The Urinary Microbiome in Health and Disease: Relevance for Bladder Cancer
Abstract
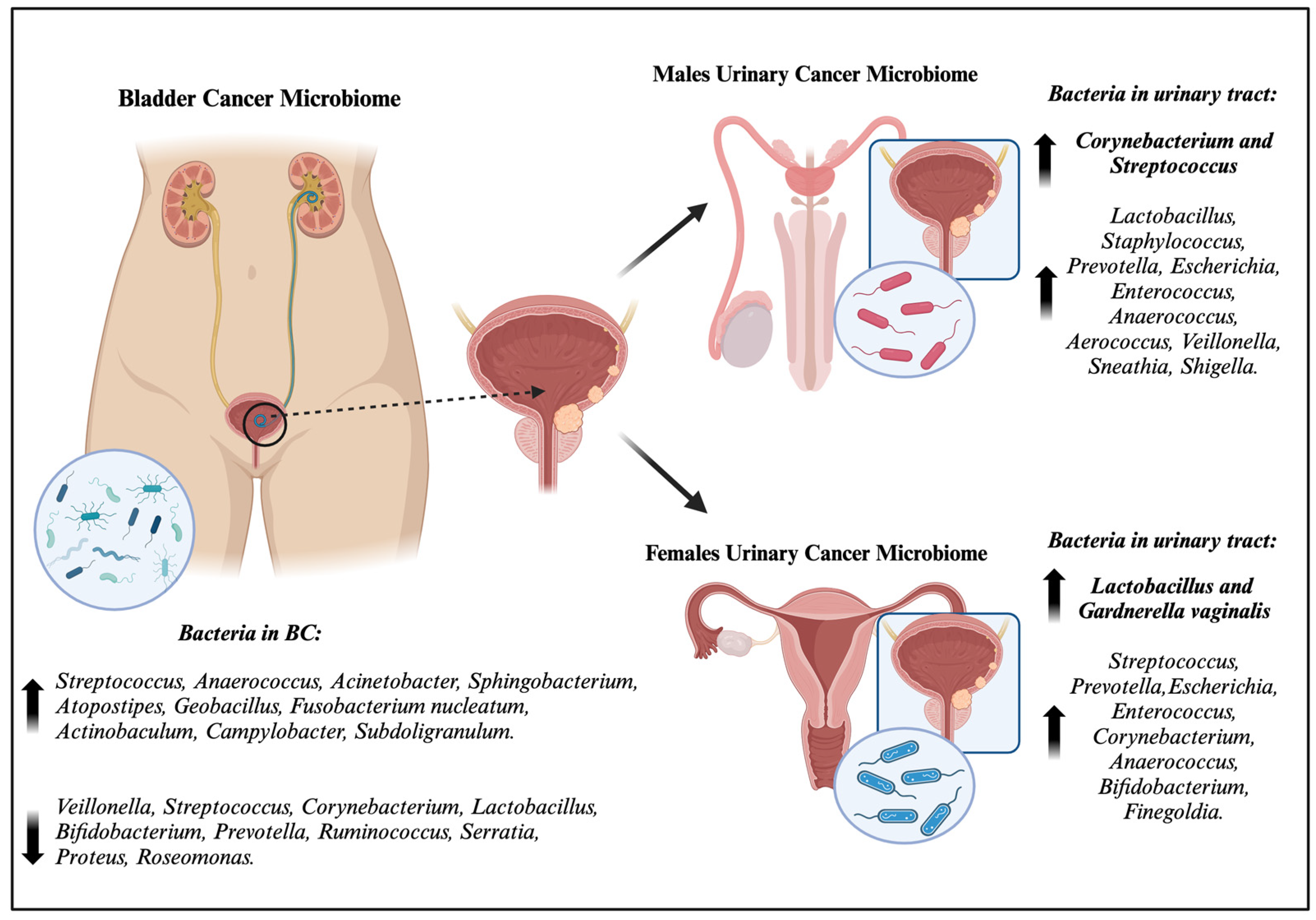
:1. Introduction
2. The Genitourinary Microbiome
2.1. The Healthy Urinary Microbiome
2.1.1. The Urinary Microbiome in Healthy Females
2.1.2. The Urinary Microbiome in Healthy Males
2.2. The Urinary Microbiome of Diseased Individuals
3. The Bladder Cancer Microbiome
| Sex (M/F) | BC/HC | Sample | Increase | Decrease | Reference |
|---|---|---|---|---|---|
| NA | 8/6 | urine | Streptoccocus, Pseudomonas, Anaerococcus | NA | [78] |
| 49M | 31/18 | urine | Acinetobacter, Anaerococcus, Sphingobacterium, Geobacillus | Serratia, Proteus, Roseomonas, Ruminiclostridium-6, Eubacterium-x | [85] |
| 33M | 12/11 | urine | Fusobacterium nucleatum, Actinobaculum, Facklamia, Campylobacter, Subdoligranulum, Ruminococcaceae | Veillonella, Streptococcus, Corynebacterium | [80] |
| 35M/20F | 29/16 | urine | Actinomyces | Streptococcus, Bifidobacterium, Lactobacillus, Veillonella | [103] |
| 18M/6F | 24/0 | urine | Enterobacteriaceae, Streptococcus, Lactobacillus, Ureaplasma, Corynebacterium, Stenotrophomonas, Enterococcus, Staphylococcus | X | [104] |
| 41M | 8/33 | urine | No differences | No differences | [105] |
| 42M/6F | 38/10 | urine | Bacteroides, Faecalibacterium | Lachnoclostridium, Burkholderiaceae | [83] |
| 14M/8F | 22/0 | urine | Tepidimonas (male) | Prevotella, Veillonella (male) | [106] |
| NA | 62/19 | urine | Micrococcus, Brachybacterium | X | [107] |
| NA | 15/11 | urine | Bacteroidaceae, Erysipelotrichales, Lachnospiraceae | X | [108] |
| 36M/7F | 43/10 | urine | MIBC: Haemophilus, Veillonela NMIBC: Cupriavidus, Serratia, Brochothrix, Negativicoccus, Escherichia-Shigella, Pseudomonas | X | [81] |
| 40M | 40/0 | urine | Pseudomonas, Staphylococcus, Corynebacterium, Acinetobacter | X | [109] |
| 56M | 32/24 | urine | Arthrobacter ginkgonis, Micrococcus sp., Hydrogenophaga aquatica, Defluviimonas pyrenivorans, Propionibacterium namnetense, Corynebacterium halotolerans, Acinetoacter celticus | X | [110] |
| 61M | 51/10 | urine | Veillonella, Corynebacterium | Ruminococcus | [86] |
| 5M/5F | 10/0 | tissue and urine | Tissue: Akkermansia, Bacteroides, Clostridium sensu stricto, Enterobacter, Klebsiella | X | [76] |
| 70M/38F | 49/59 | tissue and urine | Tissue: Burkholderia; Urine (Male): Acidobacteria, Opitutaceae; Urine (Female): Klebsiella | Urine (Male): Tissierellaceae, Alphaproteobacteria, Rhizobiales, Sphingomonadales, Pasteurellales, Streptococcaceae, Corynebacteriaceae, Patulibaceteraceae; Urine (Female): Betaproteobacteria, Burkholderiales, Pseudomonadales, Comamonadaceae, Moraxellaceae, Coriobacteriaceae, Coriobacteriia | [75] |
| 22M | 22 BC tissues 12 healthy tissue | tissue | Cupriavidus spp., Acinetobacter, Anoxybacillus, Escherichia, Shigella, Geobacillus, Pelomonas, Ralstonia, Sphingomonas | Lactobacillus, Prevotella, Ruminococcus | [82] |
4. Microbiome, Inflammation, and Bladder Cancer
5. Microbiome and Bladder Cancer Therapy
5.1. Microbiome and BCG Responsiveness
5.2. Microbiome and Immune Checkpoint Immunotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hadkhale, K.; Martinsen, J.I.; Weiderpass, E.; Kjaerheim, K.; Sparen, P.; Tryggvadottir, L.; Lynge, E.; Pukkala, E. Occupational Exposure to Solvents and Bladder Cancer: A Population-Based Case Control Study in Nordic Countries. Int. J. Cancer 2017, 140, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.C.; Gore, J.L.; Chi, A.C.; Kwan, L.; McCarthy, W.; Chamie, K.; Saigal, C.S. Impact of a Bladder Cancer Diagnosis on Smoking Behavior. J. Clin. Oncol. 2012, 30, 1871–1878. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Mao, S.; Wang, R.; Chen, H.; Ran, Y.; Liu, S.; Wu, P.; Yan, Y.; Li, W.; et al. Bladder cancer-associated microbiota: Recent advances and future perspectives. Heliyon 2023, 9, e13012. [Google Scholar] [CrossRef]
- Berdik, C. Unlocking bladder cancer. Nature 2017, 551, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M. Bladder cancer. Nature 2017, 551, S33. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2013 Guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; van de Putte, E.E.F.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncol. 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Klein, C.A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 2020, 20, 681–694. [Google Scholar] [CrossRef]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Markowski, M.C.; Boorjian, S.A.; Burton, J.P.; Hahn, N.M.; Ingersoll, M.A.; Vareki, S.M.; Pal, S.K.; Sfanos, K.S. The Microbiome and Genitourinary Cancer: A Collaborative Review. Eur. Urol. 2019, 75, 637–646. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; Del Moral, J.S.-G.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus 2018, 4, 128–138. [Google Scholar] [CrossRef]
- Bao, Y.; Al, K.F.; Chanyi, R.M.; Whiteside, S.; Dewar, M.; Razvi, H.; Reid, G.; Burton, J.P. Questions and challenges associated with studying the microbiome of the urinary tract. Ann. Transl. Med. 2017, 5, 33. [Google Scholar] [CrossRef]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Magistro, G.; Stief, C.G. The Urinary Tract Microbiome: The Answer to All Our Open Questions? Eur. Urol. Focus 2019, 5, 36–38. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar]
- Jung, C.E.; Chopyk, J.; Shin, J.H.; Lukacz, E.S.; Brubaker, L.; Schwanemann, L.K.; Knight, R.; Wolfe, A.J.; Pride, D.T. Benchmarking urine storage and collection conditions for evaluating the female urinary microbiome. Sci. Rep. 2019, 9, 13409 . [Google Scholar] [CrossRef]
