Edaravone: A Novel Possible Drug for Cancer Treatment?
Abstract
1. Introduction
2. Conventional Therapies for Cancer
2.1. Surgical Removal
2.2. Chemotherapy
2.3. Radiotherapy
2.4. Hematopoietic Stem Cell Transplantation (HSTC)
2.5. Hormone Therapy
2.6. Immunotherapy
2.7. Targeted Therapy
2.8. Precision Medicine
3. Oxidative Stress in Cancer
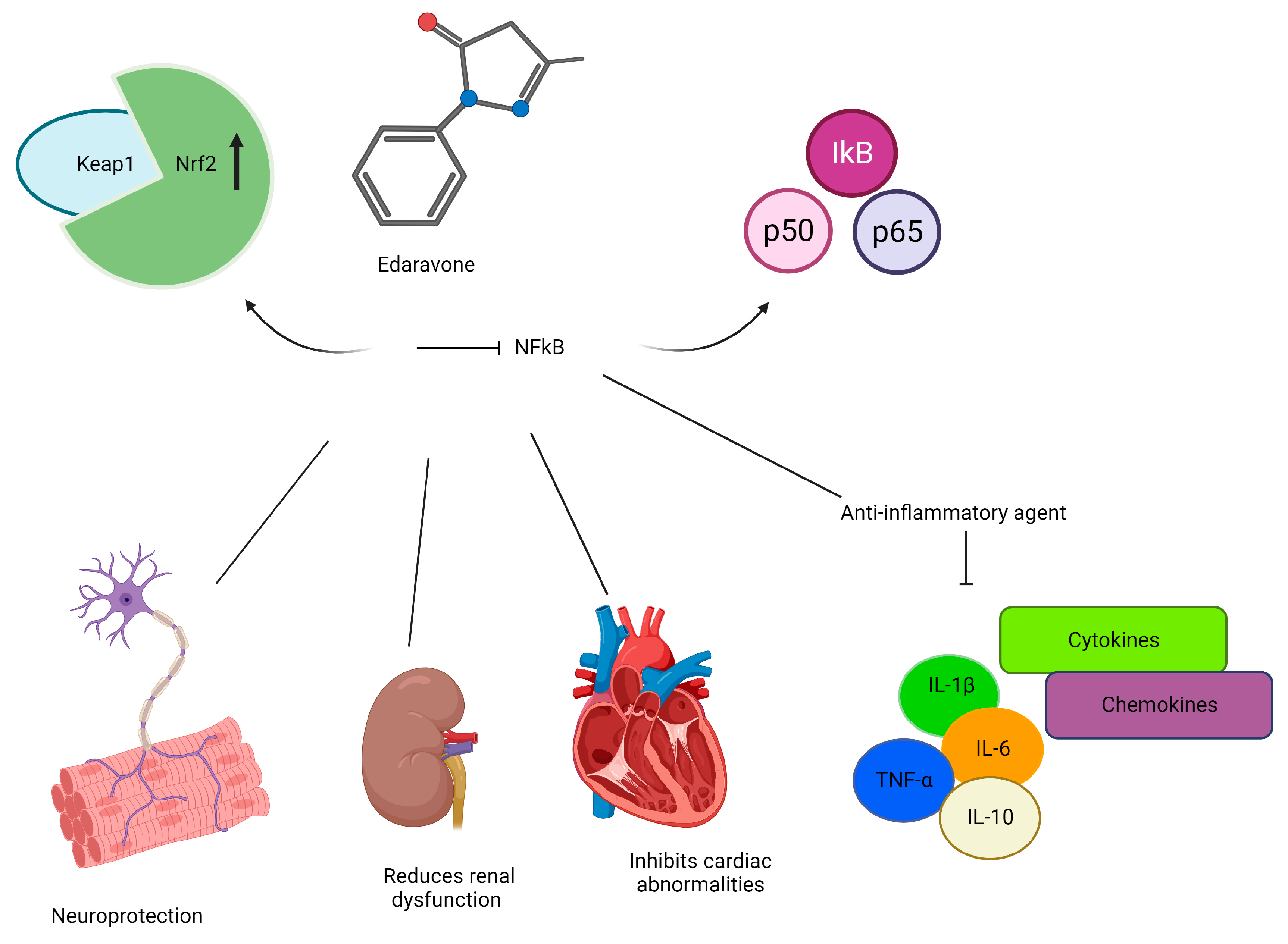
4. Edaravone
4.1. Mechanisms of Action
4.2. Anticancer Effects
4.3. Cytoprotective Effects against Conventional Cancer Therapies
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Chryplewicz, A.; Scotton, J.; Tichet, M.; Zomer, A.; Shchors, K.; Joyce, J.A.; Homicsko, K.; Hanahan, D. Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity. Cancer Cell 2022, 40, 1111–1127.e1119. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Ogrinc, N.; Saudemont, P.; Takats, Z.; Salzet, M.; Fournier, I. Cancer Surgery 2.0: Guidance by Real-Time Molecular Technologies. Trends Mol. Med. 2021, 27, 602–615. [Google Scholar] [CrossRef]
- Saidak, Z.; Piazza, C. Editorial: Oral Oncology: From Precise Surgery to Precision Medicine and Surgery. Front. Oral. Health 2022, 3, 913172. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Tan, Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med. Sci. Monit. 2019, 25, 3537–3541. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, V.; Capici, S.; Pepe, F.F.; di Mauro, P.; Riva, F.; Cicchiello, F.; Maggioni, C.; Cordani, N.; Cerrito, M.G.; Cazzaniga, M.E. How to Treat HR+/HER2- Metastatic Breast Cancer Patients after CDK4/6 Inhibitors: An Unfinished Story. Life 2022, 12, 378. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Chawla, A.; Sharma, P.; Mir, P.A.; Potoo, F.H.; Reiner, Ž.; Reiner, I.; Ateşşahin, D.A.; Sharifi-Rad, J.; Mir, R.H.; et al. Repurposing approved non-oncology drugs for cancer therapy: A comprehensive review of mechanisms, efficacy, and clinical prospects. Eur. J. Med. Res. 2023, 28, 345. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Ravi Kumar, B.V.V.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef]
- Park, K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef]
- Bailly, C. Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers. Int. Immunopharmacol. 2019, 77, 105967. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Padma, V.V. An overview of targeted cancer therapy. Biomedicine 2015, 5, 19. [Google Scholar] [CrossRef]
- Stiggelbout, A.M.; de Haes, J.C. Patient preference for cancer therapy: An overview of measurement approaches. J. Clin. Oncol. 2001, 19, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Beecher, S.M.; O’Leary, D.P.; McLaughlin, R.; Kerin, M.J. The Impact of Surgical Complications on Cancer Recurrence Rates: A Literature Review. Oncol. Res. Treat. 2018, 41, 478–482. [Google Scholar] [CrossRef] [PubMed]
- LiBrizzi, C.L.; Levin, A.S.; Strike, S.A.; Morris, C.D. Indications and outcomes of palliative major amputation in patients with metastatic cancer. Surg. Oncol. 2022, 40, 101700. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.K.; Yang, Z.L.; Peng, J.S.; Lin, H.S.; Cai, L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: Evidence from randomized and nonrandomized clinical trials. Ann. Surg. 2012, 256, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Bae, J.M.; An, J.Y.; Hyung, W.J.; Noh, S.H. Laparoscopic gastrectomy for advanced gastric cancer: Are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J. Surg. Oncol. 2013, 108, 550–556. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Heo, J.; Jeon, S.W.; Jung, M.K.; Kim, S.K.; Kim, J.; Kim, S. Endoscopic resection as the first-line treatment for early colorectal cancer: Comparison with surgery. Surg. Endosc. 2014, 28, 3435–3442. [Google Scholar] [CrossRef]
