Revisiting the Role of Serotonin in Sleep-Disordered Breathing
Abstract
1. Introduction
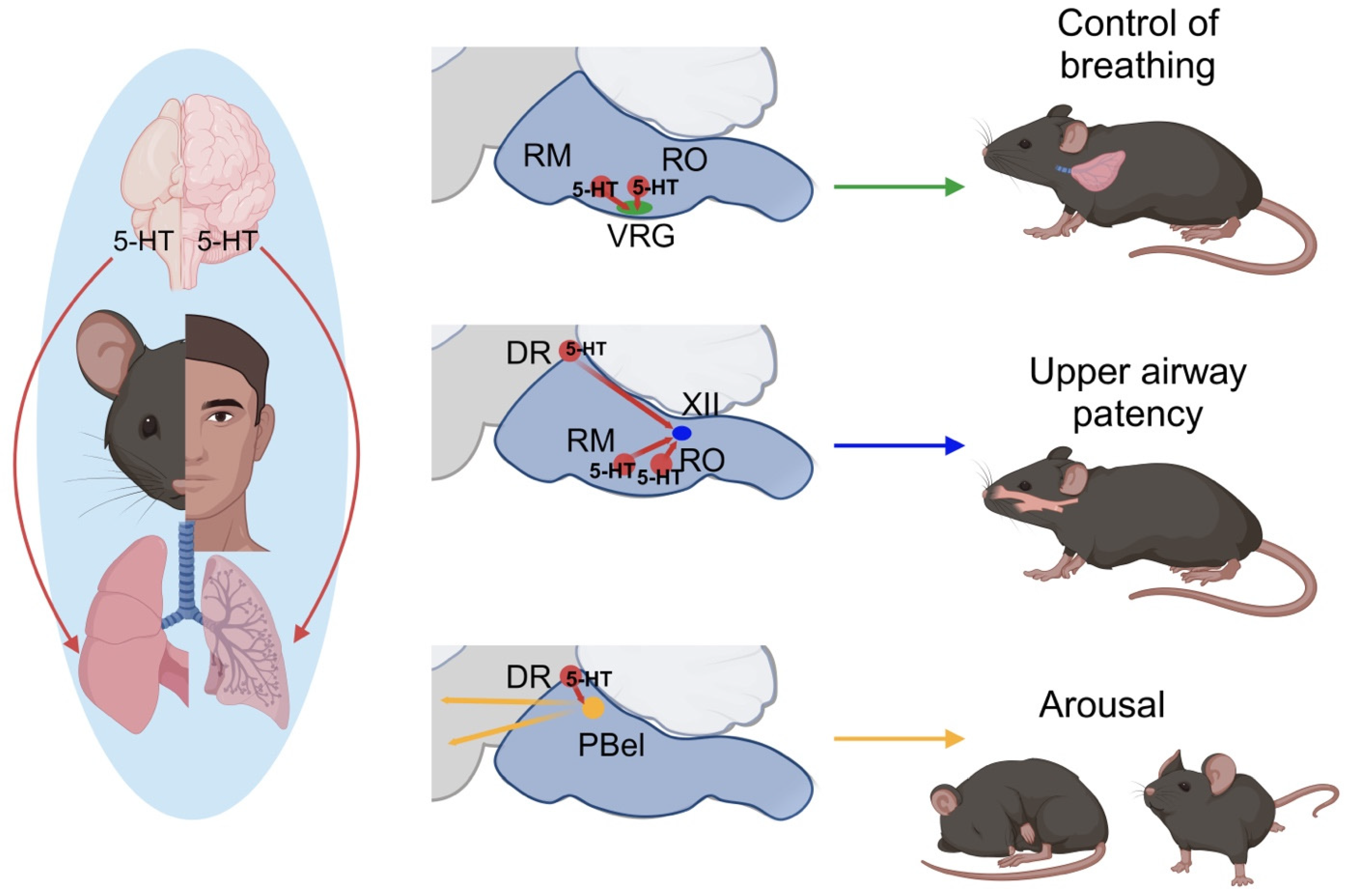
2. Serotonin and SDB
3. 5-HT Regulation: Neural Control of Breathing and Chemosensitivity in OSA
4. Anatomy and Function of the Raphe Nuclei
5. 5-HT Regulation: Upper Airway Function in OSA
6. Clinical Implications of 5-HT Studies in Treating SDB
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, C.P.; Schaub, C.D.; Haines, A.S.; Berkowitz, D.E.; Tankersley, C.G.; Schwartz, A.R.; Smith, P.L. Leptin prevents respiratory depression in obesity. Am. J. Respir. Crit. Care Med. 1999, 159, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Giusti, H.; Leite, C.M.; Anselmo-Franci, J.A.; do Carmo, J.M.; da Silva, A.A.; Hall, J.E.; Colombari, E.; Glass, M.L. Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflugers Arch. 2012, 464, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.R.; Wang, X.; Aung, O.; Bevans-Fonti, S.; Anokye-Danso, F.; Ribeiro, C.; Escobar, J.; Freire, C.; Pho, H.; Dergacheva, O.; et al. Leptin signaling in the dorsomedial hypothalamus couples breathing and metabolism in obesity. Cell Rep. 2023, 42, 113512. [Google Scholar] [CrossRef] [PubMed]
- Povitz, M.; James, M.T.; Pendharkar, S.R.; Raneri, J.; Hanly, P.J.; Tsai, W.H. Prevalence of Sleep-disordered Breathing in Obese Patients with Chronic Hypoxemia. A Cross-Sectional Study. Ann. Am. Thorac. Soc. 2015, 12, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Gastaut, H.; Tassinari, C.A.; Duron, B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the pickwick syndrome. Brain Res. 1966, 1, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum Inflammatory Markers in Obstructive Sleep Apnea: A Meta-Analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Abdala, A.P.L.; Koizumi, H.; Rybak, I.A.; Paton, J.F.R. Spatial and Functional Architecture of the Mammalian Brain Stem Respiratory Network: A Hierarchy of Three Oscillatory Mechanisms. J. Neurophysiol. 2007, 98, 3370–3387. [Google Scholar] [CrossRef] [PubMed]
- Del Negro, C.A.; Funk, G.D.; Feldman, J.L. Breathing matters. Nat. Rev. Neurosci. 2018, 19, 351–367. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.C.; Evans, K.C.; Frackowiak, R.S.J.; Corfield, D.R. Neural correlates of voluntary breathing in humans. J. Appl. Physiol. 2003, 95, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ Off. Publ. State Med. Soc. Wis. 2009, 108, 246–249. [Google Scholar]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.D.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Goldstein, N.A.; Abramowitz, T.; Weedon, J.; Koliskor, B.; Turner, S.; Taioli, E. Racial/Ethnic Differences in the Prevalence of Snoring and Sleep Disordered Breathing in Young Children. J. Clin. Sleep Med. 2011, 7, 163–171. [Google Scholar] [CrossRef]
- Ernst, G.; Mariani, J.; Blanco, M.; Finn, B.; Salvado, A.; Borsini, E. Increase in the frequency of obstructive sleep apnea in elderly people. Sleep Sci. 2019, 12, 222–226. [Google Scholar] [CrossRef]
