Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials
Abstract
1. Introduction
- Improving energy processes through recycling and the use of renewable sources.
- Optimizing waste management cycles.
- Substituting hazardous products with safer alternatives.
- Following the principles of atom economy.
- Minimizing the use of auxiliary substances (solvents, separating agents).
- Developing new catalysts.
- Reducing the E-factor.
- Increasing plant safety.
2. Classification of Polymeric Materials
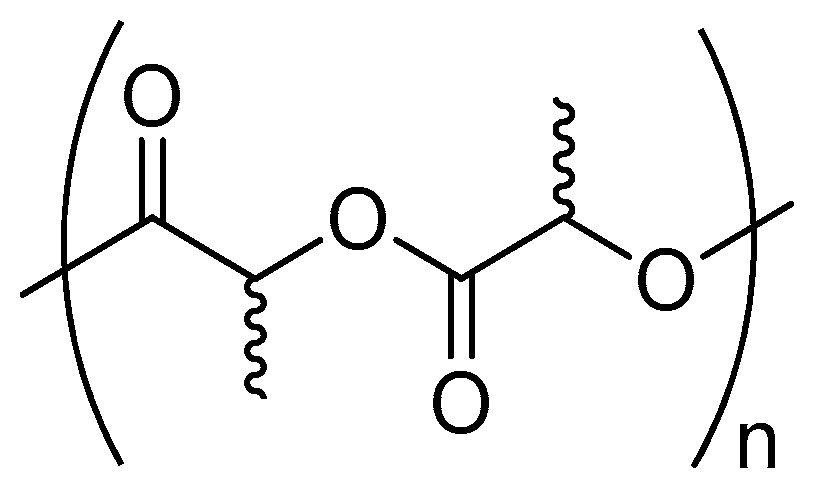
3. Poly(Lactic Acid)
3.1. Physical Properties and Applications
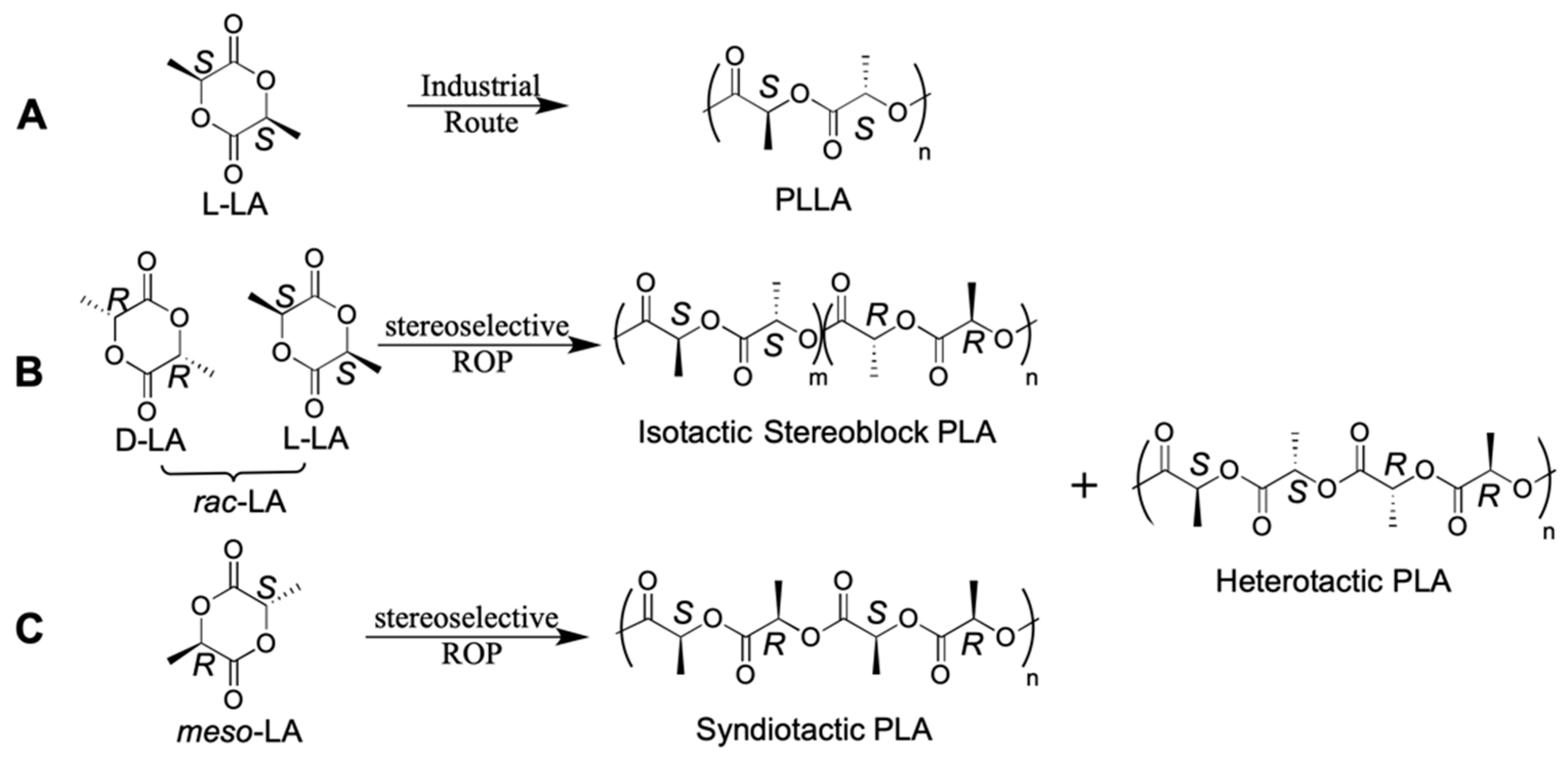
3.2. Possible Strategies for PLA Synthesis
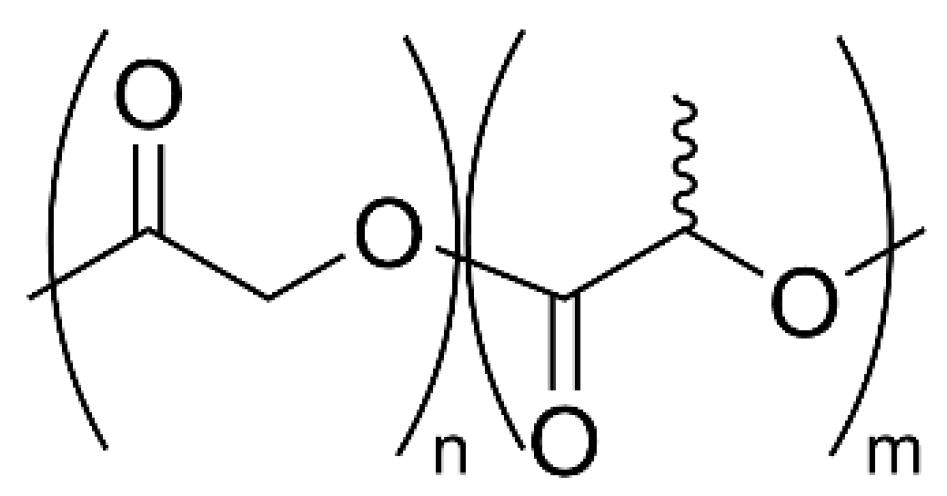
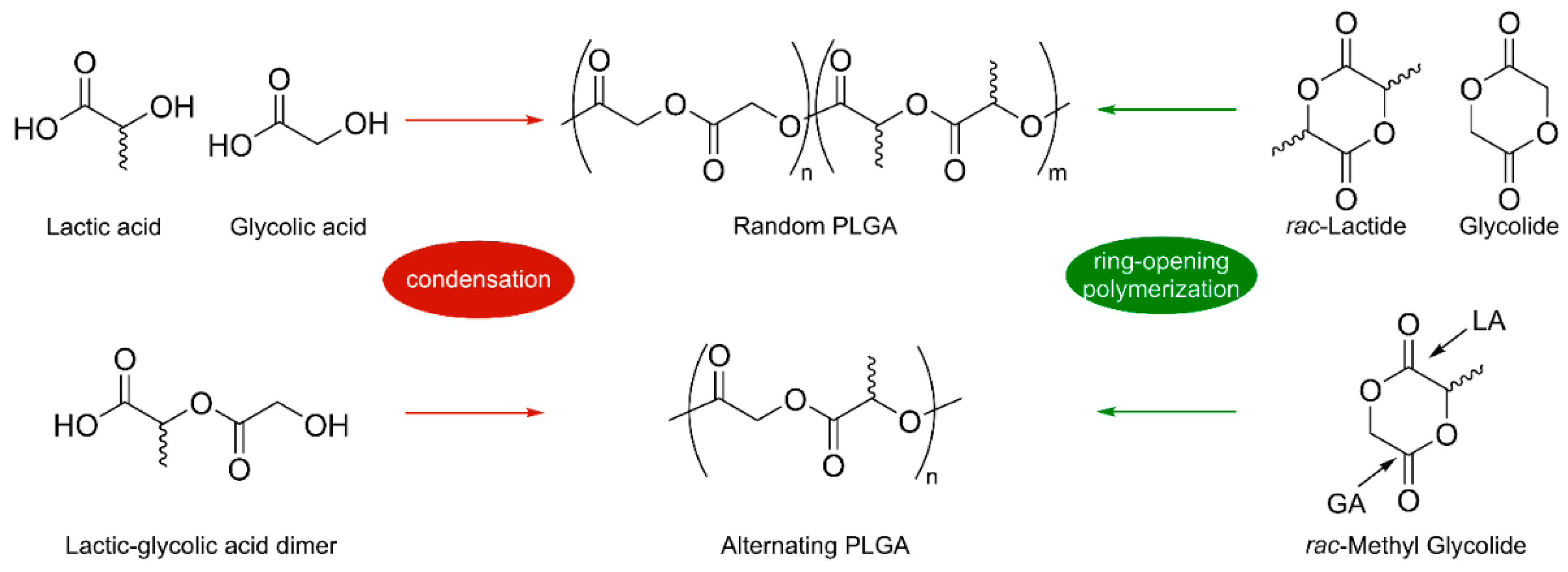
4. Poly(Lactic-co-Glycolic Acid)
4.1. Physical Properties and Applications
4.2. Possible Strategies for PLGA Synthesis
