Highlights on the Effects of Non-Coding RNAs in the Osteonecrosis of the Jaw
Abstract
:1. Osteonecrosis of the Jaw
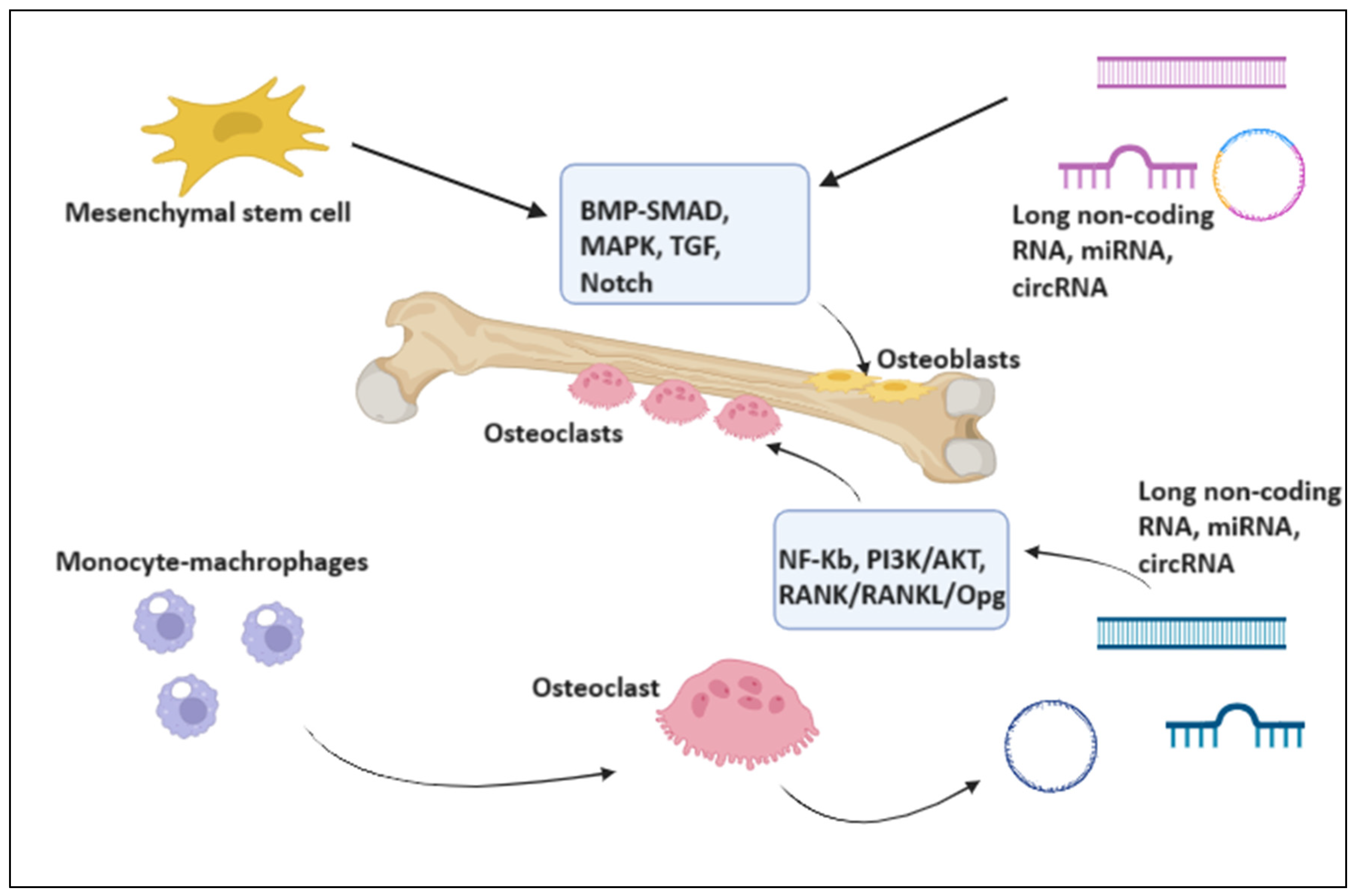
1.1. Pathogenesis
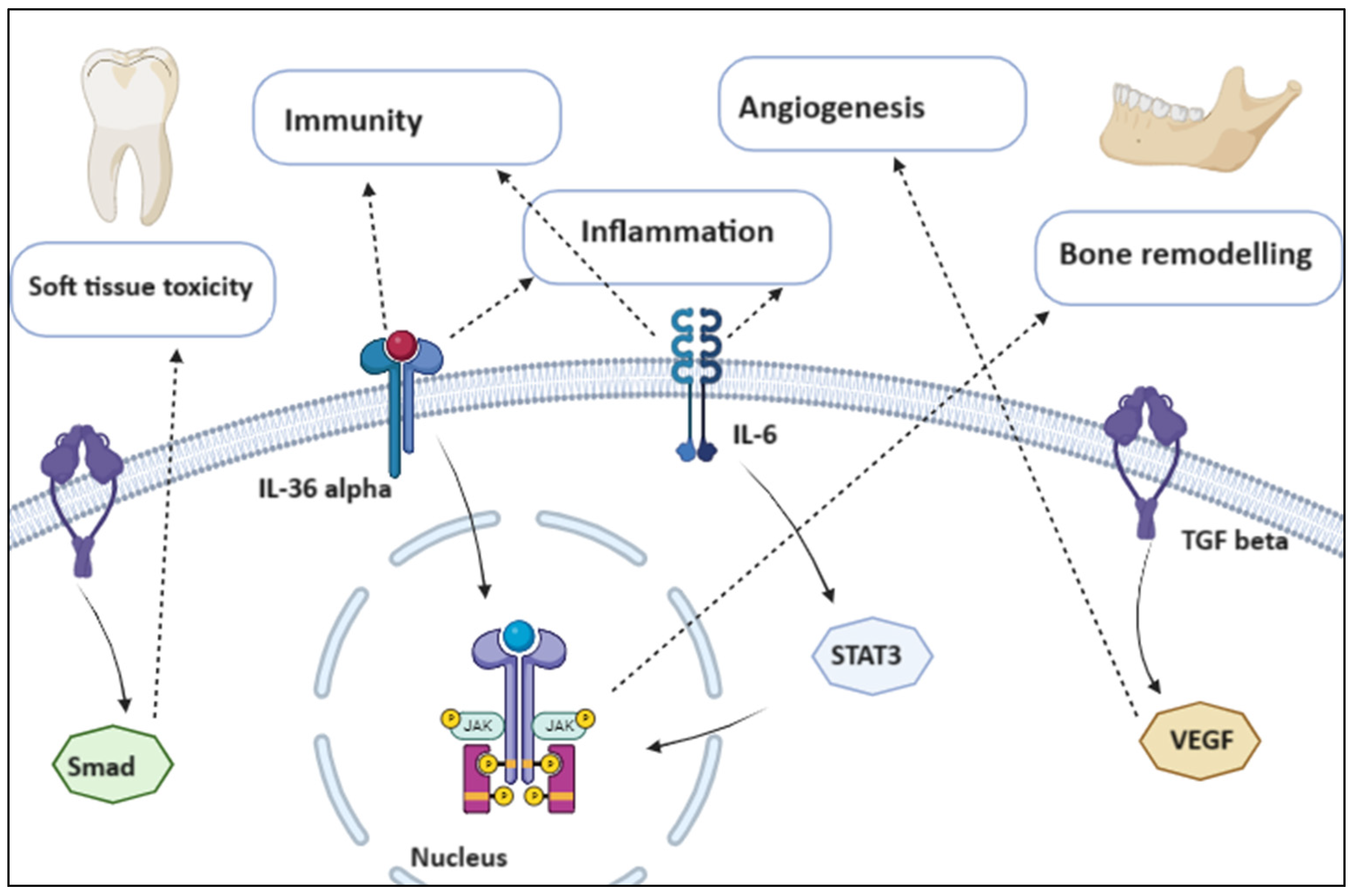
1.1.1. Inflammatory and Growth Pathways
1.1.2. Five Pathophysiology Theories
1.1.3. Gamma-Delta T Cell Impairment
2. Evidence from Non-Coding RNAs in the Osteonecrosis of the Jaw
3. miRNAs
3.1. miRNAs as Biomarkers and Therapeutic Targets of Osteoclasts Activity
3.2. mir-149-5p Modulates the Rap1a/Rap1b/VEGFR2 Pathway
4. Long Non-Coding RNAs
4.1. lncRNAs Modulate Osteogenic Proliferation and Differentiation
4.2. lncRNAs Inhibit Bone Neoangiogenesis
5. circularRNAs
mmu_circ_0001066 Attenuates the Inhibitory Action of Bisphosphonates on Osteoclastogenesis
6. New Perspectives about the Therapeutic Potential of Non-Coding RNAs
6.1. miR-29-3p Inhibitors
6.2. Targeting Maternally Expressed Gene 3 to Promote Osteogenesis
6.3. OIP5-AS1 Blocks the Pro-Oncogenic miR-410
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allegra, A.; Mania, M.; D’ascola, A.; Oteri, G.; Siniscalchi, E.N.; Avenoso, A.; Innao, V.; Scuruchi, M.; Allegra, A.G.; Musolino, C.; et al. Altered Long Noncoding RNA Expression Profile in Multiple Myeloma Patients with Bisphosphonate-Induced Osteonecrosis of the Jaw. BioMed Res. Int. 2020, 2020, 9879876. [Google Scholar] [CrossRef]
- Yunus, S.S.M.; Soh, H.Y.; Rahman, M.A.; Peng, X.; Guo, C.; Ramli, R. MicroRNA in medication related osteonecrosis of the jaw: A review. Front. Physiol. 2023, 14, 1021429. [Google Scholar] [CrossRef]
- Ji, X.; Chen, X.; Yu, X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int. J. Mol. Sci. 2016, 17, 349. [Google Scholar] [CrossRef]
- Yang, R.; Tao, Y.; Wang, C.; Shuai, Y.; Jin, L. Circulating microRNA Panel as a Novel Biomarker to Diagnose Bisphosphonate-Related Osteonecrosis of the Jaw. Int. J. Med Sci. 2018, 15, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Gangemi, S.; Murdaca, G.; Allegra, A. Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility. Int. J. Mol. Sci. 2023, 24, 11544. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Caserta, S.; Mirabile, G.; Gangemi, S. Aging and Age-Related Epigenetic Drift in the Pathogenesis of Leukemia and Lymphomas: New Therapeutic Targets. Cells 2023, 12, 2392. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Genovese, C.; Cicero, N.; Gangemi, S.; Allegra, A. The Anti-Cancer Effect of Cinnamon Aqueous Extract: A Focus on Hematological Malignancies. Life 2023, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Genovese, C.; Cicero, N.; Toscano, V.; Gangemi, S.; Allegra, A. The Interplay between Medical Plants and Gut Microbiota in Cancer. Nutrients 2023, 15, 3327. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Cicero, N.; Tonacci, A.; Musolino, C.; Gangemi, S. Circular RNA as a Novel Biomarker for Diagnosis and Prognosis and Potential Therapeutic Targets in Multiple Myeloma. Cancers 2022, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Oteri, G.; Allegra, A.; Mania, M.; D’ascola, A.; Avenoso, A.; Innao, V.; Allegra, A.G.; Campo, S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann. Hematol. 