mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles
Abstract
1. Introduction
Polymeric Architectures
2. Preparation of mRNA-Polyplex Nanoformulations
2.1. Direct Mixing
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
Direct Mixing:
|
|
| [54] |
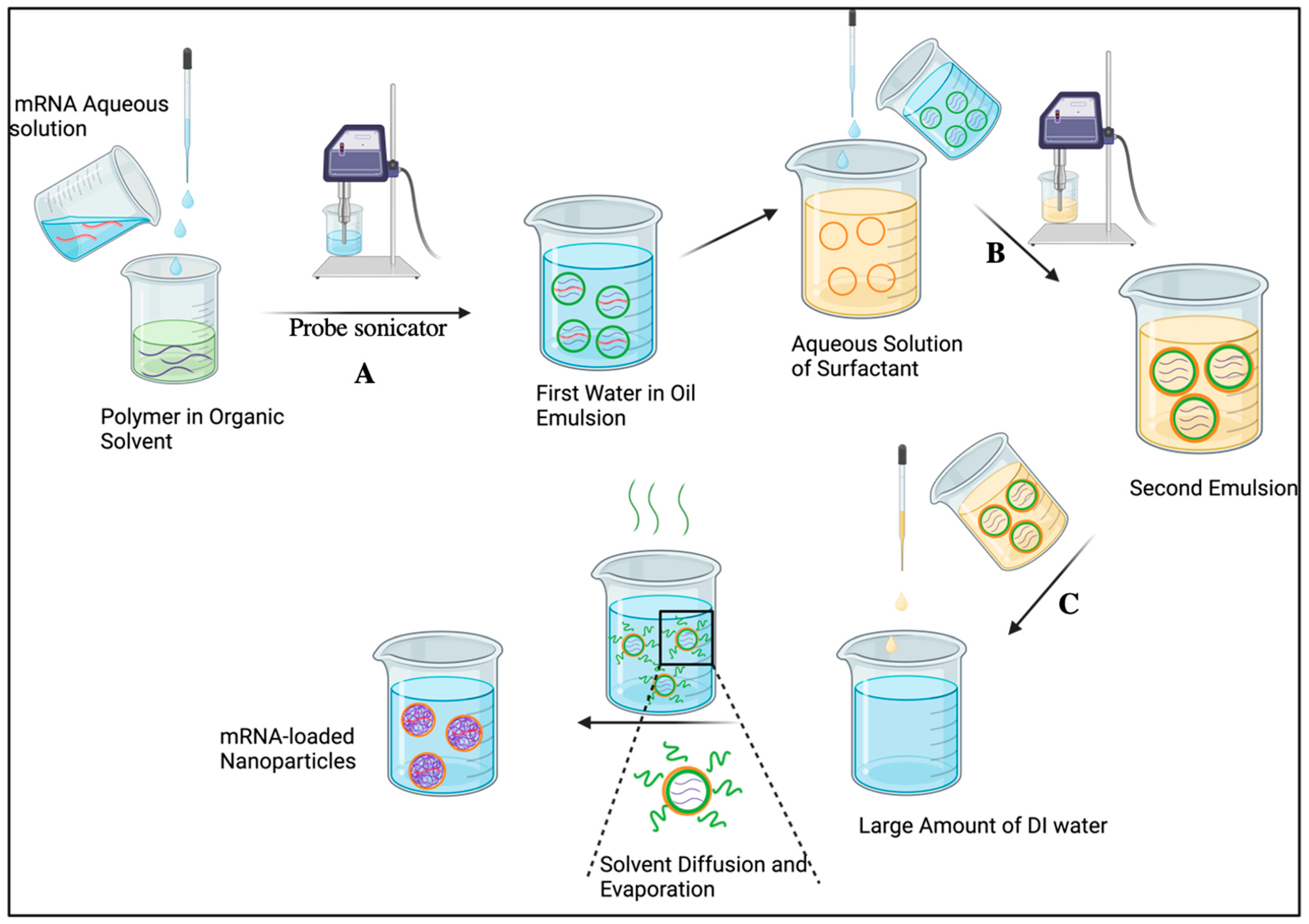
| Double emulsion/solvent evaporation | Simple, control over varied parameters, high encapsulation efficiency (especially for water-soluble cargos). | Difficult to control particle size as well as polydispersity and not easy to scale up. | [55] |
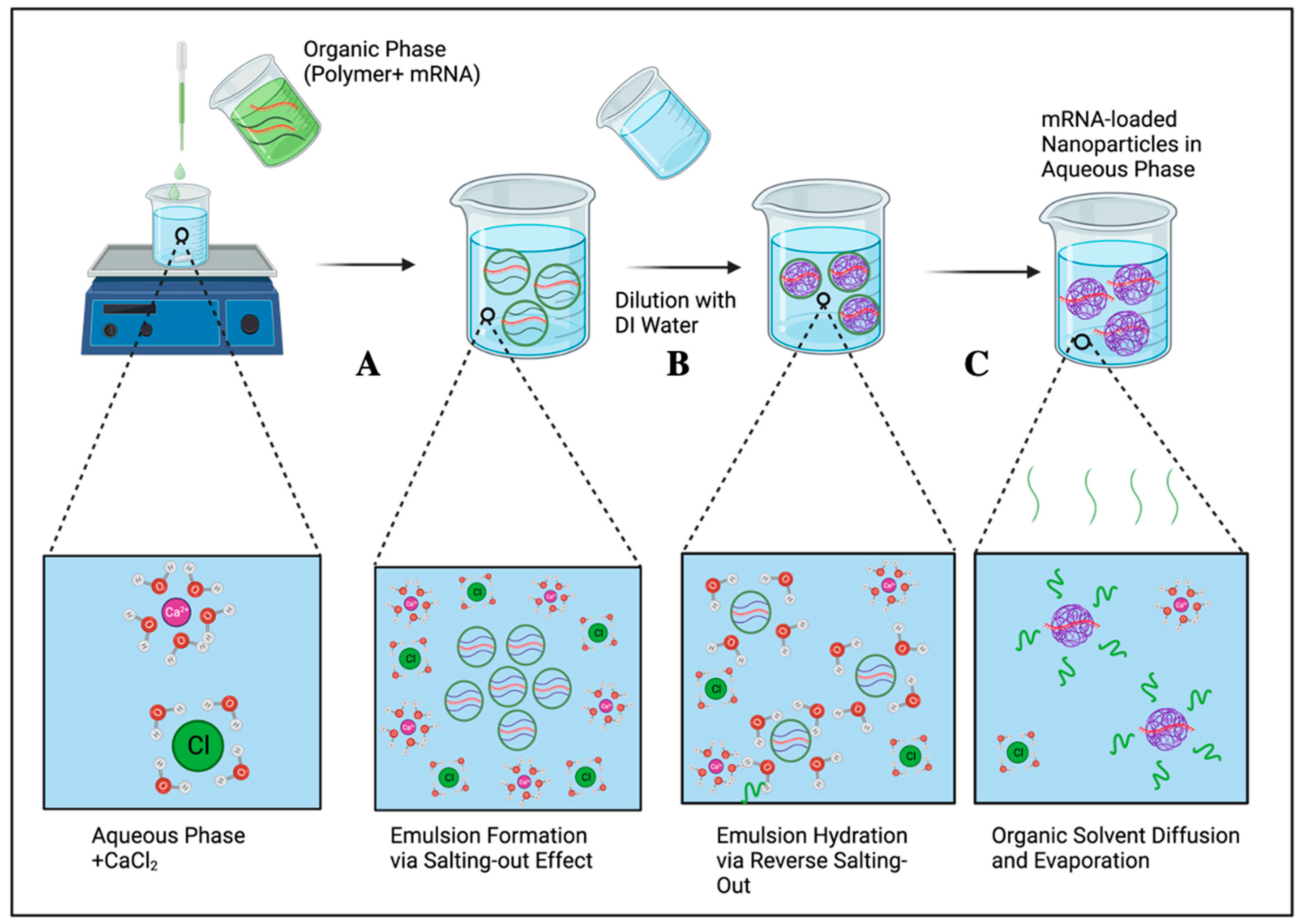
| Salting out | High reproducibility, avoids the use of chlorinated solvents, suitable for loading proteins and nucleic acids, no need for heating. | Low encapsulation efficiency for hydrophilic cargos, low scalability, time-consuming, needs the vigorous homogenizing instrument. | [56] |
| Nanoprecipitation | Easy, high reproducibility, controlled size, scalable. | Low encapsulation efficiency, high dependency of nanoparticle diameter to the stir rate. | [57] |
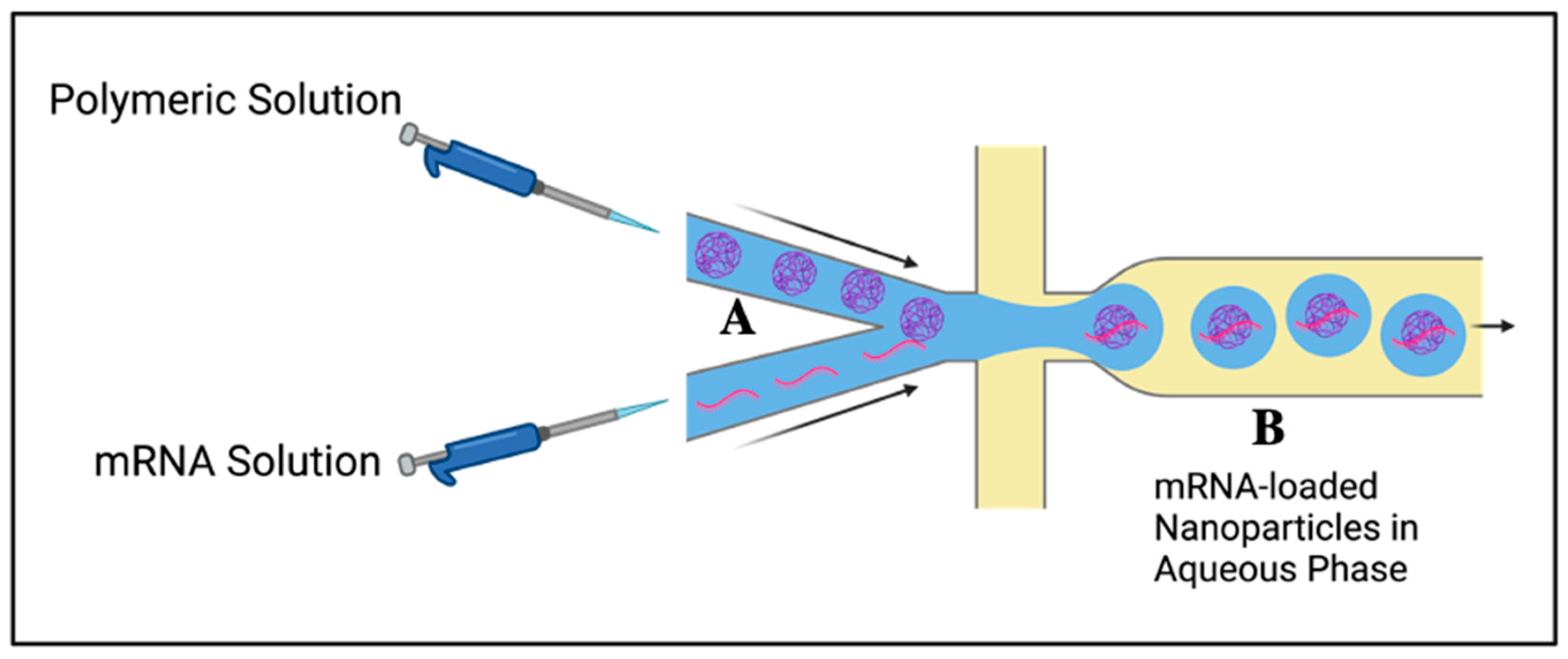
| Flash Nano-complexation | Organic solvent-free, fast, controlled particle size, scalable, and high encapsulation efficiency. | Requires control over electrostatic interactions (N:P ratio). | [58] |
2.2. Double Emulsion/Solvent Evaporation
2.3. Salting-Out
2.4. Nanoprecipitation
2.5. Flash Nano-Complexation
3. Polymeric Architectures for mRNA Delivery
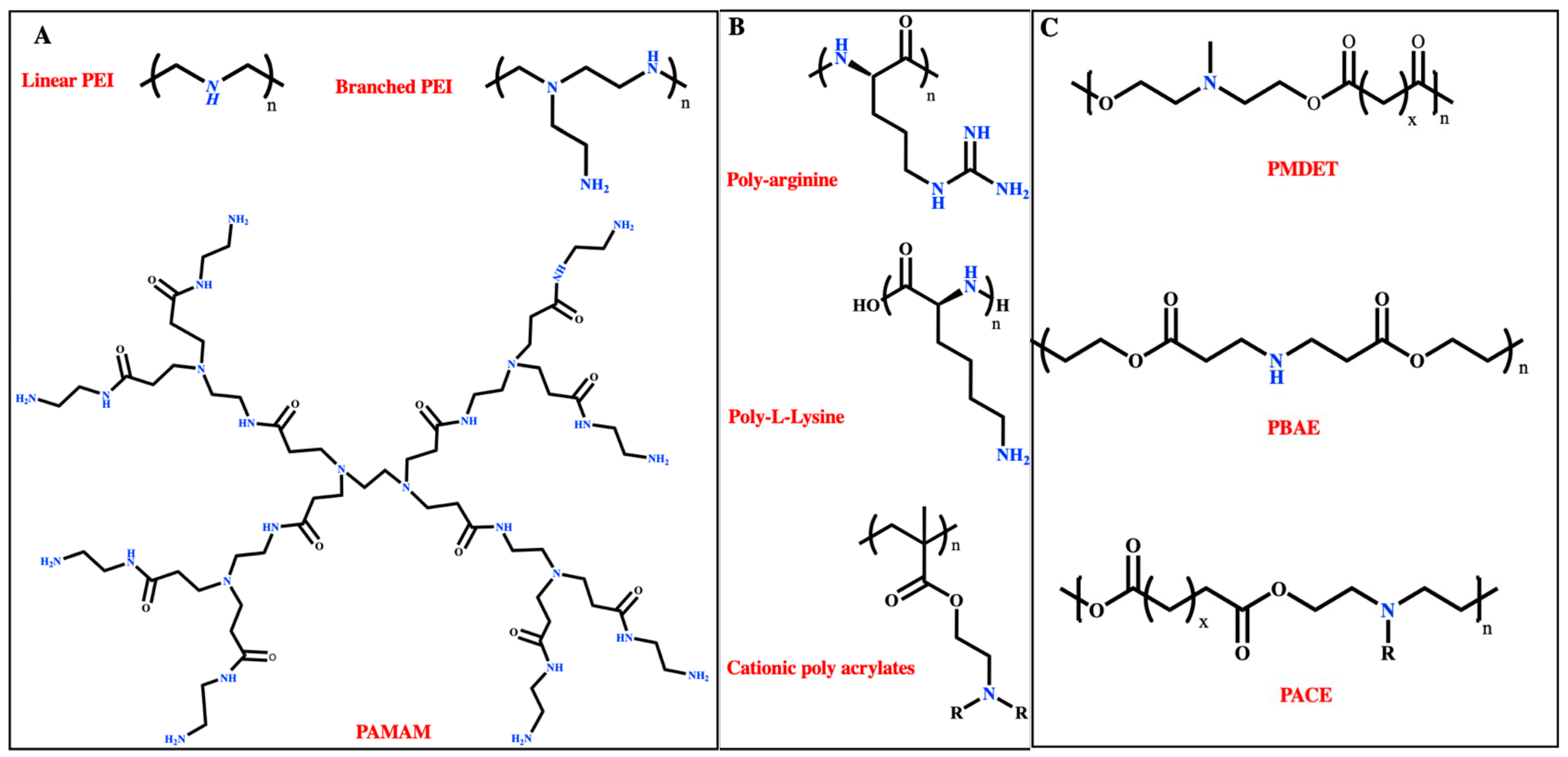
3.1. Cationic Polymers
3.1.1. Polyethyleneimine (PEI)
3.1.2. Poly (β-Amino Esters)
3.1.3. N-Methyl Diethanol Amine (MDET)-Based Polyesters
3.1.4. Poly (Amidoamine)
3.1.5. Poly Amino Co-Ester
3.1.6. Cationic Polyacrylates
3.1.7. Other Cationic Polymers
Poly Amino Acids
Poly Saccharides
3.2. Non-Cationic Polymers
3.2.1. Polyethylene Glycol (PEG)
3.2.2. Polyester
3.3. Stimuli-Responsive Polymers
3.3.1. pH-Responsive Polymers
3.3.2. Redox-Responsive Polymers
3.4. Polymer–Lipid Hybrid Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.; Kuang, G.; Li, W.; Wang, J.; Ren, H.; Zhao, Y. Stimuli-Responsive Gene Delivery Nanocarriers for Cancer Therapy. Nanomicro Lett. 2023, 15, 44. [Google Scholar] [CrossRef]
- Abtahi, N.A.; Salehi, S.; Naghib, S.M.; Haghiralsadat, F.; Edgahi, M.A.; Ghorbanzadeh, S.; Zhang, W. Multi-sensitive Functionalized Niosomal Nanocarriers for Controllable Gene Delivery in vitro and in vivo. Cancer Nanotechnol. 2023, 14, 22. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and Clinical Development of Nanoparticles for Gene Delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Mirza, Z.; Karim, S. Nanoparticles-based Drug Delivery and Gene Therapy for Breast Cancer: Recent advancements and future challenges. Semin. Cancer Biol. 2021, 69, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Cafferty, S.M.; Combes, F.; Huysmans, H.; Temmerman, J.D.; Gitsels, A.; Vanrompay, D.; Catani, J.P.; Sanders, N.N. mRNA Therapeutics Deliver a Hopeful Message. Nano Today 2018, 23, 16–39. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Francescantonio, S.D.; Marg, A.; Müthel, S.; Spuler, S.; Escobar, H. mRNA-mediated Delivery of Gene Editing Tools to Human Primary Muscle Stem Cells. Mol. Ther. Nucleic Acids 2022, 28, 47–57. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, O.; Vormehr, M.; Kranz, L.M. mRNA Therapeutics in Cancer Immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Bafleh, W.S.; Abdulsamad, H.M.R.; Al-Qaraghuli, S.M.; Khatib, R.W.E.; Elbahrawi, R.T.; Abdukadir, A.M.; Alsawae, S.M.; Dimassi, Z.; Hamdan, H.; Kashir, J. Applications of Advances in mRNA-based Platforms as Therapeutics and Diagnostics in Reproductive Technologies. Front. Cell Dev. Biol. 2023, 11, 1198848. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, Z.J.; Zhu, Y.L.; Tang, W.; Zhou, C.; Zhao, S.Q.; Wu, M.; Ming, T.; Deng, Y.Q.; Chen, Q.; et al. Rational Development of Multicomponent mRNA Vaccine Candidates Against mpox. Emerg. Microbes Infect. 2023, 12, 2192815. [Google Scholar] [CrossRef] [PubMed]
- Nitika; Wei, J.; Hui, A.M. The Delivery of mRNA Vaccines for Therapeutics. Life 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Balmayor, E.R. Synthetic mRNA—Emerging New Class of Drug for Tissue Regeneration. Curr. Opin. Biotechnol. 2022, 74, 8–14. [Google Scholar] [CrossRef]
- Vavilis, T.; Stamoula, E.; Ainatzoglou, A.; Sachinidsi, A.; Lamprinou, M.; Dardalas, I.; Vizirianakis, I.S. mRNA in the Context of Protein Replacement Therapy. Pharmaceutics 2023, 15, 166. [Google Scholar] [CrossRef]
- Bailly, A.; Milhavet, O.; Lemaitre, J.M. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics 2022, 14, 317. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 62813. [Google Scholar] [CrossRef]
- Loughrey, D.; Dahlman, J.E. Non-liver mRNA Delivery. Acc. Chem. Res. 2022, 55, 13–23. [Google Scholar] [CrossRef]
- Uludag, H.; Ubeda, A.; Ansari, A. At the Intersection of Biomaterials and Gene Therapy: Progress in Non-viral Delivery of Nucleic Acids. Front. Bioeng. Biotechnol. 2019, 7, 131. [Google Scholar] [CrossRef]
- Rhym, L.H.; Anderson, D.G. Nanoscale Delivery Platforms for RNA Therapeutics: Challenges and the Current State of the Art. Med. 2022, 3, 167–187. [Google Scholar] [CrossRef]
- Bernard, M.C.; Bazin, E.; Petiot, N.; Lemdani, K.; Commandeur, S.; Verdelet, C.; Margot, S.; Perkov, V.; Ripoll, M.; Garinot, M.; et al. The Impact of Nucleoside Based Modification in mRNA Vaccine is Influenced by the Chemistry of its Lipid Nanoparticle Delivery System. Mol. Ther. Nucleic Acids 2023, 32, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, D.; Dziembiowski, A. 5′ and 3′ Modifications Controlling RNA Degradation: From Safeguards to Executioners. Philos. Trans. R. Soc. B 2018, 373, 1762. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Kwon, S.; Kwon, M.; Im, S.; Lee, K.; Lee, H. mRNA Vaccines: The Most Recent Clinical Applications of Synthetic mRNA. Arch. Pharm. Res. 2022, 45, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R. Polyethylenimine-Based Nanocarriers in Co-Delivery of Drug and Gene: A Developing Horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Ibba, M.L.; Ciccone, G.; Esposito, C.L.; Catuogno, S.; Giangrande, P.H. Advances in mRNA Non-Viral Delivery Approaches. Adv. Drug Deliv. Rev. 2021, 177, 113930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Li, B. Nonviral Delivery of CRISPR/Cas Systems in mRNA Format. Adv. NanoBiomed Res. 2022, 2, 2200082. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The Nano Delivery Systems and Applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles Horizontal Line- From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Huang, J.; Huang, J.; Xue, H.; Liang, Z.; Wu, J.; Chen, C. Nanomedicine—A Promising Therapy for Hematological Malignancies. Biomater. Sci. 2020, 8, 2376–2393. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zhargami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Baghbanbashi, M.; Kakkar, A. Polymersomes: Soft Nanoparticles from Miktoarm Stars for Applications in Drug Delivery. Mol. Pharm. 2022, 19, 1687–1703. [Google Scholar] [CrossRef] [PubMed]