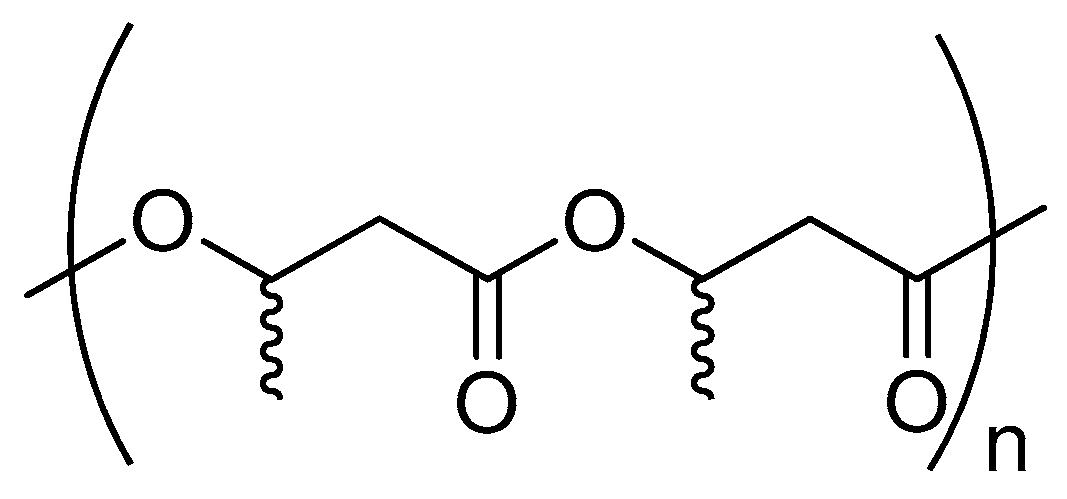
5. Poly(3-hydroxybutyrate)
5.1. Physical Properties and Applications
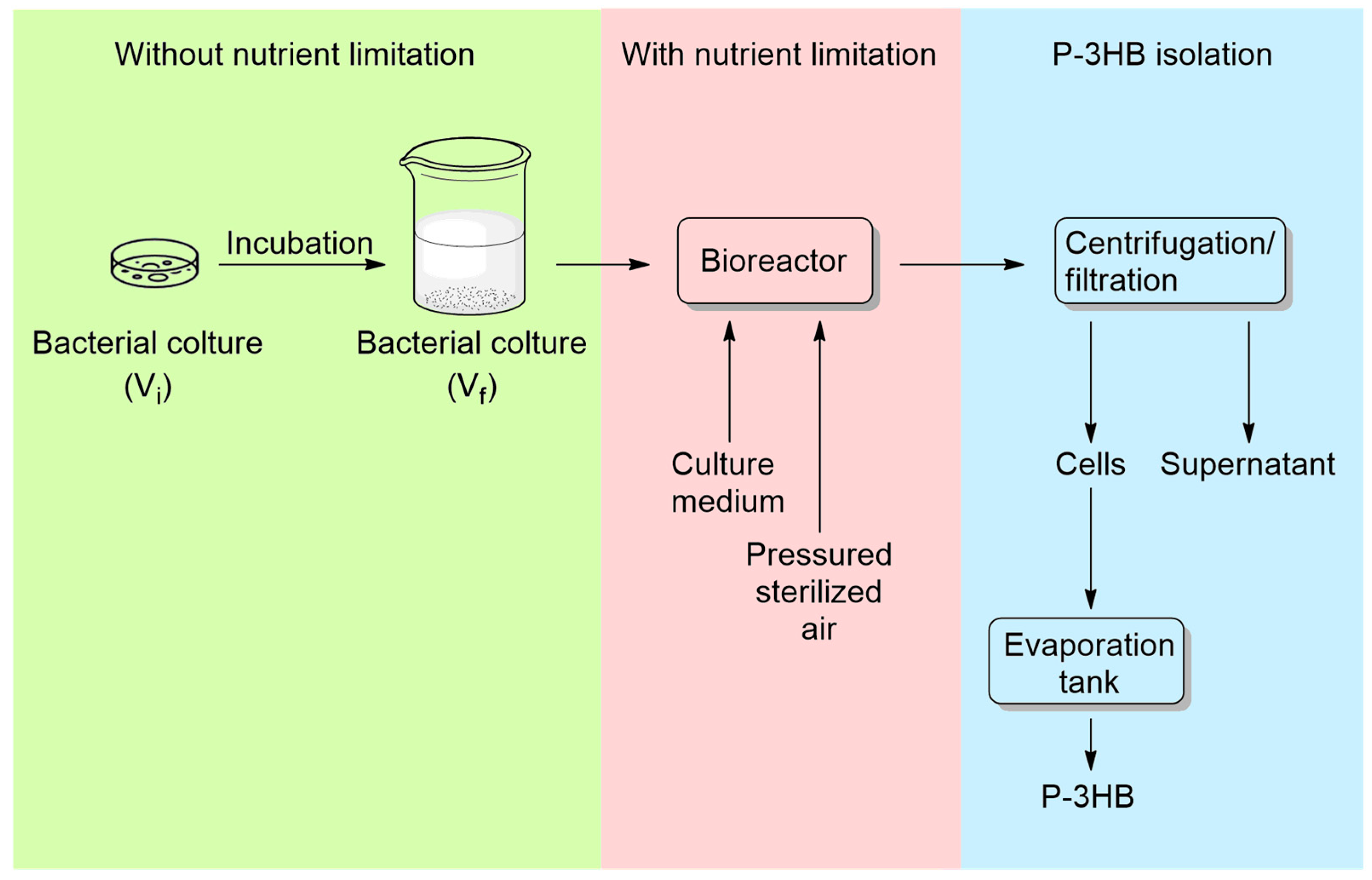
5.2. Possible Strategies for P3HB Synthesis
6. Importance of Stereo- and Regioselectivity in Cyclic Monomers ROP
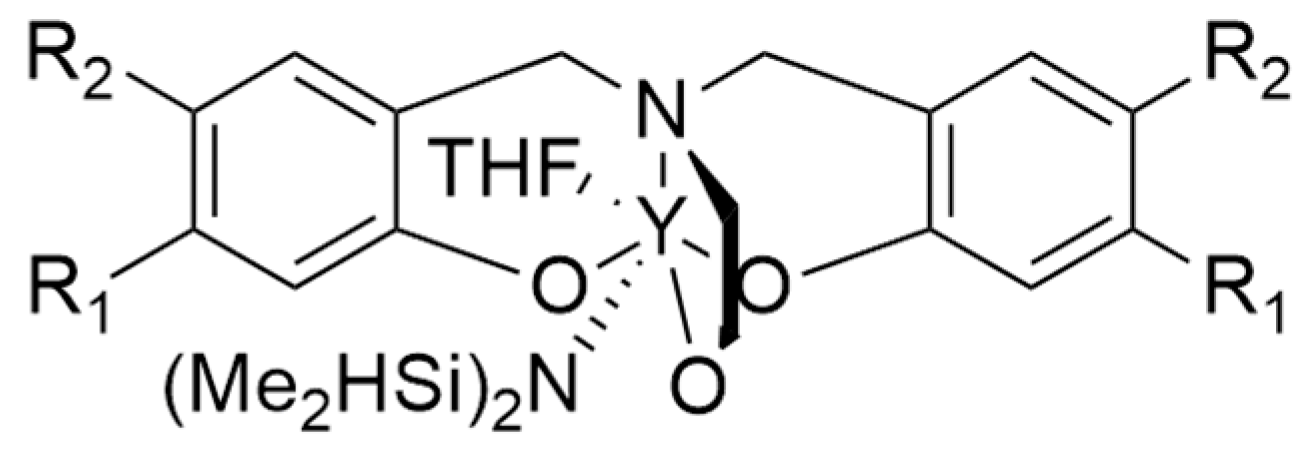
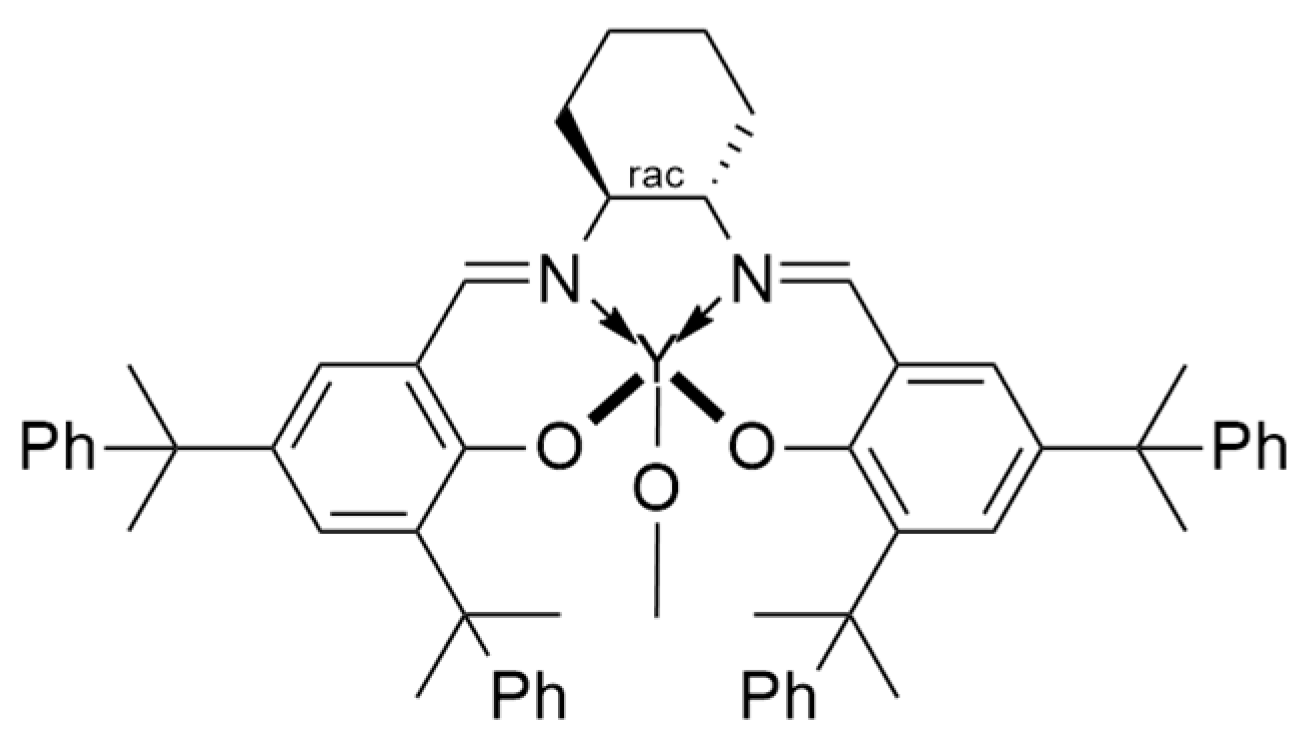
7. Salen Catalysts for Cyclic Monomers ROP Catalysis
7.1. Advantages of Salen Catalysts in Cyclic Monomers ROP Catalysis
7.2. Metal Centre Influence on Catalyst Stereoselectivity
7.3. Ligands Influence on Catalyst Stereoselectivity
7.4. Bridge Influence on Catalyst Stereoselectivity
7.5. Solvent and Initiator Influence on Catalyst Stereoselectivity
8. Future Perspectives and Challenges
- →
- Enantioselective Polymerization:
- →
- Issue: Common ROP catalysts may not be enantioselective, leading to the formation of atactic or non-stereoregular polymers.