- Wolfe, A.J.; Brubaker, L. Urobiome updates: Advances in urinary microbiome research. Nat. Rev. Urol. 2019, 16, 73–74. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.-H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Siddiqui, H.; Nederbragt, A.J.; Lagesen, K.; Jeansson, S.L.; Jakobsen, K.S. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011, 11, 244. [Google Scholar] [CrossRef]
- Modena, B.D.; Milam, R.; Harrison, F.; Cheeseman, J.A.; Abecassis, M.M.; Friedewald, J.J.; Kirk, A.D.; Salomon, D.R. Changes in Urinary Microbiome Populations Correlate in Kidney Transplants with Interstitial Fibrosis and Tubular Atrophy Documented in Early Surveillance Biopsies. Am. J. Transplant. 2016, 17, 712–723. [Google Scholar] [CrossRef]
- Brubaker, L.; Wolfe, A.J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl. Med. 2017, 5, 34. [Google Scholar] [CrossRef]
- Lewis, D.A.; Brown, R.; Williams, J.; White, P.; Jacobson, S.K.; Marchesi, J.R.; Drake, M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol. 2015, 213, 347.e1–347.e11. [Google Scholar] [CrossRef]
- Gottschick, C.; Deng, Z.-L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef]
- Nelson, D.E.; Van Der Pol, B.; Dong, Q.; Revanna, K.V.; Fan, B.; Easwaran, S.; Sodergren, E.; Weinstock, G.M.; Diao, L.; Fortenberry, J.D. Characteristic Male Urine Microbiomes Associate with Asymptomatic Sexually Transmitted Infection. PLoS ONE 2010, 5, e14116. [Google Scholar] [CrossRef]
- E Fouts, D.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.-J.; Huang, S.-T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef]
- Kogan, M.I.; Naboka, Y.L.; Ibishev, K.S.; Gudima, I.A.; Naber, K.G. Human Urine Is Not Sterile—Shift of Paradigm. Urol. Int. 2015, 94, 445–452. [Google Scholar] [CrossRef]
- Thomas-White, K.J.; Hilt, E.E.; Fok, C.; Pearce, M.M.; Mueller, E.R.; Kliethermes, S.; Jacobs, K.; Zilliox, M.J.; Brincat, C.; Price, T.K.; et al. Incontinence medication response relates to the female urinary microbiota. Int. Urogynecol. J. 2016, 27, 723–733. [Google Scholar] [CrossRef]
- Karstens, L.; Asquith, M.; Davin, S.; Stauffer, P.; Fair, D.; Gregory, W.T.; Rosenbaum, J.T.; McWeeney, S.K.; Nardos, R. Does the Urinary Microbiome Play a Role in Urgency Urinary Incontinence and Its Severity? Front. Cell. Infect. Microbiol. 2016, 6, 78. [Google Scholar] [CrossRef]
- Komesu, Y.M.; Dinwiddie, D.L.; Richter, H.E.; Lukacz, E.S.; Sung, V.W.; Siddiqui, N.Y.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; Arya, L.A.; et al. Defining the relationship between vaginal and urinary microbiomes. Am. J. Obstet. Gynecol. 2020, 222, 154.e1–154.e10. [Google Scholar] [CrossRef]
- Price, T.; Hilt, E.; Thomas-White, K.; Mueller, E.; Wolfe, A.; Brubaker, L. The urobiome of continent adult women: A cross-sectional study. Int. J. Obstet. Gynaecol. 2020, 127, 193–201. [Google Scholar] [CrossRef]
- Nickel, J.C.; Stephens, A.; Ackerman, A.L.; Anger, J.T.; Lai, H.H.; Ehrlich, G.D. The Healthy Urinary Microbiome in Asymptomatic Participants in the Mapp Network Study: Relation to Gender, Age, and Menopausal Status. Can. Urol. Assoc. J. 2022, 16, E448–E454. [Google Scholar] [CrossRef]
- Redondo-Lopez, V.; Cook, R.L.; Sobel, J.D. Emerging Role of Lactobacilli in the Control and Maintenance of the Vaginal Bacterial Microflora. Clin. Infect. Dis. 1990, 12, 856–872. [Google Scholar] [CrossRef]
- Kaewsrichan, J.; Peeyananjarassri, K.; Kongprasertkit, J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunol. Med Microbiol. 2006, 48, 75–83. [Google Scholar] [CrossRef]
- Ruiz-Gomez, M.L.; Martin-Way, D.A.; Perez-Ramirez, M.D.; Gutierrez-Fernandez, J. Male Deep Infections by Gardnerella Vaginalis. A Literature Review and a Case Report. Rev. Esp. Quimioter. 2019, 32, 469–472. [Google Scholar]
- Liu, F.; Ling, Z.; Xiao, Y.; Yang, Q.; Zheng, L.; Jiang, P.; Li, L.; Wang, W. Characterization of the urinary microbiota of elderly women and the effects of type 2 diabetes and urinary tract infections on the microbiota. Oncotarget 2017, 8, 100678–100690. [Google Scholar] [CrossRef]
- Komesu, Y.M.; Network, F.T.P.F.D.; Richter, H.E.; Carper, B.; Dinwiddie, D.L.; Lukacz, E.S.; Siddiqui, N.Y.; Sung, V.W.; Zyczynski, H.M.; Ridgeway, B.; et al. The urinary microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int. Urogynecol. J. 2018, 29, 1785–1795. [Google Scholar] [CrossRef]
- Thomas-White, K.J.; Gao, X.; Lin, H.; Fok, C.S.; Ghanayem, K.; Mueller, E.R.; Dong, Q.; Brubaker, L.; Wolfe, A.J. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int. Urogynecol. J. 2018, 29, 1797–1805. [Google Scholar] [CrossRef]
- Dubourg, G.; Morand, A.; Mekhalif, F.; Godefroy, R.; Corthier, A.; Yacouba, A.; Diakite, A.; Cornu, F.; Cresci, M.; Brahimi, S.; et al. Deciphering the Urinary Microbiota Repertoire by Culturomics Reveals Mostly Anaerobic Bacteria From the Gut. Front. Microbiol. 2020, 11, 513305. [Google Scholar] [CrossRef]
- Morand, A.; Cornu, F.; Dufour, J.-C.; Tsimaratos, M.; Lagier, J.-C.; Raoult, D. Human Bacterial Repertoire of the Urinary Tract: A Potential Paradigm Shift. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Dong, Q.; Nelson, D.E.; Toh, E.; Diao, L.; Gao, X.; Fortenberry, J.D.; Van Der Pol, B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE 2011, 6, e19709. [Google Scholar] [CrossRef]
- Nelson, D.E.; Dong, Q.; Van Der Pol, B.; Toh, E.; Fan, B.; Katz, B.P.; Mi, D.; Rong, R.; Weinstock, G.M.; Sodergren, E.; et al. Bacterial Communities of the Coronal Sulcus and Distal Urethra of Adolescent Males. PLoS ONE 2012, 7, e36298. [Google Scholar] [CrossRef]
