The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles
Abstract
1. Introduction
2. Structure of Nitroxides
3. Reactivity of Nitroxides
4. Reduction of Nitroxides
5. Cellular Effects of Nitroxides
6. Effects of Nitroxides in Animal Experiments
7. Clinical Trials of Nitroxides
8. Nitroxide-Containing Redox Nanoparticles
9. Safety and Adverse Effects of Nitroxides and Nitroxide-Containing Nanoparticles
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| A2E | bis-retinoid N-retinyl-N-retinylidene ethanolamine (A2E), a major component of lipofuscin in the retina |
| AMD | age-related macular degeneration |
| Asc | ascorbate |
| t-BHP | tert-butyl hydroperoxide |
| BSA | bovine serum albumin |
| But | butyl |
| DTNB | 5,5-dithio-bis-(2-nitrobenzoic acid) (Ellman’s reagent) |
| EPR | electron paramagnetic resonance |
| GLUT | glucose transporter |
| GSH | glutathione |
| HO-1 | heme oxygenase 1 |
| IC50 | half-inhibitory concentration |
| IL | interleukin |
| IR | ionizing radiation |
| LDH | lactate dehydrogenase |
| MMP | matrix metalloproteinase |
| mtDNA | mitochondrial DNA |
| NHE | normal hydrogen electrode |
| NMR | nuclear magnetic resonance |
| pCMB | para-chloromercuribenzoate |
| PEG | poly(ethylene glycol) |
| PMOT | poly [4-(2,2,6,6-tetramethylpiperidine-1-oxyl)oxymethylstyrene] |
| PROXYL | 2,2,5,5-tetramethylpyrrolidinyl-1-oxyl |
| RNP | redox nanoparticles |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric-acid reactive substances |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPAMINE | 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPOL | 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TEMPOL-H | hydroxylamine of TEMPOL |
References
- Dessolin, J.; Schuler, M.; Quinart, A.; De Giorgi, F.; Ghosez, L.; Ichas, F. Selective targeting of synthetic antioxidants to mitochondria: Towards a mitochondrial medicine for neurodegenerative diseases? Eur. J. Pharmacol. 2002, 447, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Trnka, J.; Blaikie, F.H.; Smith, R.A.; Murphy, M.P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 2008, 44, 1406–1419. [Google Scholar] [CrossRef]
- Zhdanov, R.; Sukhanov, V.; Shvets, V. Synthesis and Properties of Spin-Labeled Phospholipids. In Bioactive Spin Labels; Springer: Berlin/Heidelberg, Germany, 1992; pp. 297–315. [Google Scholar]
- Megli, F.M.; Conte, E.; Russo, L. Comparative 5-doxylstearoyllecithin and 3-doxylcholestane EPR spin labeling study of phospholipid bilayer perturbation by different oxidized lecithin species. Biochim. Biophys. Acta BBA Biomembr. 2010, 1798, 1886–1898. [Google Scholar] [CrossRef] [PubMed]
- Carloni, P.; Greci, L.; Stipa, P.; Eberson, L. Electron-transfer reactions. Oxidation of Grignard reagents in the presence of an aminoxyl as a radical-trapping agent. J. Org. Chem. 1991, 56, 4733–4737. [Google Scholar] [CrossRef]
- Carloni, P.; Greci, L.; Stipa, P.; Rizzoli, C.; Sgarabotto, P.; Ugozzoli, F. Antioxidants and light stabilizers. Part 1. Reactions of an indolinone nitroxide and phenoxy radicals. X-ray crystallographic analysis of 1-[O-(3,5-di-tert-butyl-4-hydroxy)-benzyl]-1,2-dihydro-2-methyl-2-phenyl-3-oxo-3H-indole and 3,5,3′5′-tetra-tert-butylstilbene-4,4′-quinone. Polym. Degrad. Stab. 1993, 39, 73–83. [Google Scholar]
- Cardellini, L.; Carloni, P.; Greci, L.; Stipa, P.; Faucitano, A. Homolytic Substitutions in Indolinone Nitroxide Radicals. Part 5. Reaction with tert.-Butylperoxy radicals. ChemInform 1990, 21, 1. [Google Scholar] [CrossRef]
- Greci, L. Homolytic substitutions in indolinone nitroxide radicals—III: Reactions with terbutoxy and methyl radicals. Tetrahedron 1982, 38, 2435–2439. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid is key to the radical-trapping antioxidant activity of nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef]
- Damiani, E.; Greci, L.; Parsons, R.; Knowland, J. Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient. Free Radic. Biol. Med. 1999, 26, 809–816. [Google Scholar] [CrossRef]
- Genovese, D.; Baschieri, A.; Vona, D.; Baboi, R.E.; Mollica, F.; Prodi, L.; Amorati, R.; Zaccheroni, N. Nitroxides as building blocks for nanoantioxidants. ACS Appl. Mater. Interfaces 2021, 13, 31996–32004. [Google Scholar] [CrossRef]
- Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Marrosu, G.; Petrucci, R.; Trazza, A. Chemical and electrochemical study on the interactions of aminoxyls with superoxide anion. Tetrahedron 1996, 52, 11257–11264. [Google Scholar] [CrossRef]
- Blinco, J.P.; Hodgson, J.L.; Morrow, B.J.; Walker, J.R.; Will, G.D.; Coote, M.L.; Bottle, S.E. Experimental and theoretical studies of the redox potentials of cyclic nitroxides. J. Org. Chem. 2008, 73, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Offer, T.; Samuni, A. Nitroxides inhibit peroxyl radical-mediated DNA scission and enzyme inactivation. Free Radic. Biol. Med. 2002, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Cimato, A.N.; Piehl, L.L.; Facorro, G.B.; Torti, H.B.; Hager, A.A. Antioxidant effects of water-and lipid-soluble nitroxide radicals in liposomes. Free Radic. Biol. Med. 2004, 37, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Carloni, P.; Biondi, C.; Greci, L. Increased oxidative modification of albumin when illuminated in vitro in the presence of a common sunscreen ingredient: Protection by nitroxide radicals. Free Radic. Biol. Med. 2000, 28, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Castagna, R.; Greci, L. The effects of derivatives of the nitroxide tempol on UVA-mediated in vitro lipid and protein oxidation. Free Radic. Biol. Med. 2002, 33, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Antosiewicz, J.; Damiani, E.; Jassem, W.; Wozniak, M.; Orena, M.; Greci, L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Radic. Biol. Med. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure−activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef]
- Carroll, R.T.; Galatsis, P.; Borosky, S.; Kopec, K.K.; Kumar, V.; Althaus, J.S.; Hall, E.D. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem. Res. Toxicol. 2000, 13, 294–300. [Google Scholar] [CrossRef]
- Bonini, M.G.; Mason, R.P.; Augusto, O. The mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem. Res. Toxicol. 2002, 15, 506–511. [Google Scholar] [CrossRef]
- Deffner, U.; Schimmack, W. Radiation effects on aqueous solutions of the nitroxyl free radical TMPN (2,2,6,6-tetramethyl-4-piperidinol-N-oxyl). Int. J. Radiat. Biol. 1976, 29, 71–75. [Google Scholar] [CrossRef]
- Lam, M.A.; Pattison, D.I.; Bottle, S.E.; Keddie, D.J.; Davies, M.J. Nitric oxide and nitroxides can act as efficient scavengers of protein-derived free radicals. Chem. Res. Toxicol. 2008, 21, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Dhouib, R.; Fairfull-Smith, K.E.; Totsika, M. Nitroxide functionalized antibiotics are promising eradication agents against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2019, 64, e01685-19. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Riesz, P.; Finkelstein, E.; Russo, A. Superoxide reaction with nitroxide spin-adducts. Free Radic. Biol. Med. 1989, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Mitchell, J.B.; Collins, C.R.; Russo, A. Superoxide reaction with nitroxides. Free Radic. Res. Commun. 1990, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.M.; Finkelstein, E.; Rauckman, E.J. A method for the detection of superoxide in biological systems. Arch. Biochem. Biophys. 1982, 215, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do nitroxide antioxidants act as scavengers of O2−˙ or as SOD mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Superoxide-dependent reduction of nitroxides by thiols. Biochim. Biophys. Acta BBA Gen. Subj. 1984, 802, 90–98. [Google Scholar] [CrossRef]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases—A review of the metal-associated mechanistic variations. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Caliceti, P.; Schiavon, O.; Sergi, M. Polyethylene glycol–superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002, 54, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins: Kinetics and mechanism. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nakano, T.; Kimoto, E. Oxidation of nitroxide radicals by the reaction of hemoglobin with hydrogen peroxide. Biochem. Biophys. Res. Commun. 1984, 120, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Rice-Evans, C.; Davies, M.; Newman, E. The formation of free radicals by cardiac myocytes under oxidative stress and the effects of electron-donating drugs. Biochem. J. 1991, 277, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.-I.; Krishna, M.C.; Mitchell, J.B. Novel pharmacokinetic measurement using electron paramagnetic resonance spectroscopy and simulation of in vivo decay of various nitroxyl spin probes in mouse blood. J. Pharmacol. Exp. Ther. 2004, 310, 1076–1083. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar]
- Yamada, K.-I.; Inoue, D.; Matsumoto, S.; Utsumi, H. In vivo measurement of redox status in streptozotocin-induced diabetic rat using targeted nitroxyl probes. Antioxid. Redox Signal. 2004, 6, 605–611. [Google Scholar] [CrossRef]
- Kasazaki, K.; Yasukawa, K.; Sano, H.; Utsumi, H. Non-invasive analysis of reactive oxygen species generated in NH4OH-induced gastric lesions of rats using a 300 MHz in vivo ESR technique. Free Radic. Res. 2003, 37, 757–766. [Google Scholar] [CrossRef]
- Zhang, Y.; Fung, L.-M. The roles of ascorbic acid and other antioxidants in the erythrocyte in reducing membrane nitroxide radicals. Free Radic. Biol. Med. 1994, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.; Van Nice, F. Influence of structure on the reduction of nitroxide MRI contrast-enhancing agents by ascorbate. Physiol. Chem. Phys. Med. NMR 1984, 16, 477–480. [Google Scholar] [PubMed]