- Kanney, M.L.; Harford, K.-L.; Raol, N.; Leu, R.M. Obstructive sleep apnea in pediatric obesity and the effects of sleeve gastrectomy. Semin. Pediatr. Surg. 2020, 29, 150887. [Google Scholar] [CrossRef]
- Dékány, L.; Molnár, V.; Molnár, A.; Bikov, A.; Lázár, Z.; Bárdos-Csenteri, O.; Benedek, P. Analysis of possible risk factors for the severity of paediatric obstructive sleep apnoea syndrome. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 5607–5614. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.L.; Schrauwen, N.; Haentjens, D.; Suys, B.; Rooman, R.P.; Van Gaal, L.; De Backer, W.A.; Desager, K.N. Sleep-disordered breathing in overweight and obese children and adolescents: Prevalence, characteristics and the role of fat distribution. Arch. Dis. Child. 2007, 92, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Tan, T.L.; Bixler, E.O.; Martin, L.F.; Shubert, D.; Kales, A. Sleep apnea and sleep disruption in obese patients. Arch. Intern. Med. 1994, 154, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Wolk, R.; Shamsuzzaman, A.S.M.; Somers, V.K. Obesity, Sleep Apnea, and Hypertension. Hypertension 2003, 42, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B. Obesity hypoventilation syndrome: A state-of-the-art review. Respir. Care 2010, 55, 1347–1362, discussion 1363–1365. [Google Scholar] [PubMed]
- Nowbar, S.; Burkart, K.M.; Gonzales, R.; Fedorowicz, A.; Gozansky, W.S.; Gaudio, J.C.; Taylor, M.R.; Zwillich, C.W. Obesity-associated hypoventilation in hospitalized patients: Prevalence, effects, and outcome. Am. J. Med. 2004, 116, 1–7. [Google Scholar] [CrossRef]
- Lopata, M.; Freilich, R.A.; Onal, E.; Pearle, J.; Lourenco, R.V. Ventilatory control and the obesity hypoventilation syndrome. Am. Rev. Respir. Dis. 1979, 119, 165–168. [Google Scholar]
- Han, F.; Chen, E.; Wei, H.; He, Q.; Ding, D.; Strohl, K.P. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2001, 119, 1814–1819. [Google Scholar] [CrossRef]
- Berger, K.I.; Ayappa, I.; Chatr-Amontri, B.; Marfatia, A.; Sorkin, I.B.; Rapoport, D.M.; Goldring, R.M. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001, 120, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.R.; Aung, O.; Mokhlesi, B.; Polotsky, V.Y. Leptin-mediated neural targets in obesity hypoventilation syndrome. Sleep 2022, 45, zsac153. [Google Scholar] [CrossRef] [PubMed]
- Macavei, V.M.; Spurling, K.J.; Loft, J.; Makker, H.K. Diagnostic Predictors of Obesity-Hypoventilation Syndrome in Patients Suspected of Having Sleep Disordered Breathing. J. Clin. Sleep Med. 2013, 9, 879–884. [Google Scholar] [CrossRef] [PubMed]
- de Athayde, R.A.B.; de Oliveira, J.R.B.; Lorenzi, G.; Genta, P.R. Obesity hypoventilation syndrome: A current review. J. Bras. Pneumol. 2018, 44, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Parthasarathy, S. Obesity Hypoventilation Syndrome. Curr. Pulmonol. Rep. 2015, 4, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Antoine, M.H.; Sankari, A.; Bollu, P.C. Obesity-Hypoventilation Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482300/ (accessed on 5 October 2023).