- Bajic, P.; Van Kuiken, M.E.; Burge, B.K.; Kirshenbaum, E.J.; Joyce, C.J.; Wolfe, A.J.; Branch, J.D.; Bresler, L.; Farooq, A.V. Male Bladder Microbiome Relates to Lower Urinary Tract Symptoms. Eur. Urol. Focus 2020, 6, 376–382. [Google Scholar] [CrossRef]
- Moustafa, A.; Li, W.; Singh, H.; Moncera, K.J.; Torralba, M.G.; Yu, Y.; Manuel, O.; Biggs, W.; Venter, J.C.; Nelson, K.E.; et al. Microbial metagenome of urinary tract infection. Sci. Rep. 2018, 8, 4333. [Google Scholar] [CrossRef]
- Groah, S.L.; Pérez-Losada, M.; Caldovic, L.; Ljungberg, I.H.; Sprague, B.M.; Castro-Nallar, E.; Chandel, N.J.; Hsieh, M.H.; Pohl, H.G. Redefining Healthy Urine: A Cross-Sectional Exploratory Metagenomic Study of People with and without Bladder Dysfunction. J. Urol. 2016, 196, 579–587. [Google Scholar] [CrossRef]
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef]
- Hooton, T.M. Clinical Practice. Uncomplicated Urinary Tract Infection. N. Engl. J. Med. 2012, 366, 1028–1037. [Google Scholar] [CrossRef]
- Khasriya, R.; Sathiananthamoorthy, S.; Ismail, S.; Kelsey, M.; Wilson, M.; Rohn, J.L.; Malone-Lee, J. Spectrum of Bacterial Colonization Associated with Urothelial Cells from Patients with Chronic Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2013, 51, 2054–2062. [Google Scholar] [CrossRef]
- Garretto, A.; Miller-Ensminger, T.; Ene, A.; Merchant, Z.; Shah, A.; Gerodias, A.; Biancofiori, A.; Canchola, S.; Canchola, S.; Castillo, E.; et al. Genomic Survey of E. coli From the Bladders of Women with and without Lower Urinary Tract Symptoms. Front. Microbiol. 2020, 11, 2094. [Google Scholar] [CrossRef]
- Croxall, G.; Weston, V.; Joseph, S.; Manning, G.; Cheetham, P.; McNally, A. Increased human pathogenic potential of Escherichia coli from polymicrobial urinary tract infections in comparison to isolates from monomicrobial culture samples. J. Med Microbiol. 2011, 60, 102–109. [Google Scholar] [CrossRef]
- Govender, Y.; Gabriel, I.; Minassian, V.; Fichorova, R. The Current Evidence on the Association Between the Urinary Microbiome and Urinary Incontinence in Women. Front. Cell. Infect. Microbiol. 2019, 9, 133. [Google Scholar] [CrossRef]
- Thomas-White, K.J.; Kliethermes, S.; Rickey, L.; Lukacz, E.S.; Richter, H.E.; Moalli, P.; Zimmern, P.; Norton, P.; Kusek, J.W.; Wolfe, A.J.; et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am. J. Obstet. Gynecol. 2017, 216, 55.e1–55.e16. [Google Scholar] [CrossRef]
- Curtiss, N.; Balachandran, A.; Krska, L.; Peppiatt-Wildman, C.; Wildman, S.; Duckett, J. A case controlled study examining the bladder microbiome in women with Overactive Bladder (OAB) and healthy controls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 31–35. [Google Scholar] [CrossRef]
- Wu, P.; Chen, Y.; Zhao, J.; Zhang, G.; Chen, J.; Wang, J.; Zhang, H. Urinary Microbiome and Psychological Factors in Women with Overactive Bladder. Front. Cell. Infect. Microbiol. 2017, 7, 488. [Google Scholar] [CrossRef]
- Fok, C.S.; Gao, X.; Lin, H.; Thomas-White, K.J.; Mueller, E.R.; Wolfe, A.J.; Dong, Q.; Brubaker, L. Urinary symptoms are associated with certain urinary microbes in urogynecologic surgical patients. Int. Urogynecol. J. 2018, 29, 1765–1771. [Google Scholar] [CrossRef]
- Nickel, J.C.; Stephens, A.; Landis, J.R.; Mullins, C.; van Bokhoven, A.; Lucia, M.S.; Ehrlich, G.D.; MAPP Research Network. Assessment of the Lower Urinary Tract Microbiota during Symptom Flare in Women with Urologic Chronic Pelvic Pain Syndrome: A MAPP Network Study. J. Urol. 2016, 195, 356–362. [Google Scholar] [CrossRef]
- Nickel, J.C.; Stephens-Shields, A.J.; Landis, J.R.; Mullins, C.; van Bokhoven, A.; Lucia, M.S.; Henderson, J.P.; Sen, B.; Krol, J.E.; Ehrlich, G.D.; et al. A Culture-Independent Analysis of the Microbiota of Female Interstitial Cystitis/Bladder Pain Syndrome Participants in the MAPP Research Network. J. Clin. Med. 2019, 8, 415. [Google Scholar] [CrossRef]
- Bresler, L.; Price, T.K.; Hilt, E.E.; Joyce, C.; Fitzgerald, C.M.; Wolfe, A.J. Female lower urinary tract microbiota do not associate with IC/PBS symptoms: A case-controlled study. Int. Urogynecol. J. 2019, 30, 1835–1842. [Google Scholar] [CrossRef]
- Meriwether, K.V.; Lei, Z.; Singh, R.; Gaskins, J.; Hobson, D.T.G.; Jala, V. The Vaginal and Urinary Microbiomes in Premenopausal Women with Interstitial Cystitis/Bladder Pain Syndrome as Compared to Unaffected Controls: A Pilot Cross-Sectional Study. Front. Cell. Infect. Microbiol. 2019, 9, 92. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Altemus, J.; Polackwich, A.S.; Tucky, B.; Wang, H.; Eng, C. The Urinary Microbiome Differs Significantly between Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls as Well as between Patients with Different Clinical Phenotypes. Urology 2016, 92, 26–32. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; bin Kim, W.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Pederzoli, F.; Ferrarese, R.; Amato, V.; Locatelli, I.; Alchera, E.; Lucianò, R.; Nebuloni, M.; Briganti, A.; Gallina, A.; Colombo, R.; et al. Sex-specific Alterations in the Urinary and Tissue Microbiome in Therapy-naïve Urothelial Bladder Cancer Patients. Eur. Urol. Oncol. 2020, 3, 784–788. [Google Scholar] [CrossRef]
- Mansour, B.; Monyók, Á.; Makra, N.; Gajdács, M.; Vadnay, I.; Ligeti, B.; Juhász, J.; Szabó, D.; Ostorházi, E. Bladder cancer-related microbiota: Examining differences in urine and tissue samples. Sci. Rep. 2020, 10, 11042. [Google Scholar] [CrossRef]