- Lin, Y.; Liu, W.; Ohno, H.; Ogata, T. Determination of ascorbate concentration in a raw leaf with electron spin resonance spectroscopy. Anal. Sci. 1999, 15, 973–977. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Bobko, A.A.; Grigor’ev, I.A.; Khramtsov, V.V. Synthesis of the tetraethyl substituted pH-sensitive nitroxides of imidazole series with enhanced stability towards reduction. Org. Biomol. Chem. 2004, 2, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, V.; Yelinova, V.; Weiner, L.; Berezina, T.; Martin, V.; Volodarsky, L. Quantitative determination of SH groups in low-and high-molecular-weight compounds by an electron spin resonance method. Anal. Biochem. 1989, 182, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Głębska, J.; Skolimowski, J.; Kudzin, Z.; Gwoździński, K.; Grzelak, A.; Bartosz, G. Pro-oxidative activity of nitroxides in their reactions with glutathione. Free Radic. Biol. Med. 2003, 35, 310–316. [Google Scholar] [CrossRef]
- Kroll, C.; Langner, A.; Borchert, H.H. Nitroxide metabolism in the human keratinocyte cell line HaCaT. Free Radic. Biol. Med. 1999, 26, 850–857. [Google Scholar] [CrossRef]
- Azuma, R.; Yamasaki, T.; Emoto, M.C.; Sato-Akaba, H.; Sano, K.; Munekane, M.; Fujii, H.G.; Mukai, T. Effect of relative configuration of TEMPO-type nitroxides on ascorbate reduction. Free Radic. Biol. Med. 2023, 194, 114–122. [Google Scholar] [CrossRef]
- Babić, N.; Orio, M.; Peyrot, F. Unexpected rapid aerobic transformation of 2,2,6,6-tetraethyl-4-oxo (piperidin-1-yloxyl) radical by cytochrome P450 in the presence of NADPH: Evidence against a simple reduction of the nitroxide moiety to the hydroxylamine. Free Radic. Biol. Med. 2020, 156, 144–156. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Pittelkow, M.R.; Wood, J.M. Free radical reduction by thioredoxin reductase at the surface of normal and vitiliginous human keratinocytes. J. Investig. Dermatol. 1986, 87, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.F.; Pou, S.; Rosen, G.M. Nitroxides as potential contrast enhancing agents for MRI application: Influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn. Reson. Med. 1987, 5, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Trubitsin, B.; Milanovsky, G.; Mamedov, M.; Semenov, A.Y.; Tikhonov, A. The interaction of water-soluble nitroxide radicals with Photosystem II. Appl. Magn. Reson. 2022, 53, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, R.; Butler, K.; Smith, I.C. Oxidant stress in malaria as probed by stable nitroxide radicals in erythrocytes infected with Plasmodium berghei. The effects of primaquine and chloroquine. Biochim. Biophys. Acta BBA Mol. Cell Res. 1987, 931, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845. [Google Scholar] [CrossRef]
- Yan, S.-X.; Hong, X.-Y.; Hu, Y.; Liao, K.-H. Tempol, one of nitroxides, is a novel ultraviolet-A1 radiation protector for human dermal fibroblasts. J. Dermatol. Sci. 2005, 37, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; DeGraff, W.; Kaufman, D.; Krishna, M.C.; Samuni, A.; Finkelstein, E.; Ahn, M.S.; Hahn, S.M.; Gamson, J.; Russo, A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys. 1991, 289, 62–70. [Google Scholar] [CrossRef]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Leathem, A.; Simone, M.; Dennis, J.M.; Witting, P.K. The cyclic nitroxide TEMPOL ameliorates oxidative stress but not inflammation in a cell model of Parkinson’s disease. Antioxidants 2022, 11, 257. [Google Scholar] [CrossRef]
- Liang, Q.; Smith, A.D.; Pan, S.; Tyurin, V.A.; Kagan, V.E.; Hastings, T.G.; Schor, N.F. Neuroprotective effects of TEMPOL in central and peripheral nervous system models of Parkinson’s disease. Biochem. Pharmacol. 2005, 70, 1371–1381. [Google Scholar] [CrossRef]
- Pichla, M.; Pulaski, Ł.; Kania, K.D.; Stefaniuk, I.; Cieniek, B.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Nitroxide radical-containing redox nanoparticles protect neuroblastoma SH-SY5Y cells against 6-hydroxydopamine toxicity. Oxidative Med. Cell. Longev. 2020, 2020, 9260748. [Google Scholar] [CrossRef] [PubMed]
- Alpert, E.; Altman, H.; Totary, H.; Gruzman, A.; Barnea, D.; Barash, V.; Sasson, S. 4-Hydroxy tempol-induced impairment of mitochondrial function and augmentation of glucose transport in vascular endothelial and smooth muscle cells. Biochem. Pharmacol. 2004, 67, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism 2021, 118, 154748. [Google Scholar] [CrossRef]
- Zarei, F.; Daghigh-Kia, H.; Masoudi, R. Supplementation of ram’s semen extender with Mito-TEMPO II: Quality evaluation and flow cytometry study of post-thawed spermatozoa. Andrologia 2022, 54, e14299. [Google Scholar] [CrossRef] [PubMed]
- Zarei, F.; Kia, H.D.; Masoudi, R.; Moghaddam, G.; Ebrahimi, M. Supplementation of ram’s semen extender with Mito-TEMPO I: Improvement in quality parameters and reproductive performance of cooled-stored semen. Cryobiology 2021, 98, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Ghosh, S.; Katiyar, R.; Gemeda, A.E.; Rautela, R.; Bisla, A.; Srivastava, N.; Kumar Bhure, S.; Devi, H.L.; Chandra, V. Supplementation of Mito TEMPO and acetovanillone in semen extender improves freezability of buffalo spermatozoa. Andrology 2022, 10, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, I.; Zare-Shahneh, A.; Goodarzi, A.; Baghshahi, H.; Fouladi-Nashta, A. The effect of Tempo and MitoTEMPO on oocyte maturation and subsequent embryo development in bovine model. Theriogenology 2021, 176, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Degraff, W.G.; Krishna, M.C.; Russo, A.; Mitchell, J.B. Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ. Mol. Mutagen. 1992, 19, 21–26. [Google Scholar] [CrossRef]
- Sies, H.; Mehlhorn, R. Mutagenicity of nitroxide-free radicals. Arch. Biochem. Biophys. 1986, 251, 393–396. [Google Scholar] [CrossRef]
- Lewinska, A.; Wnuk, M.; Slota, E.; Bartosz, G. The nitroxide antioxidant Tempol affects metal-induced cyto-and genotoxicity in human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 649, 7–14. [Google Scholar] [CrossRef]
- Maio, N.; Lafont, B.A.; Sil, D.; Li, Y.; Bollinger, J.M., Jr.; Krebs, C.; Pierson, T.C.; Linehan, W.M.; Rouault, T.A. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science 2021, 373, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jang, Y.P.; Chang, S.; Sparrow, J.R. OT-674 Suppresses Photooxidative Processes Initiated by an RPE Lipofuscin Fluorophore. Photochem. Photobiol. 2008, 84, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide pharmaceutical development for age-related degeneration and disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef]
- Mizuno, H.; Kubota, C.; Takigawa, Y.; Shintoku, R.; Kannari, N.; Muraoka, T.; Obinata, H.; Yoshimoto, Y.; Kanazawa, M.; Koshiishi, I. 2,2,6,6-Tetramethylpiperidine-1-oxyl acts as a volatile inhibitor of ferroptosis and neurological injury. J. Biochem. 2022, 172, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pinson, A.; Samuni, A. Both hydroxylamine and nitroxide protect cardiomyocytes from oxidative stress. Free Radic. Biol. Med. 1998, 24, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, D.; Damiani, E.; Greci, L.; Littarru, G.P.; Falcioni, G. Nitroxide radicals protect against DNA damage in rat epithelial cells induced by nitric oxide, nitroxyl anion and peroxynitrite. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 535, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Czepas, J.; Koceva-Chyła, A.; Gwoździński, K.; Jóźwiak, Z. Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 2008, 24, 101–112. [Google Scholar] [CrossRef]
- Castagna, R.; Davis, P.; Vasu, V.; Soucek, K.; Cross, C.; Greci, L.; Valacchi, G. Nitroxide radical TEMPO reduces ozone-induced chemokine IL-8 production in lung epithelial cells. Toxicol. In Vitro 2009, 23, 365–370. [Google Scholar] [CrossRef]
- He, S.-M.; Lei, Y.-H.; Wang, J.-M.; Geng, L.-N.; Wang, S.-P.; Zhao, J.; Hou, Y.-F. The protective effect of nitronyl nitroxide radical on peroxidation of A549 cell damaged by iron overload. Mater. Sci. Eng. C 2020, 108, 110189. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.; Mazzon, E.; Siriwardena, D.; Costantino, G.; Fulia, F.; Cucinotta, G.; Gitto, E.; Cordaro, S.; Barberi, I. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000, 875, 96–106. [Google Scholar] [CrossRef]
- Zigler, J.S., Jr.; Qin, C.; Kamiya, T.; Krishna, M.C.; Cheng, Q.; Tumminia, S.; Russell, P. Tempol-H inhibits opacification of lenses in organ culture. Free Radic. Biol. Med. 2003, 35, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Wilson, L.; Krishna, C.M.; Liebmann, J.; DeGraff, W.; Gamson, J.; Samuni, A.; Venzon, D.; Mitchell, J.B. Identification of nitroxide radioprotectors. Radiat. Res. 1992, 132, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Erker, L.; Schubert, R.; Yakushiji, H.; Barlow, C.; Larson, D.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum. Mol. Genet. 2005, 14, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G.; Pieńkowska, N.; Kut, K.; Cieniek, B.; Stefaniuk, I.; Sadowska-Bartosz, I. Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein. Int. J. Mol. Sci. 2023, 24, 16675. [Google Scholar] [CrossRef]
- Santos, G.B.; Ribeiro, A.C.; Lima, S.N.; Trostchansky, A.; Cerdeira, C.D.; Brigagão, M.R. Nitroxide Tempol down-regulates kinase activities associated with NADPH oxidase function in phagocytic cells and potentially decreases their fungicidal response. Chem. Biol. Int. 2018, 279, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mołoń, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two faces of TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl)–An antioxidant or a toxin? Biochim. Biophys. Acta BBA Mol. Cell Res. 2023, 1870, 119412. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Stylianou, M.; Lopes, J.P.; Müller, D.C.; Häggman, A.; Holmberg, S.; Grumaz, C.; Johansson, A.; Sohn, K.; Dieterich, C. Stable redox-cycling nitroxide tempol has antifungal and immune-modulatory properties. Front. Microbiol. 2019, 10, 1843. [Google Scholar] [CrossRef]
- Shi, T.Y.; Zhao, D.Q.; Wang, H.B.; Feng, S.; Liu, S.B.; Xing, J.H.; Qu, Y.; Gao, P.; Sun, X.L.; Zhao, M.G. A new chiral pyrrolyl α-nitronyl nitroxide radical attenuates β-amyloid deposition and rescues memory deficits in a mouse model of Alzheimer disease. Neurotherapeutics 2013, 10, 340–353. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Kanaan, N.M.; Yan, S.S. Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef]