2018, 97, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhu, W.; Zhang, P.; Fu, Y.; Cheng, J.; Liu, L.; Xu, R.; Jiang, H. Macrophage miR-149-5p induction is a key driver and therapeutic target for BRONJ. J. Clin. Investig. 2022, 7, e159865. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, J.; Gao, L.; Ren, W.; Li, S.; Zheng, J.; Xin, S.; Kong, X.; Zhi, K. Upregulation of mmu_circ_0001066 attenuates the inhibitory effect of bisphosphonates on osteoclastogenesis. Oral Dis. 2022, 28, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, C.; Sun, D.; Yang, S.; Wang, L. circNINL facilitates aerobic glycolysis, proliferation, invasion, and migration in lung cancer by sponging miR-3918 to mediate FGFR1 expression. Eur. J. Med. Res. 2024, 29, 67. [Google Scholar] [CrossRef] [PubMed]
- Puła, A.; Robak, T.; Dróżdż, I.; Stawiski, K.; Rycerz, A.; Misiewicz, M.; Robak, P. Circulating serum microRNAs as biomarkers of drug resistance in multiple myeloma patients treated with bortezomib-based regimens—Pilot study. Leuk. Lymphoma, 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nobili, L.; Ronchetti, D.; Agnelli, L.; Taiana, E.; Vinci, C.; Neri, A. Long Non-Coding RNAs in Multiple Myeloma. Genes 2018, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Tetradis, S.; Allen, M.R.; Ruggiero, S.L. Pathophysiology of Medication-Related Osteonecrosis of the Jaw—A Minireview. JBMR Plus 2023, 7, e10785. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.; Caldas, I.M.; Dinis-Oliveira, R.J. Bisphosphonates and osteonecrosis of the jaws: Clinical and forensic aspects. Arch. Oral Biol. 2023, 155, 105792. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, S.; Scariot, R.; Elsalanty, M. Medication-Related Osteonecrosis: Why the Jawbone? Dent. J. 2023, 11, 109. [Google Scholar] [CrossRef]
- Kalita, F.; Gupta, D.S.; Gehlot, N.; Mitra, S.; Singh, S.; Pillai, S.S. Osteonecrosis of the Jaws: An Update and Review of Literature. J. Maxillofac. Oral Surg. 2023, 22, 344–351. [Google Scholar] [CrossRef]
- Sciaccotta, R.; Murdaca, G.; Caserta, S.; Rizzo, V.; Gangemi, S.; Allegra, A. Circular RNAs: A New Approach to Multiple Sclerosis. Biomedicines 2023, 11, 2883. [Google Scholar] [CrossRef]
- Shah, M.; Sarkar, D. HCC-Related lncRNAs: Roles and Mechanisms. Int. J. Mol. Sci. 2024, 25, 597. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Singh, S.; Chen, Y.; Hamadeh, I.S.; Langaee, T.; McDonough, C.W.; Holliday, L.S.; Lamba, J.K.; Moreb, J.S.; Katz, J.; et al. Pharmacogenomics of osteonecrosis of the jaw. Bone 2019, 124, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, L.; Ren, W.; Li, S.; Zheng, J.; Li, S.; Jiang, C.; Yang, S.; Zhi, K. The Role of the Immune Response in the Development of Medication-Related Osteonecrosis of the Jaw. Front. Immunol. 2021, 12, 606043. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, I.S.; Ngwa, B.A.; Gong, Y. Drug induced osteonecrosis of the jaw. Cancer Treat. Rev. 2015, 41, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lucía, R.; María, L.-P.R.; Elisabeth, C.; De, A.L.; Gonzalo, H. New Non-Bisphosphonate Drugs that Produce Osteonecrosis of the Jaws. Oral Health Prev. Dent. 2015, 13, 385–393. [Google Scholar] [CrossRef]
- Katsarelis, H.; Shah, N.; Dhariwal, D.; Pazianas, M. Infection and Medication-related Osteonecrosis of the Jaw. J. Dent. Res. 2015, 94, 534–539. [Google Scholar] [CrossRef]