- Belderbos, T.D.; van Erning, F.N.; de Hingh, I.H.; van Oijen, M.G.; Lemmens, V.E.; Siersema, P.D. Long-term Recurrence-free Survival After Standard Endoscopic Resection Versus Surgical Resection of Submucosal Invasive Colorectal Cancer: A Population-based Study. Clin. Gastroenterol. Hepatol. 2017, 15, 403–411.e401. [Google Scholar] [CrossRef]
- Jahangeer, S.; Forde, P.; Soden, D.; Hinchion, J. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat. Rev. 2013, 39, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public. Health 2018, 47, 1218–1219. [Google Scholar]
- Ahles, T.A.; Root, J.C. Cognitive Effects of Cancer and Cancer Treatments. Annu. Rev. Clin. Psychol. 2018, 14, 425–451. [Google Scholar] [CrossRef]
- Jansen, C.E.; Miaskowski, C.; Dodd, M.; Dowling, G.; Kramer, J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 2005, 104, 2222–2233. [Google Scholar] [CrossRef]
- Blumenstein, K.G.; Brose, A.; Kemp, C.; Meister, D.; Walling, E.; DuVall, A.S.; Zhang, A. Effectiveness of cognitive behavioral therapy in improving functional health in cancer survivors: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2022, 175, 103709. [Google Scholar] [CrossRef]
- Joly, F.; Lange, M.; Dos Santos, M.; Vaz-Luis, I.; Di Meglio, A. Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors. Cancers 2019, 11, 1896. [Google Scholar] [CrossRef]
- Tamburin, S.; Park, S.B.; Alberti, P.; Demichelis, C.; Schenone, A.; Argyriou, A.A. Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S40–S51. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Keime-Guibert, F.; Napolitano, M.; Delattre, J.Y. Neurological complications of radiotherapy and chemotherapy. J. Neurol. 1998, 245, 695–708. [Google Scholar] [CrossRef]
- Cordani, N.; Lisini, D.; Coccè, V.; Paglia, G.; Meanti, R.; Cerrito, M.G.; Tettamanti, P.; Bonaffini, L.; Paino, F.; Alessandri, G.; et al. Conditioned Medium of Mesenchymal Stromal Cells Loaded with Paclitaxel Is Effective in Preclinical Models of Triple-Negative Breast Cancer (TNBC). Int. J. Mol. Sci. 2023, 24, 5864. [Google Scholar] [CrossRef]
- Coccè, V.; Bonelli, M.; La Monica, S.; Alfieri, R.; Doneda, L.; Martegani, E.; Alessandri, G.; Lagrasta, C.A.; Giannì, A.; Sordi, V.; et al. Mesenchymal stromal cells loaded with Paclitaxel (PacliMES) a potential new therapeutic approach on mesothelioma. Biochem. Pharmacol. 2023, 214, 115678. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Chaput, G.; Regnier, L. Radiotherapy: Clinical pearls for primary care. Can. Fam. Physician 2021, 67, 753–757. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dropcho, E.J. Neurotoxicity of radiation therapy. Neurol. Clin. 2010, 28, 217–234. [Google Scholar] [CrossRef]
- Wujanto, C.; Vellayappan, B.; Chang, E.L.; Chao, S.T.; Sahgal, A.; Lo, S.S. Radiotherapy to the brain: What are the consequences of this age-old treatment? Ann. Palliat. Med. 2021, 10, 936–952. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Fu, J. Treatment of Radiation-Induced Brain Necrosis. Oxid. Med. Cell Longev. 2021, 2021, 4793517. [Google Scholar] [CrossRef]
- Khaddour, K.; Hana, C.K.; Mewawalla, P. Hematopoietic Stem Cell Transplantation; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Pollyea, D.A.; Stone, R.M.; Altman, J.K.; Roboz, G.J.; Patel, M.R.; Collins, R.; Flinn, I.W.; et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 2019, 133, 676–687. [Google Scholar] [CrossRef]
- van Besien, K.; Sobocinski, K.A.; Rowlings, P.A.; Murphy, S.C.; Armitage, J.O.; Bishop, M.R.; Chaekal, O.K.; Gale, R.P.; Klein, J.P.; Lazarus, H.M.; et al. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood 1998, 92, 1832–1836. [Google Scholar]
- Philip, T.; Guglielmi, C.; Hagenbeek, A.; Somers, R.; Van der Lelie, H.; Bron, D.; Sonneveld, P.; Gisselbrecht, C.; Cahn, J.Y.; Harousseau, J.L.; et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1995, 333, 1540–1545. [Google Scholar] [CrossRef]