- →
- Challenge: Developing catalysts for the control of the chirality of the polymer chain is essential for producing materials with predictable and desirable properties.
- →
- Molecular Weight Distribution:
- →
- Issue: The ROP of racemic cyclic esters may result in broad molecular weight distributions.
- →
- Challenge: Achieving a narrow molecular weight distribution is important for ensuring consistent material properties. This often involves optimizing reaction conditions and catalyst systems.
- →
- Reaction Kinetics:
- →
- Issue: Racemic cyclic esters may exhibit different reaction kinetics compared to their pure stereoisomers.
- →
- Challenge: Understanding and controlling the reaction kinetics is essential for achieving the desired polymerization rates and preventing side reactions.
- →
- Polymerization Rate:
- →
- Issue: Racemic cyclic esters may polymerize at different rates for each enantiomer.
- →
- Challenge: Balancing the polymerization rates of different enantiomers to obtain well-defined copolymers or blends can be challenging and may require careful tuning of reaction conditions.
- →
- Mechanical Properties:
- →
- Issue: Polymers derived from racemic cyclic esters may exhibit variations in mechanical properties due to the lack of stereoselectivity.
- →
- Challenge: Optimizing reaction conditions and catalyst systems to achieve consistent mechanical properties in the resulting polymers is a significant challenge.
- →
- Biodegradability:
- →
- Issue: The racemic nature of the polymer may influence its biodegradability.
- →
- Challenge: Ensuring that the resulting polymer maintains the desired biodegradability characteristics while addressing stereochemistry challenges is a complex task.
- →
- Catalyst Design:
- →
- Issue: Many traditional catalysts may not be suitable for achieving stereochemistry control in the ROP of racemic cyclic esters.
- →
- Challenge: Designing catalysts that can effectively control stereochemistry while maintaining reactivity and selectivity is an ongoing area of research.
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| density functional theory | DFT |
| density solvation model | SMD |
| differential scanning calorimetry | DSC |
| expanded polystyrene | EPS |
| isotacticity index | Pi |
| modified organisms | GMOs |
| poly hydroxyalkanoate | PHA |
| poly(lactic acid) | PLA |
| poly(3-hydroxybutyrate) | P3HB |
| generalized gradient approximation | GGA |
| melting temperature | Tm |
| molecular weight | Mw |
| polarizable continuum model | PCM |
| the rate determining state | RDS |
| ring-opening polymerization | ROP |
| terephthalate | PET |
| transition temperature | Tg |
References
- Moriarty, P.; Honnery, D. Review: Renewable Energy in an Increasingly Uncertain Future. Appl. Sci. 2023, 13, 388. [Google Scholar] [CrossRef]
- Zebral, Y.D.; Da Silva Fonseca, J.; Marques, J.A.; Bianchini, A. Carbonic anhydrase as a biomarker of global and local impacts: Insights from calcifying animals. Int. J. Mol. Sci. 2019, 20, 3092. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Solà, M.; Poater, A. Carbon Dioxide Conversion on Supported Metal Nanoparticles: A Brief Review. Catalysts 2023, 13, 305. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Ortiz-García, T.; Solà, M.; Poater, A. Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics 2023, 11, 88. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef] [PubMed]
- Keeble, B.R. The Brundtland report: ‘Our common future’. Med. War 1988, 4, 17–25. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Panahi, F.; Bahri-Laleh, N.; Sabzi, M.; Pareras, G.; Falcone, B.N.; Poater, A. pH-Responsive Gelation in Metallo-Supramolecular Polymers Based on the Protic Pyridinedicarboxamide Ligand. Chem. Mater. 2022, 13, 6155–6169. [Google Scholar] [CrossRef]
- Ballini, R. La chimica verde. PRISMA Economia—Società—Lavoro 2012, 2011, 65–73. [Google Scholar] [CrossRef]
- Steinhäuser, K.G.; Richter, S.; Greiner, P.; Penning, J.; Angrick, M. Principles and perspectives. Environ. Sci. Pollut. Res. 2004, 11, 284–290. [Google Scholar] [CrossRef]
- Secretary-General, U.N. Implementation of Decisions from the 2005 World Summit Outcome for Action by the Secretary-General: Report of the Secretary-General; The United Nations: New York, NY, USA, 2005. [Google Scholar]
