impact of DNA intercalators on DNA and DNA-processing enzymes elucidated through force-dependent binding kinetics. Nat. Commun. 2015, 6, 7304. [Google Scholar] [CrossRef] [PubMed]
- King, G.A.; Biebricher, A.S.; Heller, I.; Peterman, E.J.G.; Wuite, G.J.L. Quantifying Local Molecular Tension Using Intercalated DNA Fluorescence. Nano Lett. 2018, 18, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, K.R.; Paramanathan, T.; McCauley, M.J.; Williams, M.C. Biophysical characterization of DNA binding from single molecule force measurements. Phys. Life Rev. 2010, 7, 299–341. [Google Scholar] [CrossRef] [PubMed]
- King, G.A.; Gross, P.; Bockelmann, U.; Modesti, M.; Wuite, G.J.; Peterman, E.J. Revealing the competition between peeled ssDNA, melting bubbles, and S-DNA during DNA overstretching using fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, H.; Le, S.; Rouzina, I.; Doyle, P.S.; Yan, J. Revealing the competition between peeled ssDNA, melting bubbles, and S-DNA during DNA overstretching by single-molecule calorimetry. Proc. Natl. Acad. Sci. USA 2013, 110, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, I.D.; McCauley, M.J.; Rouzina, L.; Williams, M.C. Mapping the phase diagram of single DNA molecule force-induced melting in the presence of ethidium. Phys. Rev. Lett. 2005, 95, 158102. [Google Scholar] [CrossRef]
- Murugesapillai, D.; Bouaziz, S.; Maher, L.J.; Israeloff, N.E.; Cameron, C.E.; Williams, M.C. Accurate nanoscale flexibility measurement of DNA and DNA-protein complexes by atomic force microscopy in liquid. Nanoscale 2017, 9, 11327–11337. [Google Scholar] [CrossRef]
- Paik, D.H.; Perkins, T.T. Dynamics and multiple stable binding modes of DNA intercalators revealed by single-molecule force spectroscopy. Angew. Chem. Int. Ed. Engl. 2012, 51, 1811–1815. [Google Scholar] [CrossRef]
- Tibbs, J.; Tabei, S.M.A.; Kidd, T.E.; Peters, J.P. Effects of Intercalating Molecules on the Polymer Properties of DNA. J. Phys. Chem. B 2020, 124, 8572–8582. [Google Scholar] [CrossRef] [PubMed]
- Kwakye-Berko, F.; Meshnick, S.R. Binding of chloroquine to DNA. Mol. Biochem. Parasitol. 1989, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Giri, P.; Kumar, G.S. DNA intercalation by quinacrine and methylene blue: A comparative binding and thermodynamic characterization study. DNA Cell Biol. 2008, 27, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, A.T.; Permogorov, V.I.; Frank-Kamenetskii, M.D.; Lazurkin, Y.S. Thermodynamic studies of DNA-dye complexes. Mol. Biol. 1972, 6, 703–708. [Google Scholar] [PubMed]
- Temerk, Y.; Ibrahim, M.; Ibrahim, H.; Schuhmann, W. Comparative studies on the interaction of anticancer drug irinotecan with dsDNA and ssDNA. RSC Adv. 2018, 8, 25387–25395. [Google Scholar] [CrossRef] [PubMed]
- Stoilova-McPhie, S.; Lynch, G.C.; Ludtke, S.; Pettitt, B.M. Domain organization of membrane-bound factor VIII. Biopolymers 2013, 99, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B. A thermodynamic signature for drug-DNA binding mode. Arch. Biochem. Biophys. 2006, 453, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343. [Google Scholar] [CrossRef]
- Accinelli, R.A.; Ynga-Meléndez, G.J.; León-Abarca, J.A.; López, L.M.; Madrid-Cisneros, J.C.; Mendoza-Saldaña, J.D. Hydroxychloroquine / azithromycin in COVID-19: The association between time to treatment and case fatality rate. Travel. Med. Infect. Dis. 2021, 44, 102163. [Google Scholar] [CrossRef]
- Peng, H.; Chen, Z.; Wang, Y.; Ren, S.; Xu, T.; Lai, X.; Wen, J.; Zhao, M.; Zeng, C.; Du, L.; et al. Systematic Review and Pharmacological Considerations for Chloroquine and Its Analogs in the Treatment for COVID-19. Front. Pharmacol. 2020, 11, 554172. [Google Scholar] [CrossRef]
