Breath Fingerprint of Colorectal Cancer Patients Based on the Gas Chromatography–Mass Spectrometry Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical Standards and Quality Benchmarks
4.2. Study Group Description and Recruitment Process
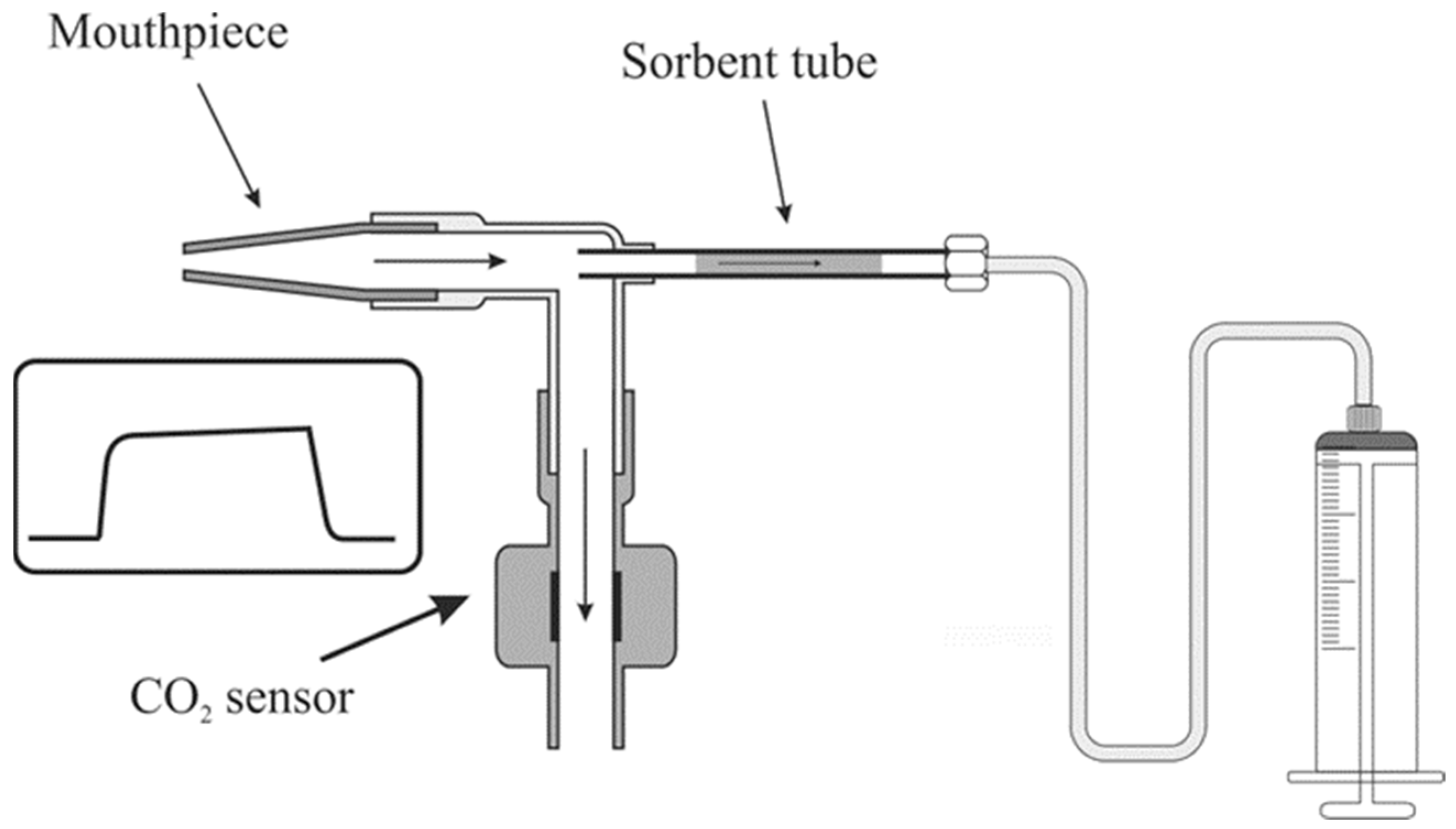
4.3. Breath Sample Collection
4.4. Gas Chromatography–Mass Spectrometry Examination of Breath Samples
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, L.G.; van Rijn, A.F.; Laheij, R.J.; van Oijen, M.G.; Fockens, P.; van Krieken, H.H.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. Random Comparison of Guaiac and Immunochemical Fecal Occult Blood Tests for Colorectal Cancer in a Screening Population. Gastroenterology 2008, 135, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wieten, E.; Schreuders, E.H.; Grobbee, E.J.; Nieboer, D.; Bramer, W.M.; Lansdorp-Vogelaar, I.; Bruno, M.J.; Kuipers, E.J.; Spaander, M.C.W. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: A systematic review and meta-analysis. Gut 2019, 68, 873–881. [Google Scholar] [CrossRef]
- Hol, L.; van Leerdam, M.E.; van Ballegooijen, M.; van Vuuren, A.J.; van Dekken, H.; Reijerink, J.C.I.Y.; van der Togt, A.C.M.; Habbema, J.D.F.; Kuipers, E.J. Screening for colorectal cancer: Randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010, 59, 62–68. [Google Scholar] [CrossRef]
- Issaka, R.B.; Chan, A.T.; Gupta, S. AGA Clinical Practice Update on Risk Stratification for Colorectal Cancer Screening and Post-Polypectomy Surveillance: Expert Review. Gastroenterology 2023, 165, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.S.; Kenny, R.P.; Rees, C.J.; Hamilton, W.; Eastaugh, C.; Richmond, C.; Sharp, L. The performance of FIT-based and other risk prediction models for colorectal neoplasia in symptomatic patients: A systematic review. eClinicalMedicine 2023, 64, 102204. [Google Scholar] [CrossRef]
- Benton, S.C.; Symonds, E.; Djedovic, N.; Jones, S.; Deprez, L.; Kocna, P.; Auge, J.M. Faecal immunochemical tests for haemoglobin: Analytical challenges and potential solutions. Clin. Chim. Acta 2021, 517, 60–65. [Google Scholar] [CrossRef]
- Widlak, M.M.; Thomas, C.L.; Thomas, M.G.; Tomkins, C.; Smith, S.; O’Connell, N.; Wurie, S.; Burns, L.; Harmston, C.; Evans, C.; et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment. Pharmacol. Ther. 2017, 45, 354–363. [Google Scholar] [CrossRef]
- Schreuders, E.; Ruco, A.; Rabeneck, L. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- Warren, J.L.; Klabunde, C.N.; Mariotto, A.B.; Meekins, A.; Topor, M.; Brown, M.L.; Ransohoff, D.F. Adverse Events After Outpatient Colonoscopy in the Medicare Population. Ann. Intern. Med. 2009, 150, 849–857. [Google Scholar] [CrossRef]
- van Hees, F.; Zauber, A.G.; Klabunde, C.N.; Goede, S.L.; Lansdorp-Vogelaar, I.; van Ballegooijen, M. The appropriateness of more intensive colonoscopy screening than recommended in Medicare beneficiaries: A modeling study. JAMA Intern. Med. 2014, 174, 1568–1576. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef]
- Church, T.R.; Wandell, M.; Lofton-Day, C.; Mongin, S.J.; Burger, M.; Payne, S.R.; Castaños-Vélez, E.; Blumenstein, B.A.; Rösch, T.; Osborn, N.; et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut 2014, 63, 317–325. [Google Scholar] [CrossRef]