- Nastasi, N.; Bruno, G.; Favre, C.; Calvani, M. Role of β3-Adrenergic Receptor in Bone Marrow Transplant as Therapeutical Support in Cancer. Front. Oncol. 2022, 12, 889634. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Gumusay, O.; Idossa, D.; Rugo, H.S. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J. Clin. 2023, 73, 480–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, R.; Zeng, F.; Zhao, J.; Peng, S.; Ma, Y.; Chen, S.; Ding, S.; Zhong, L.; Guo, W.; et al. Impact of molecular subtypes on metastatic behavior and overall survival in patients with metastatic breast cancer: A single-center study combined with a large cohort study based on the Surveillance, Epidemiology and End Results database. Oncol. Lett. 2020, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama 2005, 294, 433–439. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef]
- Storey, D.J.; McLaren, D.B.; Atkinson, M.A.; Butcher, I.; Frew, L.C.; Smyth, J.F.; Sharpe, M. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann. Oncol. 2012, 23, 1542–1549. [Google Scholar] [CrossRef]
- Grossmann, M.; Zajac, J.D. Hematological changes during androgen deprivation therapy. Asian J. Androl. 2012, 14, 187–192. [Google Scholar] [CrossRef]
- Shore, N.D.; Moul, J.W.; Pienta, K.J.; Czernin, J.; King, M.T.; Freedland, S.J. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: Treatment based on risk stratification. Prostate Cancer Prostatic Dis. 2023. [Google Scholar] [CrossRef]
- Berruti, A.; Dogliotti, L.; Terrone, C.; Cerutti, S.; Isaia, G.; Tarabuzzi, R.; Reimondo, G.; Mari, M.; Ardissone, P.; De Luca, S.; et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J. Urol. 2002, 167, 2361–2367; discussion 2367. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Vergati, M.; Intrivici, C.; Huen, N.Y.; Schlom, J.; Tsang, K.Y. Strategies for cancer vaccine development. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Cordani, N.; Bianchi, T.; Ammoni, L.C.; Cortinovis, D.L.; Cazzaniga, M.E.; Lissoni, A.A.; Landoni, F.; Canova, S. An Overview of PARP Resistance in Ovarian Cancer from a Molecular and Clinical Perspective. Int. J. Mol. Sci. 2023, 24, 11890. [Google Scholar] [CrossRef]
- Cordani, N.; Mologni, L.; Piazza, R.; Tettamanti, P.; Cogliati, V.; Mauri, M.; Villa, M.; Malighetti, F.; Di Bella, C.; Jaconi, M.; et al. TWIST1 Upregulation Is a Potential Target for Reversing Resistance to the CDK4/6 Inhibitor in Metastatic Luminal Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 16294. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar] [PubMed]
- Cortinovis, D.L.; Colonese, F.; Abbate, M.I.; Sala, L.; Meazza Prina, M.; Cordani, N.; Sala, E.; Canova, S. Harnessing DLL3 inhibition: From old promises to new therapeutic horizons. Front. Med. 2022, 9, 989405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M. Targeted therapies for cancer. BMC Med. 2022, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.G.; Cortinovis, D.; Agustoni, F.; Arosio, G.; Villa, M.; Cordani, N.; Bidoli, P.; Bisson, W.H.; Pagni, F.; Piazza, R.; et al. A Compound L1196M/G1202R ALK Mutation in a Patient with ALK-Positive Lung Cancer with Acquired Resistance to Brigatinib Also Confers Primary Resistance to Lorlatinib. J. Thorac. Oncol. 2019, 14, e257–e259. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, S.; Kumar, P.; Ramprasad, V.; Chaudhuri, A. Evolution of targeted therapies in cancer: Opportunities and challenges in the clinic. Future Oncol. 2015, 11, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Rulten, S.L.; Grose, R.P.; Gatz, S.A.; Jones, J.L.; Cameron, A.J.M. The Future of Precision Oncology. Int. J. Mol. Sci. 2023, 24, 12613. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Yang, S.R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2022, 84, 184–198. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Lopes, G.; Cho, B.C.; Kowalski, D.M.; Kasahara, K.; Wu, Y.L.; de Castro, G., Jr.; Turna, H.Z.; Cristescu, R.; Aurora-Garg, D.; et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: Pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann. Oncol. 2023, 34, 377–388. [Google Scholar] [CrossRef]