- Ulkoski, D.; Bak, A.; Wilson, J.T.; Krishnamurthy, V.R. Recent Advances in Polymeric Materials for the Delivery of RNA Therapeutics. Expert. Opin. Drug Deliv. 2019, 16, 1149–1167. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef]
- Ali, Y.; Algudah, A.; Ahmad, S.; Hamid, S.A.; Farooq, U. Macromolecules as Targeted Drugs Delivery Vehicles: An Overview. Des. Monomers Polym. 2019, 22, 91–97. [Google Scholar] [CrossRef]
- Saghebasl, S.; Akbarzadeh, A.; Gorabi, A.M.; Nikzamir, N.; Seyedsadjadi, M.; Mostafavi, E. Biodegradable Functional Macromolecules as Promising Scaffolds for Cardiac Tissue Engineering. Polym. Adv. Technol. 2022, 33, 2044–2068. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukazawa, K.; Sharma, V.; Liang, S.; Shows, A.; Dunbar, D.C.; Zheng, Y.; Ge, J.; Zhang, S.; Hong, Y.; et al. Antifouling Silicone Hydrogel Contact Lenses with a Bioinspired 2-Methacryloyloxyethyl Phosphorylcholine Polymer Surface. ACS Omega 2021, 6, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Rajewska, J.; Kowalski, J.; Matys, J.; Dobrzynski, M.; Wiglusz, R.J. The Use of Lactide Polymers in Bone Tissue Regeneration in Dentistry-A Systematic Review. J. Funct. Biomater. 2023, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Alam, B.F.; Rehman, S.U.; Ahmad, S.; Iqbal, K.; Farooq, I. Global Research on Dental Polymers and Their Application: A Bibliometric Analysis and Knowledge Mapping. Saudi Dent. J. 2023, 35, 197–205. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Selianitis, D.; Lagopati, N.; Pippa, N.; Pispas, S.; Gazouli, M. Surfactant and Block Copolymer Nanostructures: From Design and Development to Nanomedicine Preclinical Studies. Pharmaceutics 2023, 15, 501. [Google Scholar] [CrossRef]
- Ferrentino, N.; Romano, M.P.; Zappavigna, S.; Abate, M.; Vecchio, V.D.; Romano, D.; Germinario, C.; Grifa, C.; Filosa, R.; Pappalardo, D. Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers 2023, 15, 1179. [Google Scholar] [CrossRef]
- García, M.C. Stimuli-Responsive Self-Assembled Nanocarriers Based on Amphiphilic Block Copolymers for Cancer Therapy. Appl. Multifunct. Nanomater. 2023, 365–409. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kauffman, K.J.; Fenton, O.S.; Sadtler, K.; Patel, A.K.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018, 18, 6449–6454. [Google Scholar] [CrossRef]
- Duran-Mota, J.A.; Yani, J.Q.; Almquist, B.D.; Borros, S.; Oliva, N. Polyplex-Loaded Hydrogels for Local Gene Delivery to Human Dermal Fibroblasts. ACS Biomater. Sci. Eng. 2021, 7, 4347–4361. [Google Scholar] [CrossRef]