- Agarwal, P.; Rao, N.N. Bisphosphonate-associated osteonecrosis of the jaws. Indian J. Dent. Res. 2012, 23, 107. [Google Scholar] [CrossRef]
- Chamizo Carmona, E.; Gallego Flores, A.; Loza Santamaría, E.; Herrero Olea, A.; Rosario Lozano, M.P. Systematic literature review of bisphosphonates and osteonecrosis of the jaw in patients with osteoporosis. Reumatol. Clin. 2013, 9, 172–177. [Google Scholar] [CrossRef]
- Lesclous, P.; Najm, S.A.; Carrel, J.-P.; Baroukh, B.; Lombardi, T.; Willi, J.-P.; Rizzoli, R.; Saffar, J.-L.; Samson, J. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone 2009, 45, 843–852. [Google Scholar] [CrossRef]
- Gkouveris, I.; Soundia, A.; Gouveris, P.; Zouki, D.; Hadaya, D.; Tetradis, S. Macrophage Involvement in Medication-Related Osteonecrosis of the Jaw (MRONJ): A Comprehensive, Short Review. Cancers 2022, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.C.; Heredia, J.E.; da Silva, F.R.F.; Macari, S. Extracellular Vesicles in Bone Remodeling and Osteoporosis. Adv. Exp. Med. Biol. 2023, 1418, 155–168. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, F.; Li, Y.; Bai, L.; Feng, M.; Li, S.; Ma, W.; Shi, S. Functional micro-RNA drugs acting as a fate manipulator in the regulation of osteoblastic death. Nanoscale 2023, 15, 12840–12852. [Google Scholar] [CrossRef]
- Doghish, A.S.; Elballal, M.S.; Elazazy, O.; Elesawy, A.E.; Shahin, R.K.; Midan, H.M.; Sallam, A.-A.M.; Elbadry, A.M.; Mohamed, A.K.; Ishak, N.W.; et al. miRNAs as potential game-changers in bone diseases: Future medicinal and clinical uses. Pathol. Res. Pr. 2023, 245, 154440. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef] [PubMed]
- Bak, R.O.; Mikkelsen, J.G. miRNA sponges: Soaking up miRNAs for regulation of gene expression. Wiley Interdiscip. Rev. RNA 2014, 5, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, T.; Bao, Y.; Zhao, T.; Wang, J.; Wang, H.; Wang, A.; Gan, X.; Wu, Z.; Wang, L. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020, 469, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Xu, D.; Wang, Q.; Wang, D. Hypermethylation of the Promoter Region of miR-23 Enhances the Metastasis and Proliferation of Multiple Myeloma Cells via the Aberrant Expression of uPA. Front. Oncol. 2022, 12, 835299. [Google Scholar] [CrossRef]
- Russo, R.; Zito, F.; Lampiasi, N. MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation. Biology 2021, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Sung, S.-E.; Kang, K.-K.; Lee, S.; Sung, M.; Park, W.-T.; Kim, Y.I.; Seo, M.-S.; Lee, G.W. Extracellular Vesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells Suppress RANKL-Induced Osteoclast Differentiation via miR122-5p. Biochem. Genet. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Allegra, A.; Sirufo, M.M.; Pioggia, G.; Tonacci, A.; Gangemi, S. The Osteoporosis/Microbiota Linkage: The Role of miRNA. Int. J. Mol. Sci. 2020, 21, 8887. [Google Scholar] [CrossRef]
- Horita, M.; Farquharson, C.; A Stephen, L. The role of miR-29 family in disease. J. Cell. Biochem. 2021, 122, 696–715. [Google Scholar] [CrossRef]
