The Genus Cladosporium: A Prospective Producer of Natural Products
Abstract
1. Introduction
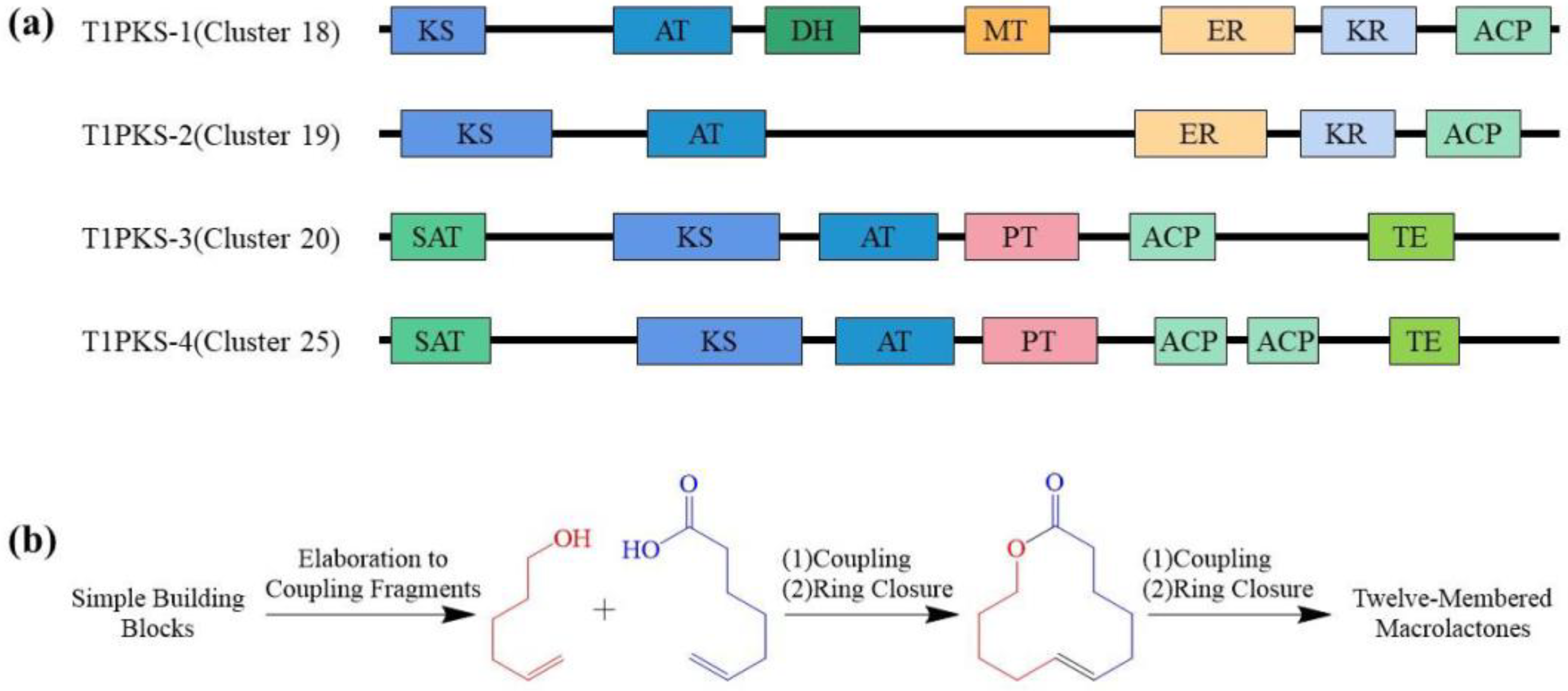
2. Polyketides
2.1. Lactones
2.2. Quinone
2.3. Linear Alkane Compounds
2.4. Other Classes of Polyketides
3. Alkaloids
4. Steroids and Terpenoids
5. Benzene Derivatives
6. Cyclic Peptides
7. Others
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X. Review of morphology classification of Cladosporium. J. Agric. Sci. 2013, 41, 6254–6255. [Google Scholar]
- De Hoog, G.S.; Guého, E.; Masclaux, F.; Gerrits Van Den Ende, A.H.G.; Kwon-Chung, K.J.; McGinnis, M.R. Nutritional physiology and taxonomy of human-pathogenic Cladosporium–Xylohypha species. Med. Mycol. 1995, 33, 339–347. [Google Scholar] [CrossRef]
- Bensch, K.; Braun, U.; Groenewald, J.; Crous, P. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Levetin, E.; Dorsey, K. Contribution of leaf surface fungi to the air spora. Aerobiologia. 2006, 22, 3–12. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Houbraken, J.; Crous, P.; Samson, R. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef]
- El-Dawy, E.G.A.E.M.; Gherbawy, Y.A.; Hussein, M.A. Morphological, molecular characterization, plant pathogenicity and biocontrol of Cladosporium complex groups associated with faba beans. Sci. Rep. 2021, 11, 14183. [Google Scholar] [CrossRef]
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Batra, N.; Kaur, H.; Mohindra, S.; Singh, S.; Shamanth, A.; Rudramurthy, S. Cladosporium sphaerospermum causing brain abscess, a saprophyte turning pathogen: Case and review of published reports. J. Med. Mycol. 2019, 29, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.; Wiederhold, N.; Cano-Lira, J.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia—Mol. Phylogeny Evol. Fungi 2016, 36, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Sellart-Altisent, M.; Torres-Rodríguez, J.M.; de Ana, S.G.; Alvarado-Ramírez, E. Microbiota fúngica nasal en sujetos alérgicos y sanos. Rev. Iberoam. Micol. 2007, 24, 125–130. [Google Scholar] [CrossRef]
- Hosoe, T.; Okada, H.; Itabashi, T.; Nozawa, K.; Okada, K.; Takaki, G.M.d.C.; Fukushima, K.; Miyaji, M.; Kawai, K.-I. A new pentanorlanostane derivative, cladosporide A, as a characteristic antifungal agent against Aspergillus fumigatus, isolated from Cladosporium sp. Chem. Pharm. Bull. 2000, 48, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Z.; Liu, X.-Z.; McKenzie, E.H.C.; Jeewon, R.; Liu, J.-K.J.; Zhang, X.-L.; Zhao, Q.; Hyde, K.D. Fungicolous fungi: Terminology, diversity, distribution, evolution, and species checklist. Fungal Divers. 2019, 95, 337–430. [Google Scholar] [CrossRef]
- Torres, D.E.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Guevara-Fefer, P.; Márquez-Guzmán, G.J.; Pérez-Martínez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, e0170782. [Google Scholar] [CrossRef] [PubMed]
- Jashni, M.K.; van der Burgt, A.; Battaglia, E.; Mehrabi, R.; Collemare, J.; de Wit, P.J.G.M. Transcriptome and proteome analyses of proteases in biotroph fungal pathogen Cladosporium fulvum. J. Plant Pathol. 2020, 102, 377–386. [Google Scholar] [CrossRef]
- Li, H.-L.; Li, X.-M.; Mándi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtán, T.; Wang, B.-G. Characterization of cladosporols from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399 and configurational revision of the previously reported cladosporol derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef]
- Wu, J.-T.; Zheng, C.-J.; Zhang, B.; Zhou, X.-M.; Zhou, Q.; Chen, G.-Y.; Zeng, Z.-E.; Xie, J.-L.; Han, C.-R.; Lyu, J.-X. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JJM22. Nat. Prod. Res. 2019, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Lin, X.; Wang, Z.; Lu, X.; Mangaladoss, F.; Yang, X.; Zhou, X.; Tu, Z.; Liu, Y. Cladosporone A, a new dimeric tetralone from fungus Cladosporium sp. KcFL6′ derived of mangrove plant Kandelia candel. J. Antibiot. 2015, 68, 213–215. [Google Scholar] [CrossRef]
- Wang, L.; Han, X.; Zhu, G.; Wang, Y.; Chairoungdua, A.; Piyachaturawat, P.; Zhu, W. Polyketides from the endophytic fungus Cladosporium sp. isolated from the mangrove plant Excoecaria agallocha. Front. Chem. 2018, 6, 344. [Google Scholar] [CrossRef]
- Yu, M.-L.; Guan, F.-F.; Cao, F.; Jia, Y.-L.; Wang, C.-Y. A new antiviral pregnane from a gorgonian-derived Cladosporium sp. fungus. Nat. Prod. Res. 2018, 32, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tomodai, H.; Tabata, N.; Miura, H.; Namikoshi, M.; Yamaguchi, Y.; Masuma, R.; Omura, S. Cladospolide D, a new 12-membered macrolide antibiotic produced by Cladosporium sp. FT-0012. J. Antibiot. 2001, 54, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, J.-T.; Zheng, C.-J.; Zhou, X.-M.; Yu, Z.-X.; Li, W.-S.; Chen, G.-Y.; Zhu, G.-Y. Bioactive cyclohexene derivatives from a mangrove-derived fungus Cladosporium sp. JJM22. Fitoterapia 2021, 149, 104823. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-Hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity potential of marine natural products from scleractinia-associated microbes and in silico anti-SARS-COV-2 evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Gao, H.; Wu, C.; Zhu, T.; Che, Q.; Gu, Q.; Guo, P.; Li, D. Lipid-lowering polyketides from a soft coral-derived fungus Cladosporium sp. TZP29. Bioorg. Med. Chem. Lett. 2015, 25, 3606–3609. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lin, T.; Wang, W.; Xin, Z.; Zhu, T.; Gu, Q.; Li, D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013, 76, 1133–1140. [Google Scholar] [CrossRef]