- Evers, M.J.W.; Kulkarni, J.A.; Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Strelkova Petersen, D.M.; Chaudhary, N.; Arral, M.L.; Weiss, R.M.; Whithead, K.A. The Mixing Method Used to Formulate Lipid Nanoparticles Affects mRNA Delivery Efficacy and Organ Tropism. Eur. J. Pharm. Biopharm. 2023, 192, 126–135. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid Nanoparticles for Delivery of RNA Therapeutics: Current Status and the Role of in vivo Imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of Pharmaceuticals and Biopharmaceuticals Loaded Microparticles Prepared by Double Emulsion Technique for Controlled Delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk Fibroin-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Sawant, K.K. Modified Nanoprecipitation Method for Preparation of Cytarabine-Loaded PLGA Nanoparticles. AAPS PharmSciTech. 2010, 11, 1456–1465. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Tian, H.; Hu, Y.; Liu, L.; Leong, K.W.; Mao, Q.; Chen, Y. Scalable Production of Core–Shell Nanoparticles by Flash Nanocomplexation to Enhance Mucosal Transport for Oral Delivery of Insulin. Nanoscale 2018, 10, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bernard, J.; Ganachaud, F. Nanoprecipitation as a Simple and Straightforward Process to Create Complex Polymeric Colloidal Morphologies. Adv Colloid Interface Sci. 2021, 294, 102474. [Google Scholar] [CrossRef]
- Panigrahi, D.; Sahu, P.K.; Swain, S.; Verma, R.K. Quality by Design Prospects of Pharmaceuticals Application of Double Emulsion Method for PLGA Loaded Nanoparticles. SN Appl. Sci. 2021, 3, 638. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.Q.; Ho, W.; Bai, X. Lipid-Polymer Hybrid “Particle-in-Particle” Nanostructure Gene Delivery Platform Explored for Lyophilizable DNA and mRNA COVID-19 Vaccines. Adv. Funct. Mater. 2022, 32, 2204462. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Tran, T.T.D.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomater 2016, 6, 26. [Google Scholar] [CrossRef]
- Dubey, S.; Mody, N.; Sharma, R.; Agrawal, U.; Vyas, S.P. Nanobiomaterials: Novel Nanoplatforms for Protein and Peptide Delivery. In Nanobiomaterials in Drug Delivery 2016; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 111–146. [Google Scholar]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomater 2022, 12, 576. [Google Scholar] [CrossRef]
- Hueppe, N.; Wurm, F.R.; Landfester, K. Nanocarriers with Multiple Cargo Load-A Comprehensive Preparation Guideline Using Orthogonal Strategies. Macromol. Rapid Commun. 2023, 44, e2200611. [Google Scholar] [CrossRef]
- Jiang, X.; Abedi, K.; Shi, J. Polymeric Nanoparticles for RNA Delivery. Ref. Modul. Mater. Sci. Mater. Eng. 2021, B978-0-12-822425-0.00017-8. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Li, M.; Shao, D.; Mao, H.-M.; Leong, K.W. Flash Technology-Based Self-Assembly in Nanoformulation: Fabrication to Biomedical Applications. Mater. Today 2021, 42, 99–116. [Google Scholar] [CrossRef]
- Ke, X.; Shelton, L.; Hu, Y.; Zhu, Y.; Chow, E.; Tang, H.; Santos, J.L.; Mao, H.-Q. Surface-Functionalized PEGylated Nanoparticles Deliver Messenger RNA to Pulmonary Immune Cells. ACS Appl. Mater. Interfaces 2020, 12, 35835–35844. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of Size on Biological Oroperties of Nanoparticles Employed in Gene Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, C.; Brown, A.; Tharmarajah, G.; Thomas, A. mrnaspark-AN-1018. Available online: https://www.precisionnanosystems.com/docs/default-source/pni-files/app-notes/spark-mrna-appnote-1018.pdf?sfvrsn=50662346_0(2018) (accessed on 14 December 2023).
