Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review
Abstract
1. Introduction
2. Ionic Liquids and Biopolymers
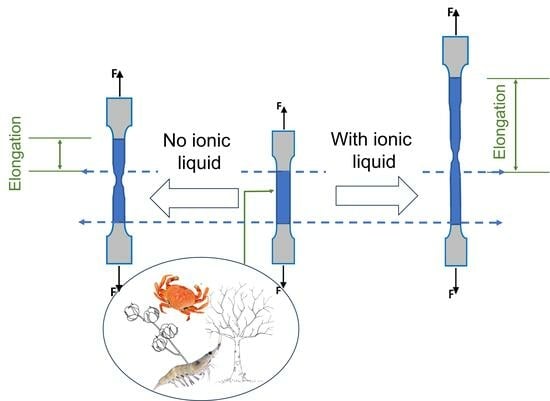
3. Ionic Liquids as Plasticizers
4. Design of Ionic Liquids and DES with Multiple Properties
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.-W.; Huber, G.W. A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.L. Will the Circular Economy Save the Planet? Sierra, 23 December 2020. Available online: https://www.sierraclub.org/sierra/2021-1-january-february/feature/will-circular-economy-save-planet (accessed on 11 December 2023).
- Ellen Macarthur Foundation. Towards the Circular Economy Vol. 1: An Economic and Business Rationale for an Accelerated Transition. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/Ellen-MacArthur-Foundation-Towards-the-Circular-Economy-vol.1.pdf (accessed on 11 December 2023).
- Boulding, K.E. The Economics of the Coming Spaceship Earth; RFF Press: Washington, DC, USA, 1966. [Google Scholar]
- National Institute of Food and Agriculture. Request for Applications. Agriculture and Food Research Initiative Competitive Grants Program. Foundational and Applied Science Program 2023–2024. Available online: https://www.nifa.usda.gov/sites/default/files/2023-02/FY23-AFRI-FAS-RFA-508.pdf (accessed on 27 December 2023).
- Global Biodegradable Plastic Market Size by Product, by Application, by Geographic Scope and Forecast. Verified Market Research® Report. Available online: https://finance.yahoo.com/news/biodegradable-plastic-market-size-worth-151500099.html (accessed on 11 December 2023).
- Plastic Became a Problem—Is Wood the Solution for Reducing Plastic? Published 27 September 2019. Available online: https://www.metsagroup.com/metsafibre/news-and-publications/news-and-releases/stories/2019/plastic-became-a-problem--is-wood-the-solution/ (accessed on 11 December 2023).
- Restore™ Calcium Alginate Dressing. Available online: https://www.hollister.com/en/products/wound-care-products/wound-dressings/calcium-alginate-dressings/restore-calcium-alginate-dressing# (accessed on 15 December 2023).
- John, R.; Ma, J.; Wong, I. Better clinicoradiological results of BST-CarGel treatment in cartilage repair compared with microfracture in acetabular chondral defects at 2 years. Am. J. Sports Med. 2020, 48, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Danimer, Chevron Expand Biopolymers Partnership. Plastics Technology, Published 21 September 23. Available online: https://www.ptonline.com/news/danimer-chevron-expand-biopolymers-partnership (accessed on 15 December 2023).