- Lee, J.M.; Cimino-Mathews, A.; Peer, C.J.; Zimmer, A.; Lipkowitz, S.; Annunziata, C.M.; Cao, L.; Harrell, M.I.; Swisher, E.M.; Houston, N.; et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women’s Cancers: A Dose-Escalation, Phase I Study. J. Clin. Oncol. 2017, 35, 2193–2202. [Google Scholar] [CrossRef]
- Schuler, M.; Awada, A.; Harter, P.; Canon, J.L.; Possinger, K.; Schmidt, M.; De Grève, J.; Neven, P.; Dirix, L.; Jonat, W.; et al. A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Res. Treat. 2012, 134, 1149–1159. [Google Scholar] [CrossRef]
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef]
- Valenti, F.; Falcone, I.; Ungania, S.; Desiderio, F.; Giacomini, P.; Bazzichetto, C.; Conciatori, F.; Gallo, E.; Cognetti, F.; Ciliberto, G.; et al. Precision Medicine and Melanoma: Multi-Omics Approaches to Monitoring the Immunotherapy Response. Int. J. Mol. Sci. 2021, 22, 3837. [Google Scholar] [CrossRef]
- Riedesser, J.E.; Ebert, M.P.; Betge, J. Precision medicine for metastatic colorectal cancer in clinical practice. Ther. Adv. Med. Oncol. 2022, 14, 17588359211072703. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Siska, P.J.; Beckermann, K.E.; Mason, F.M.; Andrejeva, G.; Greenplate, A.R.; Sendor, A.B.; Chiang, Y.J.; Corona, A.L.; Gemta, L.F.; Vincent, B.G.; et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2017, 2, e93411. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of anti therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Perera, N.D.; Beart, P.M.; Turner, B.J.; Shabanpoor, F. Amyotrophic Lateral Sclerosis and Autophagy: Dysfunction and Therapeutic Targeting. Cells 2020, 9, 2413. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A.; Lomax-Browne, H.J.; Carter, T.M.; Kinch, C.E.; Hall, D.M. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010, 112, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Chatterjee, J. ROS and oncogenesis with special reference to EMT and stemness. Eur. J. Cell Biol. 2020, 99, 151073. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kaur, J.; Vashishth, K.; Sak, K.; Sharma, U.; Choudhary, R.; Behl, T.; Singh, T.; Sharma, S.; Saini, A.K.; et al. Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis. Arch. Toxicol. 2023, 97, 103–120. [Google Scholar] [CrossRef]
- Chitty, J.L.; Filipe, E.C.; Lucas, M.C.; Herrmann, D.; Cox, T.R.; Timpson, P. Recent advances in understanding the complexities of metastasis. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef]
- Kamiya, T.; Goto, A.; Kurokawa, E.; Hara, H.; Adachi, T. Cross Talk Mechanism among EMT, ROS, and Histone Acetylation in Phorbol Ester-Treated Human Breast Cancer MCF-7 Cells. Oxid. Med. Cell Longev. 2016, 2016, 1284372. [Google Scholar] [CrossRef]
- Shin, D.H.; Dier, U.; Melendez, J.A.; Hempel, N. Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim. Biophys. Acta 2015, 1852, 2593–2602. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Inflammation: A driving force speeds cancer metastasis. Cell Cycle 2009, 8, 3267–3273. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.; Hu, X. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem. Pharmacol. 2013, 86, 253–266. [Google Scholar] [CrossRef]
- Pelicano, H.; Lu, W.; Zhou, Y.; Zhang, W.; Chen, Z.; Hu, Y.; Huang, P. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res. 2009, 69, 2375–2383. [Google Scholar] [CrossRef]