- Alfano, M.; Canducci, F.; Nebuloni, M.; Clementi, M.; Montorsi, F.; Salonia, A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 2016, 13, 77–90. [Google Scholar] [CrossRef]
- Xu, W.; Yang, L.; Lee, P.; Huang, W.C.; Nossa, C.; Ma, Y.; Deng, F.M.; Zhou, M.; Melamed, J.; Pei, Z. Mini-Review: Perspective of the Microbiome in the Pathogenesis of Urothelial Carcinoma. Am. J. Clin. Exp. Urol. 2014, 2, 57–61. [Google Scholar]
- Vollmer, P.; Walev, I.; Rose-John, S.; Bhakdi, S. Novel pathogenic mechanism of microbial metalloproteinases: Liberation of membrane-anchored molecules in biologically active form exemplified by studies with the human interleukin-6 receptor. Infect. Immun. 1996, 64, 3646–3651. [Google Scholar] [CrossRef]
- Popović, V.B.; Šitum, M.; Chow, C.-E.T.; Chan, L.S.; Roje, B.; Terzić, J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018, 8, 12157. [Google Scholar] [CrossRef]
- Hussein, A.A.; Elsayed, A.S.; Durrani, M.; Jing, Z.; Iqbal, U.; Gomez, E.C.; Singh, P.K.; Liu, S.; Smith, G.; Tang, L.; et al. Investigating the association between the urinary microbiome and bladder cancer: An exploratory study. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 370.e9–370.e19. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef]
- Chipollini, J.; Wright, J.R.; Nwanosike, H.; Kepler, C.Y.; Batai, K.; Lee, B.R.; Spiess, P.E.; Stewart, D.B.; Lamendella, R. Characterization of urinary microbiome in patients with bladder cancer: Results from a single-institution, feasibility study. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 615–621. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the Urinary Microbiota in Male Patients with Bladder Cancer in China. Front. Cell. Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef]
- Oresta, B.; Braga, D.; Lazzeri, M.; Frego, N.; Saita, A.; Faccani, C.; Fasulo, V.; Colombo, P.; Guazzoni, G.; Hurle, R.; et al. The Microbiome of Catheter Collected Urine in Males with Bladder Cancer According to Disease Stage. J. Urol. 2021, 205, 86–93. [Google Scholar] [CrossRef]
- Di Giacinto, C.; Marinaro, M.; Sanchez, M.; Strober, W.; Boirivant, M. Probiotics Ameliorate Recurrent Th1-Mediated Murine Colitis by Inducing IL-10 and IL-10-Dependent TGF-β-Bearing Regulatory Cells. J. Immunol. 2005, 174, 3237–3246. [Google Scholar] [CrossRef]
- Burrello, C.; Garavaglia, F.; Cribiù, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef]
- Svircev, Z.; Baltic, V.; Gantar, M.; Jukovic, M.; Stojanovic, D.; Baltic, M. Molecular Aspects of Microcystin-induced Hepatotoxicity and Hepatocarcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 39–59. [Google Scholar] [CrossRef]
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014, 2, 20. [Google Scholar] [CrossRef]
- Bloom, S.M.; Bijanki, V.N.; Nava, G.M.; Sun, L.; Malvin, N.P.; Donermeyer, D.L.; Dunne, W.M.; Allen, P.M.; Stappenbeck, T.S. Commensal Bacteroides Species Induce Colitis in Host-Genotype-Specific Fashion in a Mouse Model of Inflammatory Bowel Disease. Cell Host Microbe 2011, 9, 390–403. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Yurdakul, D.; Yazgan-Karataş, A.; Şahin, F. Enterobacter Strains Might Promote Colon Cancer. Curr. Microbiol. 2015, 71, 403–411. [Google Scholar] [CrossRef]
- Bossuet-Greif, N.; Vignard, J.; Taieb, F.; Mirey, G.; Dubois, D.; Petit, C.; Oswald, E.; Nougayrède, J.-P. The Colibactin Genotoxin Generates DNA Interstrand Cross-Links in Infected Cells. mBio 2018, 9, e02393-17. [Google Scholar] [CrossRef]
- Kaur, C.P.; Vadivelu, J.; Chandramathi, S. Impact of Klebsiella pneumoniae in lower gastrointestinal tract diseases. J. Dig. Dis. 2018, 19, 262–271. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Déchelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the Human Gut by E. coli and Colorectal Cancer Risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef]
- Noon, A.P.; Albertsen, P.C.; Thomas, F.; Rosario, D.J.; Catto, J.W.F. Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br. J. Cancer 2013, 108, 1534–1540. [Google Scholar] [CrossRef]
- de Jong, J.J.; Boormans, J.L.; van Rhijn, B.W.; Seiler, R.; Boorjian, S.A.; Konety, B.; Bivalacqua, T.J.; Wheeler, T.; Svatek, R.S.; Douglas, J.; et al. Distribution of Molecular Subtypes in Muscle-invasive Bladder Cancer Is Driven by Sex-specific Differences. Eur. Urol. Oncol. 2020, 3, 420–423. [Google Scholar] [CrossRef]
- Nagata, Y.; Matsukawa, T.; Goto, T.; Teramoto, Y.; Jiang, G.; Fujimoto, N.; Miyamoto, H. Protective role of mineralocorticoid receptor signaling in urothelial tumorigenesis. Am. J. Cancer Res. 2023, 13, 408–418. [Google Scholar]
- Bi, H.; Tian, Y.; Song, C.; Li, J.; Liu, T.; Chen, Z.; Chen, C.; Huang, Y.; Zhang, Y. Urinary microbiota—A potential biomarker and therapeutic target for bladder cancer. J. Med. Microbiol. 2019, 68, 1471–1478. [Google Scholar] [CrossRef]
- Mai, G.; Chen, L.; Li, R.; Liu, Q.; Zhang, H.; Ma, Y. Common Core Bacterial Biomarkers of Bladder Cancer Based on Multiple Datasets. BioMed Res. Int. 2019, 2019, 4824909. [Google Scholar] [CrossRef]