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.-U.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef]
- Peramune, D.; Manatunga, D.C.; Dassanayake, R.S.; Premalal, V.; Liyanage, R.N.; Gunathilake, C.; Abidi, N. Recent advances in biopolymer-based advanced oxidation processes for dye removal applications: A review. Environ. Res. 2022, 215, 114242. [Google Scholar] [CrossRef]
- Schick, S.; Groten, R.; Seide, G.H. Performance Spectrum of Home-Compostable Biopolymer Fibers Compared to a Petrochemical Alternative. Polymers 2023, 15, 1372. [Google Scholar] [CrossRef]
- Kunam, P.K.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Bio-based materials for barrier coatings on paper packaging. Biomass Conv. Bioref. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Appunni, S.; Chinthala, M.; No, D.-V.N. Biopolymer-supported TiO2 as a sustainable photocatalyst for wastewater treatment: A review. Environ. Chem. Lett. 2022, 20, 3071–3098. [Google Scholar] [CrossRef]
- Quintana, R.; Persenaire, O.; Lemmouchi, Y.; Sampson, J.; Martin, S.; Bonnaud, L.; Dubois, P. Enhancement of cellulose acetate degradation under accelerated weathering by plasticization with eco-friendly plasticizers. Polym. Degrad. Stab. 2013, 98, 1556–1562. [Google Scholar] [CrossRef]
- Godwin, A.D. Plasticizers. In Applied Plastics Engineering Handbook: Processing, Sustainability, Materials, and Applications; Kutz, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 533–553. [Google Scholar]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Paudel, S.; Regmi, S.; Janaswamy, S. Effect of glycerol and sorbitol on cellulose-based biodegradable films. Food Packag. Shelf Life 2023, 37, 101090. [Google Scholar] [CrossRef]
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the plasticizing effects of glycerol and peg 400 on chitosan films using solid-state NMR spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Averous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Smith, D.R.; Escobar, A.P.; Andris, M.N.; Boardman, B.M.; Peters, G.M. Understanding the molecular-level interactions of glucosamine-glycerol assemblies: A model system for chitosan plasticization. ACS Omega 2021, 6, 25227–25234. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Mechanical and thermal characteristics of pea starch films plasticized with monosaccharides and polyols. J. Food Sci. 2006, 71, 109–118. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Mekonnena, T.; Mussonea, P.; Khalilb, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Oecd Sids Triethylphosphate. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=5b64c6b3-7bf4-413d-b0b8-2bc7861f82f3 (accessed on 11 December 2023).
- Özeren, H.D.; Olsson, R.T.; Nilsson, F.; Hedenqvist, M.S. Prediction of plasticization in a real biopolymer system (starch) using molecular dynamics simulations. Mater. Des. 2020, 187, 108387. [Google Scholar] [CrossRef]
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Berton, P.; Rogers, R. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Berton, P.; Shen, X.; Rogers, R.D.; Shamshina, J.L. 110th anniversary: High-molecular-weight chitin and cellulose hydrogels from biomass in ionic liquids without chemical crosslinking. Ind. Eng. Chem. Res. 2019, 58, 19862–19876. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, G. From cellulose fibrils to single chains: Understanding cellulose dissolution in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 31592–31607. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; Salas-de la Cruz, D. Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Yu, J.; Wu, J.; Zhang, X.; He, J.; Zhang, J. Understanding cellulose dissolution: Effect of the cation and anion structure of ionic liquids on the solubility of cellulose. Sci. China Chem. 2016, 59, 1421. [Google Scholar] [CrossRef]
- Remsing, R.C.; Hernandez, G.; Swatloski, R.P.; Massefski, W.W.; Rogers, R.D.; Moyna, G. Solvation of carbohydrates in N,N′-dialkylimidazolium ionic liquids: A multinuclear NMR spectroscopy Study. Phys. Chem. B 2008, 112, 11071–11078. [Google Scholar] [CrossRef] [PubMed]