- D’Alterio, M.C.; De Rosa, C.; Talarico, G. Syndiotactic PLA from meso-LA polymerization at the Al-chiral complex: A probe of DFT mechanistic insights. Chem. Commun. 2021, 57, 1611–1614. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Monomers and Macromolecular Materials from Renewable Resources: State of the Art and Perspectives. Molecules 2022, 27, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hsu, T.-G.; Wang, J. Mechanochemical Degradation and Recycling of Synthetic Polymers. Angew. Chem. Int. Ed. 2023, 62, e202300768. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Plummer, C.M.; Li, L.; Chen, Y. Ring-Opening Polymerization for the Goal of Chemically Recyclable Polymers. Macromolecules 2023, 56, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Scoti, M.; De Stefano, F.; Di Girolamo, R.; Malafronte, A.; Talarico, G.; De Rosa, C. Model of Crystallization Behavior of Isotactic Polypropylene: The Role of Defects. Macromol. Chem. Phys. 2023, 224, 2200262. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Cohen, S.; Baño, M.C.; Cima, L.G.; Allcock, H.R.; Vacanti, J.P.; Vacanti, C.A.; Langer, R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin. Mater. 1993, 13, 3–10. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Pennings, A.J. (Co)polymers of L-lactide, 2. Mechanical properties. Macromol. Chem. Phys. 1994, 195, 1649–1663. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Abdelrazek, S.; Abou Taleb, E.; Mahmoud, A.; Hamouda, T. Utilization of Polylactic Acid (PLA) in Textile Food Packaging: A Review. Egypt. J. Chem. 2021, 65, 725–738. [Google Scholar] [CrossRef]
- Van Wouwe, P.; Dusselier, M.; Vanleeuw, E.; Sels, B. Lactide Synthesis and Chirality Control for Polylactic acid Production. ChemSusChem 2016, 9, 907–921. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef]
- Rentero, C.; Damián, J.; Medel, A.; Fernández-Millán, M.; Rusconi, Y.; Talarico, G.; Cuenca, T.; Sessini, V.; Mosquera, M.E.G. Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies. Polymers 2022, 14, 2982. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Alterio, M.C.; Tedesco, C.; Pellecchia, C. Tailor-made block copolymers of L-, D- and rac-lactides and ε-caprolactone via one-pot sequential ring opening polymerization by pyridylamidozinc(II) catalysts. RSC Adv. 2019, 9, 32771–32779. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereoselective Ring-Opening Polymerization of meso-Lactide: Synthesis of Syndiotactic Poly(lactic acid). J. Am. Chem. Soc. 1999, 121, 4072–4073. [Google Scholar] [CrossRef]
- Hador, R.; Shuster, M.; Venditto, V.; Kol, M. Stereogradient Poly(Lactic Acid) from meso-Lactide/L-Lactide Mixtures. Angew. Chem. Int. Ed. 2022, 61, e202207652. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. Controlled and Stereoselective Polymerization of Lactide: Kinetics, Selectivity, and Microstructures. J. Am. Chem. Soc. 2003, 125, 11291–11298. [Google Scholar] [CrossRef]
- Kim, K.W.; Woo, S.I. Synthesis of High-Molecular-Weight Poly(L-lactic acid) by Direct Polycondensation. Macromol. Chem. Phys. 2002, 203, 2245–2250. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic Polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; D’Auria, I.; Gaeta, L.; Tedesco, C.; Brenna, S.; Pellecchia, C. Are Well Performing Catalysts for the Ring Opening Polymerization of L-Lactide under Mild Laboratory Conditions Suitable for the Industrial Process? The Case of New Highly Active Zn(II) Catalysts. Macromolecules 2022, 55, 5115–5122. [Google Scholar] [CrossRef]
- Shinno, K.; Miyamoto, M.; Kimura, Y.; Hirai, Y.; Yoshitome, H. Solid-State Postpolymerization of l-Lactide Promoted by Crystallization of Product Polymer: An Effective Method for Reduction of Remaining Monomer. Macromolecules 1997, 30, 6438–6444. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. Single-step reactive extrusion of PLLA in a corotating twin-screw extruder promoted by 2-ethylhexanoic acid tin(II) salt and triphenylphosphine. Polymer 2000, 41, 3395–3403. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Ercan, B.; Chung, S.; Taylor, E.; Denkbaş, E.B.; Webster, T.J. Nanostructured anti-bacterial poly-lactic-co-glycolic acid films for skin tissue engineering applications. J. Biomed. Mater. Res. A 2014, 102, 4598–4608. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.A.; Swiner, D.J.; Bell, K.R.; Fedorchak, M.V.; Little, S.R.; Meyer, T.Y. The impact of monomer sequence and stereochemistry on the swelling and erosion of biodegradable poly(lactic-co-glycolic acid) matrices. Biomaterials 2017, 117, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rothstein, S.N.; Little, S.R.; Edenborn, H.M.; Meyer, T.Y. The Effect of Monomer Order on the Hydrolysis of Biodegradable Poly(lactic-co-glycolic acid) Repeating Sequence Copolymers. J. Am. Chem. Soc. 2012, 134, 16352–16359. [Google Scholar] [CrossRef] [PubMed]