- Moynihan, M.J.; Sullivan, T.; Provenzano, K.; Rieger-Christ, K. Urinary Microbiome Evaluation in Patients Presenting with Hematuria with a Focus on Exposure to Tobacco Smoke. Res. Rep. Urol. 2019, ume 11, 359–367. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Zhu, W.; Wong, W.; Clemency, N.C.; Provenzano, M.; Vilboux, T.; Niederhuber, J.E.; Deeken, J.; Chung, S.; McDaniel-Wiley, K.; et al. Studying the urine microbiome in superficial bladder cancer: Samples obtained by midstream voiding versus cystoscopy. BMC Urol. 2020, 20, 5. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, G.; Chen, C.; Li, K.; Wen, Y.; Zhao, J.; Wu, P. Alterations in Urobiome in Patients with Bladder Cancer and Implications for Clinical Outcome: A Single-Institution Study. Front. Cell. Infect. Microbiol. 2020, 10, 555508. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Shen, L.; Liu, J.; Yang, F.; Maskey, N.; Wang, H.; Zhang, J.; Yan, Y.; Yao, X. Can Smoking Cause Differences in Urine Microbiome in Male Patients with Bladder Cancer? A Retrospective Study. Front. Oncol. 2021, 11, 677605. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, Y.; Chen, C.; Xie, M.; Huang, P.; Sun, Q.; Zhou, Z.; Li, B.; Zhao, J.; Wu, P. Deciphering the influence of urinary microbiota on FoxP3+ regulatory T cell infiltration and prognosis in Chinese patients with non-muscle-invasive bladder cancer. Hum. Cell 2022, 35, 511–521. [Google Scholar] [CrossRef]
- Ahn, H.K.; Kim, K.; Park, J.; Kim, K.H. Urinary Microbiome Profile in Men with Genitourinary Malignancies. Investig. Clin. Urol. 2022, 63, 569–576. [Google Scholar] [CrossRef]
- Kuper, H.; Adami, H.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000, 248, 171–183. [Google Scholar] [CrossRef]
- Nicolaro, M.; Portal, D.E.; Shinder, B.; Patel, H.V.; Singer, E.A. The human microbiome and genitourinary malignancies. Ann. Transl. Med. 2020, 8, 1245. [Google Scholar] [CrossRef]
- Kantor, A.F.; Hartge, P.; Hoover, R.N.; Narayana, A.S.; Sullivan, J.W.; Fraumeni, J.F. Urinary Tract Infection and Risk of Bladder Cancer. Am. J. Epidemiol. 1984, 119, 510–515. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Sheweita, S.A.; O’connor, P.J. Relationship between Schistosomiasis and Bladder Cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef]
- Hicks, R.; Ismail, M.M.; Walters, C.; Beecham, P.; Rabie, M.F.; A El Alamy, M. Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans. R Soc. Trop. Med. Hyg. 1982, 76, 519–527. [Google Scholar] [CrossRef]
- Ventafridda, V. Continuing care: A major issue in cancer pain management. Pain 1989, 36, 137–143. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Gondo, T.; Nakashima, J.; Ohno, Y.; Choichiro, O.; Horiguchi, Y.; Namiki, K.; Yoshioka, K.; Ohori, M.; Hatano, T.; Tachibana, M. Prognostic Value of Neutrophil-to-lymphocyte Ratio and Establishment of Novel Preoperative Risk Stratification Model in Bladder Cancer Patients Treated with Radical Cystectomy. Urology 2012, 79, 1085–1091. [Google Scholar] [CrossRef]
- Hermanns, T.; Bhindi, B.; Wei, Y.; Yu, J.; Noon, A.P.; O Richard, P.; Bhatt, J.R.; Almatar, A.; Jewett, M.A.S.; E Fleshner, N.; et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br. J. Cancer 2014, 111, 444–451. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, K.; Wang, L.; Sun, E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol. Res. Pr. 2018, 214, 1074–1080. [Google Scholar] [CrossRef]
- Mbeutcha, A.; Shariat, S.F.; Rieken, M.; Rink, M.; Xylinas, E.; Seitz, C.; Lucca, I.; Mathieu, R.; Rouprêt, M.; Briganti, A.; et al. Prognostic significance of markers of systemic inflammatory response in patients with non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 483.e17–483.e24. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Dutta, D.; Lim, S.H. Bidirectional interaction between intestinal microbiome and cancer: Opportunities for therapeutic interventions. Biomark. Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T H 1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Raziuddin, S.; Masihuzzaman, M.; Shetty, S.; Ibrahim, A. Tumor necrosis factor alpha production in schistosomiasis with carcinoma of urinary bladder. J. Clin. Immunol. 1993, 13, 23–29. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Li, J.; Huang, J.; Xie, J.H.; Menard, L.; Shi, Y.; Zhao, X.; Xie, S.; Zang, W.; et al. Systematic characterization of the tumor microenvironment in Chinese patients with hepatocellular carcinoma highlights intratumoral B cells as a potential immunotherapy target. Oncol. Rep. 2022, 47, 38. [Google Scholar] [CrossRef]
- Chen, M.-F.; Lin, P.-Y.; Wu, C.-F.; Chen, W.-C.; Wu, C.-T. IL-6 Expression Regulates Tumorigenicity and Correlates with Prognosis in Bladder Cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef]
- Cardillo, M.R.; Sale, P.; Di Silverio, F. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer Res. 2001, 20, 4579–4583. [Google Scholar]
- Kumari, N.; Agrawal, U.; Mishra, A.K.; Kumar, A.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumor Biol. 2017, 39, 1010428317697552. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, X.; Zhu, Y.; Yao, Z.; Zhao, W.; Zhu, Y.; Sun, F.; Mu, X.; Wang, Y.; He, W.; et al. IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer. BioMed Res. Int. 2021, 2021, 5535578. [Google Scholar] [CrossRef]
- Gatta, L.B.; Melocchi, L.; Bugatti, M.; Missale, F.; Lonardi, S.; Zanetti, B.; Cristinelli, L.; Belotti, S.; Simeone, C.; Ronca, R.; et al. Hyper-Activation of STAT3 Sustains Progression of Non-Papillary Basal-Type Bladder Cancer via FOSL1 Regulome. Cancers 2019, 11, 1219. [Google Scholar] [CrossRef]
- Trikha, M.; Corringham, R.; Klein, B.; Rossi, J.-F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: A review of the rationale and clinical evidence. Clin. Cancer Res. 2003, 9, 4653–4665. [Google Scholar]
- Segain, J.P.; Raingeard de la Bletiere, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.; Galmiche, J. Butyrate Inhibits Inflammatory Responses through Nfkappab Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, T.; Wang, J. Expression of IL-23R and IL-17 and the pathology and prognosis of urinary bladder carcinoma. Oncol. Lett. 2018, 16, 4325–4330. [Google Scholar] [CrossRef]