- Messina, Z.; Patrick, H. Partial Pressure of Carbon Dioxide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551648/ (accessed on 5 October 2023).
- Ghimire, P.; Sankari, A.; Kaul, P. Pickwickian Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542216/ (accessed on 5 October 2023).
- Rawat, D.; Modi, P.; Sharma, S. Hypercapnea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK500012/ (accessed on 5 October 2023).
- Böing, S.; Randerath, W.J. Chronic hypoventilation syndromes and sleep-related hypoventilation. J. Thorac. Dis. 2015, 7, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.F.; Jerrentrup, A.; Ploch, T.; Grote, L.; Penzel, T.; Sullivan, C.E.; Peter, J.H. Effect of Nasal Continuous Positive Airway Pressure Treatment on Blood Pressure in Patients with Obstructive Sleep Apnea. Circulation 2003, 107, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pavwoski, P.; Shelgikar, A.V. Treatment options for obstructive sleep apnea. Neurol. Clin. Pract. 2017, 7, 77–85. [Google Scholar] [CrossRef]
- Howard, M.E.; Piper, A.J.; Stevens, B.; Holland, A.E.; Yee, B.J.; Dabscheck, E.; Mortimer, D.; Burge, A.T.; Flunt, D.; Buchan, C.; et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax 2017, 72, 437–444. [Google Scholar] [CrossRef]
- Nowalk, N.C.; Neborak, J.M.; Mokhlesi, B. Is bilevel PAP more effective than CPAP in treating hypercapnic obese patients with COPD and severe OSA? J. Clin. Sleep Med. 2022, 18, 5–7. [Google Scholar] [CrossRef]
- Engleman, H.M.; Asgari-Jirhandeh, N.; McLeod, A.L.; Ramsay, C.F.; Deary, I.J.; Douglas, N.J. Self-Reported Use of CPAP and Benefits of CPAP Therapy: A Patient Survey. Chest 1996, 109, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Hoekema, A. How to treat patients that do not tolerate continuous positive airway pressure. Breathe 2010, 7, 157–167. [Google Scholar] [CrossRef]
- Engleman, H.M.; Wild, M.R. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med. Rev. 2003, 7, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Mulgrew, A.T.; Nasvadi, G.; Butt, A.; Cheema, R.; Fox, N.; Fleetham, J.A.; Ryan, C.F.; Cooper, P.; Ayas, N.T. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008, 63, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Vanderveken, O.M. Oral Appliances in Obstructive Sleep Apnea. Healthcare 2019, 7, 141. [Google Scholar] [CrossRef]
- Almeida, F.R.; Bansback, N. Long-Term Effectiveness of Oral Appliance versus CPAP Therapy and the Emerging Importance of Understanding Patient Preferences. Sleep 2013, 36, 1271–1272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palotie, T.; Peltomaa, A.; Bachour, A.; Bachour, P.; Mäkitie, A.; Peltomaa, M.; Vallittu, P. Reasons for failure of mandibular advancement splint therapy in the treatment of obstructive sleep apnea. CRANIO 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fleetham, J.A. SLEEP APNEA|Oral Appliances. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 67–70. ISBN 978-0-12-370879-3. Available online: https://www.sciencedirect.com/science/article/pii/B012370879600363X (accessed on 11 January 2024).