- Greenwald, M.B.Y.; Anzi, S.; Sasson, S.B.; Bianco-Peled, H.; Kohen, R. Can nitroxides evoke the Keap1–Nrf2–ARE pathway in skin? Free Radic. Biol. Med. 2014, 77, 258–269. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Łuczak, K.; Soszyński, M.; Bartosz, G. Prooxidative effects of TEMPO on human erythrocytes. Cell Biol. Int. 2004, 28, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, K.; Gwozdzinski, L. The Effect of Piperidine Nitroxides on the Properties of Metalloproteins in Human Red Blood Cells. Molecules 2023, 28, 6174. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.; Ravizza, R.; Petterino, C.; Castagnaro, M.; Finocchiaro, G.; Monti, E. Study of in vitro and in vivo effects of the piperidine nitroxide Tempol—A potential new therapeutic agent for gliomas. Eur. J. Cancer 2003, 39, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Suy, S.; Mitchell, J.B.; Samuni, A.; Mueller, S.; Kasid, U. Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer 2005, 103, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Braunhut, S.; Medeiros, D.; Lai, L.; Bump, E. Tempol prevents impairment of the endothelial cell wound healing response caused by ionising radiation. Br. J. Cancer 1996, 27, S157. [Google Scholar]
- Czepas, J.; Matczak, K.; Koceva-Chyła, A.; Grobelski, B.; Jóźwiak, Z.; Gwoździński, K. Doxyl Nitroxide Spin Probes Can Modify Toxicity of Doxorubicin towards Fibroblast Cells. Molecules 2020, 25, 5138. [Google Scholar] [CrossRef] [PubMed]
- Sultani, H.N.; Morgan, I.; Hussain, H.; Roos, A.H.; Haeri, H.H.; Kaluđerović, G.N.; Hinderberger, D.; Westermann, B. Access to new cytotoxic triterpene and steroidal acid-TEMPO conjugates by Ugi multicomponent-reactions. Int. J. Mol. Sci. 2021, 22, 7125. [Google Scholar] [CrossRef]
- Park, W.H. Tempol Inhibits the growth of lung cancer and normal cells through apoptosis accompanied by increased O2•− levels and glutathione depletion. Molecules 2022, 27, 7341. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Celes, F.S.; Paiva, C.N.; de Oliveira, C.I. The paradoxical leishmanicidal effects of superoxide dismutase (SOD)-mimetic tempol in Leishmania braziliensis infection in vitro. Front. Cell. Infect. Microbiol. 2019, 9, 23. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Xavier, S.; DeLuca, A.M.; Sowers, A.L.; Cook, J.A.; Krishna, M.C.; Hahn, S.M.; Russo, A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic. Biol. Med. 2003, 34, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Karmeli, F.; Okon, E.; Samuni, A. A novel antiulcerogenic stable radical prevents gastric mucosal lesions in rats. Gut 1994, 35, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Gelvan, D.; Saltman, P.; Powell, S.R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc. Natl. Acad. Sci. USA 1991, 88, 4680–4684. [Google Scholar] [CrossRef]
- Behringer, W.; Safar, P.; Kentner, R.; Wu, X.; Kagan, V.E.; Radovsky, A.; Clark, R.S.; Kochanek, P.M.; Subramanian, M.; Tyurin, V.A. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J. Cereb. Blood Flow Metab. 2002, 22, 105–117. [Google Scholar] [CrossRef]
- Berber, I.; Aydin, C.; Cevahir, N.; Yenisey, C.; Gumrukcu, G.; Kocbil, G.; Tellioglu, G.; Tekin, K. Tempol reduces bacterial translocation after ischemia/reperfusion injury in a rat model of superior mesenteric artery occlusion. Surg. Today 2009, 39, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Cai, J.; Xue, P.; Zhang, Y.; Liu, S.; Gao, X.; Li, M.; Wang, Z.; Baudy-Floch, M.; Green, S.A. Protective effect of nitronyl nitroxide–amino acid conjugates on liver ischemia–reperfusion-induced injury in rats. Bioorg. Med. Chem. Lett. 2008, 18, 1788–1794. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Rodrigues, G.J.; Ceron, C.S.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009, 46, 1298–1307. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Cuzzocrea, S.; Brown, P.A.; Zacharowski, K.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Takaoka, M.; Ohkita, M.; Matsumura, Y. Tempol protects against ischemic acute renal failure by inhibiting renal noradrenaline overflow and endothelin-1 overproduction. Biol. Pharm. Bull. 2005, 28, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Li, F.; Anderson, R.E. Protection of retinal pigment epithelium by OT-551 and its metabolite TEMPOL-H against light-induced damage in rats. Exp. Eye Res. 2010, 91, 111–114. [Google Scholar] [CrossRef]
- Tanito, M.; Li, F.; Elliott, M.H.; Dittmar, M.; Anderson, R.E. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; DeLuca, A.M.; Coffin, D.; Krishna, C.M.; Mitchell, J.B. In vivo radioprotection and effects on blood pressure of the stable free radical nitroxides. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Krishna, C.M.; Wersto, N.; Venzon, D.; Russo, A.; Mitchell, J.B. Evaluation of tempol radioprotection in a murine tumor model. Free Radic. Biol. Med. 1997, 22, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, A.P.; Hyodo, F.; Matsumoto, K.-I.; Sowers, A.L.; Cook, J.A.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin. Cancer Res. 2007, 13, 4928–4933. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yamasaki, T.; Ueno, M.; Shibata, S.; Ozawa, Y.; Kamada, T.; Nakanishi, I.; Yamada, K.-I.; Aoki, I.; Matsumoto, K.-I. Radiation-induced redox alteration in the mouse brain. Free Radic. Biol. Med. 2019, 143, 412–421. [Google Scholar] [CrossRef]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Bacher, J.D.; Liebmann, J.; Krishna, M.C.; Coffin, D.; Mitchell, J.B. Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic. Biol. Med. 1999, 27, 529–535. [Google Scholar] [CrossRef]
- Adeagbo, A.S.; Joshua, I.G.; Falkner, C.; Matheson, P.J. Tempol, an antioxidant, restores endothelium-derived hyperpolarizing factor-mediated vasodilation during hypertension. Eur. J. Pharmacol. 2003, 481, 91–100. [Google Scholar] [CrossRef]
- Banday, A.A.; Marwaha, A.; Tallam, L.S.; Lokhandwala, M.F. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor–G-protein coupling and function in obese Zucker rats. Diabetes 2005, 54, 2219–2226. [Google Scholar] [CrossRef]
- Ebenezer, P.J.; Mariappan, N.; Elks, C.M.; Haque, M.; Francis, J. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity 2009, 17, 1994–2002. [Google Scholar] [CrossRef]
- DeRubertis, F.R.; Craven, P.A.; Melhem, M.F. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism 2007, 56, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, R.; Sowers, A.L.; Chandramouli, G.; Gamson, J.; Krishna, M.C.; Mitchell, J.B.; Cook, J.A. The antioxidant tempol transforms gut microbiome to resist obesity in female C3H mice fed a high fat diet. Free Radic. Biol. Med. 2022, 178, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Mitchell, J.B.; Bursill, C.A.; Sowers, A.L.; Thetford, A.; Cook, J.A.; van Reyk, D.M.; Davies, M.J. The nitroxide radical TEMPOL prevents obesity, hyperlipidaemia, elevation of inflammatory cytokines, and modulates atherosclerotic plaque composition in apoE−/− mice. Atherosclerosis 2015, 240, 234–241. [Google Scholar] [CrossRef]
- Beswick, R.A.; Zhang, H.; Marable, D.; Catravas, J.D.; Hill, W.D.; Webb, R.C. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 2001, 37, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Lan, C.; Chen, C.; Xu, Z.; Luo, H.; Zheng, S.; Gong, X.; Ren, H.; Li, Z.; Qu, S. Prenatal lipopolysaccharides exposure induces transgenerational inheritance of Hypertension. Circulation 2022, 146, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Chess, D.J.; Xu, W.; Khairallah, R.; O’Shea, K.M.; Kop, W.J.; Azimzadeh, A.M.; Stanley, W.C. The antioxidant tempol attenuates pressure overload-induced cardiac hypertrophy and contractile dysfunction in mice fed a high-fructose diet. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2223–H2230. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Filipe, H.M.; Costantino, G.; Mazzon, E.; Santagati, S.; Caputi, A.P.; Thiemermann, C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur. J. Pharmacol. 2000, 390, 209–222. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Dugo, L.; Lepore, V.; Fonti, M.T.; Ciccolo, A.; Terranova, M.L.; Caputi, A.P.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur. J. Pharmacol. 2000, 406, 127–137. [Google Scholar] [CrossRef]
- Chami, B.; San Gabriel, P.T.; Kum-Jew, S.; Wang, X.; Dickerhof, N.; Dennis, J.M.; Witting, P.K. The nitroxide 4-methoxy-tempo inhibits the pathogenesis of dextran sodium sulfate-stimulated experimental colitis. Redox Biol. 2020, 28, 101333. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Mazzon, E.; Filipe, H.M.; Lepore, V.; Terranova, M.L.; Ciccolo, A.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, on the multiple organ failure induced by zymosan in the rat. Crit. Care Med. 2001, 29, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; McDonald, M.C.; Mota-Filipe, H.; Mazzon, E.; Costantino, G.; Britti, D.; Mazzullo, G.; Caputi, A.P.; Thiemermann, C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000, 43, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Singh, I.N.; Carrico, K.M.; Hall, E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008, 28, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Mazzon, E.; Zito, D.; Maiere, D.; Britti, D.; Genovese, T.; Cuzzocrea, S. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J. Cin. Periodontol. 2005, 32, 1062–1068. [Google Scholar] [CrossRef]
- Duann, P.; Datta, P.K.; Pan, C.; Blumberg, J.B.; Sharma, M.; Lianos, E.A. Superoxide dismutase mimetic preserves the glomerular capillary permeability barrier to protein. J. Pharmacol. Exp. Ther. 2006, 316, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Elmedal, B.; De Dam, M.Y.; Mulvany, M.J.; Simonsen, U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br. J. Pharmacol. 2004, 141, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Black, H.D.; Xu, W.; Hortle, E.; Robertson, S.I.; Britton, W.J.; Kaur, A.; New, E.J.; Witting, P.K.; Chami, B.; Oehlers, S.H. The cyclic nitroxide antioxidant 4-methoxy-TEMPO decreases mycobacterial burden in vivo through host and bacterial targets. Free Radic. Biol. Med. 2019, 135, 157–166. [Google Scholar] [CrossRef]
