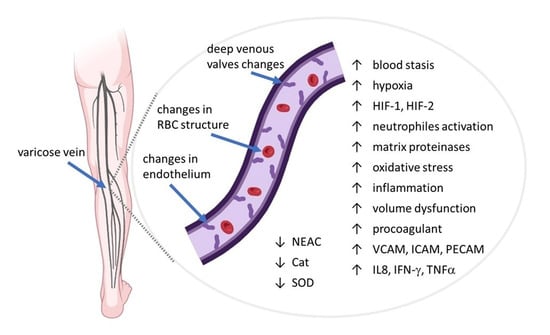
Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs
Abstract
:1. Introduction
2. Chronic Venous Insufficiency
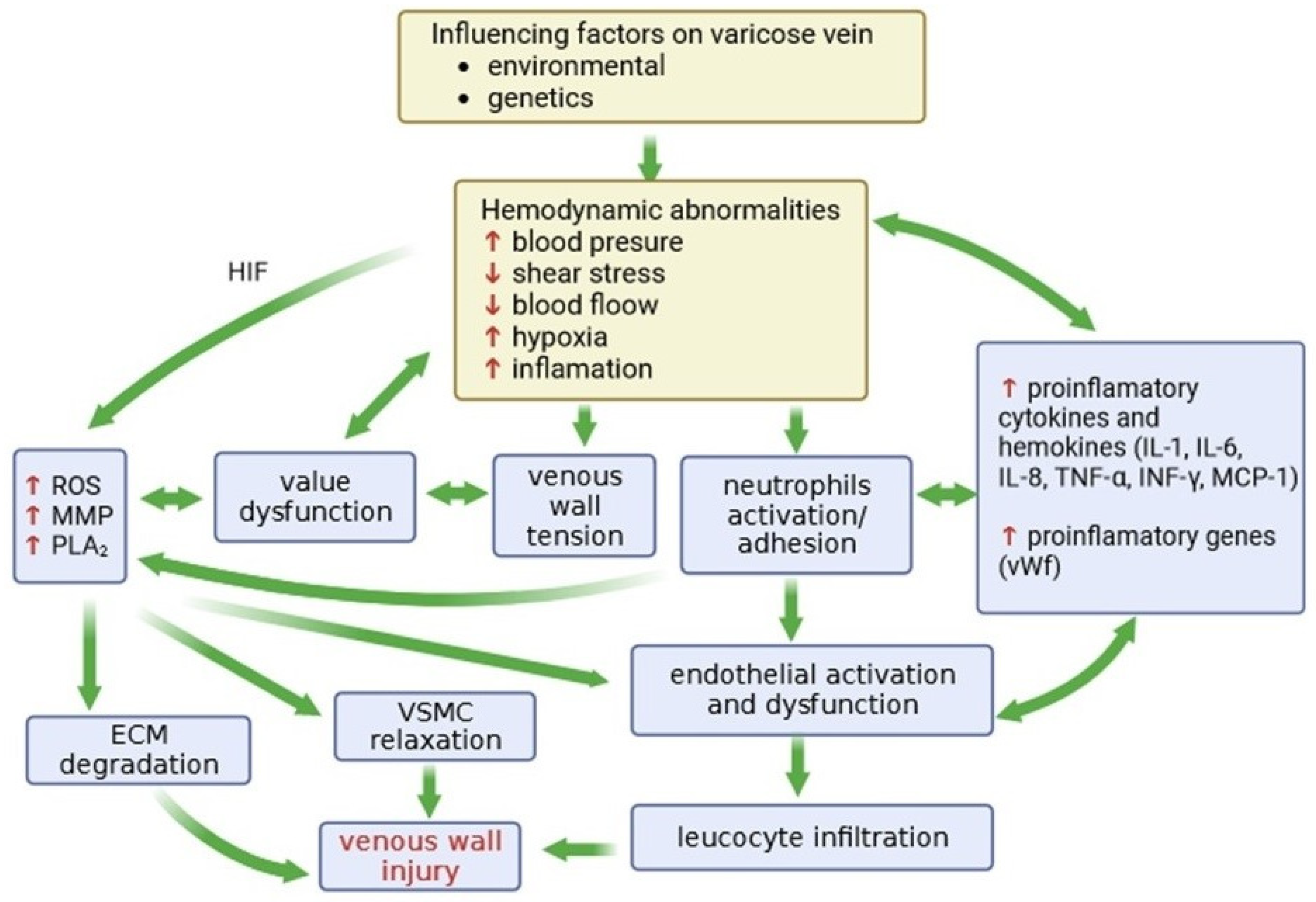
2.1. Factors Influencing the Occurrence and Development of CVeD
2.2. Changes in the Vein Wall Structure
2.3. The Role of Inflammation in CVD
2.4. Classification System for Chronic Venous Disorders
3. Vascular Endothelium
3.1. Vascular Endothelium in Inflammation
3.2. The Role of Adhesion Proteins and Cytokines in Inflammation
3.3. Cellular Response in Inflammatory Conditions
4. Hypoxia in Varicose Veins and Its Consequences
4.1. The Expression of Hypoxia-Inducing Factors
4.2. The Role of Metalloproteinases in Varicose Veins
4.3. Expression of Enzymes in Hypoxia
5. Oxidative Stress
5.1. Reactive Oxygen Species and Antioxidants
5.2. ROS and Enzymes in the Damage of Biological Material
6. Hemoglobin as an Oxidant
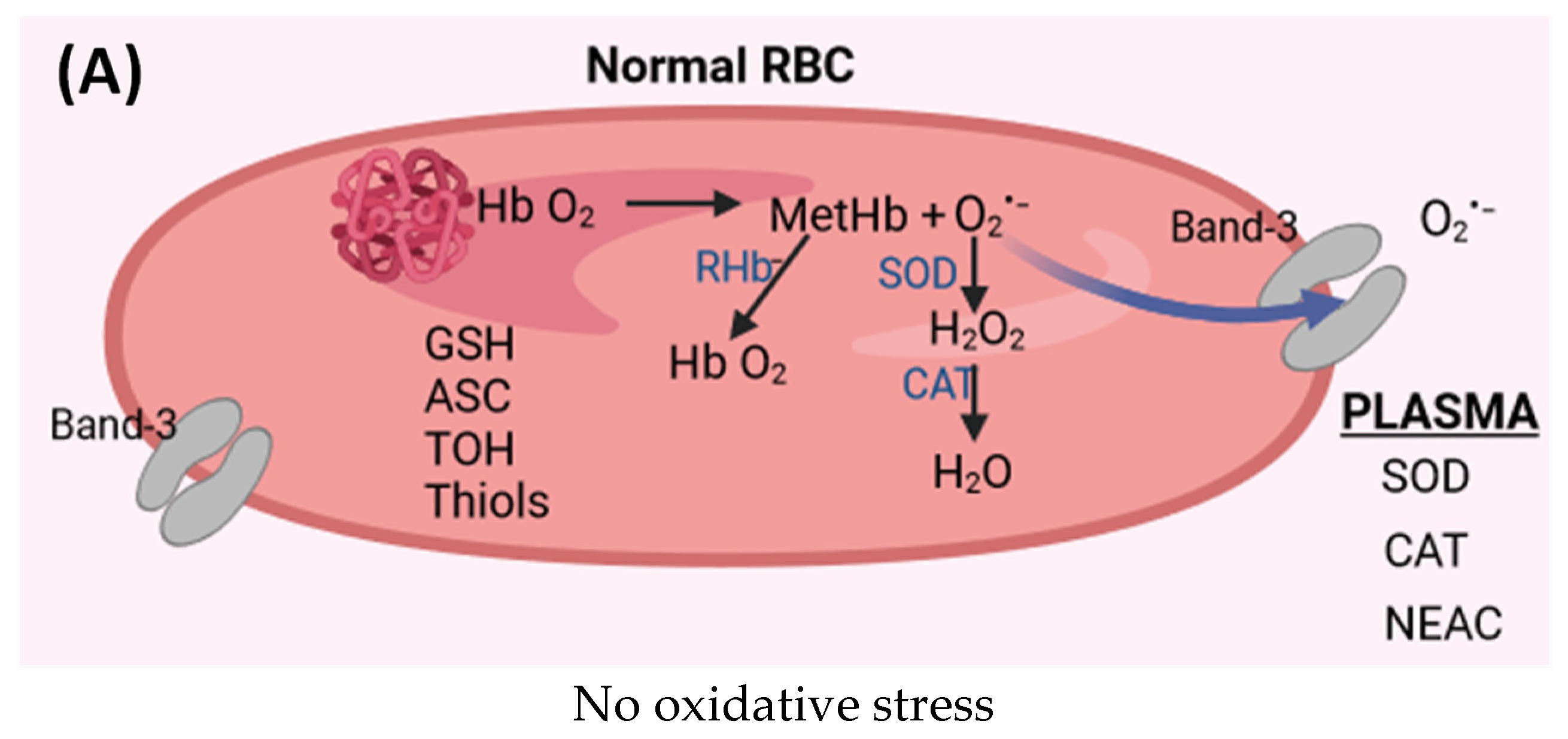
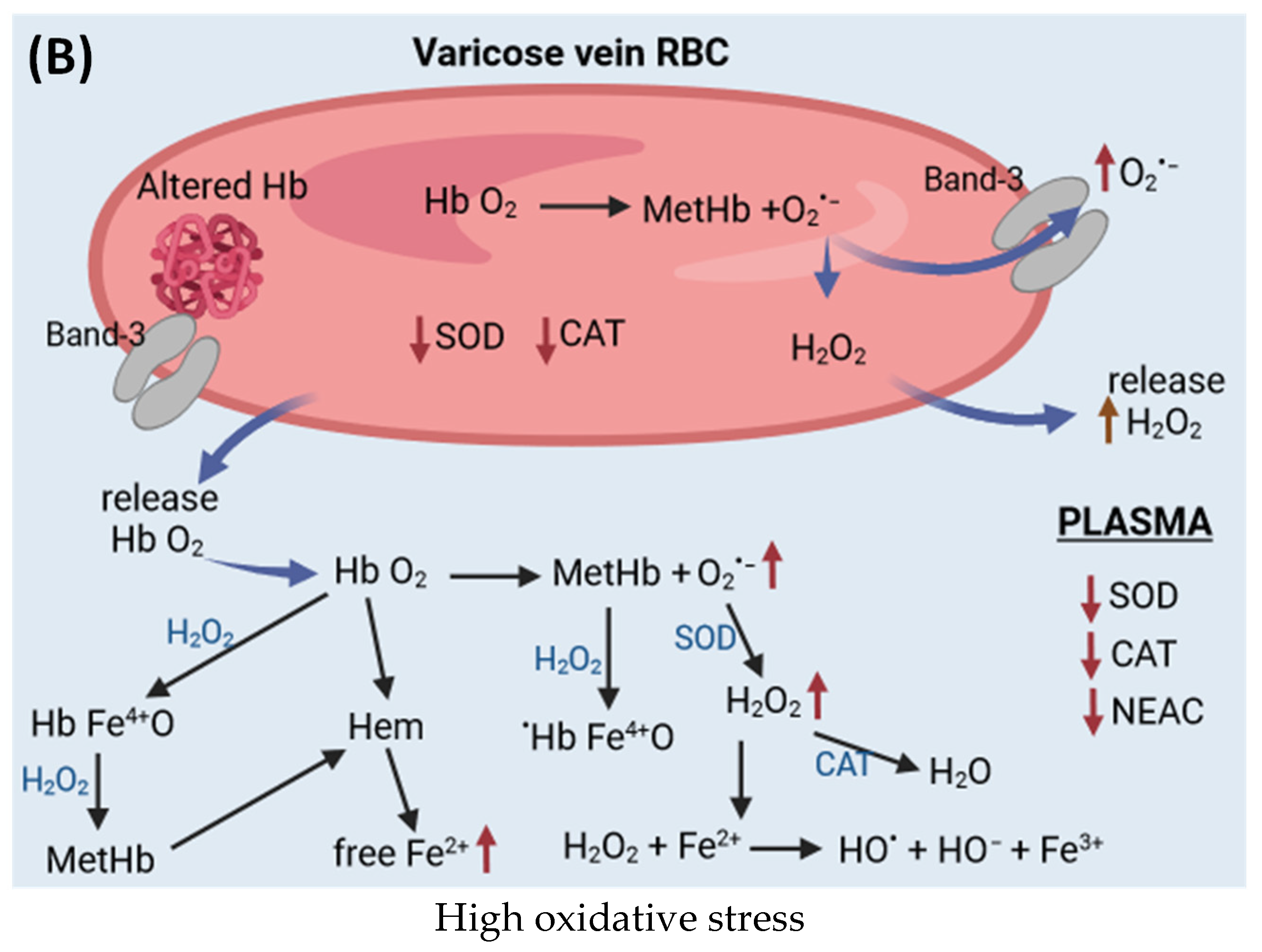
6.1. Antioxidant System in Red Blood Cells and Plasma
6.2. Heme and Forms of Hemoglobin with Iron in Higher Oxidation States
7. Plasma and Red Blood Cells in CVeD
7.1. mtDNA in Varicose Veins
7.2. Alterations in Red Blood Cells Properties in CVeD
7.3. Antioxidants and Oxidative Stress in Plasma and Red Blood Cells in CVeD
8. Varicose Veins Treatment
8.1. Flavonoid Derivatives
8.2. Coumarin Derivatives
8.3. Saponins Derivatives
8.4. Other Natural Bioactive Compounds
8.5. Synthetic Drugs
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of Lower Extremity Vein Dysfunction in Chronic Venous Disease and Implications in Management of Varicose Veins. Vessel Plus 2021, 5, 36. [Google Scholar] [CrossRef]
- Grudzińska, E.; Lekstan, A.; Szliszka, E.; Czuba, Z.P. Cytokines Produced by Lymphocytes in the Incompetent Great Saphenous Vein. Mediat. Inflamm. 2018, 2018, 7161346. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Bouaziz, N.; Remacle, J. Role of the endothelium and blood stasis in the appearance of varicose veins. Int. Angiol. 2002, 21, 18, PMID: 11941268. [Google Scholar] [PubMed]
- Rizzo, C.; La Barbera, L.; Miceli, G.; Tuttolomondo, A.; Guggino, G. The innate face of Giant Cell Arteritis: Insight into cellular and molecular innate immunity pathways to unravel new possible biomarkers of disease. Front. Mol. Med. 2022, 2, 933161. [Google Scholar] [CrossRef]
- Sayer, G.L.; Smith, P.D.C. Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Bergan, J.J.; Schmid-Schönbein, G.W.; Takase, S. Monocyte infiltration into venous valves. J. Vasc. Surg. 1998, 27, 158–166. [Google Scholar] [CrossRef]
- Knaapen, M.W.M.; Somers, P.; Bortier, H.; de Meyer, G.R.Y.; Kockx, M.M. Smooth muscle cell hypertrophy in varicose veins is associated with expression of estrogen receptor-beta. J. Vasc. Res. 2005, 42, 8–12. [Google Scholar] [CrossRef]
- Grudzińska, E.; Czuba, Z.P. Immunological aspects of chronic venous disease pathogenesis. Cent. Eur. J. Immunol. 2014, 39, 525–531. [Google Scholar] [CrossRef]
- Whiston, R.J.; Hallett, M.B.; Davies, E.V.; Harding, K.G.; Lane, I.F. Inappropriate neutrophil activation in venous disease. Br. J. Surg. 1994, 81, 695–698. [Google Scholar] [CrossRef]
- Puhr-Westerheide, D.; Schink, S.J.; Fabritius, M.; Mittmann, L.; Hessenauer, M.E.T.; Pircher, J.; Zuchtriegel, G.; Uhl, B.; Holzer, M.; Massberg, S.; et al. Neutrophils promote venular thrombosis by shaping the rheological environment for platelet aggregation. Sci. Rep. 2019, 9, 15932. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928. [Google Scholar] [CrossRef] [PubMed]
- Raetz, J.; Wilson, M.; Collins, K. Varicose Veins: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 682–688. [Google Scholar] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zahra, F. StatPearls: Chronic Venous Insufficiency; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587341/ (accessed on 15 December 2023).
- Vekilov, D.P.; Grande-Allen, K.J. Mechanical Properties of Diseased Veins. Methodist Debakey Cardiovasc. J. 2018, 14, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, A.N.; Allegra, C.; Bergan, J.; Bradbury, A.; Cairols, M.; Carpentier, P.; Comerota, A.; Delis, C.; Eklof, B.; Fassiadis, N.; et al. Management of chronic venous disorders of the lower limbs: Guidelines according to scientific evidence. Int. Angiol. 2008, 27, 1–59. [Google Scholar] [PubMed]
- Niccolini, G.; Manuello, A.; Capone, A.; Marongiu, G.; Dell’Osa, A.H.; Fois, A.; Velluzzi, F.; Concu, A. Possible Assessment of Calf Venous Pump Efficiency by Computational Fluid Dynamics Approach. Front. Physiol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Labropoulos, N.; Volteas, N.; Leon, M.; Sowade, O.; Rulo, A.; Giannoukas, A.D.; Nicolaides, A.N. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch. Surg. 1997, 132, 46–51. [Google Scholar] [CrossRef]
- Boisseau Michel-René. Chronic venous disease and the genetic influence. Phlebolymphology 2014, 21, 100–111. [Google Scholar]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arter. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Mannello, F. Pathophysiology of chronic venous disease. Int. Angiol. 2014, 33, 212–221. [Google Scholar] [PubMed]
- Atta, H.M. Varicose veins: Role of mechanotransduction of venous hypertension. Int. J. Vasc. Med. 2012, 2012, 538627. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, J.; Przywara, S.; Iłżecka, J.; Iłżecki, M. The influence of compression therapy on the level of inflammatory biomarkers in patients with chronic venous disease. Acta Angiol. 2021, 1, 32–36. [Google Scholar] [CrossRef]