- Hobson, J.C.; Robinson, S.; Antic, N.A.; McEvoy, R.D.; Windler, S.; Mackay, S.; Carney, A.S. What is “success” following surgery for obstructive sleep apnea? The effect of different polysomnographic scoring systems. Laryngoscope 2012, 122, 1878–1881. [Google Scholar] [CrossRef]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Eastwood, P.R.; Barnes, M.; Walsh, J.H.; Maddison, K.J.; Hee, G.; Schwartz, A.R.; Smith, P.L.; Malhotra, A.; McEvoy, R.D.; Wheatley, J.R.; et al. Treating Obstructive Sleep Apnea with Hypoglossal Nerve Stimulation. Sleep 2011, 34, 1479–1486. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Bennett, M.L.; Smith, P.L.; De Backer, W.; Hedner, J.; Boudewyns, A.; Van de Heyning, P.; Ejnell, H.; Hochban, W.; Knaack, L.; et al. Therapeutic Electrical Stimulation of the Hypoglossal Nerve in Obstructive Sleep Apnea. Arch. Otolaryngol. Neck Surg. 2001, 127, 1216–1223. [Google Scholar] [CrossRef]
- Woodson, B.T.; Soose, R.J.; Gillespie, M.B.; Strohl, K.P.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Badr, M.S.; Lin, H.; et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngol.–Head Neck Surg. 2016, 154, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.V.; Jun, J.; Polotsky, V.Y. Obstructive sleep apnea. Handb. Clin. Neurol. 2022, 189, 105–136. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Chen, Y.-T.; Wang, K.-L.; Su, V.Y.-F.; Wu, L.-A.; Perng, D.-W.; Chang, S.-C.; Chen, Y.-M.; Chen, T.-J.; Chou, K.-T. Comorbidities and risk of mortality in patients with sleep apnea. Ann. Med. 2017, 49, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dodds, S.; Williams, L.J.; Roguski, A.; Vennelle, M.; Douglas, N.J.; Kotoulas, S.-C.; Riha, R.L. Mortality and morbidity in obstructive sleep apnoea–hypopnoea syndrome: Results from a 30-year prospective cohort study. ERJ Open Res. 2020, 6, 00057–02020. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.V.; Schwartz, A.R. The pathogenesis of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1358–1372. [Google Scholar] [CrossRef]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin synthesis, release and reuptake in terminals: A mathematical model. Theor. Biol. Med. Model. 2010, 7, 34. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.-U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beaulieu, J.-M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef]
- Vialli, M.; Erspamer, V. Ricerche sul secreto delle cellule enterocromaffini. Z. Zellforsch. Mikrosk. Anat. 1937, 27, 81–99. [Google Scholar] [CrossRef]
- Rapport, M.M.; Green, A.A.; Page, I.H. Serum vasoconstrictor, serotonin; isolation and characterization. J. Biol. Chem. 1948, 176, 1243–1251. [Google Scholar] [CrossRef]
- Twarog, B.M.; Page, I.H. Serotonin Content of Some Mammalian Tissues and Urine and a Method for Its Determination. Am. J. Physiol.-Leg. Content 1953, 175, 157–161. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.; Piper, C.; Vogt, J.; Heintze, J.; Butz, T.; Lindner, O.; Burchert, W.; Kersting, C.; Horstkotte, D. Atrial fibrillation in carcinoid heart disease: The role of serotonin. A review of the literature. Clin. Res. Cardiol. 2007, 96, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Portas, C.M.; Bjorvatn, B.; Ursin, R. Serotonin and the sleep/wake cycle: Special emphasis on microdialysis studies. Prog. Neurobiol. 2000, 60, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Akasaka, D.; Ogasawara, H.; Sato, K.; Miyake, M.; Saito, K.; Takahashi, Y.; Kanaya, T.; Takakura, I.; Hondo, T.; et al. Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinology 2010, 151, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Bonham, A.C. Neurotransmitters in the CNS control of breathing. Respir. Physiol. 1995, 101, 219–230. [Google Scholar] [CrossRef]