- Hrdlicka, H.C.; Pereira, R.C.; Shin, B.; Yee, S.-P.; Deymier, A.C.; Lee, S.-K.; Delany, A.M. Inhibition of miR-29-3p isoforms via tough decoy suppresses osteoblast function in homeostasis but promotes intermittent parathyroid hormone-induced bone anabolism. Bone 2021, 143, 115779. [Google Scholar] [CrossRef]
- Hrdlicka, H.C.; Lee, S.-K.; Delany, A.M. MicroRNAs Are Critical Regulators of Osteoclast Differentiation. Curr. Mol. Biol. Rep. 2019, 5, 65–74. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.X.; Zboinski, E.K.; Qiu, W.; Lian, J.; Liu, S.; Van Dyke, T.E.; Johansson, H.E.; Tu, Q.; Luo, E.; et al. Long non-coding RNA APDC plays important regulatory roles in metabolism of bone and adipose tissues. RNA Biol. 2023, 20, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, T.; Kessler, C.B.; Lee, S.-K.; Delany, A.M. miR-29 Promotes Murine Osteoclastogenesis by Regulating Osteoclast Commitment and Migration. J. Biol. Chem. 2013, 288, 33347–33360. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Z.; Zhuang, Y.; Ning, X.; Zhang, H.; Shen, Z.-M.; Shang, X.-W. Artesunate inhibits osteoclastogenesis through the miR-503/RANK axis. Biosci. Rep. 2020, 40, BSR20194387. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.-P.; Shi, L.-Y.; Li, J.-P.; Zeng, Y.; Liu, W.; Tang, S.-Y.; Jia, L.-J.; Zhang, J.; Gan, G.-X. Oleanolic acid inhibits RANKL-induced osteoclastogenesis via ER alpha/miR-503/RANK signaling pathway in RAW264.7 cells. Biomed. Pharmacother. 2019, 117, 109045. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheng, P.; Xie, H.; Zhou, H.; Wu, X.; Liao, E.; Luo, X. MiR-503 Regulates Osteoclastogenesis via Targeting RANK. J. Bone Miner. Res. 2014, 29, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; An, J.; Han, X.; Zhang, X.; Wang, W.; Wang, S. Hypermethylation of microRNA-149 activates SDF-1/CXCR4 to promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 23485–23494. [Google Scholar] [CrossRef] [PubMed]
- Fasciano, S.; Luo, S.; Wang, S. Long non-coding RNA (lncRNA) MALAT1 in regulating osteogenic and adipogenic differentiation using a double-stranded gapmer locked nucleic acid nanobiosensor. Analyst 2023, 148, 6261–6273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.X.; Liu, Y.; Wang, J.; Xie, Y.; Li, R.Y.; Ma, Q.; Tu, Q.; A Melhem, N.; Couldwell, S.; El-Araby, R.E.; et al. A novel lncRNA-mediated epigenetic regulatory mechanism in periodontitis. Int. J. Biol. Sci. 2023, 19, 5187–5203. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ye, W.; Wu, X.; Huang, H.; Li, B.; Ren, Z.; Yang, Z. Long non-coding RNA MIR22HG suppresses the chondrogenic differentiation of human adipose-derived stem cells by interacting with CTCF to upregulate CRLF1. Funct. Integr. Genom. 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.A.R.; Tye, C.E.; Banerjee, B.; Ghule, P.N.; van Wijnen, A.J.; Kabala, F.S.; Page, N.A.; Falcone, M.M.; Stein, J.L.; Stein, G.S.; et al. LINC01638 sustains human mesenchymal stem cell self-renewal and competency for osteogenic cell fate. Sci. Rep. 2023, 13, 20314. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Di, S.; Xing, S.; Sun, Z.; Shen, Z.; Dou, X.; He, S.; Tang, H.; Min, J. Long non-coding RNA DANCR modulates osteogenic differentiation by regulating the miR-1301-3p/PROX1 axis. Mol. Cell. Biochem. 2021, 476, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Wang, J.; Tian, X.; Cao, G.; Gu, Y.; Shao, F.; Yan, T. Exosomes Secreted by Mesenchymal Stem Cells Induce Immune Tolerance to Mouse Kidney Transplantation via Transporting LncRNA DANCR. Inflammation 2022, 45, 460–475. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Chang, Y.-H.; Truong, V.A.; Hsu, M.-N.; Pham, N.N.; Chang, C.-W.; Wu, Y.-H.; Chang, Y.-H.; Li, H.; Hu, Y.-C. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Biomaterials 2021, 275, 120965. [Google Scholar] [CrossRef]