- Langer, H.F.; Chavakis, T. Leukocyte-endothelial interactions in inflammation. J. Cell. Mol. Med. 2009, 13, 1211–1220. [Google Scholar] [CrossRef]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; Maeseneer, M.d.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Mansilha, A.; Sousa, J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Howlader, M.H.; Smith, P.D. Increased plasma total nitric oxide among patients with severe chronic venous disease. Int. Angiol. 2002, 21, 180–186, PMID: 12110781. [Google Scholar] [PubMed]
- Hua, S. Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front. Pharmacol. 2013, 4, 127. [Google Scholar] [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Orlova, V.V.; Chavakis, T. Regulation of vascular endothelial permeability by junctional adhesion molecules (JAM). Thromb. Haemost. 2007, 98, 327–332. [Google Scholar] [CrossRef]
- Muller, W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, F.; Petri, B.; Khandoga, A.G.; Moser, C.; Khandoga, A.; Volkery, S.; Li, H.; Nasdala, I.; Brandau, O.; Fässler, R.; et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 2006, 203, 1671–1677. [Google Scholar] [CrossRef]
- Dehghani, T.; Panitch, A. Endothelial cells, neutrophils and platelets: Getting to the bottom of an inflammatory triangle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Gupta, G.S. Selectins and Associated Adhesion Proteins in Inflammatory disorders. In Animal Lectins; Gupta, G.S., Gupta, A., Gupta, R.K., Eds.; Springer: Vienna, Austria, 2012; pp. 991–1026. ISBN 978-3-7091-1064-5. [Google Scholar]
- Mosevoll, K.A.; Johansen, S.; Wendelbo, Ø.; Nepstad, I.; Bruserud, Ø.; Reikvam, H. Cytokines, Adhesion Molecules, and Matrix Metalloproteases as Predisposing, Diagnostic, and Prognostic Factors in Venous Thrombosis. Front. Med. 2018, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Golias, C.; Tsoutsi, E.; Matziridis, A.; Makridis, P.; Batistatou, A.; Charalabopoulos, K. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo 2007, 21, 757–769. [Google Scholar] [PubMed]
- Mazzone, A.; Ricevuti, G. Leukocyte CD11/CD18 integrins: Biological and clinical relevance. Haematologica 1995, 80, 161–175. [Google Scholar] [PubMed]
- Michiels, C.; Arnould, T.; Remacle, J. Endothelial cell responses to hypoxia: Initiation of a cascade of cellular interactions. Biochim. Biophys. Acta 2000, 1497, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular Neutrophil Oxidants: From Laboratory Curiosity to Clinical Reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.; Noone, D.G.; Khan, M.A.; Pluthero, F.G.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. Complement Activation Induces Neutrophil Adhesion and Neutrophil-Platelet Aggregate Formation on Vascular Endothelial Cells. Kidney Int. Rep. 2017, 2, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zeman, K.; Kantorski, J.; Paleolog, E.M.; Feldmann, M.; Tchórzewski, H. The role of receptors for tumour necrosis factor-alpha in the induction of human polymorphonuclear neutrophil chemiluminescence. Immunol. Lett. 1996, 53, 45–50. [Google Scholar] [CrossRef]
- Poredoš, P.; Šabovič, M.; Božič Mijovski, M.; Nikolajević, J.; Antignani, P.L.; Paraskevas, K.I.; Mikhailidis, D.P.; Blinc, A. Inflammatory and Prothrombotic Biomarkers, DNA Polymorphisms, MicroRNAs and Personalized Medicine for Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2022, 23, 12054. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Riva, N.; Donadini, M.P.; Ageno, W. Epidemiology and pathophysiology of venous thromboembolism: Similarities with atherothrombosis and the role of inflammation. Thromb. Haemost. 2015, 113, 1176–1183. [Google Scholar] [CrossRef]
- Chang, S.-L.; Huang, Y.-L.; Lee, M.-C.; Hu, S.; Hsiao, Y.-C.; Chang, S.-W.; Chang, C.J.; Chen, P.-C. Association of Varicose Veins with Incident Venous Thromboembolism and Peripheral Artery Disease. JAMA 2018, 319, 807–817. [Google Scholar] [CrossRef]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef]
- Schaefer, A.; Te Riet, J.; Ritz, K.; Hoogenboezem, M.; Anthony, E.C.; Mul, F.P.J.; de Vries, C.J.; Daemen, M.J.; Figdor, C.G.; van Buul, J.D.; et al. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J. Cell Sci. 2014, 127, 4470–4482. [Google Scholar] [CrossRef]
- Sakai, N.; Bain, G.; Furuichi, K.; Iwata, Y.; Nakamura, M.; Hara, A.; Kitajima, S.; Sagara, A.; Miyake, T.; Toyama, T.; et al. The involvement of autotaxin in renal interstitial fibrosis through regulation of fibroblast functions and induction of vascular leakage. Sci. Rep. 2019, 9, 7414. [Google Scholar] [CrossRef]
- Li, W.; Wang, W. Structural alteration of the endothelial glycocalyx: Contribution of the actin cytoskeleton. Biomech. Model. Mechanobiol. 2018, 17, 147–158. [Google Scholar] [CrossRef]
- Gwozdzinski, L.; Bernasinska-Slomczewska, J.; Hikisz, P.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pieniazek, A. The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study. Biomedicines 2023, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Guţu, M.; Rusu, V.; Ştefănescu, C. Fluiditatea membranară—Parametru biofizic in relaţie cu procesele de transport membranare. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 153–162. [Google Scholar] [PubMed]