- Zhou, W.; Tao, J.; Li, J.; Tao, S. Volatile organic compounds analysis as a potential novel screening tool for colorectal cancer: A systematic review and meta-analysis. Medicine 2020, 99, e20937. [Google Scholar] [CrossRef]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Non-Invasive Detection and Staging of Colorectal Cancer Using a Portable Electronic Nose. Sensors 2021, 21, 5440. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; De Vietro, N. Chemical signature of colorectal cancer: Case–control study for profiling the breath print. BJS Open 2020, 4, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mezmale, L.; Leja, M.; Lescinska, A.M.; Pčolkins, A.; Kononova, E.; Bogdanova, I.; Polaka, I.; Stonans, I.; Kirsners, A.; Ager, C.; et al. Identification of Volatile Markers of Colorectal Cancer from Tumor Tissues Using Volatilomic Approach. Molecules 2023, 28, 5990. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.; Turner, C. Breath Analysis in Disease Diagnosis: Methodological Considerations and Applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME–GC–TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Giró Benet, J.; Seo, M.; Khine, M.; Gumà Padró, J.; Pardo Martnez, A.; Kurdahi, F. Breast cancer detection by analyzing the volatile organic compound (VOC) signature in human urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef]
- Mochalski, P.; Leja, M.; Ślefarska-Wolak, D.; Mezmale, L.; Patsko, V.; Ager, C.; Królicka, A.; Mayhew, C.A.; Shani, G.; Haick, H. Identification of Key Volatile Organic Compounds Released by Gastric Tissues as Potential Non-Invasive Biomarkers for Gastric Cancer. Diagnostics 2023, 13, 335. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Fabiani, S.; Stefanelli, G.; Longo, S.; Viscido, A.; Latella, G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers 2021, 13, 2361. [Google Scholar] [CrossRef]
- Van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.J.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Haick, H. The diagnostic breathprint of cancer; the past and the future. Br. J. Cancer 2022, 128, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 606915. [Google Scholar] [CrossRef]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef]
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Lian, A.; Sun, B.; Wang, X.; Guo, L.; Chi, C.; Liu, S.; Zhao, W.; Luo, S.; et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol. Ther. 2014, 15, 200–206. [Google Scholar] [CrossRef]
- Bond, A.; Greenwood, R.; Lewis, S.; Corfe, B.; Sarkar, S.; O’Toole, P.; Rooney, P.; Burkitt, M.; Hold, G.; Probert, C. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Aliment. Pharmacol. Ther. 2019, 49, 1005–1012. [Google Scholar] [CrossRef]
