The HIF-1α/EGF/EGFR Signaling Pathway Facilitates the Proliferation of Yak Alveolar Type II Epithelial Cells in Hypoxic Conditions
Abstract
:1. Introduction
2. Results
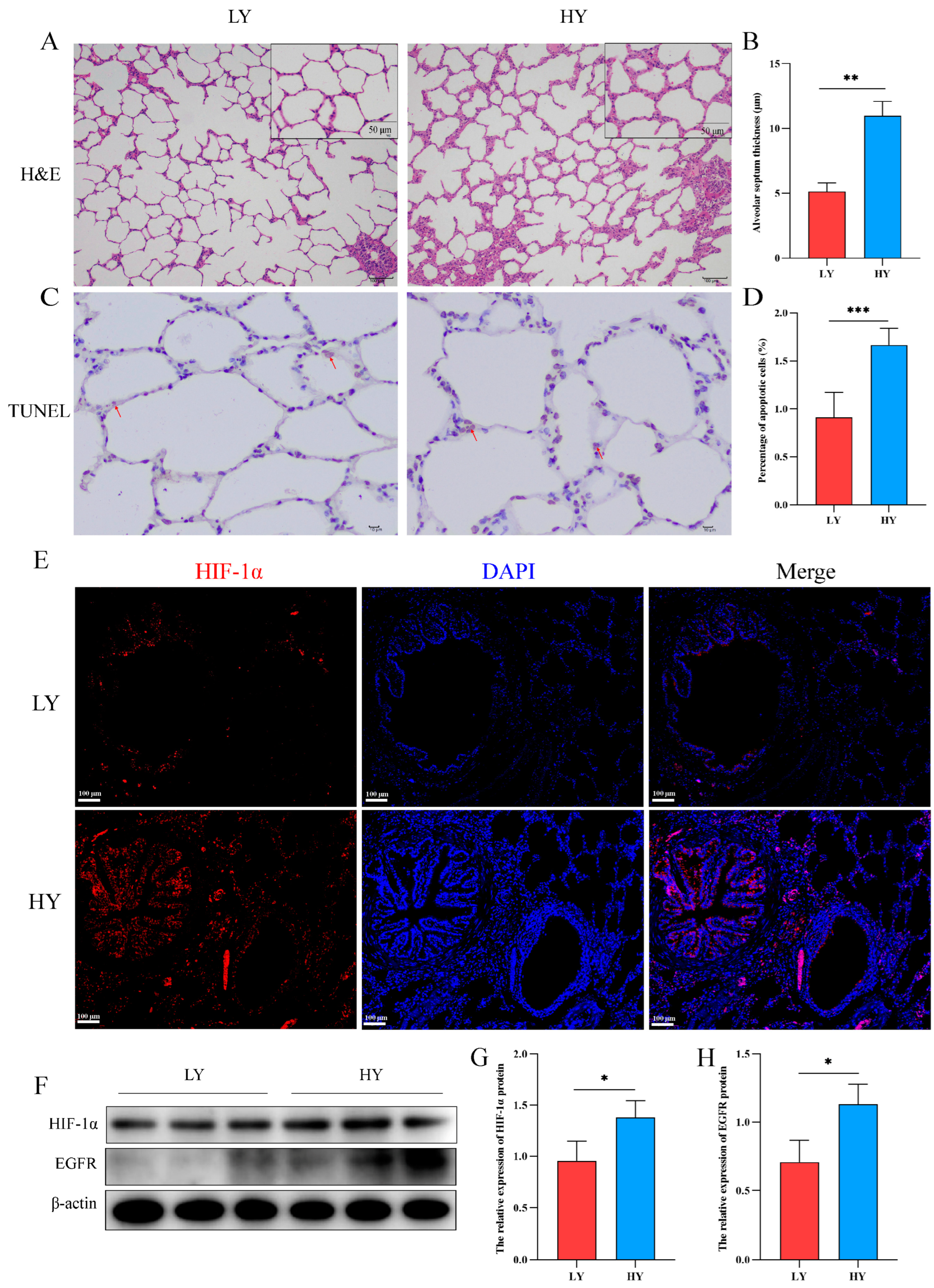
2.1. Alteration of the Alveolar Histologic Structure and Expression of HIF-1α and EGFR in 6-Month-Old Yaks by Hypoxia
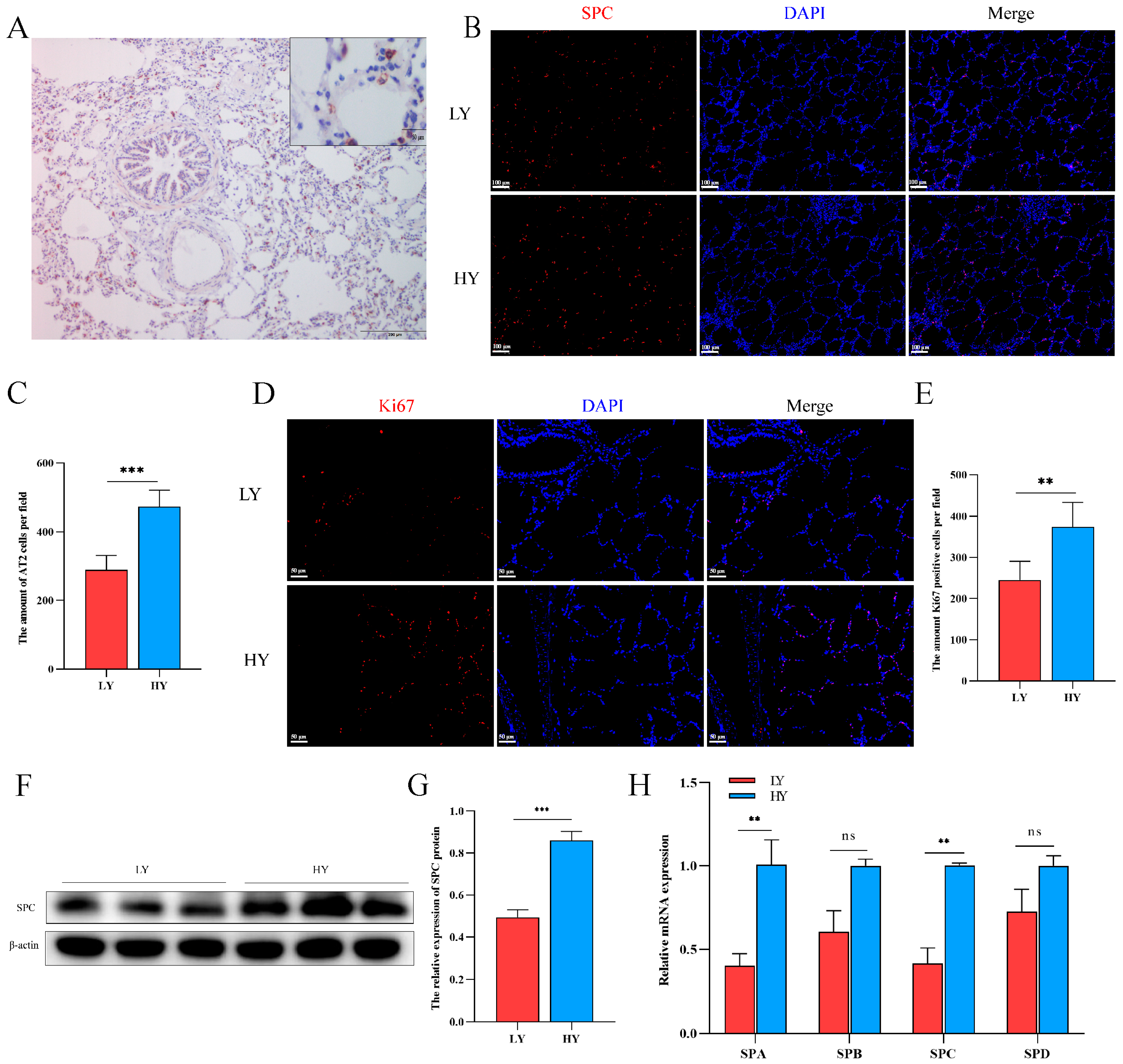
2.2. Hypoxia Alters AT2 Cell Proliferation and Alveolar Surfactant-Associated Protein (SP) Secretion in 6-Month-Old Yaks
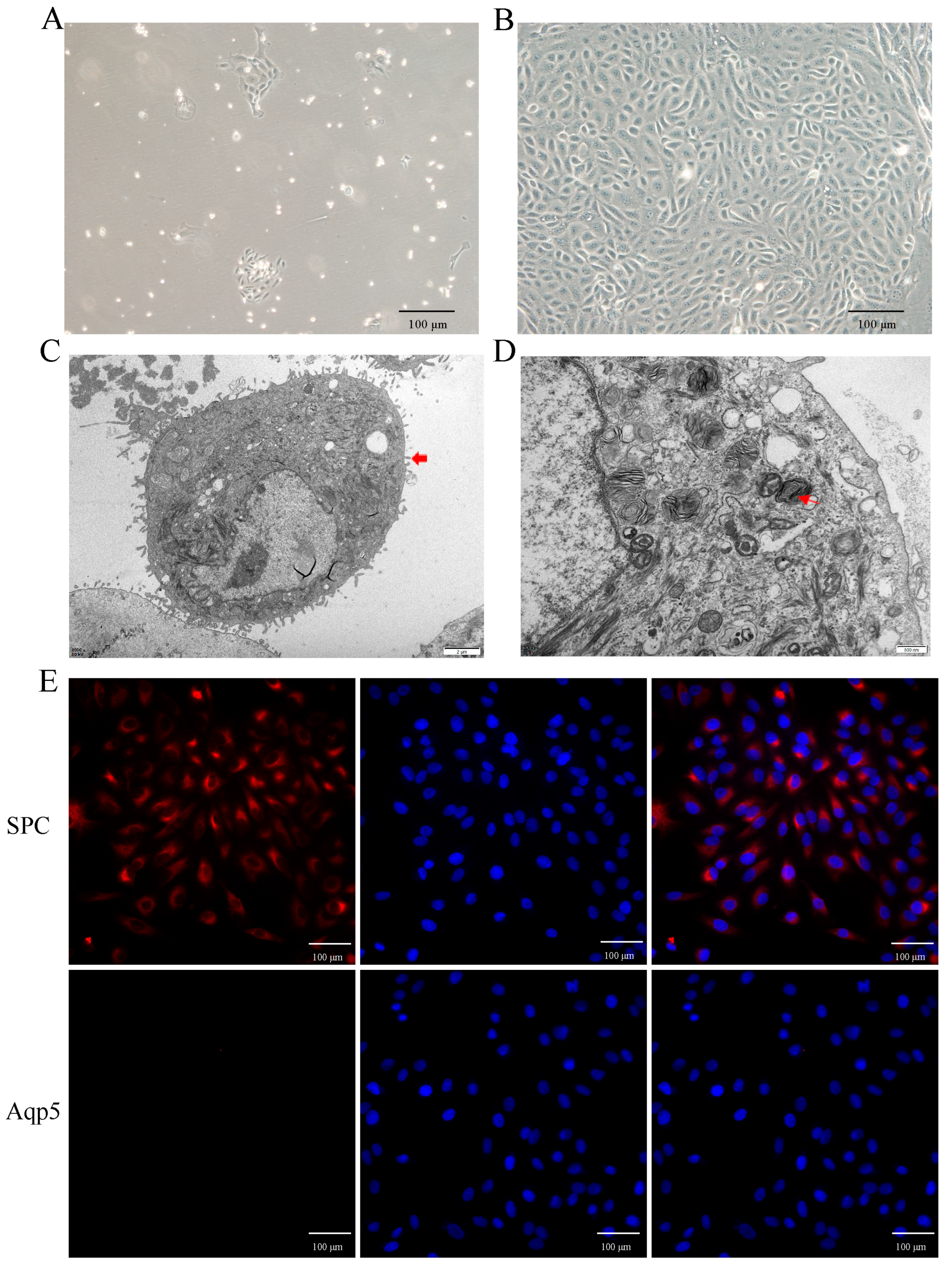
2.3. Primary Culture and Identification of Yak AT2 Cells
2.4. Hypoxia Promotes AT2 Cell Proliferation In Vitro
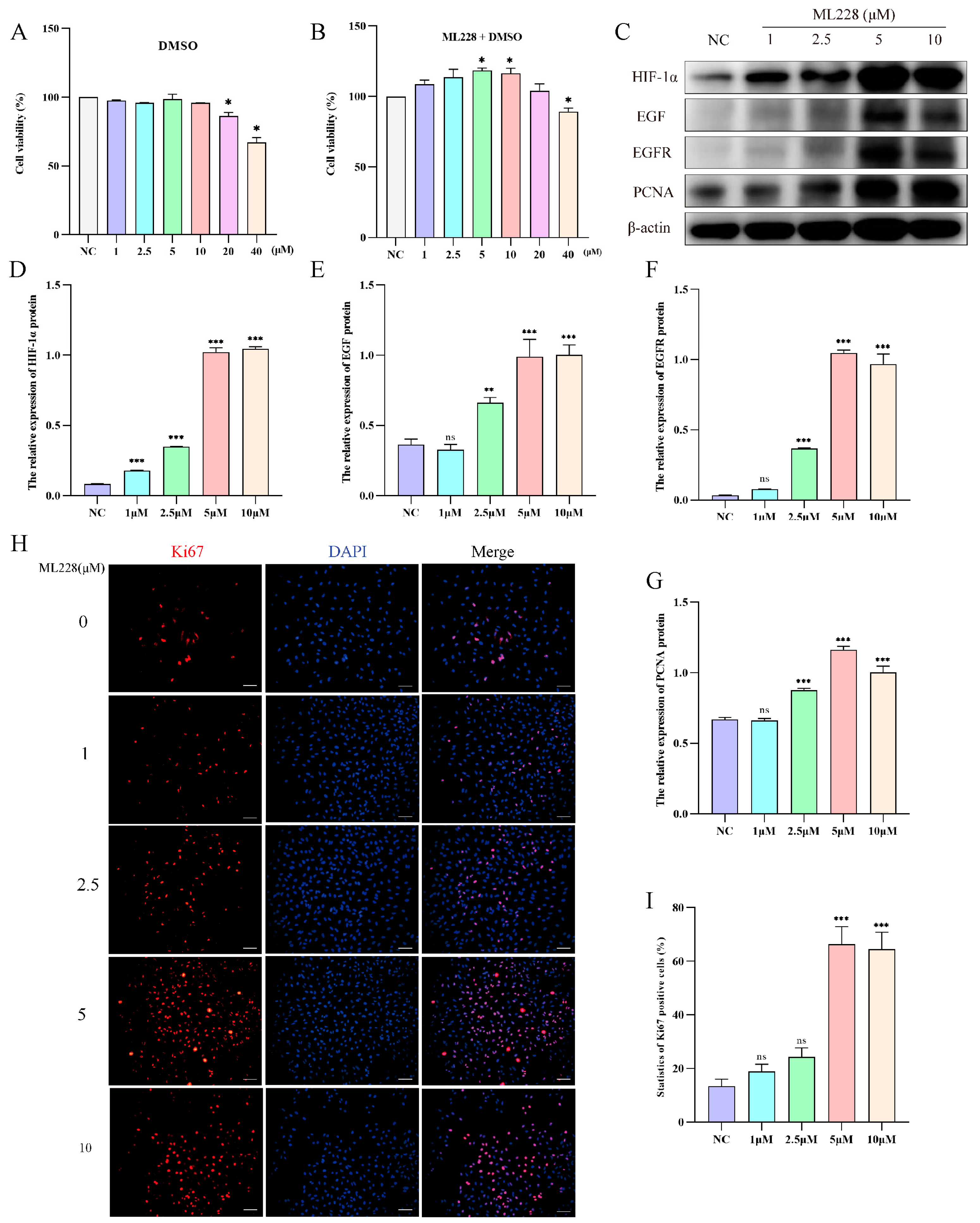
2.5. Activation of HIF-1α Promotes the Proliferation of Yak AT2 Cells
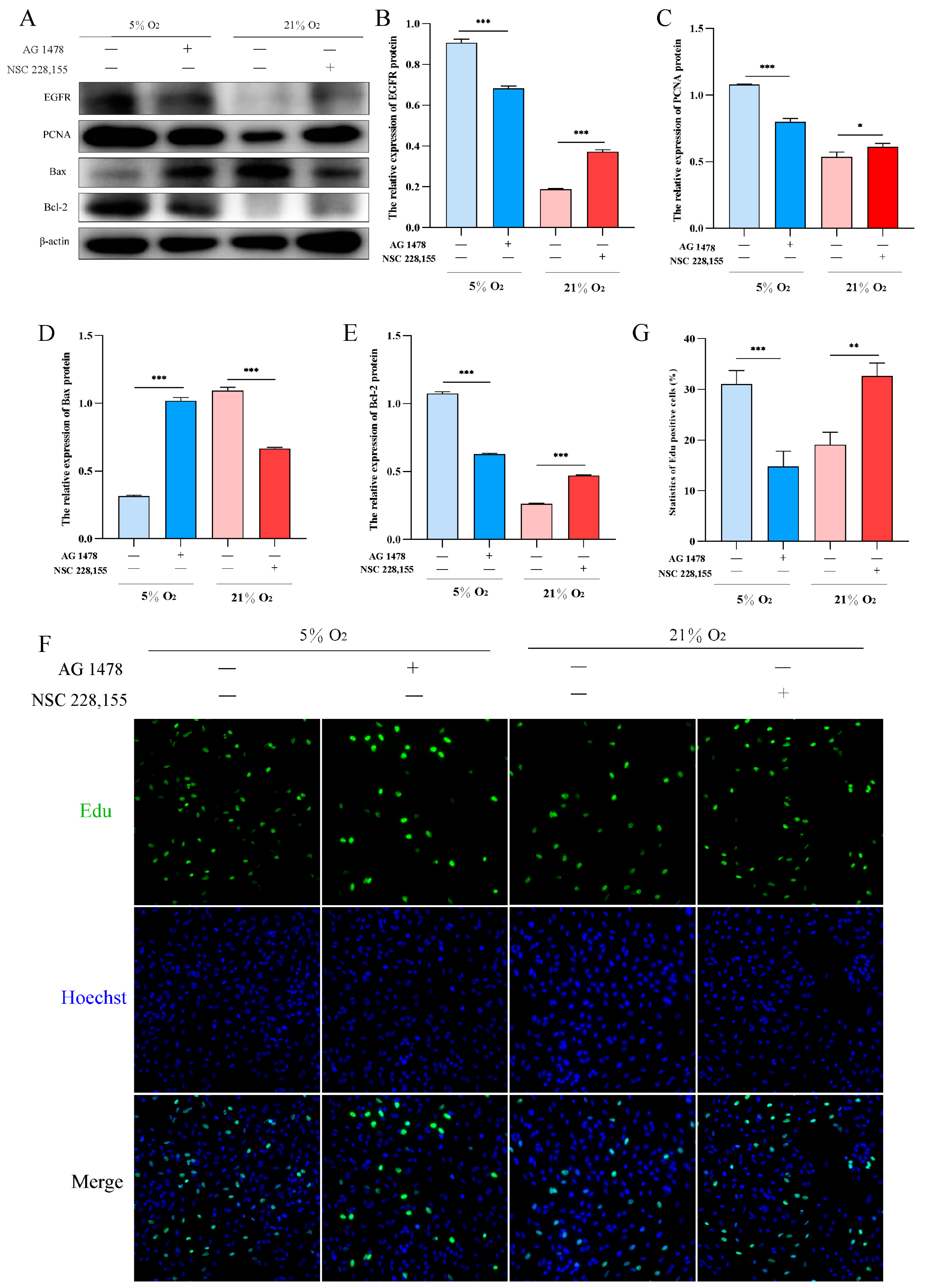
2.6. EGFR Pathway Promotes Proliferation and Inhibits Apoptosis in AT2 Cells under Hypoxia
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Immunofluorescence Assays
4.3. TUNEL Staining
4.4. RNA Isolation and Quantitative Real-Time PCR (RT-qPCR)
4.5. Protein Extraction and Western Blotting
4.6. Isolation and Culture of Yak Alveolar Type II Epithelial (AT2) Cells
4.7. Transmission Electron Microscopy (TEM)