- El-Gedamy, M.; El-Khayat, Z.; Abol-Enein, H.; El-Said, A.; El-Nahrery, E. Rs-10889677 variant in interleukin-23 receptor may contribute to creating an inflammatory milieu more susceptible to bladder tumourigenesis: Report and meta-analysis. Immunogenetics 2021, 73, 207–226. [Google Scholar] [CrossRef]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- El-Gedamy, M.; El-Khayat, Z.; Abol-Enein, H.; El-Said, A.; El-Nahrery, E. Rs-1884444 G/T variant in IL-23 receptor is likely to modify risk of bladder urothelial carcinoma by regulating IL-23/IL-17 inflammatory pathway. Cytokine 2021, 138, 155355. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.-J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.-R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Dupraz, L.; Dupraz, L.; Magniez, A.; Magniez, A.; Rolhion, N.; Rolhion, N.; Richard, M.L.; Richard, M.L.; Da Costa, G.; Da Costa, G.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 2128–2129. [Google Scholar] [CrossRef]
- Lima, L.; Oliveira, D.; Tavares, A.; Amaro, T.; Cruz, R.; Oliveira, M.J.; Ferreira, J.A.; Santos, L. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 449–457. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Tang, K.; Guan, W.; Yang, T.; Xu, H.; Zhuang, Q.-Y.; Ye, Z.-Q. OK-432 Suppresses Proliferation and Metastasis by Tumor Associated Macrophages in Bladder Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4537–4542. [Google Scholar] [CrossRef]
- Chen, C.; He, W.; Huang, J.; Wang, B.; Li, H.; Cai, Q.; Su, F.; Bi, J.; Liu, H.; Zhang, B.; et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat. Commun. 2018, 9, 3826. [Google Scholar] [CrossRef]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef]
- Boström, M.M.; Irjala, H.; Mirtti, T.; Taimen, P.; Kauko, T.; Ålgars, A.; Jalkanen, S.; Boström, P.J. Tumor-Associated Macrophages Provide Significant Prognostic Information in Urothelial Bladder Cancer. PLoS ONE 2015, 10, e0133552. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Sun, J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene 2019, 710, 91–97. [Google Scholar] [CrossRef]
- Yang, G.; Shen, W.; Zhang, Y.; Liu, M.; Zhang, L.; Liu, Q.; Lu, H.H.; Bo, J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 2017, 8, 38378–38388. [Google Scholar] [CrossRef]
- Eruslanov, E.; Neuberger, M.; Daurkin, I.; Perrin, G.Q.; Algood, C.; Dahm, P.; Rosser, C.; Vieweg, J.; Gilbert, S.M.; Kusmartsev, S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int. J. Cancer 2012, 130, 1109–1119. [Google Scholar] [CrossRef]
- Yuan, X.-K.; Zhao, X.-K.; Xia, Y.-C.; Zhu, X.; Xiao, P. Increased Circulating Immunosuppressive CD14+HLA-DR−/Low Cells Correlate with Clinical Cancer Stage and Pathological Grade in Patients with Bladder Carcinoma. J. Int. Med. Res. 2011, 39, 1381–1391. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.-L.; Li, M.-X.; Ye, S.-B.; Huang, W.-R.; Cai, T.-T.; He, J.; Peng, J.-Y.; Duan, T.-H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE 2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmstrom, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder Cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side Effects of Bacillus Calmette-Guérin (BCG) in the Treatment of Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Bladder: Results of the EORTC Genito-Urinary Cancers Group Randomised Phase 3 Study Comparing One-third Dose with Full Dose and 1 Year with 3 Years of Maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Pirzada, M.T.; Ghauri, R.; Ahmed, M.J.; Shah, M.F.; Nasir, I.U.I.; Siddiqui, J.; Ahmed, I.; Mir, K. Outcomes of BCG Induction in High-Risk Non-Muscle-Invasive Bladder Cancer Patients (NMIBC): A Retrospective Cohort Study. Cureus 2017, 9, e957. [Google Scholar] [CrossRef]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non–Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- de Queiroz, N.M.G.P.; Marinho, F.V.; de Araujo, A.C.V.S.C.; Fahel, J.S.; Oliveira, S.C. MyD88-dependent BCG immunotherapy reduces tumor and regulates tumor microenvironment in bladder cancer murine model. Sci. Rep. 2021, 11, 15648. [Google Scholar] [CrossRef]
- Jiang, S.; Redelman-Sidi, G. BCG in Bladder Cancer Immunotherapy. Cancers 2022, 14, 3073. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef]
- Luo, Y.; Knudson, M.J. Mycobacterium bovis Bacillus Calmette-Guérin-Induced Macrophage Cytotoxicity against Bladder Cancer Cells. J. Immunol. Res. 2010, 2010, 357591. [Google Scholar] [CrossRef]
- Siracusano, S.; Vita, F.; Abbate, R.; Ciciliato, S.; Borelli, V.; Bernabei, M.; Zabucchi, G. The Role of Granulocytes Following Intravesical BCG Prophylaxis. Eur. Urol. 2007, 51, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Zhang, X.; Artola-Boran, M.; Fallegger, A.; Sander, P.; Johansen, P.; Müller, A. BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 2019, 15, e1007866. [Google Scholar] [CrossRef]
- Sonoda, T.; Sugimura, K.; Ikemoto, S.-I.; Kawashima, H.; Nakatani, T. Significance of target cell infection and natural killer cells in the anti-tumor effects of bacillus Calmette-Guerin in murine bladder cancer. Oncol. Rep. 2007, 17, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Leko, V.; McDuffie, L.A.; Zheng, Z.; Gartner, J.J.; Prickett, T.D.; Apolo, A.B.; Agarwal, P.K.; Rosenberg, S.A.; Lu, Y.-C. Identification of Neoantigen-Reactive Tumor-Infiltrating Lymphocytes in Primary Bladder Cancer. J. Immunol. 2019, 202, 3458–3467. [Google Scholar] [CrossRef]