- Youngs, T.G.A.; Holbrey, J.D.; Mullan, C.L.; Norman, S.E.; Lagunas, M.C.; D’Agostino, C.; Mantle, M.D.; Gladden, L.F.; Bowron, D.T.; Hardacre, C. Neutron diffraction, NMR and molecular dynamics study of glucose dissolved in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Chem. Sci 2011, 2, 1594–1605. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. [Google Scholar] [CrossRef]
- Endo, T.; Hosomi, S.; Fujii, S.; Ninomiya, K.; Takahashi, K. Anion bridging-induced structural transformation of cellulose dissolved in ionic liquid. Phys. Chem. Lett. 2016, 7, 5156–5161. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Hosomi, S.; Fujii, S.; Ninomiya, K.; Takahashi, K. Nano-structural investigation on cellulose highly dissolved in ionic liquid: A small angle X-ray scattering study. Molecules 2017, 22, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shimo, M.; Abe, M.; Ohno, H. Functional comparison of polar ionic liquids and onium hydroxides for chitin dissolution and deacetylation to chitosan. ACS Sustain. Chem. Eng. 2016, 4, 3722–3727. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The α-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The β-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Yokoyama, R.; Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 3. Hydrogen bonding by some 2-nitroaniline derivatives. J. Am. Chem. Soc. 1976, 98, 3233–3237. [Google Scholar] [CrossRef]
- Ohno, H.; Fukaya, Y. Task specific ionic liquids for cellulose technology. Chem. Lett. 2009, 38, 2–7. [Google Scholar] [CrossRef]
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Abe, M.; Ohno, H. Production of Biofuels and Chemicals with Ionic Liquids; Fang, Z., Smith, R.L.J., Qi, X., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2013; Chapter 2; pp. 29–59. [Google Scholar]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation. Carbohydr. Polym. 2013, 94, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Lin, J.; Zhao, X. Theoretical study on interaction between ionic liquid and chitin/chitosan/cellulose. J. Chilean Chem. Soc. 2017, 62, 3668–3676. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic Liquids as Reaction Medium in Cellulose Functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef]
- Uto, T.; Idenoue, S.; Yamamoto, K.; Kadokawa, J.-I. Understanding dissolution process of chitin crystal in ionic liquids: Theoretical study. Phys. Chem. Chem. Phys. 2018, 20, 20669–20677. [Google Scholar] [CrossRef]
- Uto, T.; Yamamoto, K.; Kadokawa, J.-I. Understanding the dissolution processes of chitin in ionic liquids: A theoretical study. In Proceedings of the the 256th ACS National Meeting & Exposition, Boston, MA, USA, 19–23 August 2018. PMSE-548. [Google Scholar]
- Rahman, M.; Shoff, H.W.; Brazel, C.S. Ionic Liquids as Alternative Plasticizers for Poly(vinyl chloride): Flexibility and Stability in Thermal, Leaching, and UV Environments. In Ionic Liquids in Polymer SystemsACS Symposium Series; Brazel, C.S., Rogers, R.D., Eds.; American Chemical Society: Washington, DC, USA, 2005; pp. 103–118. [Google Scholar]
- Miri, A.; Fareghi-Alamdari, R.; Nikpour, M.; Hasanzadeh, N. Synthesis and characterization of new imidazolium ionic liquid based energetic plasticizers. Propellants Explos. Pyrotech. 2021, 46, 1547–1554. [Google Scholar] [CrossRef]
- Tyagi, V.; Wang, Y.; Bhattacharya, B. Development of ionic liquid plasticized high-tensile starch-protein-sorghum bran composite films with antimicrobial activity. J. Appl. Polym. Sci. 2022, 139, e52442. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Zhang, Z.; Ye, C.; Chen, Y.; Wei, P.; Wang, Y.; Xia, Y. Thermoplastic starch plasticized by polymeric ionic liquid. Eur. Polym. J. 2021, 148, 110367. [Google Scholar] [CrossRef]