- Moghaddam, J.A.; Jautzus, T.; Alanjary, M.; Beemelmanns, C. Recent highlights of biosynthetic studies on marine natural products. Org. Biomol. Chem. 2021, 19, 123–140. [Google Scholar] [CrossRef]
- Pang, X.; Lin, X.; Wang, J.; Liang, R.; Tian, Y.; Salendra, L.; Luo, X.; Zhou, X.; Yang, B.; Tu, Z.; et al. Three new highly oxygenated sterols and one new dihydroisocoumarin from the marine sponge-derived fungus Cladosporium sp. SCSIO41007. Steroids 2018, 129, 41–46. [Google Scholar] [CrossRef]
- AlMatar, M.; Makky, E.A. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech 2016, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 13, 3959. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped potential of marine-associated Cladosporium species: An overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 2021, 11, 645. [Google Scholar] [CrossRef]
- Höller, U.; Gloer, J.B.; Wicklow, D.T. Biologically active polyketide metabolites from an undetermined fungicolous hyphomycete resembling Cladosporium. J. Nat. Prod. 2002, 65, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Qin, S.; Li, F.; Chi, X.; Laatsch, H. Isolation, antimicrobial activity, and metabolites of fungus Cladosporium sp. associated with red alga Porphyra yezoensis. Curr. Microbiol. 2008, 56, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Gesner, S.; Cohen, N.; Ilan, M.; Yarden, O.; Carmeli, S. Pandangolide 1a, a metabolite of the sponge-associated fungus Cladosporium sp., and the absolute stereochemistry of pandangolide 1 and iso-cladospolide B. J. Nat. Prod. 2005, 68, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Yang, Q.; Shao, C.-L.; Kong, C.-J.; Zheng, J.-J.; Liu, Y.-F.; Wang, C.-Y. Bioactive 7-oxabicyclic [6.3.0] lactam and 12-membered macrolides from a gorgonian-derived Cladosporium sp. fungus. Mar. Drugs 2015, 13, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Z.; Li, X.-M.; Li, X.; Yang, S.-Q.; Meng, L.-H.; Wang, B.-G. Polyketides from the mangrove-derived endophytic fungus Cladosporium cladosporioides. Mar. Drugs 2019, 17, 296. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Kasai, Y.; Komatsu, K.; Tsuda, M.; Mikami, Y.; Kobayashi, J. Sporiolides A and B, new cytotoxic twelve-membered macrolides from a marine-derived fungus Cladosporium species. Mar. Drugs 2004, 2, 164–169. [Google Scholar] [CrossRef]
- Wuringege; Guo, Z.-K.; Wei, W.; Jiao, R.-H.; Yan, T.; Zang, L.-Y.; Jiang, R.; Tan, R.-X.; Ge, H.-M. Polyketides from the plant endophytic fungus Cladosporium sp. IFB3lp-2. J. Asian Nat. Prod. Res. 2013, 15, 928–933. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tan, H.-B.; Li, S.-N.; Chen, Y.-C.; Li, H.-H.; Qiu, S.-X.; Zhang, W.-M. Two new 12-membered macrolides from the endophytic fungal strain Cladosprium colocasiae A801 of Callistemon viminalis. J. Asian Nat. Prod. Res. 2019, 21, 696–701. [Google Scholar] [CrossRef]
- Huang, C.; Chen, T.; Yan, Z.; Guo, H.; Hou, X.; Jiang, L.; Long, Y. Thiocladospolide E and cladospamide A, novel 12-membered macrolide and macrolide lactam from mangrove endophytic fungus Cladosporium sp. SCNU-F0001. Fitoterapia 2019, 137, 104246. [Google Scholar] [CrossRef]
- Ma, C.; Meng, C.; Peng, C.; Xiong, L.; Zhou, Q. Isolation and identification of secondary metabolites of endophytic fungus Cladosporium sp. and study of antimicrobial activity. Nat. Prod. Res. 2019, 31, 69–74. [Google Scholar]
- Zhang, F.-Z.; Li, X.-M.; Meng, L.-H.; Wang, B.-G. Cladocladosin A, an unusual macrolide with bicyclo 5/9 ring system, and two thiomacrolides from the marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299. Bioorg. Chem. 2020, 101, 103950. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, H.; Sun, C.; Che, Q.; Zhang, G.; Zhu, T.; Li, D. Thiocladospolides F-J, antibacterial sulfur containing 12-membered macrolides from the mangrove endophytic fungus Cladosporium oxysporum HDN13-314. Phytochemistry 2020, 178, 112462. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A. From Total Synthesis to Diverted Total Synthesis: Case Studies in the Amphidinolide Series. Isr. J. Chem. 2011, 51, 329–345. [Google Scholar] [CrossRef]