- Li, T.; Zhang, T.; Gao, H.; Liu, R.; Gu, M.; Yang, Y.; Cui, T.; Lu, Z.; Yin, C. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021, 41, 101886. [Google Scholar] [CrossRef]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Spejo, A.B.; Teles, C.B.; Zuccoli, G.D.S.; Oliveira, A.L.R. Synapse preservation and decreased glial reactions following ventral root crush (VRC) and treatment with 4-hydroxy-tempo (TEMPOL). J. Neurosci. Res. 2019, 97, 520–534. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T. Oxidative stress mediates the fetal programming of hypertension by glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Shetty, S.; Kumar, V.; Ramesh, V.; Bharati, S. Mito-TEMPO protects against bisphenol-A-induced testicular toxicity: An in vivo study. Free Radic. Res. 2022, 56, 427–435. [Google Scholar] [CrossRef]
- Andrade, M.R.; Azeez, T.A.; Montgomery, M.M.; Caldwell, J.T.; Park, H.; Kwok, A.T.; Borg, A.M.; Narayanan, S.A.; Willey, J.S.; Delp, M.D. Neurovascular dysfunction associated with erectile dysfunction persists after long-term recovery from simulations of weightlessness and deep space irradiation. FASEB J. 2023, 37, e23246. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Xie, K.; Cao, Y.-X.; Zhang, A. Hepatoprotective effect of mitochondria-targeted antioxidant mito-TEMPO against lipopolysaccharide-induced liver injury in mouse. Med. Inflamm. 2022, 2022, 6394199. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Kaufman, A.; Berkman, N. The nitroxide/antioxidant 3-carbamoyl proxyl attenuates disease severity in murine models of severe asthma. Free Radic. Biol. Med. 2021, 177, 181–188. [Google Scholar] [CrossRef]
- Assayag, M.; Goldstein, S.; Samuni, A.; Berkman, N. 3-Carbamoyl-proxyl nitroxide radicals attenuate bleomycin-induced pulmonary fibrosis in mice. Free Radic. Biol. Med. 2021, 171, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Fan, S.; Zheng, D.; Wang, G.; Yu, Y.; Chen, R.; Song, L.-S.; Fan, G.-C.; Zhang, Z.; Peng, T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: Role of mitochondrial superoxide anion. Basic Res. Cardiol. 2019, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.C.; Ferreira, V.F.; Vaz, M.G.; Cassaro, R.A.A.; Resende, J.A.; Sacramento, C.Q.; Costa, J.; Abrantes, J.L.; Souza, T.M.L.; Jordão, A.K. Chemistry and anti-herpes simplex virus type 1 evaluation of 4-substituted-1 H-1, 2, 3-triazole-nitroxyl-linked hybrids. Mol. Divers. 2021, 25, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef]
- Shetty, S.; Kumar, R.; Bharati, S. Mito-TEMPO, a mitochondria-targeted antioxidant, prevents N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Free Radic. Biol. Med. 2019, 136, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, I.; Santos, F.; Kido, L.; Lamas, C.; Montico, F.; Cagnon, V. Tempol differential effect on prostate cancer inflammation: In vitro and in vivo evaluation. Prostate 2023, 83, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Chen, Y.; Dennehy, K.; Blau, J.; Connors, S.; Mendonca, M.; Tarpey, M.; Krishna, M.; Mitchell, J.B.; Welch, W.J.; et al. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R37–R43. [Google Scholar] [CrossRef]
- Hahn, S.M.; Krishna, M.C.; DeLuca, A.M.; Coffin, D.; Mitchell, J.B. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med. 2000, 28, 953–958. [Google Scholar] [CrossRef]
- Asghar, M.; Lokhandwala, M.F. Antioxidant tempol lowers age-related increases in insulin resistance in Fischer 344 rats. Clin. Exp. Hypertens. 2006, 28, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Pisano, B.; Dugo, L.; Ianaro, A.; Patel, N.S.; Caputi, A.P.; Thiemermann, C. Tempol reduces the activation of nuclear factor-kappaB in acute inflammation. Free Radic. Res. 2004, 38, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.F.; Vaz, S.M.; Augusto, O. Inhibition of the chlorinating activity of myeloperoxidase by tempol: Revisiting the kinetics and mechanisms. Biochem. J. 2011, 439, 423–431. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Yang, L.; Dong, X.; Han, Y.; Li, Y.; Luo, J.; Li, W. SOCE-mediated NFAT1-NOX2-NLRP1 inflammasome involves in lipopolysaccharide-induced neuronal damage and Aβ generation. Mol. Neurobiol. 2022, 59, 3183–3205. [Google Scholar] [CrossRef]
- Li, L.; Tong, X.K.; Hosseini Kahnouei, M.; Vallerand, D.; Hamel, E.; Girouard, H. impaired hippocampal neurovascular coupling in a mouse model of Alzheimer’s disease. Front. Physiol. 2021, 12, 715446. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox Signal. 2007, 9, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Brunt, V.E.; Minson, C.T. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1507–H1511. [Google Scholar] [CrossRef] [PubMed]
- Medow, M.S.; Bamji, N.; Clarke, D.; Ocon, A.J.; Stewart, J.M. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J. Appl. Physiol. 2011, 111, 20–26. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Ramick, M.G.; Farquhar, W.B.; Townsend, R.R.; Edwards, D.G. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014, 306, F1499–F1506. [Google Scholar] [CrossRef]
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of geographic atrophy by the topical administration of OT-551: Results of a phase II clinical trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.M.; Smith, D.; Mick, R.; Lustig, R.; Mitchell, J.; Cherakuri, M.; Glatstein, E.; Hahn, S.M. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004, 10, 6411–6417. [Google Scholar] [CrossRef]
- Palakkal, S.; Cortial, A.; Frušić-Zlotkin, M.; Soroka, Y.; Tzur, T.; Nassar, T.; Benita, S. Effect of cyclosporine A—Tempol topical gel for the treatment of alopecia and anti-inflammatory disorders. Int. J. Pharm. 2023, 642, 123121. [Google Scholar] [CrossRef]
- Citrin, D.; Valle, L.; Camphausen, K.; Cooley-Zgela, T.; Smart, D.; Yao, M.; Mitchell, J.B.; Thompson, W.; Sereti, I.; Uldrick, T. Pilot trial of topical MTS-01 application to reduce dermatitis in patients receiving chemoradiotherapy for stage I-III carcinoma of the anal canal. Int. J. Oncol. 2022, 60, 68. [Google Scholar] [CrossRef]
- Maurel, V.; Laferrière, M.; Billone, P.; Godin, R.; Scaiano, J. Free radical sensor based on CdSe quantum dots with added 4-amino-2, 2, 6, 6-tetramethylpiperidine oxide functionality. J. Phys. Chem. B 2006, 110, 16353–16358. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiao, C.; Oyaizu, K.; Chikushi, N.; Chen, X.; Nishide, H. Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. A Polym. Chem. 2010, 48, 5404–5410. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, X.-Q.; Wang, X.-Q.; Shi, X.; Wang, W.; Yang, H.-B. Rotaxane-branched radical dendrimers with TEMPO termini. Chem. Commun. 2022, 58, 2006–2009. [Google Scholar] [CrossRef]
- Samuelson, L.E.; Sebby, K.B.; Walter, E.D.; Singel, D.J.; Cloninger, M.J. EPR and affinity studies of mannose–TEMPO functionalized PAMAM dendrimers. Org. Biomol. Chem. 2004, 2, 3075–3079. [Google Scholar] [CrossRef] [PubMed]
- Badetti, E.; Lloveras, V.; Wurst, K.; Sebastián, R.M.; Caminade, A.-M.; Majoral, J.-P.; Veciana, J.; Vidal-Gancedo, J. Synthesis and structural characterization of a dendrimer model compound based on a cyclotriphosphazene core with TEMPO radicals as substituents. Org. Lett. 2013, 15, 3490–3493. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liang, H.; Sun, T.; Yang, D.; Cao, M. A recoverable dendritic polyamidoamine immobilized TEMPO for efficient catalytic oxidation of cellulose. Carbohydr. Polym. 2018, 202, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Boothapandi, M.; Nasar, A.S. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020, 28, 104972. [Google Scholar] [CrossRef] [PubMed]
- Swiech, O.; Bilewicz, R.; Megiel, E. TEMPO coated Au nanoparticles: Synthesis and tethering to gold surfaces. RSC Adv. 2013, 3, 5979–5986. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, Y.; Kawazoe, N.; Chen, G. TEMPO-conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl. Mater. Interfaces 2017, 9, 35683–35692. [Google Scholar] [CrossRef]
- Spillmann, C.M.; Naciri, J.; Algar, W.R.; Medintz, I.L.; Delehanty, J.B. Multifunctional liquid crystal nanoparticles for intracellular fluorescent imaging and drug delivery. ACS Nano 2014, 8, 6986–6997. [Google Scholar] [CrossRef]
- Nag, O.K.; Naciri, J.; Lee, K.; Oh, E.; Almeida, B.; Delehanty, J.B. Liquid crystal nanoparticle conjugates for scavenging reactive oxygen species in live cells. Pharmaceuticals 2022, 15, 604. [Google Scholar] [CrossRef]
- Thangavel, S.; Yoshitomi, T.; Sakharkar, M.K.; Nagasaki, Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J. Control. Release 2015, 209, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Avnir, Y.; Turjeman, K.; Tulchinsky, D.; Sigal, A.; Kizelsztein, P.; Tzemach, D.; Gabizon, A.; Barenholz, Y. Fabrication principles and their contribution to the superior in vivo therapeutic efficacy of nano-liposomes remote loaded with glucocorticoids. PLoS ONE 2011, 6, e25721. [Google Scholar] [CrossRef]
- Wasserman, V.; Kizelsztein, P.; Garbuzenko, O.; Kohen, R.; Ovadia, H.; Tabakman, R.; Barenholz, Y. The antioxidant tempamine: In vitro antitumor and neuroprotective effects and optimization of liposomal encapsulation and release. Langmuir 2007, 23, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Kizelsztein, P.; Ovadia, H.; Garbuzenko, O.; Sigal, A.; Barenholz, Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J. Nneuroimmunol. 2009, 213, 20–25. [Google Scholar] [CrossRef]
- Turjeman, K.; Bavli, Y.; Kizelsztein, P.; Schilt, Y.; Allon, N.; Katzir, T.B.; Sasson, E.; Raviv, U.; Ovadia, H.; Barenholz, Y. Nano-drugs based on nano sterically stabilized liposomes for the treatment of inflammatory neurodegenerative diseases. PLoS ONE 2015, 10, e0130442. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Pieńkowska, N.; Chmielarz, P.; Bartosz, G.; Dziedzic, A.; Sadowska-Bartosz, I. Nitroxide-containing amphiphilic polymers prepared by simplified electrochemically mediated ATRP as candidates for therapeutic antioxidants. Polymer 2023, 273, 125885. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Miyamoto, D.; Nagasaki, Y. Design of core− shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules 2009, 10, 596–601. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Ozaki, Y.; Thangavel, S.; Nagasaki, Y. Redox nanoparticle therapeutics to cancer—Increase in therapeutic effect of doxorubicin, suppressing its adverse effect. J. Control. Release 2013, 172, 137–143. [Google Scholar] [CrossRef]