- Deng, M.; Wang, Z.; Luo, J.; Cao, H.; Li, Y.; Chen, L.; Liu, G. CircZNF367 promotes osteoclast differentiation and osteoporosis by interacting with FUS to maintain CRY2 mRNA stability. J. Orthop. Surg. Res. 2023, 18, 492. [Google Scholar] [CrossRef]
- Pan, X.; Cen, X.; Zhang, B.; Pei, F.; Huang, W.; Huang, X.; Zhao, Z. Circular RNAs as potential regulators in bone remodeling: A narrative review. Ann. Transl. Med. 2021, 9, 1505. [Google Scholar] [CrossRef]
- Lin, J.; Ma, S.; Zhu, C.; Chen, C.; Lin, W.; Lin, C.; Huang, G.; Ding, Z. Circular RNA atlas in osteoclast differentiation with and without alendronate treatment. J. Orthop. Surg. Res. 2020, 15, 240. [Google Scholar] [CrossRef]
- Chen, X.; Ouyang, Z.; Shen, Y.; Liu, B.; Zhang, Q.; Wan, L.; Yin, Z.; Zhu, W.; Li, S.; Peng, D. CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. 2019, 16, 1249–1262. [Google Scholar] [CrossRef]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.R.; Balbas, M.C.d.M.; Corrêa, C.; Zanela, M.; Okamoto, R.; Pereira, R.d.S.; Homsi, N.; Hochuli-Vieira, E. The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model. Craniomaxillofacial Trauma Reconstr. 2022, 15, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, W.; Ma, H.; Zou, D.; Zhang, Z.; Wang, S. Structural insights into the binding of zoledronic acid with RANKL via computational simulations. Front. Mol. Biosci. 2022, 9, 992473. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Williams, R.; Wang, L.; Farhadfar, N.; Chen, Y.; Loiacono, A.T.; Bian, J.; Holliday, L.S.; Katz, J.; Gong, Y. Medication-Related Osteonecrosis of the Jaw in Cancer Patients: Result from the OneFlorida Clinical Research Consortium. J. Bone Miner. Res. 2022, 37, 2466–2471. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Calabrese, G.; Paterniti, I.; Campolo, M.; Lanza, M.; Capra, A.P.; Pantaleo, L.; Munaò, S.; Colarossi, L.; Forte, S.; et al. The Biological Function of MicroRNAs in Bone Tumors. Int. J. Mol. Sci. 2022, 23, 2348. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, B.; Li, J.; Yuan, H. lncRNA OIP5-AS1 attenuates the osteoarthritis progression in IL-1β-stimulated chondrocytes. Open Med. 2023, 18, 20230721. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, H.; Pan, L.; Han, Y.; Chen, Y.; Jiang, Y.; Wang, Y. LncRNA OIP5-AS1/miR-410-3p/Wnt7b axis promotes the proliferation of rheumatoid arthritis fibroblast-like synoviocytes via regulating the Wnt/β-catenin pathway. Autoimmunity 2023, 56, 2189136. [Google Scholar] [CrossRef]