- Jin, M.; Wang, J.; Ji, X.; Cao, H.; Zhu, J.; Chen, Y.; Yang, J.; Zhao, Z.; Ren, T.; Xing, J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 136. [Google Scholar] [CrossRef]
- Delierneux, C.; Kouba, S.; Shanmughapriya, S.; Potier-Cartereau, M.; Trebak, M.; Hempel, N. Mitochondrial Calcium Regulation of Redox Signaling in Cancer. Cells 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cai, Y.; Li, S.; Liu, H.; Zhou, X.; Lu, C.; Gao, X.; Qian, J.; Zhang, J.; Ju, S.; et al. Edaravone-Encapsulated Agonistic Micelles Rescue Ischemic Brain Tissue by Tuning Blood-Brain Barrier Permeability. Theranostics 2017, 7, 884–898. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Duranti, E.; Villa, C. Muscle Involvement in Amyotrophic Lateral Sclerosis: Understanding the Pathogenesis and Advancing Therapeutics. Biomolecules 2023, 13, 1582. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, E.; Ren, X.; Bai, X.; Wang, D.; Bai, L.; Luo, D.; Guo, Z.; Wang, Q.; Yang, J. Edaravone alleviates cell apoptosis and mitochondrial injury in ischemia-reperfusion-induced kidney injury via the JAK/STAT pathway. Biol. Res. 2020, 53, 28. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, M.; Miyamoto, Y.; Mitsuno, M.; Tanaka, H.; Ryomoto, M.; Fukui, S. Edaravone Suppresses Reperfusion Injury following Leg Ischemia in Rats: A Transmission Electron Microscopic Study. Int. J. Angiol. 2013, 22, 267–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Apaydin, M.; Erbas, O.; Taskiran, D. Protection by Edaravone, a Radical Scavenger, against Manganese-Induced Neurotoxicity in Rats. J. Biochem. Mol. Toxicol. 2016, 30, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Shaki, F.; Mohammadi, E.; Rezagholizadeh, N.; Ebrahimi, F. Edaravone decreases paraquat toxicity in a549 cells and lung isolated mitochondria. Iran. J. Pharm. Res. 2014, 13, 675–681. [Google Scholar] [PubMed]
- Yamamoto, Y.; Kuwahara, T.; Watanabe, K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 1996, 2, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Iwasaki, Y. Edaravone, a Free Radical Scavenger, Delayed Symptomatic and Pathological Progression of Motor Neuron Disease in the Wobbler Mouse. PLoS ONE 2015, 10, e0140316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Y.; Zhang, G.; Lin, Z.; Du, S. Protective effect of edaravone on blood-brain barrier by affecting NRF-2/HO-1 signaling pathway. Exp. Ther. Med. 2019, 18, 2437–2442. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, K. Effects of the Edaravone, a Drug Approved for the Treatment of Amyotrophic Lateral Sclerosis, on Mitochondrial Function and Neuroprotection. Antioxidants 2022, 11, 195. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, C.; Meng, X.; Li, Z.; Lv, C.; Cao, P. Neuroprotection of edaravone on the hippocampus of kainate-induced epilepsy rats through Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 159–165. [Google Scholar] [CrossRef]
- Zhang, M.; Teng, C.H.; Wu, F.F.; Ge, L.Y.; Xiao, J.; Zhang, H.Y.; Chen, D.Q. Edaravone attenuates traumatic brain injury through anti-inflammatory and anti-oxidative modulation. Exp. Ther. Med. 2019, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Som, A.T.; Pham, L.D.; Lee, B.J.; Mandeville, E.T.; Lo, E.H.; Arai, K. A free radical scavenger edaravone suppresses systemic inflammatory responses in a rat transient focal ischemia model. Neurosci. Lett. 2016, 633, 7–13. [Google Scholar] [CrossRef]
- Yi, R.; Zhizhou, Y.; Zhaorui, S.; Wei, Z.; Xin, C.; Shinan, N. Retrospective study of clinical features and prognosis of edaravone in the treatment of paraquat poisoning. Medicine 2019, 98, e15441. [Google Scholar] [CrossRef]