- Lande, M.B.; Donovan, J.M.; Zeidel, M.L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995, 106, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Gagalang, E. The effect of membrane-fluidizing agents on the adhesion of CHO cells. J. Cell. Physiol. 1979, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, B.E.; Curtis, A.S. Effects on cell adhesion and membrane fluidity of changes in plasmalemmal lipids in mouse L929 cells. J. Cell Sci. 1977, 26, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Salaita, K.; Nair, P.M.; Petit, R.S.; Neve, R.M.; Das, D.; Gray, J.W.; Groves, J.T. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science 2010, 327, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mao, H.; Joddar, B.; Umeki, N.; Sako, Y.; Wada, K.-I.; Nishioka, C.; Takahashi, E.; Wang, Y.; Ito, Y. The significance of membrane fluidity of feeder cell-derived substrates for maintenance of iPS cell stemness. Sci. Rep. 2015, 5, 11386. [Google Scholar] [CrossRef]
- Ratajczak, M.K.; Chi, E.Y.; Frey, S.L.; Cao, K.D.; Luther, L.M.; Lee, K.Y.C.; Majewski, J.; Kjaer, K. Ordered nanoclusters in lipid-cholesterol membranes. Phys. Rev. Lett. 2009, 103, 28103. [Google Scholar] [CrossRef]
- McEwan, A.J.; McArdle, C.S. Effect of hydroxyethylrutosides on blood oxygen levels and venous insufficiency symptoms in varicose veins. Br. Med. J. 1971, 2, 138–141. [Google Scholar] [CrossRef]
- Lim, C.S.; Gohel, M.S.; Shepherd, A.C.; Paleolog, E.; Davies, A.H. Venous hypoxia: A poorly studied etiological factor of varicose veins. J. Vasc. Res. 2011, 48, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Wood, J.G.; Johnson, J.S.; Mattioli, L.F.; Gonzalez, N.C. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J. Appl. Physiol. 2000, 89, 1561–1568. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-S.; Chiu, W.-T.; Hsu, P.-L.; Lin, S.-C.; Peng, I.-C.; Wang, C.-Y.; Tsai, S.-J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Wiener, C.M.; Booth, G.; Semenza, G.L. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 1996, 225, 485–488. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Yang, S.-L.; Wu, C.; Xiong, Z.-F.; Fang, X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review). Mol. Med. Rep. 2015, 12, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr. Vasc. Pharmacol. 2008, 6, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, P.; Novais, A.; Cairrão, E.; Maia, C.; Costa Almeida, C.M.; Verde, I. Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease. Int. J. Mol. Sci. 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.-P.; Cazaubon, M.; Scemama, A.; Prié, D.; Blanchet, F.; Guillin, M.-C.; Michel, J.-B. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation 2002, 106, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; Franciscis, S.d. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013, 21, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, Y.; Zhang, X.; Zhu, T.; Fu, W. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J. Surg. Res. 2011, 168, e143–e148. [Google Scholar] [CrossRef] [PubMed]
- de La Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Shields, D.A.; Andaz, S.K.; Sarin, S.; Scurr, J.H.; Coleridge Smith, P.D. Plasma elastase in venous disease. Br. J. Surg. 1994, 81, 1496–1499. [Google Scholar] [CrossRef]
- Grzela, T.; Niderla-Bielinska, J.; Litwiniuk, M.; White, R. The direct inhibition of MMP-2 and MMP-9 by an enzyme alginogel: A possible mechanism of healing support for venous leg ulcers. J. Wound Care 2014, 23, 278–285. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Kucukguven, A.; Khalil, R.A. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr. Drug Targets 2013, 14, 287–324. [Google Scholar]
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Terada, L.S.; Guidot, D.M.; Leff, J.A.; Willingham, I.R.; Hanley, M.E.; Piermattei, D.; Repine, J.E. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc. Natl. Acad. Sci. USA 1992, 89, 3362–3366. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, J.; Glowinski, S. Generation of reactive oxygen metabolites by the varicose vein wall. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Guzik, B.; Chwała, M.; Matusik, P.; Ludew, D.; Skiba, D.; Wilk, G.; Mrowiecki, W.; Batko, B.; Cencora, A.; Kapelak, B.; et al. Mechanisms of increased vascular superoxide production in human varicose veins. Pol. Arch. Med. Wewn. 2011, 121, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Horecka, A.; Biernacka, J.; Hordyjewska, A.; Dąbrowski, W.; Terlecki, P.; Zubilewicz, T.; Musik, I.; Kurzepa, J. Antioxidative mechanism in the course of varicose veins. Phlebology 2018, 33, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Kózka, M. Generation of reactive oxygen species by a sufficient, insufficient and varicose vein wall. Acta Biochim. Pol. 2011, 58, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved. Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Deshwal, S.; Di Lisa, F. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Cadenas, E.; Boveris, A.; Ragan, C.I.; Stoppani, A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977, 180, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Schmekel, B.; Karlsson, S.E.; Linden, M.; Sundstrm, C.; Tegner, H.; Venge, P. Myeloperoxidase in human lung lavage. Inflammation 1990, 14, 447–454. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Murie, J.A.; Ruckley, C.V. Role of the leucocyte in the pathogenesis of vascular disease. Br. J. Surg. 2005, 80, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.H.; Gannon, D.E.; Ward, P.A.; Karmiol, S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: Evidence of a role for elastase. Am. J. Respir. Cell Mol. Biol. 1992, 6, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Kuppusamy, P.; Thompson-Gorman, S.; Klunk, D.; Lutty, G.A. Measurement and characterization of free radical generation in reoxygenated human endothelial cells. Am. J. Physiol. 1994, 266, C700–C708. [Google Scholar] [CrossRef]