- Ferreira, L.H., Jr.; Mendonça, K.D., Jr.; Chaves de Souza, J.; Soares Dos Reis, D.C.; do Carmo Faleiros Veloso Guedes, C.; de Souza Castro Filice, L.; Bruzadelli Macedo, S.; Soares Rocha, F. Bisphosphonate-associated osteonecrosis of the jaw. Minerva Dent. Oral Sci. 2021, 70, 49–57. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, R.K. Evolution and etiopathogenesis of bisphosphonates induced osteonecrosis of the jaw. N. Am. J. Med Sci. 2013, 5, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Yamashita, J. Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice. Bone 2013, 56, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Seemann, R.; Klug, C.; Ewers, R.; Millesi, G.; Baumann, A.; Wutzl, A. Long-term success of surgery in bisphosphonate-related osteonecrosis of the jaws (BRONJs). Oral Oncol. 2013, 49, 66–70. [Google Scholar] [CrossRef]
- Myoken, Y.; Fujita, Y.; Kawamoto, K.; Toratani, S. Osteonecrosis of the jaw in a metastatic lung cancer patient with bone metastases undergoing pembrolizumab + denosumab combination therapy: Case report and literature review. Oral Oncol. 2020, 111, 104874. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mattheos, N.; Deng, C.; Su, C.; Wang, Z.; Luo, N.; Tang, H. Management of medication-related osteonecrosis of jaw: Comparison between icariin and teriparatide in a rat model. J. Periodontol. 2021, 92, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.d.B.; de Oliveira, C.; Brizeno, L.; Wong, D.; Júnior, R.L.; Gonçalves, R.; Sousa, F.; Mota, M.; Ribeiro, R.d.A.; Alves, A. Immune cellular profile of bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2016, 22, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Tasso, L.; Azambuja, A.A.; Figueiredo, M.A.; Salum, F.G.; da Silva, V.D.; Cherubini, K. Effect of hyperbaric oxygen therapy on tooth extraction sites in rats subjected to bisphosphonate therapy—Histomorphometric and immunohistochemical analysis. Clin. Oral Investig. 2017, 21, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, M.; Xing, Y.; Duan, Y.; Zhang, S.; Deng, B.; Xiang, X.; Zhou, B. Cyasterone has a protective effect on steroid-induced Osteonecrosis of the femoral head. PLoS ONE 2023, 18, e0293530. [Google Scholar] [CrossRef]
- Peng, P.; Wang, X.; Qiu, C.; Zheng, W.; Zhang, H. Extracellular vesicles from human umbilical cord mesenchymal stem cells prevent steroid-induced avascular necrosis of the femoral head via the PI3K/AKT pathway. Food Chem. Toxicol. 2023, 180, 114004. [Google Scholar] [CrossRef]
- Long, J.; Yao, Z.; Zhang, W.; Liu, B.; Chen, K.; Li, L.; Teng, B.; Du, X.; Li, C.; Yu, X.; et al. Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black Phosphorus Scaffolds for Bone Regeneration. Adv. Sci. 2023, 10, e2302539. [Google Scholar] [CrossRef]
- Yang, J.-G.; Sun, B.; Wang, Z.; Li, X.; Gao, J.-H.; Qian, J.-J.; Li, J.; Wei, W.-J.; Zhang, P.; Wang, W. Exosome-targeted delivery of METTL14 regulates NFATc1 m6A methylation levels to correct osteoclast-induced bone resorption. Cell Death Dis. 2023, 14, 738. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Chun, J.; Al-Nawas, B.; Ziebart, T.; Kwon, Y.-D. Geranylgeraniol Application in Human Osteoblasts and Osteoclasts for Reversal of the Effect of Bisphosphonates. Life 2023, 13, 1353. [Google Scholar] [CrossRef]
- Shin, B.; Hrdlicka, H.C.; Delany, A.M.; Lee, S.-K. Inhibition of miR-29 Activity in the Myeloid Lineage Increases Response to Calcitonin and Trabecular Bone Volume in Mice. Endocrinology 2021, 162, bqab135. [Google Scholar] [CrossRef]