- Nagasaki, Y. Nitroxide radicals and nanoparticles: A partnership for nanomedicine radical delivery. Ther. Deliv. 2012, 3, 165–179. [Google Scholar] [CrossRef]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–836. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Redox nanoparticles: Synthesis, properties and perspectives of use for treatment of neurodegenerative diseases. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, C.P.; Nagasaki, Y. Oral nanotherapeutics: Redox nanoparticles attenuate ultraviolet B radiation-induced skin inflammatory disorders in Kud: Hr-hairless mice. Biomaterials 2017, 142, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Shiota, K.; Hama, S.; Yoshitomi, T.; Nagasaki, Y.; Kogure, K. Prevention of UV-induced melanin production by accumulation of redox nanoparticles in the epidermal layer via iontophoresis. Biol. Pharm. Bull. 2017, 40, 941–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, A.; Yonemoto, C.; Feliciano, C.P.; Shashni, B.; Nagasaki, Y. Antioxidant nanomedicine significantly enhances the survival benefit of radiation cancer therapy by mitigating oxidative stress-induced side effects. Small 2021, 17, 2008210. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Uto, Y.; Kawasaki, A.; Noguchi, C.; Tanaka, R.; Yoshitomi, T.; Nagasaki, Y.; Endo, Y.; Hori, H. Evaluation of the in vivo antioxidative activity of redox nanoparticles by using a developing chicken egg as an alternative animal model. J. Control. Release 2014, 182, 67–72. [Google Scholar] [CrossRef] [PubMed]
- DeJulius, C.R.; Dollinger, B.R.; Kavanaugh, T.E.; Dailing, E.; Yu, F.; Gulati, S.; Miskalis, A.; Zhang, C.; Uddin, J.; Dikalov, S. Optimizing an antioxidant tempo copolymer for reactive oxygen species scavenging and anti-inflammatory effects in vivo. Bioconjug. Chem. 2021, 32, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Dao, N.V.; Ercole, F.; Li, Y.; Davis, T.P.; Kaminskas, L.M.; Sloan, E.K.; Quinn, J.F.; Whittaker, M.R. Nitroxide-functional PEGylated nanostars arrest cellular oxidative stress and exhibit preferential accumulation in co-cultured breast cancer cells. J. Mater. Chem. B 2021, 9, 7805–7820. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A. ESR method in monitoring of nanoparticle endocytosis in cancer cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef]
- Pala, R.; Barui, A.K.; Mohieldin, A.M.; Zhou, J.; Nauli, S.M. Folate conjugated nanomedicines for selective inhibition of mTOR signaling in polycystic kidneys at clinically relevant doses. Biomaterials 2023, 302, 122329. [Google Scholar] [CrossRef]
- Shashni, B.; Tamaoki, J.; Kobayashi, M.; Nagasaki, Y. Design of a new self-assembling antioxidant nanomedicine to ameliorate oxidative stress in zebrafish embryos. Acta Biomater. 2023, 159, 367–381. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Hirayama, A.; Nagasaki, Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials 2011, 32, 8021–8028. [Google Scholar] [CrossRef]
- Asanuma, H.; Sanada, S.; Yoshitomi, T.; Sasaki, H.; Takahama, H.; Ihara, M.; Takahama, H.; Shinozaki, Y.; Mori, H.; Asakura, M. Novel synthesized radical-containing nanoparticles limit infarct size following ischemia and reperfusion in canine hearts. Cardiovasc. Drugs Ther. 2017, 31, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Boonruamkaew, P.; Chonpathompikunlert, P.; Vong, L.B.; Sakaue, S.; Tomidokoro, Y.; Ishii, K.; Tamaoka, A.; Nagasaki, Y. Chronic treatment with a smart antioxidative nanoparticle for inhibition of amyloid plaque propagation in Tg2576 mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 3785. [Google Scholar] [CrossRef] [PubMed]
- Okajo, A.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C.; Endo, K. Competition of nitroxyl contrast agents as an in vivo tissue redox probe: Comparison of pharmacokinetics by the bile flow monitoring (BFM) and blood circulating monitoring (BCM) methods using X-band EPR and simulation of decay profiles. Magn. Reason. Med. 2006, 56, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Advanced biomedical applications of multifunctional natural and synthetic biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
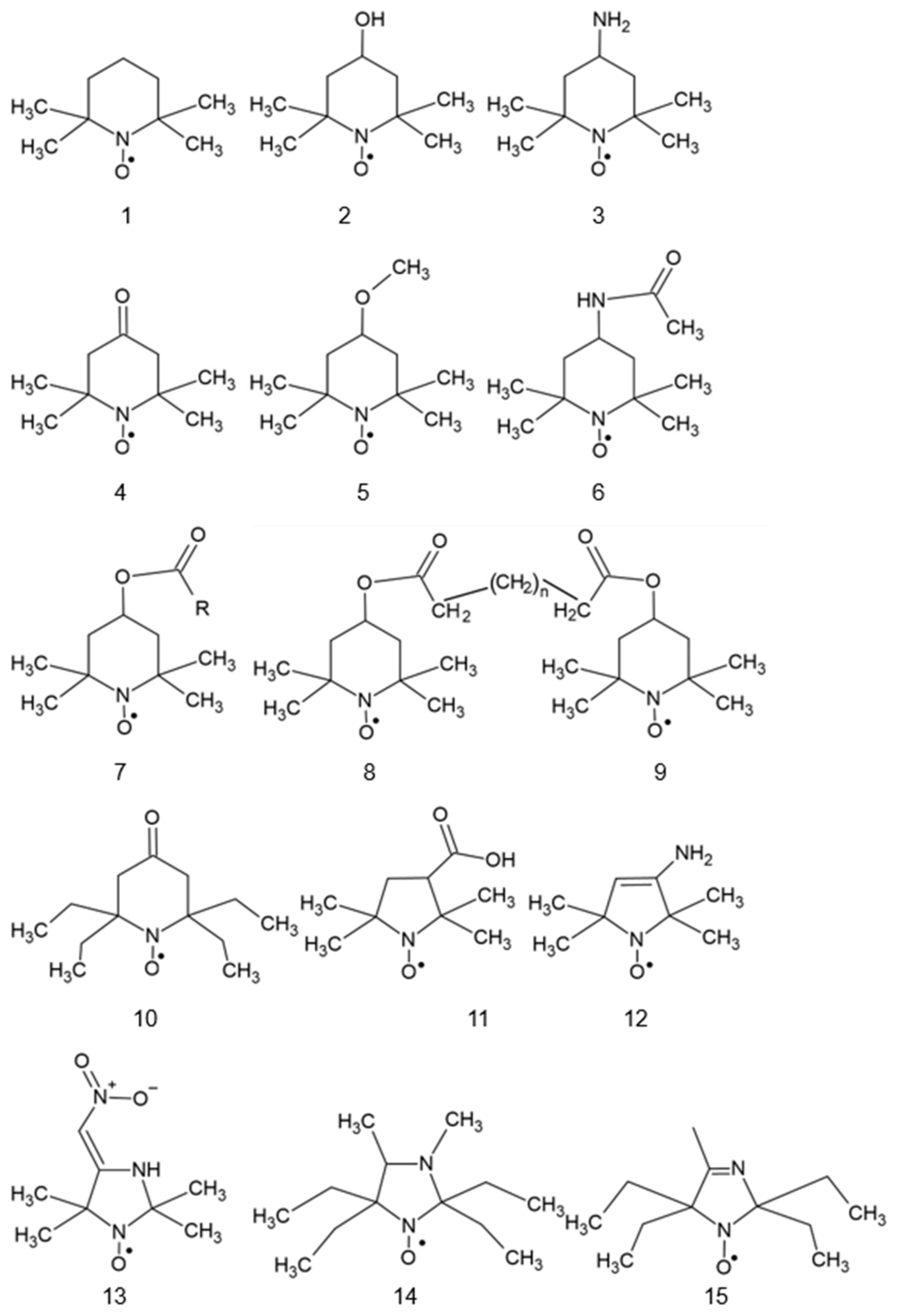
| Substituent at C4 | Reaction Rate Constant [M−1 s−1] | Standard Redox Potential with Respect to NHE [V] |
|---|---|---|
| -H | (5.1 ± 1.5) × 106 | 0.722 [11]; 0.577 [13]; 0.833 [12] |
| -OH | (1.1 ± 0.5) × 106 | 0.810 [11]; 0.603 [13]; 0.898 [12] |
| -NH2 | (5.4 ± 1.5) × 105 | 0.826 [11]; 0.789 [13] |
| -COOH | (3.7 ± 1.0) × 105 | 0.82 [11] |
| -NHCOCH3 | (1.1 ± 0.4) × 105 | 0.88 |
| -CONHBut | (1.9 ± 0.5) × 105 | |
| =O | (5.6 ± 1.2) × 104 | 0.913 |
| Trolox | (2.6 ± 0.7) × 105 |
| Cellular Model | Nitroxide, Concentration | Effect | Reference |
|---|---|---|---|
| CHO AS52 cells treated with H2O2 and hypoxanthine/xanthine oxidase | TEMPOL, 10 mM | Protection against mutagenic effects; no mutagenicity of TEMPOL alone | [69] |
| Cardiac ventricular cells from 1-day-old rats treated with hypoxanthine + xanthine oxidase | TEMPOL, 0.2 mM or hydroxylamine of TEMPOL 0.2 mM | Protection against contractability loss and LDH release by both nitroxide and hydroxylamine | [76] |
| Rat tracheal epithelial cells, exposed to NO donor S-nitrosoglutathione monoethyl ester, Angeli’s salt, SIN-1, and peroxynitrite | TEMPO, 14 and 15, 5 μM | Protection against DNA damage (tail moment in the comet assay) | [77] |
| B14 fibroblasts treated with 0.5 μM doxorubicin or 7 μM H2O2 | TEMPO, TEMPOL, TEMPAMINE, 4-acetamido-TEMPO (Figure 1, #1–3 and 6) | 0.05–3 mM nitroxides; protection against doxorubicin toxicity; 0.1–0.5 mM TEMPAMINE and 4-acetamido-TEMPO, protection against H2O2 toxicity (cell survival) | [78] |
| Human epithelial cell line A549 exposed to 0.1 ppm of O3 for 30 min | Cells pretreated with 100 μM TEMPO | Reduction in IL-8 production | [79] |
| A459 cells treated with 100 μM ferrous ammonium citrate for 24 h | 0.25 μM 2-(2,4-dimethoxyphenyl) 4,4,5,5-tetra-methylimidazoline-1-oxyl-3-oxide) (Figure 1, #20) present during the treatment | Increase in viability; decrease in lipid peroxidation; attenuation of the decrease in GSH level; decrease in apoptotic rate | [80] |
| Rat pleural macrophages treated with 1 mM H2O2 for 4 h | TEMPOL 0.03–3 mM administered 15 min before H2O2 | Attenuation of decrease in MTT reduction | [81] |
| HEK-2 cells treated with 80 μM t-BHP, or hypoxia (≤1% O2 for 24 h)/reoxygenation for 2 h) | Mito-TEMPO, 25 nM | Decreased ROS level; attenuation of the decrease in the level of mitochondrial transcription factor A and mtDNA copy number | [56] |
| Rat lenses treated with 1 mM H2O2 and incubated for 24 h | 4 mM TEMPOL hydroxylamine | Prevention of opacification | [82] |
| Human dermal fibroblasts subjected to 340–400 nm UV | TEMPOL, 0.03–8 mM | Increase in cell survival; decrease in TBARS level; inhibition of MMP-1 and MMP-3 expression | [57] |
| Human RPE-19 cells with accumulated lipofuscin fluorophore A2E | OT-674 (hydroxylamine of TEMPOL), 0.01–10 mM | Protection against the death of blue–light-exposed A2E-laden cells | [73] |
| Chinese hamster cells, X-irradiated under aerobic conditions | TEMPOL, 5, 10, 50, and 100 mM, added 10 min prior to irradiation | Protection of cell viability; protection factor of 1.25, 1.30, 2.1, and 2.5, respectively | [58] |
| Chinese hamster V79 cells, irradiated with 4 MV photons | Nitroxides, 10 mM, given 10 min before irradiation | Protection of cell viability, protection factor at 10% survival: 2.4 for 3-aminomethyl-PROXYL, 2.3 for TEMPAMINE, 1.6 for 3-cyano-PROXYL, 1.5 for 3-carbamylo-PROXYL, 1.3 for TEMPOL, and 1.2 for 4-oxo-TEMPO | [83] |
| Thymocytes from 1-month-old p53+/+ and p53−/− mice, 2.5 Gy of IR | TEMPOL, 1 mM, added 30 min before irradiation | Increase in p53 phosphorylation at Ser18 and p21 expression | [84] |
| Human fibrosarcoma HT1080 cells, ferroptosis induced by RSL3, and oxytosis induced by 5 mM glutamate | TEMPO 1, 2.5, 10, and 100 mM in a next dish, 37 °C, 5 h/8 h in the case of oxytosis | Concentration-dependent inhibition of ferroptosis by volatile TEMPO | [75] |
| Human neuroblastoma SH-SY5Y cells, differentiated, treated with 30 μM 6-hydroxydopamine (6-OHDA) (a cellular model of Parkinson’s disease) | TEMPOL, 30 μM | Attenuation of decrease in viability; decrease in necrosis; increase in mitochondrial superoxide production; increase in TBARS level; HO-1 expression; and NFκB-p65 activation | [60] |
| Human neuroblastoma SH-SY5Y cells, not differentiated, treated with 30 μM 6-OHDA | TEMPO, 100 and 150 μM; TEMPAMINE, 75–150 μM | Increase in cell survival; increase in GSH level; attenuation of decrease in mitochondrial potential | [62] |
| SH-SY5Y cells transfected with the tau protein (a model of chronic cellular oxidative stress) | TEMPO. TEMPOL and TEMPAMINE 1–10 μM | Decrease in the ROS level; increase in mitochondrial potential | [85] |
| Dopaminergic MN9D cells, differentiated, treated with 100 μM 6-OHDA for 20 min | TEMPOL, 0.15 μM, added 1 h before 6-OHDA | Protection of cell viability; activation and nuclear translocation of NF-κB | [61] |
| Bovine aortic endothelial and smooth muscle cells exposed to 23 mM glucose | 5 mM TEMPOL | Increase in GLUT1 and GLUT4 expression and in glucose uptake | [63] |
| Immortalized mouse podocytes, treated with high glucose (30 mM) for 48 h | TEMPO, 100 nM | Inhibition of triglyceride and cholesterol accumulation | [64] |
| Inflammatory neutrophils isolated from Swiss male mice administered i.p. with 12% sodium caseinate | TEMPOL 5–120 μM TEMPOL 120 μM | Inhibition of respiratory burst, IC50 = 45 μM; inhibition of protein kinase activities, inhibition of fungicidal activity | [86] |
| Buffalo bull spermatozoa | Mito-TEMPO 50 μM added to semen extender | Improvement in post-thaw semen quality; reduction in ROS and TBARS levels | [67] |
| Ram’s semen cryopreserved and thawed | Mito-TEMPO added to the cryopreservation medium, 5 and 50 μM | Improvement in sperm motility, membrane functionality, and mitochondrial activity; attenuation of apoptotic changes; increase in ROS and TBARS levels | [65] |