- Romano, S.; De Santis, S.; Martinelli, A.; Rocchi, L.A.; Rocco, D.; Sotgiu, G.; Orsini, M. Starch films plasticized by imidazolium-based ionic liquids: Effect of mono- and dicationic structures and different anions. ACS Appl. Polym. Mater. 2023, 5, 8859–8868. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Graphene oxide enhanced ionic liquid plasticisation of chitosan/alginate bionanocomposites. Carbohydr. Polym. 2021, 253, 117231. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Glycerol plasticisation of chitosan/carboxymethyl cellulose composites: Role of interactions in determining structure and properties. Int. J. Biol. Macromol. 2020, 163, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Ionic liquid (1-ethyl-3-methylimidazolium acetate) plasticization of chitosan-based bi-onanocomposites. ACS Omega 2020, 5, 19070–19081. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Cooperative effects of cellulose nanocrystals and sepiolite when combined on ionic liquid plasticised chitosan materials. Polymers 2021, 13, 571. [Google Scholar] [CrossRef]
- Aghmih, K.; Boukhriss, A.; El Bouchti, M.; Chaoui, M.A.; Majis, S.; Gmouh, S. Introduction of ionic liquids as highly efficient plasticizers and flame retardants of cellulose triacetate films. J. Polym. Environ. 2022, 30, 2905–2918. [Google Scholar] [CrossRef]
- Bendaoud, A.; Chalamet, Y. Plasticizing effect of ionic liquid on cellulose acetate obtained by melt processing. Carbohydr. Polym. 2014, 108, 75–82. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Addition of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide to improve the thermal stability of regenerated cellulose. J. Appl. Polym. Sci. 2011, 121, 750–755. [Google Scholar] [CrossRef]
- Leroy, E.; Jacquet, P.; Coavity, G.; Reguerre, A.L.; Lourdin, D. Compatibilization of starch–zein melt processed blends by an ionic liquid used as plasticizer. Carbohydr. Polym. 2012, 89, 955–963. [Google Scholar] [CrossRef]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic starch plasticized by an ionic liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Bendaoud, A.; Chalamet, Y. Effects of relative humidity and ionic liquids on the water content and glass transition of plasticized starch. Carbohydr. Polym. 2013, 97, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Spychaj, T. Ciecze jonowe jako plastyfikatory lub rozpuszczalniki skrobi. Polimery 2011, 11, 861. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Zhang, T.; Chen, L.; Li, X.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; McNally, T.; Rogers, R.D. Different characteristic effects of ageing on starch-based films plasticised by 1-ethyl-3-methylimidazolium acetate and by glycerol. Carbohydr. Polym. 2016, 146, 67–79. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.V.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of starch-based films plasticised by glycerol and by the ionic liquid 1-ethyl-3-methylimidazolium acetate: A comparative study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Shamshina, J.L.; Rogers, R.D.; McNally, T.; Wang, D.K.; Halley, P.J.; Truss, R.W.; Zhao, S.; Chen, L. Facile preparation of starch-based electroconductive films with ionic liquid. ACS Sustain. Chem. Eng. 2017, 5, 5457–5467. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.D.; Conde, J.P.; Ryder, K.S.; Wise, W.R. Salt modified starch: Sustainable, recyclable plastics. Green Chem. 2012, 14, 1302–1307. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, W.; Yang, J.; Pan, Y.; Chen, H.; Zhang, Z. The application of deep eutectic solvents systems based on choline chloride in the preparation of biodegradable food packaging films. Trends Food Sci. Technol. 2023, 139, 104124. [Google Scholar] [CrossRef]
- Galvis-Sanchez, A.C.; Sousa, A.M.M.; Hilliou, L.; Goncalves, M.P.; Souza, H.K.S. Thermo-compression molding of chitosan with a deep eutectic mixture for biofilms development. Green Chem. 2016, 18, 1571–1580. [Google Scholar] [CrossRef]
- Leroy, E.; Decaen, P.; Jacquet, P.; Coativy, G.; Pontoire, B.; Reguerre, A.-L.; Lourdin, D. Deep eutectic solvents as functional additives for starch-based plastics. Green Chem. 2012, 14, 3063–3066. [Google Scholar] [CrossRef]