- Hilaire, G.; Voituron, N.; Menuet, C.; Ichiyama, R.M.; Subramanian, H.H.; Dutschmann, M. The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 2010, 174, 76–88. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Klein, T.; Altman, R. Selective Serotonin Reuptake Inhibitors (SSRI) Pathway. Pharmacogenet. Genom. 2009, 19, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.R.; Richerson, G.B. The role of medullary serotonin (5-HT) neurons in respiratory control: Contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J. Appl. Physiol. 2010, 108, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.R.; Richerson, G.B. Contributions of 5-HT Neurons to Respiratory Control: Neuromodulatory and Trophic Effects. Respir. Physiol. Neurobiol. 2008, 164, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.; Tancredi, A.N.-; Leonardo, E.D. 5-HT1A receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 2014, 231, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Madaeva, I.M.; Berdina, O.N.; Kurashova, N.A.; Semenova, N.V.; Ukhinov, E.B.; Belskikh, A.V.; Kolesnikova, L.I. Sleep Apnea and Serum Serotonin Level Pre- and Post-PAP Therapy: A Preliminary Study. Neurol. Ther. 2021, 10, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 2008, 60, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Martynowicz, H.; Lavigne, G.; Lobbezoo, F.; Kato, T.; Winocur, E.; Wezgowiec, J.; Danel, D.; Wojakowska, A.; Mazur, G.; et al. An exploratory study on the association between serotonin and sleep breathing disorders. Sci. Rep. 2023, 13, 11800. [Google Scholar] [CrossRef]
- Ghali, M.G.Z. Respiratory rhythm generation and pattern formation: Oscillators and network mechanisms. J. Integr. Neurosci. 2019, 18, 481–517. [Google Scholar] [CrossRef]
- Alheid, G.F.; McCrimmon, D.R. The chemical neuroanatomy of breathing. Respir. Physiol. Neurobiol. 2008, 164, 3–11. [Google Scholar] [CrossRef]
- Bianchi, A.L.; Denavit-Saubié, M.; Champagnat, J. Central control of breathing in mammals: Neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 1995, 75, 1–45. [Google Scholar] [CrossRef]
- Smith, J.C.; Ellenberger, H.H.; Ballanyi, K.; Richter, D.W.; Feldman, J.L. Pre-Bötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science 1991, 254, 726–729. [Google Scholar] [CrossRef]
- Cohen, M.I.; Shaw, C.-F. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir. Physiol. Neurobiol. 2004, 143, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Baekey, D.M.; Dick, T.E.; Paton, J.F.R. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp. Physiol. 2008, 93, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Nattie, E.; Li, A. Central Chemoreceptors: Locations and Functions. Compr. Physiol. 2012, 2, 221–254. [Google Scholar] [CrossRef]
- Moreira, T.S.; Sobrinho, C.R.; Falquetto, B.; Oliveira, L.M.; Lima, J.D.; Mulkey, D.K.; Takakura, A.C. The retrotrapezoid nucleus and the neuromodulation of breathing. J. Neurophysiol. 2021, 125, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, D.K.; Stornetta, R.L.; Weston, M.C.; Simmons, J.R.; Parker, A.; Bayliss, D.A.; Guyenet, P.G. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004, 7, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.C.T.; Moreira, T.S.; Colombari, E.; West, G.H.; Stornetta, R.L.; Guyenet, P.G. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol. 2006, 572, 503–523. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.C.; Moreira, T.S.; Stornetta, R.L.; West, G.H.; Gwilt, J.M.; Guyenet, P.G. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J. Physiol. 2008, 586, 2975–2991. [Google Scholar] [CrossRef]
- Abbott, S.B.G.; Stornetta, R.L.; Fortuna, M.G.; Depuy, S.D.; West, G.H.; Harris, T.E.; Guyenet, P.G. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 2009, 29, 5806–5819. [Google Scholar] [CrossRef]
- Kumar, N.N.; Velic, A.; Soliz, J.; Shi, Y.; Li, K.; Wang, S.; Weaver, J.L.; Sen, J.; Abbott, S.B.G.; Lazarenko, R.M.; et al. PHYSIOLOGY. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 2015, 348, 1255–1260. [Google Scholar] [CrossRef]
- Salloum, A.; Rowley, J.A.; Mateika, J.H.; Chowdhuri, S.; Omran, Q.; Badr, M.S. Increased Propensity for Central Apnea in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2010, 181, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, M.; Modi, P.; Bollu, P.C. Cheyne Stokes Respirations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK448165/ (accessed on 10 January 2024).