- Herr, H.W. Age and Outcome of Superficial Bladder Cancer Treated with Bacille Calmette-Guérin Therapy. Urology 2007, 70, 65–68. [Google Scholar] [CrossRef]
- Oddens, J.R.; Sylvester, R.J.; Brausi, M.A.; Kirkels, W.J.; van de Beek, C.; van Andel, G.; de Reijke, T.M.; Prescott, S.; Witjes, J.A.; Oosterlinck, W. The Effect of Age on the Efficacy of Maintenance Bacillus Calmette-Guérin Relative to Maintenance Epirubicin in Patients with Stage Ta T1 Urothelial Bladder Cancer: Results from EORTC Genito-Urinary Group Study 30911. Eur. Urol. 2014, 66, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Gontero, P.; Sylvester, R.; Pisano, F.; Joniau, S.; Eeckt, K.V.; Serretta, V.; Larré, S.; Di Stasi, S.; Van Rhijn, B.; Witjes, A.J.; et al. Prognostic Factors and Risk Groups in T1G3 Non–Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Guérin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur. Urol. 2015, 67, 74–82. [Google Scholar] [CrossRef]
- Contieri, R.; Grajales, V.; Tan, W.S.; Martini, A.; Sood, A.; Hensley, P.; Bree, K.; Lobo, N.; Nogueras-Gonzalez, G.M.; Guo, C.C.; et al. Impact of age >70 years on oncological outcomes in patients with non-muscle-invasive bladder cancer treated with Bacillus Calmette–Guérin. BJU Int. 2024, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef] [PubMed]
- Sandes, E.; Lodillinsky, C.; Cwirenbaum, R.; Argüelles, C.; Casabé, A.; Eiján, A.M. Cathepsin B is involved in the apoptosis intrinsic pathway induced by Bacillus Calmette-Guérin in transitional cancer cell lines. Int. J. Mol. Med. 2007, 20, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Sweis, R.F. Methods to Assess Anticancer Immune Responses in Orthotopic Bladder Carcinomas. Methods Enzym. 2020, 635, 127–137. [Google Scholar]
- Knorr, J.; Adler, A.; Agudelo, J.; Ericson, K.; Murthy, P.; Campbell, R.; Bajic, P.; Almassi, N.; Weight, C.; Haber, G.-P.; et al. PD42-04 Tumor Microbiome Associated with Bcg Response in Non-Muscle Invasive Bladder Cancer. J. Urol. 2021, 206, e725–e726. [Google Scholar] [CrossRef]
- Knorr, J.; Werneburg, G.; Adler, A.; Agudelo, J.; Murthy, P.; Campbell, R.; Bajic, P.; Almassi, N.; Weight, C.; Haber, G.-P.; et al. PD12-01 Bladder Tumor Microbiome May Augment Response to Bcg in Non-Muscle Invasive Bladder Cancer. J. Urol. 2022, 207, e195. [Google Scholar] [CrossRef]
- James, C.; Gomez, K.; Desai, S.; Patel, H.D.; Rac, G.; Doshi, C.P.; Dornbier, R.; Bajic, P.; Halverson, T.; Gupta, G.N.; et al. Impact of intravesical Bacillus Calmette-Guérin and chemotherapy on the bladder microbiome in patients with non-muscle invasive bladder cancer. Front. Cell. Infect. Microbiol. 2023, 13. [Google Scholar] [CrossRef]
- Seow, S.W.; Rahmat, J.N.B.; Mohamed, A.A.K.; Mahendran, R.; Lee, Y.K.; Bay, B.H. Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium Bovis (bacillus Calmette-Guerin). J. Urol. 2002, 168, 2236–2239. [Google Scholar] [CrossRef]
- Bieri, U.; Scharl, M.; Sigg, S.; Szczerba, B.M.; Morsy, Y.; Rüschoff, J.H.; Schraml, P.H.; Krauthammer, M.; Hefermehl, L.J.; Eberli, D.; et al. Prospective observational study of the role of the microbiome in BCG responsiveness prediction (Silent-Empire): A study protocol. BMJ Open 2022, 12, e061421. [Google Scholar] [CrossRef]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef]
- Farina, M.S.; Lundgren, K.T.; Bellmunt, J. Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs 2017, 77, 1077–1089. [Google Scholar] [CrossRef]
- Teo, M.Y.; Rosenberg, J.E. Nivolumab for the treatment of urothelial cancers. Expert Rev. Anticancer. Ther. 2018, 18, 215–221. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gollwitzer, E.S.; Saglani, S.; Trompette, A.; Yadava, K.; Sherburn, R.; McCoy, K.D.; Nicod, L.P.; Lloyd, C.M.; Marsland, B.J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014, 20, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.L.; Carson, T.L. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: A narrative review. Gut Pathog. 2020, 12, 43. [Google Scholar] [CrossRef]
- Vetizou, M.; Trinchieri, G. Anti-PD1 in the wonder-gut-land. Cell Res. 2018, 28, 263–264. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Z.; Huang, P.; Li, K.; Zeng, J.; Wen, Y.; Li, B.; Zhao, J.; Wu, P. Urogenital Microbiota:Potentially Important Determinant of PD-L1 Expression in Male Patients with Non-muscle Invasive Bladder Cancer. BMC Microbiol. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Cardona, A.; Nagarkar, D.; Scala, A.D.; Gandara, D.; Rittmeyer, A.; Albert, M.; Powles, T.; Kok, M.; Herrera, F. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Pestana, R.C.; Hess, K.; Viola, G.; Subbiah, V. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann. Oncol. 2018, 29, 2396–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.E.; Hong, S.H.; Lee, M.A.; Kang, J.H.; Kim, I.-H. The effect of antibiotics on the clinical outcomes of patients with solid cancers undergoing immune checkpoint inhibitor treatment: A retrospective study. BMC Cancer 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, G.; Li, W.; Li, X.; Zhao, C.; Jiang, T.; Jia, Y.; He, Y.; Li, A.; Su, C.; et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019, 130, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Raggi, D.; Bandini, M.; Pederzoli, F.; Giannatempo, P.; Marandino, L.; Basile, G.; Gallina, A.; Briganti, A.; Montorsi, F.; Necchi, A. Concomitant antibiotics (ATBs) use and survival outcomes in patients (pts) with muscle-invasive bladder cancer (MIBC) treated with neoadjuvant pembrolizumab (PURE-01 study). J. Clin. Oncol. 2021, 39, 449. [Google Scholar] [CrossRef]