- Souza, H.K.S.; Campiña, J.M.; Sousa, A.M.M.; Silva, F.; Gonçalves, M.P. Ultrasound-assisted preparation of size-controlled chitosan nanoparticles: Characterization and fabrication of transparent biofilms. Food Hydrocolloids 2013, 31, 227–563. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Mechanical and barrier properties of starch-based films plasticized with two- or three-component deep eutectic solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Edler, K.J.; Page, A.J. Deep eutectic solvents—The vital link between ionic liquids and ionic solutions. J. Chem. Phys. 2021, 155, 150401. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, D.; Wilpiszewska, K. Deep eutectic solvents for starch treatment. Polymers 2022, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, M.; Vatanpour, V.; Castro-Munos, A.; Boczkaj, G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2022, 275, 118702. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Physicochemical and storage properties of chitosan-based films plasticized with deep eutectic solvent. Food Hydrocoll. 2020, 108, 106007. [Google Scholar] [CrossRef]
- Favero, J.; Belhabib, S.; Guessasma, S.; Decaen, P.; Reguerre, A.L.; Lourdin, D.; Leroy, E. On the representative elementary size concept to evaluate the compatibilisation of a plasticised biopolymer blend. Carbohydr. Polym. 2017, 172, 120–129. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Influence of plasticiser type and nanoclay on the properties of chitosan-based materials. Europ. Polym. J. 2021, 144, 110225. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. Ionic liquids: New generation stable plasticizers for poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Tyagi, V.; Wang, Y.; Badgujar, P.; Bhattacharya, B. Diffusion-controlled release of sorghum bran polyphenols from potato starch–zein film extends shelf-life of chicken meat. J. Polym. Environ. 2023, 31, 621–636. [Google Scholar] [CrossRef]
- Chaunier, L.; Viau, L.; Falourd, X.; Lourdin, D.; Leroy, E. A drug delivery system obtained by hot-melt processing of zein plasticized by a pharmaceutically active ionic liquid. J. Mater. Chem. B 2020, 8, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal bioavailability in rats of lidocaine in the forms of ionic liquids, salts, and deep eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Thadasack, M.; Chaunier, L.; Rabesona, H.; Viau, L.; De-Carvalho, M.; Bouchaud, G.; Lourdin, D. Release kinetics of [lidocainium][ibuprofenate] as active pharmaceutical ingredient-ionic liquid from a plasticized zein matrix in simulated digestion. Int. J. Pharm. 2022, 629, 122349. [Google Scholar] [CrossRef] [PubMed]
- Thadasack, M.; Réguerre, A.-L.; Leroy, E.; Guessasma, S.; Lourdin, D.; Weitkamp, T.; Chaunier, L. Tuning pharmaceutically active zein-based formulations for additive manufacturing. Addit. Manuf. 2023, 78, 103849. [Google Scholar] [CrossRef]
- Jouannin, C.; Tourné-Péteilh, C.; Darcos, V.; Sharkawi, T.; Devoisselle, J.-M.; Gaveau, P.; Dieudonné, P.; Vioux, A.; Viau, L. Drug delivery systems based on pharmaceutically active ionic liquids and biocompatible poly(lactic acid). J. Mater. Chem. B 2014, 2, 3133–3141. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Renewable biopolymers combined with ionic liquids for the next generation of supercapacitor materials. Int. J. Mol. Sci. 2023, 24, 7866. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Hamsan, M.H.; Aziz, S.B.; Kadir, M.F.Z.; Matmin, J.; Yusof, Y.M. Impregnation of [Emim]Br ionic liquid as plasticizer in biopolymer electrolytes for EDLC application. Electrochim. Acta 2021, 375, 137923. [Google Scholar] [CrossRef]
- Ning, W.; Xingxiang, Z.; Haihui, L.; Benqiao, H. 1-Allyl-3-methylimidazolium chloride plasticized-corn starch as solid biopolymer electrolytes. Carbohydr. Polym. 2009, 76, 482–484. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmada, A.; Hassana, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ionics 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Yaşar, Ö.; Kaya, İ. A cross-linker containing aldehyde functionalized ionic liquid for chitosan. J. Macromol. Sci. A 2019, 56, 860–870. [Google Scholar] [CrossRef]
- Baaqel, H.; Tulus, V.; Chachuat, B.; Guillén-Gosálbez, G.; Hallett, J. Uncovering the true cost of ionic liquids using monetization. Computer Aided Chem. Eng. 2020, 48, 1825–1830. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Ostadjoo, S.; Berton, P.; Shamshina, J.L.; Rogers, R.D. Scaling-up ionic liquid-based technologies: How much do we care about their toxicity? Prima facie information on 1-ethyl-3-methylimidazolium acetate. Toxicol. Sci. 2018, 161, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Abidi, N.; Shamshina, J.L. Ionic liquids: Implementing objectives of sustainability for the next generation chemical processes and industrial applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100625. [Google Scholar] [CrossRef]