- Mansukhani, M.P.; Kara, T.; Caples, S.; Somers, V.K. Chemoreflexes, Sleep Apnea, and Sympathetic Dysregulation. Curr. Hypertens. Rep. 2014, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Buyse, B.; Markous, N.; Cauberghs, M.; Van Klaveren, R.; Muls, E.; Demedts, M. Effect of obesity and/or sleep apnea on chemosensitivity: Differences between men and women. Respir. Physiol. Neurobiol. 2003, 134, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Sánchez, A.M. Sleep Apnea-Hypopnea Syndromes and Heart Failure. Rev. Esp. Cardiol. Engl. Ed. 2007, 60, 415–427. [Google Scholar] [CrossRef][Green Version]
- Steinbusch, H.W.M. Distribution of serotonin-immunoreactivity in the central nervous system of the rat—Cell bodies and terminals. Neuroscience 1981, 6, 557–618. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, R.; Nakamuta, N.; Yamamoto, Y. Hypoxia-induced increases in serotonin-immunoreactive nerve fibers in the medulla oblongata of the rat. Acta Histochem. 2016, 118, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, R.; Nakamuta, N.; Yamamoto, Y. Serotonergic projections to the ventral respiratory column from raphe nuclei in rats. Neurosci. Res. 2019, 143, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Martín-Cora, F.J.; Fornal, C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Brain Res. Rev. 2002, 40, 45–52. [Google Scholar] [CrossRef]
- DePuy, S.D.; Kanbar, R.; Coates, M.B.; Stornetta, R.L.; Guyenet, P.G. Control of Breathing by Raphe Obscurus Serotonergic Neurons in Mice. J. Neurosci. 2011, 31, 1981–1990. [Google Scholar] [CrossRef]
- Corcoran, A.E.; Richerson, G.B.; Harris, M.B. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir. Physiol. Neurobiol. 2013, 186, 214–220. [Google Scholar] [CrossRef][Green Version]
- Hodges, M.R.; Tattersall, G.J.; Harris, M.B.; McEvoy, S.D.; Richerson, D.N.; Deneris, E.S.; Johnson, R.L.; Chen, Z.-F.; Richerson, G.B. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008, 28, 2495–2505. [Google Scholar] [CrossRef]
- Mason, P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu. Rev. Neurosci. 2001, 24, 737–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tiwari, J.K.; Bradley, S.R.; Zaykin, R.V.; Richerson, G.B. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J. Neurophysiol. 2001, 85, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Richerson, G.B. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004, 5, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Meng, Y.; Fang, Y.; Sun, L.; Wang, M.; Liu, Y.; Zhao, C.; Dai, L.; Ouyang, S. Role of raphe magnus 5-HT1A receptor in increased ventilatory responses induced by intermittent hypoxia in rats. Respir. Res. 2022, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Gargaglioni, L.H.; Coimbra, N.C.; Branco, L.G.S. The nucleus raphe magnus modulates hypoxia-induced hyperventilation but not anapyrexia in rats. Neurosci. Lett. 2003, 347, 121–125. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Kaczyńska, K.; Zaremba, M. Serotonergic system in hypoxic ventilatory response in unilateral rat model of Parkinson’s disease. J. Biomed. Sci. 2017, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J. Physiol. 2006, 577, 525. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Moreira, T.S.; Takakura, A.C. Raphe Pallidus is Not Important to Central Chemoreception in a Rat Model of Parkinson’s Disease. Neuroscience 2018, 369, 350–362. [Google Scholar] [CrossRef]