- Hood, E.D.; Greineder, C.F.; Dodia, C.; Han, J.; Mesaros, C.; Shuvaev, V.V.; Blair, I.A.; Fisher, A.B.; Muzykantov, V.R. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J. Control. Release 2012, 163, 161–169. [Google Scholar] [CrossRef]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Saribal, D.; Kanber, E.M.; Hocaoglu-Emre, F.S.; Akyolcu, M.C. Effects of the oxidative stress and genetic changes in varicose vein patients. Phlebology 2019, 34, 406–413. [Google Scholar] [CrossRef]
- Page, S.; Powell, D.; Benboubetra, M.; Stevens, C.R.; Blake, D.R.; Selase, F.; Wolstenholme, A.J.; Harrison, R. Xanthine oxidoreductase in human mammary epithelial cells: Activation in response to inflammatory cytokines. Biochim. Biophys. Acta 1998, 1381, 191–202. [Google Scholar] [CrossRef]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxidative Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Takase, S.; Schmid-Schönbein, G.; Bergan, J.J. Leukocyte activation in patients with venous insufficiency. J. Vasc. Surg. 1999, 30, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Kowalska, J.; Kózka, M.; Papież, M.A.; Kwiatek, W.M. Iron content (PIXE) in competent and incompetent veins is related to the vein wall morphology and tissue antioxidant enzymes. Bioelectrochemistry 2012, 87, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Hingorani, A.; Ascher, E. Overexpression of transforming growth factor-beta1 correlates with increased synthesis of nitric oxide synthase in varicose veins. J. Vasc. Surg. 2005, 41, 523–530. [Google Scholar] [CrossRef]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.-C.; Davies, M.J.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci. Rep. 2020, 10, 17300. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Tiwary, S.K.; Kumar, A.; Mishra, S.P.; Kumar, P.; Khanna, A.K. Study of association of varicose veins and inflammation by inflammatory markers. Phlebology 2020, 35, 679–685. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Raffetto, J.D. Chronic venous insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.T.; Jeney, V. Pro-inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Hanawa, H.; Ding, L.; Ota, Y.; Yoshida, K.; Toba, K.; Ogura, M.; Ito, H.; Kodama, M.; Aizawa, Y. Free heme is a danger signal inducing expression of proinflammatory proteins in cultured cells derived from normal rat hearts. Mol. Immunol. 2011, 48, 1191–1202. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef]
- Neal, M.D.; Raval, J.S.; Triulzi, D.J.; Simmons, R.L. Innate immune activation after transfusion of stored red blood cells. Transfus. Med. Rev. 2013, 27, 113–118. [Google Scholar] [CrossRef]
- Gáll, T.; Balla, G.; Balla, J. Heme, Heme Oxygenase, and Endoplasmic Reticulum Stress—A New Insight into the Pathophysiology of Vascular Diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972, 247, 6960–6962. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.; Oudega, B.; van Gelder, B.F. Generation of superoxide radicals during the autoxidation of mammalian oxyhemoglobin. Biochim. Biophys. Acta (BBA) Enzymol. 1973, 302, 475–478. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Free-radical production and oxidative reactions of hemoglobin. Environ. Health Perspect. 1985, 64, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2014, 5, 500. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Jeney, V.; Chora, A.; Larsen, R.; Balla, J.; Soares, M.P. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J. Biol. Chem. 2009, 284, 29582–29595. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- van Dyke, B.R.; Saltman, P. Hemoglobin: A mechanism for the generation of hydroxyl radicals. Free Radic. Biol. Med. 1996, 20, 985–989. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef]
- Neote, K.; Darbonne, W.; Ogez, J.; Horuk, R.; Schall, T.J. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J. Biol. Chem. 1993, 268, 12247–12249. [Google Scholar] [CrossRef]
- Darbonne, W.C.; Rice, G.C.; Mohler, M.A.; Apple, T.; Hébert, C.A.; Valente, A.J.; Baker, J.B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J. Clin. Investig. 1991, 88, 1362–1369. [Google Scholar] [CrossRef]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J. Immunol. 2018, 201, 1343–1351. [Google Scholar] [CrossRef]
- Fukuma, N.; Akimitsu, N.; Hamamoto, H.; Kusuhara, H.; Sugiyama, Y.; Sekimizu, K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem. Biophys. Res. Commun. 2003, 303, 137–139. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Zhang, J.; Zhang, H. Immune function of erythrocytes in patients with chronic venous insufficiency of the lower extremities. Chin. Med. J. 2007, 120, 2224–2228. [Google Scholar] [CrossRef]