- Szpilman, A.M.; Carreira, E.M. Probing the biology of natural products: Molecular editing by diverted total synthesis. Angew. Chem. Int. Ed. 2010, 49, 9592–9628. [Google Scholar] [CrossRef] [PubMed]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery an underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.; Collins, I. Macrocycles in new drug discovery. Futur. Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Marsault, E.; Peterson, M.L. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef]
- Kopp, F.; Stratton, C.F.; Akella, L.B.; Tan, D.S. A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion. Nat. Chem. Biol. 2012, 8, 358–365. [Google Scholar] [CrossRef]
- Wang, F.W.; Jiao, R.H.; Cheng, A.B.; Tan, S.H.; Song, Y.C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J. Microbiol. Biotechnol. 2007, 23, 79–83. [Google Scholar] [CrossRef]
- Betina, V. Effects of the macrolide antibiotic cyanein on HeLa cells growth and metabolism. Neoplasma 1969, 16, 23–32. [Google Scholar]
- Tamura, G.; Ando, K.; Suzuki, S.; Takatsuki, A.; Arima, K. Antiviral activity of brefeldin A and verrucarin A. J. Antibiot. 1968, 21, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Li, S.; Shao, M.-W.; Xiao, X.-H.; Kong, L.-C.; Jiang, D.-H.; Zhang, Y.-L. Isolation, identification, derivatization and phytotoxic activity of secondary metabolites produced by Cladosporium oxysporum DH14, a locust-associated fungus. J. Integr. Agric. 2016, 15, 832–839. [Google Scholar] [CrossRef]
- Wang, X.; Radwan, M.M.; Taráwneh, A.H.; Gao, J.; Wedge, D.E.; Rosa, L.H.; Cutler, H.G.; Cutler, S.J. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J. Agric. Food Chem. 2013, 61, 4551–4555. [Google Scholar] [CrossRef]
- Liu, H.; Luo, D. Study on secondary metabolites of Cladosporium. Chem. Eng. Commun. 2019, 45, 2. [Google Scholar]
- Bai, M.; Wang, H.; Lian, Y.; Liu, Y.; Liu, T.; Zheng, C.; Chen, G. Studies on secondary metabolites from the endophytic fungus Cladosporium sp. JS1-2 from mangrove plant Ceriops tagal. Zhongguo Kangshengsu Zazhi 2020, 45, 1166–1169. [Google Scholar] [CrossRef]
- Nasini, G.; Arnone, A.; Assante, G.; Bava, A.; Moricca, S.; Ragazzi, A. Secondary mould metabolites of Cladosporium tenuissimum, a hyperparasite of rust fungi. Phytochemistry 2004, 65, 2107–2111. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yagi, A.; Akaishi, M.; Kirikoshi, R.; Takahashi, O.; Abe, T.; Chiba, S.; Takahashi, K.; Iwakura, N.; Namikoshi, M.; et al. Halogenated cladosporols produced by the sodium halide-supplemented fermentation of the plant-associated fungus Cladosporium sp. TMPU1621. Tetrahedron Lett. 2018, 59, 1913–1915. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, L.; Kong, F.; Ma, Q.; Xie, Q.; Li, J.; Dai, H.; Guo, L.; Zhao, Y. Altertoxins with quorum sensing inhibitory activities from the marine-derived fungus Cladosporium sp. KFD33. Mar. Drugs 2020, 18, 67. [Google Scholar] [CrossRef]
- Khan, I.H.; Sohrab, H.; Rony, S.R.; Tareq, F.S.; Hasan, C.M.; Mazid, A. Cytotoxic and antibacterial naphthoquinones from an endophytic fungus, Cladosporium sp. Toxicol. Rep. 2016, 3, 861–865. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Cai, C.; Chen, H.; Dai, Y.; Chen, P.; Kong, F.; Yuan, J.; Song, X.; Mei, W.; et al. Two new succinimide derivatives cladosporitins A and B from the mangrove-derived fungus Cladosporium sp. HNWSW-1. Mar. Drugs 2019, 17, 4. [Google Scholar] [CrossRef]
- Zurlo, D.; Leone, C.; Assante, G.; Salzano, S.; Renzone, G.; Scaloni, A.; Foresta, C.; Colantuoni, V.; Lupo, A. Cladosporol a stimulates G1-phase arrest of the cell cycle by up-regulation of p21waf1/cip1 expression in human colon carcinoma HT-29 cells. Mol. Carcinog. 2013, 52, 1–17. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.; Xu, A.; Pubu, D.; Chen, B.; Wang, J. Secondary metabolites of endophytic Cladosporium sp. J6 from endangered Chrysosplenium carnosum. Zhongshan Da Xue Xue Bao Zi Ran Ke Xue Ban 2015, 54, 84–86. [Google Scholar]
- Demuner, A.J.; Barbosa, L.C.A.; Miranda, A.C.M.; Geraldo, G.C.; da Silva, C.M.; Giberti, S.; Bertazzini, M.; Forlani, G. The fungal phytotoxin alternariol 9-methyl ether and some of its synthetic analogues inhibit the photosynthetic electron transport chain. J. Nat. Prod. 2013, 76, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, P.; Kong, F.; Mei, W.; Guo, Z.; Chen, H.; Dai, H. Secondary metabolites from the endophytic fungus Cladosporium cladosporioides JG-12 of Ceriops tagal and their biological activity. J. Trop. Ecol. 2017, 8, 29–36. [Google Scholar]