- Kato, T.; Mitsukura, Y.; Yoshida, K.; Mimura, M.; Takata, N.; Tanaka, K.F. Oscillatory Population-Level Activity of Dorsal Raphe Serotonergic Neurons Is Inscribed in Sleep Structure. J. Neurosci. 2022, 42, 7244–7255. [Google Scholar] [CrossRef]
- Smith, H.R.; Leibold, N.K.; Rappoport, D.A.; Ginapp, C.M.; Purnell, B.S.; Bode, N.M.; Alberico, S.L.; Kim, Y.-C.; Audero, E.; Gross, C.T.; et al. Dorsal Raphe Serotonin Neurons Mediate CO2-Induced Arousal from Sleep. J. Neurosci. 2018, 38, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; De Luca, R.; Khanday, M.A.; Bandaru, S.S.; Thomas, R.C.; Broadhurst, R.Y.; Venner, A.; Todd, W.D.; Fuller, P.M.; Arrigoni, E.; et al. Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals. Nat. Commun. 2020, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Pho, H.; Amorim, M.R.; Qiu, Q.; Shin, M.-K.; Kim, L.J.; Anokye-Danso, F.; Jun, J.J.; Ahima, R.S.; Branco, L.G.S.; Kuhn, D.M.; et al. The effect of brain serotonin deficiency on breathing is magnified by age. Physiol. Rep. 2022, 10, e15245. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sobrinho, C.R.; Soto-Perez, J.; Milla, B.M.; Stornetta, D.S.; Stornetta, R.L.; Takakura, A.C.; Mulkey, D.K.; Moreira, T.S.; Bayliss, D.A. 5-HT7 receptors expressed in the mouse parafacial region are not required for respiratory chemosensitivity. J. Physiol. 2022, 600, 2789–2811. [Google Scholar] [CrossRef] [PubMed]
- Kubin, L. Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms. Compr. Physiol. 2016, 6, 1801–1850. [Google Scholar] [CrossRef]
- Fleury Curado, T.; Pho, H.; Berger, S.; Caballero-Eraso, C.; Shin, M.-K.; Sennes, L.U.; Pham, L.; Schwartz, A.R.; Polotsky, V.Y. Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep 2018, 41, zsy089. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Hohenhorst, W.; de Vries, N. Drug-induced sleep endoscopy: The VOTE classification. Eur. Arch. Otorhinolaryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Remmers, J.E.; deGroot, W.J.; Sauerland, E.K.; Anch, A.M. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978, 44, 931–938. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef]
- Mezzanotte, W.S.; Tangel, D.J.; White, D.P. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Investig. 1992, 89, 1571–1579. [Google Scholar] [CrossRef]
- Veasey, S.C.; Fenik, P.; Panckeri, K.; Pack, A.I.; Hendricks, J.C. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am. J. Respir. Crit. Care Med. 1999, 160, 1659–1667. [Google Scholar] [CrossRef]
- Veasey, S.C.; Chachkes, J.; Fenik, P.; Hendricks, J.C. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep 2001, 24, 155–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jelev, A.; Sood, S.; Liu, H.; Nolan, P.; Horner, R.L. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J. Physiol. 2001, 532, 467–481. [Google Scholar] [CrossRef]
- Saponjic, J.; Radulovacki, M.; Carley, D.W. Monoaminergic system lesions increase post-sigh respiratory pattern disturbance during sleep in rats. Physiol. Behav. 2007, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mateika, J.H.; Komnenov, D.; Pop, A.; Kuhn, D.M. Genetic depletion of 5-HT increases central apnea frequency and duration and dampens arousal but does not impact the circadian modulation of these variables. J. Appl. Physiol. 2019, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Pavlinac Dodig, I.; Pecotic, R.; Valic, M.; Dogas, Z. Acute intermittent hypoxia induces phrenic long-term facilitation which is modulated by 5-HT1A receptor in the caudal raphe region of the rat. J. Sleep Res. 2012, 21, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Levitt, E.S.; Hunnicutt, B.J.; Knopp, S.J.; Williams, J.T.; Bissonnette, J.M. A selective 5-HT1a receptor agonist improves respiration in a mouse model of Rett syndrome. J. Appl. Physiol. 2013, 115, 1626–1633. [Google Scholar] [CrossRef]