4.8. Air-Liquid Interface (ALI) Cultures and Hypoxia
4.9. Cell Counting Kit-8 (CCK-8) Assays Cytotoxicity
4.10. 5-Ethynyl-20-Deoxyuridine (EdU) Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araneda, O.F.; Tuesta, M. Lung oxidative damage by hypoxia. Oxidative Med. Cell. Longev. 2012, 2012, 856918. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Peng, T.; Zepp, J.A.; Snitow, M.; Vincent, T.L.; Penkala, I.J.; Cui, Z.; Herriges, M.J.; Morley, M.P.; Zhou, S.; et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016, 17, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, H. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, M.; Niu, Y.; Wang, Y. Romance of the three kingdoms in hypoxia: HIFs, epigenetic regulators, and chromatin reprogramming. Cancer Lett. 2020, 495, 211–223. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Liu, Y.; Chen, J.; Huang, X.; Peng, H.; Liu, Z.; Tang, Y.; Zhang, K.; Xu, Q.; Li, X.; et al. Vascular peroxidase 1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation, apoptosis resistance and migration. Cardiovasc. Res. 2018, 114, 188–199. [Google Scholar] [CrossRef]
- Huang, M.; Yang, L.; Peng, X.; Wei, S.; Fan, Q.; Yang, S.; Li, X.; Li, B.; Jin, H.; Wu, B.; et al. Autonomous glucose metabolic reprogramming of tumour cells under hypoxia: Opportunities for targeted therapy. J. Exp. Clin. Cancer Res. CR 2020, 39, 185. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Q.; Liao, B.; Zhao, L.; Xiong, S.; Wang, J.; Zou, D.; Pan, J.; Wu, L.; Deng, Y.; et al. The HIF1α/HIF2α-miR210-3p network regulates glioblastoma cell proliferation, dedifferentiation and chemoresistance through EGF under hypoxic conditions. Cell Death Dis. 2020, 11, 992. [Google Scholar] [CrossRef]
- Wei, L.; Yi, Z.; Guo, K.; Long, X. Long noncoding RNA BCAR4 promotes glioma cell proliferation via EGFR/PI3K/AKT signaling pathway. J. Cell. Physiol. 2019, 234, 23608–23617. [Google Scholar] [CrossRef]
- Guan, R.; Wang, J.; Li, D.; Li, Z.; Liu, H.; Ding, M.; Cai, Z.; Liang, X.; Yang, Q.; Long, Z.; et al. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF-1α/MAPK signaling pathway. Int. Immunopharmacol. 2020, 81, 105979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, H.; Yang, T.; Li, Y.; Gao, C.; Jiao, T.; Cai, Y.; Zhao, S. The Expression Regulatory Network in the Lung Tissue of Tibetan Pigs Provides Insight Into Hypoxia-Sensitive Pathways in High-Altitude Hypoxia. Front. Genet. 2021, 12, 691592. [Google Scholar] [CrossRef] [PubMed]