- Chang, J.; Tian, X.; Fan, C.; Huang, J.; Lu, Y.; Han, Q. Secondary metabolites from the antarctic fungi Cladosporium sp. NJF4 and NJF6. Chin. J. Polar Res. 2020, 32, 8. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.-L.; Yang, R.-Y.; Zheng, C.-J.; Chen, Y.-Y.; Fu, X.-M.; Qian, P.-Y.; She, Z.-G.; de Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kong, F.; Wang, Y.; Fu, P.; Zhu, W. Cladodionen, a cytotoxic hybrid polyketide from the marine-derived Cladosporium sp. OUCMDZ-1635. Mar. Drugs 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Rotinsulu, H.; Yamazaki, H.; Sugai, S.; Iwakura, N.; Wewengkang, D.S.; Sumilat, D.A.; Namikoshi, M. Cladosporamide A, a new protein tyrosine phosphatase 1B inhibitor, produced by an Indonesian marine sponge-derived Cladosporium sp. J. Nat. Med. 2018, 72, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Zhang, X.-Y.; Xu, X.-Y.; Qi, S.-H. New citrinin derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z015. Nat. Prod. Res. 2020, 34, 1219–1226. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, P.; Zhang, Y.; Xu, Y.; Zhang, C.; Liu, X.; Che, Y. Cladoxanthones A and B, xanthone-derived metabolites with a spiro[cyclopentane-1,2′-[3,9a] ethanoxanthene]-2,4′,9′,11′-tetraone skeleton from a Cladosporium sp. J. Nat. Prod. 2022, 85, 2541–2546. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Pharmacokinetics, pharmacodynamics, toxicity, and formulations of daidzein: An important isoflavone. Phytother. Res. 2023, 37, 2578–2604. [Google Scholar] [CrossRef]
- Zheng, C.; Fang, Y.; Tong, W.; Li, G.; Wu, H.; Zhou, W.; Lin, Q.; Yang, F.; Yang, Z.; Wang, P.; et al. Synthesis and biological evaluation of novel tetrahydro-β-carboline derivatives as antitumor growth and metastasis agents through inhibiting the transforming growth factor-β Signaling Pathway. J. Med. Chem. 2014, 57, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Tonin, L.T.D.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and antiviral activity of β-carboline derivatives bearing a substituted carbohydrazide at C-3 against poliovirus and herpes simplex virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Dan, W.; Li, J.; Zhang, Q.; Bai, H.; Wang, J. Synthesis and antimicrobial activities of 3-methyl-β-carboline deriva-tives. Nat. Prod. Commun. 2015, 10, 899–902. [Google Scholar] [PubMed]
- Gu, B.; Zhang, Y.; Ding, L.; He, S.; Wu, B.; Dong, J.; Zhu, P.; Chen, J.; Zhang, J.; Yan, X. Preparative separation of sulfur-containing diketopiperazines from marine fungus Cladosporium sp. using high-speed counter-current chromatography in stepwise elution mode. Mar. Drugs 2015, 13, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-H.; Nong, X.-H.; Liang, X.; Qi, S.-H. New tetramic acid derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z0025. Tetrahedron 2018, 74, 2620–2626. [Google Scholar] [CrossRef]
- Han, X.; Bao, X.-F.; Wang, C.-X.; Xie, J.; Song, X.-J.; Dai, P.; Chen, G.-D.; Hu, D.; Yao, X.-S.; Gao, H. Cladosporine A, a new indole diterpenoid alkaloid with antimicrobial activities from Cladosporium sp. Nat. Prod. Res. 2021, 35, 1115–1121. [Google Scholar] [CrossRef]
- Hosoe, T.; Okamoto, S.; Nozawa, K.; Kawai, K.-I.; Okada, K.; Takaki, G.M.D.C.; Fukushima, K.; Miyaji, M. New pentanorlanostane derivatives, cladosporide B-D, as characteristic antifungal agents against Aspergillus fumigatus, isolated from Cladosporium sp. J. Antibiot. 2001, 54, 747–750. [Google Scholar] [CrossRef][Green Version]
- Zou, J.; Dai, J. Study on chemical constituents in marine fungus of Cladosporium cladosporioides. Chin. Pharm. J. 2009, 44, 418–421. [Google Scholar]
- Shen, G.; Oh, S.-R.; Min, B.-S.; Lee, J.; Ahn, K.S.; Kim, Y.H.; Lee, H.-K. Phytochemical investigation of Tiarella polyphylla. Arch. Pharmacal Res. 2008, 31, 10–16. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Bu, M.; Hu, L.; Du, W.W.; Jiao, C.; Pan, H.; Sdiri, M.; Wu, N.; Xie, Y.; et al. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 2016, 7, 33948–33959. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shi, W.; Liu, X.; Zhao, X.; Zhang, Z. Anticancer action and mechanism of ergosterol peroxide from paecilomyces cicadae fermentation broth. Int. J. Mol. Sci. 2018, 19, 3935. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-U.; Park, Y.-J. Ergosterol peroxide from the medicinal mushroom ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yang, Y.; Ding, J.; Guan, S.; Guo, D. Chemical constituents from the fruiting body of Ganoderma lucidum with cytotoxicity investigations. J. S. Pharm. Univ. 2014, 31, 102–106. [Google Scholar]
- Qin, W.; Jiang, W.; Li, H.; Xue, Y.; Liu, C.; Liu, S. Isolation and identification of main substance ergosterol from the en-dophytic fungus MG-9 and its antioxidant activity analysis. J of China Three Gorges Univ. (Natl Sci.) 2017, 39, 108–112. [Google Scholar]
- Mitome, H.; Shirato, N.; Hoshino, A.; Miyaoka, H.; Yamada, Y.; Van Soest, R.W. New polyhydroxylated sterols stylisterols A–C and a novel 5,19-cyclosterol hatomasterol from the Okinawan marine sponge Stylissa sp. Steroids 2005, 70, 63–70. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lim, S.W.; Kim, B.S.; Lee, J.Y.; Moon, S.S. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl. Environ. Microbiol. 2001, 67, 3739–3745. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.-Y.; Kuk, J.-H.; Moon, J.-H.; Kim, Y.-C.; Park, K.-H. Identification and antimicrobial activity of phenylacetic acid produced by Bacillus licheniformis isolated from fermented soybean, Chungkook-Jang. Curr. Microbiol. 2004, 48, 312–317. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.; Iwasaki, H. p-Hydroxyphenylacetic acid and other phenolic compounds as growth stimulators of the red alga Porphyra tenera. Plant Sci. Lett. 1976, 6, 299–307. [Google Scholar] [CrossRef]