- Stayshich, R.M.; Meyer, T.Y. New Insights into Poly(lactic-co-glycolic acid) Microstructure: Using Repeating Sequence Copolymers To Decipher Complex NMR and Thermal Behavior. J. Am. Chem. Soc. 2010, 132, 10920–10934. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Yamasaki, M.; Arakawa, Y. Stereocomplex Formation between Enantiomeric Alternating Lactic Acid-Based Copolymers as a Versatile Method for the Preparation of High Performance Biobased Biodegradable Materials. ACS Appl. Polym. Mater. 2019, 1, 1476–1484. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-M.; Qiu, K.-Y.; Gu, Z.-W.; Feng, X.-D. Synthesis of poly(D,L-lactic acid-alt-glycolic acid) from D,L-3-methylglycolide. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4179–4184. [Google Scholar] [CrossRef]
- Takojima, K.; Makino, H.; Saito, T.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. An organocatalytic ring-opening polymerization approach to highly alternating copolymers of lactic acid and glycolic acid. Polym. Chem. 2020, 11, 6365–6373. [Google Scholar] [CrossRef]
- Lu, Y.; Swisher, J.H.; Meyer, T.Y.; Coates, G.W. Chirality-Directed Regioselectivity: An Approach for the Synthesis of Alternating Poly(Lactic-co-Glycolic Acid). J. Am. Chem. Soc. 2021, 143, 4119–4124. [Google Scholar] [CrossRef]
- Lu, Y.; Coates, G.W. Pairing-Enhanced Regioselectivity: Synthesis of Alternating Poly(lactic-co-glycolic acid) from Racemic Methyl-Glycolide. J. Am. Chem. Soc. 2023, 145, 22425–22432. [Google Scholar] [CrossRef]
- Rusconi, Y.; D’Alterio, M.C.; De Rosa, C.; Lu, Y.; Severson, S.M.; Coates, G.W.; Talarico, G. Mechanism of Alternating Poly(lactic-co-glycolic acid) Formation by Polymerization of (S)- and (R)-3-Methyl Glycolide Using an Enantiopure Aluminum Complex. ACS Catal. 2024, 14, 318–323. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kreuzberger, S.; Hinrichsen, G. Influence of thermal degradation on tensile strength and Young’s modulus of poly(hydroxybutyrate). Polym. Bull. 1994, 33, 355–359. [Google Scholar] [CrossRef]
- Genovesi, A.; Aversa, C.; Barletta, M. Polyhydroxyalkanoates-based Cast Film as Bio-based Packaging for Fresh Fruit and Vegetables: Manufacturing and Characterization. J. Polym. Environ. 2023, 31, 4522–4532. [Google Scholar] [CrossRef]
- He, Y.; Shuai, X.; Cao, A.; Kasuya, K.I.; Doi, Y.; Inoue, Y. Enzymatic biodegradation of chemosynthetic atactic P(3HB) enhanced by an amorphous non-biodegradable polymer: Blend of atactic P(3HB) with PMMA. Macromol. Rapid Commun. 2000, 21, 1277–1281. [Google Scholar] [CrossRef]
- Janakiraman, V.C.; Subramani, S.; Devarajan, M.; Abdul Aziz, A. Impact of ZnO nanoparticles on dielectric and optical properties of poly(3-hydroxybutyrate) for electronics applications. Polym. Plast. Technol. Eng. 2017, 56, 1495–1504. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Ajellal, N.; Bouyahyi, M.; Amgoune, A.; Thomas, C.M.; Bondon, A.; Pillin, I.; Grohens, Y.; Carpentier, J.-F. Syndiotactic-enriched poly(3-hydroxybutyrate)s via stereoselective ring-opening polymerization of racemic β-butyrolactone with discrete yttrium catalysts. Macromolecules 2009, 42, 987–993. [Google Scholar] [CrossRef]
- Bruckmoser, J.; Pongratz, S.; Stieglitz, L.; Rieger, B. Highly Isoselective Ring-Opening Polymerization of rac-β-Butyrolactone: Access to Synthetic Poly(3-hydroxybutyrate) with Polyolefin-like Material Properties. J. Am. Chem. Soc. 2023, 145, 11494–11498. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 2018, 9, 2345. [Google Scholar] [CrossRef]
- Tang, X.; Westlie, A.H.; Watson, E.M.; Chen, E.Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 2019, 366, 754–758. [Google Scholar] [CrossRef]