- Li, M.-H.; Wu, Z.-Y.; Wang, Y.; Chen, F.-Z.; Liu, Y. Expression of miR-29 and STAT3 in osteosarcoma and its effect on proliferation regulation of osteosarcoma cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7275–7282. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Z.; Zhu, X.; Xu, R.; Xu, Y. miR-29 Family Inhibits Resistance to Methotrexate and Promotes Cell Apoptosis by Targeting COL3A1 and MCL1 in Osteosarcoma. J. Pharmacol. Exp. Ther. 2018, 24, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Ren, J.B.; Liu, M.; Yu, L. Targeting miR-29 induces apoptosis of osteosarcoma MG-63 cells via regulation of TGF-β1/PUMA signal. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3552–3560. [Google Scholar] [PubMed]
- Shen, Y.; Jiang, B.; Luo, B.; Jiang, X.; Zhang, Y.; Wang, Q. Circular RNA-FK501 binding protein 51 boosts bone marrow mesenchymal stem cell proliferation and osteogenic differentiation via modulating microRNA-205-5p/Runt-associated transcription factor 2 axis. J. Orthop. Surg. Res. 2023, 18, 782. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Yao, J.; Jin, Y.; Song, X.; Meng, Q.; Wu, J.; Liu, Q.; Liu, M.; Sun, H. Circ_0114581 promotes osteogenic differentiation of BMSCs via the MiR-155-5p/HNRNPA3 axis. Life Sci. 2023, 333, 122127. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, J.; Chen, K.; Kang, W.; Zhu, F. Circ_0000396 suppresses the proliferation and inflammation of rheumatoid arthritis synovial fibroblasts by targeting miR-574-5p/RSPO1 axis. J. Orthop. Surg. Res. 2023, 18, 718. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, Y.; Ma, C.; Lv, Q. Competitive endogenous network of circRNA, lncRNA, and miRNA in osteosarcoma chemoresistance. Eur. J. Med Res. 2023, 28, 354. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, X.; Lang, C.; Yuan, X.; Huang, J.; Li, Z.; Xu, M.; Wu, K.; Zhou, C.; Li, Q.; et al. CircFam190a: A critical positive regulator of osteoclast differentiation via enhancement of the AKT1/HSP90β complex. Exp. Mol. Med. 2023, 55, 2051–2066. [Google Scholar] [CrossRef]
- Woodruff, R.; Parekh, F.; Lamb, K.; Mekkaoui, L.; Allen, C.; Smetanova, K.; Huang, J.; Williams, A.; Toledo, G.S.; Lilova, K.; et al. Large-scale manufacturing of base-edited chimeric antigen receptor T cells. Mol. Ther. Methods Clin. Dev. 2023, 31, 101123. [Google Scholar] [CrossRef]
- Hjazi, A.; Sukmana, B.I.; Ali, S.S.; Alsaab, H.O.; Gupta, J.; Ullah, M.I.; Romero-Parra, R.M.; Alawadi, A.H.; Alazbjee, A.A.A.; Mustafa, Y.F. Functional role of circRNAs in osteogenesis: A review. Int. Immunopharmacol. 2023, 121, 110455. [Google Scholar] [CrossRef]
- Barrette, L.-X.; Suresh, N.; Salmon, M.K.; De Ravin, E.; Harris, J.; Kamdar, R.; Moreira, A.G.; Rajasekaran, K. Assessment of clinical guidelines for medication-related osteonecrosis of the jaw: Current status and future directions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 717–724. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Nørholt, S.E.; Rejnmark, L.; Langdahl, B.; Starch-Jensen, T.; Sørensen, H.T. Genome-wide association study of osteonecrosis of the jaw in Danish patients receiving antiresorptive therapy for osteoporosis: A case-control study. Bone Rep. 2022, 18, 101648. [Google Scholar] [CrossRef]