- Hotz, M.J.; Qing, D.; Shashaty, M.G.S.; Zhang, P.; Faust, H.; Sondheimer, N.; Rivella, S.; Worthen, G.S.; Mangalmurti, N.S. Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 197, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, M.A.; Oscorbin, I.P.; Shadrina, A.S.; Sevost’ianova, K.S.; Korolenya, V.A.; Gavrilov, K.A.; Shevela, A.I.; Shirshova, A.N.; Oskina, N.A.; Zolotukhin, I.A.; et al. Quantitative and structural characteristics of mitochondrial DNA in varicose veins. Vascul. Pharmacol. 2022, 145, 107021. [Google Scholar] [CrossRef]
- Chwała, M.; Spannbauer, A.; Teległów, A.; Cencora, A.; Marchewka, A.; Hardeman, M.R.; Dabrowski, Z. Red blood cell rheology in patients with chronic venous disease (CVD). Clin. Hemorheol. Microcirc. 2009, 41, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Kózka, M.; Marona, H. Red blood cell deformability and aggregation in chronic venous disease patients with varicose veins. Postep. Hig. Med. Dosw. 2013, 67, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Kózka, M.; Marona, H. Rheological properties of young and aged erythrocytes in chronic venous disease patients with varicose veins. Clin. Hemorheol. Microcirc. 2015, 60, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska, J.; Grabowski, M.; Kowalczyk, E.; Gwozdzinski, K. Erythrocytes properties in varicose veins patients. Microvasc. Res. 2017, 111, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Safeukui, I.; Buffet, P.A.; Deplaine, G.; Perrot, S.; Brousse, V.; Ndour, A.; Nguyen, M.; Mercereau-Puijalon, O.; David, P.H.; Milon, G.; et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood 2012, 120, 424–430. [Google Scholar] [CrossRef]
- McVey, M.J.; Kuebler, W.M.; Orbach, A.; Arbell, D.; Zelig, O.; Barshtein, G.; Yedgar, S. Reduced deformability of stored red blood cells is associated with generation of extracellular vesicles. Transfus. Apher. Sci. 2020, 59, 102851. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska-Slomczewska, J.; Hikisz, P.; Gwozdzinski, K. Alterations in the Plasma and Red Blood Cell Properties in Patients with Varicose Vein: A Pilot Study. Cardiol. Res. Pract. 2021, 2021, 5569961. [Google Scholar] [CrossRef]
- Kettisen, K.; Strader, M.B.; Wood, F.; Alayash, A.I.; Bülow, L. Site-directed mutagenesis of cysteine residues alters oxidative stability of fetal hemoglobin. Redox Biol. 2018, 19, 218–225. [Google Scholar] [CrossRef]
- Zhang, R.; Hess, D.T.; Qian, Z.; Hausladen, A.; Fonseca, F.; Chaube, R.; Reynolds, J.D.; Stamler, J.S. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc. Natl. Acad. Sci. USA 2015, 112, 6425–6430. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, E.M.; Wilson, M.T.; Yusof, A.; Metodiev, M.V.; Cooper, C.E. The mechanism of formation, structure and physiological relevance of covalent hemoglobin attachment to the erythrocyte membrane. Free Radic. Biol. Med. 2017, 103, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Gwozdzinski, K. Changes in the conformational state of hemoglobin in hemodialysed patients with chronic renal failure. Oxidative Med. Cell. Longev. 2015, 2015, 783073. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Rubio, M.; Arribas, S.M.; España-Caparrós, G.; Rodríguez-Rodríguez, P.; Mujica-Pacheco, E.; González, M.C. A plasma oxidative stress global index in early stages of chronic venous insufficiency. J. Vasc. Surg. 2013, 57, 205–213. [Google Scholar] [CrossRef]
- Modaghegh, M.H.S.; Saberianpour, S.; Amoueian, S.; Kamyar, M.M. Signaling pathways associated with structural changes in varicose veins: A case-control study. Phlebology 2022, 37, 33–41. [Google Scholar] [CrossRef]
- Lardinois, O.M.; Mestdagh, M.M.; Rouxhet, P.G. Reversible inhibition and irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim. Biophys. Acta 1996, 1295, 222–238. [Google Scholar] [CrossRef]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Yasim, A.; Kilinc, M.; Aral, M.; Oksuz, H.; Kabalci, M.; Eroglu, E.; Imrek, S. Serum concentration of procoagulant, endothelial and oxidative stress markers in early primary varicose veins. Phlebology 2008, 23, 15–20. [Google Scholar] [CrossRef]
- Whiteley, M.S. Current Best Practice in the Management of Varicose Veins. Clin. Cosmet. Investig. Dermatol. 2022, 15, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Gawas, M.; Bains, A.; Janghu, S.; Kamat, P.; Chawla, P. A Comprehensive Review on Varicose Veins: Preventive Measures and Different Treatments. J. Am. Nutr. Assoc. 2022, 41, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Nicolaides, A.N. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2018, 37, 143–154. [Google Scholar] [CrossRef]
- Mchale, N.G.; Hollywood, M.A. Control of Lymphatic Pumping: Interest of Daflon 500 mg. Phlebology 1994, 9, 23–25. [Google Scholar] [CrossRef]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef]
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef] [PubMed]
- das Graças C de Souza, M.; Cyrino, F.Z.; de Carvalho, J.J.; Blanc-Guillemaud, V.; Bouskela, E. Protective Effects of Micronized Purified Flavonoid Fraction (MPFF) on a Novel Experimental Model of Chronic Venous Hypertension. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Aesculus hippocastanum (Horse chestnut). Monograph. Altern. Med. Rev. 2009, 14, 278–283.
- Zaragozá, C.; Álvarez-Mon, M.Á.; Zaragozá, F.; Villaescusa, L. Flavonoids: Antiplatelet Effect as Inhibitors of COX-1. Molecules 2022, 27, 1146. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-Asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Mohammadinejad, R.; Roomiani, S.; Afshar, E.G.; Ashrafizadeh, M. Biological and Therapeutic Effects of Troxerutin: Molecular Signaling Pathways Come into View. J. Pharmacopunct. 2021, 24, 1–13. [Google Scholar] [CrossRef]