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12, e2202688. [Google Scholar] [CrossRef]
- Bhatacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 20, 12871–12934. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Xiang, J.; Wang, H.; Zhuang, Q.; Wie, T.; Cao, Z.; Gu, Q.; Liu, Z.; Peng, R. Fluoroalkane Modified Cationic Polymers for Personalized mRNA Cancer Vaccines. Chem. Eng. J. 2023, 456, 140930. [Google Scholar] [CrossRef]
- Tan, L.; Zheng, T.; Li, M.; Zhong, X.; Tang, Y.; Qin, M.; Sun, X. Optimization of an mRNA Vaccine Assisted with Cyclodextrin-Polyethyleneimine Conjugates. Drug Deliv. Transl. Res. 2020, 10, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Capasso Palmiero, U.; Kaczmarek, J.C.; Fenton, O.S.; Anderson, D.G. Poly(beta-amino ester)-co-poly(caprolactone) Terpolymers as Nonviral Vectors for mRNA Delivery In Vitro and In Vivo. Adv. Healthc. Mater. 2018, 7, e1800249. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Guerra-Rebollo, M.; Lazaro, M.A.; Castells-Sala, C.; Meca-Cortez, O.; Ramos-Perez, V.; Cascante, A.; Rubio, N.; Blanco, J.; Borros, S. mRNA Delivery System for Targeting Antigen-Presenting Cells In Vivo. Adv. Healthc. Mater. 2018, 7, e1800335. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Kaczmarek, J.C.; Bose, S.; Kauffman, K.J.; Mir, F.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv. Mater. 2019, 31, e1805116. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Patel, A.K.; Kauffman, K.J.; Fenton, O.S.; Webber, M.J.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew. Chem. Int. Ed. Engl. 2016, 55, 13808–13812. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dharmaraj, S.; Truong, N.; Pearson, R.M. Excipient-Free Ionizable Polyester Nanoparticles for Lung-Selective and Innate Immune Cell Plasmid DNA and mRNA Transfection. ACS Appl. Mater. Interfaces 2022, 14, 56440–56453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guadin, A.; Zhang, J.; Agarwal, T.; Song, E.; Kauffman, A.C.; Tietien, G.T.; Wang, Y.; Jiang, Z.; Cheng, C.J.; et al. A “Top-Down” Approach to Actuate Poly(amine-co-ester) Terpolymers for Potent and Safe mRNA Delivery. Biomaterials 2018, 176, 122–130. [Google Scholar] [CrossRef]
- Grun, M.K.; Suberi, A.; Shin, K.; Lee, T.; Gomerdinger, V.; Moscato, Z.M.; Piotrowski-Daspit, A.S.; Saltzman, W.M. PEGylation of Poly(amine-co-ester) Polyplexes for Tunable Gene Delivery. Biomaterials 2021, 272, 120780. [Google Scholar] [CrossRef]
- Kauffman, A.C.; Potrowski-Daspit, A.S.; Nakazawa, K.H.; Jiang, Y.; Datye, A.; Saltzman, W.M. Tunability of Biodegradable Poly(amine- co-ester) Polymers for Customized Nucleic Acid Delivery and Other Biomedical Applications. Biomacromolecules 2018, 19, 3861–3873. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA Nanoparticles Generate Protective Immunity Against Lethal Ebola, H1N1 Influenza, and Toxoplasma Gondii Challenges with a Single Dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, S.; Leng, Q.; Mixson, A.J. Location of a Single Histidine within Peptide Carriers Increases mRNA Delivery. J. Gene Med. 2021, 23, e3295. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Moses, A.S.; Demessie, A.A.; Singh, P.; Lee, H.; Korzun, T.; Taratula, O.R.; Alani, A.W.; Taratula, O. Poly(aspartic acid)-Based Polymeric Nanoparticle for Local and Systemic mRNA Delivery. Mol. Pharm. 2022, 19, 4696–4704. [Google Scholar] [CrossRef]
- Dong, Z.; Huang, Z.; LI, S.; Wang, Y.; Yao, Y.; Yang, X.; Xu, X. Nanoparticles (NPs)-Mediated Systemic mRNA Delivery to Reverse Trastuzumab Resistance for Effective Breast Cancer Therapy. Acta Pharm. Sin. B 2023, 13, 955–966. [Google Scholar] [CrossRef]
- Yang, W.; Chen, P.; Boonstra, E.; Hong, T.; Cabral, H. Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery. Pharmaceutics 2022, 14, 1205. [Google Scholar] [CrossRef]