| Age | No | Bacteria | Urine Sample | Reference |
|---|---|---|---|---|
| 27–67 | 8 | Lactobacillus, Prevotella, Gardnerella, Peptoniphilus, Dialister, Finegoldia, Anaerococcus, Allisonella, Streptococcus, Staphylococcus | MSU | [31] |
| 22–51 | 15 | Streptococcus, Aerococcus, Gardnerella, Prevotella, Escherichia, Enterococcus | MSU | [38] |
| NA | 12 | Lactobacillus, Actinobaculum, Aerococcus, Anaerococcus, Atopobium, Burkholderia, Corynebacterium, Gardnerella, Prevotella, Ralstonia, Sneathia, Staphylococcus, Streptococcus, Veillonella | MSU SPA TUC | [39] |
| NA | 10 | Firmicutes, Actinobacteria, Bacteroidetes | MSU | [34] |
| NA | 24 | Lactobacillus, Corynebacterium, Streptococcus, Actinomyces, Staphylococcus, Aerococcus, Gardnerella, Bifidobacterium, Actinobaculum | TUC | [30] |
| 35–65 | 58 | Lactobacillus, Gardnerella, Corynebacterium, Enterobacteriaceae, Anaerococcus, Bifidobacterium, Streptococcus, Staphylococcus, Sneathia, Peptoniphilus, Atopobium, Rhodanobacter, Trueperella, Alloscardovia, Veillonella | TUC | [27] |
| 18–25 | 24 | Lactobacillus, Staphylococci, Peptococcus, Corynebacterium, Propionibacterium, Eubacterium, Peptostreptococcus, Candida, Bacteroides, Bacillus, Veillonella, Enterobacteriaceae, Staphylococcus aureus, Enterococcus, Micrococcus, Prevotella, Actinomyces, Streptococcus | MSU | [40] |
| 35–65 | 60 | Lactobacillus, Gardnerella, Staphylococcus, Streptococcus, Enterococcus, Bifidobacterium, Atopobium, Enterobacteriaceae | TUC | [41] |
| NA | 10 | Anoxybacillus, Lactobacillus, Prevotella, Gardnerella, Arthrobacter, Escherichia, Shigella | TUC | [42] |
| 19–62 | 49 | Prevotella amnii, Gardnerella vaginalis, Atopobium vaginae, Lactobacillus iners, Shigella sonnei, Escherichia coli, Enterococcus faecalis, Streptococcus agalacticie, Citrobacter murliniae, Lactobacillus crispatus | MSU | [36] |
| NA | 10 | Lactobacillus, Corynebacterium, Gardnerella, Prevotella, Bacillus | MSU | [32] |
| NA | 60 | Staphylococcus epidermidis, Micrococcus luteus, Lactobacillus gasseri, Escherichia coli, Streptococcus oralis, Neisseria perflava, Lactobacillus crispatus, Lactobacillus jensenii, Gardnerella vaginalis, Rothia mucilaginosa, Lactobacillus delbrueckii, Lactobacillus rhamnosus, Bacillus infantis, Actinomyces odontolyticus, Bacillus idriensis, Corynebacterium amycolatum, Streptococcus anginosus, Streptococcus agalactiae, Gordonia terrae, Staphylococcus warneri, Lactobacillus iners, Streptococcus mitis, Bifidobacterium bifidum, Streptococcus gordonii, Aerococcus urinae, Actinomyces neuii, Enterococcus faecalis, Streptococcus salivarius, Streptococcus sanguinis, Corynebacterium aurimucosum, Actinomyces naeslundii, Streptococcus equinus, Alloscardovia omnicolens, Corynebacterium tuscaniense, Bifidobacterium longum | TUC | [29] |
| ≈53 | 84 | Lactobacillus, Streptococcus, Tepidimonas, Prevotella, Flavobacterium, Escherichia, Ureaplasma, Shuttleworthia, Aerococcus, Gardnerella, Veillonella, Bacteroides, Enterobacter, Acidovorax, Sneathia, Clostridium, Fusobacterium, Sphingobium, Proteus, Trabulsiella | TUC | [43] |
| NA | 224 | Predominant urotypes: Lactobacillus, Gardnerella, Streptococcus, EscherichiaOther: Aerococcus, Alloscardovia, Anaerococcus, Bifidobacterium, Corynebacterium, Enterococcus, Finegoldia, Klebsiella, Prevotella, Staphylococcus | TUC | [44] |
| ≈38 | 110 | Bifidobacterium, Staphylococcus, Lactobacillus, Corynebacterium | MSU | [45] |
| Age | No | Bacteria | Urine Sample | Reference |
|---|---|---|---|---|
| ≈18 | 9 | Lactobacillus, Corynebacterium, Escherichia, Streptococcus | FC | [37] |
| ≈28 | 22 | Lactobacillus, Sneathia, Veillonella, Corynebacterium, Prevotella, Streptococcus, Ureaplasma, Mycoplasma, Anaerococcus, Atopobium, Aerococcus, Staphylococcus, Gemella, Enterococcus, Finegoldia | FC | [54] |
| 24–50 | 11 | Lactobacillus, Klebsiella, Corynebacterium, Staphylococcus, Streptococcus, Aerococcus, Gardnerella, Prevotella, Escherichia, Enterococcus | MSU | [38] |
| 14–17 | 18 | Corynebacterium, Lactobacillus, Staphylococcus, Gardnerella, Streptococcus, Anaerococcus, Veillonella, Prevotella, Escherichia | FC | [55] |
| 39–86 | 6 | Firmicutes | MSU | [34] |
| 18–25 | 28 | Staphylococci, Eubacterium, Corynebacterium, Peptostreptococcus, Enterococcus, Bacteroides, Peptococcus, Megasphaera, Mobiluncus, Enterobacteriaceae, S. aureus, Propionibacterium, Veillonella, Fusobacterium | MSU | [40] |
| 23–58 | 31 | Prevotella amnii, Sneathia amnii, Shigella sonnei, Enterococcus faecalis, Streptococcus agalacticie, Citrobacter murliniae | MSU | [36] |
| NA | 10 | Streptococcus, Lactobacillus, Prevotella, Corynebacterium, Pseudomonas | MSU | [32] |
| ≈43 | 97 | Staphylococcus, Propionibacterium | MSU | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustrimovic, N.; Bilato, G.; Mortara, L.; Baci, D. The Urinary Microbiome in Health and Disease: Relevance for Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 1732. https://doi.org/10.3390/ijms25031732
Kustrimovic N, Bilato G, Mortara L, Baci D. The Urinary Microbiome in Health and Disease: Relevance for Bladder Cancer. International Journal of Molecular Sciences. 2024; 25(3):1732. https://doi.org/10.3390/ijms25031732
Chicago/Turabian StyleKustrimovic, Natasa, Giorgia Bilato, Lorenzo Mortara, and Denisa Baci. 2024. "The Urinary Microbiome in Health and Disease: Relevance for Bladder Cancer" International Journal of Molecular Sciences 25, no. 3: 1732. https://doi.org/10.3390/ijms25031732