- Celik, S.; Ozel, A.E.; Akyuz, S. Comparative study of antitumor active cyclo(Gly-Leu) dipeptide: A computational and molecular modeling study. Vib. Spectrosc. 2016, 83, 57–69. [Google Scholar] [CrossRef]
- Wen, L.; Wei, Q.; Chen, G.; Cai, J.; She, Z. Chemical constituents from the mangrove endophytic fungus Sporothrix sp. Chem. Nat. Compd. 2013, 49, 137–140. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]

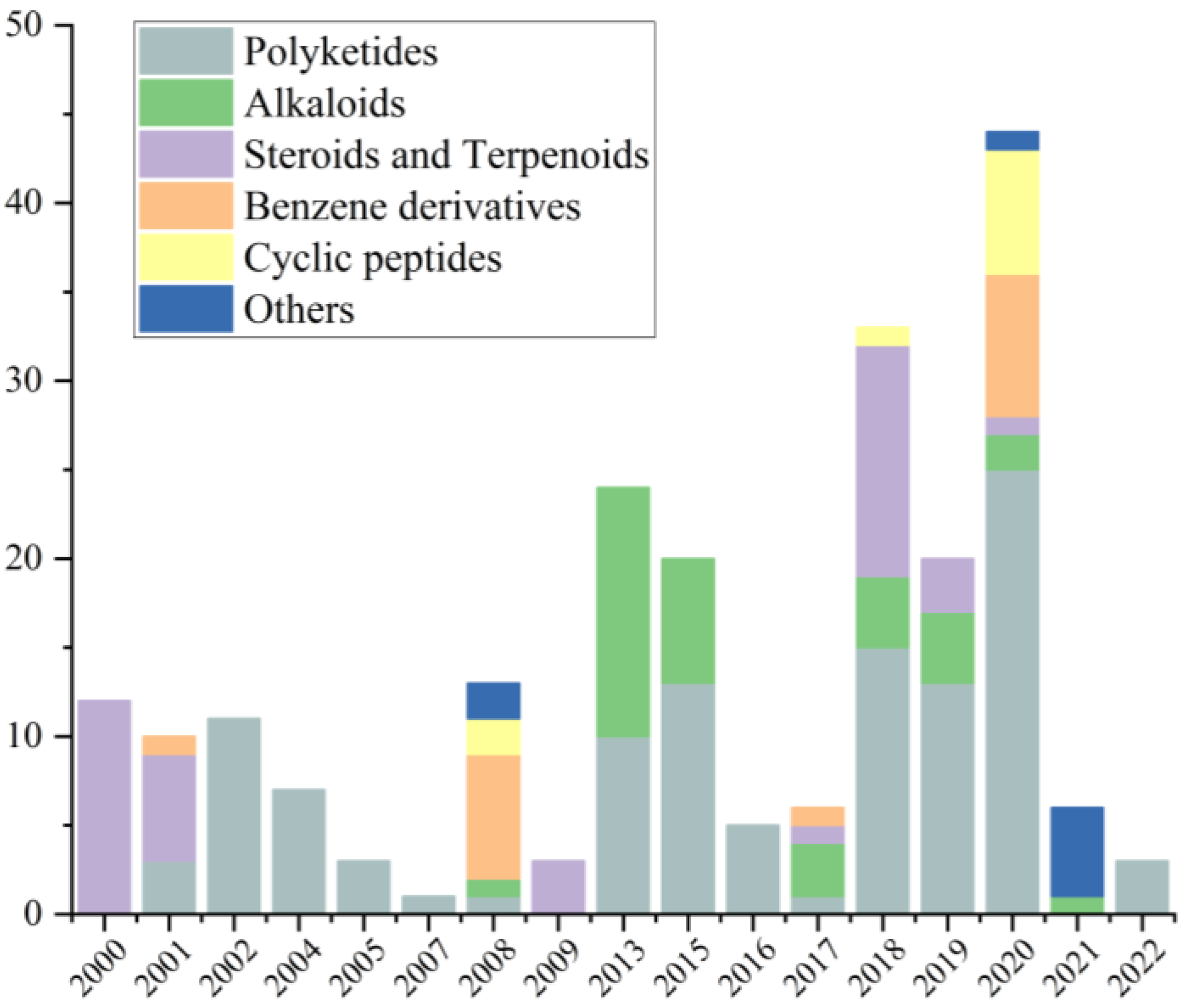
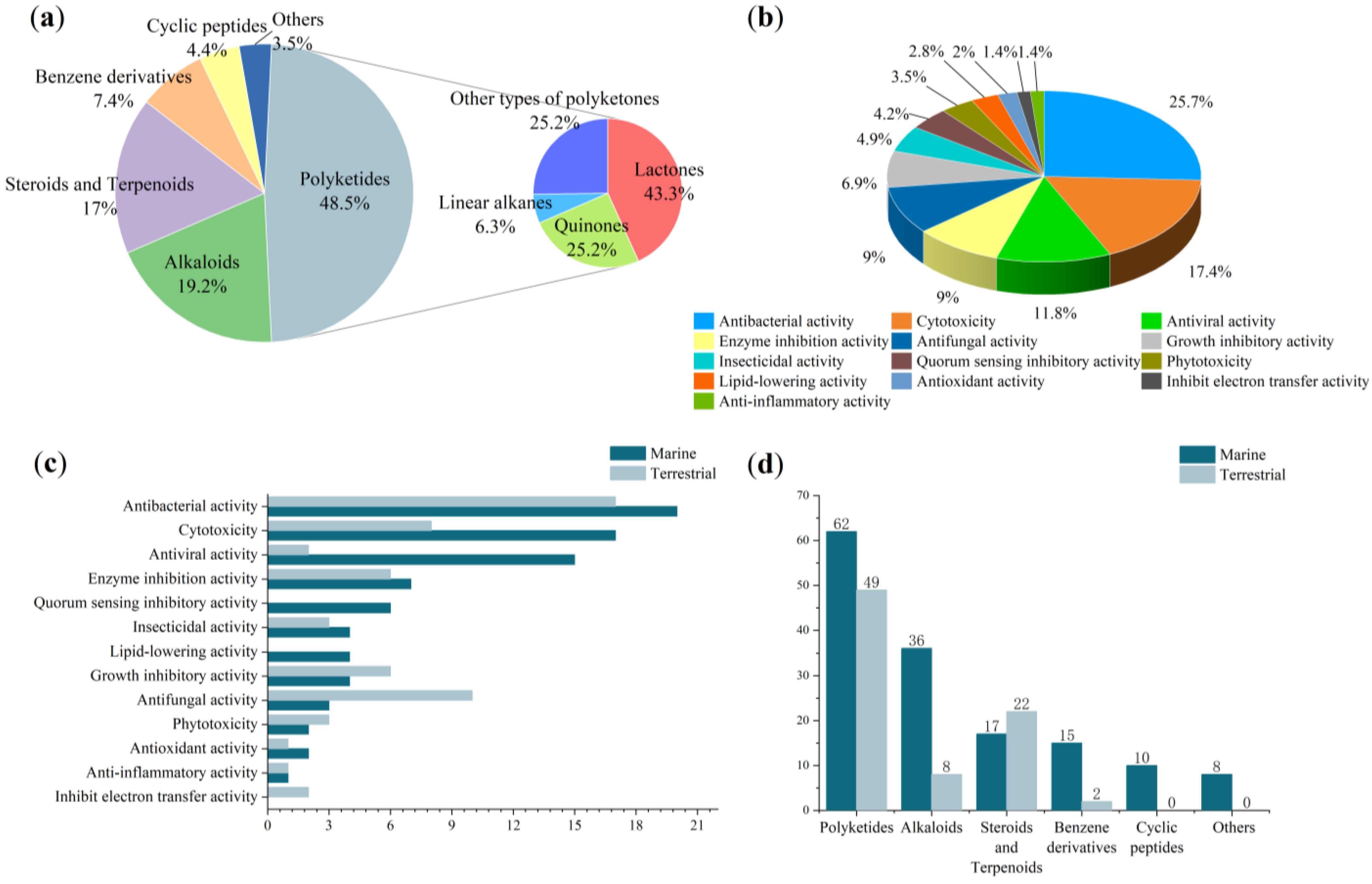
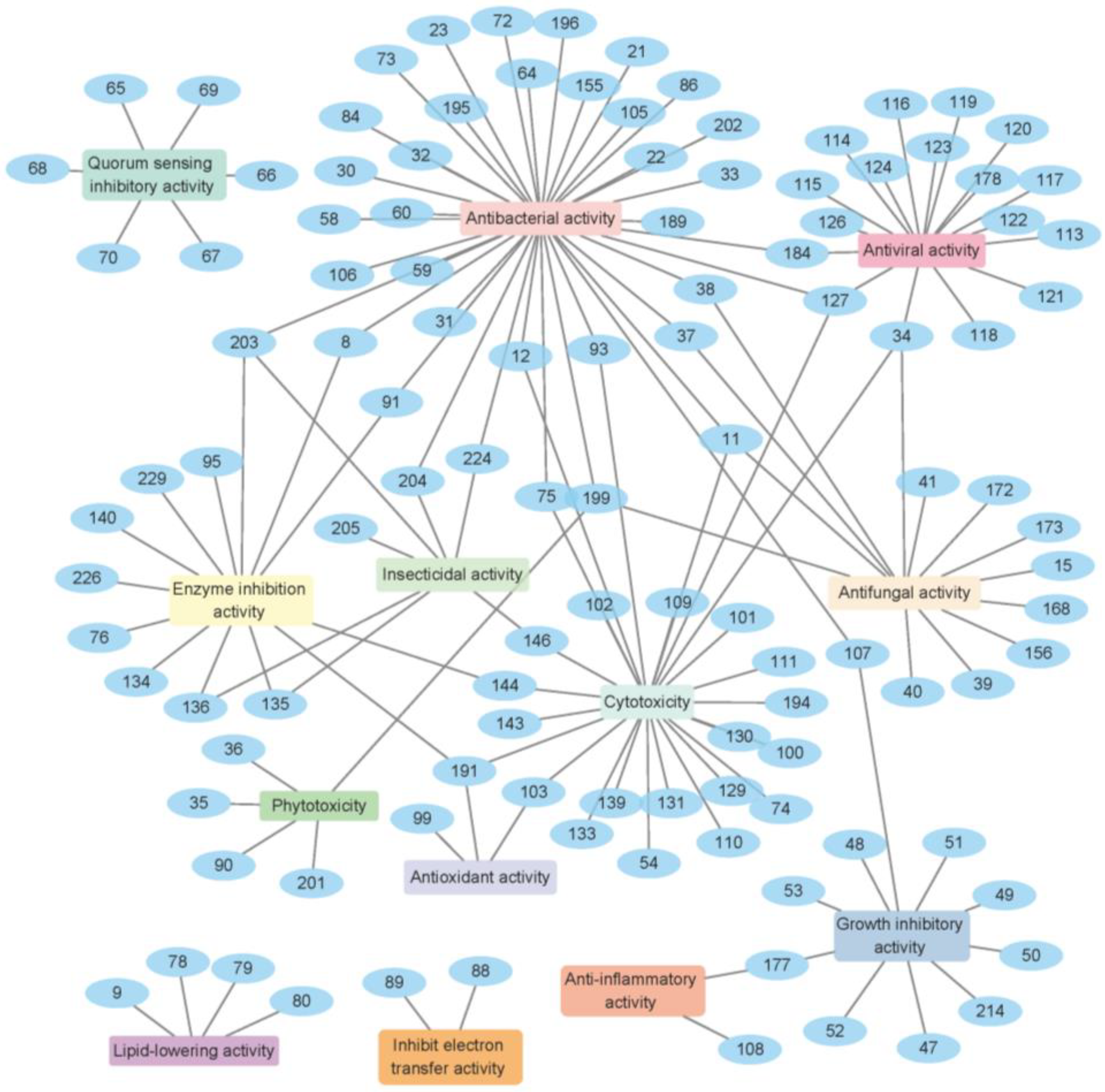
| Types | Comps. | Sources | Distribution | Years | Refs. |
|---|---|---|---|---|---|
| Polyketides | 1–4 | Cladosporium sp. NRRL 29097 | Malette | 2002 | [32] |
| 5 | Cladosporium sp. N5 | Jiangsu China | 2008 | [33] | |
| 6 | Sponge Niphatesrowi sp. derived Cladosporium sp. DQ100370 | the Red Sea | 2005 | [34] | |
| 7 | Gorgonian Anthogorgia ochracea (GXWZ-07)-derived Cladosporium sp. RA07-1 (GenBank No. KP720581) | the South China Sea | 2015 | [35] | |
| 8–10 | Soft coral-derived fungus Cladosporium sp. TZP-29 (GenBank No. KR817674) | 2019 | [36] | ||
| 11–12 | Marine-derived fungus Cladosporium sp. L037 | Okinawa Island | 2004 | [37] | |
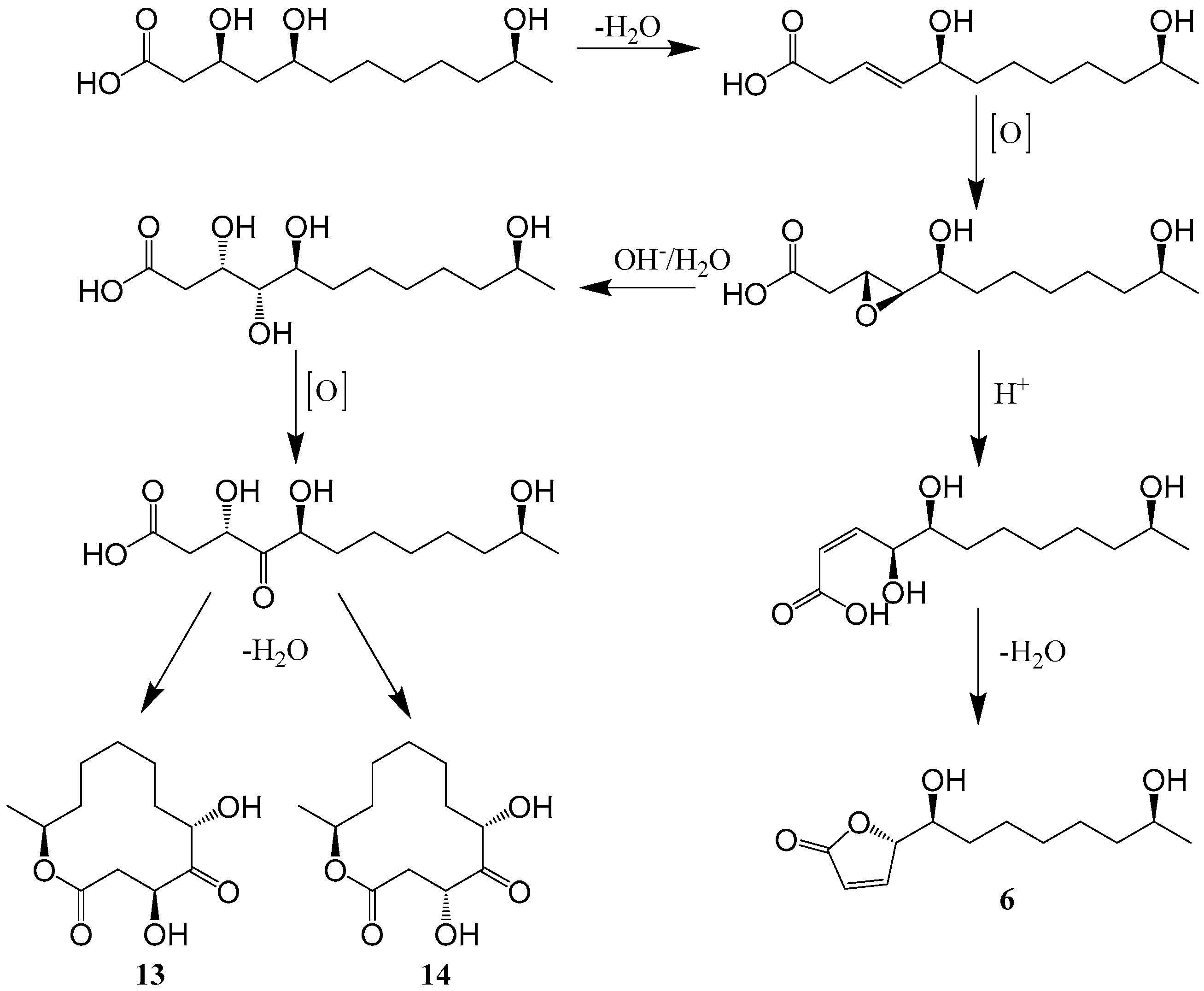
| 13–14 | Sponge Niphatesrowi sp. derived Cladosporium sp. DQ100370 | the Red Sea | 2005 | [34] | |
| 15–17 | Sponge-derived Cladosporium sp. FT-0012 | Pohnpei island | 2001 | [22] | |
| 18–19 | Plant Rhizophora stylosa-derived endophytic fungus Cladosporium sp. IFB3lp-2 | Hainan China | 2013 | [38] | |
| 20–24 | Gorgonian Anthogorgia ochracea (GXWZ-07)-derived Cladosporium sp. RA07-1 (GenBank No. KP720581) | the South China Sea | 2015 | [35] | |
| 25–26 | plant Callistemon viminalis-derived fungus Cladosporium sp. A801 (GenBank No.MF138133) | Guangzhou China | 2019 | [39] | |
| 27–29 | Mangrove endophytic fungus Cladosporium sp. SCNU-F0001 (GenBank NO. NG062723.1) | Guangzhou China | 2019 | [40] | |
| 30 | Endophytic fungus Cladosporium sp. IS384 (GenBank NO. KU158172) | Sichuan China | 2019 | [41] | |
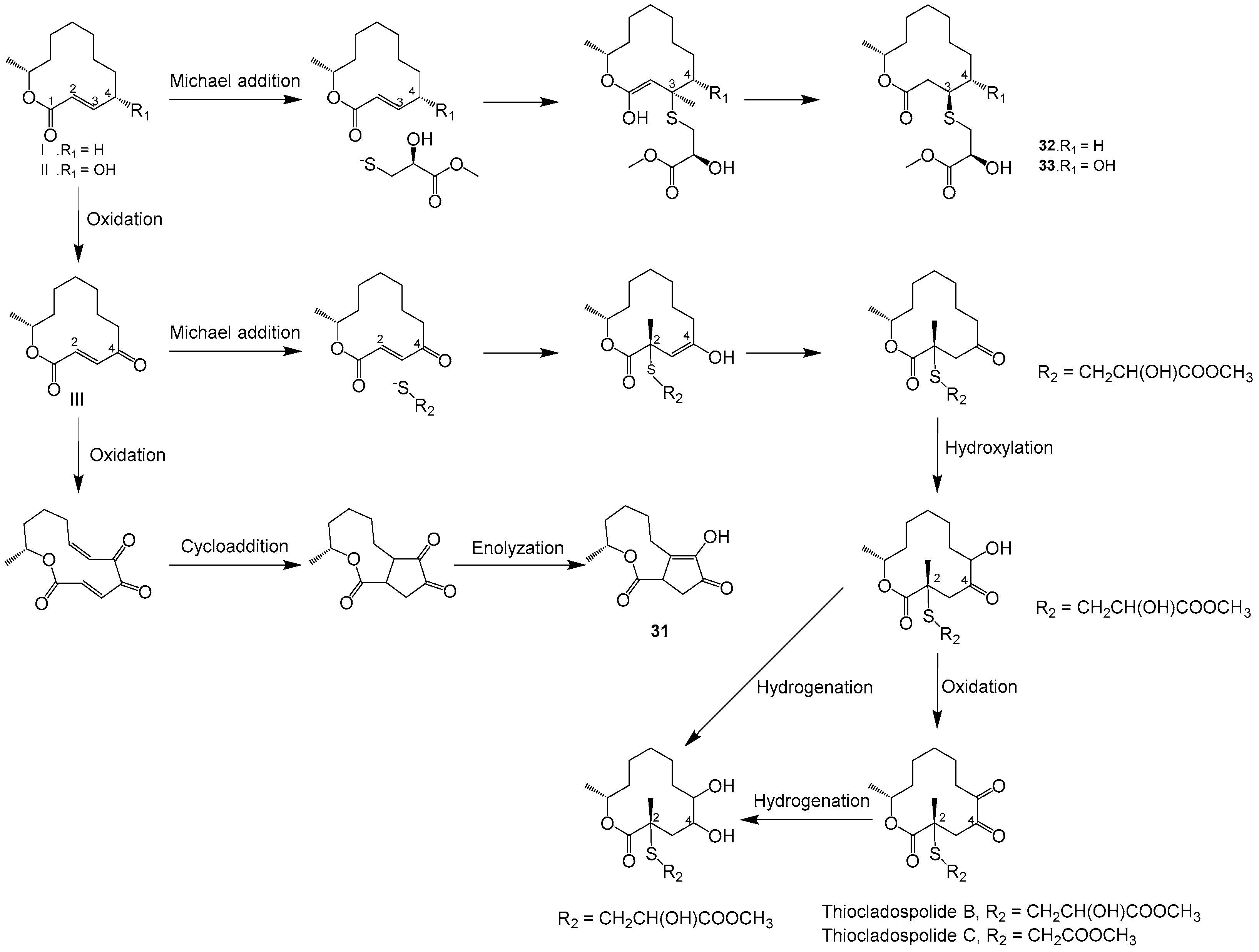
| 31–33 | Marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299 (GenBank No. MH822624) | Hainan China | 2020 | [42] | |
| 34 | Cladosporium sp. I(R)9-2 | Nanjing China | 2007 | [50] | |
| 35–36 | Cladosporium oxysporum DH14 (GenBank No. JN887339.1) | Jiangsu China | 2016 | [53] | |
| 37–41 | Endophytic fungus Cladosporium cladosporioides | Tifton | 2013 | [54] | |
| 42–43 | Plant Ammopiptanthus mongolicus-derived fungus Cladosporium cladosporioides | 2019 | [55] | ||
| 44–48 | Plant Ceriops tagal-derived fungus Cladosporium sp. JS1-2 | Hainan China | 2020 | [56] | |
| 49–53 | Cladosporium tenuissimum ITT21 | Tuscany Italy | 2004 | [57] | |
| 54–57 | Mangrove plant Kandelia candel-derived Cladosporium sp. KcFL6′ | Guangzhou China | 2015 | [19] | |
| 58–64 | Cladosporium sp. TMPU1621 | Okinawa Japan | 2018 | [58] | |
| 65–70 | Cladosporium sp. KFD33 (GenBank No.MN737504) | Hainan China | 2020 | [59] | |
| 71–73 | Cladosporium sp. NRRL 29097 | Red River | 2002 | [32] | |
| 74–75 | Cladosporium sp. RSBE-3 | 2016 | [60] | ||
| 76 | Mangrove-derived fungus Cladosporium sp. HNWSW-1 (GenBank No. MH 535968) | Hainan China | 2019 | [61] | |
| 77 | Plant Rhizophora stylosa-derived endophytic fungus Cladosporium sp. IFB3lp-2 | Hainan China | 2013 | [38] | |
| 78–80 | Soft coral-derived fungus Cladosporium sp. TZP-29 (GenBank NO.KR817674) | 2015 | [25] | ||
| 81–82 | Cladosporium sp. OUCMDZ-302 | Hainan China | 2018 | [20] | |
| 83 | Plant Ammopiptanthus mongolicus-derived fungus Cladosporium cladosporioides | 2019 | [55] | ||
| 84–87 | Cladosporium sp. NRRL 29097 | Red River | 2002 | [31] | |
| 88–89 | Plant endangered Chrysosplenium carnosum-derived fungus Cladosporium sp. J6 (GenBank NO.KR492688) | Tibet China | 2013 | [64] | |
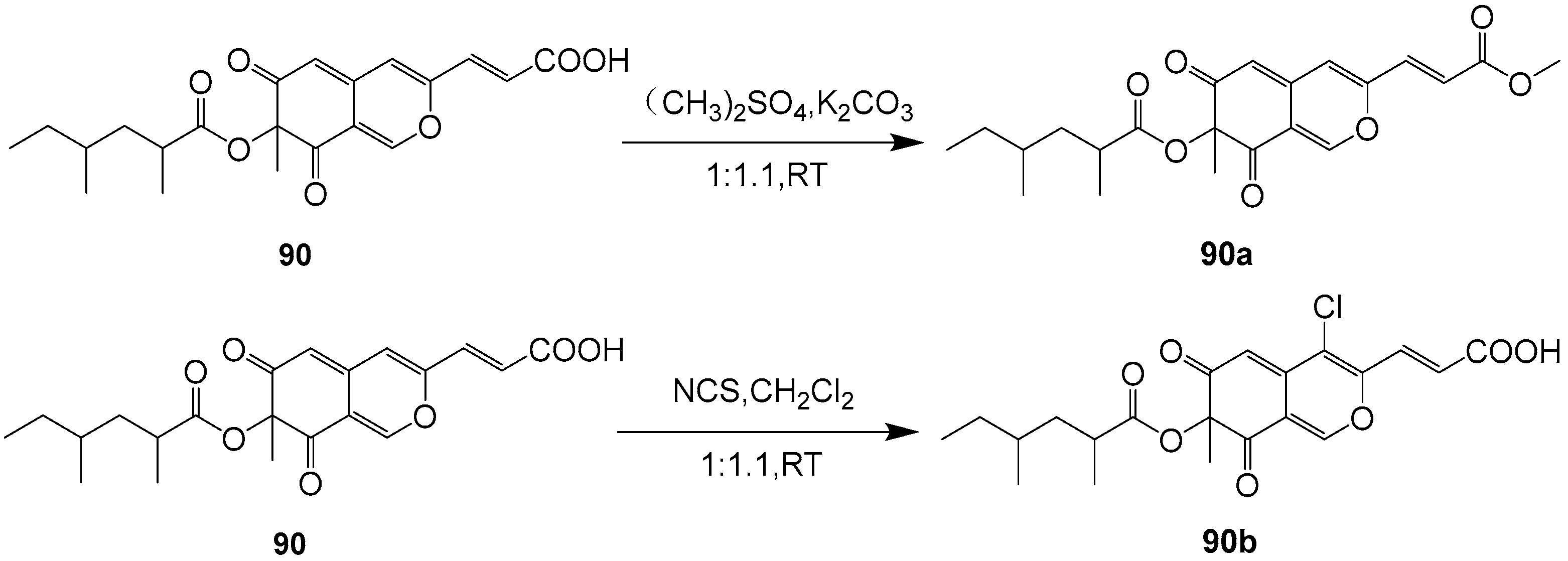
| 90 | Cladosporium oxysporum DH14 (GenBank NO. JN887339.1) | 2016 | [53] | ||
| 91 | Cladosporium cladosporioides JG-12 | Hainan China | 2017 | [65] | |
| 92–93 | Marine sediment-derived Cladosporium sp. NJF6 | Antarctica | 2020 | [66] | |
| 94 | Marine-derived Cladosporium sp. OUCMDZ-1635 (GenBank No. KT336457) | Xisha Islands of China | 2018 | [68] | |
| 95 | Marine sponge-derived Cladosporium sp. TPU1507 | Manado, Indonesia | 2018 | [69] | |
| 96–99 | Cladosporium sp. OUCMDZ-302 | Hainan, China | 2018 | [20] | |
| 100–103 | Deep sea-derived fungus Cladosporium sp. SCSIO z015 (GenBank No. KX258800) | Okinawa Trough | 2020 | [70] | |