- Yan, Y.; Xiong, H.; Zhang, Z.; Cheng, Q.; Siegwart, D.J. Systemic mRNA Delivery to the Lungs by Functional Polyester-based Carriers. Biomacromolecules 2017, 18, 4307–4315. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Uchida, S.; Naito, M.; Osada, K.; Cabral, H.; Kataoka, K. Induced Packaging of mRNA into Polyplex Micelles by Rregulated Hybridization with a Small Number of Cholesteryl RNA Oligonucleotides Directed Enhanced in vivo Transfection. Biomaterials 2019, 197, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; Haabeth, O.A.W.; Blake, T.R.; Castillo, T.J.D.; Czerwinski, D.K.; Rajapaksa, R.; Wender, P.A.; Waymouth, R.M.; Levy, R. Fingolimod-Conjugated Charge-Altering Releasable Transporters Efficiently and Specifically Deliver mRNA to Lymphocytes In Vivo and In Vitro. Biomacromolecules 2022, 23, 2976–2988. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yu, X.; Cheng, Q.; Johnson, L.T.; Chatterjee, S.; Zhang, D.; Lee, S.M.; Sun, Y.; Lin, T.C.; et al. Zwitterionic Phospholipidation of Cationic Polymers Facilitates Systemic mRNA Delivery to Spleen and Lymph Nodes. J. Am. Chem. Soc. 2021, 143, 21321–21330. [Google Scholar] [CrossRef]
- Jeandupeux, E.; Alameh, M.J.; Ghattas, M.; Crescenzo, G.D.; Lavertu, M. Poly(2-Propylacrylic Acid) Increases In Vitro Bioactivity of Chitosan/mRNA Nanoparticles. J. Pharm. Sci. 2021, 110, 3439–3449. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, R.; Chen, X.; Yang, X.; Wu, S.; Xiao, H.; Dong, W. A Targeted and Stable Polymeric Nanoformulation Enhances Systemic Delivery of mRNA to Tumors. Mol. Ther. 2017, 25, 92–101. [Google Scholar] [CrossRef]
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The Roles of Polymers in mRNA Delivery. Matter 2022, 5, 1670–1699. [Google Scholar] [CrossRef]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric Vehicles for Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef]
- Uchida, S.; Perche, F.; Pichon, C.; Cabral, H. Nanomedicine-Based Approaches for mRNA Delivery. Mol. Pharm. 2020, 17, 3654–3684. [Google Scholar] [CrossRef] [PubMed]
- Sharifnia, Z.; Bandehpour, M.; Hamishehkar, H.; Mosaffa, N.; Kazemi, B.; Zarghami, N. In-vitro Transcribed mRNA Delivery Using PLGA/PEI Nanoparticles into Human Monocyte-derived Dendritic Cells. Iran. J. Pharm. Res. 2019, 18, 1659–1675. [Google Scholar] [PubMed]
- Wang, H.; Liu, X.; Ai, X.; Remant-Bahadur, K.C.; Dick, T.A.; Yan, B.; Lu, T.; Zhou, X.; Luo, R.; Liu, M.; et al. Safe and Effective Delivery of mRNA Using Modified PEI-Based Lipopolymers. Pharmaceutics 2023, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, M.; Liang, D.; Yang, J.; Tang, X. Vitamin E-Labeled Polyethylenimine for in vitro and in vivo Gene Delivery. Biomacromolecules 2016, 17, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, C.; Sabirsh, A.; Ye, L.; Wu, X.; Lu, H.; Liu, J. A Novel Graphene Quantum Dot-Based mRNA Delivery Platform. ChemistryOpen 2021, 10, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lacerda, C.; Patil, V.S.; Biswal, D.; Wattamwar, P.; Hilt, J.Z.; Dziubla, T.S. Degradation of Poly (β-amino ester) Gels in Alcohols Through Transesterification: Method to Conjugate Drugs to Polymer Matrices. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly (beta-amino ester)s as Gene Delivery Vehicles: Challenges and Opportunities. Expert. Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Lotocki, V.; Yazdani, H.; Zhang, Q.; Gran, E.R.; Nyrko, A.; Maysinger, D.; Kakkar, A. Miktoarm Star Polymers with Environment-Selective ROS/GSH Responsive Locations: From Modular Synthesis to Tuned Drug Release through Micellar Partial Corona Shedding and/or Core Disassembly. Macromol. Biosci. 2021, 21, 2000305. [Google Scholar] [CrossRef]
- Nehzad, M.S. Poly (beta-amino ester) as an in vivo Nanocarrier for Therapeutic Nucleic Acids. Biotechnol. Bioeng. 2023, 120, 95–113. [Google Scholar]
- Rai, D.B.; Pooja, D.; Kulhari, H. Dendrimers in gene delivery. In Pharmaceutical Applications of Dendrimers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 211–231. [Google Scholar]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-Engineered Dendrimers in Gene Delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Fanelli, A.; Botta, L.; Zippilli, C.; Cesarini, S.; Saladino, R. Dendrimeric Structures in the Synthesis of Fine Chemicals. Mater 2021, 14, 5318. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Viltres, H.; Lopez, Y.C.; Leyva, C.; Gupta, N.K.; Naranjo, A.G.; Acevedo-Pena, P.; Sanchez-Diaz, A.; Bae, J.; Kim, K.S. Polyamidoamine Dendrimer-Based Materials for Environmental Applications: A review. J. Mol. Liq. 2021, 334, 116017. [Google Scholar] [CrossRef]
- Li, X.; Ta, W.; Hua, R.; Song, J.; Lu, W. A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy. Biomedicines 2022, 10, 2455. [Google Scholar] [CrossRef]