- Aziz, Z.; Tang, W.L.; Chong, N.J.; Tho, L.Y. A systematic review of the efficacy and tolerability of hydroxyethylrutosides for improvement of the signs and symptoms of chronic venous insufficiency. J. Clin. Pharm. Ther. 2015, 40, 177–185. [Google Scholar] [CrossRef]
- Nonikashvili, Z.; Jūratė Gerbutavičienė, R. Ginkgo biloba, troxerutin and heptaminol chlorhydrate combined treatment for the management of venous insufficiency and hemorrhoidal crises. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5200–5209. [Google Scholar] [CrossRef] [PubMed]
- Bernardczyk-Meller, J. Trokserutyna w terapii chorób oczu—Sprawdzona substancja odkrywana na nowo. OphthaTher. Ther. Ophthalmol. 2023, 10, 201–204. [Google Scholar] [CrossRef]
- Wermeille, M.; Chollet, D. O-(beta-hydroxyethyl)-rutosides-chemical aspects. Phlebology 1993, 8, 3–9. [Google Scholar]
- Yun, S. Practical Use of Venoactive Drugs for Chronic Venous Disease in Korea. Ann. Phlebol. 2022, 20, 1–5. [Google Scholar] [CrossRef]
- Schmid-Schönbein, H.; Volger, E.; Weiss, J.; Brandhuber, M. Effect of o-(beta-hydroxyethyl)-rutosides on the microrheology of human blood under defined flow conditions. Vasa 1975, 4, 263–270. [Google Scholar] [PubMed]
- Lopes de Almeida, J.P.; Oliveira, S.; Saldanha, C. Erythrocyte as a biological sensor. Clin. Hemorheol. Microcirc. 2012, 51, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Ledda, A.; Cacchio, M.; Ruffini, I.; Ricci, A.; Ippolito, E.; Di Renzo, A.; Dugall, M.; Corsi, M.; et al. 5-Year control and treatment of edema and increased capillary filtration in venous hypertension and diabetic microangiopathy using O-(beta-hydroxyethyl)-rutosides: A prospective comparative clinical registry. Angiology 2008, 59 (Suppl. 1), 14S–20S. [Google Scholar] [CrossRef]
- Roland, I.H.; Bougelet, C.; Ninane, N.; Arnould, T.; Michiels, C.; Remacle, J. Effect of hydroxyethylrutosides on hypoxial-induced neutrophil adherence to umbilical vein endothelium. Cardiovasc. Drugs Ther. 1998, 12, 375–381. [Google Scholar] [CrossRef]
- Akbulut, B. Calcium dobesilate and oxerutin: Effectiveness of combination therapy. Phlebology 2010, 25, 66–71. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Leal, D.B.R.; Doleski, P.H.; Ledur, P.C.; Ecker, A. Peripheral blood mononuclear cells from rat model of pleurisy: The effects of hesperidin on ectoenzymes activity, apoptosis, cell cycle and reactive oxygen species production. Biomed. Pharmacother. 2017, 91, 278–286. [Google Scholar] [CrossRef]
- Hosawi, S. Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life 2023, 13, 937. [Google Scholar] [CrossRef]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef]
- Enseleit, F.; Sudano, I.; Périat, D.; Winnik, S.; Wolfrum, M.; Flammer, A.J.; Fröhlich, G.M.; Kaiser, P.; Hirt, A.; Haile, S.R.; et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: A double-blind, randomized, placebo-controlled, cross-over study. Eur. Heart J. 2012, 33, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Zolfaghari, B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res. Pharm. Sci. 2011, 6, 1–11. [Google Scholar] [PubMed]
- Schäfer, A.; Chovanová, Z.; Muchová, J.; Sumegová, K.; Liptáková, A.; Duracková, Z.; Högger, P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed. Pharmacother. 2006, 60, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Jameel, K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011, 133, 261–277. [Google Scholar] [CrossRef]
- Farinola, N.; Piller, N. Pharmacogenomics: Its role in re-establishing coumarin as treatment for lymphedema. Lymphat. Res. Biol. 2005, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Girolami, A.; Cosi, E.; Ferrari, S.; Girolami, B. Heparin, coumarin, protein C, antithrombin, fibrinolysis and other clotting related resistances: Old and new concepts in blood coagulation. J. Thromb. Thrombolysis 2018, 45, 135–141. [Google Scholar] [CrossRef]
- Trailokya, A. Acenocoumarol in Thromoembolic Disorders. Cardiol. Pharmacol 2015, 4, 4. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.-J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef]
- Tien, Y.-C.; Liao, J.-C.; Chiu, C.-S.; Huang, T.-H.; Huang, C.-Y.; Chang, W.-T.; Peng, W.-H. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int. J. Mol. Sci. 2011, 12, 4053–4067. [Google Scholar] [CrossRef]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. Dev. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef]
- Masullo, M.; Pizza, C.; Piacente, S. Ruscus Genus: A Rich Source of Bioactive Steroidal Saponins. Planta Medica 2016, 82, 1513–1524. [Google Scholar] [CrossRef]
- Balica, S.; Bulai Livideanu, C.; Fournié, P.; Fortenfant, F.; Soler, V.; Barbarot, S.; Paul, C. Is conjunctival mucous involvement a marker of severity in pemphigus vulgaris? J. Eur. Acad. Dermatol. Venereol. 2013, 27, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Rudofsky, G.; Haussler, K.F.; Künkel, H.P.; Schneider-May, H.; Spengel, F.; Symann, O.; Werner, H.J. Intravenous treatment of chronic peripheral occlusive arterial disease: A double-blind, placebo-controlled, randomized, multicenter trial of pentoxifylline. Angiology 1989, 40, 639–649. [Google Scholar] [CrossRef]
- Bouaziz, N.; Michiels, C.; Janssens, D.; Berna, N.; Eliaers, F.; Panconi, E.; Remacle, J. Effect of Ruscus extract and hesperidin methylchalcone on hypoxia-induced activation of endothelial cells. Int. Angiol. 1999, 18, 306–312. [Google Scholar] [PubMed]