- Kokura, S.; Yoshida, N.; Sakamoto, N.; Ishikawa, T.; Takagi, T.; Higashihara, H.; Nakabe, N.; Handa, O.; Naito, Y.; Yoshikawa, T. The radical scavenger edaravone enhances the anti-tumor effects of CPT-11 in murine colon cancer by increasing apoptosis via inhibition of NF-kappaB. Cancer Lett. 2005, 229, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Nonogawa, M.; Arai, T.; Endo, N.; Pack, S.P.; Kodaki, T.; Makino, K. Reactive oxygen species generation through NADH oxidation by pterin derivatives. Nucleic Acids Symp. Ser. 2008, 52, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Nonogawa, M.; Makino, K.; Endo, N.; Mori, H.; Miyoshi, T.; Yamashita, K.; Sasada, M.; Kakuyama, M.; Fukuda, K. The radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) reacts with a pterin derivative and produces a cytotoxic substance that induces intracellular reactive oxygen species generation and cell death. J. Pharmacol. Exp. Ther. 2008, 324, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Gopalrao, R.K.; Maeda, H.; Rao, P.; Yamamoto, M.; Xing, Y.; Mizobuchi, S.; Sasaguri, S. MCI-186 inhibits tumor growth through suppression of EGFR phosphorylation and cell cycle arrest. Anticancer. Res. 2005, 25, 1131–1138. [Google Scholar]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Duarte, D.; Guerreiro, I.; Vale, N. Novel Strategies for Cancer Combat: Drug Combination Using Repurposed Drugs Induces Synergistic Growth Inhibition of MCF-7 Breast and HT-29 Colon Cancer Cells. Curr. Issues Mol. Biol. 2022, 44, 4930–4949. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, E.; Fukazawa, R.; Kanbe, M.; Watanabe, M.; Abe, M.; Kamisago, M.; Hajikano, M.; Katsube, Y.; Ogawa, S. Edaravone, a potent free radical scavenger, prevents anthracycline-induced myocardial cell death. Circ. J. 2007, 71, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zhang, S.; Gu, L.; Liu, S.; Gao, H.; You, Z.; Zhou, G.; Wen, L.; Yu, J.; Xuan, Y. Electrocardiographic and biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol. Pharm. Bull. 2011, 34, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, K.; Mishima, K.; Makino, K.; Itoh, Y.; Tsuruya, K.; Hirakata, H.; Oishi, R. Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur. J. Pharmacol. 2002, 451, 203–208. [Google Scholar] [CrossRef]
- Satoh, M.; Kashihara, N.; Fujimoto, S.; Horike, H.; Tokura, T.; Namikoshi, T.; Sasaki, T.; Makino, H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 2003, 305, 1183–1190. [Google Scholar] [CrossRef]
- Iguchi, T.; Nishikawa, M.; Chang, B.; Muroya, O.; Sato, E.F.; Nakatani, T.; Inoue, M. Edaravone inhibits acute renal injury and cyst formation in cisplatin-treated rat kidney. Free Radic. Res. 2004, 38, 333–341. [Google Scholar] [CrossRef]
- Koike, N.; Sasaki, A.; Murakami, T.; Suzuki, K. Effect of edaravone against cisplatin-induced chronic renal injury. Drug Chem. Toxicol. 2021, 44, 437–446. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Mercer, E.; Tian, H.S.; Thompson, V.; Cheung, J.M.; Dorso, M.; Kubala, J.M.; Gudas, L.J.; de Stanchina, E.; et al. Kidney-Targeted Redox Scavenger Therapy Prevents Cisplatin-Induced Acute Kidney Injury. Front. Pharmacol. 2021, 12, 790913. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Kwatra, M.; Singh, T.; Pant, R.; Kushwah, P.; Ahmed, S.; Dwivedi, D.; Saroha, B.; Lahkar, M. Edaravone alleviates cisplatin-induced neurobehavioral deficits via modulation of oxidative stress and inflammatory mediators in the rat hippocampus. Eur. J. Pharmacol. 2016, 791, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Im, G.J.; Chang, J.; Lee, S.; Choi, J.; Jung, H.H.; Lee, H.M.; Ryu, S.H.; Park, S.K.; Kim, J.H.; Kim, H.J. Protective role of edaravone against cisplatin-induced ototoxicity in an auditory cell line. Hear. Res. 2015, 330, 113–118. [Google Scholar] [CrossRef]
- Hong, S.J.; Im, G.J.; Chang, J.; Chae, S.W.; Lee, S.H.; Kwon, S.Y.; Jung, H.H.; Chung, A.Y.; Park, H.C.; Choi, J. Protective effects of edaravone against cisplatin-induced hair cell damage in zebrafish. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1025–1031. [Google Scholar] [CrossRef]