- Sheldon, R. Metal-Catalyzed Oxidations of Organic Compounds: Mechanistic Principles and Synthetic Methodology Including Biochemical Processes; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Xie, X.; Huo, Z.; Jang, E.; Tong, R. Recent advances in enantioselective ring-opening polymerization and copolymerization. Commun. Chem. 2023, 6, 202. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do new century catalysts unravel the mechanism of stereocontrol of old Ziegler−Natta catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef]
- Talarico, G.; Budzelaar, P.H.M. Analysis of Stereochemistry Control in Homogeneous Olefin Polymerization Catalysis. Organometallics 2014, 33, 5974–5982. [Google Scholar] [CrossRef]
- Cavallo, L. The evolution of stereoselective olefin polymerization modeling. Polymer 2024, 291, 126589. [Google Scholar] [CrossRef]
- Tang, X.; Shi, C.; Zhang, Z.; Chen, E.Y.-X. Toughening Biodegradable Isotactic Poly(3-hydroxybutyrate) via Stereoselective Copolymerization of a Diolide and Lactones. Macromolecules 2021, 54, 9401–9409. [Google Scholar] [CrossRef]
- Tschan, M.J.L.; Gauvin, R.M.; Thomas, C.M. Controlling polymer stereochemistry in ring-opening polymerization: A decade of advances shaping the future of biodegradable polyesters. Chem. Soc. Rev. 2021, 50, 13587–13608. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef]
- Chile, L.-E.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. A Comparison of the Rheological and Mechanical Properties of Isotactic, Syndiotactic, and Heterotactic Poly(lactide). Macromolecules 2016, 49, 909–919. [Google Scholar] [CrossRef]
- Urayama, H.; Moon, S.I.; Kimura, Y. Microstructure and thermal properties of polylactides with different L-and D-unit sequences: Importance of the Helical nature of the L-sequenced segments. Macromol. Mater. Eng. 2003, 288, 137–143. [Google Scholar] [CrossRef]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; Pugh, R.I.; White, A.J.P. Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminum salen-type initiators. Proc. Natl. Acad. Sci. USA 2006, 103, 15343–15348. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Poater, A.; Childers, M.I.; Widger, P.C.B.; LaPointe, A.M.; Lobkovsky, E.B.; Coates, G.W.; Cavallo, L. Enantioselective Polymerization of Epoxides Using Biaryl-Linked Bimetallic Cobalt Catalysts: A Mechanistic Study. J. Am. Chem. Soc. 2013, 135, 18901–18911. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective ring-opening polymerization of a racemic lactide by using achiral salen- and homosalen-aluminum complexes. Chem. Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Karroonnirun, O. Stereoselective ring-opening polymerization of rac-lactides catalyzed by chiral and achiral aluminum half-salen complexes. Organometallics 2010, 29, 5627–5634. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Xie, X.; Liu, Y.; Huo, Z.; Lin, M.; Xin, H.; Tong, R. Bayesian-optimization-assisted discovery of stereoselective aluminum complexes for ring-opening polymerization of racemic lactide. Nat. Commun. 2023, 14, 3647. [Google Scholar] [CrossRef]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Remarkable Stereocontrol in the Polymerization of Racemic Lactide Using Aluminum Initiators Supported by Tetradentate Aminophenoxide Ligands. J. Am. Chem. Soc. 2004, 126, 2688–2689. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shakaroun, R.M.; Guillaume, S.M.; Carpentier, J.-F. Recent Advances in Metal-Mediated Stereoselective Ring-Opening Polymerization of Functional Cyclic Esters towards Well-Defined Poly(hydroxy acid)s: From Stereoselectivity to Sequence-Control. Chem. Eur. J. 2020, 26, 128–138. [Google Scholar] [CrossRef]
- Spassky, N.; Wisniewski, M.; Pluta, C.; Le Borgne, A. Highly stereoelective polymerization of rac-(D,L)-lactide with a chiral Schiff’s base/aluminium alkoxide initiator. Macromol. Chem. Phys. 1996, 197, 2627–2637. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; De Rosa, C.; Talarico, G. Stereoselective Lactide Polymerization: The Challenge of Chiral Catalyst Recognition. ACS Catal. 2020, 10, 2221–2225. [Google Scholar] [CrossRef]
- Rusconi, Y.; D’Alterio, M.C.; Grillo, A.; Poater, A.; De Rosa, C.; Talarico, G. The metal role on the activity and stereoselectivity of ring-opening polymerization of racemic lactide promoted by Salen catalysts. Polymer 2024, 292, 126639. [Google Scholar] [CrossRef]