| Biopolymer(s) or Derivative—Additive | Ionic Liquid | Tensile Strength (MPa) | Elongation at Break (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Without IL | With IL | Without IL | With IL | |||
| Starch-zein | [C4mim]Cl | 1.38 ± 0.45 | 8.93 ± 1.59 | 8.06 ± 0.05 | 29.53 ± 0.86 | [64] |
| Starch | Polymeric IL | 10.37 ± 0.5 a | 4.91 ± 1 a | 2.25 ± 0.05 a | 6.81 ± 0.1 a | [65] |
| Starch | [C4mim]Cl | 1.9 ± 0.1 b | 0.6 ± 0.1 | 88 ± 7 b | 392 ± 27 | [65] |
| Starch | [C4mim][SO4Et] | ND c | 70± 8 | ND c | 35 ± 4 | [66] |
| Starch | [C4mim][OAc] | ND c | 53 ± 8 | ND c | 42 ± 4 | [66] |
| Starch | [C4mim][N(CN2)] | ND c | 45 ± 4 | ND c | 40 ± 3 | [66] |
| Starch | [C4mim]Cl | ND c | 14 ± 1 | ND c | 22 ± 1 | [66] |
| Starch | [(C1mim)2]Cl2 | ND c | 1600 ± 400 | ND c | 4 ± 1 | [66] |
| Chitosan-GO d | [C2mim][OAc] | 33 ± 3 a,b | 28 ± 2 a | 163 ± 16 a,b | 225 ± 25 a | [67] |
| Chitosan-rGO e | [C2mim][OAc] | 33 ± 2 a,b | 33 ± 3 a | 155 ± 12 a,b | 285 ± 20 a | [67] |
| Chitosan-alginate (50/50)-GO d | [C2mim][OAc] | 20 ± 1 a,b | 28 ± 4 a | 62 ± 1 a,b | 75 ± 3 a,b | [67] |
| Chitosan-alginate (50/50)-rGO e | [C2mim][OAc] | 20 ± 1 a,b | 21 ± 3 a | 62 ± 3 a,b | 50 ± 8 a,b | [67] |
| Chitosan-clay composite films | [C4mim]Cl | 12 ± 2 | 5.2 ± 1.1 | 1.5 ± 0.2 | 3.1 ± 1.2 | [68] |
| Chitosan | [C2mim][OAc] | 27 ± 2 b | 28 ± 2 a,f/12 ± 2 a,g | 72 ± 8 a,b | 70 ± 8 a,f/82 ± 12 a,g | [69,70] |
| Chitosan-carboxymethylcellulose | [C2mim][OAc] | 38 ± 3 a,b | 38 ± 3 a,f/23 ± 8 a,g | 70 ± 10 a,b | 35 ± 10 a,f/92 ± 20 a,g | [67,69] |
| Chitosan-carboxymethylcellulose (50/50)-GO d | [C2mim][OAc] | 33 ± 3 a,b | 37 ± 3 a,f/17 ± 4 a,g | 63 ± 10 a,b | 22 ± 12 a,f/110 ± 6 a,g | [67,69] |
| Chitosan-carboxymethylcellulose (50/50)-rGO e | [C2mim][OAc] | 34 ± 3 a,b | 40 ± 3 a,f/16 ± 4 a,g | 60 ± 10 a,b | 38 ± 11 a,f/100 ± 20 a,g | [67,69] |
| Chitosan-Sepolite | [C2mim][OAc] | 43 ± 1 a | 27 ± 1 a | 15 ± 1 a | 65 ± 15 a | [71] |
| Chitosan-CNC h | [C2mim][OAc] | 54 ± 2 a | 25 ± 2 a | 15 ± 2 a | 52 ± 8 a | [70] |
| Chitosan-Sepolite-CNC h | [C2mim][OAc] | 56 ± 0.5 a | 25 ± 2 a | 14 ± 2 a | 64 ± 11 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-Sepolite | [C2mim][OAc] | 57 ± 3 a | 37 ± 2 a | 20 ± 2 a | 30 ± 6 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-CNC h | [C2mim][OAc] | 57 ± 2 a | 36 ± 2 a | 22 ± 8 a | 34 ± 10 a | [70] |
| Chitosan-carboxymethylcellulose (50/50)-Sepolite-CNC h | [C2mim][OAc] | 56 ± 2 a | 46 ± 2 a | 24 ± 6 a | 35 ± 8 a | [70] |
| Cellulose triacetate | [C6mim][OAc] | 17.5 ± 2 a,i | 20 ± 1 a,i | 1.5 ± 0.1 a,i | 3 ± 0.1 a,i | [72] |
| Cellulose triacetate | [C6mim][PF6] | 17.5 ± 2 a,i | 12.5 ± 2 a,i | 1.5 ± 0.1 a,i | 15 ± 0.1 a,i | [72] |
| Cellulose triacetate | [N2226][OAc] | 17.5 ± 2 a,i | 15 ± 2 a,i | 1.5 ± 0.1 a,i | 2 ± 0.1 a,i | [72] |
| Cellulose triacetate | [N2226][PF6] | 17.5 ± 2 a,i | 8 ± 2 a,i | 1.5 ± 0.1 a,i | 11 ± 0.1 a,i | [72] |
| Cellulose acetate | [C4mim]Cl | 18.2 ± 0.1 a | 9 ± 2 a | 11.9 ± 2 a | 6.5 ± 0.2 a | [73] |
| Cellulose | [C4mim][TFSI] k | 41.5 j | 29 | ND | ND | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamshina, J.L.; Berton, P. Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review. Int. J. Mol. Sci. 2024, 25, 1720. https://doi.org/10.3390/ijms25031720
Shamshina JL, Berton P. Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review. International Journal of Molecular Sciences. 2024; 25(3):1720. https://doi.org/10.3390/ijms25031720
Chicago/Turabian StyleShamshina, Julia L., and Paula Berton. 2024. "Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review" International Journal of Molecular Sciences 25, no. 3: 1720. https://doi.org/10.3390/ijms25031720
APA StyleShamshina, J. L., & Berton, P. (2024). Ionic Liquids as Designed, Multi-Functional Plasticizers for Biodegradable Polymeric Materials: A Mini-Review. International Journal of Molecular Sciences, 25(3), 1720. https://doi.org/10.3390/ijms25031720