- Han, N.; Li, Z. Non-coding RNA Identification in Osteonecrosis of the Femoral Head Using Competitive Endogenous RNA Network Analysis. Orthop. Surg. 2021, 13, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wei, W.; Zhao, B.; Guo, X.; Liu, S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS ONE 2017, 12, e0169097. [Google Scholar] [CrossRef] [PubMed]
- Gonzálvez-García, M.; Rodríguez-Lozano, F.; Villanueva, V.; Segarra-Fenoll, D.; Rodríguez-González, M.; Oñate-Sánchez, R.; Blanquer, M.; Meseguer-Olmo, L.; Moraleda, J. Mesenchymal stem cells and bisphosphonate-related osteonecrosis of the jaw: The future? Oral Dis. 2012, 18, 823–824. [Google Scholar] [CrossRef]
- Otto, M.; Neff, A.; Ziebart, T.; Halling, F. A large animal model of periodontal defects in bisphosphonate-related osteonecrosis of the jaw: A comparison of clinical and radiological findings. J. Periodontal Implant. Sci. 2023, 53, e43. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Sawada, S.; Kojima, Y. Medication-related osteonecrosis of the jaw without osteolysis on computed tomography: A retrospective and observational study. Sci. Rep. 2023, 13, 12890. [Google Scholar] [CrossRef] [PubMed]
- Wichelmann, T.A.; Ahdi, H.S.; Pandravada, S.; Ehrenpreis, E.D. A Summary of the Rare Reports of Osteonecrosis of the Jaw Associated With Tumor Necrosis-α Inhibitors in the United States Food and Drug Administration’s Adverse Event Reporting System Database. J. Oral Maxillofac. Surg. 2023, 81, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Koka, S. Osteonecrosis of the jaw and biomarkers: What do we tell our patients? Int. J. Oral Maxillofac. Implant. 2008, 23, 179–180. [Google Scholar]
- Kim, J.; Cha, I.; Kim, S.; Kim, M. Biomarkers for Bisphosphonate-Related Osteonecrosis of the Jaw. Clin. Implant. Dent. Relat. Res. 2016, 18, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.; Sayáns, M.P.; Gonzalez-Palanca, S.; Chamorro-Petronacci, C.; Bagan, J.; Garcia-Garcia, A. Biomarkers to predict the onset of biphosphonate-related osteonecrosis of the jaw: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, E26–E36. [Google Scholar] [CrossRef] [PubMed]

| miRNA | Target Genes | Over/Down Expression | Effect on Apoptosis |
|---|---|---|---|
| miR-29 | SRGAP2, NFIA, CD93, CALCR | Over | Promotion |
| miR-31-5p | RhoA | Over | Promotion |
| miR-183-5p | HO-1 | Over | Promotion |
| miR-7b-5p | DC-STAMP | Down | Inhibition |
| miR-125a-5p | TRAF6 | Down | Inhibition |
| miR-503-5p | RANK | Down | Inhibition |
| miR-214-3p | Pten | Over | Promotion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caserta, S.; Stagno, F.; Gangemi, S.; Allegra, A. Highlights on the Effects of Non-Coding RNAs in the Osteonecrosis of the Jaw. Int. J. Mol. Sci. 2024, 25, 1598. https://doi.org/10.3390/ijms25031598
Caserta S, Stagno F, Gangemi S, Allegra A. Highlights on the Effects of Non-Coding RNAs in the Osteonecrosis of the Jaw. International Journal of Molecular Sciences. 2024; 25(3):1598. https://doi.org/10.3390/ijms25031598
Chicago/Turabian StyleCaserta, Santino, Fabio Stagno, Sebastiano Gangemi, and Alessandro Allegra. 2024. "Highlights on the Effects of Non-Coding RNAs in the Osteonecrosis of the Jaw" International Journal of Molecular Sciences 25, no. 3: 1598. https://doi.org/10.3390/ijms25031598