- Takaro, T.; Gaddy, L.R.; Parra, S. Thin alveolar epithelial partitions across connective tissue gaps in the alveolar wall of the human lung: Ultrastructural observations. Am. Rev. Respir. Dis. 1982, 126, 326–331. [Google Scholar] [PubMed]
- Jing, X.; Ding, L.; Zhou, J.; Huang, X.; Degen, A.; Long, R. The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2022, 9, 249–258. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Wang, L.; Yu, Y.; Yu, H.; Wei, Q. Changes in the expression levels of elastic fibres in yak lungs at different growth stages. BMC Dev. Biol. 2021, 21, 9. [Google Scholar] [CrossRef]
- Kugler, M.C.; Loomis, C.A.; Zhao, Z.; Cushman, J.C.; Liu, L.; Munger, J.S. Sonic Hedgehog Signaling Regulates Myofibroblast Function during Alveolar Septum Formation in Murine Postnatal Lung. Am. J. Respir. Cell Mol. Biol. 2017, 57, 280–293. [Google Scholar] [CrossRef]
- Enomoto, N.; Suda, T.; Kono, M.; Kaida, Y.; Hashimoto, D.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Imokawa, S.; Funai, K.; et al. Amount of elastic fibers predicts prognosis of idiopathic pulmonary fibrosis. Respir. Med. 2013, 107, 1608–1616. [Google Scholar] [CrossRef]