- Śmiełowska, M.; Ligor, T.; Kupczyk, W.; Szeliga, J.; Jackowski, M.; Buszewski, B. Screening for volatile biomarkers of colorectal cancer by analyzing breath and fecal samples using thermal desorption combined with GC-MS (TD-GC-MS). J. Breath Res. 2023, 17, 047102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Klieber, M.; Unterkofler, K.; Hinterhuber, H.; Baumann, M.; Amann, A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst 2013, 138, 2134–2145. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, M.; Jahangiri-Manesh, A.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Detection of hexanal gas as a volatile organic compound cancer biomarker using a nanocomposite of gold nanoparticles and selective polymers. J. Electroanal. Chem. 2022, 905, 115962. [Google Scholar] [CrossRef]
- Kure, S.; Satoi, S.; Kitayama, T.; Nagase, Y.; Nakano, N.; Yamada, M.; Uchiyama, N.; Miyashita, S.; Iida, S.; Takei, H.; et al. A prediction model using 2-propanol and 2-butanone in urine distinguishes breast cancer. Sci. Rep. 2021, 11, 19801. [Google Scholar] [CrossRef] [PubMed]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Salehi, L.B.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef] [PubMed]
- Van Vorstenbosch, R.; Cheng, H.R.; Jonkers, D.; Penders, J.; Schoon, E.; Masclee, A.; van Schooten, F.-J.; Smolinska, A.; Mujagic, Z. Systematic Review: Contribution of the Gut Microbiome to the Volatile Metabolic Fingerprint of Colorectal Neoplasia. Metabolites 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]

| Cancer Group, n (%) | Control Group, n (%) | Total, n (%) | p-Value | ||
|---|---|---|---|---|---|
| Gender | Females | 31 (44.0%) | 40 (56.0%) | 71 (100.0%) | 0.836 |
| Males | 47 (58.0%) | 34 (42.0%) | 81 (100.0%) | ||
| Total included | 78 (51.0%) | 74 (49.0%) | 152 (100.0%) | - | |
| Cancer stage | I | 16 (21.0%) | - | - | - |
| II | 37 (47.0%) | - | - | ||
| III | 19 (24.0%) | - | - | ||
| IV | 6 (8.0%) | - | - | ||
| Cancer differentiation grade | 1 | 23 (29.0%) | - | - | - |
| 2 | 43 (55.0%) | - | - | ||
| 3 | 12 (15.0%) | - | - | ||
| Chemical Class | Compound Name (CAS, Occurrence of Cancer/Non-Cancer (%)) |
|---|---|
| Aldehydes | hexanal (66-25-1; 87/93), dodecanal (112-54-9; 73/82) |
| Esters | diethyl phthalate (84-66-2; 87/93) |
| Ketones | 6-methyl-5-hepten-2-one, (110-93-0; 83/92), cyclohexanone (108-94-1; 74/76) |
| Hydrocarbons | hexadecane (544-76-3; 86/92), tetradecane (629-59-4; 81/84), nonane (111-84-2; 78/82), 1-nonene (124-11-8; 63/59) |
| Alcohols | 1-butanol (71-36-3; 87/92), benzyl alcohol (100-51-6; 81/82), 1-dodecanol (112-53-8; 50/58) |
| Aromatics | ethylbenzene (100-41-4; 79/90), toluene (108-88-3; 85/88), p-xylene (106-42-3; 73/80), benzene (71-43-2; 73/80) |
| Heterocyclic | 2-methyl-1,3-dioxolane (497-26-7; 58/54) |
| Compound Name | CAS | p-Value | Median (Q25,Q75) Breath Gradient | ||
|---|---|---|---|---|---|
| Level in CRC Group Compared to Control Group | Controls | CRC Patients | |||
| p-xylene | 106-42-3 | 0.0005 | ↑ | 1,504,742 (936,665–2,127,623) | 2,289,566 (1,418,724–3,064,362) |
| hexanal | 66-25-1 | 0.0012 | ↑ | 1,091,070 (823,273–1,314,062) | 1,369,804 (1,076,601–1,605,449) |
| 2-methyl-1,3-dioxolane | 497-26-7 | 0.0024 | ↑ | 331,109 (198,932–454,532) | 484,592 (335,398–768,482) |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | 0.0025 | ↑ | 1,030,684 (742,621–1,417,747) | 1,341,632 (1,038,423–1,807,558) |
| hexadecane | 544-76-3 | 0.0026 | ↑ | 838,697 (631,009–1,890,079) | 1,598,483 (905,689–1,989,248) |