- Domarecka, E.; Skarzynska, M.; Szczepek, A.J.; Hatzopoulos, S. Use of zebrafish larvae lateral line to study protection against cisplatin-induced ototoxicity: A scoping review. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420959554. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A. Protective Effect of Edaravone on Cyclophosphamide Induced Oxidative Stress and Neurotoxicity in Rats. Curr. Drug Saf. 2019, 14, 209–216. [Google Scholar] [CrossRef]
- Yoneda, K.; Fujii, M.; Imaoka, A.; Kobayashi, R.; Hayashi, R.; Yoshida, Y.; Kohno, T.; Tsuji, T. Preventive effect of edaravone ointment on cyclophosphamide-chemotherapy induced alopecia. Support. Care Cancer 2021, 29, 6127–6134. [Google Scholar] [CrossRef]
- Sasano, N.; Enomoto, A.; Hosoi, Y.; Katsumura, Y.; Matsumoto, Y.; Shiraishi, K.; Miyagawa, K.; Igaki, H.; Nakagawa, K. Free radical scavenger edaravone suppresses x-ray-induced apoptosis through p53 inhibition in MOLT-4 cells. J. Radiat. Res. 2007, 48, 495–503. [Google Scholar] [CrossRef]
- Hong, Z.; Kase, Y.; Moritake, T.; Gerelchuluun, A.; Sun, L.; Suzuki, K.; Terunuma, T.; Yasuoka, K.; Kumada, H.; Anzai, K.; et al. Lineal energy-based evaluation of oxidative DNA damage induced by proton beams and X-rays. Int. J. Radiat. Biol. 2013, 89, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sasano, N.; Enomoto, A.; Hosoi, Y.; Katsumura, Y.; Matsumoto, Y.; Morita, A.; Shiraishi, K.; Miyagawa, K.; Igaki, H.; Nakagawa, K. Edaravone, a known free radical scavenger, enhances X-ray-induced apoptosis at low concentrations. Cancer Lett. 2010, 293, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Natsume, A.; Wakabayashi, T.; Takeuchi, H.; Hasegawa, H.; Kim, S.U.; Yoshida, J. The free-radical scavenger edaravone restores the differentiation of human neural precursor cells after radiation-induced oxidative stress. Neurosci. Lett. 2007, 423, 225–230. [Google Scholar] [CrossRef]
- Anzai, K.; Furuse, M.; Yoshida, A.; Matsuyama, A.; Moritake, T.; Tsuboi, K.; Ikota, N. In vivo radioprotection of mice by 3-methyl-1-phenyl-2-pyrazolin-5-one (edaravone; Radicut), a clinical drug. J. Radiat. Res. 2004, 45, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Rong, X.; Hu, W.; Li, G.; Yang, X.; Yang, J.; Xu, P.; Luo, J. Effect of edaravone on radiation-induced brain necrosis in patients with nasopharyngeal carcinoma after radiotherapy: A randomized controlled trial. J. Neurooncol. 2014, 120, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Watanabe, S.; Kiyoi, T.; Tanaka, A.; Suemaru, K.; Araki, H. Evaluation of edaravone against radiation-induced oral mucositis in mice. J. Pharmacol. Sci. 2015, 127, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Min, J.; Mao, X.; Wang, X.; Yang, Y.; Chen, Y. Edaravone ameliorates experimental autoimmune thyroiditis in rats through HO-1-dependent STAT3/PI3K/Akt pathway. Am. J. Transl. Res. 2018, 10, 2037–2046. [Google Scholar] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Galetta, F.; Citi, E.; Benvenga, S.; Antonelli, A. Thyroid disorders induced by checkpoint inhibitors. Rev. Endocr. Metab. Disord. 2018, 19, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mengíbar, J.L.; Capel, I.; Bonfill, T.; Mazarico, I.; Espuña, L.C.; Caixàs, A.; Rigla, M. Simultaneous onset of type 1 diabetes mellitus and silent thyroiditis under durvalumab treatment. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19–45. [Google Scholar] [CrossRef]