- Huang, Y.-L.; Kou, J.-P.; Ma, L.; Song, J.-X.; Yu, B.-Y. Possible mechanism of the anti-inflammatory activity of ruscogenin: Role of intercellular adhesion molecule-1 and nuclear factor-kappaB. J. Pharmacol. Sci. 2008, 108, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Medica 2020, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Stroemgaard, K.; Nakanishi, K. Chemistry and Biology of Terpene Trilactones from Ginkgo Biloba. Angew. Chem. Int. Ed. 2004, 43, 1640–1658. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, A. Use of Ginkgo biloba phytosome in chronic venous disease: A pilot study. Acta Phlebol. 2015, 16, 83–92. [Google Scholar]
- Shang, Q.; Zhou, X.; Yang, M.-R.; Lu, J.-G.; Pan, Y.; Zhu, G.-Y.; Jiang, Z.-H. Amide Derivatives of Ginkgolide B and Their Inhibitory Effects on PAF-Induced Platelet Aggregation. ACS Omega 2021, 6, 22497–22503. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, D.; Ribaudo, G.; Pompermaier, N.; Madabeni, A.; Bortoli, M.; Orian, L. Radical Scavenging Potential of Ginkgolides and Bilobalide: Insight from Molecular Modeling. Antioxidants 2023, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Scholtyssek, H.; Damerau, W.; Wessel, R.; Schimke, I. Antioxidative activity of ginkgolides against superoxide in an aprotic environment. Chem. Biol. Interact. 1997, 106, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, Z.; Cao, S.; Xu, Y.; Yu, J. Transformation of multi-component ginkgolide into ginkgolide B by Coprinus comatus. BMC Biotechnol. 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Vayssairat, M. Placebo-Controlled Trial of Naftazone in Women with Primary Uncomplicated Symptomatic Varicose Veins. Phlebology 1997, 12, 17–20. [Google Scholar] [CrossRef]
- Gohel, M.S.; Davies, A.H. Pharmacological agents in the treatment of venous disease: An update of the available evidence. Curr. Vasc. Pharmacol. 2009, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Yu, W.; Wang, X.; Calanni, F.; Mattana, P.; Khalil, R.A. Sulodexide Improves Contraction and Decreases Matrix Metalloproteinase-2 and -9 in Veins Under Prolonged Stretch. J. Cardiovasc. Pharmacol. 2020, 75, 211–221. [Google Scholar] [CrossRef]
| Group | Substance | Pharmacological Action |
|---|---|---|
| Flavonoids | ||
| Diosmin | 7-disaccharide derivative of diosmetin. The drug is available in the form of micronized purified flavonoid fraction (MPFF, Daflon-90% and 10% other active flavonoids: hesperidin, diosmetin, linarin and isoorhoifolin from Rutaceae aurantiae. | Diosmin acts on vessels, increasing venous tone, lymphatic flow, and improving vascular elasticity. It also has an anti-edema effect, reduces the permeability of blood vessel walls, and improves blood circulation in the capillaries. It has anti-inflammatory and antioxidant properties and has positive effects on the elasticity of the vessels. It inhibits leukocyte adhesion, activation of platelets and the complement system, and decreases the COX-1 activity. |
| Rutin | 3-disaccharide derivative of quercetin found in citrus fruits, buckwheat, asparagus, peaches, green tea, and capers. | It has anti-inflammatory and antioxidant properties. It inhibits pro-inflammatory signaling of VCAM-1, ICAM-1, and E-selectin. It inhibits PAF but induces NOS, increasing NO release. It inhibits adhesion and migration of leucocyte to inflamed endothelium and neutrophils adhesion and migration. It reduces the expression of NF-κB and of the pro-inflammatory cytokines IL-6 and TN-α. It has antihypertensive properties. |
| Troxerutin | 3-rutoside, disaccharide derivative. It occurs in citrus fruits, tea, coffee cereal grains, and vegetables. | It improves the rheological properties of blood, inhibits the aggregation of erythrocytes and platelets, improves the deformability and aggregation of erythrocytes, as well as the viscosity of plasma and microcirculation in the retina. It has antithrombotic properties, increases the tension of venous walls, and regulates their permeability. It is used in the treatment of venous and lymphatic circulation disorders, especially in the lower limbs, phlebitis, post-thrombotic syndrome, varicose veins of the lower limbs and anus. |
| Oxerutin | 3-disaccharide derivative hydroxyethylrutoside. Oxerutines are semi-synthetic derivatives of rutin. The standardized mixture used as a pharmacological preparation contains 5% mono-, 34% di-, 46% tri-, and 5% O-b-hydroxyethyl tetrarutosides. Oxerutins are obtained from the Sophora japonica plant. | Oxerutins are used in the treatment of chronic venous disease. Hydroxyethylrutosides have a protective effect on the vascular endothelium and also have a strong affinity for the venous wall. They inhibit the permeability of microvessels and reduce swelling. They have a positive effect on red blood cell membranes, their deformation and aggregation, and improve the rheological properties of RBCs. In addition to their anti-edema effect, they inhibit the synthesis of prostaglandins. They inhibit the recruitment and activation of neutrophils by stimulating the endothelium during blood stasis. |