| Ram’s semen in the cooling medium stored at 5 °C for 24 and 48 h | Mito-TEMPO added to the medium, 5 and 50 μM | Improved sperm viability, motility, and mitochondrial membrane potential; decreased TBARS level, elevated pregnancy, parturition, and lambing rates | [66] |
| Bovine oocytes in the maturation medium | 1 μM mito-TEMPO in the medium | Increase in the proportion of developing oocytes and expression of Bcl2 and GSH level; decrease in the ROS level and expression of Bax; better effect of mito-TEMPO than TEMPO | [68] |
| Baker’s yeast Saccharomyces cerevisiae | TEMPO, 3 mM | Reduction in the number of double-strand DNA breaks; increase in metabolic rate; change in the pattern of gene expression; slowing down the aging of postmitotic cells; protection against H2O2 toxicity | [87] |
| Candida albicans | TEMPOL, 0.344 mg/mL | Upregulation of genes involved in iron homeostasis, mitochondrial stress, steroid synthesis, and amino acid metabolism; IC90: 0.5–0.68 mg/mL | [88] |
| Biofilms of Staphylococcus aureus | Nitroxides conjugated to antibiotics, e.g., fluoroquinolone-TEMPO or coadministration of 4-carboxy-TEMPO with ciprofloxacin | Penetration of biofilms and into the cells; dispersal of biofilms; no toxicity to human cells | [24] |
| Mouse primary neurons | 0.1–10 μM L-NNNBP | Protection from the toxicity of 5 Aβ1–42 and Aβ1–42-induced caspase activation and apoptosis, protein nitration, and depolarization of mitochondria | [89] |
| Cytoplasmic hybrids (cybrids) of SH-SY5Y cells containing mitochondria from platelets of patients with mild cognitive impairment and cortical neurons from tau mice | Mito-TEMPO (concentration not indicated) | Protection of mitochondrial respiratory function; suppression of tau oligomer accumulation | [90] |
| Cellular Model | Nitroxide, Concentration | Effect | Reference |
|---|---|---|---|
| HaCaT cells | Nitroxides, 24 h treatment | Cytotoxicity, IC50 values: TEMPO, 2.66 mM; TEMPOL, 11.4 mM; TEMPAMINE, 9.5 mM | [49] |
| Human erythrocytes, hematocrit 20%, 3-h incubation at 37 °C | TEMPO, 0.5 mM | Decrease in GSH level down to 30% after 3 h, increase in GSH export to the extracellular medium, increase in methemoglobin content | [92] |
| Human erythrocytes, hematocrit 10%, 1-h incubation at room temperature | TEMPO 0.2–2 mM | Human erythrocytes, hematocrit 10%, 1 h incubation at room temp. | [93] |
| Rat glioma C6 cells | TEMPOL, 1 mM | 60 and 67% apoptosis after 24 and 72 h, respectively | [94] |
| Human prostate carcinoma PC-3, LNCaP and DU-145 cells | TEMPO 0.25–5 mM | Induction of apoptosis; increase in activities of caspases 3 and 9; chromatin fragmentation; viability loss | [95] |
| MCF-7. HL-60, HepG2 cells | 5 mM TEMPO | Increase in the intracellular level of H2O2 | [95] |
| Bovine aortic endothelial and smooth muscle cells, 5 and 23 mM glucose | 0.2–5 mM TEMPOL 5 mM TEMPOL | Increase in ROS level; increase in protein carbonylation | [63] |
| Microvascular cells derived from bovine adrenals, X-irradiated (8 Gy) | 0.5 and 2 mM TEMPOL, 10 min before and 1 h after irradiation | Partial prevention of cell mobility loss in a wound healing assay | [96] |
| B14 fibroblasts | 5-DS 0.5–500 μM, Methyl-12-DS, and 16-DS, 0.5 μM–2 mM, 24-h incubation | Reduction in viability | [97] |
| Vero E6 cells | TEMPOL, 0.2 and 0.5 mM | Loss of the Fe-S clusters of nsp12; no effect on the activities of several mitochondrial Fe-S enzymes, including the respiratory complexes, mitochondrial aconitase, and cytosolic dihydropyrimidine dehydrogenase | [72] |
| Immortalized human keratinocytes, HaCaT | TEMPOL, TEMPOL-H, TEMPAMINE 5 mM; TEMPO and TEMPO+, 16 μM TEMPOL, TEMPOL-H, TEMPAMINE 5 mM; TEMPO, 1 mM; TEMPO+, 16 μM TEMPOL, TEMPOL-H, TEMPAMINE 5 mM; TEMPO, 1 mM; TEMPO+, 4 μM | Increase in the level of ROS. Activation of Nrf2; (Small) protection against UV (30 mJ/cm2) | [91] |
| PC3 human prostate cancer cells and HT29 human colon cancer cells | TEMPAMINE conjugates of betulinic, fusidic, and cholic acids | Significant cytotoxicity, 6.0 and 7.4 μM, respectively, for the fusidic acid conjugate, a mitochondria-targeted fusidic acid derivative constructed | [98] |
| Human squamous lung carcinoma Calu-6 cells, non-squamous lung carcinoma A549 cells, and normal lung WI-38 VA-13 subclone 2RA cells | TEMPOL, 48 h | IC50 of 1–2 mM, no difference between carcinoma and normal cells Increase in ROS, GSH depletion (2 mM TEMPOL) | [99] |
| Baker’s yeast Saccharomyces cerevisiae | TEMPO | Growth inhibition of Δsod1 strain (from 0.1 mM TEMPO and Δsod2 strain (from 1 mM TEMPO; wild type yeast from 3 mM TEMPO | [87] |
| Promastigotes of Leishmania braziliensis in macrophages | TEMPOL | Killing of promastigotes with IC50 of 0.66 mM | [100] |
| Animal Model | Nitroxide, Dose | Effect | Reference |
|---|---|---|---|
| C3H mice | TEMPOL administered in chow, 10 mg/g food (ca. 58 mM) | Weight reduction (28.2 ± 0.8 g vs. 41.9 ± 0.6 g over 30–75 weeks) Increase in mean life span (123 vs. 92.6 weeks) Longer persistence of activity and coat color Decreased tumor incidence (10% vs. 40%) Elevated levels of UCP-2 and HSP70 in the skeletal muscle | [104] |
| Atm−/− mice, in 129SvEv background, a mouse model of ataxia telangiectasia, displaying accelerated oxidative damage and stress | TEMPOL administered in chow, 10 mg/g food | No decrease in food intake, metabolic rate, or physical activity, but reduced body mass, decreased ROS, and increased mitochondrial potential in thymocytes, reduced proliferation of thymocytes and splenocytes, attenuation of increased HO-1 expression in the brain and thymus, and increased protein carbonyls | [105] |
| p53−/− mice | TEMPOL administered in chow, 10 mg/g food | Increase in mean tumor-free survival (21.4 to 25 w), no reduction of oxidative stress | [84] |
| Sprague Dawley male rats administered intragastrically with 1 mL 96% ethanol, subcutaneously with indomethacin (30 mg/kg b.w.), or intragastrically with aspirin (0.1 g/kg b.w.) | TEMPOL 0.1 g/kg b.w. 5 min before induction of the damage | Reduction in mucosal damage and the level of leukotriene B4 in the mucosa | [106] |
| Male C57BL/6 mice, renal ischemia induced by bilateral clamping of the renal pedicles for 30 min | Mito-TEMPO, 25 μL of 5 μM solution injected into each kidney after reperfusion, and then 5 mg/kg each day, i.p. for 5 days | Improvement in renal functions decreased Bax expression | [56] |
| Hearts isolated from male Sprague Dawley rats, 10 min of ischemia, 5 min reperfusion | TEMPO, 0.4 and 1 mM in the perfusion fluid from 10 min before reperfusion | Protection against reperfusion injury and LDH release | [106] |
| Male Sprague Dawley rats, heart ischemia (5 min) and reperfusion (30 min) | TEMPOL 30 mg/kg and 100 mg/kg, 5 min before occlusion, 60 s before reperfusion, or 60 s after onset of reperfusion | Protection against ventricular tachycardia and ventricular fibrillation when administered before ischemia or before reperfusion | [107] |
| Dogs subjected to 20-min cardiac arrest | TEMPOL 300 mg/kg in saline flush | Improved cerebral performance, better neurologic scores | [108] |
| Male Wistar Albino rats, a 60 min occlusion of the superior mesenteric artery | TEMPOL 30 mg/kg in saline solution during the first 60 min of reperfusion | Attenuation of increase in myeloperoxidase activity, TBARS level, bacterial translocation, and decrease in GSH level | [109] |
| Male Wistar rats, subjected to 30-min liver ischemia and 2-h reperfusion | NNR (Figure 1, #21), 30 mg/kg 10 min before reperfusion and 1 h after the onset of reperfusion | Decrease in hepatic TBARS level and serum level of ALAT and ASPAT | [110] |
| Male Wistar rats, two-kidney, one-clip (2K-1C; 8 w) hypertension | TEMPOL 18 mg/kg/day by gavage | Attenuation of increase in systolic blood pressure, reduction in endothelium-dependent vasorelaxation and MMP-2 activity, vascular remodeling, ROS, and TBARS levels | [111] |
| Wistar rats subjected to bilateral renal occlusion for 45 min | TEMPOL 30 mg/kg | Attenuation of increase in total severity score plasma urea and creatinine and LDH release | [112] |
| Male Mongolian gerbils, ischemia-reperfusion injury induced by 5-min bilateral occlusion of the common carotid arteries | TEMPOL, 30 mg/kg i.p., 30 min before and 1, 2, and 6 h after the onset of reperfusion | Increased survival, reduced hyperactivity, reduced nitrotyrosine staining, reduced loss of neurons from the pyramidal layer of the CA1 region | [81] |
| Male Sprague Dawley rats, ischemic acute renal failure induced by renal artery and vein were occlusion for 45 min | TEMPOL 100 mg/kg, i.v, 5 min before ischemia | Attenuated the ischemia/reperfusion-induced renal dysfunction | [113] |
| C.B-17/Icr-+/+Jcl mice, focal cerebral ischemia induced by electrocoagulation | 0.1 g of cotton soaked in 5 mL of 0.03–1 mM TEMPO in mouse cage 15 min after infarction for 8 h | Reduction in ischemic damage | [75] |
| Female Sprague Dawley rats, acute retinal ischemia by elevation of intraocular pressure for 60 min | 5,6-dicarboxy-1,1,3,3-tetraethyllisoindolin-2-yloxyl, 2 μL of 2.5 mM solution injected into eye 30 min before reperfusion, 2 i.p. injections (20 mg/kg) at the beginning of experiment and after 60 min reperfusion | Protection of the retina from I/R-induced damage, maintaining retinal function, and decrease in the number of “activated” microglia, particularly in the outer retina | [113] |
| Sprague Dawley rats exposed to 700 lux of white fluorescent light for 6 h | OT-551, 25, 50, or 100 mg/kg; TEMPOL-H (OT-674, 100 mg/kg | Reduction in RPE damage index, more significant for all concentrations of OT-551 than TEMPOL-H | [114] |
| Sprague Dawley rats exposed to 700 lux of white fluorescent light for 6 h | OT-551, 25, 50, or 100 mg/kg; TEMPOL-H (OT-674, 100 mg/kg TEMPOL-H (OT-674, 100 mg/kg | Reduction in increase in the levels of 4-HNE- and 4-HNE-protein adducts, increased electroretinogram b-wave amplitudes, and increased outer nuclear layer thickness, more significant for all concentrations of OT-551 than TEMPOL-H | [115] |