- Hyung, S.; Jeong, Y.S.; Yeo, J.; Song, Y.K.; Kim, M.S.; Im, Y.J.; Maeng, H.J.; Chung, S.J. Identification of the primary determining factor(s) governing the oral absorption of edaravone in rats. Eur. J. Pharm. Sci. 2018, 123, 312–320. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Yuan, B.; Wang, W.; Xu, C.; Zhao, W.; Zhao, P.; Wang, Y.; Zhao, X. Bioavailability of Edaravone Sublingual Tablet Versus Intravenous Infusion in Healthy Male Volunteers. Clin. Ther. 2018, 40, 1683–1691. [Google Scholar] [CrossRef]
- Li, Q.; Huang, W.; Yang, J.; Wang, J.; Hu, M.; Mo, J.; Cheng, Y.; Ou, Z.; Zhang, Z.J.; Guan, S. Gastric retention pellets of edaravone with enhanced oral bioavailability: Absorption mechanism, development, and in vitro/in vivo evaluation. Eur. J. Pharm. Sci. 2018, 119, 62–69. [Google Scholar] [CrossRef]
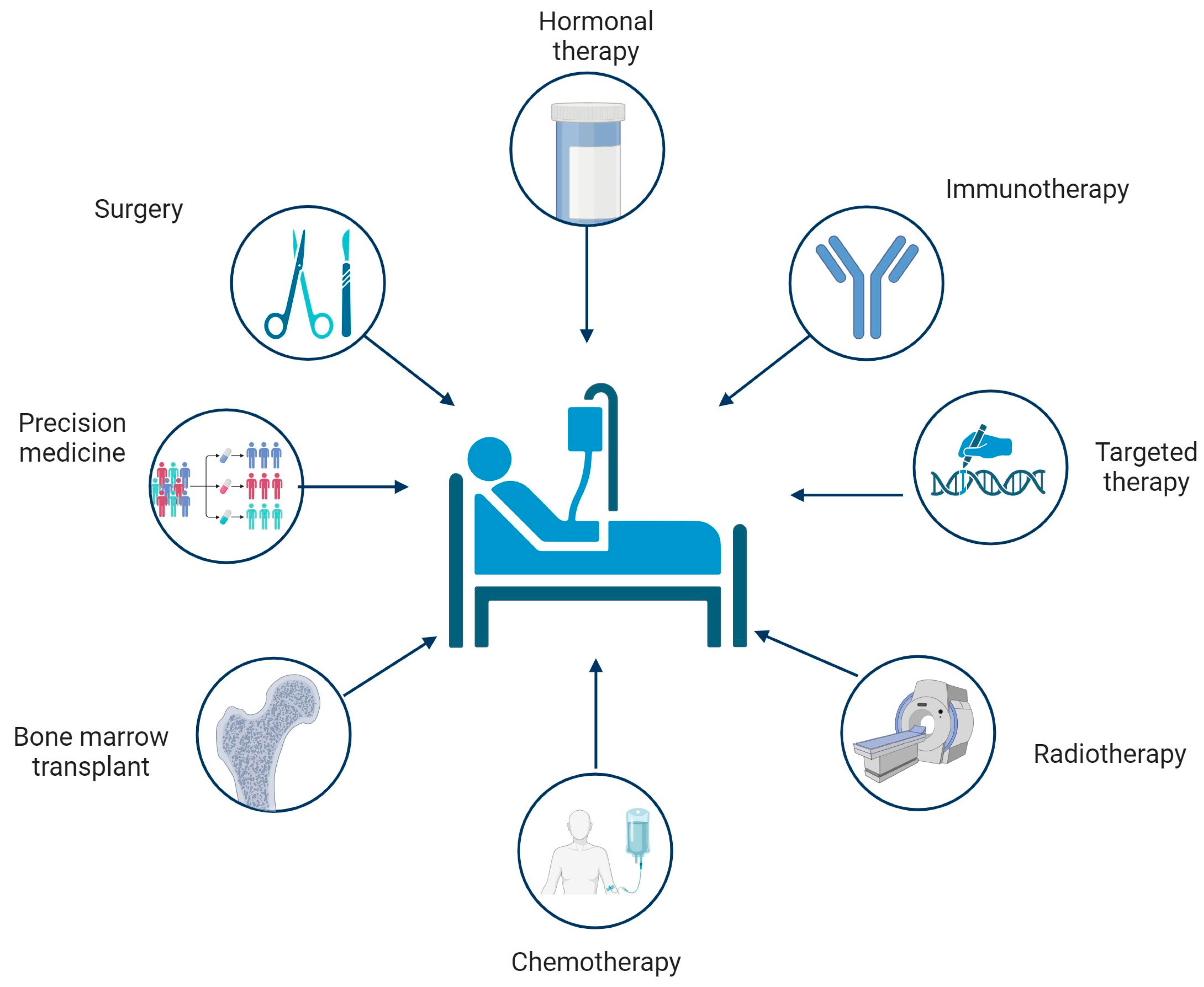

| Kind of Therapy | Beneficial Effects | Side Effects |
|---|---|---|
| Surgery | Tumor removal, symptom relief, local control | Increased pain, recurrence, inappropriateness for some cancer types, limitations in case of metastases |
| Chemotherapy | Broad coverage, apoptosis of tumor cells, utilization in various cancer stages | Damage to healthy cells, possible ineffectiveness, impact on quality of life |
| Radiotherapy | Localized treatment, preserving the functionality of nearby sites, treatment flexibility | Potential impairment of target organ functionality, limitations in case of metastases |
| Hematopoietic stem cell transplantation | Cell replacement, curative for some blood cancers, potential long-term cure | Complications and risks, limited number of compatible donors, extended recovery period, risk of rejection |
| Hormone therapy | Targeted tumor cells, various administration modes, maintenance treatment | Variable response, hormonal side effects, potential long-term issues, limited efficacy in advanced stages |
| Immunotherapy | Target specificity, long-lasting response, application to various cancers, minimal side effects | Risk of autoimmune reactions, possible insufficiency in response, variable response among patients |
| Targeted therapy | Precision in targeting, efficacy, fewer side effects, quick response, specific cancer types | Limitations in non-targeted tumors, genetic complexity, specific side effects |
| Precision therapy | Treatment personalization, good efficacy, minimal side effects, potential to improve quality of life | Development of resistance, genetic complexity, limitations in non-mutated tumors, variable response |
| The Actions of Edaravone In Vitro | References | In Vivo | References |
|---|---|---|---|
| Inhibition of HepG2, MSTO-211H, TMK-1, and MCF-7 tumor growth in a dose-dependent manner | [105,106,107,108] | Cytoprotective: reduces cardiotoxicity, renal failure, and neurotoxicity | [111,112,113,114,115,116,117] |
| Weak cytotoxic drug | [106,107] | Antioxidant | [115] |
| Blocking NFκB activation | [109] | Limiting ROS production | [121] |
| Has an anticancer effect in MCF-7 and HT-29 in combination with paclitaxel or doxorubicin | [110] | Radioprotective | [124,125,127,128] |
| Reduces the occurrence of thyroid dysfunctions | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duranti, E.; Cordani, N.; Villa, C. Edaravone: A Novel Possible Drug for Cancer Treatment? Int. J. Mol. Sci. 2024, 25, 1633. https://doi.org/10.3390/ijms25031633
Duranti E, Cordani N, Villa C. Edaravone: A Novel Possible Drug for Cancer Treatment? International Journal of Molecular Sciences. 2024; 25(3):1633. https://doi.org/10.3390/ijms25031633
Chicago/Turabian StyleDuranti, Elisa, Nicoletta Cordani, and Chiara Villa. 2024. "Edaravone: A Novel Possible Drug for Cancer Treatment?" International Journal of Molecular Sciences 25, no. 3: 1633. https://doi.org/10.3390/ijms25031633
APA StyleDuranti, E., Cordani, N., & Villa, C. (2024). Edaravone: A Novel Possible Drug for Cancer Treatment? International Journal of Molecular Sciences, 25(3), 1633. https://doi.org/10.3390/ijms25031633