| Hesperidin | 7-disaccharide derivative of flavanon. It occurs in large concentrations in citrus fruits. | It has anti-inflammatory, antioxidant, and antimicrobial properties. The anti-inflammatory effect of Hesperidin was associated with the inhibition of the p38 MAPK signaling pathway, and the expression of pro-inflammatory cytokines. This flavonoid reduces platelet aggregation and increases the expression of antioxidant enzymes CAT and SOD. Hesperidin can inhibit inflammatory mediators, NF-κB, iNOS, and COX-2, and activate the ERK/Nrf2 signaling pathway, improving cellular antioxidant defense. |
| Pycnogenol | Extracts from pine tree bark (Pinus pinaster ssp. atlantica) containing many flavonoids, 65–75% of proanthocyanidins, and phenolic acid. | Pycnogenol exerts antioxidative, anti-inflammatory, and anti-platelet effects. It increases the resistance of capillaries and improves blood flow, increases the release of NO from vascular endothelial cells and protects the endothelium against the damaging effects of ROS. It regenerates the ascorbyl radical and has a protective role against endogenous a-tokoferol and GSH in the cellular antioxidant system. It inhibits nuclear factor (NF-κB), and modulates NO metabolism by scavenging NO and inhibiting iNOS mRNA expression and iNOS activity. PG inhibits the gene responsible for the expression of pro-inflammatory cytokines (IL-1 and IL-2) and inhibits the activities of COX-1 and COX-2. It dilates blood vessels, has antithrombotic properties, and stabilizes collagen. |
| Coumarin | chromen-2-on derivatives. They occur in Melilot (Melilotus officinalis) and Woodruff (Asperula odorata). Synthetic derivatives: acenocoumarol, warfarin, fraxetin, fraxin, esculetin. | Coumarin derivatives, have broad pharmacological and therapeutic properties, such as anti-inflammatory, antioxidant, antiviral, antibacterial, anti-coagulant, anti-edema, and anti-cancer effects. Esculetin coumarin derivatives scavenge free radicals generated during lipid peroxidation, improving the levels of antioxidant enzymes such as CAT, SOD, and GPX. Esculetin inhibits the synthesis of leukotrienes B4, activation of the NF-κB and MPAK pathways and the expression of inflammatory cytokines and chemokines, such as TNF-α, IL-1β, IL-6, CCL2, and iNOS. |
| Saponins | ||
| Aescin | Horse chestnut seed extracts (Aesculus hippocastanum L.) | Aescin has anti-inflammatory properties, reduces vascular permeability in inflamed tissues, which leads to inhibition of edema. This saponin prevents disruption of the normal expression and distribution of the platelet endothelial cell adhesion molecule in hypoxia, which may help explain its protective effect on vascular permeability. Aescin induces iNOS, which increases the permeability of EC to calcium ions, and induces the release of prostaglandin F2α. Aescin reduces the content of TNF-α and IL-1β. |
| Steroid saponins | Ruscus aculeatus (Butcher’s broom) and Centella asiatica extracts | The extract inhibits endothelial cell activation due to hypoxia, a condition occurring in venous blood stasis. This effect is associated with a decrease in ATP concentration and activation of phospholipase A2, and a subsequent increase in neutrophil adhesion. These observations explain the beneficial therapeutic effects of the extract in the treatment of chronic venous disease. Ruscogenin has strong anti-inflammatory and antithrombotic effects. Ruscogenin significantly inhibits leukocyte migration into the peritoneum. Ruscogenin also inhibits TNF-α-induced ICAM-1 overexpression at the mRNA and protein levels. It significantly inhibits NF-κB activation, reducing NF-κB p65 translocation and DNA binding activity. |
| Ginkgo biloba (Gb) extract | Extracts of Ginkgo biloba contain flavonoid glycosides, terpenes, lactones, proanthocyanidins, carboxylic acids, and high molecular weight compounds, as well as sterols, carotenoids, polyphenols, and long-chain hydrocarbons. Compounds with interesting properties are diterpenes with a caged skeleton, such as ginkgolides A, B, C, J, and M, and sesquiterpenoids (bilobalide) | Gb inhibits the expression of ICAM1 and VCAM, and also performs an important role in peripheral circulation and microcirculation. Ginkgolide B (GB) is the most potent antagonist of platelet-activating factor (PAF) and an intracellular mediator involved in the process of platelet aggregation and inflammatory reactions. Ginkgolide A and B have antioxidant properties, they inhibit the formation of free radicals, and protect against OS. Ginkgolides B, C, J, M, and bilobalide react with superoxide and its protonated form. Ginkgolide B has anti-ischemic and antioxidant effects, and it is an anticonvulsant and controls inflammation. It is used to treat thrombosis in clinical practice. GB inhibits PLA2 activity and the production of TNF-α. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwozdzinski, L.; Pieniazek, A.; Gwozdzinski, K. Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs. Int. J. Mol. Sci. 2024, 25, 1560. https://doi.org/10.3390/ijms25031560
Gwozdzinski L, Pieniazek A, Gwozdzinski K. Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs. International Journal of Molecular Sciences. 2024; 25(3):1560. https://doi.org/10.3390/ijms25031560
Chicago/Turabian StyleGwozdzinski, Lukasz, Anna Pieniazek, and Krzysztof Gwozdzinski. 2024. "Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs" International Journal of Molecular Sciences 25, no. 3: 1560. https://doi.org/10.3390/ijms25031560
APA StyleGwozdzinski, L., Pieniazek, A., & Gwozdzinski, K. (2024). Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs. International Journal of Molecular Sciences, 25(3), 1560. https://doi.org/10.3390/ijms25031560