- Monreal-Corona, R.; Pla-Quintana, A.; Poater, A. Predictive catalysis: A valuable step towards machine learning. Trends Chem. 2023, 5, 935–946. [Google Scholar] [CrossRef]
- Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. SambVca: A web application for the calculation of the buried volume of N-heterocyclic carbene ligands. Eur. J. Inorg. Chem. 2009, 2009, 1759–1766. [Google Scholar] [CrossRef]
- Escayola, S.; Bahri-Laleh, N.; Poater, A. %VBur index and steric maps: From predictive catalysis to machine learning. Chem. Soc. Rev. 2024, 53, 853–882. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef]
- Bakewell, C.; Cao, T.-P.-A.; Long, N.; Le Goff, X.F.; Auffrant, A.; Williams, C.K. Yttrium phosphasalen initiators for rac-lactide polymerization: Excellent rates and high iso-selectivities. J. Am. Chem. Soc. 2012, 134, 20577–20580. [Google Scholar] [CrossRef] [PubMed]
- Bakewell, C.; White, A.J.P.; Long, N.J.; Williams, C.K. Metal-Size Influence in Iso-Selective Lactide Polymerization. Angew. Chem. Int. Ed. 2014, 53, 9226–9230. [Google Scholar] [CrossRef] [PubMed]
- Coudane, J.; Ustariz-Peyret, C.; Schwach, G.; Vert, M. More about the stereodependence of DD and LL pair linkages during the ring-opening polymerization of racemic lactide. J. Polym. Sci. A Polym. Chem. 1997, 35, 1651–1658. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. [(salen)Al]-Mediated, Controlled and Stereoselective Ring-Opening Polymerization of Lactide in Solution and without Solvent: Synthesis of Highly Isotactic Polylactide Stereocopolymers from Racemic D,L-Lactide. Angew. Chem. Int. Ed. 2002, 41, 4510–4513. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Gallucci, J.C.; Quisenberry, K.T.; Zhou, Z. Complexities in the Ring-Opening Polymerization of Lactide by Chiral Salen Aluminum Initiators. Inorg. Chem. 2008, 47, 2613–2624. [Google Scholar] [CrossRef]
- Xu, J.; Hadjichristidis, N. Heteroatom-containing degradable polymers by ring-opening metathesis polymerization. Prog. Polym. Sci. 2023, 139, 101656. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Hadjichristidis, N. Poly(amino ester)s as an emerging synthetic biodegradable polymer platform: Recent developments and future trends. Prog. Polym. Sci. 2023, 136, 101634. [Google Scholar] [CrossRef]
- Dove, A.P. Controlled ring-opening polymerisation of cyclic esters: Polymer blocks in self-assembled nanostructures. Chem. Commun. 2008, 48, 6446–6470. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsuo, Y.; Kouno, I. Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int. J. Mol. Sci. 2010, 11, 14–40. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T.; Rajpurohit, J.; Lipstman, S.; Venditto, V.; Kol, M. Isoselective Polymerization of rac-Lactide by Highly Active Sequential {ONNN} Magnesium Complexes. Chem. Eur. J. 2020, 26, 17183–17189. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Westlie, A.H.; Caporaso, L.; Cavallo, L.; Falivene, L.; Chen, E.Y.-X. Biodegradable Polyhydroxyalkanoates by Stereoselective Copolymerization of Racemic Diolides: Stereocontrol and Polyolefin-Like Properties. Angew. Chem. Int. Ed. 2020, 59, 7881–7890. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Hollmann, F.; Luque, R.; Song, I.K.; Talarico, G.; Tatsumi, T.; Yan, N. Molecular Catalysis for the Chemistry of the future: A perspective. Mol. Catal. 2022, 522, 112233. [Google Scholar] [CrossRef]
- Poater, A. Never too late: Stereoselective alkyne semi-hydrogenation by Ir catalysis. Chem Catal. 2022, 2, 1256. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Talarico, G. Expanding the Origin of Stereocontrol in Propene Polymerization Catalysis. ACS Catal. 2016, 6, 3767–3770. [Google Scholar] [CrossRef]
- Pang, X.; Duan, R.; Li, X.; Hu, C.; Wang, X.; Chen, X. Macromolecules, Breaking the Paradox between Catalytic Activity and Stereoselectivity: Rac-Lactide Polymerization by Trinuclear Salen–Al Complexes. Macromolecules 2018, 51, 906–913. [Google Scholar] [CrossRef]

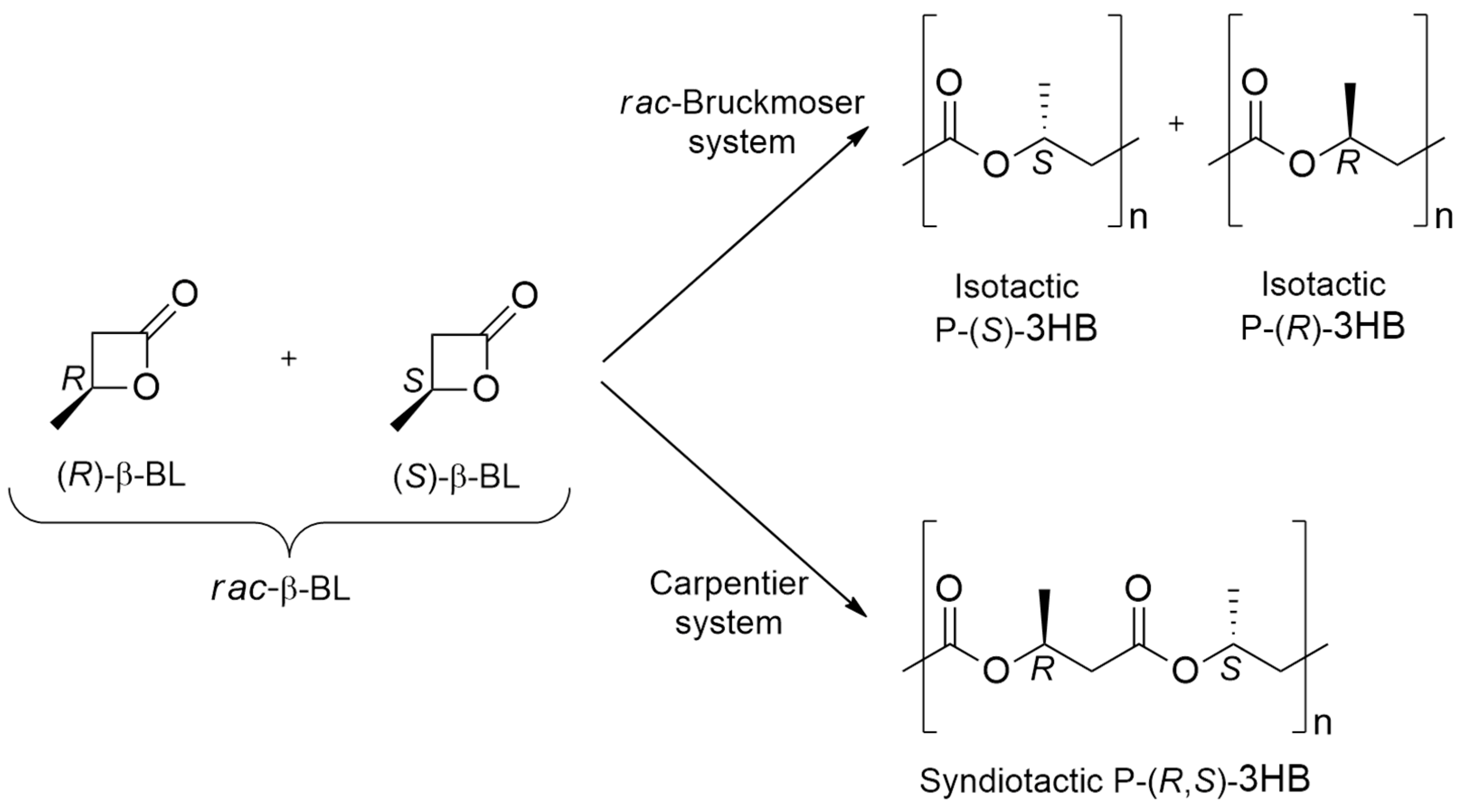
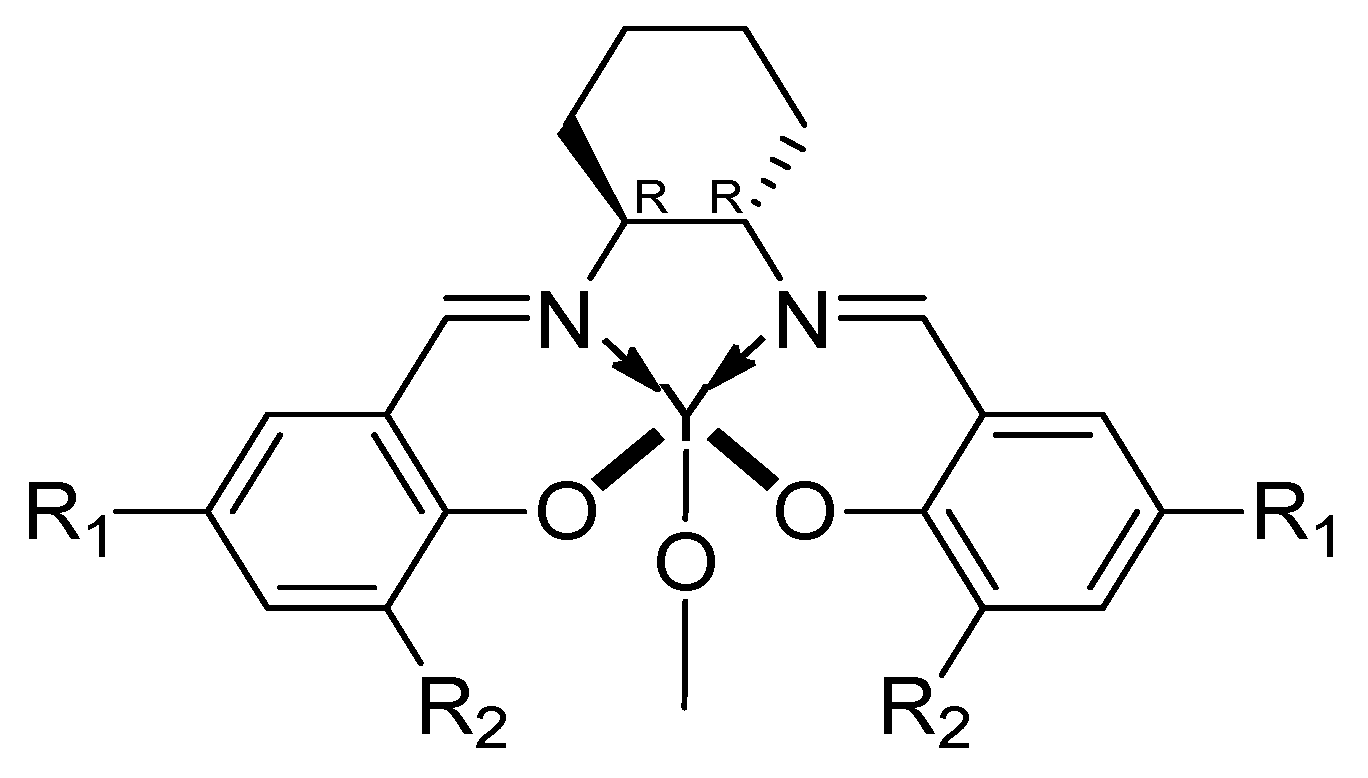

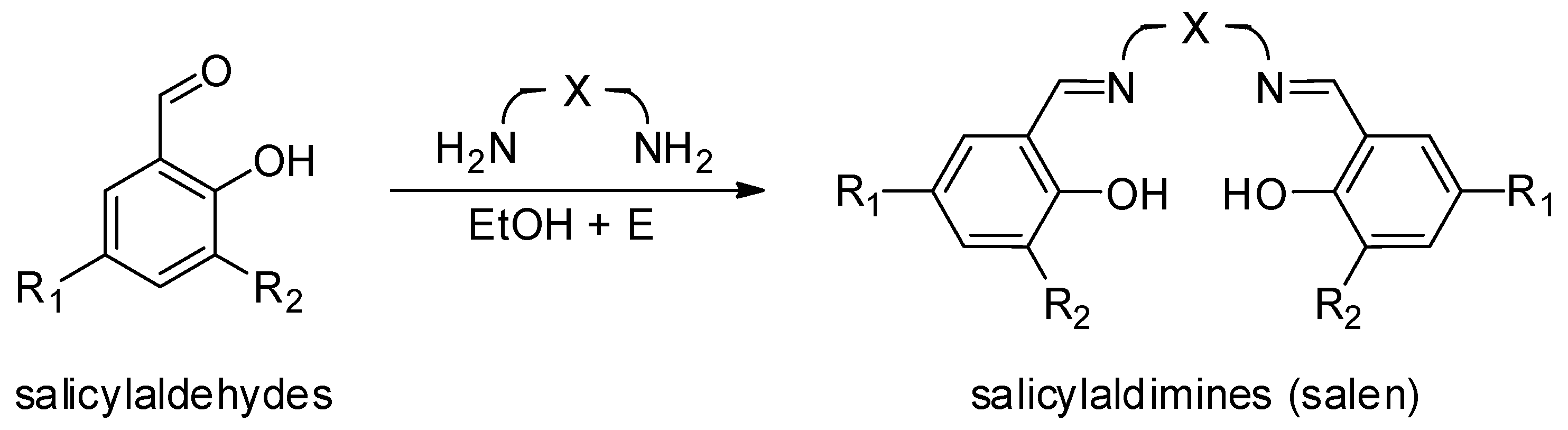
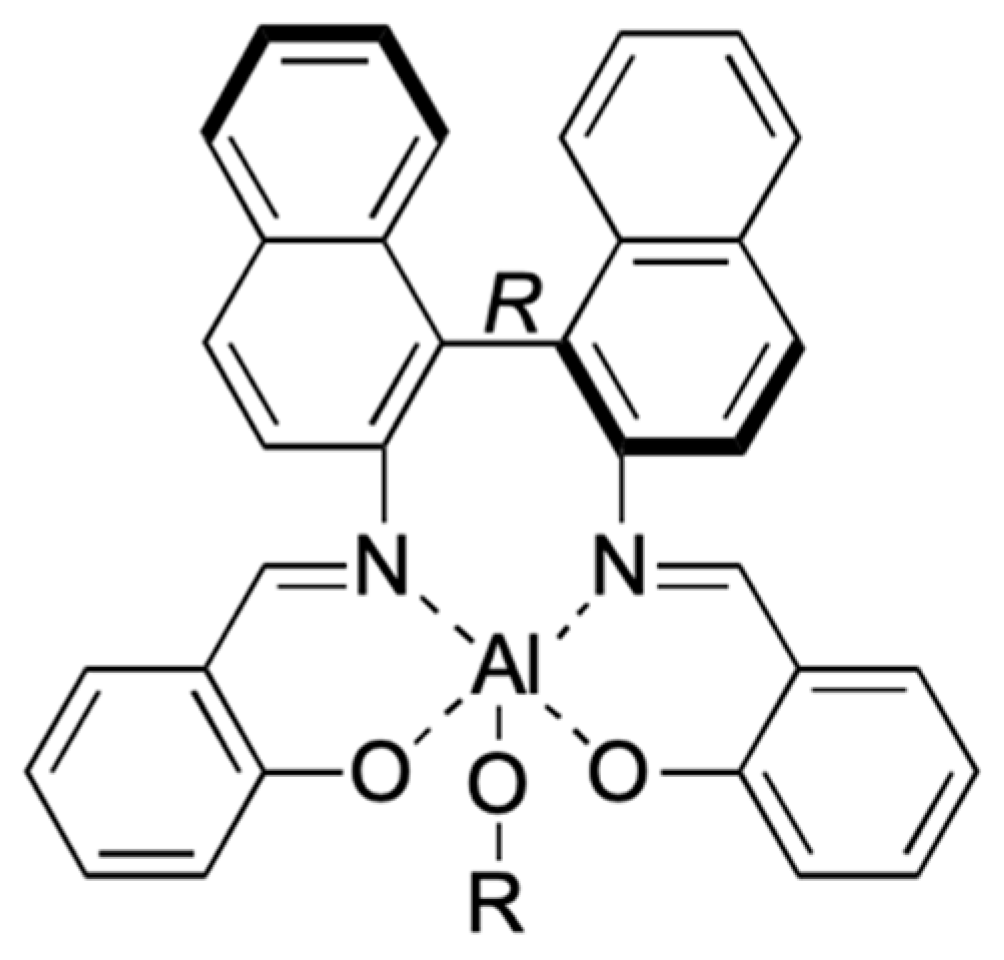
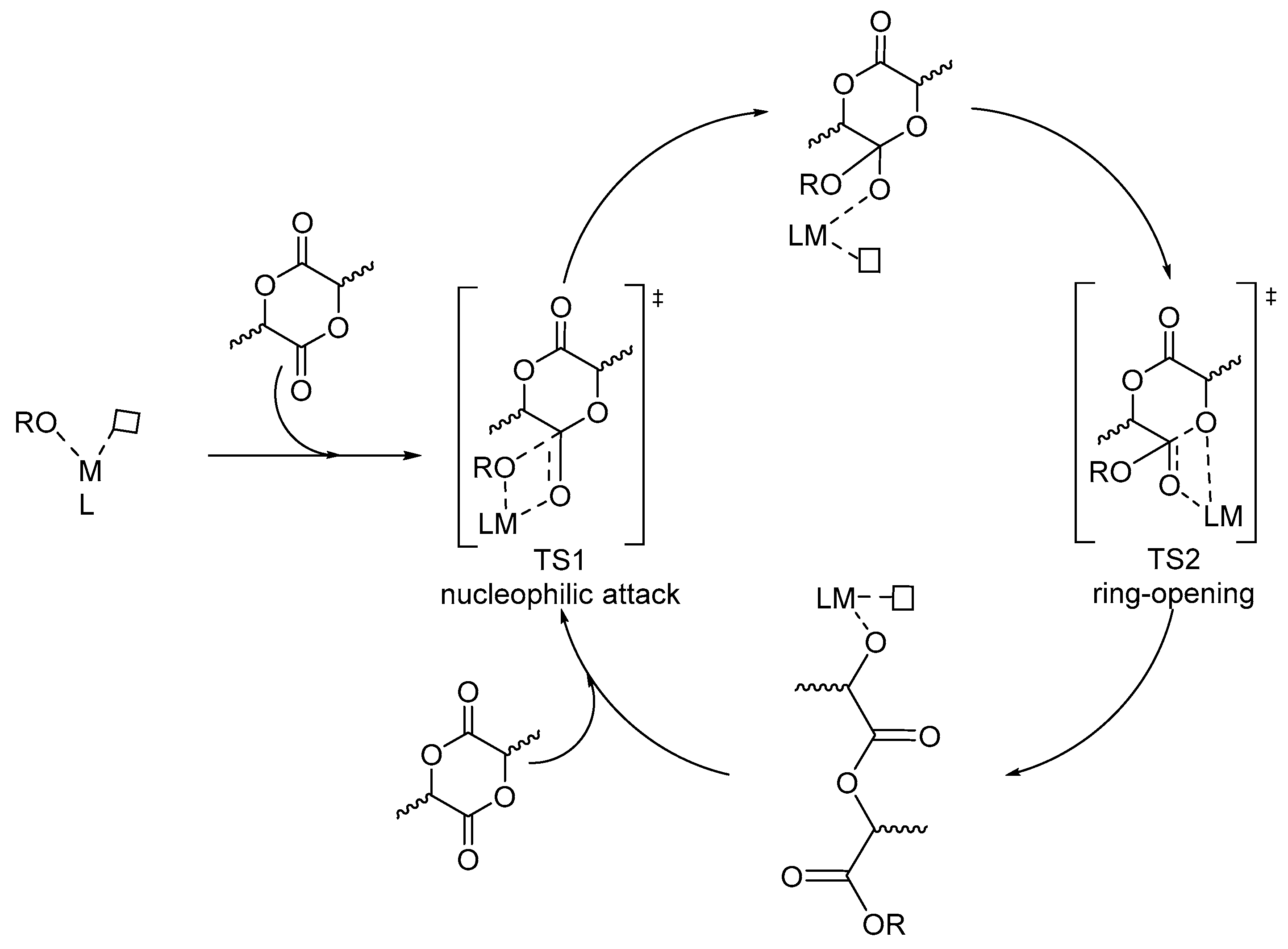
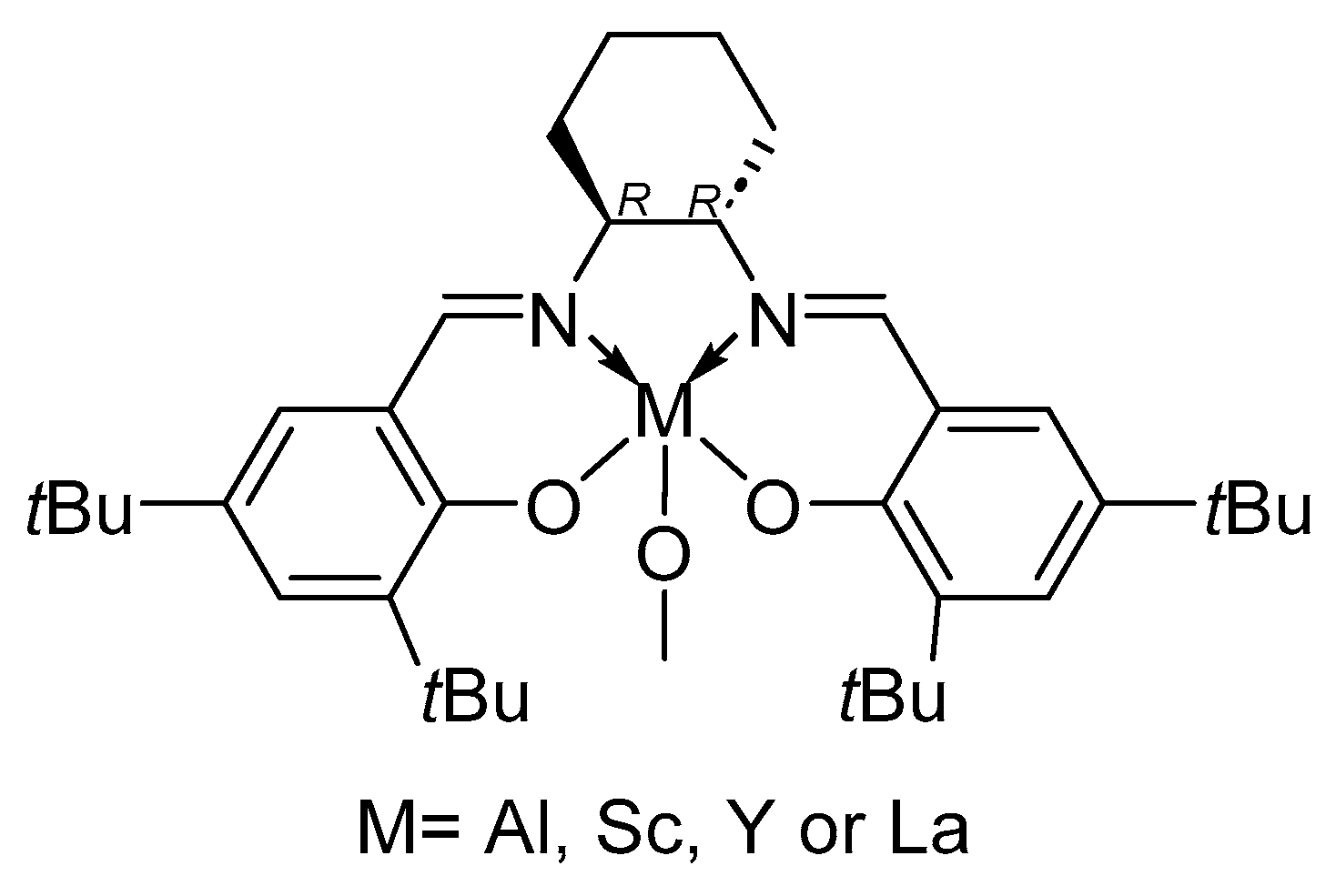