| Female C3H mice subjected to 137Cs gamma irradiation (dose rate: 1 Gy/min) | TEMPOL administered i.p. 5–10 min prior to irradiation | Radioprotective effect: increase in LD50 from 7.84 Gy to 9.97 Gy | [59] |
| Female C3H mice irradiated with 9 kGy of γ radiation (137Cs source) | TEMPOL 275 mg/kg, TEMPAMINE 250 mg/kg, 3-aminomethyl-PROXYL 225–275 mg/kg, 3-carbamoyl-PROXYL 300–500 mg/kg, 4-oxo-TEMPO 225 mg/kg, and 3-CTPO 400 mg/kg given i.p. 5–10 min before irradiation | Increase in survival, 3-AM > TEMPOL > 3-CTPO > 3-CP > TEMPAMINE > 4-oxo-TEMPO. Decrease in blood pressure over 60 min, smallest for 3-CP | [116] |
| Female C3H mice with RIF-1 tumor | Whole-body irradiation, 10–60 Gy with 9 Me electrons TEMPOL i.p. injection (275 mg/kg, 10 min prior to irradiation | No protection of tumor cells, radioprotection of bone marrow cells, probably due to greater reduction to hydroxylamine in cancer cells | [117] |
| Female C3H mice subjected to localized X irradiation, 5 × 6 Gy to head Female C3H/Hen mice with propagated squamous cell carcinoma, X-irradiated 5 × 3 Gy or with HT-29 adenocarcinoma, X-irradiated 5 × 2 Gy | TEMPOL i.p. injection (275 mg/kg) 10 min before irradiation + 50 μL TEMPOL gel to oral cavity TEMPOL i.p. injection (275 mg/kg) 10 min before irradiation + 50 μL TEMPOL gel to oral cavityo oral cavity | Salivary gland radioprotection No tumor radioprotection; 2× faster reduction to hydroxylamine in the cancer | [118] |
| Female C3H mice, head irradiated with X-rays or carbon-ion beam | TEMPOL 150 mM injected i.v. | Changes in redox status of the brain, reduction followed by reoxidation | [119] |
| Miniature pigs of both sexes (30–80 kg) | TEMPOL 25–35 mg/kg and 3-CP (10, Figure 1) 30–300 mg/kg given i.v. | Decrease in arterial blood pressure (maximal after 5–10 min) accompanied by increased heart rate. No hypotensive effect of 3-CP | [120] |
| Male Sprague Dawley rats, experimental hypertension induced by deoxycorticosterone acetate | TEMPOL, 15 mg/kg, i.p., 21 days | Alleviation of hypertension, improvement in acetylcholine-induced EDHF-mediated vasodilation | [121] |
| Obese Zucker rats | 1 mM TEMPOL in drinking water for 15 d | Decreased body mass and the levels of insulin, triglycerides, and TBARS, improvement in insulin sensitivity | [122] |
| Zucker rats fed a high-fat diet for 10 w | TEMPOL, 1 mM in the drinking water for 10 w | Decrease in the levels of blood glucose, triglycerides, cholesterol, VLDL, CRP, insulin, and urinary albumin, increase in blood HDL, attenuation of the expression of genes coding for desmin, TNF-α, NFκB, and NOX-1 | [123] |
| SOD1−/+ C57BL/6 mice, streptozotocin-induced diabetes | TEMPOL 80 mg/kg/d for 35 days | Suppression of albuminuria increases in glomerular transforming growth factor β, collagen α1(IV), nitrotyrosine, and glomerular superoxide | [124] |
| Female C3H/Hen− TacMT mice fed high-fat diet | TEMPOL in the diet (10 g/kg) | Restriction of body mass gain and lipid accumulation, alteration of gut microbiome, downregulation of fatty acid synthesis genes, and upregulation of fatty acid oxidation genes | [125] |
| ApoE−/− mice, fed standard and high-fat diet (HFD) | TEMPOL, 10 mg/g food, up to 90 d | Reduction in body mass gain in mice fed standard diet but, especially, HFD, decrease in plasma triglycerides and cholesterol and inflammatory markers. | [126] |
| Male Sprague Dawley rats, experimental hypertension induced by 28-d treatment with deoxycorticosterone | TEMPOL 1 mM administered in drinking water during the experiment | Amelioration of hypertension (142 ± 5 vs. 199 ± 3 mm Hg) | [127] |
| Sprague Dawley rats intraperitoneally injected with LPS to induce hypertension | TEMPOL 1 mM in drinking water | Prevention of hypertension in the first-generation offspring and the transgenerational inheritance of hypertension | [128] |
| C57BL/6J mice, 20–25 g, fed high fructose (8 weeks), subjected to transverse aortic constriction | TEMPOL, 0.1% in feed (ca 150 mg/kg/day), 8 weeks | Attenuation of cardiac hypertrophy, decrease in LV area; decrease in TBARS and 4-hydroxyalkenals | [129] |
| Male Sprague Dawley rats carrageenan-induced pleurisy | TEMPOL, 10, 30, and 100 mg kg−1 given i.p. 15 min before carrageenan | Dose-dependent attenuation of lung injury histology, increase in tissue myeloperoxidase and TBARS, decrease in nitrotyrosine content and peroxynitrite formation | [130] |
| Male Sprague Dawley rats, dinitrobenzene sulfonic acid-induced colitis | TEMPOL i.p., 15 mg/kg daily for 7 d | Decrease in mortality, damage score, myeloperoxidase activity, and TBARS level in the colon | [131] |
| C57BL/6 mice, experimental colitis induced by 3% w/v dextran-sodium-sulfate (DSS) in drinking water over 9-days | 4-Methoxy-TEMPO, 15 mg/kg, i.p., twice daily | Decreased clinical index, attenuation of body mass loss, crypt loss, mucin loss; decreased cellular infiltrate and serum content of lipid peroxidation products | [132] |
| Male Sprague Dawley rats, zymosan-induced generalized inflammation | TEMPOL (100 mg/kg i.p.) at 1 and 6 h after zymosan administration | Decreased mortality, toxicity score, myeloperoxidase activity, and TBARS level in lung, intestine, and liver | [133] |
| Male Lewis rats, collagen-induced arthritis | TEMPOL, 10 mg/kg/d, i.p., days 23–34 | Decrease in % of arthritic rats, reduced hind paw swelling, histological damage score, radiograph score, and plasma level of TBARS | [134] |
| Male CF-1 mice subjected to controlled cortical focal traumatic brain injury | TEMPOL 300 mg/kg i.p. 15 min after the injury TEMPOL 300 mg/kg i.p. 15 min, 3, 6, 9, and 12 h) | Suppression of 3-nitrotyrosine formation in injured cortical tissue 1 h after injury Suppression α-spectrin degradation by 45% at 24 h | [135] |
| Male Sprague Dawley rats, periodontitis induced by ligation of the 1st molar for 8 d | TEMPOL, 10 mg/kg daily, i.p., for 8 days | Decreased neutrophil infiltration, tissue permeability, nitrotyrosine level, poly-(ADP-ribose)polymerase (PARP) activation | [136] |
| Lewis rats, glomerular immune injury induced by an antiglomerular basement membrane antibody or TNF | TEMPOL, 230 mg/kg, i.p. | Decrease in of urine protein and total isoprostane excretion | [137] |
| Male Wistar rats subjected to hypoxic, hypobaric conditions for 2 weeks | TEMPOL, 1 mM in the drinking water during the experiment | Prevention of increase in the right ventricular systolic pressure, amelioration of right ventricular hypertrophy | [138] |
| A/J mice, 6-OHDA administered to the striatum (a model of Parkinson’s disease) | TEMPOL, 200 mg/kg, given i.p. 60 min before the treatment | Reduced ptosis score, increased activity score, decreased fractional mortality | [61] |
| Zebrafish (Danio rerio) microinfected with Mycobacterium marinum (a model of tuberculosis) | 4-Metoxy-TEMPO, 1 and 5 mM in the medium | Inhibition of production of mitochondrial ROS decreased infection-induced granuloma cell death, disruption of the NADH: NAD+ balance in M. marinum | [139] |
| Female prepuberal Sprague Dawley rats treated with dehydroepiandrosterone for 21 d | TEMPOL 30 mg/kg daily for 12 d | A significant reduction in intestinal oxidative stress in polycystic ovary syndrome rats without affecting the ovarian redox state. Changes in gut microbiota composition and serum metabolite profiles | [140] |
| Female C57BL/6 mice injected with 0.52 × 106 cells of Candida albicans | TEMPOL, 1.6 mg/g of mouse/day | Partial protection, reduction in fungal burden in the kidneys of infected animals during infection onset, improvement in animal fitness | [88] |
| C57BL/6N mice | TEMPOL administered by gavage, 250 mg kg −1 per day | Alteration in the gut microbiome, preferential reduction in Lactobacillus and its bile salt hydrolase activity, and anti-obesity effects | [141] |
| Female Sprague Dawley rats subjected to ventral root crush (VRC) at the lumbar intumescence | TEMPOL 250 mg/kg, 10 min and 24 h after injury and then every 48 h for 14 days | Preservation of proprioceptive glutamatergic inputs without exacerbating the rate of motoneuron degeneration | [142] |
| Female Wistar Kyoto (WKY) rats given dexamethasone (0.1 mg/kg per day) s.c. from gestational day 15 to 21) | TEMPOL, 1 mM in drinking water during pregnancy plus 100 μg/kg s.c., days 15–21 | Attenuation of Dex-induced increases in blood pressure, adrenal mRNA, and protein levels of catecholamine biosynthetic enzymes in the offspring | [143] |
| Male Wistar rats administered bisphenol A, 10 mg/kg b.w., orally, once a week for 4 w | Mito-TEMPO 0.1 mg/kg b.w, i.p. twice a week | Normalization of sperm parameters and preserved histoarchitecture of the testis, inhibition of increase in mitochondrial ROS level and lipid peroxidation | [144] |
| Male Fisher 344 rats, 4-week simulated weightlessness, 0.75 or 1.5 Gy of cosmic radiation, 12–13-month recovery | Tissues (distal internal pudendal artery and corpus cavernosum) incubated with 5 μM mito-TEMPO for 30 min | Improvement in neurovascular erectile function | [145] |
| C57BL/6 mice, sepsis induced by i.p. injection of LPS, 5 mg/kg b.w. | LPS, 20 mg/kg, i.p., body,1h prior to LPS injection | Inhibition of inflammation, attenuation of LPS-induced liver injury, prevention of increase in serum TBARS level, attenuation of increase in mitochondrial ROS production | [146] |
| Male C57Bl/6 and Balb/c mice, asthma induced by chicken ovoalbumin or house dust mites | 3-CP in chow (1% w/w) during the experiment | Decrease in the inflammatory cell count, pulmonary collagen, TGF-β and 3-nitrotyrosine, improved baseline pulmonary functions | [147] |
| C57Bl/6 mice, bleomycin-induced lung injury | 3-CP in chow (1% w/w) during the experiment | Decrease in the inflammatory cell count, pulmonary collagen, fibrosis, TGF-β and 3-nitrotyrosine, improved baseline pulmonary functions and weight loss | [148] |
| C57BL/6 mice transfected with a vector coding for cardiomyocyte-specific mitochondrially targeted calpain 1 | Daily i.p. injections of mito-TEMPO, 0.7 mg/kg/day, for 30 d | Inhibition of progression of progression of dilated heart failure, adverse myocardial remodeling, reduced mortality | [149] |