- Ebeid, K.; Geary, M.S.; Salem, A.K. Preparation and Characterization of a Liver Targeted, Poly(amidoamine) Based, Gene Delivery System. Methods Mol. Biol. 2022, 2455, 319–332. [Google Scholar] [PubMed]
- Han, M.; Beon, J.; Lee, J.Y.; Oh, S.S. Systematic Combination of Oligonucleotides and Synthetic Polymers for Advanced Therapeutic Applications. Macromol. Res. 2021, 29, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Kintzing, J.R.; Hanna, A.; Shannon, J.M.; Gupta, M.K.; Duvall, C.L. Balancing Cationic and Hydrophobic Content of PEGylated siRNA Polyplexes Enhances Endosome Escape, Stability, Blood Circulation Time, and Bioactivity in vivo. ACS Nano 2013, 7, 8870–8880. [Google Scholar] [CrossRef] [PubMed]
- Olden, B.R.; Cheng, Y.; Yu, J.L.; Pun, S.H. Cationic Polymers for Non-Viral Gene Delivery to Human T cells. J. Control Release 2018, 282, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ulkoski, D.; Munson, M.J.; Jacobson, M.E.; Palmer, C.R.; Carson, C.S.; Sabirsh, A.; Wilson, J.T.; Krishnamurthy, V.R. High-Throughput Automation of Endosomolytic Polymers for mRNA Delivery. ACS Appl. Bio Mater. 2021, 4, 1640–1654. [Google Scholar] [CrossRef]
- Cheng, C.; Convertine, A.J.; Stayton, P.S.; Bryers, J.D. Multifunctional Triblock Copolymers for Intracellular Messenger RNA Delivery. Biomaterials 2012, 33, 6868–6876. [Google Scholar] [CrossRef]
- Okay, S.; Özge Özcan, Ö.; Karahan, M. Nanoparticle-Based Delivery Platforms for mRNA Vaccine Development. AIMS Biophys. 2020, 7, 323–338. [Google Scholar] [CrossRef]
- Yokoo, H.; Oba, M.; Uchida, S. Cell-Penetrating Peptides: Emerging Tools for mRNA Delivery. Pharmaceutics 2021, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Oldenhuis, N.J.; Eldredge, A.C.; Burts, A.O.; Ryu, K.H.; Chung, J.; Johnson, M.E.; Guan, Z. Biodegradable Dendronized Polymers for Efficient mRNA Delivery. ChemistrySelect 2016, 1, 4413–4417. [Google Scholar] [CrossRef]
- Gao, C.; Cheng, K.; Li, Y.; Gong, R.; Zhao, X.; Nie, G.; Ren, H. Injectable Immunotherapeutic Hydrogel Containing RNA-Loaded Lipid Nanoparticles Reshapes Tumor Microenvironment for Pancreatic Cancer Therapy. Nano Lett. 2022, 22, 8801–8809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Thayumanavan, S. Non-cationic Material Design for Nucleic Acid Delivery. Adv. Ther. 2020, 3, 1900206. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine's most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, Y.; Liu, X.; Zhu, D. Polyester Materials for mRNA Delivery. Explor. Target. Antitumor Ther. 2022, 3, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wang, Q.; Luo, X.; Mao, L.; Wang, Z.; Ye, E.; Loh, X.J.; Li, Z.; Wu, Y.L. Recent Advances in Stimuli-Rresponsive Polymeric Carriers for Controllable CRISPR/Cas9 Gene Editing System Delivery. Biomater. Sci. 2023, 11, 5078–5094. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Qi, X. Advances and Challenges of Stimuli-Responsive Nucleic Acids Delivery System in Gene Therapy. Pharmaceutics 2023, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Reichel, L.S.; Traeger, A. Stimuli-Responsive Non-viral Nanoparticles for Gene Delivery. In Handbook of Experimental Pharmacology; Springer: Berlin, Heidelberg, 2023. [Google Scholar] [CrossRef]
- Monaco, A.; Barbieri, B.D.; Yilmaz, G.; Shattock, R.J.; Becer, C.R. Degradable Glycopolymers for saRNA Transfection. Polym. Chem. 2023, 14, 2750–2761. [Google Scholar] [CrossRef]
- Guo, J.; Wan, T.; Li, B.; Pan, Q.; Xin, H.; Qiu, Y.; Ping, Y. Rational Design of Poly(disulfide)s as a Universal Platform for Delivery of CRISPR-Cas9 Machineries toward Therapeutic Genome Editing. ACS Cent. Sci. 2021, 7, 990–1000. [Google Scholar] [CrossRef]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid Polymer Hybrid Nanoparticles as Potent Vehicles for Drug Delivery in Cancer Therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]

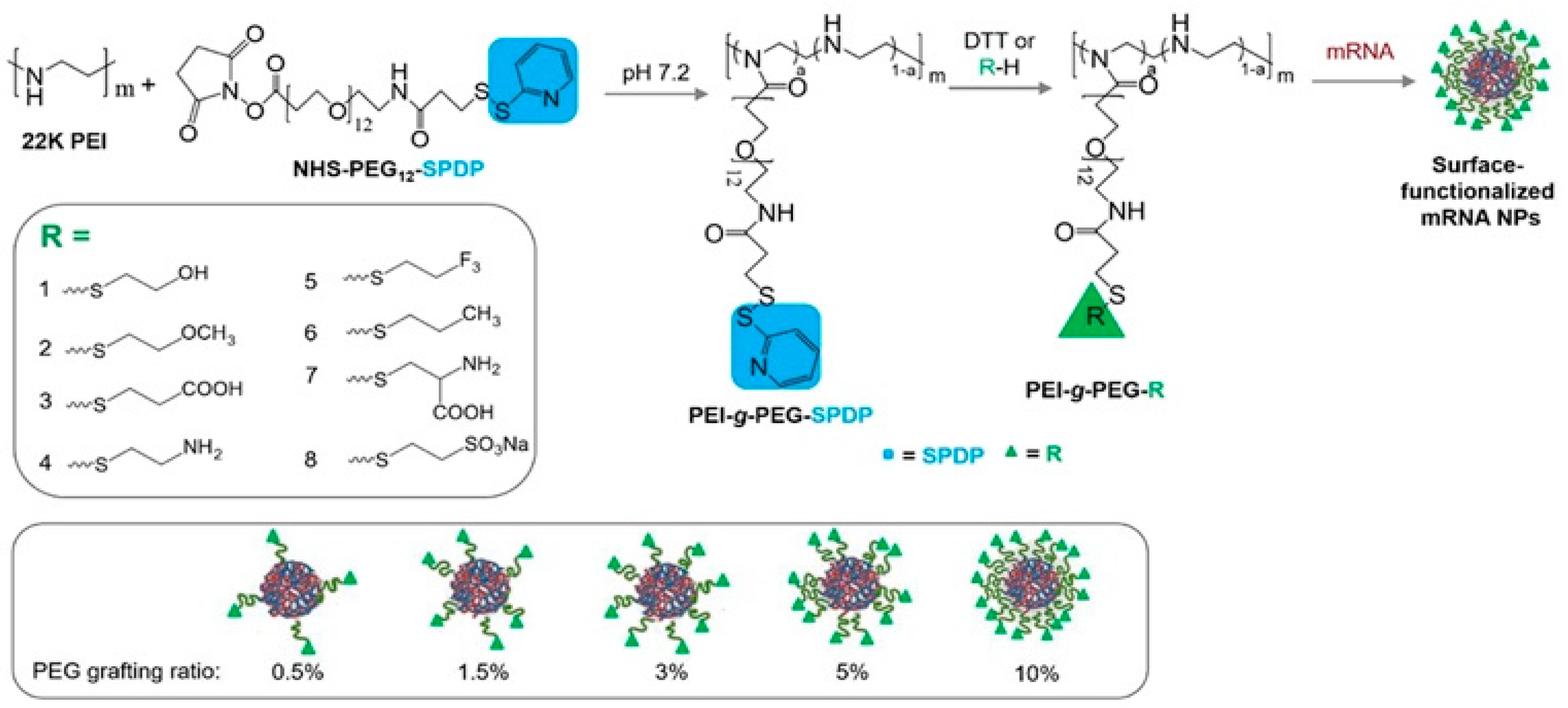
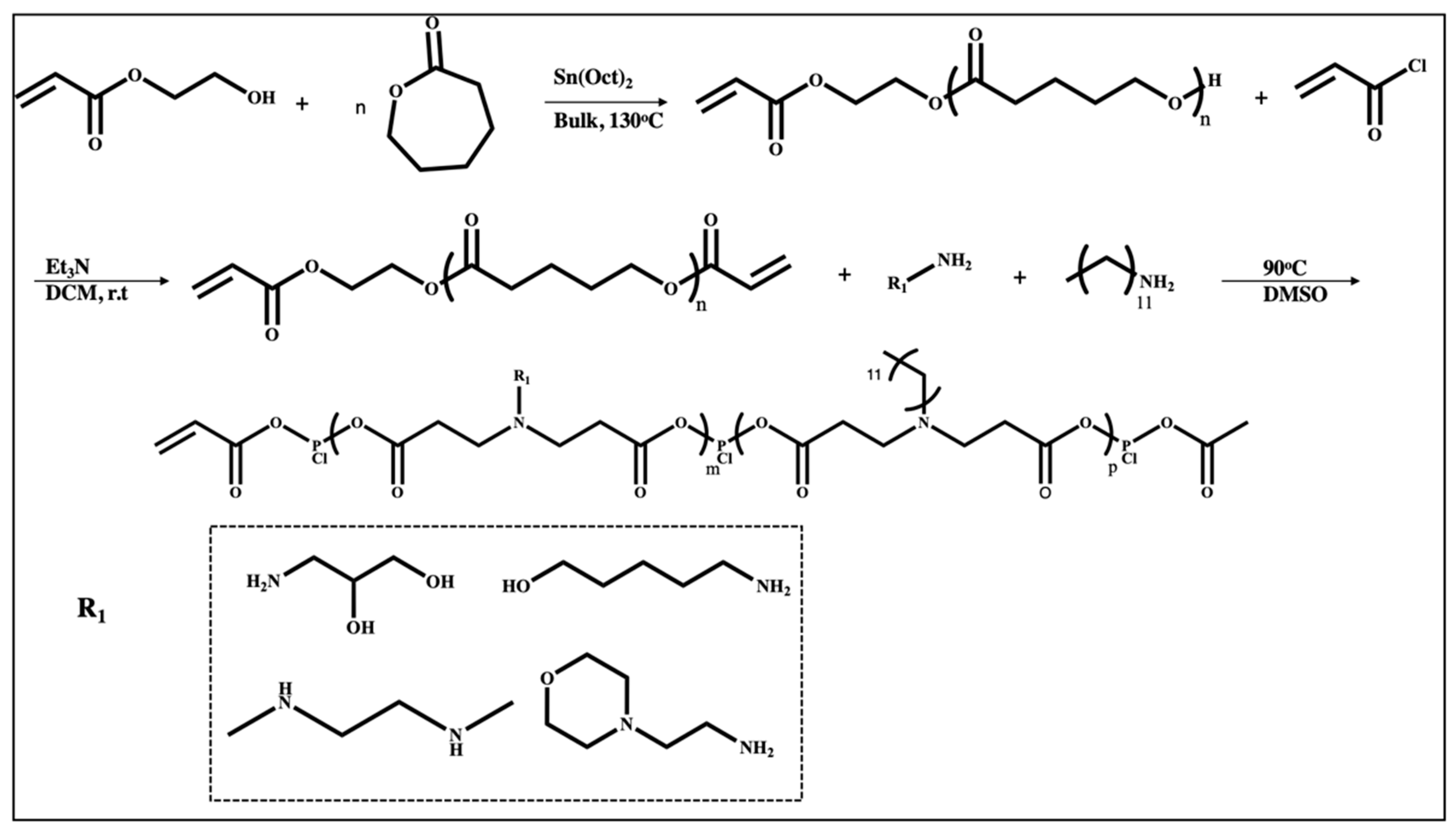


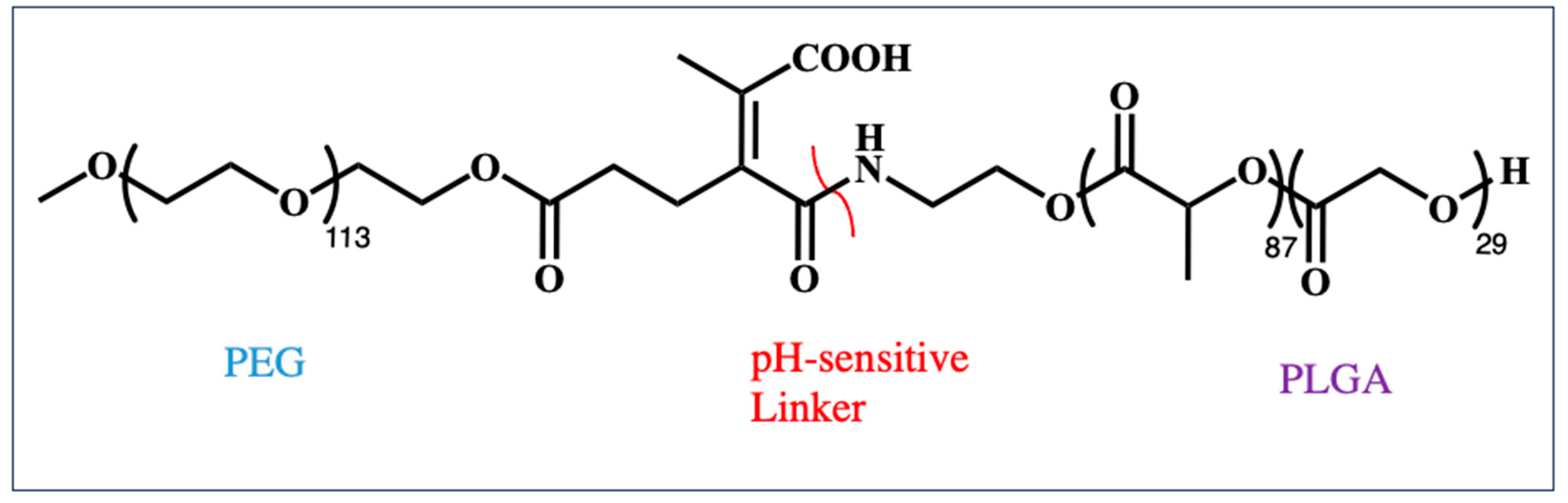
| Polymer | Molecular Weight of mRNA-Interacting Polymer | N:P Ratio | NP Formation Method | Characterization Methods | Reference |
|---|---|---|---|---|---|
| PEI-g-PEG | PEI (22 kDa) | 8 | Flash nanocomplexation Solvent: Water | DLS, Zeta Potential, TEM | [70] |