| 104–107 | Cladosporium sp. JS1-2 | Hainan China | 2020 | [56] | |
| 108 | Marine sediment-derived Cladosporium sp. NJF6 | Antarctica | 2020 | [66] | |
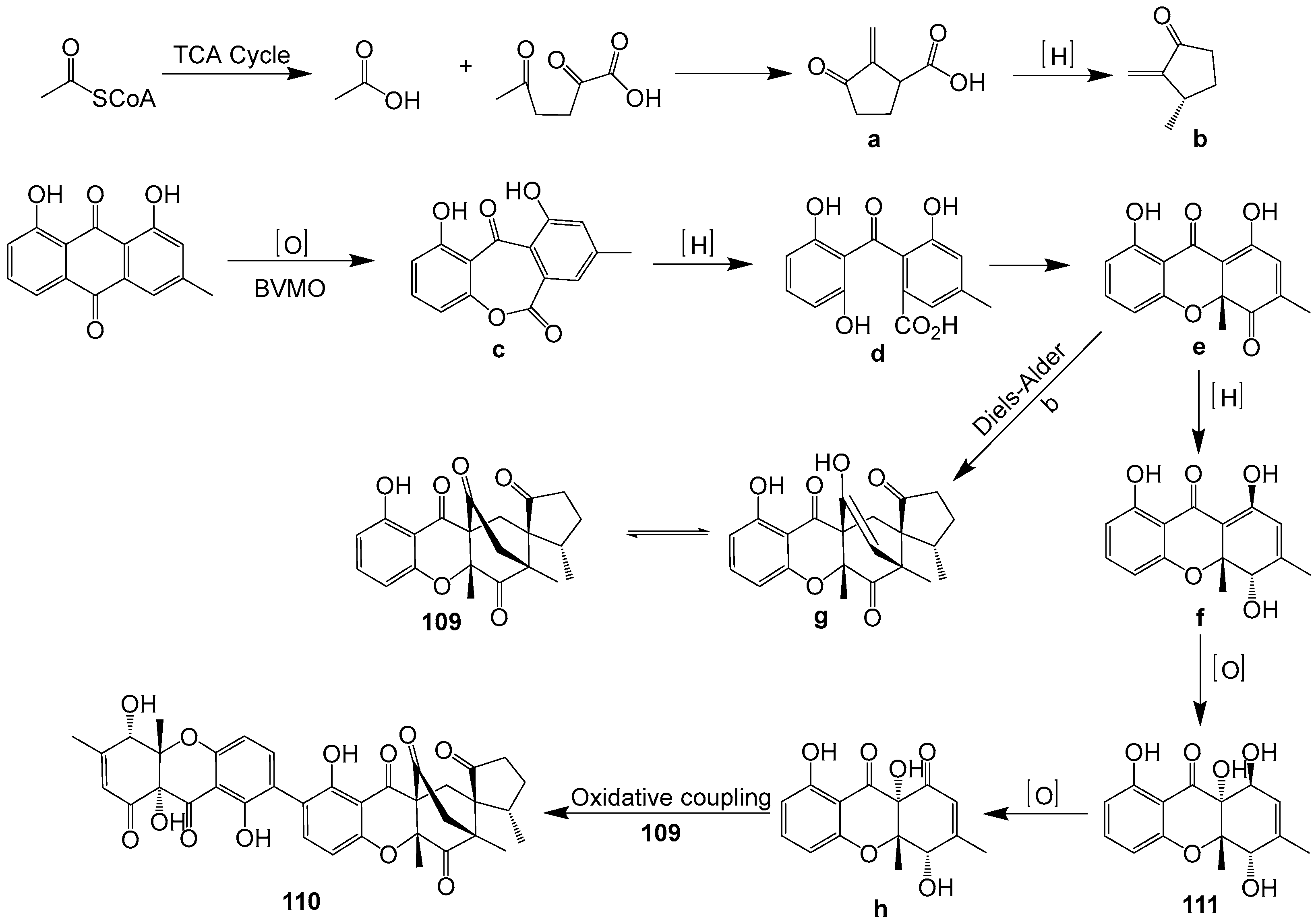
| 109–111 | Cladosporium sp. (GenBank No. QH071013) | Qinghai China | 2022 | [71] | |
| Alkaloids | 112 | Cladosporium sp. N5(GenBank No. EF424419) | Lianyungang China | 2008 | [33] |
| 113–126 | Mangrove-derived fungus Cladosporium sp. PJX-41 (GenBank No. KC589122) | Guangzhou China | 2013 | [26] | |
| 127–128 | Plant endangered Chrysosplenium carnosum-derived fungus Cladosporium sp. J6 (GenBank NO. KR492688) | Tibet China | 2015 | [63] | |
| 129–131 | Marine sediment-derived Cladosporium sp. | Zhejiang China | 2015 | [76] | |
| 132 | Plant endangered Chrysosplenium carnosum-derived fungus Cladosporium sp. J6 (GenBank NO. KR492688) | Tibet China | 2015 | [63] | |
| 133 | Gorgonian Anthogorgia ochracea (GXWZ-07)-derived Cladosporium sp.RA07-1 (GenBank NO. KP720581) | the South China Sea | 2015 | [35] | |
| 134–136 | Cladosporium cladosporioides JG-12 | Hainan China | 2017 | [65] | |
| 137–138 | Marine sponge Callyspongia sp. derived fungus Cladosporium sp. scsio41007 (GenBank NO.MF188197) | Guangzhou China | 2018 | [28] | |
| 139 | Marine-derived fungus Cladosporium sp. OUCMDZ-1635 (GenBank No. KT336457) | Xisha Islands of China | 2018 | [68] | |
| 140 | Marine sponge-derived Cladosporium sp. TPU1507 | Indonesian | 2018 | [69] | |
| 141 | Plant Ammopiptanthus mongolicus-derived fungus Cladosporium cladosporioides | 2019 | [55] | ||
| 142–144 | Mangrove-derived fungus Cladosporium sp. HNWSW-1 (GenBank access No. MH 535968) | Hainan China | 2019 | [61] | |
| 145–146 | Marine sediment-derived Cladosporium sp. NJF6 | Antarctica | 2020 | [66] | |
| 147–154 | Deep sea sediment-derived Cladosporium sp. SCSIO z0025 | Okinawa | 2018 | [78] | |
| 155 | Cladosporium sp. JNU17DTH12-9-01 (GenBank no. MK994007) | 2021 | [79] | ||
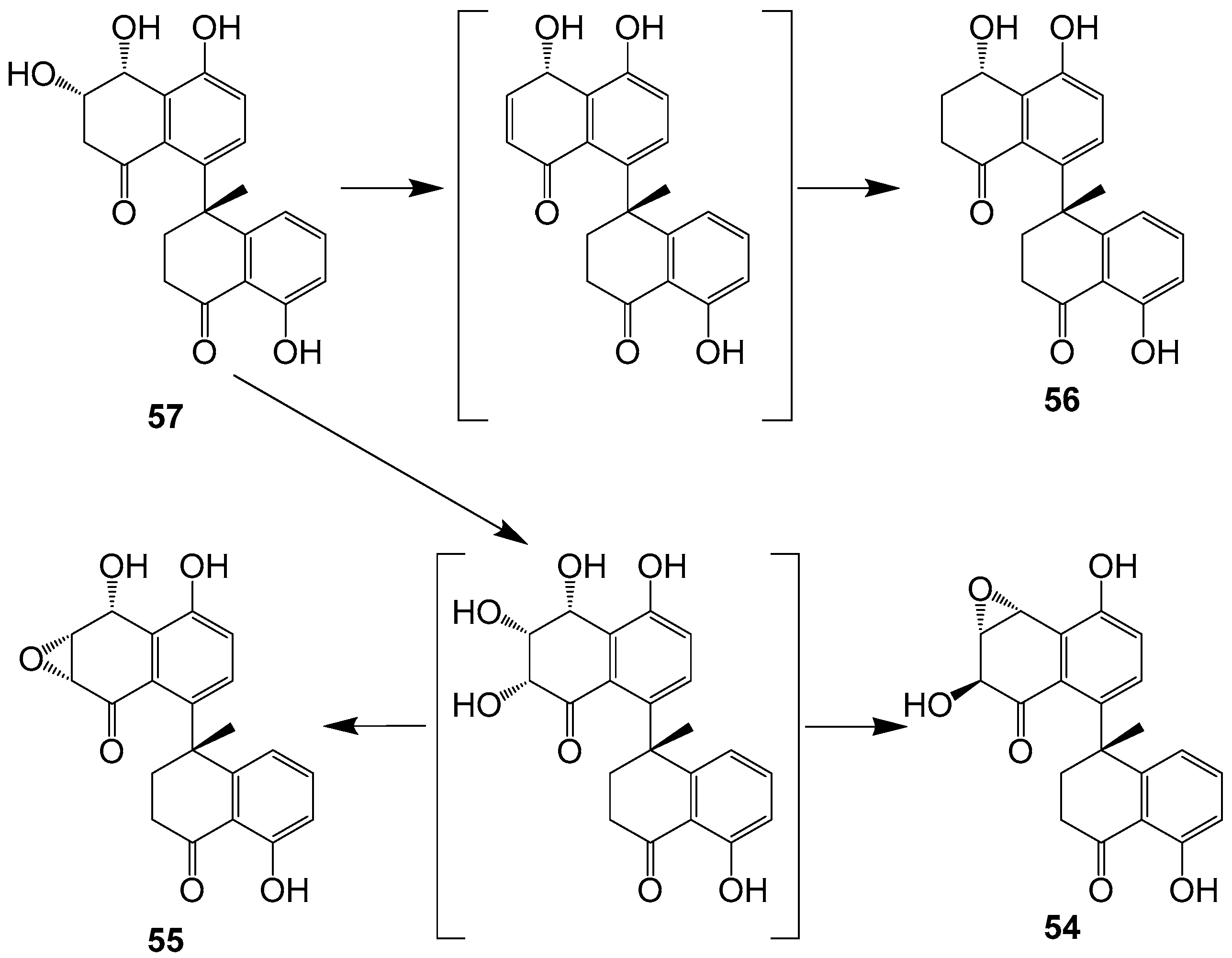
| Steroids and Terpenoids | 156–167 | Cladosporium sp. IFM49189 | 2000 | [13] | |
| 168–173 | Cladosporium sp. IFM49189 | 2001 | [80] | ||
| 174–176 | Marine mangrove-derived fungus Cladosporium cladosporioides | Guangzhou China | 2009 | [81] | |
| 177 | Cladosporium cladosporioides JG-12 | Hainan China | 2017 | [65] | |
| 178–183 | Marine sponge-derived fungus Cladosporium sp. SCSIO41007 | Guangzhou China | 2018 | [28] | |
| 184–190 | Gorgonian-derived fungus Cladosporium sp. WZ-2008-0042 | the South China Sea | 2018 | [21] | |
| 191 | Endophytic fungus Cladosporium sp. IS384 | Sichuan China | 2019 | [41] | |
| 192–193 | Plant Ammopiptanthus mongolicus-derived fungus Cladosporium cladosporioides | 2019 | [55] | ||
| 194 | Marine sediment-derived Cladosporium sp. NJF4 | 2020 | [66] | ||
| Benzene derivatives | 195 | Cladosporium sp. NRRL 29097 | Red River | 2001 | [32] |
| 196–202 | Cladosporium sp. N5 (GenBank No. EF424419) | Lianyungang China | 2008 | [33] | |
| 203 | Cladosporium cladosporioides JG-12 | Hainan China | 2017 | [65] | |
| 204–205 | Cladosporium sp. JS1-2 | Hainan China | 2020 | [56] | |
| 206–211 | Marine sediment-derived Cladosporium sp. NJF6 | Antarctica | 2020 | [66] | |
| Cyclic peptides | 212–213 | Cladosporium sp. N5 (GenBank No. EF424419) | Lianyungang China | 2008 | [33] |
| 214 | Marine sponge Callyspongia sp. derived fungus Cladosporium sp. scsio41007 (GenBank NO.MF188197) | Guangzhou China | 2018 | [28] | |
| 215–221 | Marine sediment-derived Cladosporium sp. NJF4 | Antarctica | 2020 | [66] | |
| Others | 222–223 | Cladosporium sp. N5 | Lianyungang China | 2008 | [33] |
| 224 | Cladosporium sp. JS1-2 | Hainan China | 2020 | [56] | |
| 225–229 | Mangrove-derived fungus Cladosporium sp. JJM22 (GenBank No.MF593626) | Hainan China | 2021 | [23] |
| Strains | Comps. | Values (MIC) | Values of Positive Controls (MIC) | Pros | Cons |
|---|---|---|---|---|---|
| Staphylococcus aureus (MRSA) | 8 (μg/mL) [36] | 8.0 | 1.0 | Moderate inhibitory activity | |
| Micrococcus luteus | 11–12 (μg/mL) [37] | 16.7 | Moderate activities | ||
| Bacillus cereus | 21–23 (μM) [35] | 12.5/25.0/6.25 | 1.56 | Broad-spectrum antibacterial activity; compounds 21 and 22 exhibited the strongest activities against T. halophilus | Moderate activity |
| Tetrag enococcus halophilus | 3.13/3.13/25.0 | 1.56 | |||
| S. epidermidis | 6.25/25.0/25.0 | 0.78 | |||
| MRSA | 6.25/25.0/12.5 | 0.39 | |||
| Escherichia coli | 12.5/12.5/25.0 | 1.56 | |||
| Pseudomonas putida | 12.5/25.0/6.25 | 0.39 | |||
| Nocardia brasiliensis | 6.25/12.5/25.0 | 0.78 | |||
| Vibrio parahaemolyticus | 12.5/25.0/25.0 | 1.56 | |||
| Enterococcus faecalis ATCC 29212 | 30 (μg/mL) [41] | 0.31 | 20.83 | Strong activity | |
| Edwardsiella tarda | 31–33 (μg/mL) [42] | 1.0/2.0/2.0 | 0.5 | Strong activities | |
| V. anguillarum | 2.0/2.0/4.0 | 1.0 | |||