- Sarber, K.M.; Howard, J.J.M.; Dye, T.J.; Pascoe, J.E.; Simakajornboon, N. Sleep-Disordered Breathing in Pediatric Patients With Rett Syndrome. J. Clin. Sleep Med. 2019, 15, 1451–1457. [Google Scholar] [CrossRef]
- Popa, D.; Léna, C.; Fabre, V.; Prenat, C.; Gingrich, J.; Escourrou, P.; Hamon, M.; Adrien, J. Contribution of 5-HT2 Receptor Subtypes to Sleep–Wakefulness and Respiratory Control, and Functional Adaptations in Knock-Out Mice Lacking 5-HT2A Receptors. J. Neurosci. 2005, 25, 11231–11238. [Google Scholar] [CrossRef]
- Nakano, H.; Magalang, U.J.; Lee, S.D.; Krasney, J.A.; Farkas, G.A. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am. J. Respir. Crit. Care Med. 2001, 163, 1191–1197. [Google Scholar] [CrossRef]
- Carley, D.W.; Depoortere, H.; Radulovacki, M. R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats. Pharmacol. Biochem. Behav. 2001, 69, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Spinazzi, A.; Santangelo, G.; Steinijans, V.W.; Wurst, W.; Solleder, P.; Girbino, G. Acute Effects of Urapidil on Airway Response in Hypertensive Patients with Chronic Obstructive Pulmonary Disease. Drugs 1990, 40, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Hanzel, D.A.; Proia, N.G.; Hudgel, D.W. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest 1991, 100, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Yamaura, E.M.; Gill, K.; Reist, C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 1999, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stradling, J.; Smith, D.; Radulovacki, M.; Carley, D. Effect of ondansetron on moderate obstructive sleep apnoea, a single night, placebo-controlled trial. J. Sleep Res. 2003, 12, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, W.B.; Maczaj, M.; Holt, J. Buspirone administration to sleep apnea patients. J. Clin. Psychopharmacol. 1991, 11, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Haponik, E.F.; Allen, R.P.; Bleecker, E.R. The effects of protriptyline in sleep-disordered breathing. Am. Rev. Respir. Dis. 1983, 127, 8–13. [Google Scholar] [CrossRef]
- Robillard, R.; Saad, M.; Ray, L.B.; BuJáki, B.; Douglass, A.; Lee, E.K.; Soucy, L.; Spitale, N.; De Koninck, J.; Kendzerska, T. Selective serotonin reuptake inhibitor use is associated with worse sleep-related breathing disturbances in individuals with depressive disorders and sleep complaints: A retrospective study. J. Clin. Sleep Med. 2021, 17, 505–513. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, O.; Amorim, M.R.; Mendelowitz, D.; Polotsky, V.Y. Revisiting the Role of Serotonin in Sleep-Disordered Breathing. Int. J. Mol. Sci. 2024, 25, 1483. https://doi.org/10.3390/ijms25031483
Aung O, Amorim MR, Mendelowitz D, Polotsky VY. Revisiting the Role of Serotonin in Sleep-Disordered Breathing. International Journal of Molecular Sciences. 2024; 25(3):1483. https://doi.org/10.3390/ijms25031483
Chicago/Turabian StyleAung, O, Mateus R. Amorim, David Mendelowitz, and Vsevolod Y. Polotsky. 2024. "Revisiting the Role of Serotonin in Sleep-Disordered Breathing" International Journal of Molecular Sciences 25, no. 3: 1483. https://doi.org/10.3390/ijms25031483
APA StyleAung, O., Amorim, M. R., Mendelowitz, D., & Polotsky, V. Y. (2024). Revisiting the Role of Serotonin in Sleep-Disordered Breathing. International Journal of Molecular Sciences, 25(3), 1483. https://doi.org/10.3390/ijms25031483