| HSV-1 virus in Vero (African green monkey kidney) cells | 4-Substituted-1,2,3-1H-1,2,3-triazole linked TEMPOL derivatives | New derivatives are less cytotoxic than acyclovir, one more virucidal than acyclovir | [150] |
| C57BL/6 J mice subjected to 5/6 nephrectomy | Mito-TEMPO 1 mg/kg/d, i.p., 12 w | Improvement in impaired renal function and renal fibrosis, attenuation of kidney disease-induced muscle atrophy, suppression of inflammatory cytokines and ROS level, mitochondrial dysfunction, and endoplasmic reticulum stress in skeletal muscles | [151] |
| Male BALB/c mice, hepatocarcinogenesis induced by N-nitrosodiethylamine (i.p.) | Mito-TEMPO, 0.1 mg/kg, i.p., once a week until the end of the experiment | Increased animal survival (by 30%), decreased tumor incidence (by 25%), and tumor multiplicity (by 39%). | [152] |
| Athymic female nude mice injected with C6 glioma cells | TEMPOL, 0.25 g/mL (TPL B) receiving 0.375 g/mL via osmotic pump (0.5 μL/h) for 14 days plus 2 daily i.p. injections, 100 mg/kg), 5 days/week | Dose-dependent decrease in the xenograft growth | [94] |
| Male BALB/c nu/nu mice inoculated with LNCaP, DU-145, or PC-3 cells | TEMPO given intratumorally, once daily, 100 mg/kg per dose, Days 1–8, and then 200 mg/kg per dose, Days 9–23 | Decreased tumor growth | [95] |
| Male TRAMP mice in the early and late stages of prostate cancer | TEMPOL 50 or 100 mg/kg diluted in water five times a week for 4 w | Decrease in NFB total protein and TNFα levels; decreased tumor progression | [153] |
| Male APP/PS1 double-transgenic mice (model for Alzheimer’s disease) | L-NNNBP, 1 mM in drinking water (55–100 mg/kg) for 1 m | Attenuated brain Aβ deposition and tau phosphorylation, decreased astrocyte activation, and improved spatial learning and memory | [89] |
| Male spontaneously hypertensive (SHR) rats | Pipridine and pyrrolidine nitroxides, i.v. | Piperidine but not pyrrolidine nitroxide dose-dependently decreased mean arterial pressure (by more than 40 mm Hg at 270 μmol/kg TEMPOL) | [154] |
| Examined Subjects | Nitroxide, Administration | Effect | Reference |
|---|---|---|---|
| Ten young smokers (19–26 y) | TEMPOL 10 μM, infused (2.0 μL/min) via microdialysis fibers to the ventral side of the forearm in the dermal layer of the skin for at least 75 min | Increase in the NO-dependent cutaneous vascular conductance plateau, indicative of enhanced NO availability | [164] |
| Eight healthy volunteers (24.5–29.5 y) | TEMPOL 10 μM, administered via a microdialysis catheter | Partial reversal of attenuation of the heat response (measure of local blood flow) caused by angiotensin-II, apparently due to superoxide scavenging | [165] |
| Twelve patients with metastatic cancer to the brain, irradiated with 3000 cGy delivered in fractions of 300 cGy/d | TEMPOL 70 mg/mL, administered topically to the scalp, 30–45 min each day | TEMPOL blood levels averaging from 0.4 to 0.7 mol/L; full scalp hair retention in 60% of the patients | [168] |
| Ten patients with chronic kidney disease, stages 3–4 | TEMPOL 10 μM administered via intradermal microdialysis fibers | Augmentation of NO-dependent cutaneous vasodilation ascribed to superoxide scavenging | [166] |
| Ten patients with geographic atrophy, the advanced atrophic form of age-related macular degeneration (AMD) | Topical 0.45% OT-551 was administered in one randomly assigned eye three times daily for 2 y | The drug was well tolerated, with few adverse events; smaller decrease in the best corrected visual acuity | [167] |
| Five patients with anal carcinoma received X radiation, 42–45 Gy in 28 fractionsered as a single daily fraction | TEMPOL administered in a topical gel (70 mg/mL) 15–30 min prior to each fraction of radiation | Amelioration of dermatitis in the irradiated skin | [170] |
| Nanoparticle Composition | Structure | Model | Effect | Reference |
|---|---|---|---|---|
| Random dimethylacrylamide (DMA)- -TEMPO) copolymers | MW 1–19 kDa; Optimal composition: 40 mol % TEMPO/60 mol % DMA (MW 17.1 kDa) | ATDC5 chondrogenic cells | Protection from 1 mM SIN-1 induced cytotoxicity | [197] |
| TEMPAMINE conjugated to POEGA-pentafluorophenyl (PFP) functional nanostars | MW 94.1–130.8 kDa, diameter 10–20 nm | BJ-5ta fibroblasts and MCF-7 cells | No cytotoxicity up to 1 mg/mL; mitochondrial localization; decrease in cellular ROS level | [198] |
| TEMPAMINE conjugated to (POEGA) or poly(2-hydroxyethyl acrylate) (PHEA) | MW 4.5–33.4 kDa; average diameter 31 and 27 nm, respectively | MRC-5 fibroblasts Erythrocytes | No effect on viability up to 15 μM No hemolysis or change in osmotic fragility | [187] |
| RPN | Mean diameter of ca. 40 nm | SH-SY5Y cells treated with 6-OHDA | Protection of cell viability, attenuation of ROS increase, decrease in mitochondrial potential and ATP level | [62] |
| Magnetic silica nanoparticles, Fe3O4@SiO2 functionalized with (3-isocyanatopropyl) triethoxysilan or with fluorescein isothiocyanate, covered with dextran functionalized by TEMPOL, 1–25 μM in TEMPO | Size < 50 nm | Human microvascular endothelial cells, MDA-MB-231 breast cancer cells, yeast S. cerevisiae | Nanoparticles as a useful tool to study endocytosis | [199,200] |
| TEMPAMINE-dimethylacrylamide copolymer | MW of 1–19 kDa | ATDC5 chondrocyte-like cells treated with SIN-1 | Polymer uptake, cytoprotection | [197] |
| Perylene (PY)-loaded liquid crystal NPs (PY-LCNPs) surface functionalized with poly (ethylene glycol) (PEG) and TEMPO | Mean diameter 145 nm; zeta potential of −1 mV; ca. 1880 TEMPO molecules per nanoparticle | HeLa cells treated with 0.5 mM H2O2 or t-BHP | Decreased level of intracellular ROS and lipid peroxidation | [181] |
| Composition | Structure | Model | Effect | Reference |
|---|---|---|---|---|
| Poly(D,L-lactide-co-glycolide)-poly(ethylene glycol)-bis(amine)-folate conjugated with TEMPO or rapamycin and TEMPO | Mean size 153 nm, zeta potential of −28.2 mV | PKD (KspCre•Pkd2flox/flox) mice (animal model of bilateral renal cyst formation) | Increase in the efficacy, potency, and tolerability of rapamycin, increased survival rate, and improved kidney function | [201] |
| Nano sterically stabilized egg phosphatidylcholine-based liposomes loaded with TEMPAMINE | Mean size 74.3 nm; 7 mM TEMPAMINE, encapsulation >85%, drug to lipid ratio 0.16 | SJL/J mice, acute encephalomyelitis (EAE) model | Limited therapeutic efficacy | [186] |
| PEGylated nano sterically stabilized egg phosphatidylcholine-based liposomes loaded with TEMPAMINE | Diameter about 80 nm | Female mice, acute EAE induced with proteolipid protein (PLP139–151). Chronic EAE induced with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide | Improvement in clinical score in both models, decrease in mRNA of pro-inflammatory cytokines IFNγ and TNFα (chronic EAE) | [185] |
| RNPO | Diameter ca. 40 nm | Chicken embryo, 14 d old, treated with AAPH (and RNP), 4 mg/egg) effects studied after 72 h Treated with sodium hydrocortisone hemisuccinate sodium, 300 μg, RNPO administered after 15 h, effects studied after 48 h | RNPO, 57 μg, protection against lethality RNPO, 300 μg, decreased TBARS in blood serum | [196] |
| RNP | Hydrodynamic diameter: 36.6 ± 0.1 nm, zeta potential: −16.2 ± 2.1 | Zebrafish larvae exposed to hydrogen peroxide (1.5 and 4 mM) or AAPH (10 or 20 mM) | No discernible toxicity, significant improvement in survival under oxidative stress | [202] |
| RNP | Diameter ca. 40 nm | Male ICR mice, ischemia induced by left renal pedicle clamping for 50 min; RNPN renal injection, 3 mg/kg or RNPO 1.5 mg/kg, 5 min after reperfusion | Suppression of increase in superoxide, lipid peroxidation, and IL-6 in renal tissue and changes in blood pressure, the much stronger effect of RNPN | [203] |
| RNP | Diameter ca. 40 nm | hos: HRM2 hairless mice irradiated once a day for 5 d with UVB (302 nm); RNPN introduced into the skin by iontophoresis 3 times every 3 d before irradiation | Decreased UV-induced melanin production in the skin | [194] |
| RNPO and RNPN | Diameter ca. 40 nm | Beagle dogs, cardiac ischemia (occlusion of the left anterior descending coronary artery for 90 min, followed by reperfusion (6 h); RNPO (3 mg/kg) injected into a vein 5 min before reperfusion | Reduction in infarct size and myocardial apoptosis, increase in however coronary venous NOx | [204] |
| RNPN | Diameter ca. 40 nm | Male BALB/c mice inoculated with C26 murine colon cancer cells, X-irradiated; RNPN (200 mg TEMPO/kginjected s.c. 24 h before irradiation | Increased survival (10–30 Gy), prevention of kidney and liver damage. Intestine and bone marrow | [195] |
| RNPN | Diameter ca. 40 nm | Male BALB/c mice, male ICR mice injected (s.c.) with colon-26 colon adenocarcinoma cells; RNPN (300 mg/kg) injected i.v. | Decreased generation of superoxide and TNFα in the tumor tissue, reduction in tumor volume growth, prevention of increase in plasma creatine kinase and MDA levels | [189] |
| RNPN | Diameter 30–40 nm | Tg2576 Alzheimer’s disease mice; RPPN, 5 mg/mL drinking water for 6 m | Improvement in memory and learning parameters, Attenuation of increase in MDA superoxide and 8-OHdG levels, decrease in GPX activity Aβ(1–40) levels in brain and plasma, and γ-secretase activity in brain | [205] |
| RNPN | Diameter ca. 40 nm | Kud: Hr-hairless mice exposed to intense UVB (302 nm); RNPN 300 mg/kg (total) given in the drinking water (0.5 mg/mL) for 37 d | Significant reduction in UVB-induced skin aging, epidermal thickening, edema, erythema and skin lesions | [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1446. https://doi.org/10.3390/ijms25031446
Sadowska-Bartosz I, Bartosz G. The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles. International Journal of Molecular Sciences. 2024; 25(3):1446. https://doi.org/10.3390/ijms25031446
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2024. "The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles" International Journal of Molecular Sciences 25, no. 3: 1446. https://doi.org/10.3390/ijms25031446
APA StyleSadowska-Bartosz, I., & Bartosz, G. (2024). The Cellular and Organismal Effects of Nitroxides and Nitroxide-Containing Nanoparticles. International Journal of Molecular Sciences, 25(3), 1446. https://doi.org/10.3390/ijms25031446