| MRSA ATCC43300 | 58/59/60/64 (μg/mL) [58] | 25/3.13/25/25 | 0.78 | Compound 59 displayed strong activity of MRSA | Compounds 58 and 60 showed weak activities |
| MRSA ATCC700698 | 25/12.5/50/25 | 1.56 | |||
| B. subtilis | 72–73 (inhibition zone) (mm) [32] | 33/23 | Moderate activities | ||
| MRSA | 31/20 | ||||
| MRSA | 75 (inhibition zone) (mm) [60] | 27 | 32 | Strong and broad-spectrum activity | |
| E. coli | 25 | 30 | |||
| Pseudomonas aeruginosa | 24 | 30 | |||
| B. megaterium | 22 | 32 | |||
| MRSA | 84 (inhibition zone) (mm) [32] | 13 | Moderate activity | ||
| B. subtilis | 86 (inhibition zone) (mm) [32] | 8 | Weak activity | ||
| Ralstonia solanacearum | 91 (inhibition zone) (mm) [65] | 6.29 ± 0.10 | 25.16 ± 0.06 | Weak activity | |
| MRSA | 6.45 ± 0.11 | 17.62 ± 0.08 | |||
| B. subtilis | 93 (μM) [66] | 2.50 | 1.25 | Broad-spectrum activity | |
| Sarcina lutea | 2.50 | 2.50 | |||
| E. coli | 5.00 | 0.625 | |||
| M. tetragenus | 20.0 | 0.312 | |||
| V. parahaemolyticus | 10.0 | 0.160 | |||
| V. anguillarum | 5.0 | 0.160 | |||
| MRSA | 105–107 (μg/mL) [56] | 12.5 | 3.12 | Moderate activities | |
| MRSA 209P | 155 (μg/mL) [79] | 4 | 0.13 | Weak activity | |
| Candida albicans FIM709 | 16 | 0.06 | |||
| Shigella dysenteriae | 189 (μM) [21] | 3.13 | Moderate activity | ||
| B. subtilis | 195 (inhibition zone) (mm) [32] | 22 | Weak activity | ||
| C. albicans | 203 (inhibition zone) (mm) [65] | 7.43 ± 0.12 | 12.00 ± 0.09 | Strong activity | |
| MRSA | 204/224 (μg/mL) [56] | 12.5/12.5 | 3.12 | Moderate activities |
| Cells | Comps. | Values (IC50) | Values of Positive Controls (IC50) | Pros | Cons |
|---|---|---|---|---|---|
| L1210 | 11–12 (μg/mL) [37] | 0.13/0.81 | Strong activities | ||
| MCF–7 | 34 (μM) [50] | 0.2 | Strong and broad-spectrum cytotoxicity | ||
| A549 | 0.3 | ||||
| HCT116 | 0.5 | ||||
| 786-O | 0.7 | ||||
| PC3 | 1.5 | ||||
| K562 | 54 (μM) [19] | 14.3 | 0.24 | Weak activity | |
| A549 | 15.7 | 0.05 | |||
| Huh-7 | 29.9 | 0.08 | |||
| H1975 | 40.6 | 0.09 | |||
| MCF-7 | 21.3 | 0.78 | |||
| U937 | 10.5 | 0.06 | |||
| BGC823 | 17.0 | 0.09 | |||
| HL60 | 10.1 | 0.09 | |||
| Hela | 53.7 | 0.11 | |||
| MOLT-4 | 14.6 | 0.03 | |||
| K-562 | 74–75 (μg/mL) [60] | 3.97/3.58 | 12.0 | Potential cytotoxicities against human leukemia cells (K-562) | |
| HL-60 | 93 (μM) [66] | >50 | Weak activity | ||
| A-549 | >50 | ||||
| Brine shrimp naupalii | 100–103 (μM) [70] | 72.0/81.7/49.9/81.4 | 21.2 | Moderate activities | |
| MB49 | 109–110 (μM) [71] | 13.9 ± 2.5/41.7 ± 7.5 | 2.7 ± 0.6 | Broad-spectrum cytotoxicity | Weak activities |
| J82 | 25.0 ± 6.1/24.7 ± 4.4 | 0.6 ± 0.1 | |||
| 4T1 | 38.7 ± 4.2/27.5 ± 2.8 | 4.5 ± 1.6 | |||
| Huh7 | 24.3 ± 3.5/46.4 ± 9.3 | 5.1 ± 1.2 | |||
| MCF-7 | 127 (μM) [63] | 20 | Broad-spectrum cytotoxicity | Moderate activity | |
| A549 | 15 | ||||
| HT-29 | 10 | ||||
| HepG2 | 10 | ||||
| HepG2 | 129–131 (μg/mL) [76] | 21/42/48 | Moderate activities | ||
| HeLa | 133 (μM) [35] | 0.76 | Potent cytotoxicity against a series of cancer cell lines and broad-spectrum cytotoxicity | ||
| P388 | 1.35 | ||||
| HT-29 | 2.48 | ||||
| A549 | 3.11 | ||||
| MCF-7 | 139 (μM) [68] | 18.7 | 0.67 | Broad-spectrum cytotoxicity | Moderate activity |
| HeLa | 19.1 | 0.32 | |||
| HCT-116 | 17.9 | 0.21 | |||
| HL-60 | 9.1 | 0.02 | |||
| BEL-7042 | 143–144 (μM) [60] | 29.4 ± 0.35/26.7 ± 1.1 | 11.9 ± 0.37 | Broad-spectrum cytotoxicities | Moderate activities |
| K562 | 25.6 ± 0.47/- | 14.2 ± 0.66 | |||
| SGC-7901 | 41.7 ± 0.71/- | 6.66 ± 0.2 | |||
| Hela | -/14.9 ± 0.21 | 11.5 ± 0.18 | |||
| Vero | 146 (μM) [66] | 87 | Weak activity | ||
| HeLa | 191 (μM) [41] | 22 | Weak activity | ||
| HeLa | 194 (μM) [66] | 14.1 | Moderate activity |
| Bioactivities | Cells/Stains/Enzyme | Comps. | Values | Values of Positive Controls | Pros | Cons |
|---|---|---|---|---|---|---|
| Antiviral activity | HBV | 34 (μM) [50] | 0.5 | Strong and broad-spectrum antiviral activity | ||
| HIV-1 | 1.0 | |||||
| HCMV | 1.5 | |||||
| IAV | 0.5 | |||||
| H1N1 | 113–126 (μM) [26] | 80–150 | 87 | Compounds 115, 118, 120–122 and 124 showed noteworthy antiviral activities | Compounds 113, 114, 116, 117, 119, 123 and 126 showed weak antiviral activities | |
| HBV | 127 (μM) [63] | 5 | Broad-spectrum antiviral activity | |||
| HIV-1 | 5 | |||||
| HCMV | 5 | |||||
| IAV | 5 | |||||
| H3N2 | 178 (μM) [28] | 16.2 | 34.0 | Weak activity | ||
| RSV | 184 (mM) [21] | 0.12 | 0.08 | Potent antiviral activity | ||
| Antifungal activity | Candida albicans | 11 (μg/mL) [37] | 16.7 | Strong and broad-spectrum antifungal activity | ||
| Cryptococcus neoformans | 8.4 | |||||
| Aspergillus niger | 16.7 | |||||
| Neurospora crassa | 8.4 | |||||
| Pyricularia oryzae | 15 (μg/mL) [22] | 0.15 | Strong antifungal activity | |||
| Muco rracemosus | 29 | |||||
| A. niger | 34 (μg/mL) [50] | 0.97 | Strong activity | |||
| C. albicans | 1.9 | |||||
| Trichophyton rubrum | 1.9 | |||||