| nonane | 111-84-2 | 0.0028 | ↑ | 354,633 (244,656–529,638) | 484,690 (330,122–799,694) |
| ethylbenzene | 100-41-4 | 0.0028 | ↑ | 357,817 (161,941–570,524) | 596,334 (278,377–840,950) |
| cyclohexanone | 108-94-1 | 0.0045 | ↑ | 164,463 (95,647–245,373) | 210,847 (154,565–373,447) |
| diethyl phthalate | 84-66-2 | 0.0060 | ↑ | 2,372,295 (1,244,073–4,668,721) | 3,810,126 (2,186,709–6,749,612) |
| 6-methyl-5-hepten-2-one | 110-93-0 | 0.0076 | ↑ | 655,879 (349,775–1,046,236) | 852,666 (581,498–1,227,127) |
| tetrahydro-2h-pyran-2-one | 542-28-9 | 0.0093 | ↑ | 183,202 (159,649–232,003) | 274,253 (198,146–419,828) |
| 2-butanone | 78-93-3 | 0.0109 | ↑ | 485,529 (338,060–609,519) | 690,855 (445,338–901,846) |
| benzaldehyde | 100-52-7 | 0.0126 | ↑ | 4721459 (3,876,629–5,921,829) | 5,998,416 (4,588,636–7,960,831) |
| dodecanal | 112-54-9 | 0.0127 | ↑ | 561,576 (413,502–769,154) | 735,169 (519,829–876,106) |
| benzothiazole | 95-16-9 | 0.0148 | ↑ | 158,701 (133,720–195,510) | 199,243 (155,950–273,625) |
| tetradecane | 629-59-4 | 0.0178 | ↑ | 1,098,813 (760,748–1,927,047) | 1,521,759 (1,143,601–2,151,569) |
| 1-dodecanol | 112-53-8 | 0.0202 | ↑ | 544,799 (426,605–684,038) | 712,005 (489,257–908,544) |
| benzene | 71-43-2 | 0.0280 | ↑ | 1,148,309 (769,348–2,120,718) | 1,830,674 (1,112,987–2,508,011) |
| 3-methylcyclopentyl acetate | 24070-70-0 | 0.0322 | ↑ | 319,377 (270,008–397,761) | 406,271 (298,582–480,658) |
| 1-nonene | 124-11-8 | 0.0342 | ↑ | 247,535 (177,798–337,746) | 318,057 (226,743–477,655) |
| toluene | 108-88-3 | 0.0457 | ↑ | 1,192,237 (793,122–2,220,208) | 1,553,576 (1,114,674–2,548,652) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononova, E.; Mežmale, L.; Poļaka, I.; Veliks, V.; Anarkulova, L.; Vilkoite, I.; Tolmanis, I.; Ļeščinska, A.M.; Stonāns, I.; Pčolkins, A.; et al. Breath Fingerprint of Colorectal Cancer Patients Based on the Gas Chromatography–Mass Spectrometry Analysis. Int. J. Mol. Sci. 2024, 25, 1632. https://doi.org/10.3390/ijms25031632
Kononova E, Mežmale L, Poļaka I, Veliks V, Anarkulova L, Vilkoite I, Tolmanis I, Ļeščinska AM, Stonāns I, Pčolkins A, et al. Breath Fingerprint of Colorectal Cancer Patients Based on the Gas Chromatography–Mass Spectrometry Analysis. International Journal of Molecular Sciences. 2024; 25(3):1632. https://doi.org/10.3390/ijms25031632
Chicago/Turabian StyleKononova, Elīna, Linda Mežmale, Inese Poļaka, Viktors Veliks, Linda Anarkulova, Ilona Vilkoite, Ivars Tolmanis, Anna Marija Ļeščinska, Ilmārs Stonāns, Andrejs Pčolkins, and et al. 2024. "Breath Fingerprint of Colorectal Cancer Patients Based on the Gas Chromatography–Mass Spectrometry Analysis" International Journal of Molecular Sciences 25, no. 3: 1632. https://doi.org/10.3390/ijms25031632
APA StyleKononova, E., Mežmale, L., Poļaka, I., Veliks, V., Anarkulova, L., Vilkoite, I., Tolmanis, I., Ļeščinska, A. M., Stonāns, I., Pčolkins, A., Mochalski, P., & Leja, M. (2024). Breath Fingerprint of Colorectal Cancer Patients Based on the Gas Chromatography–Mass Spectrometry Analysis. International Journal of Molecular Sciences, 25(3), 1632. https://doi.org/10.3390/ijms25031632