- Vergote, V.; Laenen, L.; Mols, R.; Augustijns, P.; Van Ranst, M.; Maes, P. Chloroquine, an Anti-Malaria Drug as Effective Prevention for Hantavirus Infections. Front. Cell Infect. Microbiol. 2021, 11, 580532. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Singh, B.; Ryan, H.; Kredo, T.; Chaplin, M.; Fletcher, T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 2, Cd013587. [Google Scholar] [CrossRef]
- Cervantes-Salguero, K.; Biaggne, A.; Youngsman, J.M.; Ward, B.M.; Kim, Y.C.; Li, L.; Hall, J.A.; Knowlton, W.B.; Graugnard, E.; Kuang, W. Strategies for Controlling the Spatial Orientation of Single Molecules Tethered on DNA Origami Templates Physisorbed on Glass Substrates: Intercalation and Stretching. Int. J. Mol. Sci. 2022, 23, 7690. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.J.; Rueter, E.M.; Rouzina, I.; Maher, L.J., III; Williams, M.C. Single-molecule kinetics reveal microscopic mechanism by which High-Mobility Group B proteins alter DNA flexibility. Nucleic Acids Res. 2012, 41, 167–181. [Google Scholar] [CrossRef]
- Ciak, J.; Hahn, F.E. Chloroquine: Mode of action. Science 1966, 151, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Candelli, A.; Hoekstra, T.P.; Farge, G.; Gross, P.; Peterman, E.J.; Wuite, G.J. A toolbox for generating single-stranded DNA in optical tweezers experiments. Biopolymers 2013, 99, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gien, H.; Morse, M.; McCauley, M.J.; Kitzrow, J.P.; Musier-Forsyth, K.; Gorelick, R.J.; Rouzina, I.; Williams, M.C. HIV-1 Nucleocapsid Protein Binds Double-Stranded DNA in Multiple Modes to Regulate Compaction and Capsid Uncoating. Viruses 2022, 14, 235. [Google Scholar] [CrossRef]
- Cai, W.; Jäger, M.; Bullerjahn, J.T.; Hugel, T.; Wolf, S.; Balzer, B.N. Anisotropic Friction in a Ligand-Protein Complex. Nano Lett. 2023, 23, 4111–4119. [Google Scholar] [CrossRef]
- Evans, E.; Ritchie, K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997, 72, 1541–1555. [Google Scholar] [CrossRef]
- Odijk, T. Stiff Chains and Filaments under Tension. Macromolecules 1995, 28, 7016–7018. [Google Scholar] [CrossRef]
| Na+ Concentration (mM) | KD (µM) | Experimental Method |
|---|---|---|
| 20 | 90 ± 10 | thermal melting |
| 30 | 360 ± 20 | isothermal titration calorimetry |
| 100 | 650 ± 150 | thermal melting |
| 100 | 170 ± 90 | optical tweezers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, J.; McCauley, M.J.; Morse, M.; Muccio, M.R.; Kanlong, J.G.; Rocha, M.S.; Rouzina, I.; Musier-Forsyth, K.; Williams, M.C. Mechanism of DNA Intercalation by Chloroquine Provides Insights into Toxicity. Int. J. Mol. Sci. 2024, 25, 1410. https://doi.org/10.3390/ijms25031410
Joshi J, McCauley MJ, Morse M, Muccio MR, Kanlong JG, Rocha MS, Rouzina I, Musier-Forsyth K, Williams MC. Mechanism of DNA Intercalation by Chloroquine Provides Insights into Toxicity. International Journal of Molecular Sciences. 2024; 25(3):1410. https://doi.org/10.3390/ijms25031410
Chicago/Turabian StyleJoshi, Joha, Micah J. McCauley, Michael Morse, Michael R. Muccio, Joseph G. Kanlong, Márcio S. Rocha, Ioulia Rouzina, Karin Musier-Forsyth, and Mark C. Williams. 2024. "Mechanism of DNA Intercalation by Chloroquine Provides Insights into Toxicity" International Journal of Molecular Sciences 25, no. 3: 1410. https://doi.org/10.3390/ijms25031410
APA StyleJoshi, J., McCauley, M. J., Morse, M., Muccio, M. R., Kanlong, J. G., Rocha, M. S., Rouzina, I., Musier-Forsyth, K., & Williams, M. C. (2024). Mechanism of DNA Intercalation by Chloroquine Provides Insights into Toxicity. International Journal of Molecular Sciences, 25(3), 1410. https://doi.org/10.3390/ijms25031410