| Colletotrichum acutatum | 37–41 (tested at 30 µM) (%) [54] | 92.7/38.3/-/-/- | 99.7 | Compound 37 showed broad-spectrum antifungal activity, and the activity is significant | ||
| Co. fragariae | 90.1/50.4/-/-/- | 99.6 | ||||
| Co. gloeosporioides | 95.4/60.2/-/-/- | 96.1 | ||||
| Phomopsis viticola | 79.9/83.0/53.9/35.1/79.4 | 94.2 | ||||
| P. obscurans | 22.1/22.5/25.6/-/10.3 | 96.2 | ||||
| A. Fumigatus IFM 4942/40849/41375/41382/46075/47064/47078/47031/47032 | 156 (μg/disc) [13] | 0.5–4 | Strong and exhibited specific antifungal activity toward A. fumigatus | |||
| A. fumigatus IFM 4942/40819/41375/46075/47064/47078 | 168, 172–173 (μg/disc) [80] | 9–17 | Compounds 172 and 173 retained weak antifungal activities against A. fumigatus | |||
| C. albicans | 199 (mM) [33] | 1.5 | Broad-spectrum antifungal activity | Weak activity | ||
| Pseudomonas aeruginosa | 3.1 | |||||
| Staphylococcus aureus | 0.78 | |||||
| Enzyme inhibition activity | Acetylcholinesterase | 8 (μM) [36] | 40.26 | Potent inhibitory activity against acetylcholinesterase | ||
| α-glycosidase | 76 (μM) [61] | 49.3 ± 10.6 | 275.7 ± 2.7 | Strong activity | ||
| Acetylcholinesterase | 91 (tested at 1 g/L) (%) [65] | 23.54 ± 0.70 | 77.43 ± 1.47 | Weak activity | ||
| PTP1B | 95 (μM) [69] | 11 | 0.9 | Moderate inhibitory activity | ||
| Acetylcholinesterase | 134–136 (tested at 1 g/L) (%) [65] | 37.20 ± 1.31/26.94 ± 5.64/26.35 ± 1.55 | 77.43 ± 1.47 | Weak activity | ||
| PTP1B | 140 (μM) [69] | 48 | 0.9 | Strong activity | ||
| TCPTP | 54 | 0.8 | ||||
| α-glycosidase | 144 (μM) [61] | 78.2 ± 2.1 | 275.7 ± 2.7 | Strong activity | ||
| Acetylcholinesterase | 203 (tested at 1 g/L) (%) [65] | 25.43 ± 1.08 | 77.43 ± 1.47 | Weak activity | ||
| α-glucosidase | 226, 229 (μM) [23] | 2.95/2.05 | 2.35 | Potent inhibitory activities against α-glucosidase | ||
| Growth inhibitory activity | Growth inhibition activity against newly hatched larvae of Helicoverpa armigera Hubner | 47–48, 107, 204–205, 224 (μg/mL) [56] | 100/100/100/100/100/100 | 50 | Moderate inhibitory activities | |
| Inhibition of Uromyces appendiculatus urediniospore germination | 49–53 (tested at 100 ppm) (%) [57] | 84.2/100/77.6/69.4/74.8 | 20.9 | Strong inhibitory activities | ||
| Anti-inflammatory activity | 177 (μM) [65] | 27.9 | 61.7 | Potent anti-inflammatory activity | ||
| Insecticidal activity | Panagrellus redivivus | 135–136, 203 (tested at 2.5 g/L) (%) [65] | 78.2 ± 0.7/80.7 ± 0.4/89.6 ± 0.9 | 37.2 ± 0.3 | Significant and comparable to that of the positive control | |
| Trypanosoma cruzi | 146 (μM) [66] | 26.9 | Moderate activity | |||
| Quorum sensing inhibitory activity | Chromobacterium violaceum CV026 | 65–70 (μg/well) [59] | 30/30/20/30/20/30 | Strong activities | ||
| Phytotoxicity | Amaranthus retroflexus L. | 35–36, 90 (μg/mL) [53] | 4.80/8.16/4.51 | 1.95 | Potent phytotoxic activities against the radicle growth of Amaranthus retroflexus L. | |
| Porphyra tenera | 199, 201 (mm) [33] | 1.57 ± 0.06/0.95 ± 0.04 | 0.98 ± 0.05 | Strong activities | ||
| Lipid-lowering activity | HepG2 hepatocytes | 9, 78–80 (μM) [36] | 8.3/12.1/8.4/13.1 | 8.3 | Potent lipid-lowering activities in HepG2 hepatocytes | |
| Antioxidant activity | DPPH free radical scavenging | 99 (μM) [71] | 2.65 | Significant radical scavenging activity against DPPH | ||
| DPPH free radical scavenging | 103 (μM) [70] | 16.4 | 4.9 | Significant antioxidant activity against DPPH radicals | ||
| SH-SY5Y | 191 (μg/mL) [41] | 6.25 | Weak activity | |||
| Inhibit electron transfer activity | Photosynthetic electron transport in spinach | 88–89 (μM) [64] | 29.1 ± 6.5/22.8 ± 8.8 | Strong activities | ||
| Anti-inflammatory activity | NF-kB, TGF-β, TNF-α, IL-6, IL-8 and COX-2; caspase-3, caspase-8, Bcl-2 and Bax | 108 [66] | Potential candidate for the therapy of different vascular inflammatory diseases | |||
| Breast cancer cells, colon cancer cells | 177 [65] | Potential candidate for inhibiting cancer cell proliferation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Wang, H.; Shi, T.; Wang, B. The Genus Cladosporium: A Prospective Producer of Natural Products. Int. J. Mol. Sci. 2024, 25, 1652. https://doi.org/10.3390/ijms25031652
Li Y, Wang Y, Wang H, Shi T, Wang B. The Genus Cladosporium: A Prospective Producer of Natural Products. International Journal of Molecular Sciences. 2024; 25(3):1652. https://doi.org/10.3390/ijms25031652
Chicago/Turabian StyleLi, Yanjing, Yifei Wang, Han Wang, Ting Shi, and Bo Wang. 2024. "The Genus Cladosporium: A Prospective Producer of Natural Products" International Journal of Molecular Sciences 25, no. 3: 1652. https://doi.org/10.3390/ijms25031652
APA StyleLi, Y., Wang, Y., Wang, H., Shi, T., & Wang, B. (2024). The Genus Cladosporium: A Prospective Producer of Natural Products. International Journal of Molecular Sciences, 25(3), 1652. https://doi.org/10.3390/ijms25031652


