- Kingma, P.S.; Zhang, L.; Ikegami, M.; Hartshorn, K.; McCormack, F.X.; Whitsett, J.A. Correction of pulmonary abnormalities in Sftpd-/- mice requires the collagenous domain of surfactant protein D. J. Biol. Chem. 2006, 281, 24496–24505. [Google Scholar] [CrossRef]
- Nogee, L.M. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu. Rev. Physiol. 2004, 66, 601–623. [Google Scholar] [CrossRef]
- Sherman, M.A.; Suresh, M.V.; Dolgachev, V.A.; McCandless, L.K.; Xue, X.; Ziru, L.; Machado-Aranda, D.; Shah, Y.M.; Raghavendran, K. Molecular Characterization of Hypoxic Alveolar Epithelial Cells after Lung Contusion Indicates an Important Role for HIF-1α. Ann. Surg. 2018, 267, 382–391. [Google Scholar] [CrossRef]
- Mollereau, B.; Perez-Garijo, A.; Bergmann, A.; Miura, M.; Gerlitz, O.; Ryoo, H.D.; Steller, H.; Morata, G. Compensatory proliferation and apoptosis-induced proliferation: A need for clarification. Cell Death Differ. 2013, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Dial, C.F.; Tune, M.K.; Doerschuk, C.M.; Mock, J.R. Foxp3(+) Regulatory T Cell Expression of Keratinocyte Growth Factor Enhances Lung Epithelial Proliferation. Am. J. Respir. Cell Mol. Biol. 2017, 57, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Klausen, C.; Leung, P.C. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett. 2013, 329, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, L.; Gong, S.; Xiong, S.; Wang, J.; Zou, D.; Pan, J.; Deng, Y.; Yan, Q.; Wu, N.; et al. HIF1α/HIF2α-Sox2/Klf4 promotes the malignant progression of glioblastoma via the EGFR-PI3K/AKT signalling pathway with positive feedback under hypoxia. Cell Death Dis. 2021, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.T.; Garvalov, B.K.; Seidel, S.; Cuesta, A.M.; Ritter, M.; Filatova, A.; Foss, F.; Dopeso, H.; Essmann, C.L.; Maxwell, P.H.; et al. Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat. Commun. 2014, 5, 5582. [Google Scholar] [CrossRef]
- Secades, P.; de Santa-María, I.S.; Merlo, A.; Suarez, C.; Chiara, M.D. In vitro study of normoxic epidermal growth factor receptor-induced hypoxia-inducible factor-1-alpha, vascular endothelial growth factor, and BNIP3 expression in head and neck squamous cell carcinoma cell lines: Implications for anti-epidermal growth factor receptor therapy. Head Neck 2015, 37, 1150–1162. [Google Scholar] [PubMed]
- Zheng, H.L.; Wang, L.H.; Sun, B.S.; Li, Y.; Yang, J.Y.; Wu, C.F. Oligomer procyanidins (F2) repress HIF-1α expression in human U87 glioma cells by inhibiting the EGFR/AKT/mTOR and MAPK/ERK1/2 signaling pathways in vitro and in vivo. Oncotarget 2017, 8, 85252–85262. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.S.; Nolan, D.J.; Guo, P.; Babazadeh, A.O.; Cao, Z.; Rosenwaks, Z.; Crystal, R.G.; Simons, M.; Sato, T.N.; Worgall, S.; et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2011, 147, 539–553. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hoshikawa, E.; Saito, T.; Suebsamarn, O.; Naito, E.; Suzuki, A.; Ishihara, S.; Haga, H.; Tomihara, K.; Izumi, K. The EGF/EGFR axis and its downstream signaling pathways regulate the motility and proliferation of cultured oral keratinocytes. FEBS Open Bio 2023, 13, 1469–1484. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Zhao, P. The Silkworm Carboxypeptidase Inhibitor Prevents Gastric Cancer Cells’ Proliferation through the EGF/EGFR Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 1078. [Google Scholar] [CrossRef]

| Genes | Primer Sequences (5’–3’) | TM/°C |
|---|---|---|
| SPA | F: 5′-GTCTTCTCTACCAATGGGCAGTCAG-3′ R: 5′-GGCAGCAATGTGTCCACCTACTC-3′ | 58 |
| SPB | F: 5′-GCTGCCACAAGACTCTGACTGC-3′ R: 5′-CTCCTCCACAAACCGCTCACAC-3′ | 58 |