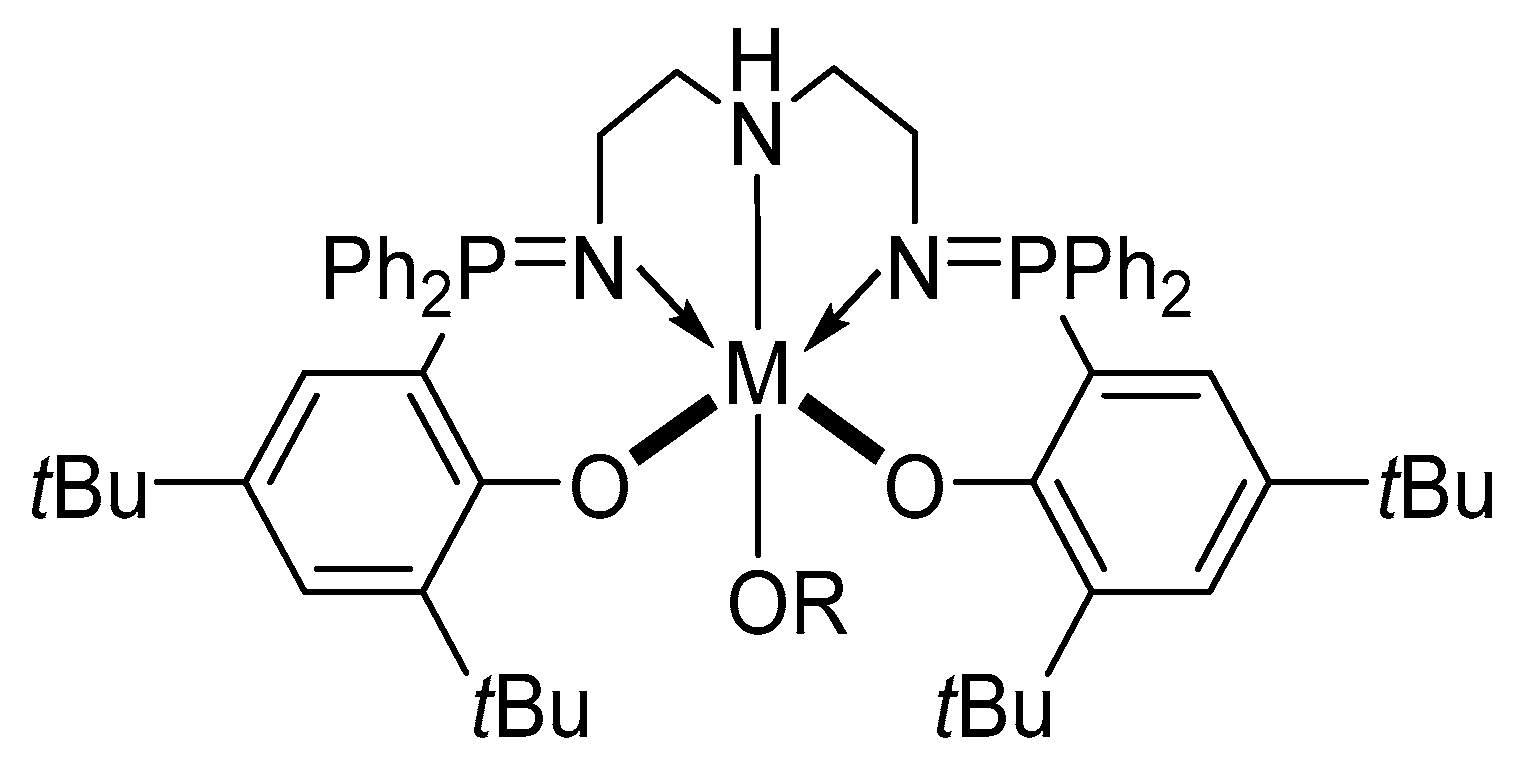
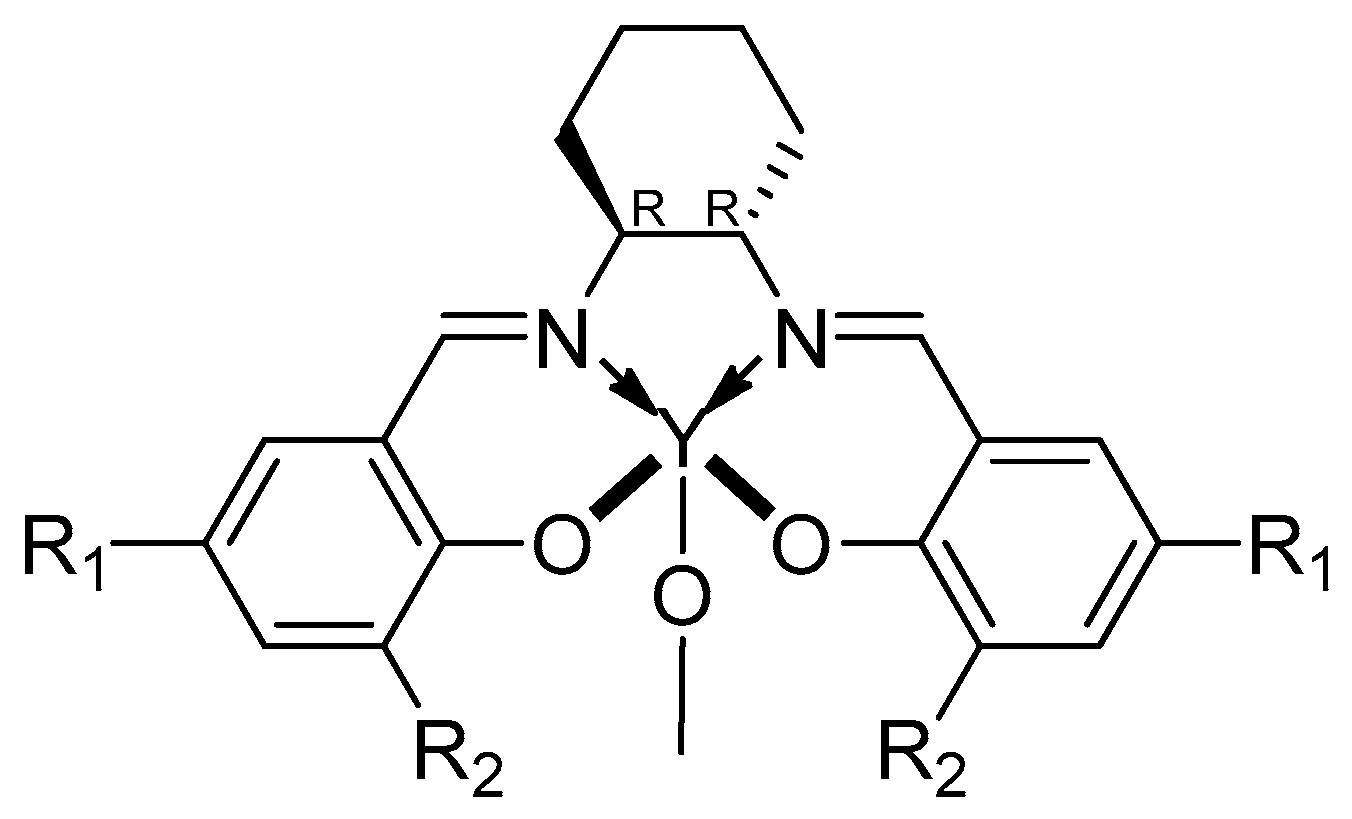
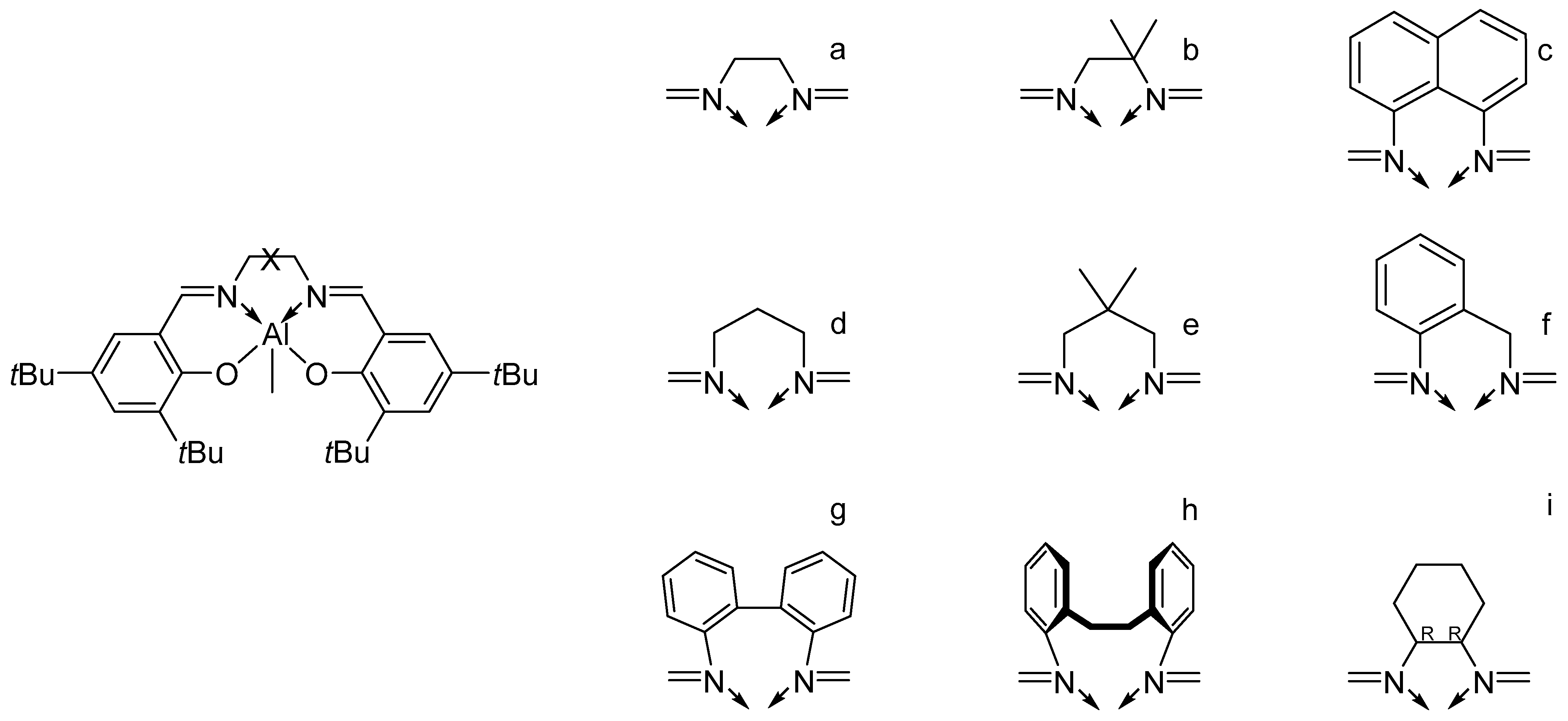
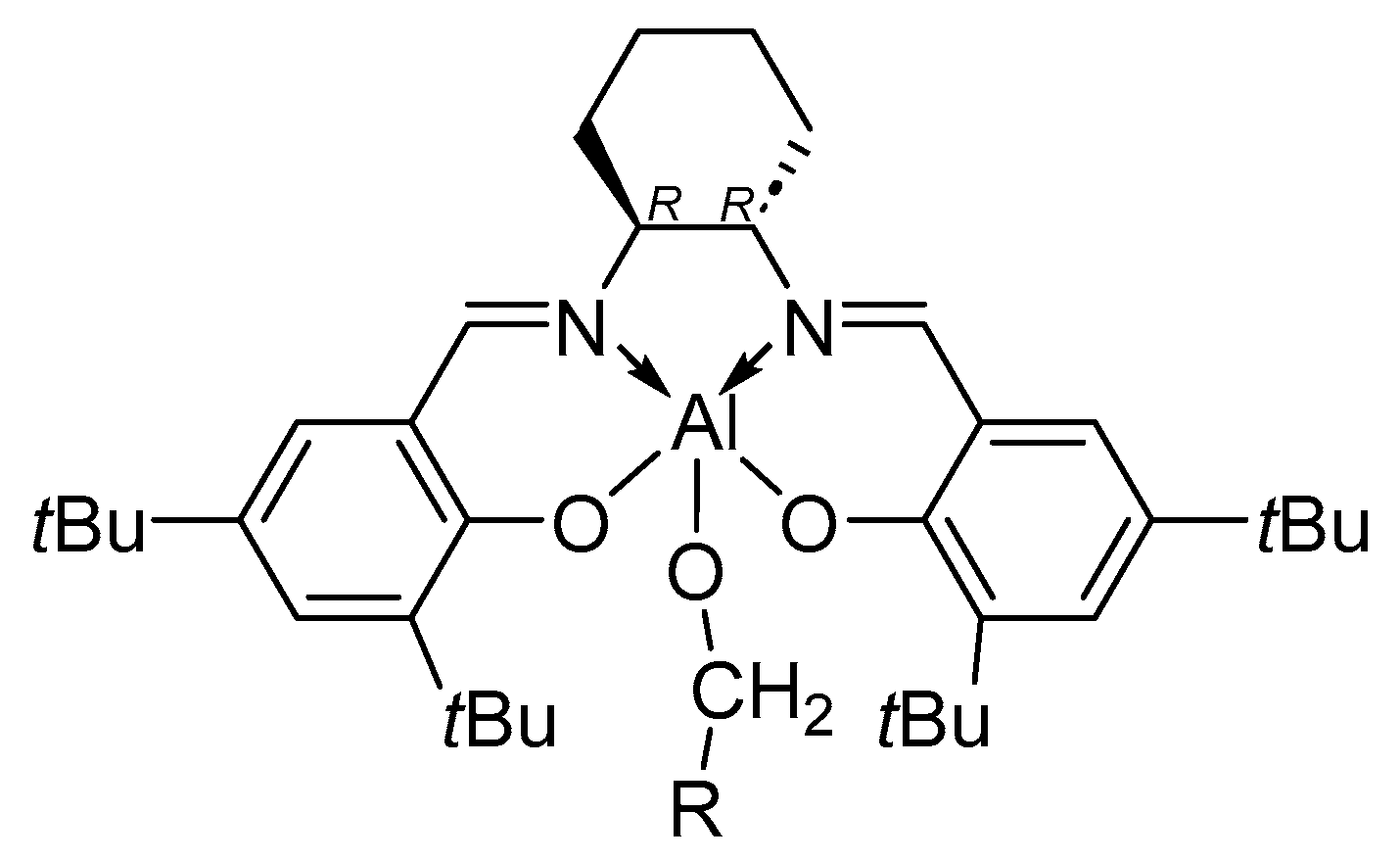
| [LA]:[Cat.] | T [K] | t [h] | Conv. [%] | Mnexp·10−3 [kg/mol] | Mncalc·10−3 [kg/mol] | OP | |
|---|---|---|---|---|---|---|---|
| 75:1 | 343 | 5 | 19.0 | 2.7 | 2.0 | 137 | 0.88 |
| 75:1 | 343 | 3.5 | 38.0 | 2.5 | 4.0 | 125 | 0.80 |
| 75:1 | 343 | 22 | 62.5 | 4.8 | 7.0 | 106 | 0.68 |
| 75:1 | 343 | 42.5 | 72.0 | 5.8 | 8.1 | 80 | 0.51 |
| 75:1 | 343 | 113 | 90.0 | 6.8 | 9.7 | 20 | 0.13 |
| 75:1 | 343 | 281 | 97.5 | 5.9 | 1.1 | 5 | 0.30 |
| Metal | [Cat.]:[iPrOH]:[LA] | T [K] | t [h] | Conv. [%] | Mnexp·10−3 [kg/mol] | Mncalc·10−3 [kg/mol] | PDI | Pi | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Y | 1:0:500 | 298 | 0.5 | 87 | 210.0 | 62.6 | 1.09 | 0.76 | [83] |
| 1:1:500 | 298 | 1 | 92 | 41.0 | 66.2 | 1.05 | 0.74 | [83] | |
| 1:1:100 | 298 | 0.3 | 92 | 12.8 | 13.2 | 1.03 | 0.77 | [83] | |
| 1:1:100 | 298 | 3 | 77 | 16.0 | 11.1 | 1.01 | 0.84 | [83] | |
| Lu | 1:0:500 | 298 | 8 | 81 | 101.7 | 58.3 | 1.06 | 0.80 | [91] |
| 1:1:500 | 298 | 9 | 84 | 38.9 | 60.5 | 1.07 | 0.75 | [91] | |
| 1:0.5:500 | 257 | 72 | 84 | 69.6 | 60.5 | 1.02 | 0.84 | [91] | |
| 1:0.5:200 | 257 | 48 | 90 | 36.0 | 26.0 | 1.02 | 0.83 | [91] | |
| La | 1:1:500 | 298 | 20 s | 98 | 57.3 | 70.6 | 1.05 | 0.28 | [91] |
| 1:2:1000 | 298 | 20 s | 93 | 50.0 | 67.0 | 1.03 | 0.28 | [91] |
| Cat. | [rac-DL]:[Cat.] | t [min] | Conv. [%] | Mnexp·10−3 [kg/mol] | PDI | I* | Pm | [mm] [%] | Tm [°C] |
|---|---|---|---|---|---|---|---|---|---|
| a | 20/1 | 20 | 100 | 4.77 | 1.17 | 74 | 0.91 | 87 | 128/136 |
| 50/1 | 20 | 100 | 10.9 | 1.05 | 80 | 0.93 | 87 | 133/143 | |
| 100/1 | 20 | 100 | 23.0 | 1.04 | 75 | 0.94 | 89 | 136/145 | |
| 200/1 | 20 | 100 | 32.0 | 1.03 | 108 | 0.93 | 89 | 146 | |
| b | 100/1 | 20 | 100 | 25.1 | 1.03 | 69 | 0.95 | 89 | 147 |
| 200/1 | 20 | 100 | 37.3 | 1.01 | 93 | 0.95 | 88 | 147 | |
| c | 100/1 | 20 | 100 | 25.7 | 1.11 | 67 | 0.96 | 93 | 153/157 |
| 200/1 | 20 | 100 | 52.7 | 1.14 | 66 | 0.96 | 94 | 156 | |
| d | 100/1 | 20 | 100 | 20.1 | 1.07 | 86 | 0.99 | 98 | 161 |
| 200/1 | 20 | 100 | 37.4 | 1.07 | 92 | >0.99 | >99 | 164 | |
| 400/1 | 20 | 100 | 64.3 | 1.02 | 107 | >0.99 | >99 | 169 | |
| 800/1 | 60 | 98 | 119 | 1.03 | 113 | >0.99 | >99 | 170 | |
| 1200/1 | 30 | 71 | 154 | 1.01 | 95 | >0.99 | >99 | 171 |
| Cat. | [LA]:[Cat.] | T [K] | t [d] | Conv. [%] | Mnexp·10−3 [kg/mol] | Mncalc·10−3 [kg/mol] | PDI | Pi | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a | 50:1 | 343 | - | >90 | 5.9 | 7.2 | 1.27 | 0.83 | [76] |
| b | 50:1 | 343 | - | >90 | 7.2 | 7.2 | 1.31 | 0.77 | [76] |
| c | 62:1 | 343 | 2 | 21.1 | 2.5 | 1.9 | 1.04 | 0.92 | [95] |
| 62:1 | 343 | 4 | 36.3 | 3.5 | 3.2 | 1.04 | - | [95] | |
| 62:1 | 343 | 24 | 87.8 | 8.4 | 7.8 | 1.08 | - | [95] | |
| 100:1 | 383 | 6 | 94.0 | 13.9 | 13.5 | 1.31 | - | [95] | |
| 200:1 | 403 | 2 | 86.4 | 23.7 | 24.7 | 1.18 | - | [95] | |
| d | 50:1 | 343 | - | >90 | 9.5 | 7.2 | 1.07 | 0.88 | [76] |
| e | 50:1 | 343 | - | >90 | 8.1 | 7.2 | 1.19 | 0.64 | [76] |
| f | 50:1 | 343 | - | >90 | 7,9 | 7.2 | 1.08 | 0.86 | [76] |
| g | 50:1 | 343 | - | >90 | 6.5 | 7.2 | 1.26 | 0.63 | [76] |
| h | 50:1 | 343 | - | >90 | 8.1 | 7.2 | 1.20 | 0.65 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillo, A.; Rusconi, Y.; D'Alterio, M.C.; De Rosa, C.; Talarico, G.; Poater, A. Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. Int. J. Mol. Sci. 2024, 25, 1647. https://doi.org/10.3390/ijms25031647
Grillo A, Rusconi Y, D'Alterio MC, De Rosa C, Talarico G, Poater A. Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. International Journal of Molecular Sciences. 2024; 25(3):1647. https://doi.org/10.3390/ijms25031647
Chicago/Turabian StyleGrillo, Andrea, Yolanda Rusconi, Massimo Christian D'Alterio, Claudio De Rosa, Giovanni Talarico, and Albert Poater. 2024. "Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials" International Journal of Molecular Sciences 25, no. 3: 1647. https://doi.org/10.3390/ijms25031647
APA StyleGrillo, A., Rusconi, Y., D'Alterio, M. C., De Rosa, C., Talarico, G., & Poater, A. (2024). Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. International Journal of Molecular Sciences, 25(3), 1647. https://doi.org/10.3390/ijms25031647