| F-PEI | 1.8 kDa | w:w (1:1) | Direct mixing Solvent: Water | DLS, Zeta Potential, TEM | [76] |
| CD-PEI | bPEI (2 kDa) | 16 (Optimized) | Direct mixing Solvent: Water | DLS, Zeta Potential, TEM | [77] |
| PBAE@PEG-lipid | PBAE (2354 Da) | 50 (Optimized) | 1. Pipette mixing or 2. Microfluidic mixing or microfluidic device mixing Dialysis method Solvents: PBAE in DMSO, PEG-lipid in ethanol, mRNA in sodium acetate buffer | DLS | [50] |
| PBAE NP-doped PEG-PBAE | - | w:w 25:1 and 50:1 | Direct mixing, PBAE in DMSO, mRNA in water Both phases diluted in acetate buffer before mixing | DLS, SEM | [51] |
| PNP (PEG-PLGA@Lipid-PBAE | PBAE (4137 Da) | - | Double emulsion–solvent evaporation Solvent: polymers in DCM, mRNA in water | DLS, Zeta Potential, TEM | [61] |
| PBAE-co-PCL terpolymer | 850–2800 Da | 50 | Direct mixing Polymer in DMSO diluted in acetate buffer, mRNA in acetate buffer | DLS, Zeta potential, Cryo TEM | [78] |
| Oligopeptide end modified PBAE | - | 25 | Direct mixing Solvent: acetate buffer | DLS, NTA, Zeta potential | [79] |
| hPBAE | 20 kDa | 50 | Direct mixing Solvent: acetate buffer | DLS, Zeta potential, TEM | [80] |
| PBAE terpolymer@PEG-Lipid | 2376 Da | 57 | Direct mixing Solvent: pH = 5.2 buffer | DLS, Cryo TEM | [81] |
| MDET@Cp | 4760–5050 Da | 55 | Direct mixing Solvent: polymer in DMSO diluted in acetate buffer+ mRNA | DLS, Zeta potential | [82] |
| PACE terpolymer | 2–20 kDa | 100 | Direct mixing Solvent: polymer in DMSO diluted in acetate buffer, mRNA in acetate buffer | DLS, Zeta potential | [83] |
| PACE@PEG | 17,500 Da | 100 | Direct mixing Solvent: polymer in DMSO diluted in acetate buffer, mRNA in acetate buffer | DLS, Zeta Potential, TEM | [84] |
| PACE | 7 kDa | 100 | 1. Polyplex formation: direct mixing Solvent: polymer in DMSO diluted in acetate buffer, mRNA in Acetate buffer 2. Solid NP formation: single or double emulsion-solvent evaporation method Polymer in DCM, mRNA in acetate buffer, PVA | DLS, Zeta Potential, TEM, SEM | [85] |
| PAMAM@PEG-lipid | - | 5.22 | Microfluidic device, dialysis, polymer and PEG-lipid in ethanol, mRNA in citrate buffer pH = 3 | DLS, TEM | [86] |
| Histidine rich Peptide | - | 4 | Direct mixing, Solvent: Opti-MEM | DLS, Zeta potential | [87] |
| (DET)-P(Asp)@PEG | 18,747 Da | 32 | Direct mixing, Solvent: HEPES | DLS, Zeta potential | [88] |
| Meo-PEG-Dlinkm-PLGA | Total polymer: 16 kDa | - | Nanoprecipitation, Polymer and G0-C14 in DMF, mRNA in water | DLS, Zeta potential, TEM | [89] |
| PEG-pLL (CAA) | 6725 Da | 4 | Direct mixing, Solvent: HEPES | DLS | [90] |
| PE4K-A17 0.33C12 | - | 30 | Direct mixing, Solvent: Polymer in DMSO, mRNA in citrate buffer | DLS, Zeta potential | [91] |
| PEG-PAsp (DET)-Chol (+) @Chol (+)-OligoRNA | - | 3 | Direct mixing, Solvent: HEPES buffer | DLS, Zeta potential, TEM | [92] |
| FTY720-CART | 9700 Da | 10 | Direct mixing, Solvent: polymer in DMSO, mRNA in PBS buffer | DLS, Zeta potential | [93] |
| zwitterionic phospholipidated polymers | 3 kDa | 20 | Dialysis Solvent: PEG-lipid in Ethanol, mRNA in citrate buffer Dialyzed with PBS | DLS, Zeta potential, TEM | [94] |
| CS/mRNA/PPAA | 5 and 10 kDa | 5 | Direct mixing Solvent: CS, PPAA stock solutions in water or buffer, mRNA in buffer. | DLS, Zeta potential | [95] |
| cRGD-PEG@(PEG)-(PLys) (thiol)@(PNIPAM)-PLys(thiol) | 8000 | 1.5 | Direct mixing Solvent: HEPES buffer, dialysis | AFM, TEM, DLS, Zeta potential | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi Adlsadabad, S.; Hanrahan, J.W.; Kakkar, A. mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1739. https://doi.org/10.3390/ijms25031739
Yousefi Adlsadabad S, Hanrahan JW, Kakkar A. mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles. International Journal of Molecular Sciences. 2024; 25(3):1739. https://doi.org/10.3390/ijms25031739
Chicago/Turabian StyleYousefi Adlsadabad, Samaneh, John W. Hanrahan, and Ashok Kakkar. 2024. "mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles" International Journal of Molecular Sciences 25, no. 3: 1739. https://doi.org/10.3390/ijms25031739
APA StyleYousefi Adlsadabad, S., Hanrahan, J. W., & Kakkar, A. (2024). mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles. International Journal of Molecular Sciences, 25(3), 1739. https://doi.org/10.3390/ijms25031739