| SPC | F: 5′-AAGATGGATGTGGGCAGCAAAGAG-3′ R: 5′-GTTCACGGGACAGCAAGGGATG-3′ | 58 |
| SPD | F: 5′-GAGCATGAGCGACACCAGGAAG-3′ R: 5′-CACACAGTTCTCTGAGCCACCATC-3′ | 58 |
| β-actin | F: 5′-TCCTGCGGCATTCACGAAACTAC-3′ R: 5′-GTGTTGGCGTAGAGGTCCTTGC-3′ | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; He, J.; Cui, Y.; Yu, S.; Zhang, H.; Wei, P.; Zhang, Q. The HIF-1α/EGF/EGFR Signaling Pathway Facilitates the Proliferation of Yak Alveolar Type II Epithelial Cells in Hypoxic Conditions. Int. J. Mol. Sci. 2024, 25, 1442. https://doi.org/10.3390/ijms25031442
Wang B, He J, Cui Y, Yu S, Zhang H, Wei P, Zhang Q. The HIF-1α/EGF/EGFR Signaling Pathway Facilitates the Proliferation of Yak Alveolar Type II Epithelial Cells in Hypoxic Conditions. International Journal of Molecular Sciences. 2024; 25(3):1442. https://doi.org/10.3390/ijms25031442
Chicago/Turabian StyleWang, Biao, Junfeng He, Yan Cui, Sijiu Yu, Huizhu Zhang, Pengqiang Wei, and Qian Zhang. 2024. "The HIF-1α/EGF/EGFR Signaling Pathway Facilitates the Proliferation of Yak Alveolar Type II Epithelial Cells in Hypoxic Conditions" International Journal of Molecular Sciences 25, no. 3: 1442. https://doi.org/10.3390/ijms25031442





